1. Introduction
Periodontal diseases are infections caused by microorganisms that colonize the gingival margin, leading to inflammation and resulting in destruction of tooth- supporting tissues. These microorganisms accumulate in structures called dental biofilms, which protect the bacteria against the body’s immune system (Haffajee et al., 2003).
One of the main causes of tooth loss in the adult population is periodontitis, which may be classified into several categories. Among these, aggressive periodontitis (AgP) is the most destructive type of periodontitis. Three factors generally characterize periodontitis: one or more sites with inflammation (bleeding on probing), radiographic bone loss, and reduction in clinical attachment level (CAL) (Catunda et al., 2018).
AgP affects young healthy individuals, exhibits fast progression, and exhibits both localized and generalized forms with respect to etiology and pathogenesis. Moreover, the quantity of biofilm and calculus deposited at the affected site is not consistent with the progression of the disease (Armitage, 1999, Armitage, 2004). It is characterized by severe destruction of the supporting tissues of the teeth, which can lead to edentulism in the early stages of life. This characteristic can be used with other criteria to differentiate AgP from chronic periodontitis (Catunda et al., 2018, Griffiths et al., 2011).
The treatment using scaling and root planning (SRP) alone does not achieve good results in individuals with AgP (Herrera et al., 2002, Xajigeorgiou et al., 2006). Thus, complementary use of systemic antibiotics is recommended in the treatment of this pathology (Mestnik et al., 2012). Some authors have argued that the use of antimicrobial therapy might help in the treatment of AgP and would provide additional benefits. In patients with AgP, a combination of metronidazole (MTZ) and amoxicillin (AMX) was demonstrated to be the best antimicrobial protocol for clinical and microbiological benefits (Haffajee et al., 2003, Heitz-Mayfield, 2009). Previous studies have presented better results after using antimicrobials in the treatment of AgP (Buchmann et al., 2002, Guerrero et al., 2005, Machtei and Younis, 2008, Akincibay et al., 2008).
The aim of this systematic review was to evaluate whether MTZ plus AMX in combination with SRP would lead to better results in terms of gain in CAL and reduction in probing depth (PD) when compared with SRP alone. The null hypotheses of this study were: [1] the use of MTZ and AMX results in increased CAL when used concomitantly with SRP and [2] the use of MTZ and AMX reduces the probing depth when used concomitantly with SRP.
2. Materials and methods
This systematic review and meta-analysis was conducted according to the checklist guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (Moher et al., 2009) and was registered in the International Prospective Record of Systematic Reviews (CRD42018111595).
2.1. Eligibility criteria
The conductive question of this study was, “Does the use of systemic MTZ and AMX improve the treatment response in patients with aggressive periodontitis?” The question was based on population, intervention, control, and outcomes (PICO) criteria. The population (P) was composed of patients with AgP, the intervention (I) was the combination of systemic MTZ and AMX with SRP, the comparison (C) was patients undergoing SRP alone, and the evaluated outcomes (O) were gain in CAL and reduction in PD.
The inclusion criteria were randomized clinical trials (RCTs), studies with a minimum follow-up of 6 months, studies involving patients with AgP, studies comparing mechanical therapy alone and mechanical therapy in combination with MTZ and AMX, and studies involving humans with no age limit and including both genders. The exclusion criteria were prospective studies without randomization, retrospective studies, case reports, reviews, in vitro studies, animal studies, studies involving patients with systemic diseases and associated syndromes, studies comparing the use of MTZ with other therapies, studies associated with surgical therapy, studies in deciduous dentition, and letters to the editors.
2.2. Search strategy
The searches were performed by two independent authors (C.L.M. and C.P.P.A.) using the following databases: PubMed/MEDLINE, Scopus, Web of Science, and the Cochrane Library. The authors used the MeSH terms “aggressive periodontitis,” “metronidazole,” and “scaling root.” To complement this review, the same authors conducted a manual search for articles published in the following journals: Saudi Dental Journal, Brazilian Dental Journal, Journal of Periodontal Research, Oral Diseases, Journal of Clinical Periodontology, Journal of Periodontology, Annals of Periodontology, Quintessence International, Journal of the Canadian Dental Association, Periodontal Clinical Investigation, Trials, Periodontology 2000, Brazilian Journal of Microbiology, and British Journal of Pharmaceutical Research. In addition, OpenGrey database (www.opengrey.eu) was used to search the literature. The electronic searches were conducted till December 2019, including only the articles published in English, without imposing limits on the year of publication.
2.3. Selection of studies
The studies were selected and classified according to the inclusion and the exclusion criteria by reading the title and the abstract. Studies that did not clearly fit the inclusion or the exclusion criteria were downloaded and read in full and then the decision about inclusion or exclusion was made. A third author (R.B.) analyzed all the differences in choices between the authors and a consensus was reached through discussion.
2.4. Data extraction
One author (C.L.M.) collected important information from the articles, while another author (C.P.P.A.) reviewed the collected information. A careful analysis was performed to verify disagreements between the authors. A third author (R.B.) was consulted when there was no consensus.
2.5. Methodological quality assessment
Two authors (C.L.M and C.P.P.A) evaluated the methodological quality of the studies according to the Cochrane risk-of-bias tool for RCTs to verify the level of evidence from the studies included in the review.
2.6. Data synthesis
The meta-analysis was based on the inverse variance methods. The Mean deviations (MD) and 95% confidence intervals (CI) were calculated for each study. The MD values were considered significant at a p-value < 0.05. The extracted data were analyzed using Review Manager Software 5.3 (Cochrane, London, UK) (Higgins and Green, 2011).
2.7. Additional analysis
The Kappa score (Landis and Kock, 1977) test was used to calculate the level of agreement between the authors during the selection process of the articles in PubMed/MEDLINE, Scopus, Web of Science, and the Cochrane Library databases. Any disagreements were resolved by discussion until a consensus among all authors was reached.
3. Results
3.1. Literature search
The initial search in the databases resulted in 137 references including 64 in PubMed/MEDLINE, 35 in Web of Science, 21 in Scopus, 16 in the Cochrane Library, and one in OpenGrey. After duplicate references were eliminated, a detailed review of the titles and the abstracts of the selected studies was performed. The remaining 101 articles were subjected to a detailed analysis including application of the inclusion and the exclusion criteria. After this process, 17 complete articles were downloaded and selected for further analysis. After reading the articles completely, 13 were excluded and four articles were selected for this systematic review and meta-analysis (Fig. 1). The level of agreement among authors during the initial selection process of articles in the databases produced the following results: PubMed/MEDLINE = 0.89, Scopus = 1.0, Web of Science = 1.0, and the Cochrane Library = 1.0. The values indicated a high level of agreement among authors according to the Kappa criterion.
Fig. 1.
Flow diagram of manuscripts screened through the review process.
3.2. Characteristics of the included studies
Characteristics of the included studies are presented in Table 1. All four articles selected in this study were RCTs (Mestnik et al., 2012, Berglundh et al., 1998, Taiete et al., 2016, Yek et al., 2010), in which 109 patients aged up to 58 years were evaluated and followed up for a period of 6–24 months.
Table 1.
Characteristics of included studies (n = 4).
| Author Year Reference |
Patients | Average (years-old) | Follow-up | Interventions | Outcomes |
|---|---|---|---|---|---|
| Berglundh et al. 199817 | n = 16 | 35–58 age | 12/24 months | SRP + Mtz (250 mg × 3/day) + Amx (375 mg 2x/day) (n = 8) SRP + Placebo (3x/day) (n = 8) |
ΔCAL = 0.8 mm [+ − 0.4]/1.1 mm [+ − 0.3] ΔPD = 3.1 mm [+ − 0.3]/2.7 mm [+ − 0.2] ΔCAL = 0.7 mm [+ − 0.3]/0.8 mm [+ − 0.4] ΔPD = 3.1 mm [+ − 0.4]/2.9 mm [+ − 0.6] |
| Mestnik et al. 20127 | n = 26 | ≤ 20 age | 6/12 months | SRP + Mtz (400 mg/14 days) + Amx (500 mg/14 days) (n = 13) SRP (n = 13) |
ΔCAL = 1.24 mm [+ − 0.54]/1.15 mm [+ − 0.74] ΔPD = 1.63 mm [+ − 0.35]/1.63 mm [+ − 0.42] ΔCAL = 0.80 mm [+- 0.63]/0.60 mm [+ − 0.58] ΔPD = 0.93 mm [+ − 0.52]/0.92 mm [+ − 0.46] |
| Taiete et al. 201618 | n = 39 | <35 age | 6 months | SRP + Mtz (250 mg × 3/day/7days) + Amx (375 mg × 3/day/7 days) (n = 21) SRP + Placebo (pills 3/day/7days) (n = 18) |
ΔCAL = 2.3 mm [+ − 1.2] ΔPD = 3.8 mm [+ − 1.3] ΔCAL = 2.2 mm [+ − 1.0] ΔPD = 3.0 mm [+ − 0.9] |
| Yek et al. 201019 | n = 28 | N.R. | 6 months | SRP + MTZ (500 mg/3 × 1) + AMX (500 mg/3 × 1) (n = 12) SRP + Placebo (n = 16) |
ΔCAL = 0.99 mm [+ − 1.15] ΔPD = 1.47 mm [+ − 0.50] ΔCAL = 0.91 mm [+ − 1.26] ΔPD = 1.22 mm [+ − 0.62] |
Δ mean change from baseline in mm (standard deviation).
Amx, amoxicillin; CAL, clinical attachment level; MTZ, metronidazole; PD, probing depth; SRP, scaling roof planning.
One of these studies (Berglundh et al., 1998) evaluated the effect of systemic administration of MTZ (250 mg thrice daily) and AMX (375 mg twice daily) in combination with mechanical therapy (n = 8) when compared with the effect of placebo-associated mechanical therapy (thrice daily) (n = 8) in 16 subjects (10 women and 6 men) with AgP. These patients (aged 35 to 58 years) were followed up for 12 months (Δ CAL = 0.8 ± 0.4 mm/1.1 ± 0.3 mm and Δ PD = 3.1 ± 0.3 mm/2.7 ± 0.2 mm) and 24 months (Δ CAL = 0.7 ± 0.3 mm/0.8 ± 0.4 mm and Δ PD = 3.1 ± 0.4 mm/2.9 ± 0.6 mm) after the completion of active therapy.
In another study (Mestnik et al., 2012), the authors evaluated the clinical effects of complementary use of MTZ (400 mg for 14 days) and AMX (500 mg for 14 days) in combination with mechanical therapy (n = 13) when compared with mechanical therapy alone (n = 13) in 26 individuals (18 women and 8 men) with AgP. These patients (younger than 20 years) were monitored at 6 months (Δ CAL = 1.24 ± 0.54 mm/1.15 ± 0.74 mm and Δ PD = 1.63 ± 0.35 mm/1.63 ± 0.42 mm) and at 1 year (Δ CAL = 0.80 ± 0.63 mm/0.60 ± 0.58 mm and Δ PD = 0.93 ± 0.52 mm/0.92 ± 0.46 mm) after the therapy.
Another study (Taiete et al., 2016) compared patients with AgP treated with MTZ (250 mg thrice daily for 7 days) and AMX (375 mg thrice daily for 7 days) in combination with mechanical therapy (n = 21) and patients treated with placebo-associated mechanical therapy (pills thrice daily for 7 days) (n = 18). The patients (n = 39, 27 women and 12 men) were aged < 35 years. They were reassessed at 3 months (Δ CAL = 2.3 mm ± 1.2 mm and Δ PD = 3.8 ± 1.3 mm) and at 6 months (Δ CAL = 2.2 ± 1.0 mm and Δ PD = 3.0 ± 0.9 mm) after the therapy.
The final study included in this review (Yek et al., 2010) evaluated the effects of the combination of MTZ (500 mg thrice daily for 1 day) and AMX (500 mg thrice daily for 1 day) in combination with mechanical therapy (n = 12) when compared with the effects of placebo-associated mechanical therapy (n = 16) in patients with AgP. The patients (n = 28, 19 women and 9 men) were evaluated at an initial time (Δ CAL = 0.99 ± 1.15 mm and Δ PD = 1.47 ± 0.50) and reassessed at 6 months (Δ CAL = 0.91 ± 1.26 mm and Δ PD = 1.22 ± 0.62 mm) after the proposed treatment.
All the included studies evaluated CAL and PD among other clinical parameters.
The risk of bias was assessed using the Cochrane risk-of-bias tool to verify the level of evidence from the studies. The results indicated a high risk for allocation bias (Berglundh et al., 1998, Yek et al., 2010), blinding bias (Yek et al., 2010), and other biases (Berglundh et al., 1998). Risks included uncertain bias for random sequence generation (Berglundh et al., 1998), blinding of participants and personnel (Berglundh et al., 1998), and blinding of outcome assessment (Berglundh et al., 1998). Low risk was detected for other biases, suggesting that the studies were of high quality (Fig. 2).
Fig. 2.

Risk of bias of the study.
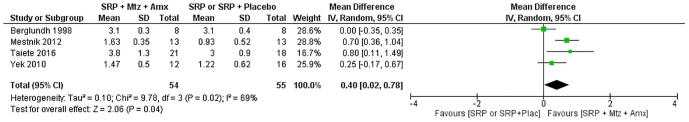
Four studies (Mestnik et al., 2012, Berglundh et al., 1998, Taiete et al., 2016, Yek et al., 2010) were selected for the analysis comparing SRP + MTZ + AMX versus SRP/SRP + Placebo. The meta-analysis showed no statistically significant difference in CAL (p = 0.52, MD: 0.21, 95% CI: −0.04–0.46) (Fig. 3). However, there was a statistically significant difference in PD (p = 0.02, MD: 0.40, 95% CI: 0.02–0.78), indicating reduction in PD when MTZ and AMX were used in combination with SRP (Fig. 4).
Fig. 3.
Forest plot of random effects meta-analysis evaluating the difference in clinical attachment level charge between patients submited to SRP/SRP + Placebo or SRP + Metronidazole (MTZ) + Amoxicilin (AMX).
Fig. 4.
Forest plot of randon effects meta-analysis evaluating the difference in depth probing charge between patients submited to SRP/SRP + Placebo or SRP + Metronidazole (MTZ) + Amoxicilin (AMX).
4. Discussion
The first null hypothesis of this study suggested that the use of MTZ plus AMX concomitant with SRP favors the gain in CAL. This hypothesis was rejected. The present meta-analysis showed that there was no statistically significant difference in CAL gain. These data are consistent with data from other studies in the literature (Haffajee et al., 2003, Xajigeorgiou et al., 2006, Feres et al., 2018). There was a favorable shift toward the SRP + MTZ + AMX group in all the studies (Fig. 3), but there was no statistically significant difference between the intervention and the comparison groups in the meta-analysis (p = 0.52 MD: 0.21, 95% CI: −0.04–0.46). On the other hand, some studies have reported statistically significant difference in CAL gain (Griffiths et al., 2011, Feres et al., 2018, Keestra et al., 2015, Sgolastra et al., 2012, Zandbergen et al., 2016). The second null hypothesis of this study suggested that MTZ + AMX combination results in reduced PD when used concomitantly with SRP. This hypothesis was accepted. The present meta-analysis showed that there was a statistically significant difference in PD reduction. These data are consistent with data from other studies in the literature (Griffiths et al., 2011, Xajigeorgiou et al., 2006, Feres et al., 2018, Keestra et al., 2015, Sgolastra et al., 2012). There was a favorable shift toward the SRP + MTZ + AMX group (Fig. 4) with a statistically significant difference between the intervention and the comparison groups in the meta-analysis (p = 0.02 MD: 0.40, 95% CI: 0.02–0.78). All studies included in this systematic review reported a non-significant difference in CAL gain and a significant difference in PD reduction when systemic antibiotics were used concomitantly with non-surgical periodontal therapy (Table 1).
The findings of the present meta-analysis are consistent with other systematic reviews (Haffajee et al., 2003; Herrera et al,. 2002; Keestra et al., 2015, Sgolastra et al., 2012, Zandbergen et al., 2013, Zandbergen et al., 2016) that suggested the clinical efficacy of MTZ + AMX in the treatment of AgP (Sgolastra et al., 2012, Zandbergen et al., 2016). However, the literature is still quite heterogeneous regarding the AgP treatment protocol wherein systematic reviews show some disagreements regarding parameters such as study design, dosages of medications administered, and duration of administration (Sgolastra et al., 2012). The present systematic review highlights the fact that the combination of MTZ and AMX concomitant with SRP improves the condition of patients with AgP when compared with the conventional therapy. Several studies have reported the complementary use of other antibiotics (both local and systemic) such as metronidazole, topical chlorhexidine, tetracycline (doxycycline and tetracycline), and macrolides (azithromycin) in treatment of AgP (Xajigeorgiou et al., 2006, Keestra et al., 2015, Aimetti et al., 2011). However, most of the studies corroborate the use of AMX + MTZ as they are among the first antibiotics of choice in the treatment of oral conditions (Haffajee et al., 2003, Feres et al., 2018, Sgolastra et al., 2012, Zandbergen et al., 2013).
All the studies included in this review evaluated CAL and PD. However, other clinical parameters such as percentage of sites with plaque accumulation, gingival bleeding, bleeding on probing, suppuration, bone level, number of remaining teeth, biopsy, microbial sampling, levels of interferon γ, prostaglandin E2, interleukin 6, gingival crevicular fluid, and microorganism counts were not common among the studies included in the meta-analysis. Therefore, it is necessary to standardize the protocols for future RCTs without affecting the treatment of AgP (Mestnik et al., 2012, Berglundh et al., 1998, Taiete et al., 2016, Yek et al., 2010).
The findings in the articles included in this meta-analysis and systematic review (Berglundh et al., 1998, Taiete et al., 2016, Yek et al., 2010) are consistent with the findings of other studies (Xajigeorgiou et al., 2006, Feres et al., 2018, Aimetti et al., 2011) regarding reduction in the microbiological, immunological, and biochemical parameters of the patients with shallow, medium, or deep periodontal pockets treated with SRP + MTZ + AMX. However, these findings raise questions about whether AMX + MTZ can induce microbial resistance of pathogens to antibiotics and alteration of the microflora itself or whether the combination allows the growth of opportunistic pathogens (Sgolastra et al., 2012, Zandbergen et al., 2013).
Possible adverse effects in patients were assessed during the course of the study. These included diarrhea, vomiting, mild gastrointestinal discomfort, nausea, rashes, headache, and metallic taste (Griffiths et al., 2011, Xajigeorgiou et al., 2006, Guerrero et al., 2005, Zandbergen et al., 2013), suggesting the importance of the cost/benefit analysis of these antibiotics and analysis of their side effects in future RCTs (Sgolastra et al., 2012, Zandbergen et al., 2013).
Results of the present meta-analysis regarding the relevant benefits of MTZ + AMX in the treatment of AgP should be interpreted with caution due to the small number of clinical trials evaluated. Although the short-term effects of antibiotics used to treat AgP (AMX + MTZ) are known (Berglundh et al., 1998), the concentration of each drug and the dosage can interfere with the plasma concentration and consequently, with its effect (Mestnik et al., 2012, Berglundh et al., 1998, Taiete et al., 2016, Yek et al., 2010). This fact makes the creation of treatment protocols extremely important. Hence, more RCTs with longer observation periods are needed in the future.
5. Conclusion
The present systematic review and meta-analysis concluded that there was no statistically significant difference in CAL gain between the use of systemic antibiotics with SRP and the use of SRP alone in the treatment of AgP. However, there was a statistically significant difference in PD, favoring the combination of systemic antibiotics and SRP.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Cácio Lopes Mendes, Email: caciolopes@yahoo.com.br.
Rodivan Braz, Email: rodivan.braz@upe.br.
References
- Aimetti M., Romano F., Guzzi N. One-stage full-mouth disinfection as a therapeutic approach for generalized aggressive periodontitis. J Periodontol. 2011;82:845–853. doi: 10.1902/jop.2010.100468. [DOI] [PubMed] [Google Scholar]
- Akincibay H., Orsal S.O., Sengun D. Systemic administration of doxycycline versus metronidazole plus amoxicillin in the treatment of localized aggressive periodontitis: A clinical and microbiologic study. Quintessence Int. 2008;39:e33–e39. [PubMed] [Google Scholar]
- Armitage G.C. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- Armitage G.C. Periodontal diagnoses and classification of periodontal diseases. Periodontol. 2004;2000(34):9–21. doi: 10.1046/j.0906-6713.2002.003421.x. [DOI] [PubMed] [Google Scholar]
- Berglundh T., Krok L., Liljenberg B. The use of metronidazole and amoxicillin in the treatment of advanced periodontal disease. A prospective, controlled clinical trial. J Clin Periodontol. 1998;25:354–362. doi: 10.1111/j.1600-051x.1998.tb02455.x. [DOI] [PubMed] [Google Scholar]
- Buchmann R., Nunn M.E., Van Dyke T.E. Aggressive periodontitis: 5-year follow-up of treatment. J Periodontol. 2002;73:675–683. doi: 10.1902/jop.2002.73.6.675. [DOI] [PubMed] [Google Scholar]
- Catunda R.Q., Levin L., Kornerup I. Diagnosis of aggressive periodontitis: a dilema? Quintessence Int. 2018;49:173–180. doi: 10.3290/j.qi.a39743. [DOI] [PubMed] [Google Scholar]
- Feres M., Retamal-Valdes B., Mestnik M.J. The ideal time of systemic metronidazole and amoxicillin administration in the treatment of severe periodontitis: study protocol for a randomized controlled trial. Trials. 2018;19:201. doi: 10.1186/s13063-018-2540-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G.S., Ayob R., Guerrero A. Amoxicillin and metronidazole as an adjunctive treatement in generalized aggressive periodontitis at initial therapy or re-treatment: a randomized controlled clinical trial. J Clin Periodontol. 2011;38:43–49. doi: 10.1111/j.1600-051X.2010.01632.x. [DOI] [PubMed] [Google Scholar]
- Guerrero A., Griffiths G.S., Nibali L. Adjunctive benefits of systemic amoxicillin and metronidazole in non-surgical treatment of generalized aggressive peri- odontitis: A randomized placebo-controlled clinical trial. J Clin Periodontol. 2005;32:1096–1107. doi: 10.1111/j.1600-051X.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- Haffajee A.D., Socransky S.S., Gunsolley J.C. Systemic anti-infective periodontal therapy. A systematic review. Ann Periodontol. 2003;8:115–181. doi: 10.1902/annals.2003.8.1.115. [DOI] [PubMed] [Google Scholar]
- Heitz-Mayfield L.J. Systemic antibiotics in periodontal therapy. Aust Dent J. 2009;54:S96–S101. doi: 10.1111/j.1834-7819.2009.01147.x. [DOI] [PubMed] [Google Scholar]
- Herrera D., Sanz M., Jepsen S. A systematic review on the effects of systemic antimicrobials as an adjuntct to scaling and root planning in periodontitis patients. J Clin Periodontol. 2002;29:136–159. doi: 10.1034/j.1600-051x.29.s3.8.x. discussion 160–2. [DOI] [PubMed] [Google Scholar]
- Higgins J.P.T., Green S. 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2011] The Cochrane Collaboration. Available from www.cochrane-handbook.org (accessed and downloaded in November 11, 2018).
- Keestra J.A., Grosjean I., Coucke W. Non-surgical periodontal therapy with systemic antibiotics in patients with untreated aggressive periodontitis: a systematic review and meta-analysis. J Periodontal Res. 2015;50:689–706. doi: 10.1111/jre.12252. [DOI] [PubMed] [Google Scholar]
- Landis J.R., Kock G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Machtei E.E., Younis M.N. The use of 2 antibiotic regimens in aggressive periodontitis: Comparison of changes in clinical parameters and gingival crevicular fluid biomarkers. Quintessence Int. 2008;39:811–819. [PubMed] [Google Scholar]
- Mestnik M.J., Feres M., Figueiredo L.C. The effects of adjuntctive metronidazole plus amoxicillin in the treatment of generalized aggressive periodontitis: a 1-year double-blinded, placebo-controlled, randomized clinical trial. J Clin Periodontol. 2012;39:955–961. doi: 10.1111/j.1600-051X.2012.01932.x. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgolastra F., Petrucci A., Gatto R. Effetiveness of systemic amoxicillin/metronidazole as an adjunctive therapy to full-mouth scaling and root planning in the treatment of aggressive periodontitis: a systematic review and meta-analysis. J Periodontol. 2012;83:731–743. doi: 10.1902/jop.2011.110432. [DOI] [PubMed] [Google Scholar]
- Taiete T., Casati M.Z., Ribeiro E.D.P. Amoxicillin/metronidazole associated with nonsurgical therapy did not promote additional benefits in immunologic parameters in generalized aggressive periodontitis: a randomized controlled clinical trial. Quintessence Int. 2016;47:281–292. doi: 10.3290/jqi.a34723. [DOI] [PubMed] [Google Scholar]
- Xajigeorgiou C., Sakellari D., Slini T. Clinical and microbiological effects of differents antimicrobials on generalized aggressive periodontitis. J Clin Periodontol. 2006;33:254–264. doi: 10.1111/j.1600-051X.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- Yek E.C., Cintan S., Topcuoglu N. Efficacy of amoxicillin and metronidazole combination for the management of generalized aggressive periodontitis. J Periodontol. 2010;81:964–974. doi: 10.1902/jop.2010.090522. [DOI] [PubMed] [Google Scholar]
- Zandbergen D., Slot D.E., Cobb C.M. The clinical effect of scaling and root planning and the concomitant administration of systemic amoxicillin and metronidazole: a systematic review. J Periodontol. 2013;84:332–351. doi: 10.1902/jop.2012.120040. [DOI] [PubMed] [Google Scholar]
- Zandbergen D., Slot D.E., Niederman R. The concomitant administration of systemic amoxicillin and metronidazole compared to scaling and root planning alone in treating periodontitis: a systematic review. BMC Oral Health. 2016 doi: 10.1186/s12903-015-0123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]