Abstract
Objectives
To determine the reporting quality of published randomised controlled trial (RCT) protocols before and after the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement (2013), and any association with author, trial or journal factors.
Design
Methodological study.
Data sources
MEDLINE, Embase and CENTRAL were electronically searched using optimised search strategies.
Eligibility criteria
Protocols written for an RCT of living humans, published in full text in a peer-reviewed journal and published in the English language.
Main outcome
Primary outcome was the overall proportion of checklist items which were adequately reported in RCT protocols published before and after the SPIRIT statement.
Results
300 RCT protocols were retrieved; 150 from the period immediately before the SPIRIT statement (9 July 2012 to 28 December 2012) and 150 from a recent period after the SPIRIT statement (25 January 2019 to 20 March 2019). 47.9% (95% CI, 46.5% to 49.3%) of checklist items were adequately reported in RCT protocols before the SPIRIT statement and 56.7% (95% CI, 54.9% to 58.5%) after the SPIRIT statement. This represents an 8.8% (95% CI, 6.6% to 11.1%; p<0.0001) mean improvement in the overall proportion of checklist items adequately reported since the SPIRIT statement. While 40% of individual checklist items had a significant improvement in adequate reporting after the SPIRIT statement, 11.3% had a significant deterioration and there were no RCT protocols in which all individual checklist items were complete. The factors associated with higher reporting quality of RCT protocols in multiple regression analysis were author expertise or experience in epidemiology or statistics, multicentre trials, longer protocol word length and publicly reported journal policy of compliance with the SPIRIT statement.
Conclusion
The overall reporting quality of RCT protocols has significantly improved since the SPIRIT statement, although a substantial proportion of individual checklist items remain poorly reported. Continued and concerted efforts are required by journals, editors, reviewers and investigators to improve the completeness and transparency of RCT protocols.
Keywords: statistics & research methods, epidemiology, clinical trials, protocols & guidelines, quality in health care
Strengths and limitations of this study.
We conducted a methodological study in accordance with a prospectively registered protocol (PROSPERO CRD42019126522).
We assessed the reporting quality of two equal, arbitrary samples of 150 randomised controlled trial (RCT) protocols published before and after the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statement.
Data extraction of the first 100 RCT protocols was independently duplicated by two investigators and any issues with data extraction were discussed at fortnightly round-table meetings attended by five investigators.
The design of this study is limited by the lack of blinding of data collectors to the date of publication of RCT protocols and by the inclusion of only RCT protocols published in the English language.
The associations found in this study may not be causal, and the improvements in overall reporting quality may be due to underlying secular trends whereby RCT protocol quality improves over time, unrelated to the introduction of the SPIRIT statement.
Introduction
Background
Randomised controlled trial (RCT) protocols should permit prospective assessment of trial methodology, scientific integrity, ethical standards and safety considerations, public documentation of protocol changes and approved amendments, and retrospective validation of trial conduct and subsequent reporting.1 A well-written RCT protocol is an critical component of a high-quality RCT as it allows comparison between the initial inception, possible amendments and final publication. This supports RCT investigators and sponsors by improving research quality, ethics committees and journals by improving research completeness, and participants and the public by improving research transparency.2
However, studies have frequently reported concerning inconsistencies between RCT protocols and their corresponding publications,3–6 and serious deficiencies in the content of RCT protocols.5–13 Incomplete, inaccurate or undisclosed reporting of RCT protocols can result in research misrepresentation, selective outcome reporting and other biases which undercut the credibility and validity of health research and scientific knowledge.2 The SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statement was published in January 2013 and describes a 33-item minimum set of scientific, methodological, ethical and administrative components that should be routinely included in a trial protocol.1 It aims to address long-standing issues with the completeness and transparency of many trial protocols by providing a standardised structure to trial plans, promoting strict accountability to trial conduct, improving the reliability and validity of trial outcomes, and facilitating the assessment of risk of bias, methodological quality and reporting quality.1
Objectives
The impact of the SPIRIT statement on the reporting quality of RCT protocols in health research is unknown. The primary objectives of this study are to (1) determine the reporting quality of published RCT protocols before and after the SPIRIT statement and (2) determine whether author, trial or journal factors are associated with the reporting quality of published RCT protocols.
Methods
Study design
We conducted a methodological study in accordance with a prospectively registered protocol (PROSPERO CRD42019126522).
Setting
RCT protocols were identified by electronically searching the bibliographic databases MEDLINE, Embase and CENTRAL using a search strategy formulated by an experienced medical librarian (online supplementary appendix A). All searches were performed independently by two investigators on 29 March 2019.
bmjopen-2020-038283supp001.pdf (88.5KB, pdf)
Included protocols
RCT protocols were eligible for inclusion if they were (a) written for an RCT of living humans, (b) published in full text in a peer-reviewed journal and (c) published in the English language. RCT protocols were excluded if they were (a) published only in protocol databases or online registries, or (b) reported any study results.
We screened RCT protocols until we retrieved two equal, arbitrary samples of 150 RCT protocols published before and after the SPIRIT statement. The sample of 150 RCT protocols published immediately before the SPIRIT statement were retrieved by searching for RCT protocols published from 28 December 2012 and proceeding retrospectively until 150 eligible RCT protocols were selected. Similarly, the sample of 150 RCT protocols published recently since the SPIRIT statement were selected by searching for RCT protocols published from 20 March 2019 and proceeding retrospectively until 150 eligible RCT protocols were retrieved. The titles and abstracts of all retrieved RCT protocols were independently screened by two investigators and the full texts of relevant RCT protocols were independently assessed for eligibility by two investigators. Any disagreements were resolved by discussion between the two investigators and, if required, arbitration by a third investigator. All eligible RCT protocols were imported into EndNote X9 (Clarivate Analytics) software. Duplicates were removed by manually screening by author, year, title and journal.
Variables
The primary variables of interest were the checklist items from the SPIRIT statement, defined in the SPIRIT statement explanation and elaboration.1 A data extraction form was developed based on the checklist items from the SPIRIT statement. Two checklist items (items 4 and 12) were subcategorised to reflect binary criterion and provide appropriate granularity. The checklist item ‘funding’ was split into ‘funding source’, defined as sources of financial, material and other support (eg, name and location of the funder), and ‘funding type’, defined as type of financial, material and other support (eg, funds, equipment, drugs and services). The checklist item ‘outcomes’ was split into ‘primary, secondary and other outcomes’ (eg, the specific measurement variable, analysis metric, method of aggregation and time point for each outcome), and ‘explanation of clinical relevance of chosen efficacy and harm outcomes’. This resulted in a total of 53 individual checklist items. Each checklist item was assessed as either adequate or inadequate/unclear. The data extraction form and assessment criteria were independently piloted for ten randomly selected RCT protocols by five investigators. Disagreements were resolved by fortnightly round-table meetings attended by five investigators and the definitions of adequate and inadequate/unclear for each checklist item were revised accordingly.
The secondary variables of interest related to author, trial and journal factors. Author factors included the number of authors per protocol and the presence of authors with expertise or experience in epidemiology or statistics (defined as one or more authors with either a degree in clinical epidemiology, public health or biostatistics, or an affiliation to a clinical epidemiology, public health or biostatistics department14 15). Trial factors included the total planned sample size, centre status (ie, multicentre or single centre), protocol word length (ie, greater or less than 3500 words) and funding source (ie, industry or non-industry funding). Protocol report of compliance with the SPIRIT statement and publicly reported journal policy of compliance with the SPIRIT statement in the instructions to authors on the journal website, as of 2019, was also collected for RCT protocols published after the SPIRIT statement.
Data measurement
Data extraction was performed on the 300 RCT protocols. Data extraction of the first 100 RCT protocols was independently duplicated by two investigators and data extraction of the remaining 200 RCT protocols was completed once between two investigators. Any issues with data extraction were discussed at fortnightly round-table meetings attended by five investigators.
Statistical methods
The final data points used for analysis were the results of the duplicate data collection and discussion of disagreements. We performed descriptive analysis of the primary outcome by calculating the proportion (percentage) of checklist items which were adequately reported in RCT protocols. This was considered a measure of the overall reporting quality of RCT protocols. We also calculated the proportion (percentage) of RCT protocols which adequately reported each checklist item. Inter-rater agreement and kappa scores were calculated on the initial data points extracted by independent duplicate data collection (ie, before discussion of disagreements) of the first 100 RCT protocols. We performed exploratory multiple linear regression analysis to determine whether author, trial or journal factors were associated with the reporting quality of RCT protocols. Stepwise backward linear regression was performed, using p<0.25 as the criterion for inclusion in a multiple regression model, and R2 as the criterion for removal of variables in the backward elimination model. A p value <0.05 was considered statistically significant, and the R2 value was used as a measure of the final model goodness of fit. All statistical analyses were stratified by publication before or after the SPIRIT statement and were performed using Stata software (StataCorp 2019, Stata Statistical Software: Release 16, College Station, Texas, USA, StataCorp LP).
Patient and public involvement
As this was a study of RCT protocols, there was no patient or public involvement in the conception, design or conduct of the study, or the writing or editing of this paper.
Results
Included protocols
A total of 300 RCT protocols were retrieved; 150 from before the SPIRIT statement (9 July 2012 to 28 December 2012) and 150 from after the SPIRIT statement (25 January 2019 to 20 March 2019). In the full-text eligibility assessment of RCT protocols published before the SPIRIT statement, 25 articles were excluded because they did not describe an RCT protocol, two because they had been retracted, one because it included study results, and one because it was not published in full-text. In the full-text eligibility assessment of RCT protocols published after the SPIRIT statement, six articles were excluded because they did not describe an RCT protocol. All excluded articles were replaced with eligible studies. The final 300 RCT protocols were published across 45 peer-reviewed journals, with 46% (138/300) published in Trials.
The inter-rater agreement for data extraction of the first 100 RCT protocols ranged from 64.5% to 100%, with kappa scores provided in online supplementary appendix B. The individual checklist items with the lowest and highest inter-rater agreement were ‘statistical methods: statistical methods to handle missing data’ and ‘background and rationale: explanation for choice of comparators’, respectively. The checklist items with the lowest and highest Kappa scores were ‘research ethics approval’ and ‘background and rationale: explanation for choice of comparators’, respectively.
Descriptive data
Author and trial characteristics were similar before and after the SPIRIT statement (table 1).
Table 1.
Author and trial characteristics before and after the SPIRIT statement
| Before the SPIRIT statement | After the SPIRIT statement | |
| Author characteristics | ||
| Authors per protocol (median, range) | 8, 1 to 90 | 8, 2 to 80 |
| One or more authors with expertise or experience in epidemiology or statistics (n, %) | 50, 33.3% | 48, 32% |
| Trial characteristics | ||
| Total planned sample size (median) | 214.5 | 200 |
| Multicentre status (n, %) | 70, 46.7% | 64, 42.7% |
| Protocol word length >3500 (n, %) | 105, 70% | 106, 70.7% |
| Industry funding (n, %) | 8, 6% | 10, 7% |
SPIRIT, Standard Protocol Items: Recommendations for Interventional Trials.
Of RCT protocols published after the SPIRIT statement, 42.7% (64/150) self-reported compliance with the SPIRIT statement, and 88% (132/150) were published in a peer-reviewed journal with a publicly reported policy of compliance with the SPIRIT statement. Additionally, only 17/300 (6%) of RCT protocols were published in journals which published in print, while the remainder (94%) were published in journals which published exclusively online. The mean word count of RCT protocols published in online journals and print journals was 4387 words and 3581 words, respectively, with an 806 word difference in mean word count (95% CI, 26 to 1586 words, p=0.04).
Outcome data
Of the 150 RCT protocols published before the SPIRIT statement, an average of 47.9% of checklist items per RCT protocol were adequately reported (95% CI, 46.5% to 49.3%). Comparably, of the 150 RCT protocols published after the SPIRIT statement, an average of 56.7% of checklist items were adequately reported (95% CI, 54.9% to 58.5%). This represents an 8.8% (95% CI, 6.6% to 11.1%; p<0.0001) mean improvement in the overall proportion of checklist items adequately reported since the SPIRIT statement.
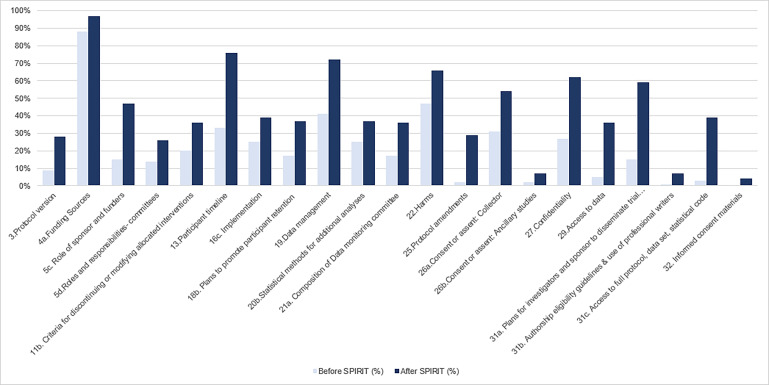
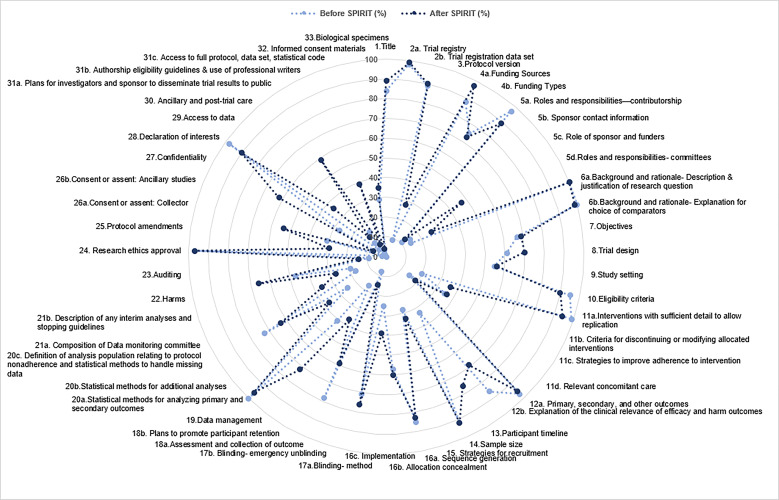
Of the 53 individual checklist items, 21 (40%) had a significant increase (p<0.05) in adequate reporting since the SPIRIT statement (figure 1) and 6 (11.3%) had a significant decrease (p<0.05) in adequate reporting since the SPIRIT statement (online supplementary appendix C). Twenty-three individual checklist items were inadequately or not reported in more than half of all RCT protocols (figure 2). Only one checklist item was adequately reported in all 300 RCT protocols—‘background and rationale: description and justification of research question’. None of the 300 RCT protocols adequately reported all individual checklist items from the SPIRIT statement and no individual checklist items were inadequately or not reported in all 300 RCT protocols.
Figure 1.
Checklist items with a significant increase in adequate reporting after the SPIRIT statement. SPIRIT, Standard Protocol Items: Recommendations for Interventional Trials.
Figure 2.
Completeness of randomised controlled trial protocols by checklist items, before and after the SPIRIT statement. SPIRIT, Standard Protocol Items: Recommendations for Interventional Trials.
Table 2 shows the multiple regression analysis of the association between author, trial and journal factors and the reporting quality of RCT protocols. The final model had an adjusted R2 value of 0.37, indicating that 37% of the variability in SPIRIT score was explained in our model. Author self-reported compliance with the SPIRIT statement was not associated with actual compliance with the SPIRIT statement. However, publicly reported journal policy of compliance with the SPIRIT statement was associated with significantly improved reporting quality. Industry funding was not associated with compliance with the SPIRIT statement, with only a 0.3% (95% CI, −4.9% to –5.6%, p=0.9) difference in mean SPIRIT scores between industry-funded and non-industry-funded trials. Similarly, publication type (either print or exclusively online) was not associated with compliance with the SPIRIT statement (p=0.29). As such, industry funding and publication type were not included in the regression analysis as our preplanned regression modelling limited the inclusion of variables to only those with potential statistical influence.
Table 2.
Multiple regression analysis of author, trial and journal characteristics associated with the reporting quality of RCT protocols
| Increase in proportion of adequately reported checklist items from the SPIRIT statement | P value | |
| Author characteristics | ||
| Number of authors per protocol | 0.2% | 0.004 |
| One or more authors with expertise or experience in epidemiology or statistics | 2.6% | 0.016 |
| Trial characteristics | ||
| Multicentre status | 4.6% | 0.000 |
| Protocol word length >3500 | 6.5% | 0.000 |
| Protocols self-reporting compliance with the SPIRIT statement | – | 0.145 |
| Journal characteristics | ||
| Journal policy of compliance with the SPIRIT statement | 6.2% | 0.000 |
RCT, randomised controlled trial; SPIRIT, Standard Protocol Items: Recommendations for Interventional Trials.
Discussion
Key results
We assessed the reporting quality of published RCT protocols before and after the SPIRIT statement. We found a significant improvement in the completeness of RCT protocols published since the SPIRIT statement. Although our study suggests significant improvements in the overall reporting quality of RCT protocols published after the SPIRIT statement, these significant improvements were only seen in 40% (21/53) of individual checklist items, and there were no RCT protocols in which all individual checklist items were complete.
Limitations
Our study is limited by the lack of blinding of data collectors to the date of publication of RCT protocols, introducing the possibility for researcher bias. This was minimised through strict adherence to predefined parameters for the assessment of the checklist items from the SPIRIT statement, fortnightly round-table meetings and duplication of data collection for one-third of RCT protocols. Our study was also limited by the inclusion of only RCT protocols published in the English language.
The associations found in this study may not be causal, and the improvements in overall reporting quality may be due to underlying secular trends whereby RCT protocol quality improves over time, unrelated to the introduction of the SPIRIT statement. However, the association between specific journal requirement for the SPIRIT statement, and reporting to that requirement, suggests some degree of causation. Additionally, while none of the 300 RCT protocols adequately reported all individual checklist items from the SPIRIT statement, some checklist items may not be relevant to all RCT protocols and thus the level of under-reporting observed here may be a slight overestimate.
Interpretation
Despite the significant improvement in the overall reporting quality of RCT protocols suggested by our study, three individual checklist items from the SPIRIT statement were inadequately or not reported by more than 90% of RCT protocols: ‘consent or assent: ancillary studies’, ‘dissemination policy: authorship eligibility guidelines and any intended use of professional writers’ and ‘informed consent materials’.
The low completeness of checklist item ‘consent or assent: ancillary studies’ may be related to a misperception by authors that it is not necessary to report the decision that participant data or biological specimens will not be used in ancillary studies. However, deciding and reporting on the provisions of additional consent for ancillary studies is important, particularly given the increasing emphasis on data sharing plans. A similar sentiment may explain the low completeness of checklist item ‘informed consent materials: model consent form and other related documentation given to participants and authorised surrogates’, as authors may consider it sufficient to describe a plan to obtain informed consent and not necessary to provide the model consent form. However, providing the model consent form is important in determining that the relevant information is delivered with sufficient detail at an appropriate literacy level for the target population. Additionally, the low completeness of checklist item ‘dissemination policy: authorship eligibility guidelines and any intended use of professional writers’ may be underpinned by an underappreciation of the importance of disclosing the use of professional writers. A study of industry-initiated RCTs reported that 91% of 44 RCT protocols had evidence of ghost authorship.12
The factors associated with higher reporting quality of RCT protocols in multiple regression analysis were one or more authors with expertise or experience in epidemiology or statistics, multicentre trials, longer protocol word length and publicly reported journal policy of compliance with the SPIRIT statement. The association between author expertise or experience in epidemiology or statistics, and higher reporting quality has previously been reported16 and may be related to education in the importance of transparency and experience in writing RCT protocols. In a similar way, the association between multicentre trials and higher reporting quality may be explained by the larger nature of these studies and, by extension, the greater level of support available to these studies for writing the protocol and greater importance of transparently and completely reporting the protocol. Additionally, the association between longer protocol word lengths and higher reporting quality may be underpinned by the capacity to more completely describe a planned RCT with more permitted words. This would support a more discretionary, individualised approach to determining appropriate word lengths of RCT protocols, rather than arbitrary, blanket cut-offs.
Interestingly, protocol report of compliance with the SPIRIT statement was not a significant predictor of reporting quality after adjusting for publicly reported journal policy of compliance with the SPIRIT statement. A possible explanation for this finding is that some authors who are aware of either the SPIRIT statement or the journal’s policy of compliance with the SPIRIT statement may decide to self-report compliance with the SPIRIT statement without actually applying the checklist. This suggests that author self-report of compliance with the SPIRIT statement cannot be relied on as a proxy indicator of reporting quality as awareness of the SPIRIT statement does not translate into application of the checklist. Rather, the association between publicly reported journal policy of compliance with the SPIRIT statement and higher reporting quality supports the role of journals and editors in checking adherence to the SPIRIT statement to improve the completeness and transparency of RCT protocols. Some possible aids for journals and editors checking adherence to the SPIRIT statement include mandated author completed presubmission checklists, structured online manuscript submission systems and automated manuscript reporting quality checks. Other avenues include incorporating the SPIRIT statement into the mandatory fields required by clinical trial registries (eg, ClinicalTrials.gov, ANZCTR and ISRCTN). This is particularly relevant, given many trials may be registered but do not have published protocols.
The findings from our research expand on those of Gao et al (2016), who assessed the reporting quality of 142 RCT protocols in acupuncture using the checklist items from the SPIRIT statement.17 However, we found a substantially larger number of checklist items whose completeness significantly improved after the SPIRIT statement (5 in Gao et al (2016) and 21 in our study).17 This difference may be explained by the time since the SPIRIT statement; while Gao et al (2016) assessed RCT protocols published 1 to 2 years after the SPIRIT statement, our study assessed RCT protocols published 6 to 7 years after the SPIRIT statement. This could suggest increasing awareness and adoption of the SPIRIT statement over time. More recently, Yang et al (2018) assessed the reporting quality of 126 trial protocols in anaesthesia against the SPIRIT statement, and found no significant improvement in the completeness of trial protocols published after the SPIRIT statement and substantially more checklist items which were inadequately or not reported by more than 90% of included trial protocols (18 by Yang et al (2018) and 3 in our study). However, their findings were limited by the small sample size of 18 trial protocols from after the SPIRIT statement.18
Overall, there remains substantial opportunity for further improvement. A study of emergency medicine journals found that reporting guidelines, including the SPIRIT statement, were infrequently endorsed,19 and a scoping review of systematic reviews of adherence to other reporting guidelines reported insufficient adherence.20 These findings suggest that the challenges to improving adherence to the SPIRIT statement are shared with other reporting guidelines. The focus should be on increasing the awareness of the SPIRIT statement throughout the research community, particularly among trial investigators, and promoting the adoption of the SPIRIT statement in the editorial community, specifically by advocating for mandated adherence to reporting guidelines. Improving the reporting quality of RCT protocols is necessary to improve the completeness and transparency of RCTs, and, by extension, the validity and reliability of RCT outcomes which ultimately contribute to informing patient care. It is likely that continued and concerted efforts by journals, editors, reviewers and investigators to advocate for adherence to the SPIRIT statement would improve the completeness and transparency of RCT protocols.
Supplementary Material
Footnotes
Twitter: @mabpinheiro
Contributors: The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. ZWT contributed to curating the data, analysed the data, administrated the project and contributed to reviewing the manuscript. ACT drafted the original manuscript and edited the reviewed manuscript. MS and JvT contributed to curating the data and reviewing the manuscript. TL, IH, JMN, MP, LH and KC contributed to reviewing the manuscript. SA conceptualised the study, designed the methodology and contributed to reviewing the manuscript. All authors met the ICMJE (International Committee of Medical Journal Editors) criteria for authorship and contributed to the revision of the manuscript. ZWT is the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: All authors have completed the ICMJE (International Committee of Medical Journal Editors) uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer-reviewed.
Data availability statement: No data are available.
References
- 1.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. . Spirit 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan A-W, Upshur R, Singh JA, et al. . Research protocols: waiving confidentiality for the greater good. BMJ 2006;332:1086–9. 10.1136/bmj.332.7549.1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan A-W, Hróbjartsson A, Haahr MT, et al. . Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291:2457–65. 10.1001/jama.291.20.2457 [DOI] [PubMed] [Google Scholar]
- 4.Dwan K, Altman DG, Arnaiz JA, et al. . Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLoS One 2008;3:e3081. 10.1371/journal.pone.0003081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernández AV, Steyerberg EW, Taylor GS, et al. . Subgroup analysis and covariate adjustment in randomized clinical trials of traumatic brain injury: a systematic review. Neurosurgery 2005;57:1244–53. 10.1227/01.NEU.0000186039.57548.96 [DOI] [PubMed] [Google Scholar]
- 6.Chan A-W, Hróbjartsson A, Jørgensen KJ, et al. . Discrepancies in sample size calculations and data analyses reported in randomised trials: comparison of publications with protocols. BMJ 2008;337:a2299. 10.1136/bmj.a2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pildal J, Chan A-W, Hróbjartsson A, et al. . Comparison of descriptions of allocation concealment in trial protocols and the published reports: cohort study. BMJ 2005;330:1049. 10.1136/bmj.38414.422650.8F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mhaskar R, Djulbegovic B, Magazin A, et al. . Published methodological quality of randomized controlled trials does not reflect the actual quality assessed in protocols. J Clin Epidemiol 2012;65:602–9. 10.1016/j.jclinepi.2011.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hróbjartsson A, Pildal J, Chan A-W, et al. . Reporting on blinding in trial protocols and corresponding publications was often inadequate but rarely contradictory. J Clin Epidemiol 2009;62:967–73. 10.1016/j.jclinepi.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 10.Scharf O, Colevas AD. Adverse event reporting in publications compared with sponsor database for cancer clinical trials. J Clin Oncol 2006;24:3933–8. 10.1200/JCO.2005.05.3959 [DOI] [PubMed] [Google Scholar]
- 11.Al-Marzouki S, Roberts I, Evans S, et al. . Selective reporting in clinical trials: analysis of trial protocols accepted by the Lancet. Lancet 2008;372:201. 10.1016/S0140-6736(08)61060-0 [DOI] [PubMed] [Google Scholar]
- 12.Gøtzsche PC, Hróbjartsson A, Johansen HK, et al. . Ghost authorship in industry-initiated randomised trials. PLoS Med 2007;4:e19. 10.1371/journal.pmed.0040019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundh A, Krogsbøll LT, Gøtzsche PC. Access to data in industry-sponsored trials. Lancet 2011;378:1995–6. 10.1016/S0140-6736(11)61871-0 [DOI] [PubMed] [Google Scholar]
- 14.Adie S, Harris IA, Naylor JM, et al. . Consort compliance in surgical randomized trials: are we there yet? A systematic review. Ann Surg 2013;258:872–8. 10.1097/SLA.0b013e31829664b9 [DOI] [PubMed] [Google Scholar]
- 15.Adie S, Ma D, Harris IA, et al. . Quality of conduct and reporting of meta-analyses of surgical interventions. Ann Surg 2015;261:685–94. 10.1097/SLA.0000000000000836 [DOI] [PubMed] [Google Scholar]
- 16.Adie S, Harris IA, Naylor JM, et al. . Are outcomes reported in surgical randomized trials patient-important? A systematic review and meta-analysis. Can J Surg 2017;60:86–93. 10.1503/cjs.010616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao L, Sha T, Chen B, et al. . Study protocols on randomized clinical trials of acupuncture: an assessment of reporting quality with the spirit statement. Eur J Integr Med 2016;8:881–7. 10.1016/j.eujim.2016.10.003 [DOI] [Google Scholar]
- 18.Yang L, Chen S, Yang D, et al. . A quality analysis of clinical anaesthesia study protocols from the Chinese clinical trials registry according to the spirit statement. Oncotarget 2018;9:24830–6. 10.18632/oncotarget.24982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sims MT, Henning NM, Wayant CC, et al. . Do emergency medicine journals promote trial registration and adherence to reporting guidelines? A survey of ‘Instructions for Authors’. Scand J Trauma Resusc Emerg Med 2016;24:137. 10.1186/s13049-016-0331-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samaan Z, Mbuagbaw L, Kosa D, et al. . A systematic scoping review of adherence to reporting guidelines in health care literature. J Multidiscip Healthc 2013;6:169–88. 10.2147/JMDH.S43952 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-038283supp001.pdf (88.5KB, pdf)