Abstract
Culture-independent diagnostics have revealed a larger burden of Shigella among children in low-resource settings than previously recognized. We further characterized the epidemiology of Shigella in the first two years of life in a multisite birth cohort. We tested 41,405 diarrheal and monthly non-diarrheal stools from 1,715 children for Shigella by quantitative PCR. To assess risk factors, clinical factors related to age and culture positivity, and associations with inflammatory biomarkers, we used log-binomial regression with generalized estimating equations. The prevalence of Shigella varied from 4.9%-17.8% in non-diarrheal stools across sites, and the incidence of Shigella-attributable diarrhea was 31.8 cases (95% CI: 29.6, 34.2) per 100 child-years. The sensitivity of culture compared to qPCR was 6.6% and increased to 27.8% in Shigella-attributable dysentery. Shigella diarrhea episodes were more likely to be severe and less likely to be culture positive in younger children. Older age (RR: 1.75, 95% CI: 1.70, 1.81 per 6-month increase in age), unimproved sanitation (RR: 1.15, 95% CI: 1.03, 1.29), low maternal education (<10 years, RR: 1.14, 95% CI: 1.03, 1.26), initiating complementary foods before 3 months (RR: 1.10, 95% CI: 1.01, 1.20), and malnutrition (RR: 0.91, 95% CI: 0.88, 0.95 per unit increase in weight-for-age z-score) were risk factors for Shigella. There was a linear dose-response between Shigella quantity and myeloperoxidase concentrations. The burden of Shigella varied widely across sites, but uniformly increased through the second year of life and was associated with intestinal inflammation. Culture missed most clinically relevant cases of severe diarrhea and dysentery.
Author summary
Shigella is the second leading cause of diarrhea morbidity and mortality among children in low and middle-income countries. We characterized the epidemiology of Shigella using highly sensitive diagnostic methods in 41,405 diarrheal and monthly non-diarrheal stools from the first two years of life in a multisite birth cohort. The prevalence of Shigella varied from 4.9%-17.8% across sites, and the incidence of Shigella-attributable diarrhea was 31.8 cases (95% CI: 29.6, 34.2) per 100 child-years. Shigella diarrhea episodes were more likely to be severe and less likely to be culture positive in younger children. Older age, unimproved sanitation, low maternal education, initiating complementary foods before 3 months, and malnutrition were risk factors for Shigella. There was a linear dose-response between Shigella quantity and myeloperoxidase, a marker of intestinal inflammation, which suggests a potential mechanism for the impact of Shigella on child growth. Because culture missed most clinically relevant cases of severe diarrhea and dysentery, molecular diagnostics may be important tools in upcoming Shigella vaccine trials.
Introduction
Shigella is the second leading cause of diarrhea morbidity and mortality among children in low and middle-income countries, accounting for approximately 60,000 deaths in 2016 [1]. An invasive Gram-negative rod, Shigella has a low infectious inoculum, and both fecal-oral and direct person-to-person transmission can occur [2]. Shigella is strongly associated with dysentery; correspondingly, the WHO guidelines recommend treatment of all pediatric cases of dysentery with ciprofloxacin or azithromycin for presumed Shigella infection [3].
The recent use of quantitative PCR for Shigella detection revealed a more than five times higher burden of Shigella-attributable diarrhea among children in low-resource settings than previously recognized using culture-based diagnostics [4–6]. Importantly, the majority of Shigella burden was associated with watery diarrhea, not dysentery [5]. A recent meta-analysis showed that the proportion of Shigella infections that present with dysentery has been decreasing, and that Shigella infections overall had a stronger association with mortality than Shigella-associated cases of dysentery [7]. Furthermore, even in the absence of diarrheal symptoms, Shigella has been associated with impaired linear growth [6,8,9]. WHO treatment guidelines do not currently recommend treatment for the majority of Shigella infections that may be associated with adverse outcomes, such that there may be missed treatment opportunities. Increasing rates of fluoroquinolone and macrolide resistance have highlighted the need for novel interventions, and particularly increased the urgency of the development of a Shigella vaccine [10], which may offer a more sustainable solution.
Given our recently revised understanding of the magnitude of Shigella disease burden and in preparation for Shigella vaccine trials, a better understanding of the epidemiology of Shigella infections among children in low-resource settings is needed. We describe the burden, diagnostic and clinical characteristics, risk factors, and seasonality of Shigella in the first two years of life in 8 low-resource settings.
Methods
The MAL-ED study was conducted in eight sites: Dhaka (Bangladesh), Vellore (India), Bhaktapur (Nepal), Naushero Feroze (Pakistan), Venda (South Africa), Haydom (Tanzania), Fortaleza (Brazil), and Loreto (Peru), as previously described [11]. Briefly, between November 2009 and February 2012, children were recruited within 17 days of birth if maternal age was ≥ 16 years, their family intended to remain in the area for 6+ months, the child was a singleton pregnancy, birthweight was ≥ 1500 g, the child was not diagnosed with severe disease, and their siblings were not in the study. Fieldworkers conducted active surveillance for child illnesses, antibiotic use, and feeding practices twice weekly until two years of age. Anthropometry was measured monthly. Diarrheal stool samples were collected during diarrhea defined by maternal report of three or more loose stools in 24 hours or one stool with visible blood. Clinical characteristics, including blood observed in stool, were caregiver-reported. Severe diarrhea was defined using the CODA score, which has been previously validated against hospitalization [12,13] and is more appropriate for Shigella than the Vesikari score, which was validated for rotavirus. Non-diarrheal stool samples were collected monthly (at least 3 days distant to a diarrhea episode). Weight-for-age (WAZ) and length-for-age z-scores (LAZ) were calculated using 2006 WHO child growth standards [14]. Socioeconomic status (SES) was summarized using a construct of water, assets, maternal education, and income and was averaged over 4 biannual surveys [15].
Ethics statement
The study was approved by the Institutional Review Board for Health Sciences Research, University of Virginia, USA as well as the respective governmental, local institutional, and collaborating institutional ethical review boards at each site: Ethical Review Committee, ICDDR,B (Bangladesh); Committee for Ethics in Research, Universidade Federal do Ceara; National Ethical Research Committee, Health Ministry, Council of National Health (Brazil); Institutional Review Board, Christian Medical College, Vellore; Health Ministry Screening Committee, Indian Council of Medical Research (India); Institutional Review Board, Institute of Medicine, Tribhuvan University; Ethical Review Board, Nepal Health Research Council; Institutional Review Board, Walter Reed Army Institute of Research (Nepal); Institutional Review Board, Johns Hopkins University; PRISMA Ethics Committee; Health Ministry, Loreto (Peru); Ethical Review Committee, Aga Khan University (PKN); Health, Safety and Research Ethics Committee, University of Venda; Department of Health and Social Development, Limpopo Provincial Government (South Africa); Medical Research Coordinating Committee, National Institute for Medical Research; Chief Medical Officer, Ministry of Health and Social Welfare (Tanzania). Informed written consent was obtained from the parent or guardian of each participating child on their behalf.
Analysis of stool specimens
Total nucleic acid was extracted from stool specimens from children who completed 2 years of follow-up using the QIAmp Fast DNA Stool Mini Kit (Qiagen), as previously described [16]. Extrinsic controls phocine herpesvirus and bacteriophage MS2 monitored the efficiency of extraction and amplification. Quantitative PCR with custom-designed TaqMan Array Cards was used to detect 29 enteropathogens using the AgPath One Step realtime PCR kit (ThermoFisher), as described elsewhere [5,17]. Shigella was detected using the ipaH gene, and as in previous work [5,6], we interpret ipaH detections as diagnostic of Shigella even though both Shigella and enteroinvasive E. coli are detected using the ipaH target. Shigella species were detected using periplasmic protein, O-antigen, and type 3 restriction enzyme genes (S. flexneri) and a methylase gene (S. sonnei), as previously described [4]. Shigella infection was defined by ipaH qPCR cycle threshold (Cq) < 35, and quantity defined by log10-copy numbers per gram of stool based on the Cq, as previously [6]. Among ipaH positive stools, speciation assays were considered positive when Cq < 40 since the speciation assays target single copy genes and are therefore less sensitive than the ipaH assay. Shigella-attributable diarrhea episodes were identified using attributable fractions (AFe) to adjust for subclinical pathogen infections, as previously [4,5]. We defined Shigella-attributable episodes when the Shigella quantity-derived AFe ≥ 0.5 (i.e. majority attribution). Shigella was also previously detected by culture using standard protocols across sites in all diarrheal stools and non-diarrheal stools collected monthly in the first year and quarterly in the second year [18].
Fecal intestinal biomarkers were analyzed in the same subset of non-diarrheal stools, as described previously [19,20]. Myeloperoxidase (MPO; log [ng/ml]) was measured as marker of neutrophil activation and infiltration, neopterin (NEO; log[nmol/L]) as a marker of Th1 inflammation, and α-1-anti-trypsin (AAT; log[mg/g]) as a marker of intestinal permeability. Urinary lactulose and mannitol were measured at 3, 6, 9, and 15 months, and were converted into sample-based lactulose:mannitol excretion ratio z-scores (LMZs) using the Brazil cohort as the internal reference population [21]. α-1-acid glycoprotein (AGP), a marker of systemic inflammation, was measured in serum from 7, 15, and 24 months.
Statistical analysis
We analyzed all stool samples with valid qPCR results for Shigella (97.1%, n = 41,450 of 42,630 samples with sufficient stool collected). We estimated the incidence of Shigella-attributable diarrhea (AFe ≥ 0.5) using Poisson regression and reweighted estimates from the number of episodes tested to the total number of episodes identified by surveillance. We estimated the relative risks of an episode presenting with each clinical characteristic in the first versus second year of life, and for children’s first versus subsequent Shigella diarrhea episodes, using log-binomial regression with generalized estimating equations (GEE) to account for correlated episodes within children, adjusting for site.
We assessed diagnostic test characteristics of Shigella culture compared to qPCR as the gold standard among all stools, among attributable diarrheal stools, and among attributable dysenteric stools by site. We further estimated the associations between clinical characteristics of attributable episodes and culture positivity using log-binomial regression with GEE and in univariable (adjusting for site) and multivariable (adjusting for site and other clinical characteristics) models.
To identify risk factors for Shigella infection in both non-diarrheal and diarrheal stools, we included sociodemographics, household, and child-level variables as potential risk factors in univariable log-binomial regression models with GEE, adjusting only for site. We then estimated adjusted associations in a multivariable model with a subset of risk factors that were either statistically significant (p<0.05) or had a risk ratio with magnitude greater than 1.2 or less than 0.83 in the univariable models, overall and by site. For covariates measuring similar constructs (e.g. anthropometric measurements and recent antibiotic use), we included the variables with complete data and/or larger magnitudes of effect in the multivariable model.
We modeled the seasonality of Shigella detections in non-diarrheal stools at each site using predictions from logistic regression models with linear and quadratic terms for the week of the year (w), and the terms sin(2πw/52), cos(2πw/52), sin(4πw/52), and cos(4πw/52). We estimated the associations of historical monthly average temperature and rainfall from 1982–2012 for towns nearest each site [22] with Shigella using log-binomial regression with effects scaled to compare high versus low temperature and rainfall at each site, defined by the 90th and 10th percentiles of the site-specific distributions.
Finally, we assessed the associations between concurrent measurements of biomarkers and Shigella infection and quantity, both in quartiles and continuously per log10 increase in copy numbers per gram of stool based on the Cq value, using linear regression models with GEE and adjusting for site, age, sex, and stool consistency.
Results
Shigella burden
Among 1715 children with 2 years of follow up, a total of 41,405 stool samples (6,751 diarrheal and 34,654 non-diarrheal samples) were tested for Shigella by qPCR (Table 1). The prevalence of Shigella was 11.5% (n = 4744) overall, and was higher in diarrheal stools than non-diarrheal stools (18.4% vs. 10.1%; p<0.0001). Shigella prevalence in non-diarrheal stools varied from 4.9%-17.8% across sites. Almost half (611/1407, 43.4%) of children with Shigella detected in one or more non-diarrheal stools never had a diarrhea episode in which Shigella was detected, and almost two-thirds (900/1407, 64.0%) never experienced a Shigella-attributable diarrhea episode. The quantity of Shigella detected was approximately 1 log higher in diarrheal (6.13 log10-copy numbers per gram of stool) compared to non-diarrheal (5.56 log10-copy numbers) stools, and quantities were lower in Pakistan and South Africa compared to the other sites. The prevalence and quantity of Shigella infection increased with age (Table 1).
Table 1. Prevalence of Shigella infection, quantity detected, and incidence of Shigella-attributable diarrhea among 1,715 children in the MAL-ED study.
| Prevalence in diarrheal stools (N = 6,751) n (%) | Quantity1 in diarrheal stools Mean (SD) | Prevalence in non-diarrheal stools (N = 34,654) n (%) | Quantity1 in non-diarrheal stools Mean (SD) | Number of Shigella-attributable diarrhea episodes | Incidence of Shigella-attributable diarrhea1 (95% CI) | Number of Shigella-attributable dysentery episodes | Incidence of Shigella-attributable dysentery2 (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Overall | 1239 (18.35) | 6.13 (1.54) | 3505 (10.11) | 5.56 (1.28) | 755 | 31.8 (29.6, 34.2) | 111 | 4.85 (4.02, 5.84) |
| Site | ||||||||
| Dhaka, Bangladesh | 402 (29.03) | 6.19 (1.46) | 564 (13.06) | 5.61 (1.19) | 275 | 75.1 (66.7, 84.5) | 30 | 8.23 (5.75, 11.77) |
| Fortaleza, Brazil | 21 (23.08) | 6.18 (1.73) | 139 (4.90) | 5.14 (1.24) | 12 | 6.7 (3.8, 11.8) | 0 | 0 |
| Vellore, India | 141 (22.07) | 6.43 (1.46) | 592 (12.42) | 5.47 (1.20) | 101 | 33.9 (27.9, 41.1) | 23 | 8.32 (5.53, 12.52) |
| Bhaktapur, Nepal | 118 (12.98) | 6.69 (1.81) | 290 (5.75) | 5.64 (1.26) | 80 | 21.0 (16.9, 26.2) | 16 | 3.93 (2.41, 6.41) |
| Loreto, Peru | 305 (19.01) | 6.28 (1.66) | 574 (13.63) | 5.81 (1.43) | 162 | 47.9 (41, 55.8) | 25 | 7.47 (5.04, 11.04) |
| Naushero Feroze, Pakistan | 195 (10.57) | 5.29 (1.00) | 268 (5.77) | 4.89 (0.89) | 102 | 39.0 (32.1, 47.4) | 12 | 4.51 (2.56, 7.93) |
| Venda, South Africa | 20 (16.81) | 5.47 (1.22) | 321 (7.01) | 5.05 (0.94) | 10 | 5.4 (2.9, 10.0) | 0 | 0 |
| Haydom, Tanzania | 37 (23.13) | 6.12 (1.47) | 757 (17.80) | 5.91 (1.37) | 13 | 10.8 (6.2, 18.5) | 5 | 3.81 (1.58, 9.14) |
| Age (months) | ||||||||
| 0–5 | 44 (2.67) | 5.31 (1.34) | 144 (1.87) | 4.97 (1.08) | 16 | 2.9 (1.8, 4.8) | 4 | 0.78 (0.29, 2.09) |
| 6–11 | 253 (12.22) | 5.79 (1.55) | 620 (7.39) | 5.50 (1.23) | 128 | 21.8 (18.3, 25.9) | 19 | 3.31 (2.11, 5.18) |
| 12–17 | 426 (25.10) | 6.17 (1.55) | 1096 (12.26) | 5.52 (1.27) | 265 | 44.8 (39.7, 50.5) | 39 | 7.00 (5.11, 9.58) |
| 18–23 | 516 (38.62) | 6.33 (1.50) | 1645 (17.08) | 5.67 (1.31) | 346 | 53.3 (48.0, 59.2) | 49 | 7.66 (5.79, 10.14) |
1log10-copy numbers per gram of stool based on the Cq value
2Episodes with AFe ≥ 0.5 per 100 child years, reweighted from episodes tested to total number of episodes surveilled
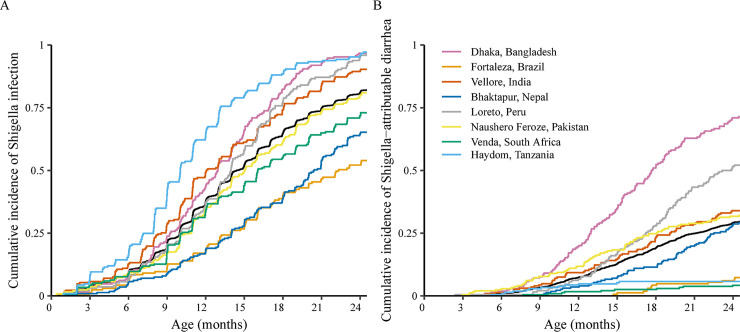
Shigella was detected at quantities high enough to attribute the episode to Shigella in 11.2% (n = 755) of diarrheal stools. The overall incidence of Shigella-attributable diarrhea was 31.8 cases (95% CI: 29.6, 34.2) per 100 child-years. Tanzania had the third lowest incidence (10.8 cases per 100 child-years, 95% CI: 6.2, 18.5) of Shigella-attributable diarrhea despite having the highest prevalence in stools overall. Incidence of Shigella-attributable diarrhea was higher in older age groups (incidence rate ratio: 1.75, 95% CI: 1.70, 1.81 per 6-month increase in age). Incidence of Shigella-attributable dysentery was lower (4.85 cases per 100 child-years, 95% CI: 4.02, 5.84), but followed similar patterns by age and site. Incidence estimates by disease severity and diagnostic are reported in S1 Table. By two years of age, 82.0% (n = 1407) of children had been infected with Shigella (Fig 1A), and 29.6% (n = 507) had at least one episode of Shigella-attributable diarrhea (Fig 1B). Median time to first infection was 14.4 (95% CI: 14.0, 15.0) months.
Fig 1.
Cumulative incidence of Shigella infection (A) and Shigella-attributable diarrhea episodes (B) among 1715 children in the MAL-ED cohort.
Because the Shigella speciation assays were less sensitive than that for ipaH, results were available for only 31.3% (n = 1245) of ipaH positive stools with speciation testing (n = 3980, 83.9% of ipaH positives). ipaH quantity was 6.4 log10-copy numbers per gram of stool in speciated detections compared to 5.4 log10-copy numbers in non-speciated detections. Among the speciated Shigella-attributable diarrheal stools (n = 258), 58.9% (n = 152) were S. flexneri and 43.8% (n = 113) were S. sonnei. The majority of Shigella-attributable diarrheal stools were S. flexneri in all sites except Brazil, Nepal, and South Africa. The ratio of S. flexneri to S. sonnei (71.9% vs. 31.7%) observed in all (diarrheal and non-diarrheal) stools was similar to that in diarrheal stools (S2 Table).
Clinical characteristics of Shigella diarrhea
Among Shigella-attributable diarrheal episodes, 28.3% (n = 214) were severe and 14.7% (n = 111) had bloody stools. Approximately a third (n = 235, 31.1%) were accompanied by fever, 18.5% (n = 140) by vomiting, 10.1% (n = 76) by dehydration, and 18.9% (n = 143) lasted 7 days or longer. The majority of episodes were antibiotic treated (456, 60.4%), and of these, the majority were treated with a macrolide, cephalosporin, or fluoroquinolone (n = 311/456, 68.2%). However, there was substantial site variability; 97.0% of all macrolide treatment was given in Bangladesh and Peru, and antibiotic treatment was rare in Brazil and South Africa. There were differences in the clinical presentation of shigellosis by age (Table 2). Episodes of Shigella-attributable diarrhea in the first year of life were more likely to be prolonged (aRR: 1.24, 95% CI: 0.88, 1.74), with vomiting (aRR: 1.72, 95% CI: 1.26, 2.35) and with more than 6 loose stools in 24 hours (aRR: 1.41, 95% CI: 1.06, 1.86) compared to episodes in the second year. Episodes in the first year were also more likely to be treated with cephalosporins and less likely to be treated with fluoroquinolones. Adjusting for age, a child’s first episode of Shigella-attributable diarrhea was more likely to be accompanied by dehydration (aRR: 1.41, 95% CI: 0.84, 2.36) compared to subsequent episodes. First episodes were also slightly more likely to be prolonged and with vomiting and high frequency of stools, but less likely to be bloody (S3 Table).
Table 2. Clinical characteristics of Shigella-attributable diarrhea episodes in the first and second years of life and the associations between age and clinical characteristics among 755 episodes.
| Episode characteristic | Year 1 (0–11 months; N = 144) N (%) | Year 2 (12–23 months; N = 611) N (%) | Risk ratio1 for characteristic in year 1 vs. year 2 (95% CI) |
|---|---|---|---|
| Severe (score ≥4) | 47 (32.6) | 167 (27.3) | 1.16 (0.89, 1.50) |
| Blood | 23 (16.0) | 88 (14.4) | 1.06 (0.69, 1.63) |
| Fever | 48 (33.3) | 187 (30.6) | 1.04 (0.81, 1.33) |
| Prolonged (≥7 days) | 35 (24.3) | 108 (17.7) | 1.24 (0.88, 1.74) |
| Persistent (≥14 days) | 8 (5.6) | 18 (2.9) | 1.32 (0.59, 2.93) |
| Dehydration | 18 (12.5) | 58 (9.5) | 1.11 (0.72, 1.72) |
| Vomiting | 44 (30.6) | 96 (15.7) | 1.72 (1.26, 2.35) |
| High frequency (>6 loose stools in 24 hours) | 43 (29.9) | 137 (22.4) | 1.41 (1.06, 1.86) |
| Hospitalization | 1 (0.7) | 1 (0.2) | — |
| Any antibiotic treatment2 | 88 (61.1) | 368 (60.2) | 0.97 (0.83, 1.12) |
| Macrolide treatment2 | 29 (20.1) | 136 (22.3) | 1.05 (0.76, 1.45) |
| Cephalosporin treatment2 | 15 (10.4) | 44 (7.2) | 1.21 (0.72, 2.02) |
| Fluoroquinolone treatment2 | 10 (6.9) | 97 (15.9) | 0.46 (0.25, 0.85) |
1Adjusted for site; excludes sites with no Shigella-attributable diarrhea episodes with characteristic (Brazil for severe, dehydration, and high frequency; Brazil and South Africa for blood; Brazil, South Africa, and Tanzania for persistent diarrhea, macrolide, cephalosporin, and fluoroquinolone treatment).
2Treatment on at least one day during the diarrhea episode.
Coinfections were detected in almost all Shigella-attributable diarrheal stools (S4 Table). However, a second etiology of diarrhea (i.e. a coinfecting pathogen was detected in a quantity high enough to be associated with diarrhea) was identified in only 38.5% of these episodes (n = 289). The coinfecting pathogen had a higher AFe than Shigella (i.e. potentially the primary etiology) in 12.3% of episodes (n = 92). Episodes with a viral co-etiology were less likely to be bloody (aRR: 0.60, 95% CI: 0.38, 0.96) and more likely to include vomiting (aRR: 1.79, 95% CI: 1.31, 2.46) compared to episodes with Shigella as the only etiology identified. Episodes with another bacterial co-etiology were also less likely to be bloody (aRR: 0.62, 95% CI: 0.35, 1.11; S5 Table).
Performance of Shigella culture
Of 30,678 stools tested by both qPCR and culture, 3,372 (11.0%) were positive by qPCR and 280 (0.9%) were positive by culture. Considering qPCR the gold standard, the overall sensitivity of culture was 6.6% (Table 3). Specificity was uniformly high, at more than 99% at all sites. In the subset of Shigella-attributable diarrheal stools (n = 736), which have higher Shigella quantity detected than all stools, sensitivity improved to 17.5%, and ranged from 0% in South Africa and Tanzania to 24.0% in Peru (Table 3). Sensitivity was even higher in dysentery episodes (27.8%). The sensitivity of culture among Shigella-attributable diarrheal stools without another attributable pathogen identified (20.6%, n = 94/457) was higher than that among Shigella-attributable diarrheal stools with another attributable pathogen identified (12.5%, n = 35/279). Culture positivity was strongly associated with age, such that attributable diarrhea stools among younger children were less likely to be culture positive (Table 4). Presence of blood had the strongest association with culture positivity (aRR: 1.84, 95% CI: 1.28, 2.65), but culture still missed more than 70% of dysentery cases. Diarrhea severity was not associated with culture positivity, and caregiver-reported fever was inversely associated with culture positivity. Recent macrolide treatment was also associated with reduced detection by culture (aRR: 0.57, 95% CI: 0.30, 1.08; Table 4).
Table 3. Performance of Shigella culture compared to quantitative PCR among 30,678 diarrheal and non-diarrheal stools tested by both diagnostics.
| All sites n (%) | Dhaka, Bangladesh n (%) | Fortaleza, Brazil n (%) | Vellore, India n (%) | Bhaktapur, Nepal n (%) | Loreto, Peru n (%) | Naushero Feroze, Pakistan n (%) | Venda, South Africa n (%) | Haydom, Tanzania n (%) | |
|---|---|---|---|---|---|---|---|---|---|
| All stools | 30678 | 4305 | 1895 | 3722 | 4176 | 5809 | 4716 | 3158 | 2897 |
| qPCR positive | 3372 (11.0) | 667 (15.5) | 95 (5.0) | 448 (12.0) | 258 (6.2) | 879 (15.1) | 323 (6.8) | 204 (6.5) | 498 (17.2) |
| Culture positive | 280 (0.9) | 34 (0.8) | 5 (0.3) | 45 (1.2) | 53 (1.3) | 56 (1) | 79 (1.7) | 3 (0.1) | 5 (0.2) |
| Sensitivity1 | 6.6 | 3.6 | 4.2 | 8.7 | 16.7 | 6.3 | 17.3 | 0.5 | 0.2 |
| Specificity1 | 99.8 | 99.7 | 99.9 | 99.8 | 99.7 | 99.9 | 99.5 | 99.9 | 99.8 |
| Positive predictive value1 | 79.6 | 70.6 | 80.0 | 86.7 | 81.1 | 98.2 | 70.9 | 33.3 | 20.0 |
| Negative predictive value1 | 89.6 | 84.9 | 95.2 | 88.9 | 94.8 | 85.7 | 94.2 | 93.6 | 82.8 |
| Shigella-attributable diarrhea episodes | 736 | 267 | 11 | 101 | 76 | 162 | 97 | 10 | 12 |
| Culture positive2 | 129 (17.5) | 14 (10.9) | 3 (2.3) | 24 (18.6) | 29 (22.5) | 31 (24.0) | 28 (21.7) | 0 | 0 |
| Shigella-attributable dysentery episodes | 108 | 29 | 0 | 23 | 15 | 25 | 11 | 0 | 5 |
| Culture positive2 | 30 (27.8) | 3 (10.3) | 0 | 6 (26.1) | 6 (40.0) | 11 (44.0) | 4 (36.4) | 0 | 0 |
1qPCR considered the gold standard
2Number of Shigella-attributable episodes (defined by qPCR positive with AFe ≥ 0.5) that were also culture positive; percent positive is the sensitivity of culture compared to qPCR as the gold standard
Table 4. Associations between characteristics of Shigella-attributable diarrhea episodes and culture positivity among 736 attributable diarrhea episodes tested by qPCR and culture1.
| Episode characteristic | N episodes | N (%) culture positive | Univariable2 Risk ratio (95% CI) |
Multivariable3 Risk ratio (95% CI) |
|---|---|---|---|---|
| Age (months) | ||||
| 0–5 | 15 | 1 (6.7) | 0.25 (0.04, 1.70) | 0.19 (0.02, 1.51) |
| 6–11 | 116 | 13 (11.2) | 0.53 (0.31, 0.92) | 0.51 (0.30, 0.88) |
| 12–17 | 246 | 44 (17.9) | 0.82 (0.59, 1.14) | 0.86 (0.62, 1.20) |
| 18–23 | 326 | 68 (20.9) | 1. | 1. |
| Severe (score ≥4) | 211 | 35 (16.6) | 0.91 (0.64, 1.28) | --3 |
| Blood | 103 | 30 (29.1) | 1.53 (1.09, 2.15) | 1.84 (1.28, 2.65) |
| Fever | 225 | 35 (15.6) | 0.69 (0.47, 1.01) | 0.66 (0.45, 0.95) |
| Prolonged (≥7 days) | 137 | 29 (21.2) | 0.88 (0.60, 1.29) | 0.84 (0.55, 1.29) |
| Persistent (≥14 days) | 26 | 6 (23.1) | 0.89 (0.40, 1.96) | --3 |
| Dehydration | 71 | 20 (28.2) | 1.22 (0.80, 1.86) | 1.17 (0.77, 1.78) |
| Vomiting | 131 | 22 (16.8) | 0.89 (0.59, 1.36) | 1.13 (0.73, 1.75) |
| No. loose stools (mean; SD) | 5.8 (2.64) | 6.0 (2.37) | 1.01 (0.96, 1.06) | 1.03 (0.97, 1.09) |
| Any antibiotic treatment in the last 15 days | 420 | 65 (15.5) | 0.87 (0.63, 1.20) | --3 |
| Macrolide treatment in the last 15 days | 146 | 10 (6.9) | 0.64 (0.33, 1.22) | 0.57 (0.30, 1.08) |
| Cephalosporin treatment in the last 15 days | 66 | 14 (21.2) | 1.04 (0.64, 1.69) | 0.95 (0.57, 1.60) |
| Fluoroquinolone treatment in the last 15 days | 77 | 9 (11.7) | 0.95 (0.52, 1.73) | 0.71 (0.38, 1.32) |
1Analysis excludes Brazil, South Africa, and Tanzania, which had 5 or fewer culture-positive attributable diarrhea episodes
2Adjusted for site
3Adjusted for other characteristics included in the table; estimates are excluded for composite (collinear) variables
Risk factors for Shigella
In univariable analysis, unimproved sanitation, crowding (2+ people living in a single room), <10 years of maternal education, having 3+ live children, initiating complementary foods before 3 months, recent diarrhea, antibiotic use (particularly fluoroquinolone use), and lower anthropometric measurements prior to sample collection were also associated with higher risk of Shigella infection (Table 5). Of these variables, unimproved sanitation (aRR: 1.15, 95% CI: 1.03, 1.29), less than 10 years of maternal education (aRR: 1.14, 95% CI: 1.03, 1.26), and child WAZ at the most recent measurement prior to diarrhea (aRR: 0.91, 95% CI: 0.88, 0.95 per z-score increase in weight) were the strongest risk factors in multivariable analysis (Table 5). There was some variability in associations by site; for example, crowding and recent diarrhea were strong risk factors in Brazil, low maternal education had the largest associations in Brazil, India, and South Africa, and recent fluoroquinolone use was only a risk factor in India and Pakistan (S1 Fig).
Table 5. Risk factors for Shigella infection among 41,405 diarrheal and non-diarrheal stools.
| Univariate1 analysis | Multivariate2 analysis | |||||
|---|---|---|---|---|---|---|
| Risk ratio (95% CI) | P-value | Risk ratio (95% CI) | P-value | |||
| Diarrheal stool (Ref: non-diarrheal stool) | 1.86 | (1.74, 1.99) | <0.0001 | 1.97 | (1.85, 2.10) | <0.0001 |
| Household level factors | ||||||
| Exchange rate adjusted income < $150 dollar (Ref: income > = $150) | 1.07 | (0.97, 1.17) | 0.2 | |||
| Earth, sand, clay, mud, dung floor (Ref: Wooden, concrete, vinyl etc.) | 1.09 | (0.98, 1.23) | 0.1 | |||
| Unimproved sanitation | 1.21 | (1.09, 1.35) | 0.0005 | 1.15 | (1.03, 1.29) | 0.01 |
| Unimproved source of drinking water | 1.05 | (0.93, 1.18) | 0.4 | |||
| Crowding (2+ people per room) | 1.09 | (1.00, 1.19) | 0.05 | 1.04 | (0.95, 1.14) | 0.4 |
| Ownership of cattle | 1.01 | (0.90, 1.13) | 0.9 | |||
| Ownership of chicken | 0.98 | (0.88, 1.09) | 0.7 | |||
| Maternal characteristics | ||||||
| <10 years of maternal education | 1.19 | (1.08, 1.32) | 0.0005 | 1.14 | (1.03, 1.26) | 0.01 |
| Maternal BMI (Ref. 18.5–22.9 kg/m2) | ||||||
| <18.5 kg/m2 | 1.10 | (0.96, 1.25) | 0.2 | |||
| ≥23 kg/m2 | 1.02 | (0.95, 1.11) | 0.6 | |||
| 3 or more living children | 1.08 | (1.00, 1.17) | 0.05 | 1.01 | (0.94, 1.10) | 0.7 |
| Child characteristics | ||||||
| Age (months) (Ref. 0–5 months) | ||||||
| 6–11 | 4.13 | (3.49, 4.88) | <0.0001 | 3.93 | (3.32, 4.64) | <0.0001 |
| 12–17 | 7.04 | (5.95, 8.32) | <0.0001 | 6.84 | (5.78, 8.09) | <0.0001 |
| 18–23 | 9.78 | (8.30, 11.53) | <0.0001 | 9.66 | (8.19, 11.39) | <0.0001 |
| Female sex (Ref: Male) | 1.00 | (0.93, 1.07) | 1.0 | |||
| Exclusive breastfeeding < 3 months | 1.09 | (1.00, 1.19) | 0.06 | |||
| Complementary foods initiated < 3 months | 1.11 | (1.02, 1.21) | 0.02 | 1.10 | (1.01, 1.20) | 0.03 |
| WAZ at enrollment | 0.99 | (0.96, 1.03) | 0.7 | |||
| LAZ at enrollment3 | 0.99 | (0.96, 1.03) | 0.7 | |||
| WLZ3 | 0.80 | (0.77, 0.83) | <0.0001 | |||
| WAZ | 0.79 | (0.76, 0.82) | <0.0001 | 0.91 | (0.88, 0.95) | <0.0001 |
| LAZ3 | 0.73 | (0.71, 0.76) | <0.0001 | |||
| Days with diarrhea (past 3 months; per 7 day increase) | 1.09 | (1.05, 1.13) | <0.0001 | 1.06 | (1.02, 1.09) | 0.002 |
| Antibiotic use (past 15 days) | 1.13 | (1.06, 1.20) | 0.0002 | |||
| Antibiotic use (past 30 days) | 1.02 | (0.96, 1.09) | 0.6 | |||
| Macrolide use (past 15 days) | 1.01 | (0.89, 1.14) | 0.9 | |||
| Macrolide use (past 30 days) | 0.92 | (0.81, 1.05) | 0.2 | |||
| Cephalosporin use (past 15 days) | 0.97 | (0.85, 1.11) | 0.7 | |||
| Cephalosporin use (past 30 days) | 0.89 | (0.77, 1.01) | 0.08 | |||
| Fluoroquinolone use (past 15 days)4 | 1.67 | (1.43, 1.94) | <0.0001 | |||
| Fluoroquinolone use (past 30 days) | 1.54 | (1.29, 1.86) | <0.0001 | 1.10 | (0.93, 1.31) | 0.3 |
1Adjusted for site.
2Adjusted for site and other variables in table with multivariate estimates.
3Excludes Pakistan.
4Not included in multivariable model since collinear with fluoroquinolone use in past 30 days.
The seasonality of Shigella infections differed by site (Fig 2). Peak prevalence was observed in May/June in Bangladesh, June in Nepal, July/August in India, November in South Africa, and February in Tanzania. Two peaks were observed in Pakistan and Peru, and there was little seasonality in Brazil. Among climactic factors that could explain these heterogeneities, temperature was more strongly associated with Shigella detection than rainfall, though temperature was collinear with rainfall at most sites (Fig 2, S6 Table). Higher temperature was most strongly associated with Shigella in Nepal (aRR: 2.71, 95% CI: 1.96, 3.75) and Tanzania (aRR: 1.92, 95% CI: 1.63, 2.27). Uniquely, higher rainfall was protective in Pakistan (aRR: 0.78, 95% CI: 0.61, 1.00; S6 Table).
Fig 2. Seasonality of Shigella detections in non-diarrheal stools by site.
Top plots: weekly incidence (bars) and modeled incidence (solid dark lines); bottom plots: historical monthly averages for rainfall (bars) and temperature (dotted lines) at each sites.
Association of Shigella with biomarkers of environmental enteropathy
Myeloperoxidase levels were 0.33 log(ng/mL) (95% CI: 0.27, 0.40) higher in stools with Shigella, and the association was greater in diarrheal stools (mean difference: 0.65, 95% CI: 0.30, 1.00 log(ng/ml)) than in non-diarrheal stools (mean difference: 0.32, 95% CI: 0.26, 0.39; p for heterogeneity: 0.08). There was a linear dose-response with Shigella quantity; myeloperoxidase levels increased by 0.21 logs (0.16, 0.26) per log increase in Shigella quantity (Table 6). Associations with myeloperoxidase were observed in all sites, but were highest in Brazil (0.70, 95% CI: 0.29, 1.11) and Peru (0.56, 95% CI: 0.40, 0.71; S7 Table). The dose response relationship was also consistent across sites (S2 Fig). α-1-acid glycoprotein was slightly elevated in stools with Shigella (7.53 mg/dL difference, 95% CI: 3.51, 11.55), and higher Shigella quantity was associated with higher concentrations. There were no consistent associations with neopterin, α-1-antitrypsin, the lactulose:mannitol z-score, or its components (Table 6).
Table 6. Associations between Shigella and biomarkers of environmental enteropathy among 19,148 diarrheal and non-diarrheal stools with biomarker measurements.
| Adjusted1 concentration difference (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
| Myeloperoxidase (log[ng/mL]) | Neopterin (log[nmol/L]) | α-1-antitrypsin (log[mg/g]) | α-1-acid glycoprotein (mg/dL)2 | Lactulose:mannitol z-score3 | Lactulose z-score3 | Mannitol z-score3 | |
| Any Shigella | 0.33 (0.27, 0.40) | 0.00 (-0.06, 0.06) | -0.01 (-0.07, 0.05) | 7.53 (3.51, 11.55) | 0.09 (-0.02, 0.21) | -0.05 (-0.19, 0.08) | -0.12 (-0.24, -0.01) |
| Shigella quantity | |||||||
| 1st quartile | 0.09 (-0.02, 0.21) | -0.02 (-0.14, 0.10) | -0.03 (-0.14, 0.07) | 1.58 (-4.07, 7.23) | 0.17 (0.01, 0.34) | 0.03 (-0.19, 0.25) | -0.14 (-0.33, 0.06) |
| 2nd quartile | 0.19 (0.07, 0.30) | 0.01 (-0.10, 0.12) | -0.05 (-0.16, 0.05) | 7.60 (0.82, 14.38) | 0.07 (-0.16, 0.30) | -0.19 (-0.43, 0.05) | -0.20 (-0.40, 0.01) |
| 3rd quartile | 0.43 (0.31, 0.54) | -0.03 (-0.16, 0.09) | -0.02 (-0.12, 0.09) | 9.28 (1.70, 16.86) | -0.11 (-0.37, 0.14) | -0.07 (-0.33, 0.19) | 0.05 (-0.17, 0.26) |
| 4th quartile | 0.81 (0.66, 0.95) | 0.07 (-0.05, 0.20) | 0.10 (-0.01, 0.22) | 12.90 (4.81, 21.00) | 0.26 (0.06, 0.45) | 0.01 (-0.25, 0.28) | -0.23 (-0.45, -0.02) |
| Per log increase | 0.21 (0.16, 0.26) | 0.06 (0.01, 0.11) | 0.03 (-0.01, 0.07) | 2.19 (-0.35, 4.74) | 0.04 (-0.03, 0.12) | 0.07 (-0.02, 0.16) | -0.01 (-0.08, 0.06) |
1Adjusted for site, age, sex, and stool consistency.
2N = 4147 at 7, 15, and 24 months of age; estimates adjusted for site, age, and sex.
3Brazil cohort was the internal reference population; N = 6110 at 3, 6, 9, and 15 months of age; estimates adjusted for site, age, and sex.
Discussion
The burden of Shigella among children under two was heterogeneous across eight sites with the absolute burden of infection and illness was higher in the South Asian sites and Peru. The burden of Shigella diarrhea relative to subclinical infections also varied. Shigella diarrhea episodes were accompanied by blood in only a minority of episodes, and episodes were generally more severe in the younger children. In a minority of Shigella diarrhea episodes that were also attributable to another pathogen, clinical phenotypes were often mixed; for example, episodes with viral co-etiologies predictably presented with more vomiting.
An 11-fold higher detection of Shigella was observed with qPCR compared to culture, including a 3-fold increase for Shigella-attributable dysentery. While higher sensitivity of culture among more severe cases has been previously noted [23], culture still missed the majority of cases of Shigella diarrhea, severe diarrhea, and dysentery, and culture had the lowest sensitivity among young children who are at highest risk for poor outcomes. These results highlight the need for more sensitive diagnostic tools.
The analyses of Shigella risk factors were consistent with prior work, which identified maternal education, exclusive breastfeeding, and larger WAZ as protective [24–26], and found similar trends with age [24,26,27]. Undernourished children were more likely to be infected, and interestingly, the seasonality of Shigella in Tanzania mirrored the seasonality of malnutrition, previously described [28]. The identification of unimproved sanitation as a risk factor alongside seasonal patterns that correlate strongly with average temperature and rainfall suggest environmental transmission pathways may be important. The seasonal patterns also support the potential implication of houseflies as a mechanical vector, as housefly population densities are seasonal and have been shown to correlate with Shigella [29]. Surprisingly, recent antibiotic exposure, including to macrolides and fluoroquinolones which are recommended for the treatment of shigellosis [3], was not associated with reduced Shigella detection.
Among several biomarkers that indirectly measure environmental enteric dysfunction (EED), especially MPO, but also AGP, were elevated during Shigella infections with a dose-response with Shigella quantity. Several previous studies found that high levels of MPO were most predictive of linear growth decrements compared to other biomarkers, including in previous analyses of data from the Bangladesh [30] and Peru [31] MAL-ED sites, and in a birth cohort in Pakistan [32]. The associations between Shigella and MPO, and MPO and growth faltering, suggest a potential mechanism for the impact of Shigella on linear growth previously characterized [6].
This study was limited by the fact that stool samples were not collected and/or tested from all diarrhea episodes [5], such that we may have underestimated the incidence of Shigella diarrhea. Because a second etiology of diarrhea was frequently identified, we were unable to determine whether Shigella was the primary cause in a substantial subset of Shigella-attributable diarrhea episodes. Both Shigella and enteroinvasive E. coli can be detected using the ipaH gene. However, previous speciation [4] and metagenomic work [33] supports the interpretation of these detections as Shigella. In addition, the Shigella speciation assays were insensitive, such that species data were available for only a third of infections. Improvements to the speciation assays have been made since the MAL-ED study; validated real time PCR assays that can differentiate >80% ipaH positives regardless of culture positivity and identify a panel of S. flexneri serotypes (including 2a, 3a, and 6) are now available for future studies. Because deaths were rare in this community-based cohort, we could not assess the associations between Shigella and mortality, as in the GEMS study [34]. Finally, site-specific estimates were relatively imprecise given the low numbers of children at each site. Because burden varied substantially by site, the incidence estimates may not be generalizable to other low-resource settings.
The high burden of Shigella disease documented in MAL-ED highlights the potential utility of Shigella vaccines. Almost all children were exposed to Shigella by two years of age in most sites, which suggests a pathogen-specific population-based prevention strategy is warranted. Furthermore, because the incidence of Shigella diarrhea was higher in the second year of life, the vaccine could potentially be given later in infancy and prevent the majority of cases. However, younger children presented with more severe symptoms, suggesting protection early in infancy may be important. Continued monitoring of Shigella epidemiology is needed since incidence trends may change as macrolide antibiotics become more available globally. More than 15 Shigella vaccines are currently in development [10], with some rapidly advancing to evaluation in target populations. Because of the poor sensitivity of culture, use of molecular diagnostics to define outcomes in future vaccine efficacy studies could limit misclassification of the outcome and reduce the sample size required to estimate significant effects.
Supporting information
(PDF)
Estimates are adjusted for age, diarrheal vs. non-diarrheal stool, and all other factors included in the figure. Estimates are excluded for specific sites for factors with no variability at that site.
(PDF)
Estimates are adjusted for age, sex, and stool consistency.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability
All relevant data including individual participant data are available by request from the ClinEpiDB database (https://clinepidb.org/ce/app/record/dataset/DS_3dbf92dc05).
Funding Statement
The Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED) was carried out as a collaborative project supported by the Bill and Melinda Gates Foundation (https://www.gatesfoundation.org/; OPP1131125), the Foundation for the National Institutes of Health, and the National Institutes of Health, Fogarty International Center. This work was supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (https://www.niaid.nih.gov/; grant K01AI130326 to ETRM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Khalil IA, Troeger C, Blacker BF, Rao PC, Brown A, Atherly DE, et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990–2016. Lancet Infect Dis. 2018. 10.1016/S1473-3099(18)30475-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotloff KL, Riddle MS, Platts-Mills JA, Pavlinac P, Zaidi AKM. Shigellosis. The Lancet. 2018;391: 801–812. 10.1016/S0140-6736(17)33296-8 [DOI] [PubMed] [Google Scholar]
- 3.Williams PCM, Berkley JA. Guidelines for the treatment of dysentery (shigellosis): a systematic review of the evidence. Paediatr Int Child Health. 2018;38: S50–S65. 10.1080/20469047.2017.1409454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet. 2016;388: 1291–1301. 10.1016/S0140-6736(16)31529-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platts-Mills JA, Liu J, Rogawski ET, Kabir F, Lertsethtakarn P, Siguas M, et al. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health. 2018;6: e1309–e1318. 10.1016/S2214-109X(18)30349-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogawski ET, Liu J, Platts-Mills JA, Kabir F, Lertsethtakarn P, Siguas M, et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Health. 2018;6: e1319–e1328. 10.1016/S2214-109X(18)30351-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tickell KD, Brander RL, Atlas HE, Pernica JM, Walson JL, Pavlinac PB. Identification and management of Shigella infection in children with diarrhoea: a systematic review and meta-analysis. Lancet Glob Health. 2017;5: e1235–e1248. 10.1016/S2214-109X(17)30392-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black RE, Brown KH, Becker S. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics. 1984;73: 799–805. [PubMed] [Google Scholar]
- 9.Lee G, Paredes Olortegui M, Peñataro Yori P, Black RE, Caulfield L, Banda Chavez C, et al. Effects of Shigella-, Campylobacter- and ETEC-associated Diarrhea on Childhood Growth. Pediatr Infect Dis J. 2014;33: 1004–1009. 10.1097/INF.0000000000000351 [DOI] [PubMed] [Google Scholar]
- 10.Mani S, Wierzba T, Walker RI. Status of vaccine research and development for Shigella. Vaccine. 2016;34: 2887–2894. 10.1016/j.vaccine.2016.02.075 [DOI] [PubMed] [Google Scholar]
- 11.MAL-ED Network Investigators. The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clin Infect Dis. 2014;59 Suppl 4: S193–206. 10.1093/cid/ciu653 [DOI] [PubMed] [Google Scholar]
- 12.Lee GO, Richard SA, Kang G, Houpt ER, Seidman JC, Pendergast LL, et al. A Comparison of Diarrheal Severity Scores in the MAL-ED Multisite Community Based Cohort Study. J Pediatr Gastroenterol Nutr. 2016. 10.1097/MPG.0000000000001286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee G, Yori PP, Olortegui MP, Caulfield LE, Sack DA, Fischer-Walker C, et al. An instrument for the assessment of diarrhoeal severity based on a longitudinal community-based study. BMJ Open. 2014;4: e004816 10.1136/bmjopen-2014-004816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age, Methods and development. 2006. Available: http://www.who.int/childgrowth/standards/Technical_report.pdf?ua=1 [Google Scholar]
- 15.Psaki SR, Seidman JC, Miller M, Gottlieb M, Bhutta ZA, Ahmed T, et al. Measuring socioeconomic status in multicountry studies: results from the eight-country MAL-ED study. Population Health Metrics. 2014;12: 8 10.1186/1478-7954-12-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Kabir F, Manneh J, Lertsethtakarn P, Begum S, Gratz J, et al. Development and assessment of molecular diagnostic tests for 15 enteropathogens causing childhood diarrhoea: a multicentre study. Lancet Infect Dis. 2014;14: 716–724. 10.1016/S1473-3099(14)70808-4 [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Gratz J, Amour C, Nshama R, Walongo T, Maro A, et al. Optimization of Quantitative PCR Methods for Enteropathogen Detection. PLoS ONE. 2016;11: e0158199 10.1371/journal.pone.0158199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houpt E, Gratz J, Kosek M, Zaidi AKM, Qureshi S, Kang G, et al. Microbiologic Methods Utilized in the MAL-ED Cohort Study. Clin Infect Dis. 2014;59: S225–S232. 10.1093/cid/ciu413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormick BJJ, Lee GO, Seidman JC, Haque R, Mondal D, Quetz J, et al. Dynamics and Trends in Fecal Biomarkers of Gut Function in Children from 1–24 Months in the MAL-ED Study. Am J Trop Med Hyg. 2017;96: 465–472. 10.4269/ajtmh.16-0496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosek M, Guerrant RL, Kang G, Bhutta Z, Yori PP, Gratz J, et al. Assessment of Environmental Enteropathy in the MAL-ED Cohort Study: Theoretical and Analytic Framework. Clin Infect Dis. 2014;59: S239–S247. 10.1093/cid/ciu457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosek MN, Lee GO, Guerrant RL, Haque R, Kang G, Ahmed T, et al. Age and Sex Normalization of Intestinal Permeability Measures for the Improved Assessment of Enteropathy in Infancy and Early Childhood: Results From the MAL-ED Study. J Pediatr Gastroenterol Nutr. 2017;65: 31–39. 10.1097/MPG.0000000000001610 [DOI] [PubMed] [Google Scholar]
- 22.Data sources—Climate-Data.org. [cited 3 Oct 2019]. Available: https://en.climate-data.org/info/sources/
- 23.Quinn E, Najjar Z, Huhtinen E, Jegasothy E, Gupta L. Culture-positive shigellosis cases are epidemiologically different to culture-negative/PCR-positive cases. Aust N Z J Public Health. 2019;43: 41–45. 10.1111/1753-6405.12844 [DOI] [PubMed] [Google Scholar]
- 24.Kosek M, Yori PP, Pan WK, Olortegui MP, Gilman RH, Perez J, et al. Epidemiology of highly endemic multiply antibiotic-resistant shigellosis in children in the Peruvian Amazon. Pediatrics. 2008;122: e541–549. 10.1542/peds.2008-0458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vubil D, Acácio S, Quintò L, Ballesté-Delpierre C, Nhampossa T, Kotloff K, et al. Clinical features, risk factors, and impact of antibiotic treatment of diarrhea caused by Shigella in children less than 5 years in Manhiça District, rural Mozambique. Infect Drug Resist. 2018;11: 2095–2106. 10.2147/IDR.S177579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu-Elyazeed RR, Wierzba TF, Frenck RW, Putnam SD, Rao MR, Savarino SJ, et al. Epidemiology of Shigella-associated diarrhea in rural Egyptian children. Am J Trop Med Hyg. 2004;71: 367–372. [PubMed] [Google Scholar]
- 27.Black RE, Brown KH, Becker S, Alim ARMA, Huq I. Longitudinal Studies of Infectious Diseases and Physical Growth of Children in Rural Bangladesh Ii. Incidence of Diarrhea and Association with Known Pathogens. Am J Epidemiol. 1982;115: 315–324. 10.1093/oxfordjournals.aje.a113308 [DOI] [PubMed] [Google Scholar]
- 28.Rogawski McQuade ET, Clark S, Bayo E, Scharf RJ, DeBoer MD, Patil CL, et al. Seasonal Food Insecurity in Haydom, Tanzania, Is Associated with Low Birthweight and Acute Malnutrition: Results from the MAL-ED Study. Am J Trop Med Hyg. 2019;100: 681–687. 10.4269/ajtmh.18-0547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farag TH, Faruque AS, Wu Y, Das SK, Hossain A, Ahmed S, et al. Housefly Population Density Correlates with Shigellosis among Children in Mirzapur, Bangladesh: A Time Series Analysis. PLOS Neglected Tropical Diseases. 2013;7: e2280 10.1371/journal.pntd.0002280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arndt MB, Richardson BA, Ahmed T, Mahfuz M, Haque R, John-Stewart GC, et al. Fecal Markers of Environmental Enteropathy and Subsequent Growth in Bangladeshi Children. Am J Trop Med Hyg. 2016;95: 694–701. 10.4269/ajtmh.16-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colston JM, Peñataro Yori P, Colantuoni E, Moulton LH, Ambikapathi R, Lee G, et al. A methodologic framework for modeling and assessing biomarkers of environmental enteropathy as predictors of growth in infants: an example from a Peruvian birth cohort. Am J Clin Nutr. 2017;106: 245–255. 10.3945/ajcn.116.151886 [DOI] [PubMed] [Google Scholar]
- 32.Iqbal NT, Sadiq K, Syed S, Akhund T, Umrani F, Ahmed S, et al. Promising Biomarkers of Environmental Enteric Dysfunction: A Prospective Cohort study in Pakistani Children. Sci Rep. 2018;8: 2966 10.1038/s41598-018-21319-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Almeida M, Kabir F, Shakoor S, Qureshi S, Zaidi A, et al. Direct Detection of Shigella in Stool Specimens by Use of a Metagenomic Approach. J Clin Microbiol. 2018;56 10.1128/JCM.01374-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine MM, Nasrin D, Acácio S, Bassat Q, Powell H, Tennant SM, et al. Diarrhoeal disease and subsequent risk of death in infants and children residing in low-income and middle-income countries: analysis of the GEMS case-control study and 12-month GEMS-1A follow-on study. Lancet Glob Health. 2020;8: e204–e214. 10.1016/S2214-109X(19)30541-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Estimates are adjusted for age, diarrheal vs. non-diarrheal stool, and all other factors included in the figure. Estimates are excluded for specific sites for factors with no variability at that site.
(PDF)
Estimates are adjusted for age, sex, and stool consistency.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data including individual participant data are available by request from the ClinEpiDB database (https://clinepidb.org/ce/app/record/dataset/DS_3dbf92dc05).