Abstract
Due to the high recurrence and progression rate of non-muscle invasive bladder cancer after transurethral resection of bladder tumor, some new optical imaging technologies have arisen as auxiliary imaging modes for white light cystoscopy to improve the detection rate of small or occult tumor lesions, such as photodynamic diagnosis, narrow-band imaging, and molecular imaging. White light cystoscopy is inadequate and imperfect for bladder cancer detection, and thus residual tumors or coexisting flat malignant lesions, especially carcinoma in situ, would be ignored during conventional resection. The bladder, a hollow organ with high compliance, provides an ideal closed operation darkroom for endoscopic molecular imaging free from interference of external light sources. Also, intravesical instillation of a molecular fluorescent tracer is simple and convenient before surgery through the urethra. Molecular fluorescent tracer has high sensitivity and specificity to tumor cells, and its mediated molecular imaging allows small or occult tumor lesion detection while minimizing false-positive results. Meanwhile, endoscopic molecular imaging provides a real-time and dynamic image during surgery, which helps urologists to perform high-quality and complete tumor resection through accurate judgment of tumor boundaries and depth of invasion. Photoimmunotherapy is a novel molecular targeted therapeutic pattern of photodynamic therapy that kills malignant cells selectively and minimizes the cytotoxicity to normal tissues. The combination of endoscopic molecular imaging and photoimmunotherapy used in initial treatment may avoid the need of repeat transurethral resection in strictly selected patients and improve oncological outcomes such as recurrence-free survival and overall survival after operation.
Keywords: bladder cancer, endoscopic molecular imaging, photoimmunotherapy, diagnosis and treatment, precision medicine
Graphical Abstract
Due to the high recurrence and progression rate of non-muscle invasive bladder cancer after transurethral resection of bladder tumor, some new optical imaging technologies have arisen as auxiliary imaging modes for white light cystoscopy to improve the detection rate of small or occult tumor lesions, such as photodynamic diagnosis, narrow-band imaging, and molecular imaging. White light cystoscopy is inadequate and imperfect for bladder cancer detection, and thus residual tumors or coexisting flat malignant lesions, especially carcinoma in situ, would be ignored during conventional resection. The bladder, a hollow organ with high compliance, provides an ideal closed operation darkroom for endoscopic molecular imaging free from interference of external light sources. Also, intravesical instillation of a molecular fluorescent tracer is simple and convenient before surgery through the urethra. Molecular fluorescent tracer has high sensitivity and specificity to tumor cells, and its mediated molecular imaging allows small or occult tumor lesion detection while minimizing false-positive results. Meanwhile, endoscopic molecular imaging provides a real-time and dynamic image during surgery, which helps urologists to perform high-quality and complete tumor resection through accurate judgment of tumor boundaries and depth of invasion. Photoimmunotherapy is a novel molecular targeted therapeutic pattern of photodynamic therapy that kills malignant cells selectively and minimizes the cytotoxicity to normal tissues. The combination of endoscopic molecular imaging and photoimmunotherapy used in initial treatment may avoid the need of repeat transurethral resection in strictly selected patients and improve oncological outcomes such as recurrence-free survival and overall survival after operation.
Main Text
Recently, molecular fluorescent tracer-mediated molecular imaging and photoimmunotherapy (PIT) have shown some potential advantages in the management of cancers.1, 2, 3, 4 Molecular imaging refers to a subject of qualitative and quantitative study of pathological processes at the cellular and molecular levels using imaging technologies, such as optical molecular imaging, magnetic resonance molecular imaging, ultrasound, and nuclear medicine molecular imaging, which may indicate diseases prior to macrostructure changes.5 Unlike magnetic and nuclear medicine molecular imaging, endoscopic molecular imaging provides a real-time and dynamic image during operation. PIT is a novel molecular-targeted therapeutic pattern of photodynamic therapy (PDT) that kills malignant cells selectively and minimizes the cytotoxicity to normal tissues simultaneously.6 The urinary bladder, as a hollow organ with high compliance, is appropriate to perform endoscopic molecular imaging and PIT after intravesical instillation of tumor-specific fluorescent tracer.
Non-muscle invasive bladder cancer (NMIBC) is confined to the mucosa (stage Ta and carcinoma in situ [CIS]) or submucosa (stage T1), and it comprised nearly 75% of newly diagnosed bladder cancer (BC).7 Transurethral resection of bladder tumor (TURBT) followed by tailored adjuvant treatment strategy is the contemporary cornerstone in the management of NMIBC. Although it plays a central role during conventional TURBT, white light cystoscopy (WLC) has some recognized limitations. First, small or occult tumor lesions, particularly CIS in the bladder, are not easy to visualize and diagnose.8 Second, the morphological characteristics of CIS under WLC appear as erythematous areas, and thus it is difficult to distinguish CIS from inflammatory lesions due to no histopathological information provided during an operation.9 Finally, the depth of tumor invasion and surgical margin status identified by two-dimensional cystoscopic images and the operator’s experience are often subjective and inaccurate, even among senior urologists.10 In a systematic review that involved 8,409 cases with high-grade (HG) Ta and T1 bladder tumor who accepted repeat transurethral resection (reTUR), residual tumors were found in 17%–67% of patients with stage Ta and in 20%–71% of patients with T1 bladder tumor, and 36%–86% of residual tumors were located in the initial resection site.11 Besides the quality of TURBT, it is verified that the number of tumors, tumor diameter, prior recurrence rate, concurrent CIS, pathological stage, and histologic grade also influence the recurrence and progression rate substantially.12 Thus, new imaging technologies that serve as auxiliary imaging modes for WLC have been developed recently to enhance visualization of tumor lesions and diagnostic accuracy, which will contribute to complete tumor resection and reduction of recurrence rate.13
The procedure of piecemeal resection during conventional TURBT may cause dissemination and seeding of exfoliated tumor cells.14 Tailored intravesical chemotherapy or immunotherapy corresponding to the individual tumor risk stratification is recommended as an adjuvant treatment strategy.7 Many studies have evaluated the therapeutic effect of chemotherapeutic drugs, such as mitomycin C, epirubicin, pirarubicin, and gemcitabine. The results showed that patients with NMIBC benefit from intravesical therapy in terms of oncological outcomes.15, 16, 17 However, poor absorption of drug in tumor tissue due to its non-cancer-specific characteristic and related adverse effects (AEs) reduce the efficacy and safety of intravesical chemotherapy.18 Comparatively, bacillus Calmette-Guérin (BCG) immunotherapy has more AEs than does chemotherapy, and even some patients may not respond to this therapy.19,20 Meanwhile, due to the shortage of BCG, not every eligible patient has access to immunotherapy.21 PIT is a new molecular targeted therapeutic model that relies on a conjugate of tumor-specific monoclonal antibody with highly hydrophilic phthalocyanine dye, which targets malignant tissue and reduces nonspecific accumulation in normal tissue.
Recently, a systematic review analyzed the latest developments in molecular targeted agents for fluorescence image-guided cancer surgery.22 This molecular imaging technology used in cancer surgery mainly focuses on breast and hollow viscus, such as the respiratory and gastrointestinal tracts.23, 24, 25 Optical molecular imaging can specifically show the pathological process related to tumorigenesis and provide real-time macroscopic imaging of tumor tissue in vivo. Therefore, it could be used to improve the quality of NMIBC management, such as detection of small or occult tumor lesions and evaluation of the status of surgical margin and depth of tumor invasion during tumor resection. Meanwhile, PIT might serve as a supplement for optimizing the adjuvant intravesical therapy after operation. In this review, we focus on the evidence about endoscopic molecular imaging and PIT used in bladder tumor, and point out their potential future applications.
Different Molecular Tracers Used in BC Management
Molecular tracers can be divided into five broad categories as follows: monoclonal antibodies, antibody fragments, protein scaffolds, peptides, and small molecules.26 Antibodies are the largest class of molecular tracers used in clinical trials due to the relatively mature production strategy of new antibodies. For example, the CD47 antibody and carbonic anhydrase IX (CAIX) antibody, after conjugating with Qdot625, have been explored in bladder tumor detection.27,28 The result of preclinical trials indicated their effectiveness in small or occult tumor lesion detection, and the diagnostic accuracy was verified by histopathological evidence. The therapeutic epidermal growth factor receptor (EGFR) monoclonal antibodies cetuximab and panitumumab, and the human EGFR-2 (HER-2) antibody trastuzumab, after binding to IRDye 700DX (IR700), have been used in PIT of BC management.29, 30, 31 However, fluorophore-antibody conjugate-based optical molecular imaging has some recognized limitations, such as the long half-life of blood circulating and poor penetration into tumor tissue due to its large molecular size (150 kDa).32 Furthermore, due to nonspecific tissue accumulation of molecular tracer by the enhanced permeability and retention (EPR) effect, the added clinical value of fluorescence-guided surgery (FGS) will be discounted by false-positive results and lower tumor-to-background ratio (TBR) signals.33
To overcome the limitations associated with monoclonal antibodies and obtain a more rapid pharmacokinetic, antibody fragments have been emerged in our sight with the development of protein engineering. Antibody fragments include minibodies, diabodies, single-chain variable fragments, and Fab fragments, which retain the cancer-specific binding properties, but have a smaller molecular size (15–80 kDa).34 The application of antibody fragments can decrease blood retention times and increase the specificity of molecular fluorescent agent and the depth of tumor tissue penetration, thus mitigating potential drug-related toxicities.35,36 Nanoparticles conjugated with minibodies of anti-EGFR showed therapeutic benefits in orthotopic bladder tumor models.37
Among molecular tracers, peptides and small molecules have the smallest molecular size (0.5–2.0 kDa) and can be synthesized through routine manufacturing protocols with a reduced cost compared with biologicals. However, because there is a huge difference in structure and physicochemical characteristic of peptides and small molecules, the general pharmacokinetic profile of them is hard to predict. CSNRDARRC, CSSPIGRHC, and CSDRIMRGC peptides were generated by the technique of phage-display peptide libraries. The previous studies demonstrated that they have the ability to specifically bind to bladder tumor tissue.38, 39, 40 CQDGRMGFC peptide was synthesized by a one-bead, one-compound combinatorial chemistry approach. After conjugating with Cy5.5, the molecular fluorescent tracer selectively accumulated in bladder tumor tissue of patient-derived xenograft mouse models.41 The pH low insertion peptide (pHLIP) was synthesized by reversed phase chromatography. When labeled with indocyanine green (ICG), it showed a high diagnostic accuracy in BC detection (Table 1).42
Table 1.
Comprehensive Overview of Potential Molecular Tracers in Bladder Cancer
| Tracer Type | Compound Name | Molecular Target | Reporter Fluorophore | Application | Study Design | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|
| Antibodies | CD47 antibody27 | CD47 | Qdot625 | molecular imaging | in vitro: fresh intact bladder specimen | 82.9 | 90.5 |
| CD47 antibody82 | CD47 | Alexa Fluor 790 | molecular imaging | in vitro: fresh bladder tumor specimen | NA | NA | |
| CAIX antibody28 | CAIX | Qdot625 | molecular imaging | in vitro: fresh intact bladder specimen | 88.00 | 93.75 | |
| CD47 antibody86 | CD47 | IR700 | PIT |
in vitro: bladder tumor cell lines in vivo: xenograft mouse model |
NA | NA | |
| panitumumab29 | EGFR | IR700 | PIT |
in vitro: bladder tumor cell lines in vivo: xenograft mouse model |
NA | NA | |
| panitumumab trastuzumab30 |
EGFR HER-2 |
IR700 | PIT |
in vitro: bladder tumor cell lines in vivo: xenograft mouse model |
NA | NA | |
| Peptides | CSNRDARRC38 | NA | fluorescein | molecular imaging |
in vitro: human bladder cancer tissue in vivo: orthotopic mouse model |
NA | NA |
| NYZL139 | NA | FITC | molecular imaging |
in vitro: human bladder cancer tissue in vivo: xenograft mouse model |
NA | NA | |
| PLSWT740 | NA | IRDye 800CW | molecular imaging | in vivo: fresh intact bladder specimen | 84.0 | 86.7 | |
| PLZ441 | NA | Cy5.5 | molecular imaging |
in vitro: bladder tumor cell lines in vivo: xenograft mouse model |
NA | NA | |
| pHLIP42 | NA | ICG | molecular imaging | in vitro: fresh intact bladder specimen | 97 | 100a | |
NA, not applicable; IR700, IRDye 700DX; PIT, photoimmunotherapy; EGFR, epidermal growth factor receptor; HER-2, human epidermal growth factor receptor 2; FITC, fluorescein isothiocyanate; ICG, indocyanine green.
If necrotic and previously chemotherapy tissues were considered as false positive, the specificity was reduced to 80%.
Endoscopic Molecular Imaging for Precision Medicine
At present, the decision for BC management is mainly dependent on the results of radiological imaging and histopathological assessment of sampled tissue.43,44 Radiological imaging, such as intravenous pyelography (IVP), computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography/computed tomography (PET/CT), plays a significant role in assessing the extent of invasion and preoperative clinical stage of bladder tumor.45 CT is the first choice for evaluation of tumor stage due to the advantages of relatively low cost, widespread use, high-speed image acquisition, and acceptable accuracy in diagnosis of locally advanced and metastatic disease in the abdomen. However, radiologists cannot assess whether detrusor muscle (DM) is invaded through a CT image, and thus differentiate Ta, T1, and T2 stage bladder tumor with great difficulty.46,47 Compared with CT, multiparametric MRI (mpMRI) has a high diagnostic value in differentiating the absence or presence of DM invasion in patients, and then distinguishing muscle invasive BC (MIBC) from NMIBC through the Vesical Imaging-Reporting and Data System (VI-RADS).48, 49, 50 However, MRI is more time-consuming and not suitable for patients who have a medical history of metal foreign body implantation. PET/CT is the combination of function-based PET imaging and anatomy-based CT imaging that is mainly used in the detection of metastatic disease and positive lymph nodes with normal size.51 The clinical utility of all of the above-mentioned radiological imaging technologies is limited by high number of false-negative results and lack of real-time dynamic judgment of residual tumors during surgery.52
During conventional TURBT, urologists mainly rely on two-dimensional images on the monitor and their own experience alone to assess the depth of tumor invasion and distinguish the boundaries between malignant tissue and benign tissue, a technique with poor accuracy. Therefore, incomplete tumor resection or excessive removal of adjacent normal tissues may occur, resulting in residual tumors or bladder perforation. The residual tumors are responsible for high recurrence and progression rates after operations.53 Also, bladder perforation provides the favorable condition for extravesical implantation of exfoliated tumor cells and delays the time of first intravesical instillation treatment, leading to poor oncological outcomes.54,55 The application of new optical imaging technology in transurethral tumor resection is the main unmet clinical need. Endoscopic molecular imaging using a cancer-specific tracer-targeted fluorophore with a paired detection medical device has shown potential to improve tumor detection and provide clear surgical margins in several preclinical studies.27,28 Simultaneously, this diagnostic molecular imaging technology can be applied directly as an adjuvant PIT to improve oncological outcomes and, thus, recurrence-free and overall survival rates.
BC Detection
Based on the field of imaging view, optical imaging technologies are classified as two different models, i.e., macroscopic and microscopic modalities. Photodynamic diagnosis (PDD) and narrow-band imaging (NBI) belong to macroscopic imaging modalities that survey a wide region of bladder mucosa in a way similar to cystoscopy and highlight suspicious malignant lesions by additional contrast enhancement.56 Considering their nature of non-tumor specificity, although PDD and NBI improve the rate of papillary lesions and CIS detection, false-positive results can occur in prior intravesical therapy, inflammation, and acute bleeding.57 Confocal laser endomicroscopy (CLE) and optical coherence tomography (OCT), as real-time microscopic imaging technologies, provide pathological information on tissue microstructure and cell morphological changes by producing high-resolution images of tissue in vivo.58,59 Unfortunately, CLE and OCT only survey a limited area of bladder mucosa tissue. Therefore, the use of microscopic imaging technologies need to combine with other macroscopic imaging methods (e.g., WLC, PDD, or NBI) to define the boundaries of malignant tissue first.60
Unlike PDD, molecular fluorescent tracer instead of photosensitizer is used in optical molecular imaging to decrease the incidence of false-positive results. CD47 is overexpressed on the membrane of more than 80% of bladder tumor cells but not on normal urothelium. The interaction between CD47 and signal regulatory protein α (SIRPα) on phagocytic cells transmits a signal that inhibits phagocytosis of macrophages on bladder tumor cells.61 Pan et al.27 evaluated the diagnostic accuracy of anti-CD47-Qdot625-mediated endoscopic molecular imaging for BC detection. After incubation with anti-CD47-Qdot625, 119 suspicious bladder regions from 21 fresh intact bladder specimens were examined under blue light, and the overall sensitivity and specificity were 82.9% and 90.5%, respectively. CAIX is a member of the carbonic anhydrase family that plays a role in the regulation of intracellular pH value under hypoxia, and then changes the biological characteristics of tumors in adhesion, proliferation, and progression.62 Through a similar strategy, eight fresh intact bladder specimens were incubated with anti-CAIX-Qdot625 and then examined under white and blue light cystoscopy. The sensitivity and specificity for bladder tumor detection were 76.0% and 90.5% under WLC. However, tumor detection under CAIX-targeted endoscopic molecular imaging obtained a high diagnostic accuracy, and the overall sensitivity and specificity were 88.00% and 93.75%, respectively.28
Peptides that target specific bladder tumor tissue can be selected through the technique of phage-display peptide libraries or a one-bead, one-compound combinatorial chemistry approach. CSNRDARRC peptide represents the first peptide identified by phage-display library technology that binds to human bladder tumor cell lines (HT-1376) specifically. After intravesical instillation of the corresponding fluorophore-peptide conjugate in the N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN)-induced rat models of bladder tumor, it selectively targeted the luminal epithelium of bladder tumor lesions but not that of normal bladder tissues.38 CSSPIGRHC peptide, named NYZL1, was also identified by a phage- display technique that binds to human bladder tumor cell lines (BIU-87). After NYZL1-targeted fluorophore injection into the tail vein of human bladder tumor xenografts in nude mice, the tracer bound to tumor tissue, but not to normal organs or tissues.39 CSDRIMRGC peptide is a bladder tumor-specific peptide named after PLSWT7. Its diagnostic accuracy for bladder tumor detection was explored in a preclinical study in eight patients who accepted radical cystectomy (RC). The result showed that PLSWT7-mediated molecular imaging yielded a high sensitivity (84%) and specificity (86.7%) for detection of bladder malignant lesions.40 CQDGRMGFC peptide, PLZ4 for short, is another bladder tumor-specific ligand synthesized by the combinatorial chemistry approach. An in vivo molecular imaging study showed that the PLZ4-Cy5.5 conjugate selectively accumulated in tumor tissues of patient-derived xenograft mouse models after intravenous administration.41 Intratumor heterogeneity significantly compromises the diagnostic accuracy of optical molecular imaging.63 Many studies have confirmed that malignant cells, including bladder tumor cells, have an increased anaerobic glycolysis activity, which leads to an acidic tumor microenvironment.64,65 The pHLIPs first target the acidity on the cancer cell surfaces, and then cross through the membrane of cancer cells. This effect is triggered by the acidic tumor microenvironment.66,67 22 fresh intact bladder specimens were obtained after RC and incubated with ICG-pHLIP molecular probe, and then examined under near-infrared (NIR) imaging equipment. The sensitivity of malignant tissue detection was 97%, and the specificity was 100% regardless of pathological stage. However, when necrotic and previously chemotherapy-treated tissues were included, the number of false-positive results increased and the specificity was reduced to 80%.42
A series of molecular fluorescent tracers have been used in molecular imaging and have shown the potential to highlight bladder tumor lesions in animal models of urothelial cancer and tumor specimens from patients. Thus, we question how to choose the best molecular fluorescent tracer to perform endoscopic molecular imaging in a particular patient with BC. The phenomena of intratumor heterogeneity and different expression patterns between patients or even within the same patient complicate the process of molecular fluorescent tracer selection.68 Fortunately, before TURBT, it was strongly recommended that patients with suspected malignant disease should undergo cystoscopy-guided biopsy followed by histopathological assessment of obtained sample tissue as the initial diagnostic procedure according to the European Association of Urology (EAU) guidelines on NMIBC.7 Histopathological assessment and next-generation sequencing (NGS) of tumor specimens can reveal potential molecular imaging targets and thus help urologists to select the most suitable molecular fluorescent tracer for candidates who are most likely to benefit from endoscopic molecular imaging.69
BC Resection
TURBT is the contemporary cornerstone in the management of NMIBC, and urologists depend on their own experience and indirect visual feedback to assess tumor number, boundary, and depth of tumor invasion. Unfortunately, residual tumors occurred in 51% of patients with a T1 bladder tumor.7 Then complete resection of malignant tissues is pivotal for prolonged recurrence-free survival and overall survival. Simultaneously, avoiding unnecessary deep resection and extended resection to preserve as much adjacent vital normal tissues as possible, urologists need additional intraoperative imaging guidance to perform tumor resection, regardless of the operator’s experience in endoscopic surgery. Besides relying on subjective visual assessment and indirect tactile feedback, surgeons may use optical molecular imaging as an auxiliary imaging mode for WLC in tumor resection. The mouse xenograft models were randomly divided into two groups in a preclinical study: bladder tumor resection was performed under natural light and optical molecular imaging in the control group (n = 20) and experimental group (n = 20), respectively. One week later, the tumor recurrence rate in the control group and experimental group was 95% and 5%, respectively. Also, 30 days after operation, the corresponding overall survival rate in two groups was 0% and 90%.40
Histopathological assessment of tumor specimens obtained after conventional TURBT may be inaccurate because the tumor tissue has been cut into pieces during surgery. To overcome this drawback, en bloc resection of bladder tumor (ERBT) as a “no touch” technique for treatment of NMIBC has obtained increasing interests among urologists globally.70, 71, 72 Endoscopic molecular imaging-guided en bloc tumor resection may provide a clear vision of surgical margin and tumor base for urologists. Also, after surgery, a fresh intact tumor specimen labeled with a molecular fluorescent tracer will help the pathologist to make a more accurate histopathological evaluation with the assistance of a fluorescence microscope. If a positive surgical margin or MIBC is identified in the tumor specimen, the patient will be advised to undergo reTUR or RC accordingly.
Decision-Making for reTUR
The ultimate goal in the management of NMIBC is to completely remove all visible tumors, with negative surgical margins and DM available in the specimen. Residual tumors found at reTUR reflect the poor quality of the initial transurethral resection. Cumberbatch et al.11 showed that residual tumors were found at reTUR in 17%–67% of patients with initial Ta and in 20%–71% of patients with initial T1 bladder tumor, and the vast majority of residual tumors (36%–86%) were located in the initial resection site. Naselli et al.10 demonstrated that the pooled prevalence rate of residual tumors was 61% and upstaging to muscle-invasive disease was 15% at reTUR in T1 cases. Even in the subgroup with DM presented in the specimen from initial transurethral resection, the corresponding rates were 58% and 11%, respectively. In order to detect occult residual tumors and achieve an accurate assessment of tumor stage, according to the EAU guidelines, the patients with incomplete initial tumor resection, no DM in the specimen of high-grade Ta (TaHG) tumors and all T1 tumors are recommended to accept reTUR within 2–6 weeks after initial transurethral resection.7 Therefore, after initial transurethral resection, a large proportion of patients with NMIBC require surgical intervention of reTUR. Unfortunately, from a European telephone interview survey, the proportion of patients with low- and high-risk NMIBC who accepted reTUR were only 25%–75% and 55%–98%, respectively.73 Also, another systematic review enrolled 932 patients with high-risk NMIBC, with the result showing that 463 (49.7%) patients underwent reTUR within 6 month, and only 55 patients (11.9%) among them accepted reTUR within 6 weeks of diagnosis.74
Although enhanced imaging technologies (e.g., PDD, NBI, and high-definition cameras) and advanced resection techniques (e.g., bipolar or plasmakinetic) have been applied as a supplement to conventional monopolar TURBT,75,76 the intrinsic characteristic of piecemeal resection of tumor tissue has not changed for nearly 60 years.77 Therefore, histopathological analyses of resected tumor specimens are considered to be very challenging. Also, assessment of surgical margin status and depth of tumor invasion are confused by electrocautery injuries, crush artifacts, tangential sections, and lack of spatial orientation.78 To follow the general principles of oncological surgery and obtain an intact tumor specimen, ERBT as a potential alternative to conventional TURBT has gained growing interest among urologists.79
A two-center prospective phase 1 trial (ClinicalTrials.gov: NCT01508572) has been completed. The trial observed the drug safety and clinical efficacy in terms of positive surgical margins in 20 patients with primary breast cancer. The results showed that systemically administered microdosing (4.5 mg) bevacizumab-IRDye 800CW during surgery was safe. Also, although the status of surgical margin could not be assessed in situ, a clear fluorescent signal could be detected at the positive surgical margin of the excised specimen. Therefore, the surgeon could obtain quick and valuable feedback on a removed specimen from fluorescence-guided intraoperative biopsy pathology and then make a right decision to improve the quality of cancer care.80 Koller et al.81 reported a novel analytical framework for fluorescence image analysis from the macroscopic to microscopic level. An increase of 88% intraoperative detection of tumor-positive surgical margins was achieved when the author used this novel analytical framework to evaluate the clinical efficacy of FGS for invasive primary breast cancer patients. Similarly, to evaluate the status of surgical margins in BC patients, our group combined the advantages of en bloc resection and NIR imaging technology, and the mean TBR of resected specimens ranged from 1.70 to 4.09.82 Thus, on the one hand, en bloc resection enables accurate pathological interpretation of excised samples through better protection of tumor architecture and spatial orientation during surgery.83 On the other hand, optical molecular imaging of a fresh intact specimen is a useful complement that provides accurate information about both the depth of tumor invasion and the status of tumor surgical margin. By following this strategy, the status of the surgical margin, pathological stage, histologic grade, and whether DM exists in the specimen can be assessed more objectively and precisely, so that the surgeon can choose the right patient to perform reTUR decisively.
PIT for BC
PDT is an effective treatment modality for NMIBC. Due to the AEs associated with PDT, it has not yet become a conventional therapy method.84 PIT is a novel molecular targeted therapeutic model that depends on a conjugate of tumor-specific monoclonal antibody with a highly hydrophilic phthalocyanine dye, IR700, which can be activated by NIR light energy.85 The natural hydrophilic of IR700 prevents its nonspecific accumulation in bladder tumor and normal urothelium. When IR700 is conjugated with a monoclonal antibody, the conjugate selectively targets to tumor cell membranes where the monoclonal antibody-specific surface protein is overexpressed. Thus, PIT can play a targeted therapeutic role in the tumor area and minimize the cytotoxicity to normal urothelium under NIR radiation.
Proteins such as EGFR, HER-2, and CD47 are overexpressed on the tumor cell membranes and have been used as excellent targets for PIT therapy in vitro and in vivo. Panitumumab is a humanized anti-EGFR monoclonal antibody. In a proof-of-concept study, panitumumab-IR700-mediated PIT accelerated the death of human bladder tumor cell lines (e.g., UMUC-5) and restrained tumor growth in bladder tumor xenograft models compared with single panitumumab-IR700 immunotherapy.29 Panitumumab and trastuzumab can target EGFR and HER2 enriched on the bladder tumor cell surfaces, respectively. Panitumumab-IR700- and trastuzumab-IR700-mediated combination PIT therapy has a higher cytotoxicity to human bladder tumor cell lines (e.g., SW780) and a stronger anti-tumor effect in bladder tumor xenograft models than either single agent.30 In another study, anti-CD47-IR700-mediated PIT increased cytotoxicity to human bladder tumor cell lines and primary bladder tumor cells significantly in a light dose-dependent manner. Compared with the NIR irradiation group and blank control group, the tumor growth rate in the NIR-PIT group was significantly decreased. Also, after NIR-PIT therapy for 5 consecutive weeks, statistical significance was reached in inhibition of tumor growth compared to repeated anti-CD47 immunotherapy.86
PDT induces immunogenic cell death as characterized by the production of damage-associated molecular patterns (DAMPs) from the dead cells, and in turn primes the anti-tumor immune system.87 Cetuximab is a chimeric human-mouse monoclonal antibody that binds to the EGFR. The combination therapy of PDT and cetuximab dramatically inhibited the tumor growth in nude mouse xenograft models than the other two groups treated with only PDT or cetuximab.88 Thus, this result shows the possibility that patients with BC may benefit more from the combination therapy of PIT with immunotherapy in future clinical trials.
Challenges and Future Perspectives
Endoscopic molecular imaging has shown the potential to improve the detection rate of tumor lesions in fresh intact bladder specimens. Also, NIR-PIT therapy has the ability to kill human bladder tumor cell lines in vitro and restrain the tumor growth of bladder tumor xenograft models in vivo. However, this current evidence was limited to preclinical studies. There are still some scientific and technical challenges that need to be addressed. For example, in order to strike a balance between image quality and safety, it is necessary to conduct dose optimization studies. Meanwhile, the applications of bright fluorescent molecules and sensitive imaging devices are the guarantee for obtaining high-resolution images. So far, the only imaging instrument approved by the US Food and Drug Administration (FDA) for clinical use is designed based on the optical characteristics of ICG.89 However, some newly designed fluorophores might have unique photophysical properties that would be incompatible with the existing clinically approved instruments, and thus a customized imaging device may be needed to obtain high-resolution images and TBR. Compared to WLC, additional optical imaging instrumentation and molecular fluorescent agents are needed to perform endoscopic molecular imaging and PIT. When we assess the cost-effectiveness of these new technologies, the extra costs of fluorescence agents, amortization of the optical imaging equipment, and the benefit from improved oncological outcomes should be considered together. Analogously, PDD and NBI are enhanced cystoscopy technologies used in TURBT and require additional optical imaging equipment. Considering the reduction of recurrence rate and related reoperation, PDD-guided TURBT saved €168 per patient per year,90 and the biggest economic benefit was acquired among patients with intermediate-risk NMIBC.91 For patients who received NBI-guided TURBT, $230–$500 was saved per patient per year compared with conventional TURBT.92
Molecular fluorescent tracers and optical imaging instruments are basic necessities for endoscopic molecular imaging and PIT. However, no general consensus is reached on the standardized clinical evaluation of molecular fluorescent tracer that makes it difficult to conduct a multicenter study and compare the experimental results from different research centers. For example, fluorescence signal intensity and TBR are the most important reference to evaluate the clinical value of a targeted fluorescent agent. The fluorescence intensity of the inspection area is not only affected by the dosage, pharmacokinetics, and time interval between administration and examination of the fluorescent tracers being investigated, but also by the technical parameters, performance, and manufacturers of the selected imaging instrument.93 Furthermore, although a high TBR could be obtained, data acquisition might be influenced by the selected region of interest (ROI).
The ultimate goal is the clinical translation of molecular fluorescent tracers. Hence, a promising tracer has to go through a security analysis before being investigated in clinical trails. Unfortunately, the current good production process and toxicity analysis are time-consuming and expensive, and thus they are still the main obstacles to the development of new tracers and fluorophores. Ideally, molecular fluorescent agents should have certain characteristics, such as appropriate pharmacokinetics, high detection accuracy, good safety, and structural stability in vivo. Although endoscopic molecular imaging currently cannot provide real-time histopathological information in transurethral tumor resection, it has great potential value for real-time qualitative and localized diagnosis of bladder malignant lesions that makes tumor resection and histopathological assessment of specimens more accurate. Subsequently, PIT may be used as an adjuvant therapy to kill exfoliated tumor cells and residual tumors, and thus reduce recurrence rates (Figure 1).
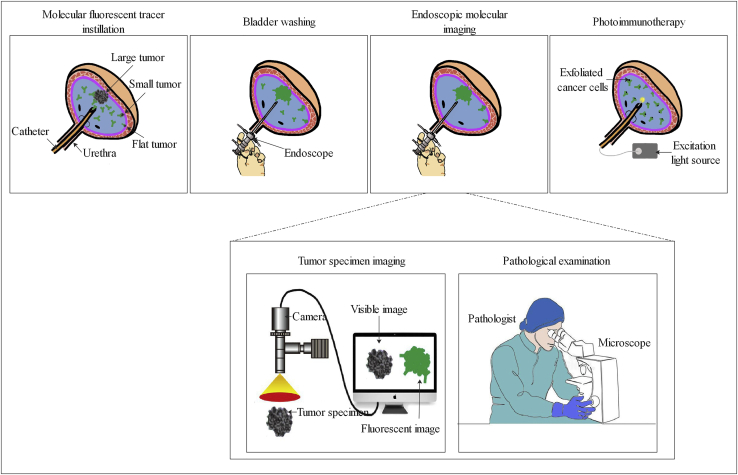
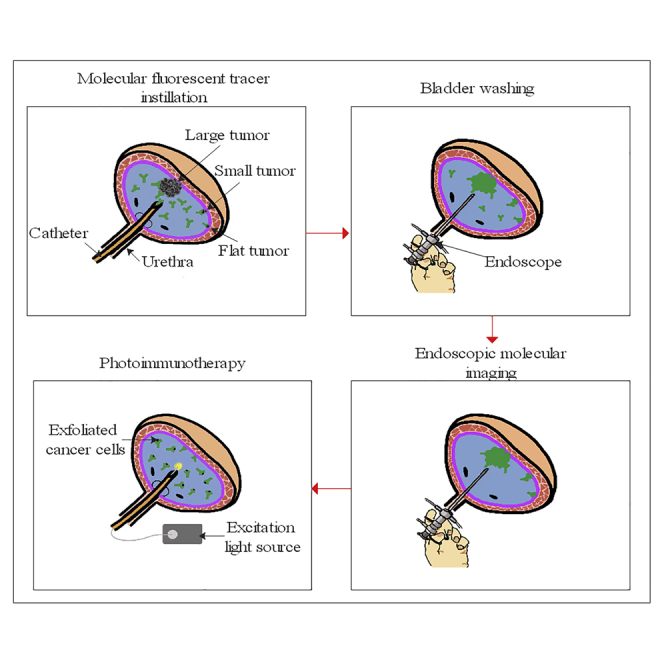
Figure 1.
Principle of Endoscopic Molecular Imaging-Guided Tumor Resection and Photoimmunotherapy
(Top) Schematic diagram of molecular fluorescent tracer-mediated endoscopic molecular imaging and photoimmunotherapy. (Bottom) Fluorescence imaging and histopathological analysis of a fresh intact tumor specimen labeled with molecular fluorescent tracer. After intravesical instillation of the tracer (from 0.5 h to a few days before en bloc resection depending on the pharmacokinetics of the tracer), the bladder is flushed and then examined with a paired imaging detection device. After en bloc resection of the bladder tumor, the patient can accept photoimmunotherapy in a post-anesthesia care unit or ward immediately. Simultaneously, the fresh intact tumor specimen is sent to the pathology department and imaged with a fluorescence camera and microscope. The pathologist can make a more accurate assessment of the status of the surgical margin and depth of tumor invasion, then provide urologists with useful histopathological information to guide which patients need repeat transurethral resection or radical cystectomy.
Conclusions
Even in their infancy stage, endoscopic molecular imaging and PIT have shown their potential to revolutionize the overall management of patients with bladder tumors by improving the positive detection rate of malignant tissues, highlighting the boundaries between tumor and normal tissue, and reducing the rates of tumor recurrence. However, there are still some obstacles that hinder the clinical translation of these promising results. Therefore, multidisciplinary collaboration between clinicians, chemists, physicists, biologists, and engineers is necessary to develop new molecular fluorescent agents and optical imaging instruments, translate them into clinical practice, and realize their full potential value in cancer detection and therapy. and realize its full potential value in cancer detection and therapy.
Author Contributions
Y.Y. and C.L. conceived the idea, designed the study, drafted the manuscript; X.Y. and Y.Y. made critical revisions to this manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (grant 81172444 to X.Y.).
References
- 1.Zhang R.R., Schroeder A.B., Grudzinski J.J., Rosenthal E.L., Warram J.M., Pinchuk A.N., Eliceiri K.W., Kuo J.S., Weichert J.P. Beyond the margins: real-time detection of cancer using targeted fluorophores. Nat. Rev. Clin. Oncol. 2017;14:347–364. doi: 10.1038/nrclinonc.2016.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werner R.A., Derlin T., Lapa C., Sheikbahaei S., Higuchi T., Giesel F.L., Behr S., Drzezga A., Kimura H., Buck A.K. 18F-labeled, PSMA-targeted radiotracers: leveraging the advantages of radiofluorination for prostate cancer molecular imaging. Theranostics. 2020;10:1–16. doi: 10.7150/thno.37894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mondal S., O’Brien C., Bishop K., Fields R., Margenthaler J., Achilefu S. Repurposing molecular imaging and sensing for cancer image-guided surgery. J. Nucl. Med. 2020;61:1113–1122. doi: 10.2967/jnumed.118.220426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nath S., Saad M.A., Pigula M., Swain J.W.R., Hasan T. Photoimmunotherapy of ovarian cancer: a unique niche in the management of advanced disease. Cancers (Basel) 2019;11:1887. doi: 10.3390/cancers11121887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stammes M.A., Bugby S.L., Porta T., Pierzchalski K., Devling T., Otto C., Dijkstra J., Vahrmeijer A.L., de Geus-Oei L.F., Mieog J.S.D. Modalities for image- and molecular-guided cancer surgery. Br. J. Surg. 2018;105:e69–e83. doi: 10.1002/bjs.10789. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi H., Choyke P.L. Near-infrared photoimmunotherapy of cancer. Acc. Chem. Res. 2019;52:2332–2339. doi: 10.1021/acs.accounts.9b00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babjuk M., Burger M., Compérat E.M., Gontero P., Mostafid A.H., Palou J., van Rhijn B.W.G., Rouprêt M., Shariat S.F., Sylvester R. European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ)—2019 update. Eur. Urol. 2019;76:639–657. doi: 10.1016/j.eururo.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Subiela J.D., Rodríguez Faba Ó., Guerrero-Ramos F., Aumatell J., Breda A., Palou J. Carcinoma in situ of the bladder: why is it underdetected? Curr. Opin. Urol. 2020;30:392–399. doi: 10.1097/MOU.0000000000000758. [DOI] [PubMed] [Google Scholar]
- 9.Subiela J.D., Rodríguez Faba O., Guerrero Ramos F., Vila Reyes H., Pisano F., Breda A., Palou J. Carcinoma in situ of the urinary bladder: a systematic review of current knowledge regarding detection, treatment, and outcomes. Eur. Urol. Focus. 2020;6:674–682. doi: 10.1016/j.euf.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Naselli A., Hurle R., Paparella S., Buffi N.M., Lughezzani G., Lista G., Casale P., Saita A., Lazzeri M., Guazzoni G. Role of restaging transurethral resection for T1 Non-muscle invasive bladder cancer: a systematic review and meta-analysis. Eur. Urol. Focus. 2018;4:558–567. doi: 10.1016/j.euf.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Cumberbatch M.G.K., Foerster B., Catto J.W.F., Kamat A.M., Kassouf W., Jubber I., Shariat S.F., Sylvester R.J., Gontero P. Repeat transurethral resection in non-muscle-invasive bladder cancer: a systematic review. Eur. Urol. 2018;73:925–933. doi: 10.1016/j.eururo.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Sylvester R.J., van der Meijden A.P., Oosterlinck W., Witjes J.A., Bouffioux C., Denis L., Newling D.W., Kurth K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 2006;49:466–477. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Chang T.C., Marcq G., Kiss B., Trivedi D.R., Mach K.E., Liao J.C. Image-guided transurethral resection of bladder tumors—current practice and future outlooks. Bladder Cancer. 2017;3:149–159. doi: 10.3233/BLC-170119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erman A., Kapun G., Novak S., Pavlin M., Dražić G., Drobne D., Veranič P. How cancer cells attach to urinary bladder epithelium in vivo: study of the early stages of tumorigenesis in an orthotopic mouse bladder tumor model. Histochem. Cell Biol. 2019;151:263–273. doi: 10.1007/s00418-018-1738-x. [DOI] [PubMed] [Google Scholar]
- 15.Oosterlinck W., Decaestecker K. Update on early instillation of chemotherapy after transurethral resection of non-muscle-invasive bladder cancer. Expert Rev. Anticancer Ther. 2018;18:437–443. doi: 10.1080/14737140.2018.1451748. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg R.L., Thomas L.J., Brooks N., Mott S.L., Vitale A., Crump T., Rao M.Y., Daniels M.J., Wang J., Nagaraju S. Multi-institution evaluation of sequential gemcitabine and docetaxel as rescue therapy for nonmuscle invasive bladder cancer. J. Urol. 2020;203:902–909. doi: 10.1097/JU.0000000000000688. [DOI] [PubMed] [Google Scholar]
- 17.Tanimoto R., Saika T., Ebara S., Kobayashi Y., Nasu R., Yamada D., Takamoto H., Miyaji Y., Nasu Y., Tsushima T., Kumon H. Prospective randomized controlled trial of postoperative early intravesical chemotherapy with pirarubicin (THP) for solitary non-muscle invasive bladder cancer comparing single and two-time instillation. World J. Urol. 2018;36:889–895. doi: 10.1007/s00345-018-2196-8. [DOI] [PubMed] [Google Scholar]
- 18.Joice G.A., Bivalacqua T.J., Kates M. Optimizing pharmacokinetics of intravesical chemotherapy for bladder cancer. Nat. Rev. Urol. 2019;16:599–612. doi: 10.1038/s41585-019-0220-4. [DOI] [PubMed] [Google Scholar]
- 19.Yadav S., Tomar V., Yadav S.S., Priyadarshi S., Banerjee I. Role of oral pentosan polysulphate in the reduction of local side effects of BCG therapy in patients with non-muscle-invasive bladder cancer: a pilot study. BJU Int. 2016;118:758–762. doi: 10.1111/bju.13489. [DOI] [PubMed] [Google Scholar]
- 20.Li R., Sundi D., Zhang J., Kim Y., Sylvester R.J., Spiess P.E., Poch M.A., Sexton W.J., Black P.C., McKiernan J.M. Systematic review of the therapeutic efficacy of bladder-preserving treatments for non-muscle-invasive bladder cancer following intravesical bacillus Calmette- Guérin. Eur. Urol. 2020 doi: 10.1016/j.eururo.2020.02.012. Published online March 3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guallar-Garrido S., Julián E. Bacillus Calmette-Guérin (BCG) therapy for bladder cancer: an update. ImmunoTargets Ther. 2020;9:1–11. doi: 10.2147/ITT.S202006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernot S., van Manen L., Debie P., Mieog J.S.D., Vahrmeijer A.L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019;20:e354–e367. doi: 10.1016/S1470-2045(19)30317-1. [DOI] [PubMed] [Google Scholar]
- 23.Smith B.L., Gadd M.A., Lanahan C.R., Rai U., Tang R., Rice-Stitt T., Merrill A.L., Strasfeld D.B., Ferrer J.M., Brachtel E.F., Specht M.C. Real-time, intraoperative detection of residual breast cancer in lumpectomy cavity walls using a novel cathepsin-activated fluorescent imaging system. Breast Cancer Res. Treat. 2018;171:413–420. doi: 10.1007/s10549-018-4845-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Predina J.D., Newton A., Corbett C., Xia L., Sulyok L.F., Shin M., Deshpande C., Litzky L., Barbosa E., Low P.S. Localization of pulmonary ground-glass opacities with folate receptor-targeted intraoperative molecular imaging. J. Thorac. Oncol. 2018;13:1028–1036. doi: 10.1016/j.jtho.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boogerd L.S.F., Hoogstins C.E.S., Schaap D.P., Kusters M., Handgraaf H.J.M., van der Valk M.J.M., Hilling D.E., Holman F.A., Peeters K.C.M.J., Mieog J.S.D. Safety and effectiveness of SGM-101, a fluorescent antibody targeting carcinoembryonic antigen, for intraoperative detection of colorectal cancer: a dose-escalation pilot study. Lancet Gastroenterol. Hepatol. 2018;3:181–191. doi: 10.1016/S2468-1253(17)30395-3. [DOI] [PubMed] [Google Scholar]
- 26.Debie P., Hernot S. Emerging fluorescent molecular tracers to guide intra-operative surgical decision-making. Front. Pharmacol. 2019;10:510. doi: 10.3389/fphar.2019.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan Y., Volkmer J.P., Mach K.E., Rouse R.V., Liu J.J., Sahoo D., Chang T.C., Metzner T.J., Kang L., van de Rijn M. Endoscopic molecular imaging of human bladder cancer using a CD47 antibody. Sci. Transl. Med. 2014;6:260ra148. doi: 10.1126/scitranslmed.3009457. [DOI] [PubMed] [Google Scholar]
- 28.Wang J., Fang R., Wang L., Chen G., Wang H., Wang Z., Zhao D., Pavlov V.N., Kabirov I., Wang Z. Identification of carbonic anhydrase IX as a novel target for endoscopic molecular imaging of human bladder cancer. Cell. Physiol. Biochem. 2018;47:1565–1577. doi: 10.1159/000490875. [DOI] [PubMed] [Google Scholar]
- 29.Railkar R., Krane L.S., Li Q.Q., Sanford T., Siddiqui M.R., Haines D., Vourganti S., Brancato S.J., Choyke P.L., Kobayashi H., Agarwal P.K. Epidermal growth factor receptor (EGFR)-targeted photoimmunotherapy (PIT) for the treatment of EGFR-expressing bladder cancer. Mol. Cancer Ther. 2017;16:2201–2214. doi: 10.1158/1535-7163.MCT-16-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siddiqui M.R., Railkar R., Sanford T., Crooks D.R., Eckhaus M.A., Haines D., Choyke P.L., Kobayashi H., Agarwal P.K. Targeting epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) expressing bladder cancer using combination photoimmunotherapy (PIT) Sci. Rep. 2019;9:2084. doi: 10.1038/s41598-019-38575-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagaya T., Okuyama S., Ogata F., Maruoka Y., Knapp D.W., Karagiannis S.N., Fazekas-Singer J., Choyke P.L., LeBlanc A.K., Jensen-Jarolim E., Kobayashi H. Near infrared photoimmunotherapy targeting bladder cancer with a canine anti-epidermal growth factor receptor (EGFR) antibody. Oncotarget. 2018;9:19026–19038. doi: 10.18632/oncotarget.24876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freise A.C., Wu A.M. In vivo imaging with antibodies and engineered fragments. Mol. Immunol. 2015;67(2 Pt A):142–152. doi: 10.1016/j.molimm.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tummers W.S., Miller S.E., Teraphongphom N.T., Gomez A., Steinberg I., Huland D.M., Hong S., Kothapalli S.R., Hasan A., Ertsey R. Intraoperative pancreatic cancer detection using tumor-specific multimodality molecular imaging. Ann. Surg. Oncol. 2018;25:1880–1888. doi: 10.1245/s10434-018-6453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xenaki K.T., Oliveira S., van Bergen En Henegouwen P.M.P. Antibody or antibody fragments: implications for molecular imaging and targeted therapy of solid tumors. Front. Immunol. 2017;8:1287. doi: 10.3389/fimmu.2017.01287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernhard W., El-Sayed A., Barreto K., Gonzalez C., Fonge H., Geyer C.R. Near infrared imaging of epidermal growth factor receptor positive xenografts in mice with domain I/II specific antibody fragments. Theranostics. 2019;9:974–985. doi: 10.7150/thno.30835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long N.E., Sullivan B.J., Ding H., Doll S., Ryan M.A., Hitchcock C.L., Martin E.W., Jr., Kumar K., Tweedle M.F., Magliery T.J. Linker engineering in anti-TAG-72 antibody fragments optimizes biophysical properties, serum half-life, and high-specificity tumor imaging. J. Biol. Chem. 2018;293:9030–9040. doi: 10.1074/jbc.RA118.002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen C.H., Wu Y.J., Chen J.J. Photo-thermal therapy of bladder cancer with anti-EGFR antibody conjugated gold nanoparticles. Front. Biosci. 2016;21:1211–1221. doi: 10.2741/4451. [DOI] [PubMed] [Google Scholar]
- 38.Lee S.M., Lee E.J., Hong H.Y., Kwon M.K., Kwon T.H., Choi J.Y., Park R.W., Kwon T.G., Yoo E.S., Yoon G.S. Targeting bladder tumor cells in vivo and in the urine with a peptide identified by phage display. Mol. Cancer Res. 2007;5:11–19. doi: 10.1158/1541-7786.MCR-06-0069. [DOI] [PubMed] [Google Scholar]
- 39.Yang X., Zhang F., Luo J., Pang J., Yan S., Luo F., Liu J., Wang W., Cui Y., Su X. A new non-muscle-invasive bladder tumor-homing peptide identified by phage display in vivo. Oncol. Rep. 2016;36:79–89. doi: 10.3892/or.2016.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng L., Shang W., Guo P., He K., Wang H., Han Z., Jiang H., Tian J., Wang K., Xu W. Phage display-derived peptide-based dual-modality imaging probe for bladder cancer diagnosis and resection postinstillation: a preclinical study. Mol. Cancer Ther. 2018;17:2100–2111. doi: 10.1158/1535-7163.MCT-18-0212. [DOI] [PubMed] [Google Scholar]
- 41.Zhang H., Aina O.H., Lam K.S., de Vere White R., Evans C., Henderson P., Lara P.N., Wang X., Bassuk J.A., Pan C.X. Identification of a bladder cancer-specific ligand using a combinatorial chemistry approach. Urol. Oncol. 2012;30:635–645. doi: 10.1016/j.urolonc.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golijanin J., Amin A., Moshnikova A., Brito J.M., Tran T.Y., Adochite R.C., Andreev G.O., Crawford T., Engelman D.M., Andreev O.A. Targeted imaging of urothelium carcinoma in human bladders by an ICG pHLIP peptide ex vivo. Proc. Natl. Acad. Sci. USA. 2016;113:11829–11834. doi: 10.1073/pnas.1610472113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cipollari S., Carnicelli G., Bicchetti M., Campa R., Pecoraro M., Panebianco V. Utilization of imaging for staging in bladder cancer: is there a role for MRI or PET-computed tomography? Curr. Opin. Urol. 2020;30:377–386. doi: 10.1097/MOU.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 44.Flaig T.W., Spiess P.E., Agarwal N., Bangs R., Boorjian S.A., Buyyounouski M.K., Chang S., Downs T.M., Efstathiou J.A., Friedlander T. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2020;18:329–354. doi: 10.6004/jnccn.2020.0011. [DOI] [PubMed] [Google Scholar]
- 45.(2017). Bladder cancer: diagnosis and management of bladder cancer. BJU Int. 120, 755–765. [DOI] [PubMed]
- 46.Salmanoglu E., Halpern E., Trabulsi E.J., Kim S., Thakur M.L. A glance at imaging bladder cancer. Clin. Transl. Imaging. 2018;6:257–269. doi: 10.1007/s40336-018-0284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKibben M.J., Woods M.E. Preoperative imaging for staging bladder cancer. Curr. Urol. Rep. 2015;16:22. doi: 10.1007/s11934-015-0496-8. [DOI] [PubMed] [Google Scholar]
- 48.Wang H., Luo C., Zhang F., Guan J., Li S., Yao H., Chen J., Luo J., Chen L., Guo Y. Multiparametric MRI for bladder cancer: validation of VI-RADS for the detection of detrusor muscle invasion. Radiology. 2019;291:668–674. doi: 10.1148/radiol.2019182506. [DOI] [PubMed] [Google Scholar]
- 49.Woo S., Panebianco V., Narumi Y., Del Giudice F., Muglia V.F., Takeuchi M., Ghafoor S., Bochner B.H., Goh A.C., Hricak H. Diagnostic performance of vesical imaging reporting and data system for the prediction of muscle-invasive bladder cancer: a systematic review and meta-analysis. Eur. Urol. Oncol. 2020;3:306–315. doi: 10.1016/j.euo.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang N., Wang X., Wang C., Chen S., Wu J., Zhang G., Zhu W., Liu J., Xu B., Du M., Chen M. Diagnostic accuracy of multi-parametric magnetic resonance imaging for tumor staging of bladder cancer: meta-analysis. Front. Oncol. 2019;9:981. doi: 10.3389/fonc.2019.00981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crozier J., Papa N., Perera M., Ngo B., Bolton D., Sengupta S., Lawrentschuk N. Comparative sensitivity and specificity of imaging modalities in staging bladder cancer prior to radical cystectomy: a systematic review and meta-analysis. World J. Urol. 2019;37:667–690. doi: 10.1007/s00345-018-2439-8. [DOI] [PubMed] [Google Scholar]
- 52.Yan H., Zhou X., Wang X., Li R., Shi Y., Xia Q., Wan L., Huang G., Liu J. Delayed 18F FDG PET/CT imaging in the assessment of residual tumors after transurethral resection of bladder cancer. Radiology. 2019;293:144–150. doi: 10.1148/radiol.2019190032. [DOI] [PubMed] [Google Scholar]
- 53.Tao K., Liu S., Wang L., Qiu H., Li B., Zhang M., Guo M., Liu H., Zhang X., Liu Y. Targeted multifunctional nanomaterials with MRI, chemotherapy and photothermal therapy for the diagnosis and treatment of bladder cancer. Biomater. Sci. 2019;8:342–352. doi: 10.1039/c9bm01377f. [DOI] [PubMed] [Google Scholar]
- 54.Comploj E., Dechet C.B., Mian M., Trenti E., Palermo S., Lodde M., Mian C., Ambrosini-Spaltro A., Horninger W., Pycha A. Perforation during TUR of bladder tumours influences the natural history of superficial bladder cancer. World J. Urol. 2014;32:1219–1223. doi: 10.1007/s00345-013-1197-x. [DOI] [PubMed] [Google Scholar]
- 55.Bosschieter J., Nieuwenhuijzen J.A., van Ginkel T., Vis A.N., Witte B., Newling D., Beckers G.M.A., van Moorselaar R.J.A. Value of an immediate intravesical instillation of mitomycin C in patients with non-muscle-invasive bladder cancer: a prospective multicentre randomised study in 2243 patients. Eur. Urol. 2018;73:226–232. doi: 10.1016/j.eururo.2017.06.038. [DOI] [PubMed] [Google Scholar]
- 56.Chen C., Huang H., Zhao Y., Liu H., Luo Y., Sylvester R.J., Li J.P., Lam T.B., Lin T., Huang J. Diagnostic accuracy of photodynamic diagnosis with 5-aminolevulinic acid, hexaminolevulinate and narrow band imaging for non-muscle invasive bladder cancer. J. Cancer. 2020;11:1082–1093. doi: 10.7150/jca.34527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J.J., Droller M.J., Liao J.C. New optical imaging technologies for bladder cancer: considerations and perspectives. J. Urol. 2012;188:361–368. doi: 10.1016/j.juro.2012.03.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liem E.I.M.L., Freund J.E., Savci-Heijink C.D., de la Rosette J.J.M.C.H., Kamphuis G.M., Baard J., Liao J.C., van Leeuwen T.G., de Reijke T.M., de Bruin D.M. Validation of confocal laser endomicroscopy features of bladder cancer: the next step towards real-time histologic grading. Eur. Urol. Focus. 2020;6:81–87. doi: 10.1016/j.euf.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 59.Xiong Y.Q., Tan J., Liu Y.M., Li Y.Z., You F.F., Zhang M.Y., Chen Q., Zou K., Sun X. Diagnostic accuracy of optical coherence tomography for bladder cancer: a systematic review and meta-analysis. Photodiagn. Photodyn. Ther. 2019;27:298–304. doi: 10.1016/j.pdpdt.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 60.Kiss B., Marcq G., Liao J.C. Optical and cross-sectional imaging technologies for bladder cancer. Cancer Treat. Res. 2018;175:139–163. doi: 10.1007/978-3-319-93339-9_7. [DOI] [PubMed] [Google Scholar]
- 61.Matlung H.L., Szilagyi K., Barclay N.A., van den Berg T.K. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol. Rev. 2017;276:145–164. doi: 10.1111/imr.12527. [DOI] [PubMed] [Google Scholar]
- 62.Klatte T., Seligson D.B., Rao J.Y., Yu H., de Martino M., Kawaoka K., Wong S.G., Belldegrun A.S., Pantuck A.J. Carbonic anhydrase IX in bladder cancer: a diagnostic, prognostic, and therapeutic molecular marker. Cancer. 2009;115:1448–1458. doi: 10.1002/cncr.24163. [DOI] [PubMed] [Google Scholar]
- 63.Griffin J.L. Devil in the detail: intratumour heterogeneity and personalised medicine for bladder cancer. Eur. Urol. 2019;75:23–24. doi: 10.1016/j.eururo.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 64.Corbet C., Feron O. Tumour acidosis: from the passenger to the driver’s seat. Nat. Rev. Cancer. 2017;17:577–593. doi: 10.1038/nrc.2017.77. [DOI] [PubMed] [Google Scholar]
- 65.Granja S., Tavares-Valente D., Queirós O., Baltazar F. Value of pH regulators in the diagnosis, prognosis and treatment of cancer. Semin. Cancer Biol. 2017;43:17–34. doi: 10.1016/j.semcancer.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 66.Weerakkody D., Moshnikova A., Thakur M.S., Moshnikova V., Daniels J., Engelman D.M., Andreev O.A., Reshetnyak Y.K. Family of pH (low) insertion peptides for tumor targeting. Proc. Natl. Acad. Sci. USA. 2013;110:5834–5839. doi: 10.1073/pnas.1303708110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wyatt L.C., Moshnikova A., Crawford T., Engelman D.M., Andreev O.A., Reshetnyak Y.K. Peptides of pHLIP family for targeted intracellular and extracellular delivery of cargo molecules to tumors. Proc. Natl. Acad. Sci. USA. 2018;115:E2811–E2818. doi: 10.1073/pnas.1715350115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Witjes J.A., Babjuk M., Bellmunt J., Bruins H.M., De Reijke T.M., De Santis M., Gillessen S., James N., Maclennan S., Palou J. EAU-ESMO consensus statements on the management of advanced and variant bladder cancer—an international collaborative multistakeholder effort: under the auspices of the EAU-ESMO guidelines committees. Eur. Urol. 2020;77:223–250. doi: 10.1016/j.eururo.2019.09.035. [DOI] [PubMed] [Google Scholar]
- 69.Pietzak E.J., Bagrodia A., Cha E.K., Drill E.N., Iyer G., Isharwal S., Ostrovnaya I., Baez P., Li Q., Berger M.F. Next-generation sequencing of nonmuscle invasive bladder cancer reveals potential biomarkers and rational therapeutic targets. Eur. Urol. 2017;72:952–959. doi: 10.1016/j.eururo.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Z., Zeng S., Zhao J., Lu X., Xu W., Ma C., Wang Y., Chen X., Jia G., Zhou T. A pilot study of vela laser for en bloc resection of papillary bladder cancer. Clin. Genitourin. Cancer. 2017;15:e311–e314. doi: 10.1016/j.clgc.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 71.Zhang J., Wang L., Mao S., Liu M., Zhang W., Zhang Z., Guo Y., Huang B., Yan Y., Huang Y., Yao X. Transurethral en bloc resection with bipolar button electrode for non-muscle invasive bladder cancer. Int. Urol. Nephrol. 2018;50:619–623. doi: 10.1007/s11255-018-1830-0. [DOI] [PubMed] [Google Scholar]
- 72.Li K., Xu Y., Tan M., Xia S., Xu Z., Xu D. A retrospective comparison of thulium laser en bloc resection of bladder tumor and plasmakinetic transurethral resection of bladder tumor in primary non-muscle invasive bladder cancer. Lasers Med. Sci. 2019;34:85–92. doi: 10.1007/s10103-018-2604-8. [DOI] [PubMed] [Google Scholar]
- 73.Hendricksen K., Aziz A., Bes P., Chun F.K., Dobruch J., Kluth L.A., Gontero P., Necchi A., Noon A.P., van Rhijn B.W.G., Young Academic Urologists Urothelial Carcinoma Group of the European Association of Urology Discrepancy between European Association of Urology guidelines and daily practice in the management of non-muscle-invasive bladder cancer: results of a European survey. Eur. Urol. Focus. 2019;5:681–688. doi: 10.1016/j.euf.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 74.Gordon P.C., Thomas F., Noon A.P., Rosario D.J., Catto J.W.F. Long-term outcomes from re-resection for high-risk non-muscle-invasive bladder cancer: a potential to rationalize use. Eur. Urol. Focus. 2019;5:650–657. doi: 10.1016/j.euf.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 75.Chen C., Huang H., Zhao Y., Liu H., Sylvester R., Lin T., Huang J. Diagnostic performance of image technique based transurethral resection for non-muscle invasive bladder cancer: systematic review and diagnostic meta-analysis. BMJ Open. 2019;9:e028173. doi: 10.1136/bmjopen-2018-028173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liem E.I.M.L., McCormack M., Chan E.S.Y., Matsui Y., Geavlete P., Choi Y.D., de Reijke T.M., Farahat Y., Inman B.A., de la Rosette J.J.M.C.H., Naito S. Monopolar vs. bipolar transurethral resection for non-muscle invasive bladder carcinoma: a post-hoc analysis from a randomized controlled trial. Urol. Oncol. 2018;36:338.e1–338.e11. doi: 10.1016/j.urolonc.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 77.Mostafid H., Babjuk M., Bochner B., Lerner S.P., Witjes F., Palou J., Roupret M., Shariat S., Gontero P., van Rhijn B. Transurethral resection of bladder tumour: the neglected procedure in the technology race in bladder cancer. Eur. Urol. 2020;77:669–670. doi: 10.1016/j.eururo.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 78.Herrmann T.R., Wolters M., Kramer M.W. Transurethral en bloc resection of nonmuscle invasive bladder cancer: trend or hype. Curr. Opin. Urol. 2017;27:182–190. doi: 10.1097/MOU.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 79.Kramer M.W., Altieri V., Hurle R., Lusuardi L., Merseburger A.S., Rassweiler J., Struck J.P., Herrmann T.R.W. Current evidence of transurethral en-bloc resection of nonmuscle invasive bladder cancer. Eur. Urol. Focus. 2017;3:567–576. doi: 10.1016/j.euf.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 80.Lamberts L.E., Koch M., de Jong J.S., Adams A.L.L., Glatz J., Kranendonk M.E.G., Terwisscha van Scheltinga A.G.T., Jansen L., de Vries J., Lub-de Hooge M.N. Tumor-specific uptake of fluorescent bevacizumab-IRDye800CW microdosing in patients with primary breast cancer: a phase I feasibility study. Clin. Cancer Res. 2017;23:2730–2741. doi: 10.1158/1078-0432.CCR-16-0437. [DOI] [PubMed] [Google Scholar]
- 81.Koller M., Qiu S.Q., Linssen M.D., Jansen L., Kelder W., de Vries J., Kruithof I., Zhang G.J., Robinson D.J., Nagengast W.B. Implementation and benchmarking of a novel analytical framework to clinically evaluate tumor-specific fluorescent tracers. Nat. Commun. 2018;9:3739. doi: 10.1038/s41467-018-05727-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang Y., Yang X., Liu C., Li J. Preliminary study on the application of en bloc resection combined with near-infrared molecular imaging technique in the diagnosis and treatment of bladder cancer. World J. Urol. 2020 doi: 10.1007/s00345-020-03143-w. Published online March 4, 2020. [DOI] [PubMed] [Google Scholar]
- 83.Liang H., Yang T., Wu K., He D., Fan J. En bloc resection improves the identification of muscularis mucosae in non-muscle invasive bladder cancer. World J. Urol. 2019;37:2677–2682. doi: 10.1007/s00345-019-02672-3. [DOI] [PubMed] [Google Scholar]
- 84.Lee J.Y., Diaz R.R., Cho K.S., Lim M.S., Chung J.S., Kim W.T., Ham W.S., Choi Y.D. Efficacy and safety of photodynamic therapy for recurrent, high grade nonmuscle invasive bladder cancer refractory or intolerant to bacille Calmette-Guérin immunotherapy. J. Urol. 2013;190:1192–1199. doi: 10.1016/j.juro.2013.04.077. [DOI] [PubMed] [Google Scholar]
- 85.Railkar R., Agarwal P.K. Photodynamic therapy in the treatment of bladder cancer: past challenges and current innovations. Eur. Urol. Focus. 2018;4:509–511. doi: 10.1016/j.euf.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 86.Kiss B., van den Berg N.S., Ertsey R., McKenna K., Mach K.E., Zhang C.A., Volkmer J.P., Weissman I.L., Rosenthal E.L., Liao J.C. CD47-targeted near-infrared photoimmunotherapy for human bladder cancer. Clin. Cancer Res. 2019;25:3561–3571. doi: 10.1158/1078-0432.CCR-18-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qi S., Lu L., Zhou F., Chen Y., Xu M., Chen L., Yu X., Chen W.R., Zhang Z. Neutrophil infiltration and whole-cell vaccine elicited by N-dihydrogalactochitosan combined with NIR phototherapy to enhance antitumor immune response and T cell immune memory. Theranostics. 2020;10:1814–1832. doi: 10.7150/thno.38515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bhuvaneswari R., Gan Y.Y., Soo K.C., Olivo M. Targeting EGFR with photodynamic therapy in combination with Erbitux enhances in vivo bladder tumor response. Mol. Cancer. 2009;8:94. doi: 10.1186/1476-4598-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DSouza A.V., Lin H., Henderson E.R., Samkoe K.S., Pogue B.W. Review of fluorescence guided surgery systems: identification of key performance capabilities beyond indocyanine green imaging. J. Biomed. Opt. 2016;21:80901. doi: 10.1117/1.JBO.21.8.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burger M., Zaak D., Stief C.G., Filbeck T., Wieland W.F., Roessler W., Denzinger S. Photodynamic diagnostics and noninvasive bladder cancer: is it cost-effective in long-term application? A Germany-based cost analysis. Eur. Urol. 2007;52:142–147. doi: 10.1016/j.eururo.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 91.Daneshmand S., Schuckman A.K., Bochner B.H., Cookson M.S., Downs T.M., Gomella L.G., Grossman H.B., Kamat A.M., Konety B.R., Lee C.T. Hexaminolevulinate blue-light cystoscopy in non-muscle-invasive bladder cancer: review of the clinical evidence and consensus statement on appropriate use in the USA. Nat. Rev. Urol. 2014;11:589–596. doi: 10.1038/nrurol.2014.245. [DOI] [PubMed] [Google Scholar]
- 92.Raharja P.A.R., Hamid A.R.A.H., Mochtar C.A., Umbas R. Recent advances in optical imaging technologies for the detection of bladder cancer. Photodiagn. Photodyn. Ther. 2018;24:192–197. doi: 10.1016/j.pdpdt.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 93.Koch M., Symvoulidis P., Ntziachristos V. Tackling standardization in fluorescence molecular imaging. Nat. Photonics. 2018;12:505–515. [Google Scholar]