Abstract
Adult-onset diffuse leukoencephalopathy with axonal spheroids and pigmented glia (ALSP) is a rare progressive degenerative white matter disease caused by mutations in the colony-stimulating factor-1 receptor gene. Patients commonly present in the 4th or 5th decade with variable clinical presentations including behavioral changes, dementia, parkinsonism, and motor dysfunctions, eventually leading to death within a few years. Although the disease is typically hereditary, sporadic cases are known to occur. The classic MRI features of ALSP include T2 hyperintensities in the frontal and parietal white matter, scattered foci of restricted diffusion in the white matter, age-advanced cerebral involutional changes, thinning and signal changes in the corpus callosum, absence of infratentorial involvement and lack of enhancement. CT commonly shows tiny calcifications in the corpus callosum and deep white matter.
We report a unique case of sporadic ALSP that initially presented as young stroke with acute onset of left-sided hemiparesis and no preceding history of cognitive decline. However, subsequent cognitive and behavioral changes lead to the consideration of an alternative diagnosis. Stroke-like symptoms is a very rare primary presentation of this disease entity. We have highlighted the classic MRI and CT features that helped to guide its diagnosis in our patient and prompted early corroborative genetic testing.
Keywords: Young stroke, ALSP, Cognitive dysfunction, T2 white matter hyperintensity, CSF1R gene
Introduction
Adult-onset diffuse leukoencephalopathy with axonal spheroids and pigmented glia (ALSP) is a hereditary autosomal dominant leukodystrophy associated with progressive cognitive, psychiatric and motor dysfunction. Nonhereditary sporadic cases are also known to occur. The onset of symptoms typically occurs in the 4th or 5th decade, eventually leading to dementia and death within a few years. ALSP is now known to be caused by mutations in the colony-stimulating factor 1 receptor (CSF1R) gene [1], [2], [3], [4], [5], [6], [7], [8], [9], [10].
We present a unique case report of sporadic ALSP presenting with acute stroke-like symptoms, albeit with no previous cognitive complaints. We aim to highlight the unique first clinical presentation of acute-onset hemiparesis causing an initial diagnostic dilemma, which was subsequently solved by carefully correlating the progressive cognitive decline with classic imaging features (on both MRI and CT) and prompt genetic testing.
Case report
A 32-year-old Chinese male presented to the Emergency Department with acute onset of left arm and left leg weakness that had started the same morning. He had no prior medical conditions. His right arm and right leg power was not affected and sensation was intact in all 4 limbs. MRI stroke study at this time (Fig. 1) showed foci of restricted diffusion in bilateral corona radiata, on the right more than the left. These foci of restricted diffusion were seen on a background of periventricular and deep white matter T2 hyperintensities, again more extensive on the right side. There was no parenchymal hemorrhage, mass effect or large vessel occlusion. The clinical and radiological impression at that point of time was acute ischemic stroke. Vasculitis was considered as a possible aetiology, considering the age-advanced background white matter signal changes. Young stroke work-up (which included screening for venereal, immunological and hematological disorders) was unremarkable. Ultrasound of the carotid arteries did not reveal any significant stenosis. Cardiac workup including transthoracic echocardiogram and holter monitoring was unremarkable for any cardioembolic causes of stroke. The patient was started on aspirin and discharged after 5 days with some improvement in motor power. He was able to walk independently despite some residual left-sided weakness.
Fig. 1.
MRI brain study at initial presentation (baseline study). Axial diffusion weighted (DWI) image (a) and ADC map (b) show scattered foci of restricted diffusion (arrows) in bilateral corona radiata (right > left). Axial T1W image (c) shows hypointense signal in some of these foci. Axial T2W image (d) and axial FLAIR image (e) show hyperintense signal in the periventricular and deep white matter of the frontal and parietal lobes, more confluent on the right. MR angiogram maximum intensity projection image (f) shows unremarkable intracranial arteries.
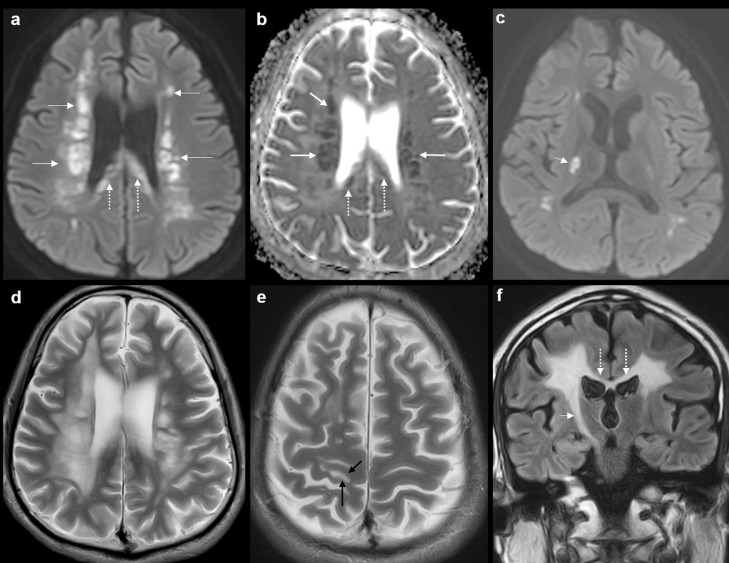
Over the next 5 months, the patient started developing behavioral and cognitive changes. The patient's family reported that the patient had become less motivated, more distracted, and required more assistance for activities of daily living. A clinical review at 5 months showed reduced fluency of speech and word finding difficulties. There was a corresponding decline in Montreal Cognitive Assessment (MOCA) score from an initial 26 (out of 30) to 23, with deficits in visuospatial, delay recall and fluency components. The patient continued to have gait unsteadiness and progressive left-sided spastic hemiparesis. At this point, the patient was again admitted for further evaluation and inpatient rehabilitation. On examination, the left hand was held in flexed spastic resting posture and the left ankle was in plantarflexion, with co-contraction of the left quadriceps and hamstring muscles. There was an asymmetrical increase in tone with sustained ankle clonus. A repeat MRI study of the brain at this time (Fig. 2) showed interval appearance of striking cerebral involutional changes, including in the corpus callosum. There was interval progression and confluence of T2/FLAIR hyperintensities in the periventricular and deep white matter of bilateral frontal and parietal lobes (right > left). Subcortical white matter involvement was now seen in the right precentral and postcentral gyri. Also, there was corresponding T1 hypointensity in the involved regions. As compared to the baseline MR scan, the areas of restricted diffusion in the deep white matter had increased in size and number. Multiple foci of restricted diffusion were also seen in the corpus callosum. New T2/FLAIR hyperintensity and restricted diffusion was noted along the entire right corticospinal tract up to the right cerebral peduncle. No abnormal contrast enhancement was seen. The constellation of imaging findings and the progressive cognitive and motor dysfunctions at this stage prompted an alternative diagnosis as against the initial red herring picture of stroke. Adult-onset leukoencephalopathy and vasculitis were considered as likely differentials. Absence of anterior temporal and external capsule involvement ruled out cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) [4,9,11]. The striking areas of restricted diffusion in the deep white matter, corpus callosum and right corticospinal tract raised suspicion for ALSP [3,[6], [7], [8], [9], [10],[12], [13], [14], [15], [16], [17]. The subsequent noncontrast enhanced CT (NECT) study (Fig. 3; with 1mm thick sagittal reconstructions) detected tiny specks of calcification in the corpus callosum, adjacent to the frontal horns of the lateral ventricles, confirming the radiological diagnosis of ALSP [9,10,14,16].
Fig. 2.
Second MRI brain study (at 5 months). Axial DWI image (a) and ADC map (b) show interval increase in size and number of foci of restricted diffusion (arrows) in the deep white matter of bilateral frontal and parietal lobes. Note the striking involvement of the splenium of the corpus callosum (dotted arrows). Axial DWI image at the level of the internal capsule (c) shows restricted diffusion in the right corticospinal tract (short arrow). Axial T2W images (d, e) show cerebral involutional changes and sulcal widening. The periventricular and deep white matter T2 hyperintense signal is more extensive than in the baseline study. Note the involvement of subcortical white matter of right precentral gyrus (black arrows in (e). Coronal FLAIR image (f) shows marked atrophy and hyperintense signal in the body of the corpus callosum (dotted arrows). Extensive involvement of the right corticospinal tract (short arrow) is seen up to the midbrain.
Fig. 3.
NECT brain study (at 5 months). Thin-section sagittal reconstructions showing characteristic punctate calcifications (arrows) in the white matter adjacent to the frontal horns of the right lateral ventricle (a) and left lateral ventricle (b).
Whole genome sequencing revealed a novel intronic variant located next to exon 18 within the CSF1R gene. This variant was deemed to be potentially pathogenic given the consistent clinical phenotype, and that it was located next to a previously reported pathogenic variant associated with ALSP (1). Functional validation of this variant is underway. Family counseling revealed that there was no history of similar illness, and none of his siblings have displayed similar symptoms.
Over the next 3 months, the patient showed further rapid progression in executive and cognitive dysfunction. Disinhibition, emotional lability and urinary incontinence ensued at this time, eventually leading to him becoming bedbound and uncommunicative. A repeat third MRI study at this time showed further progression of all the imaging features mentioned in the second MRI study.
The last imaging study was a NECT (Fig. 4) performed 19 months after the first presentation. It showed marked global involutional changes, confluent white matter hypodensity and appearance of coarse calcific specks in the left corona radiata, again a well-described imaging feature of ALSP [14,16].
Fig. 4.
NECT brain study (at 19 months). Coronal images show discrete new calcifications in the white matter adjacent to the frontal horn of the left lateral ventricle (a) and in the left corona radiata (b).
Discussion
ALSP is a rare progressive degenerative white matter disorder. In recent years, it has been included in the disease spectrum of previously separate entities, namely, hereditary diffuse leukoencephalopathy with axonal spheroids and pigmentary orthochromatic leukodystrophy. This group of leukoencephalopathies is associated with mutations affecting the tyrosine kinase domain of the CSF1R gene. Since CSF1R is an important mediator of microglial proliferation in the brain, it is likely that microglial dysfunction plays a critical role in the pathogenesis of these leukoencephalopathies, also labeled as microgliopathies. Their neuropathological hallmark includes severe axonal and myelin loss, axonal swelling (spheroids) and pigmented macrophages and microglia [1], [2], [3], [4].
ALSP exhibits autosomal dominant inheritance, with patients typically presenting in the 4th decade (mean age of onset is 43 years). Sporadic cases as ours may occur due to genetic mosaicism, incomplete penetrance or de novo mutations [1], [2], [3], [4], [5], [6]. Disease onset is usually marked by neuropsychiatric features such as behavioral changes, cognitive decline, executive dysfunction and depression. Concurrent or subsequent motor and gait dysfunctions (apraxia, ataxia), pyramidal dysfunctions, dysarthria, parkinsonism, urinary and fecal incontinence are commonly seen. Stroke-like primary presentation with no previous neuropsychiatric features (as seen in our case) is extremely rare with only a few cases reported in literature [7,8,10]. Seizures and spasticity occur in the later stages of the disease. ALSP runs a rapid and dismal clinical course, with death occurring within 6-7 years [1], [2], [3], [4], [5], [6], [7], [8], [9], [10].
ALSP is known to be under-recognized clinically. Histologically-confirmed cases of ALSP at autopsy have been shown to have been clinically misdiagnosed as vascular dementia (especially in sporadic cases), CADASIL, multiple sclerosis, frontotemporal dementia, Alzheimer's disease, and Parkinsonism [1,4,8,15]. In an original study including 83 confirmed ALSP cases with CSF1R mutations [9], Konno T. et al concluded that the ‘core diagnostic features’ of ALSP could be summarized as follows: (i) age of onset under 60 years, (ii) 2 or more clinical findings out of cognitive/psychiatric symptoms, pyramidal signs, parkinsonism and epilepsy, (iii) autosomal dominant or sporadic occurrence, (iv) classic brain MR and CT findings, and (v) exclusion of vascular dementia, other adult-onset leukodystrophies and multiple sclerosis.
We performed an extensive review of literature to highlight the characteristic imaging features of ALSP [1], [2], [3], [4], [5], [6], [7], [8], [9], [10],[12], [13], [14], [15], [16], [17]. Bilateral T2/FLAIR hyperintensities in the periventricular and deep white matter of the frontal and parietal lobes are the hallmarks of ALSP on MRI. The T2/FLAIR hyperintensities may be asymmetric initially, but eventually grow confluent. Corresponding T1 hypointensity is commonly seen in the involved regions. Subcortical white matter deep to pre and postcentral gyri and corticospinal tracts are often involved. Temporal and occipital lobe involvement is uncommon. Infratentorial involvement is not seen. Other characteristic features include diffuse cerebral atrophy (especially in the frontal or frontoparietal regions) and dilatation of lateral ventricles. Multifocal T2/FLAIR hyperintense lesions with thinning of the corpus callosum is another hallmark feature; the volume loss is most marked in the splenium. ALSP classically shows persistent foci of restricted diffusion in the involved white matter (in more than two-third cases), which helps to differentiate it from other leukodystrophies such as metachromatic leukodystrophy, X-linked adrenoleukodystrophy and Krabbe's disease. CADASIL may show foci of restricted diffusion in the white matter, however its typical anterior temporal and external capsule involvement as well as parenchymal microhemorrhages are not seen in ALSP. Contrast enhancement is not seen in ALSP.
Thin section (1 mm) NECT images are useful to demonstrate tiny characteristic calcifications which are reported in approximately 54% cases of ALSP. These calcifications may occur in a symmetric “stepping stone” pattern in the pericallosal regions (adjacent to the frontal horns of the lateral ventricles) or scattered in the frontal and parietal white matter. ALSP does not show calcifications in the basal ganglia, thalamus or cerebellum which helps to differentiate it from other metabolic and genetic conditions [4,9,10,13,14,16]. MR spectroscopy studies have shown decreased N-acetylaspartate and increased choline and myoinositol in involved regions, however, these are nonspecific findings [4,7,17]. FDG-PET imaging in a few reported cases revealed diffuse hypometabolism in the frontal and parietal lobes [4,8].
In our case, the acute hemiparesis and deep water-shed territory restricted diffusion were both red-herrings, leading to an initial confounding diagnosis. We believe that the initial sites of neurodegeneration in our patient could have possibly been in the corona radiata (as seen on the first MRI study). This possibly resulted in motor deficit as the first symptom. Subsequently the patient developed the characteristic constellation of clinical signs of ALSP. These corroborated with the MRI findings (second MRI study) of diffuse cerebral atrophy, ventricular prominence, bilateral confluent T2/FLAIR white matter hyperintensities, persistent and new foci of restricted diffusion in the white matter, atrophy and restricted diffusion in the corpus callosum, involvement of the right corticospinal tract, absence of infratentorial involvement and absence of contrast enhancement. Tiny characteristic calcifications in the corpus callosum seen on NECT further cemented the diagnosis of ALSP. A prompt genetic test was sought thereby circumventing the need for brain biopsy. The patient's subsequent rapid clinical decline was associated with worsening of findings in the third MRI study. The last NECT study showed new appearance of discrete calcifications in the corona radiata, again an expected radiological feature.
ALSP is currently untreatable. Supportive management is used to control symptoms like seizures and spasticity. Multiple authors have advocated early screening of CSF1R mutations in patients with a clinico-radiological picture of undefined CNS vasculitis or adult-onset leukodystrophy especially with a background of neuropsychiatric signs or dementia. Genetic fingerprinting will allow for clarification of broader family risk and for predictive testing in younger family members.
Conclusion
Our case describes acute-onset hemiparesis as a primary clinical presentation of ALSP. The initial clinico-radiological workup suggested ischemic stroke, which was actually a distractor. We hope that clinicians and radiologists could learn from our experience, and that this case serves as a reminder to keep an open mind while considering various differentials of “young stroke.” Also, awareness of the classic MRI and CT features of ALSP in the appropriate clinical setting would aid in early genetic testing and avoiding a biopsy. MRI and NECT may be useful screening tools in identifying presymtpomatic individuals in familial cases of ALSP, thereby improving counseling and prognostication.
Acknowledgments
Consent to publish
No ethics committee approval is required for case report publication as per our institutional policy. No personally identifiable data or photos are in the case report.
Author contributions
Bela Purohit: Study concept, drafted manuscript, literature review, prepared images. Fasial Johandi: Data collection, drafted manuscript, literature review. Yih Yian Sitoh: Literature review. Adeline Ng: Genetic analysis. Carol Tham: Edited manuscript.
Footnotes
A version of this manuscript was presented as an electronic poster at the 5th Annual European Stroke Organisation Conference (ESOC) in Milan, May 2019.
Grants information: No grants were applied or received. No conflict of interests.
Acknowledgments: The authors would like to acknowledge and thank Ms. Jayne Tan (Dept. of Neurology, National Neuroscience Institute) for her kind assistance in collection of samples for genetic analysis and Dr. Lim Weng Khong (Singhealth Duke-NUS Institute of Precision Medicine, Singapore; Cancer & Stem Cell Biology Program, Duke-NUS Medical School, Singapore) for his kind assistance with genetic analysis.
References
- 1.Rademakers R., Baker M., Nicholson A.M., Rutherford N.J, Finch N., Soto-Ortolaza A. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat Genet. 2011;44(2):200–205. doi: 10.1038/ng.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foulds N., Pengelly R.J., Hammans S.R., Nicoll J.A.R., Ellison D.W., Ditchfield A. Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia caused by a novel R782G mutation in CSF1R. Sci Rep. 2015;5:10042. doi: 10.1038/srep10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch D.S., Jaunmuktane Z., Sheerin U.M., Phadke R., Brandner S., Milonas I. Hereditary leukoencephalopathy with axonal spheroids: a spectrum of phenotypes from CNS vasculitis to parkinsonism in an adult onset leukodystrophy series. J Neurol Neurosurg Psychiatry. 2016;87(5):512–519. doi: 10.1136/jnnp-2015-310788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams S.J, Kirk A., Auer R.N. Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP): Integrating the literature on hereditary diffuse leukoencephalopathy with spheroids (HDLS) and pigmentary orthochromatic leukodystrophy (POLD) J Clin Neurosci. 2018;48:42–49. doi: 10.1016/j.jocn.2017.10.060. [DOI] [PubMed] [Google Scholar]
- 5.Karle K.N., Biskup S., Schüle R., Schweitzer K.A., Krüger R., Bauer P. De novo mutations in hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS) Neurology. 2013;81(23):2039–2044. doi: 10.1212/01.wnl.0000436945.01023.ac. [DOI] [PubMed] [Google Scholar]
- 6.Kleinfeld K., Mobley B., Hedera P., Wegner A., Sriram S., Pawate S. Adult-onset leukoencephalopathy with neuroaxonal spheroids and pigmented glia: report of five cases and a new mutation. J Neurol. 2013;260(2):558–571. doi: 10.1007/s00415-012-6680-6. [DOI] [PubMed] [Google Scholar]
- 7.Battisti C., Donato I.D., Bianci S., Monti L., Formichi P., Rufa A. Hereditary diffuse leukoencephalopathy with axonal steroids: three patients with stroke-like presentations carrying new mutations in CSF1R gene. J Neurol. 2014;261(4):768–772. doi: 10.1007/s00415-014-7257-3. [DOI] [PubMed] [Google Scholar]
- 8.Freeman S.H., Hyman B.T., Sims K.B., Hedley-Whyte E.T., Vossough A., Frosch M.P. Adult onset leukoencephalopathy with neuroaxonal spheroids: clinical, neuroimaging and neuropathological observations. Brain Pathol. 2009;19(1):39–47. doi: 10.1111/j.1750-3639.2008.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konno T., Yoshida K., Mizuta I., Mizuno T., Kawarai T., Tada M. Diagnostic criteria for adult-onset leukoencephalopathy with axonal spheroids and pigmented glia due to CSF1R mutation. Eur J Neurol. 2018;25:142–147. doi: 10.1111/ene.13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konno T., Yoshida K., Mizuno T., Kawarai T., Tada M., Nozaki H. Clinical and genetic characterization of adult-onset leukoencephalopathy with axonal spheroids and pigmented glia associated with CSF1R mutation. Eur J Neurol. 2017;24:37–45. doi: 10.1111/ene.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoemaker D., Quiroz Y.T., Torrico-Teave H., Arboleda-Velasquez J.F. Clinical and research applications of magnetic resonance imaging in the study of CADASIL. Neurosci Lett. 2019;298:173–179. doi: 10.1016/j.neulet.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mateen F.J., Keegan B.M., Krecke K., Parisi J.E., Trenerry M.R., Pittock S.J. Sporadic leukodystrophy with neuroaxonal spheroids: persistence of DWI hangs and neurocognitive profiles: a case study. J Neurol Neurosurg Psychiatry. 2010;81:619–622. doi: 10.1136/jnnp.2008.169243. [DOI] [PubMed] [Google Scholar]
- 13.Codjia P., Ayrignac X., Mochel F., Mouzat K., Carra-Dalliere C., Castelnovo G. Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia: an MRI study of 16 French cases. Am J Neuroradiol. 2018;39(9):1657–1661. doi: 10.3174/ajnr.A5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konno T., Broderick D.F., Mezaki N., Isami A., Kaneda D., Tashiro Y. Diagnostic value of brain calcifications in adult-onset leukoencephalopathy with axonal spheroids and pigmented glia. Am J Neuroradiol. 2017;38(1):77–83. doi: 10.3174/ajnr.A4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makary M.S., Awan U., Kisanuki Y.Y., Slone H.W. Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia: clinical and imaging characteristics. Neuradiol J. 2019;32(2):139–142. doi: 10.1177/1971400918822136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang S., Kim D.M., Lee I.H., Song C.J. Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia: a case report. Radiol Case Rep. 2019;14:514–517. doi: 10.1016/j.radcr.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bender B., Klose U., Lindig T., Biskup S., Nagele T., Schols L. Imaging features in conventional MRI, spectroscopy and diffusion weighted images of hereditary diffuse leukoencephlopathy with axonal spheroids (HDLS) J Neurol. 2014;261:2351–2359. doi: 10.1007/s00415-014-7509-2. [DOI] [PubMed] [Google Scholar]