Abstract
Purpose
To describe multimodal imaging findings with focus on retinal and choroidal vascular features in acute macular neuroretinopathy (AMN).
Observations
Three eyes from 3 patients (1 man, 2 women) with average age of 31 were included in this retrospective case series at a single institution. Each case showed petaloid hyporeflective areas on infrared images (IR) with variable levels of outer retinal defects on spectral domain optical coherence tomography (OCT). En face OCT angiography (OCT-A) images showed quantifiable reduction in vessel density at levels of the deep capillary plexus (DCP) and choriocapillaris (CC) layers. In 2 of the cases with near-infrared autofluorescence imaging (NIRAF), there were subtle areas of hypoautofluorescence corresponding in location to the lesions seen on IR. In one case, fluorescein angiography (FA) showed a small area of retinal vascular leakage in the area of the IR lesion, and in other 2 cases, there were paracentral areas of hypofluorescence in the area of the IR lesions. En face structural OCT image at the retinal pigment epithelium (RPE) level in each case showed no evidence of projection artifact from the retina.
Conclusions and Importance
The pathogenesis of AMN is suspected to involve a vasogenic insult. However, the precise localization of the vascular insult has been controversial and unclear. Our findings demonstrate that concurrent vascular flow defects in both DCP and CC could be possible in AMN and suggest that an inflammatory and vascular etiology in concert could underlie the pathogenesis of AMN.
Keywords: Acute macular neuroretinopathy, Optical coherence tomography angiography, Deep capillary plexus, Choriocapillaris
1. Introduction
Acute macular neuroretinopathy (AMN) was initially described in 1975 using clinical exam and fluorescein angiogram findings.1 Due to associations with use of vasoactive agents and birth control pills, vasogenic etiology was suspected. With the advent of spectral domain optical coherence tomography (OCT), a hyper reflectant band in the outer retina was demonstrated in the absence of apparent retinal pigment epithelium or choroid changes, and a defect at the level of the deep capillary plexus (DCP) was initially hypothesized.2 Availability of OCT angiography (OCT-A) later allowed visualization of vascular networks in the retina and choroid. However, the exact location of the vascular defect within these layers has been an area of controversy. Studies utilizing projection artifact-removed spectral domain (SD) OCT-A images have revealed localized vascular signal attenuation in the DCP.3,4 On the contrary, there have recently been reports showing localized reduction in flow signal in the choriocapillaris (CC) using SD OCT-A.5,6 Furthermore, these reports described no apparent decrease in vascular flow in the DCP layer. However, it was not clear whether the signal attenuation seen in the CC layer was primarily a projection artifact due to the overlying hyperreflective band typically seen in AMN.
Herein, we describe 3 patients with AMN who showed evidence of concurrent flow deficits in both the DCP and CC on SD OCT-A. We also show using en face structural OCT images at the RPE layer to show that the signal attenuation detected in the CC layer is less likely to be due to a projection artifact.
Medical records of the 3 patients who were evaluated under an institutional review board-approved protocol in the Uveitis and Ocular Immunology Clinic at the National Eye Institute were reviewed.
2. Findings
2.1. Case 1
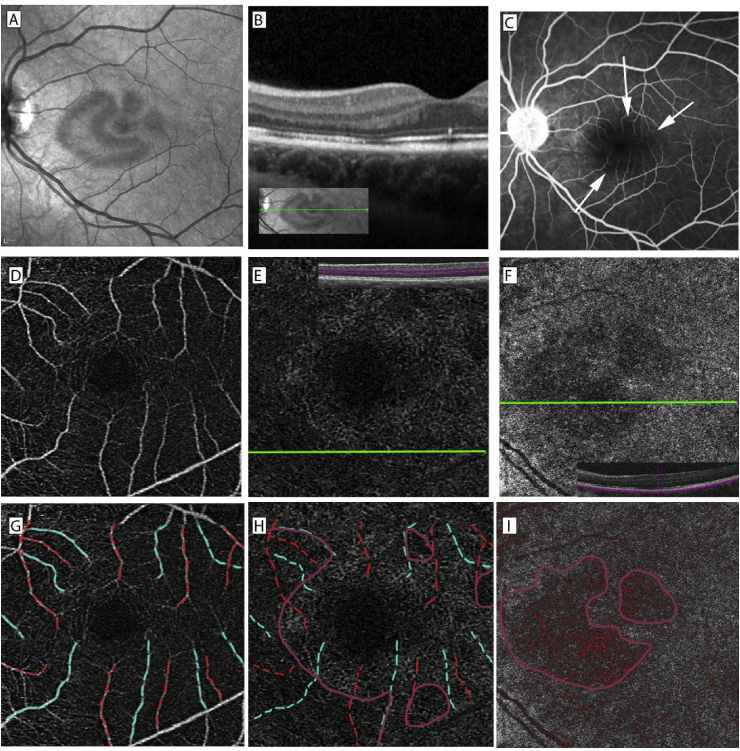
A 30-year-old male with a history of attention deficit hyperactivity disorder on amphetamine/dextroamphetamine presented with a one-week history of paracentral scotoma in the left eye. Best-corrected visual acuity was 20/20 OU, and formal visual field testing showed left paracentral scotoma. Anterior segment exam was normal. Dilated fundus exam (DFE) revealed multi-lobular brown lesion, which was visualized most prominently with infrared reflectance imaging (IR) (Fig. 1A). OCT showed loss of ellipsoid and RPE interdigitation zones (EZ and RZ, respectively) with outer retinal layer thinning (Fig. 1B). OCT-A revealed flow signal reductions in both the DCP and CC layers, corresponding to the lesion seen in IR (Fig. 1F–H). There was no visible projection artifact on the en face structural OCT image at the level of RPE (Fig. 4D). Near-infrared autofluorescence (NIRAF) revealed a subtle area of hypoautofluorescence in the parafovea (Fig. 1K). Late-phase fluorescein angiography (FA) showed focal areas of leakage involving two vessels over the superior lobule of the lesion (Fig. 1C), and OCT-A showed that these two vessels represented an arteriole and venule that anastomosed through the same vascular vortex in the DCP (Fig. 1G).
Fig. 1.
Multimodal imaging of patient 1. IR demonstrates multi-lobular hyporeflective parafoveal lesion of the left eye (A). OCT line scan demonstrates thinning of the outer nuclear layer and disruption of the ellipsoid and RPE interdigitation zones (B). Late-phase FA of the left eye shows an area of parafoveal leakage (C), which is highlighted in red square and visualized at higher magnification (D). NIRAF reveals a subtle area of hypoautofluorescence (arrow) in the parafovea (K). 3 × 3 OCT-A of the SCP (E), DCP (F), and CC (H) of the left eye reveal areas of vascular flow signal attenuation at the DCP and CC levels. Anastomosis (arrow) between the leaking venule and arteriole is highlighted in red square (F) and visualized at higher magnification (G). The arterioles and venules of interest in the SCP are colored in red and blue using Adobe Photoshop (Adobe Systems, Inc., San Jose, CA, USA) (I), respectively, which is overlayed on the DCP image (J). Areas of vascular signal attenuation in the DCP × and CC are outlined manually in purple using Adobe Photoshop (J and L, respectively). Areas of vascular flow voids in CC were highlighted in red, using Matlab (R2018; MathWorks, Inc., Natick, MA, USA) custom algorithm. (L). *Using Mean auto thresholding and binarization algorithm in ImageJ (National Institutes of Health, Bethesda, Maryland, USA), vascular density of the DCP was 11% reduced in the affected eye compared to the unaffected eye. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
Cross-sectional (A, B, C) and en face (D, E, F) structural OCT images for cases 1, 2, and 3, respectively, do not show signal attenuation at the level of RPE that could suggest projection artifact from the retina.
2.2. Case 2
A 15-year-old female with a history of Takayasu arteritis on prednisone, methotrexate, and tocilizumab presented with 7-month history of decreased vision in the left eye. Best-corrected visual acuity was 20/16 OD and 20/800 OS. Anterior segment exam was normal. DFE revealed paracentral multi-lobular brown lesion in the left eye, which was best seen with IR (Fig. 2A). OCT showed loss of EZ and RZ with outer retinal layer thinning (Fig. 2B). Mid-phase FA showed areas of subtle hypofluorescence corresponding to the IR lesions in the parafovea (Fig. 2C). OCT-A revealed flow signal reductions in both the DCP and CC layers, corresponding to the IR lesions (Fig. 2E–F). There was no visible projection artifact on the en face structural OCT image at the level of RPE (Fig. 4E).
Fig. 2.
Multimodal imaging of patient 2. IR demonstrates a multi-lobular hyporeflective parafoveal lesion of the left eye (A). OCT line scan demonstrates thinning of the outer nuclear layer and disruption of the ellipsoid and RPE interdigitation zones (B). Mid-phase FA of the left eye (C) shows areas of hypofluorescence (arrows). 3 × 3 OCT-A of the SCP (D) and DCP (E) and 6 × 6 OCT-A of the CC (F) of the left eye reveal areas of vascular flow signal attenuation at the DCP and CC levels. The arterioles and venules of interest in the SCP are colored in red and blue (G), respectively, which is overlayed on the DCP image (H). Areas of vascular signal attenuation in the DCP × and CC are highlighted manually in purple (H and I, respectively). Areas of vascular flow voids in CC were highlighted in red, using Matlab (R2018; MathWorks, Inc., Natick, MA, USA) custom algorithm. (I). *Vascular density of the DCP was 30% reduced in the affected eye compared to the unaffected eye. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.3. Case 3
A 48-year-old female with history of blepharitis on minocycline and oral contraceptive pill presented with a 1-month history of paracentral scotoma in the right eye. Best-corrected visual acuity was 20/16 OD and 20/20 OS. Anterior segment exam was normal. DFE revealed a lobular reddish lesion in the macula of the right eye, which was best seen with IR (Fig. 3A). OCT showed loss of EZ and RZ with outer retinal layer thinning (Fig. 3B). NIRAF revealed a subtle area of hyperautofluorescence with a hypofluorescent border (Fig. 3L). Mid-phase FA of the right eye showed a subtle area of hypofluorescence corresponding to the IR lesion (Fig. 3C). OCT-A revealed flow deficits in both the DCP and CC layers, corresponding to the IR lesion (Fig. 3F and G). There was no visible projection artifact on the en face structural OCT image at the level of RPE (Fig. 4F).
Fig. 3.
Multimodal imaging of patient 3. IR demonstrates a lobular hyporeflective lesion of the right eye (A). OCT line scan demonstrates thinning of the outer nuclear layer and disruption of the ellipsoid and RPE interdigitation zones (B). Mid-phase FA of the right eye shows an area of hypofluorescence (arrow) (C), which is highlighted in red square and visualized at higher magnification (D). NIRAF reveals a subtle area of hyperautofluorescence with a hypofluorescent border (arrow) (L). 6 × 6 OCT-A of the SCP (E), DCP* (F), and CC (G) of the right eye reveal subtle areas of vascular flow signal attenuation at the DCP and CC levels. The subtle area of flow signal attenuation in the CC is highlighted in red square and visualized in higher magnification (H). The arterioles and venules of interest in the SCP are colored in red and blue (I), respectively, which is overlayed on the DCP image (J). Areas of vascular signal attenuation in the DCP** and CC are outlined manually in purple (J and K, respectively). * The area of flow signal attenuation was outside the margin of 3 × 3 OCT-A of the DCP, and thus, 6 × 6 OCT-A of the DCP was used for analysis instead. ** Because the area of flow signal attenuation in DCP was relatively small compared to the 6 × 6 OCT-A image, a 1.5 × 1.5 cropped image that included the area of flow signal attenuation was compared to an analogous area in the unaffected eye. The vascular density of the 1.5 × 1.5 cropped image of DCP was 22% reduced in the affected eye compared to the analogous area in the unaffected eye. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
A vascular etiology has been suspected in the pathogenesis of AMN, but the exact location, specifically between the DCP and CC, has been under debate. Traditionally, the DCP had been thought to be the location of a vascular insult in AMN. The DCP provides 10–15% vascular supply to the photoreceptors in animal models,7 and photoreceptor axons replete with mitochondria are in the watershed area supplied by the DCP and are thought to be especially vulnerable to ischemic injury from the DCP.8 Using cross-sectional B-scans analyzed by projection-resolved OCT-A, Chu and coworkers showed DCP flow signal reduction in AMN cases corresponding to the area of the IR lesion.4 However, it was not entirely clear why other retinal conditions involving the DCP such as parafoveal acute middle maculopathy4 would not exhibit similar features as those seen in AMN. Additionally, abnormal autofluorescence (AF) seen in NIRAF2 and subtle hypofluorescence corresponding to the location of AMN lesions in the late phases of FA9 in some AMN cases could not be explained sufficiently by the DCP hypothesis alone.
Alternatively, CC ischemia was recently hypothesized to underlie the pathogenesis of AMN. In 2016, Thanos and coworkers showed 3 AMN cases with CC flow signal reduction on en face SD OCT-A images, but no visible flow signal reduction in 6 × 6 en face SD OCT-A of the DCP.5 However, it was not clear whether the reduced signal was due to true flow voids or an artifact from the hyper-reflective band in the outer retina. In 2017, after longitudinally following 7 patients over a mean period of 11 weeks, Lee and coworkers using SD OCT-A showed that the CC flow signal reduction became more pronounced while the hyper-reflective band on OCT either decreased significantly or resolved completely over time,6 demonstrating that the CC signal reduction was less likely to be an artifact. Anatomically, the CC is arranged in series of polygonal mosaic lobules,10 and Lee and coworkers hypothesized that the lobular shape of AMN lesions could represent ischemic injury to one or more of these lobules in the CC.6
In this report, we show that vascular flow defects could occur in both the DCP and CC. Unlike the SCP and MCP, the DCP has a flat laminar structure and is arranged in vortices.11,12 Using en face SD OCT-A, Nesper and Fawzi demonstrated that an arteriole terminating in the DCP connects to a draining venule in SCP via DCP capillaries and draining channel in vortices.12 In case 1, the parafoveal leakage of two vessels on FA occurred in the superior lobule of the AMN lesion. More importantly, the two vessels were demonstrated on OCT-A to be an arteriole and venule that were supplying and draining the same vortex in the DCP. Analogous to the CC lobule ischemia hypothesis, these data suggest that the DCP ischemia could also lead to lobular presentation by affecting the DCP vortices.
As demonstrated by Lee and coworkers, our cases also support the view that the vascular flow signal reduction in the CC is less likely derived from an artifact. The OPL hyper-reflective band on OCT typically appears acutely and resolves rapidly within 1–2 weeks.2 Patient 2 and patient 3 demonstrated CC flow signal reductions 7 months and 1 month post-symptom onset, respectively, when the hyper-reflective band had resolved with no visible projection artifact on en face structural OCT images at the level of RPE. Additionally, both cases showed hypofluorescent areas corresponding to the AMN lesion on FA, which could indicate CC flow deficits as hypothesized previously by Yeh and coworkers.9
The exact pathogenesis of AMN has been elusive. In a comprehensive review of the literature spanning a 40-year period, Bhavsar and coworkers showed that a preceding febrile illness was associated with nearly 50% of the cases and was the most commonly associated factor.13 Moreover, numerous cases of AMN have been reported in the setting of dengue fever, as high as 55% of patients with dengue fever-associated maculopathies.14 Furthermore, AMN has been reported to occur post vaccine administration.3 In our series, different pathophysiology may have served as contributing risk factors to a final common pathway of DCP and CC ischemia. For instance, in case 1, amphetamine could induce ischemia through vasoconstrictive effects. Takayasu arteritis, in patient 2, could impact oxygenation status globally (as this is a large-vessel disease), and retinal and/or choroidal vascular disease could subsequently be observed. In case 3, OCP use could lead to ischemia secondary to endothelial cell dysfunction. These distinct etiologies appear to converge through the shared pathway of microvascular insults in DCP and CC.
Around the time our manuscript was submitted for publication, Casalino and coworkers15 published a study that demonstrated areas of signal attenuation at the DCP and CC layers on OCT-A corresponding to the AMN lesions. However, the authors did not address the central question of our current report of whether the areas of OCT-A signal attenuation were true vascular flow deficit or simply artifacts. Our study adds valuable insight to the literature by demonstrating that real vascular flow voids at the levels of DCP and CC could concurrently exist in AMN. To prevent creating an artifactual area of DCP signal attenuation in areas of outer retinal atrophy, we manually corrected the OCT-A software-generated segmentation lines for DCP so that the segmentation lines would capture the entire DCP. To ensure that there was no detectable projection artifact arising from the outer retina, we obtained en face OCT image at the RPE layer and confirmed the absence of projection artifacts for all 3 of our cases. We also utilized additional imaging studies such as NIFAF and FA to show findings that indicate possible presence of RPE defect and CC flow deficits.
Our findings demonstrating concurrent flow deficits in two independent vascular networks (retinal and choroidal) could collectively suggest that an inflammatory and vascular etiology in concert could underlie the pathogenesis of AMN. Our cases support the hypothesis that microvascular insult occurred initially followed by a possible inflammatory insult. It is possible that a primary insult could occur initially in either the DCP or CC with secondary vascular changes in the other layer at a later time point through an inflammatory etiology, but this hypothesis was not specifically tested in this report. Additionally, due to the small sample size in this report, future studies with larger sample sizes are needed to confirm our findings.
4. Conclusions
Vascular etiology has been suspected in the pathogenesis of AMN. Localization of the vascular defect between the DCP and CC in the pathogenesis of AMN has been under debate. Our cases show that concurrent vascular insults in both the DCP and CC are possible in AMN and could suggest that an inflammatory and vascular etiology in concert could underlie the pathogenesis of AMN.
Consent to publish this case report has been obtained from the patient(s) in writing.
Funding
The Heed Ophthalmic Foundation (CKH), National Eye Institute Intramural Research Program (CKH, HNS).
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
None to disclose.
Acknowledgements
None.
References
- 1.Bos P.J., Deutman A.F. Acute macular neuroretinopathy. Am J Ophthalmol. 1975;80(4):573–584. doi: 10.1016/0002-9394(75)90387-6. [DOI] [PubMed] [Google Scholar]
- 2.Fawzi A.A., Pappuru R.R., Sarraf D. Acute macular neuroretinopathy: long-term insights revealed by multimodal imaging. Retina. 2012;32(8):1500–1513. doi: 10.1097/IAE.0b013e318263d0c3. [DOI] [PubMed] [Google Scholar]
- 3.Liu J.C., Nesper P.L., Fawzi A.A., Gill M.K. Acute macular neuroretinopathy associated with influenza vaccination with decreased flow at the deep capillary plexus on OCT angiography. Am J Ophthalmol. 2018;10:96–100. doi: 10.1016/j.ajoc.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu S., Nesper P.L., Soetikno B.T., Bakri S.J., Fawzi A.A. Projection-resolved OCT angiography of microvascular changes in paracentral acute middle maculopathy and acute macular neuroretinopathy. Invest Ophthalmol Vis Sci. 2018;59(7):2913–2922. doi: 10.1167/iovs.18-24112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thanos A., Faia L.J., Yonekawa Y., Randhawa S. Optical coherence tomographic angiography in acute macular neuroretinopathy. JAMA Ophthalmol. 2016;134(11):1310–1314. doi: 10.1001/jamaophthalmol.2016.3513. [DOI] [PubMed] [Google Scholar]
- 6.Lee S.Y., Cheng J.L., Gehrs K.M. Choroidal features of acute macular neuroretinopathy via optical coherence tomography angiography and correlation with serial multimodal imaging. JAMA Ophthalmol. 2017;135(11):1177–1183. doi: 10.1001/jamaophthalmol.2017.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birol G., Wang S., Budzynski E., Wangsa-Wirawan N.D., Linsenmeier R.A. Oxygen distribution and consumption in the macaque retina. Am J Physiol Heart Circ Physiol. 2007;293(3):H1696–H1704. doi: 10.1152/ajpheart.00221.2007. [DOI] [PubMed] [Google Scholar]
- 8.Stone J., van Driel D., Valter K., Rees S., Provis J. The locations of mitochondria in mammalian photoreceptors: relation to retinal vasculature. Brain Res. 2008;1189:58–69. doi: 10.1016/j.brainres.2007.10.083. [DOI] [PubMed] [Google Scholar]
- 9.Yeh S., Hwang T.S., Weleber R.G., Watzke R.C., Francis P.J. Acute macular outer retinopathy (AMOR): a reappraisal of acute macular neuroretinopathy using multimodality diagnostic testing. Arch Ophthalmol. 2011;129(3):365–368. doi: 10.1001/archophthalmol.2011.22. [DOI] [PubMed] [Google Scholar]
- 10.Hayreh S.S. The choriocapillaris. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1974;192(3):165–179. doi: 10.1007/BF00416864. [DOI] [PubMed] [Google Scholar]
- 11.Hirano T., Chanwimol K., Weichsel J., Tepelus T., Sadda S. Distinct retinal capillary plexuses in normal eyes as observed in optical coherence tomography angiography axial profile Analysis. Sci Rep. 2018;8(1):9380. doi: 10.1038/s41598-018-27536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nesper P.L., Fawzi A.A. Human parafoveal capillary vascular anatomy and connectivity revealed by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2018;59(10):3858–3867. doi: 10.1167/iovs.18-24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhavsar K.V., Lin S., Rahimy E. Acute macular neuroretinopathy: a comprehensive review of the literature. Surv Ophthalmol. 2016;61(5):538–565. doi: 10.1016/j.survophthal.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Li M., Zhang X., Ji Y., Ye B., Wen F. Acute macular neuroretinopathy in dengue fever: short-term prospectively followed up case series. JAMA Ophthalmol. 2015;133(11):1329–1333. doi: 10.1001/jamaophthalmol.2015.2687. [DOI] [PubMed] [Google Scholar]
- 15.Casalino G., Arrigo A., Romano F., Munk M.R., Bandello F., Parodi M.B. Acute macular neuroretinopathy: pathogenetic insights from optical coherence tomography angiography. Br J Ophthalmol. 2019;103(3):410–414. doi: 10.1136/bjophthalmol-2018-312197. [DOI] [PubMed] [Google Scholar]