Abstract
Background
Most implants for total knee arthroplasty (TKA) are comprised of alloys that contain nickel. Controversy exists whether metal allergies produce negative effects and affect clinical outcomes. The purpose of this study was to retrospectively review a minimum 2-year follow-up with an ion-bombarded titanium TKA implant in patients with reported metal sensitivity.
Methods
A retrospective review of patients who underwent primary TKA with the ion-bombarded titanium Vanguard (Zimmer Biomet, Warsaw, IN) implant with 2-year minimum follow-up was performed from 2008 through 2017. The query revealed 346 patients (451 knees) with minimum 2-year follow-up. The mean age was 64.7 years, the mean body mass index was 35.1 kg/m2, and 95% of patients were women.
Results
The mean follow-up was 4.6 years. The mean range of motion improved from 109° to 112° (P = .03), University of California Los Angeles activity scale from 4.1 to 5.1 (P < .001), Knee Society Clinical scores from 36 to 89 (P < .001), and Knee Society Functional scores from 48 to 73 (P < .001). There were 5 (1.1%) revisions: 2 infections (2-staged exchange), 1 tibial revision for aseptic loosening after a fall, and 2 bearing exchanges for instability. Other surgeries were open reduction internal fixation of periprosthetic fracture, 1 arthroscopic release of snapping popliteus, and 4 local wound incision and debridement (2 superficial infections and 2 nonhealing wounds). Manipulation under anesthesia was required in 27 (6%) patients.
Conclusions
These early results are encouraging for the use of alternative metal titanium alloy implants in metal-sensitive patients undergoing primary TKA. At 4.6 years of mean follow-up, patients had substantial improvement in the range of motion and clinical outcomes with a low frequency of revision.
Keywords: Total knee arthroplasty, Metal allergy, Titanium, Nickel
Introduction
Total knee arthroplasty (TKA) remains a successful surgical treatment for patients with end-stage osteoarthritis, but despite advances in the procedure, there still remain approximately 20% of patients with unsatisfactory results [1]. The cause of patient dissatisfaction is multifactorial. In an otherwise well-aligned, balanced knee, some have attributed worse outcomes to potential implant metal allergies [1]. The first documented case of metal sensitivity was reported in 1966, and to date, there is still debatable correlation as to whether metal sensitivities lead to increased implant failure [2]. The prevalence of metal sensitivity in the general population is 6%-15% [[3], [4], [5]] and higher in patients who had undergone joint replacement surgery than other orthopaedic surgeries such as fracture cases [6].
The patch test is the most frequently used method to diagnose metal hypersensitivity reactions but faces significant controversy because of its specificity and sensitivity [7], and questions arise regarding the correlation of dermatologic metal sensitivity and deep peri-implant sensitivity [[8], [9], [10], [11]]. Many in vivo tests such as lymphocyte transformation test (LTT), modified lymphocyte stimulation test, and leukocyte migration inhibition test (LMIT) have been developed, which have improved sensitivity but are timely and expensive and yield false-positive results [12]. To add to this dilemma, Yang et al. [13] demonstrated the LTT not to correlate with prerevision or postrevision functional scoring or even histopathologic assessments taken intraoperatively.
Prior studies have shown no correlation between metal sensitivity and dermatologic or orthopaedic complications despite implantation of devices to which the patients reported sensitivity [[14], [15], [16]]. However, alternative bearing options have been developed to address the potential concern of metal allergy. The most common metal-bearing alternatives are Oxinium (Smith and Nephew, Memphis, TN) and titanium alloys. The purpose of this study is to evaluate the short-term outcomes after primary TKA with an ion-impregnated titanium implant in patients reporting a metal allergy. We hypothesized that outcomes in this group of patients would be similar to those reported in prior TKA studies.
Material and methods
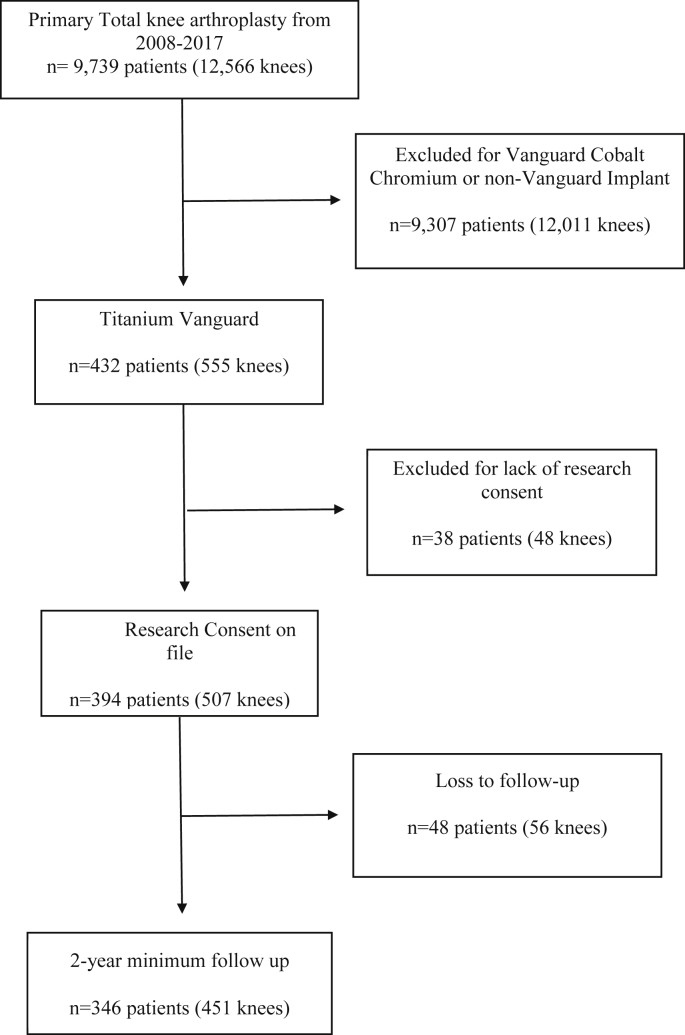
We performed a retrospective review of our institutional arthroplasty registry of all patients with a self-reported metal allergy who underwent primary TKA with the alternative bearing Vanguard (Zimmer Biomet, Warsaw, IN) ion-impregnated titanium implant from 2008 through 2017. The inclusion criterion was end-stage osteoarthritis, post-traumatic arthritis, or inflammatory arthropathy of the knee along with patient-reported metal allergy. The flow sheet for subject selection is shown in Figure 1. This resultant cohort consisted of 346 consented patients (451 knees) with a minimum 2-year follow-up or revision.
Figure 1.
Flowchart for subject enrollment.
Demographics, including gender, age, height, weight, body mass index, and length of follow-up, were recorded. Ninety-five percent of patients were female with a mean age of 65 years (range, 36-89 years) and a mean body mass index of 36 kg/m2 (range, 16-64 kg/m2). Surgical reports and clinic visits were reviewed for implant data, range of motion (ROM), Knee Society Clinical (KSC) Score, Knee Society Functional (KSF) Score, and Knee Society Pain (KSP) Score, University of California Los Angeles activity score, complications, and revisions. Follow-up was performed at 6 weeks, 1 year, and annually thereafter. All outcome data represent the most recent follow-up. Patients who were lost to follow-up were called a minimum of 2 times, referring and primary care physicians contacted, as well as online death index lists and obituaries queried for patient deaths.
All surgeries were performed by one of 4 fellowship-trained adult reconstruction surgeons. The Vanguard complete knee system (Zimmer Biomet, Warsaw, IN) with the alternative bearing ion-bombarded titanium implant was used in all cases. Measured resection technique with cement fixation was used in all surgeries. Cruciate-retaining inserts were used in 58% of cases, anterior-stabilized inserts in 40% of cases, and posterior substituting in 2% of cases. The choice of insert was made at the surgeon’s discretion based on the preferred surgical technique and balancing. All patients were prescribed physical therapy 3 times per week for 6 weeks postoperatively. The institution did not have a standardized protocol for metal allergy testing.
All patients signed a general research consent, approved and monitored by an independent institutional review board (Western IRB, Puyallup, Washington), which allows inclusion in retrospective reviews.
Statistical analysis
Statistical analysis was performed using Microsoft Excel (Microsoft Corporation, Redmond, Washington) and MedCalc Statistical Software, version 18.6 (MedCalc Software bvba, Ostend, Belgium). Paired t-test was used for statistical analysis of demographic differences and outcome measures between groups. The Kaplan-Meier survival analysis was performed, with failure being defined as revision of any component. Patients with minimum 2-year follow-up and those with any revision regardless of time of revision were included in survivorship analysis.
Results
The mean follow-up was 4.6 years (range, 2 to 11 years, SD ± 2). The ROM improved from 109° (range, 20°-135°, SD ± 15) to 112° (range, 6°-135°, SD ± 13) (P = .03). Forty-six percent of patients had improvement in ROM, 16% had no change, and 38% had worse postoperative ROM. The University of California Los Angeles activity scale improved from 4.1 (range, 2-10, SD ± 1.5) to 5.1 (range, 1-10, SD ± 2) (P < .001). KSP scores improved from 4.5 (range, 0-50, SD ± 8.5) to 42.9 (range, 0 to 50, SD ± 14) (P < .001), KSC scores improved from 36 (range, 8-95, SD ± 145) to 89 (range, 32°-100°, SD ± 15) P < .001), and KSF scores improved from 48 (range, 0-95, SD ± 145) to 73 (range, 0-100, SD ± 28) (P < .001).
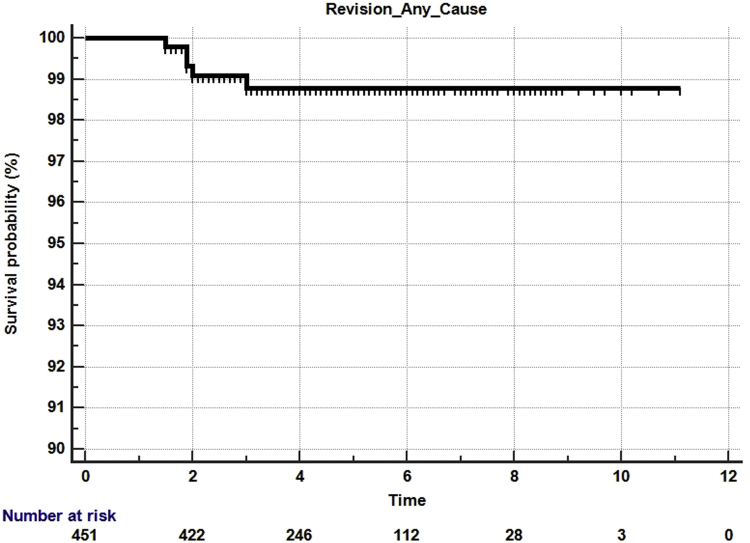
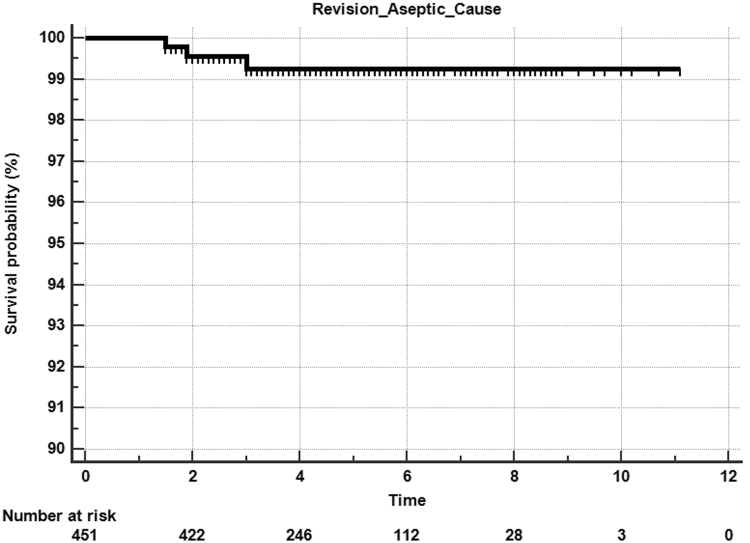
There was a 6% manipulation rate, with 3 patients requiring a second manipulation for residual knee stiffness. Revisions were performed in 5 (1.1%) knees, with 2 patients requiring a 2-stage revision for infection, 2 patients requiring a poly insert change due to instability, and one patient sustaining aseptic loosening of the tibia after a traumatic fall. Other subsequent surgeries included the following: 1 open reduction internal fixation of periprosthetic fracture, 1 arthroscopic release of snapping popliteus, and 4 superficial wound incision and debridement (2 superficial infections and 2 wound dehiscence). The Kaplan-Meier 5-year all-cause survival was 99% (95% confidence interval, 93.%-100%) (Fig. 2), and 5-year aseptic survival was 99.3% (95% confidence interval, 94%-100%) (Fig. 3).
Figure 2.
Kaplan-Meier survival curve for all-cause survivorship.
Figure 3.
Kaplan-Meier survival curve for aseptic survivorship.
Discussion
Metal sensitivity remains a controversial topic among joint replacement surgeons. Currently, there is no universally accepted implant allergy test that accurately predicts poor outcomes or early implant failure [16]. Skin patch tests are the most commonly used method to test for metal sensitivities. Benefits of the patch test are the ability to perform large-scale screenings of multiple immunogenic substances [11,17], they are also quick and inexpensive [16]. Patch testing has shown specificity to be 71% but sensitivity to be only 77% in some studies so that negative skin patch test results are much more beneficial [7,16]. Routine skin patch testing is not recommended [18]. Alternative tests include the LTT or the LIF test. One study has shown swelling, pain, and dermatologic reactions are most closely associated with a positive LIF test [19]. Contrary to skin testing, the LIF test will turn negative if the offending substance is removed, whereas the skin patch testing will remain positive [11,18].
To add to this confusion, Rooker and Wilkinson [19] have reported a certain level of metal tolerance over time. These authors noted that from the 6 patients who tested positive for metal sensitivity in their cohort preoperatively, 5 of the 6 tested negative postoperatively after receiving an implant that contained metal compounds to which the patients were allergic. Another report in 2013 reviews a case in which a single patient with documented cobalt-chrome-nickel allergy received bilateral TKAs, one with a hypoallergenic titanium-niobium implant and the other with a standard cobalt-chromium implant. At 2-year follow-up, the knee with the standard cobalt-chrome implant demonstrated no signs of radiographic loosening, a Knee Society Score of 98, and no signs of atopic dermatitis [16].
Alternate bearing options are growing. Two of the most common alternate bearing options are Oxinium and titanium alloys. Oxinium is an oxidized zirconium that has the benefits from the improved wear characteristics of ceramics while maintaining the mechanical resiliency of metal alloys [20]. It is noted to have less than 0.0035% nickel and is lighter and stronger than cobalt-chrome alloys. In 2010, Innocenti et al. [21] reported 5-year minimum follow-up with an Oxinium knee and found a mean KSC score of 89 and KSF score of 86. The ROM in this study improved from 92° to 118. A 10-year minimum follow-up study by the same group demonstrated a mean KSC score of 86 and KSF score of 83 at 10-year follow-up. The 10-year aseptic survivorship was 97.8% [22]. While biomechanical testing demonstrates Oxinium total knee replacements to have improved wear characteristics, 12-year registry data have failed to show a difference in revision rates or improved survivability over the cobalt-chrome counterpart [23].
Titanium alloys, specifically ion-impregnated titanium alloys, are noted to have improved wear rates, have resistance to corrosion, and have mechanical properties that make them harder than regular titanium alone [24,25]. Ion implantation is performed by a graded energy technique that produces approximately 20% atomic nitrogen ion concentration at a thickness of 1000 angstroms. This process allows for a harder and more scratch-resistant metal and gives the material a resistance to corrosion while not containing nickel, which is the most common metal associated with sensitivity [26]. This becomes important when reviewing previous articles demonstrating that even a single scratch to the metal components may accelerate polyethylene wear exponentially [27]. Biomechanical testing of ion-implanted titanium implants demonstrates a 98% reduction in wear when compared with their cobalt-chrome counterparts [28]. Survivorship of this alternate bearing implant remains high with over 95% survivorship at 10 years, for any reason, and similar KSF scores, ROM, and postoperative pain as traditional implants [29]. The present study demonstrates 4.6-year mean follow-up with substantial improvement in the ROM and clinical outcomes and a very low incidence of all-cause and aseptic revision.
A benefit of the implant used in this study is that it has the same design and uses the same instrumentation as its cobalt-chromium version. This is important for surgeons who are familiar with this implant system as they would not need to change to a product for which they may have limited experience. The authors have previously published on the long-term outcomes of the cobalt-chromium version of the Vanguard Knee [30]. At a mean of 11.9-year follow-up, there was an average improvement of 3.9° of ROM, KSC Score improvement of 48 points, KSF Score improvement of 15.1 points, and KSP Score improvement of 35.8 points. Manipulations were performed in 7.6% of knees, and the 10-year aseptic survival was 96.4%. Although the present study evaluating the titanium version of the Vanguard was not a direct comparison to the cobalt-chromium version, the results are encouraging when viewed to historical data.
This study has several limitations. Our study may be limited by selection bias because we relied on self-reported metal allergy without confirmatory testing to guide our decision to use this implant. However, determining whether a patient has a true metal allergy is clinically difficult because of a lack of standardized tests and the absence of a universally accepted metal allergy testing algorithm. The retrospective analysis may also underestimate the number of revisions or complications in this population as patients may have had procedures performed at other institutions. Another limitation was that there was the lack of a comparison group in this present study of patients with self-reported metal allergy receiving cobalt-chromium implants.
Conclusion
Although further long-term follow-up is needed, these early to midterm results show that this ion-implanted titanium implant demonstrated encouraging results and is a potential implant option for patients with self-reported metal sensitivity.
Conflict of interest
Direct funding for the study was provided by Zimmer Biomet (Warsaw, IN); K.R. Berend receives royalties from Zimmer Biomet, is a paid consultant for Zimmer Biomet, owns minority interest in SPR Therapeutics, ElutiBone, and Joint Development Corporation, receives research support from Zimmer Biomet and SPR Therapeutics, is a member of the editorial/governing board of the Journal of Arthroplasty; Journal of Bone and Joint Surgery–American; Clinical Orthopaedics and Related Research; Orthopedics; and Reconstructive Review, and is a board member/part of the committee appointments for The Knee Society; D.A. Crawford is a paid consultant for Ossio Ltd., is an unpaid consultant for SPR Therapeutics, receives research support from KCI USA, Inc., and is a member of the editorial/governing board of the American Journal of Sports Medicine; A.V. Lombardi receives royalties from Zimmer Biomet, is a paid consultant for Zimmer Biomet, owns minority interest in SPR Therapeutics, ElutiBone, and Joint Development Corporation, receives research support from Zimmer Biomet and SPR Therapeutics, is a member of the editorial/governing board of the Journal of Arthroplasty, Journal of Bone and Joint Surgery – American; Journal of the American Academy of Orthopaedic Surgeons; Journal of Orthopaedics and Traumatology; Surgical Technology International; The Knee; and Clinical Orthopaedics and Related Research, and is a board member/part of the committee appointments for Operation Walk USA, The Hip Society, The Knee Society; Mount Carmel Education Foundation at New Albany; J.M. Hurst receives royalties from Total Joint Orthopedics, is a paid consultant for Total Joint Orthopedics and Zimmer Biomet, and receives research support from Zimmer Biomet and SPR Therapeutics; M.J. Morris receives royalties from Total Joint Orthopedics, is a paid consultant for Total Joint Orthopedics and Zimmer Biomet, and receives research support from Zimmer Biomet and SPR Therapeutics; and J.I. Law has declares no potential conflicts of interest.
Appendix A. Supplementary data
References
- 1.Bourne R.B., Chesworth B.M., Davis A.M., Mahomed N.N., Charron K.D. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57. doi: 10.1007/s11999-009-1119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foussereau J., Laugier P. Allergic eczemas from metallic foreign bodies. Trans St Johns Hosp Dermatol Soc. 1966;52:220. [PubMed] [Google Scholar]
- 3.Basko-Plluska J.L., Thyssen J.P., Schalock P.C. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22(2):65. [PubMed] [Google Scholar]
- 4.Bergschmidt P., Bader R., Mittelmeier W. Metal hypersensitivity in total knee arthroplasty: revision surgery using a ceramic femoral component: a case report. Knee. 2012;19(2):144. doi: 10.1016/j.knee.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Caicedo M.S., Desai R., McAllister K., Reddy A., Jacobs J.J., Hallab N.J. Soluble and particulate Co-Cr-Mo alloy implant metals activate the inflammasone danger signaling pathway in human macrophages: a novel mechanism for implant debris reactivity. J Orthop Res. 2009;27:847. doi: 10.1002/jor.20826. [DOI] [PubMed] [Google Scholar]
- 6.Granchi C., Cenni E., Giunti A., Baldini N. Metal hypersensitivity testing in patients undergoing joint replacement: a systematic review. J Bone Joint Surg Br. 2012;94-B:1126. doi: 10.1302/0301-620X.94B8.28135. [DOI] [PubMed] [Google Scholar]
- 7.Akil S., Newman J.M., Shah N.V., Ahmed N., Deshmukh A.J., Maheshwari A.V. Metal hypersensitivity in total hip and knee arthroplasty: current concepts. J Clin Orthop Trauma. 2018;9:3. doi: 10.1016/j.jcot.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beecker J., Gordon J., Pratt M. An interesting case of joint prosthesis allergy. Dermatitis. 2009;20:4. [PubMed] [Google Scholar]
- 9.Frigerio E., Pigatto P.D., Guzzi G., Altomare G. Metal sensitivity in patients with orthopedic implants: a prospective study. Contact Dermat. 2011;64:273. doi: 10.1111/j.1600-0536.2011.01886.x. [DOI] [PubMed] [Google Scholar]
- 10.Granchi D., Cenni E., Tigani D., Trisolino G., Baldini N., Giunti A. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29:1494. doi: 10.1016/j.biomaterials.2007.11.038. [DOI] [PubMed] [Google Scholar]
- 11.Schalock P.C., Menne ´ T., Johansen J.D. Hypersensitivity reactions to metallic implants-diagnostic algorithm and suggested patch test series for clinical use. Contact Dermat. 2012;66:4. doi: 10.1111/j.1600-0536.2011.01971.x. [DOI] [PubMed] [Google Scholar]
- 12.Saccomanno M., Sircana G., Masci G. Allergy in total knee replacement surgery: is it a real problem? World J Orthop. 2019;10(2):63. doi: 10.5312/wjo.v10.i2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang S., Dipane M., Lu C., Schmalzried T.P., McPherson E.J. Lymphocyte transformation testing (LTT) in cases of pain following total knee arthroplasty: little relationship to histopathologic findings and revision outcomes. J Bone Joint Surg Am. 2019;101(3):257. doi: 10.2106/JBJS.18.00134. [DOI] [PubMed] [Google Scholar]
- 14.Carlsson A., Moller H. Implantation of orthopaedic devices in patients with metal allergy. Acta Derm Venereol. 1989;69(1):62. [PubMed] [Google Scholar]
- 15.Webley M., Kates A., Snaith M.L. Metal sensitivity in patients with a hinge arthroplasty of the knee. Ann Rheum Dis. 1978;37(4):373. doi: 10.1136/ard.37.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thienpont E., Berger Y. No allergic reaction after TKA in a cobalt – chrome nickel sensitive patient: case report and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):636. doi: 10.1007/s00167-012-2000-z. [DOI] [PubMed] [Google Scholar]
- 17.Niki Y., Matsumoto H., Otani T. Screening for symptomatic metal sensitivity: a prospective study of 92 patients undergoing total knee arthroplasty. Biomaterials. 2005;26(9):1019. doi: 10.1016/j.biomaterials.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 18.Thyssen J.P., Menne ´ T., Schalock P.C., Taylor J.S., Maibach H.I. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164(3):473. doi: 10.1111/j.1365-2133.2010.10144.x. [DOI] [PubMed] [Google Scholar]
- 19.Rooker G.D., Wilkinson J.D. Metal sensitivity in patients undergoing hip replacement. A prospective study. J Bone Joint Surg Br. 1980;62-B(4):502. doi: 10.1302/0301-620X.62B4.7430234. [DOI] [PubMed] [Google Scholar]
- 20.Hunter G., Jones W., Spector M. Springer; Berling, Heidelberg: 2005. Oxidized zirconium. Total knee arthroplasty; p. 370. [Google Scholar]
- 21.Innocenti M., Civinini R., Carulli C., Matassi F., Villano M. The 5-year results of an oxidized zirconium femoral component for TKA. Clin Orthop Relat Res. 2010;468(5):1258. doi: 10.1007/s11999-009-1109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Innocenti M., Matassi F., Carulli C., Nistri L., Civinini R. Oxidized zirconium femoral component for TKA: a follow-up note of a previous report at a minimum of 10 years. Knee. 2014;21(4):858. doi: 10.1016/j.knee.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Vertullo C., Lewis P., Graves S., Kelly L., Lorimer M., Myers P. Twelve-year outcomes of an Oxinium total knee replacement compared with the same cobalt-chrome design. J Bone joint Surg Am. 2017;99(4):275. doi: 10.2106/JBJS.16.00092. [DOI] [PubMed] [Google Scholar]
- 24.Williams J., Buchanan R., Rigney E. Proceedings, ASM conference on Applications of ion plating and implantation to materials, Atlanta, Georgia. 1985. Improvement in wear performance of surgical Ti-6A1-4V alloy by ion implantation of nitrogen or carbon. [Google Scholar]
- 25.Sioshansi P., Oliver R., Matthews F. Proceedings, MRS Symposium on Biomedical materials, Boston. 1985. “Wear improvement of surgical titanium alloys by ion implantation. [Google Scholar]
- 26.Buchanan R., Rigney E., Williams J. Wear Accelerated corrosion of Ti-6A1-4V and nitrogen-ion-implantedTi-6Al-4V: mechanisms and influence of fixed-stress magnitude. J Biomed Mater Res. 1987;21(3):367. doi: 10.1002/jbm.820210309. [DOI] [PubMed] [Google Scholar]
- 27.Dowson D., Taheri S., Wallbridge N.C. The role of counterface imperfections in the wear of polyethylene. Wear. 1987;119(3):277. [Google Scholar]
- 28.Pappas M.J., Makris G., Buechel F.F. Titanium nitride ceramic film against polyethylene. A 48 million cycle wear test. Clin Orthop Relat Res. 1995;317:64. [PubMed] [Google Scholar]
- 29.van Hove R.P., Brohet R.M., van Royen B.J., Nolte P.A. No clinical benefit of titanium nitride coating in cementless mobile-bearing total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23(5):1833. doi: 10.1007/s00167-014-3359-9. [DOI] [PubMed] [Google Scholar]
- 30.Crawford D., Adams J., Hurst J., Berend K., Lombardi A. Ten-year minimum outcomes and survivorship with a high flexion knee system. J Arthroplasty. 2019;34(9):1975. doi: 10.1016/j.arth.2019.04.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.