Abstract
In Colombia, dogs and opossum are the most important mammals in domestic and sylvatic T. cruzi transmission. However, the role of both species has not been evaluated in areas where both species converge in the peridomestic area. To evaluate the infection status of domestic and wild mammals in peridomestic habitats of Puerto Valdivia, Antioquia Department. The infection of domestic dogs and small wild mammals was performed by hemoculture, molecular and serological methods. Additionally, the infection in children under 15 years old and triatomine searches was carried out. We found that 16.07% and 34% dogs, and 59.1% and 61.1% Didelphis marsupialis were found positive by molecular and serological methods respectively. Moreover, in 25% and 75% of the infected dogs were detected TcIDom and TcI sylvatic, respectively, while all the D. marsupialis were infected with TcI. Six Rattus rattus and three Proechimys semispinosus were captured but without T. cruzi infection. Finally, none of the 82 children were positive and no triatomine bugs were captured. D. marsupialis and domestics dogs have an important role in the transmission of T. cruzi suggesting a potential risk in T. cruzi transitions areas.
Keywords: Chagas disease, Colombia, Didelphis marsupialis, Dogs, Reservoirs, DTUI
1. Background
Chagas disease is a zoonosis caused by the protozoan hemoflagellate Trypanosoma cruzi that affects around six million people, resulting in approximately 12,000 deaths annually (WHO, 2019). In Colombia, it has been estimated that between 1.67 and 2% of the population is infected with this parasite (Olivera et al., 2019). T. cruzi is transmitted to humans mainly by triatomine vectors (Hemiptera: Reduviidae) throughout skin contact and mucous membranes with feces and other secretions of these insects (Chagas, 1909). However, the importance of other ways of transmission in free areas or without a history of vectorial domestic transmission has increased in some countries (Rueda et al., 2014). Oral outbreaks are of growing concern, in scenarios where vectors in domiciliation process such as Panstrongylus geniculatus and wild reservoir hosts like Didelphis marsupialis have active participation (Caicedo-Garzon et al., 2019; Deane et al., 1984).
T. cruzi presents tremendous genetic diversity and has been divided into six Discrete Typing Units (DTUs) from TcI to TcVI, associated in some countries and regions with different clinical manifestations, geographical distribution, and transmission cycles (Domestic, Peridomestic, and Sylvatic) (Zingales et al., 2009). TcI is the most widely distributed DTU in Colombia (Guhl and Ramírez, 2013), and It has been divided into two genotypes, TcIDom and Sylvatic TcI, associated with domestic and sylvatic foci, respectively (Ramirez et al., 2012). Moreover, Sylvatic TcI is the most common within oral outbreaks (Ramirez et al., 2013a).
Around 150 mammalians distributed from the southern USA to the south of Argentina are infected by T. cruzi (Jansen and Roque, 2010). The role that different species of mammals and triatomine vectors play in T. cruzi transmission to humans depends on the local conditions that facilitate contact with humans in the different types of transmission cycles (Miles et al., 2003). In Colombia, Canis lupus familiaris (domestic dogs) and Didelphis marsupialis (common opossum) are considered the mammals' species with the highest epidemiological importance (Guhl and Ramírez, 2013). Domestic dogs have been described as with the highest pooled prevalence in Colombia and active participation in the domestic cycle (Rodríguez-Monguí et al., 2019). However, this species represents a heterogeneous role in the ecoepidemiology of Chagas disease in this country, with importance as synanthropic reservoirs of T. cruzi in the Boyaca department to a secondary role in the Caribbean region (Cantillo-Barraza et al., 2015; Ramirez et al., 2013b).
On the other hand, D. marsupialis is a generalist species with great potential as T. cruzi reservoir, especially in highly disturbed environments due to its persistent high infection rates, and the highly adaptive behavior that allows it to live close to both domestic and sylvatic habitats and to transport infection between households (Roque et al., 2013). In Colombia, D. marsupialis is the species with the highest prevalence and reports of infection with an active role in the sylvatic cycle and outbreaks resulting from oral transmission (Guhl and Ramírez, 2013; Rodríguez-Monguí et al., 2019; Hernandez et al., 2016). However, the role of this species has not been extensively studied yet in the domestic and peridomestic T. cruzi transmission cycles despite its well-known synanthropic behavior.
In rural Colombia's zones, domestic dogs are mostly used for house protection and hunting activities, increasing the potential risk of infection with T. cruzi, especially in the peridomestic ecotope (Cantillo-Barraza et al., 2015; Ramirez et al., 2013b). Besides, the synanthropic behavior of common opossum also facilitates the presence of this species in the peridomestic area (Jansen and Roque, 2010; Roque et al., 2013). In this sense, the ecological behavior features of both species allow the evaluation of infection as one alternative for the estimation of the potential risk as well as T. cruzi transmission hotspots, especially in areas with previous reports of triatomine species associated with oral outbreaks. The present study was conducted in Puerto Valdivia, Antioquia department in Colombia, where the presence of P. geniculatus has previously been reported (Guhl et al., 2007). This work aimed to evaluate T. cruzi infection in domestic dogs and peridomestic opossums as a measure of the potential risk of Chagas disease transmission in this area of Colombia.
2. Materials and methods
2.1. Study area
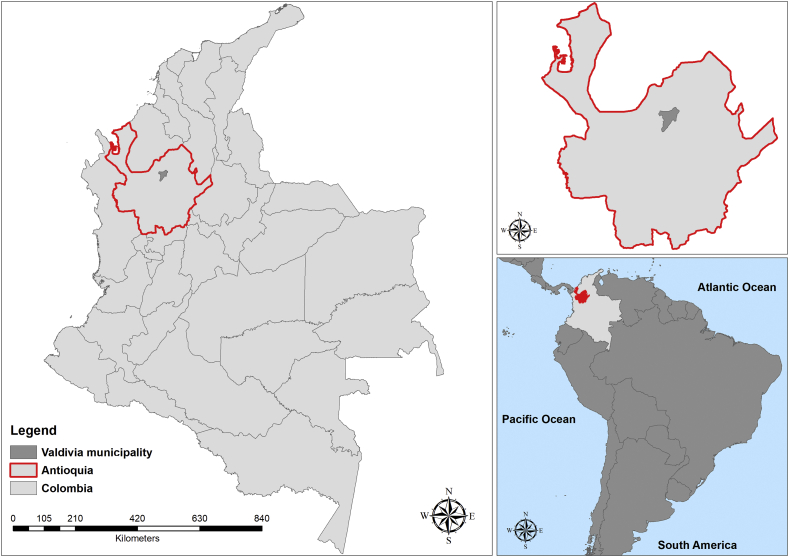
This study was conducted between 2012 and 2014 on the municipality of Valdivia at the edge of the Cauca River in Antioquia Department, Colombia. The population of approximately 22.179 inhabitants, 15.627 persons living in urban areas, and 6.552 in rural areas. The village has an altitude of 400 m.a.s.l. and an average temperature of 21 °C with precipitation levels between 3000 and 3510 mm. The specific study zone was located in the rural area of the Valdivia municipality, known as Puerto Valdivia (Fig. 1).
Fig. 1.
Study area located in the Puerto Valdivia, Valdivia municipality and Colombia.
2.2. T. cruzi seroprevalence in children
2.2.1. Blood sampling and serology analysis
With previous informed consent signed by one or both parents and following the requirement of the University of Antioquia (License 08–012-185), blood samples were collected from 85 children under 15 years of age. The procedure was carried out in rural schools by trained personnel. Approximately 5 mL of whole blood was collected by venipuncture. Blood was centrifuged and serum was stored – 30 °C until processing.
Anti T- cruzi IgG in children were detected by two serological tests: (i) all samples were subject to one initial screening by ELISA based on crude parasites extracted from two T. cruzi isolates, as described above. The optical density (OD) values of previously confirmed positive and negative control samples were used to define the limits of seropositivity in the assay. OD values higher than 3 SD above the OD average for negative controls were considered ELISA-positive. (ii) All ELISA-positive samples were later evaluated by indirect immunofluorescence (IFAT) with a titer of 1/40 used as the positive cut off. Samples were considered positive if both tests were reactive (Camargo, 1972).
2.3. Mammals hosts: domestic and sylvatic samples collection
2.3.1. Dog surveys
Blood sampling was conducted through a house-to-house strategy. The inclusion criteria for selected dogs were as follows: (i) born and rose in the study area, and (ii) having a recognizable owner who signed informed consent for them. After owner consent, five mL of peripheral blood was obtained from the cephalic vein in an EDTA containing vacutainer. The age was based on the owner's information and confirmed with the dental condition status.
2.3.2. Sylvatic mammals capture
Small wild mammals were captured using Tomahawk® live traps arranged linearly. The capture points were baited with a peanut, banana, oat, and fish mixture, and ten live traps were placed at 10 m intervals. The traps were placed in peridomestic areas that we defined as the total area around the house, including permanent or temporal structures built and used by humans or their domestic animals. Caught wild mammals were anesthetized intramuscularly (9:1 ketamine 10% and xylazine 2%) and 0.1–0.0.3 mL of blood collected by cardiac puncture (Rocha et al., 2013).
2.4. Trypanosoma cruzi infection in mammals
We used three procedures for detection infection in domestic dogs and sylvatic mammals: parasitological, serological, and molecular approaches. (i) 0.2–0.4 mL of blood was cultured in two tubes containing Novy-McNeal-Nicole (NNN) medium with Liver Infusion Tryptose (LIT) overlay (hemoculture). (ii) The remaining blood was centrifuged at 5000 g for 10 min and serum was stored at −20 °C until serological assays were performed; (iii) finally the remaining samples were stored for DNA extraction and molecular diagnosis (see below). If insufficient blood was collected, priority was given to hemoculture and molecular diagnosis (Roque et al., 2013; Rocha et al., 2013).
2.4.1. Parasitological diagnostic
Hemocultures was analyzed every 15 days for three months. Positive hemocultures were amplified for molecular characterization and cryopreserved in the collection of Trypanosomas spp. of the BCEI-UdeA research group.
2.4.2. Serological diagnostic test
Serological diagnosis in dogs: detection of anti-T. cruzi antibodies (IgG) in dogs was conducted using an Enzyme-Linked Immunosorbent Assay (ELISA) and an Indirect Immunofluorescence Antibody Test (IFAT). For both techniques, the antigen was prepared from harvested epimastigotes of T. cruzi Colombian strains (I.RHO/CO/00/CAS-15.CAS; I. TRI/CO/03/MG-8.MAG), previously characterized as TcI (Cantillo-Barraza et al., 2015). For ELISA, a whole lysate extracted from epimastigotes was used as antigen, while for IFAT it was obtained from complete epimastigotes fixed in 1% formaldehyde as reported elsewhere (Roque et al., 2013). The cut off was determined as optical absorbance ≥0.200 (mean ± SD of negative control) for ELISA and sera dilution of ≥1/40 for IFAT as reported elsewhere (Camargo, 1972). Animals were defined as positive when samples were reactive to both tests, which have a 100% sensitivity and 98.7% specificity concerning the ELISA and IFAT from Bio-Manguinhos, FIOCRUZ, Rio de Janeiro, RJ Brazil (Rocha et al., 2013). Cross-reaction and/or mixed infections by T. cruzi and Leishmania spp. in serum were also assayed with antigens derived from a mixture of Leishmania infantum and L. panamensis using IFAT. The IFAT cut-off value adopted for T. cruzi infection was 1/40 when IFAT results for Leishmania spp were negative. On the other hand, in Leishmania spp. positive dogs, positive T. cruzi infection was considered only when the IFAT titer was 1/80 or higher (Camargo, 1972).
Serological diagnosis in opossum: detection of anti-T. cruzi antibodies (IgG) were obtained using an adapted version of the IFAT described by (Guhl et al., 2007). The antigen used was an equal mixture of complete parasites derived from the strains I00/BR/00F (TcI) and MHOM/BR/1957/Y (TcII). Marsupials were tested with specific intermediary antibodies anti-opossum (IgG), raised in rabbits, and the reaction was revealed by an anti-rabbit IgG conjugate coupled to fluorescein isothiocyanate, the cut-off value adopted was 1:40 (Camargo, 1972; Rocha et al., 2013). Serological diagnoses for rodents were not performed.
2.4.3. Molecular detection test
Genomic DNA was extracted from 200 μL of blood and hemoculture positives using the Genomic DNA purification kit (DNeasy Blood & Tissue kit Quiagen®) following the manufacturer's instructions. T. cruzi molecular detection was performed by direct amplification of a 180 bp fragment of satellite DNA using primers TcZ1 and TcZ2 (Moser et al., 1989) T. cruzi samples were submitted for molecular discrimination of T. cruzi DTUs based on the amplification of the intergenic spacer of the spliced leader gene (SL-IR) amplified with primers TCC, TC1 and TC2 (Burgos et al., 2007). Amplification products were visualized on a 1.5% agarose gel stained with ethidium bromide under UV light. For direct sequencing of the SL-IR region, PCR products were purified and sequenced using the Sanger methodology at Macrogen sequencing service, Seoul, South Korea. For the TcI genotype identification, the microsatellite motif of the spliced leader gene (positions ranking between ~14 to ~40) was omitted as suggested (Tomasini et al., 2011). All nucleotide sequences were aligned using CLUSTALW as implemented in BioEdit v.7.1.9. The highest nucleotide identity value of the sequences based on optimal global pairwise alignments of each SL-RI sequence against reference strains reported for Colombia (Herrera et al., 2009) was calculated in BioEdit v.7.1.9.
2.5. Entomological investigation
Triatomine insect searches were carried out in collaboration with local personnel from the Ministry of health. Domiciliary and Peridomestic areas were covered carrying out the traditional manual collection method using a dislodging spray (Gurtler et al., 1999). Moreover, workshops and individual visits to households were organized in Puerto Valdivia, to provide the population with information about Chagas disease and insect vectors. Households were additionally instructed to collect triatomines found inside or around their houses in plastic vials/bags labeled with their name, address, and date of capture. All participants received biosafety training and gloves for self-protection. A total of 55 houses were inspected in this locality.
2.6. Statistical analyses
The association between the dogs´ ages, sex, sleep areas, activities, and infection status were calculated using a 2 × 2 contingency tables and chi-square test with SPSS (Statistical Package for the Social Science) V 15.0 (IL, USA). The level of significance was set as (P < .05).
2.7. Ethics statement for animal evaluations
All procedures were designed to reduce animal suffering. All owners were informed about the risk of the procedure and the risk of Chagas disease, both for the human and canine population. All animals were handled in strict accordance with the Colombian code of practice for the care and use of animals for scientific purpose, established by law 84 of 1989. Ethical approval (Act No 2223) for analyzing animal species was obtained from the animal ethics committee of the Antioquia University.
3. Results
3.1. Seroprevalence in children less than 15 years of age
A total of 82 children under 15-year-old who lived in Puerto Valdivia were tested, and no one was positive to both serological tests.
3.2. Trypanosoma cruzi infection in dogs
The majority of dogs sampled for this study live at non-delimited houses by fences made of solid material allowing the animals to roam outside freely. Dogs have access inside the house without any restriction; however, they spend the night outdoors in improvised places such as barns, sheds, or roofed-over areas that protect them from inclement weather.
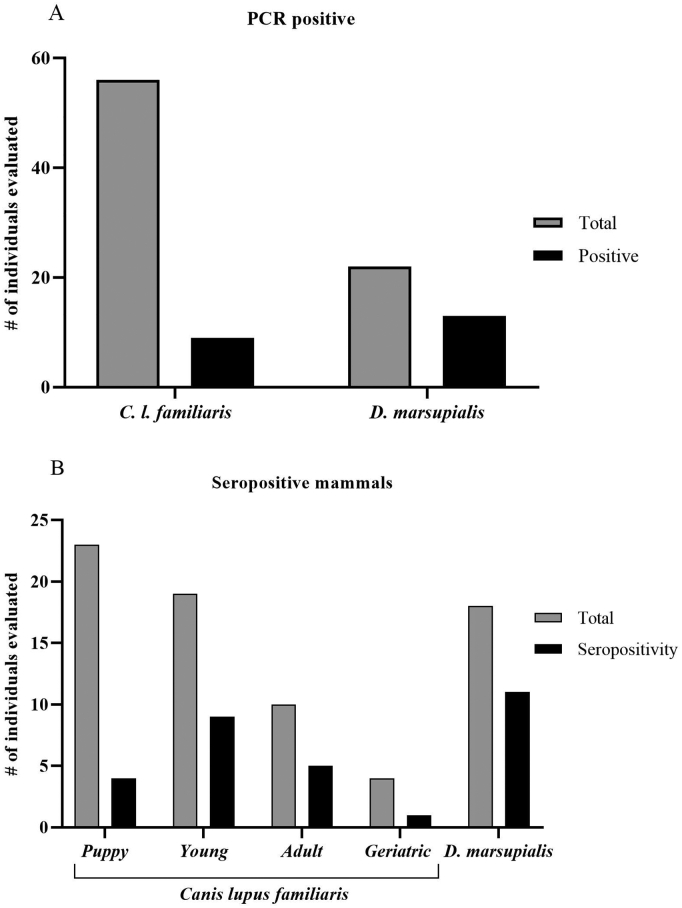
A total of 56 dogs, with a mean age of 2.8 ± 2.4 years (range from 6 months to 10 years old) were analyzed. One dog (1.78%) was positive by hemoculture, while the amplification of T. cruzi satellite DNA in blood showed a prevalence of infection of 16.07% (9/56) (Fig. 2A). Discrimination between DTUs by SL-IR analysis revealed only the presence of DTUI in all the samples. Four samples, all of the domestic dogs were sequenced and analyzed for TcI genotypes. Both TcI Dom and sylvatic TcI were detected. However, TcI Dom was found only in one sample (25%) while TcI sylvatic was found in 3 samples (75%). Both genotypes had a sequence identity higher than 97% with reference sequences for each genotype described in Genbank.
Fig. 2.
Trypanosoma cruzi infection in domestics dogs and small mammals captured in Puerto Valdivia, Antioquia, Colombia. PCR (A) and Serology (B) tests.
Concerning the serological test, 19 dogs (34%) were seropositive for T. cruzi by both ELISA and IFAT. Moreover, four (17.3%) of under one-year-old evaluated dogs were seropositive (Table 1 and Fig. 2B). We found significant differences in the T. cruzi infection rates between <2 years old and older dogs (13/32 versus 10/24, chi-square = 0.937 P =0 .0061). However, no difference between other variables and T. cruzi infected dogs were observed.
Table 1.
Infection in domestics and wild mammals captured in Puerto Valdivia, Colombia.
| Mammals | # samples | Seropositivity | PCR positive | Hemoculture positive |
|---|---|---|---|---|
| Cannis lupus familiaris | ||||
| Puppy (3 m to <1y) | 23 | 4 (17.3) | ||
| Young (1 > y to 4 y) | 19 | 9 (47.3) | 5 (26.3) | 1 |
| Adult (>4 y to 7 y) | 10 | 5 (50) | ||
| Geriatric (> 7 y) | 4 | 1 (25) | ||
| Total Cannis familiaris | 56 | 19 (33.9) | ||
| Didelphis marsupialis | 22 | 11(61.15)a | 13(59.1) | 2 |
| P. semispinosus | 3 | ND | ||
| Rattus rattus | 6 | ND |
n = 18 D. marsupialis.
3.3. T. cruzi infection in wild mammals
Thirty-one wild mammals were captured in the study area. D. marsupialis was the most abundant species (70.9%), with n = 22 specimens captured, followed by n = 6 Rattus rattus and n = 3 Proechimys semispinosus. Two blood samples resulted in a positive hemoculture, while T. cruzi DNA was detected in 13 samples of opossum, obtaining a prevalence by molecular tools of 59.1% (13/22) (Fig. 2A). Eighteen D. marsupialis from which was possible to obtain serum were also evaluated by IFAT. Of this group, eleven individuals were reactive getting seropositivity of 61% (Fig. 2B). No infection was detected in P. semispinosus and R. rattus by hemoculture or PCR, and no serological tests were developed for these species. Moreover, the T. cruzi DNA SL-IR analysis conducted in the 13 positive samples of D. marsupialis showed that all specimens were infected with DTUI (Table 1). We did not sequence and analyze the TcI genotypes for this species.
3.4. Entomological investigation
Fifty-five houses were evaluated for the presence of triatomine bugs inside the home, and around the peridomestic areas. No triatomine bugs were collected during this study in both spaces. However, several households manifested the intrusion of triatomines at least one time during the last two years. Some features related to them such as hematophagy suggested that local people differentiate triatomines from other Reduviidae bugs.
4. Discussion
Historically, domestic and peridomestic T. cruzi transmission cycles are the most important in the epidemiology of Chagas disease in Colombia (Guhl and Ramírez, 2013; Coura et al., 2014). However, in the last decade, several studies in regions without the presence of primary vectors, and with reports of acute Chagas disease outbreaks have shown the relevance of sylvatic T. cruzi transmission in this country (Cantillo-Barraza et al., 2015; Ramirez et al., 2013b; Tovar Acero et al., 2017). In this study, we performed an eco-epidemiological survey in one area of the Colombian Andean region that had reported the intrusion of P. geniculatus. Our results showed that both, domestic dogs and sylvatic D. marsupialis are synanthropic or autochthonous vehicles for T. cruzi between sylvatic and peridomestic transmission cycles. Due to these eco-epidemiological roles, the evaluation of infection in both mammalian species could be adopted to determine the potential risk of sylvatic T. cruzi transmission.
Domestic dogs may play different roles in different epidemiological scenarios of T. cruzi transmission within the continent. In some countries, dogs are considered the main reservoirs in domestic transmission (Gurtler et al., 2007; Enriquez et al., 2014). While, in Brazil and the United States, this species has been shown to have high rates of T. cruzi infection, despite having little epidemiological relevance (Jansen and Roque, 2010; Camargo, 1972; Xavier et al., 2012; Curtis-Robles et al., 2017). A different situation seems to be occurring in Colombia where domestic dogs constitute the link between the domestic and sylvatic environments (Ramirez et al., 2013b; Jaimes-Duenez et al., 2017). The results of the present study support the role of dogs as a synanthropic reservoir in Colombia. The infection with both genotypes of TcI (TcI Dom and sylvatic TcI), as well as observations made during fieldwork, confirmed that in the study area dogs roam freely on the streets near homes and in the extra-domestic area at any given time of day. This constitutes one link between extra- and peridomestic environments (Ramirez et al., 2013b).
The serological evaluation of T. cruzi infection in mammals reflects the intensity of T. cruzi transmission. Some authors have reported that the T. cruzi infection rate in Colombian dogs is modulated by the dynamics of vector populations, which are considered the main source of infection (Rodríguez-Monguí et al., 2019; Cantillo-Barraza et al., 2015; Ramirez et al., 2013b; Jaimes-Duenez et al., 2017). Here we reported a moderate seroprevalence (34%) in dogs from the department of Antioquia, where P. geniculatus is the only reported species (Guhl et al., 2007). However, the seroprevalence reported here is higher than the prevalence of infection reported in other departments, such as Cundinamarca (29.49%), and Meta (25.6%), where the presence of P. geniculatus is widely known (Mesa-Arciniegas et al., 2020; Turriago et al., 2008). The higher seroprevalence in this area could be a result of both vector and oral transmission by the ingestion of or contact with infected sylvatic mammals such as D. marsupialis.
The presence of T. cruzi DNA in the blood of dogs suggests the initial phase of infection and their potential infect blood-feeding insect vectors (Rocha et al., 2013; Curtis-Robles et al., 2017). Our results showed a higher active infection rate (16.07%) than in dogs in enzootic areas of Colombia (Jaimes-Duenez et al., 2017; Turriago et al., 2008). The active transmission of T. cruzi is also supported by seroprevalence of 21.05% in dogs under one-year-old. On the other hand, regarding the infectivity ability, a poor transmissibility competence was observed in these dogs since only one isolate was obtained by hemoculture (1.78%), while the PCR analyses of blood revealed that 16.07% of dogs harbored parasites in their blood (Jansen and Roque, 2010). This incongruence between the PCR and hemoculture results has been related to the low circulation of the infective forms of TcI in some mammalian species, such as dogs and rodents (Enriquez et al., 2014; Orozco et al., 2014).
A prolonged exposure of dogs to T. cruzi transmission in Puerto Valdivia was observed in this study. The statistical analysis suggested a significant difference in the infection rates between dogs younger than 2 years and those older than 2 years. These characteristics have been previously reported in many settings in Latin America where the association between increased age and T. cruzi seroprevalence could reflect the cumulative exposure to parasitic infections (Pineda et al., 2011; Ocana-Mayorga et al., 2010).
D. marsupialis is considered the main reservoir in the sylvatic transmission cycle of T. cruzi. Nonetheless, few studies have investigated the role of this species in the peridomestic area, although its synanthropic behavior is well known (Rodríguez-Monguí et al., 2019). The results of this work showed some evidence that suggests the important role of the common opossum in the active transmission of T. cruzi around of household: (i) this species was the most common sylvatic mammals in the peridomestic area (70.9%), (ii) of evaluated dogs, 59.1% had T. cruzi DNA in their blood samples, (iii) positive hemocultures showed transmissibility competence in opossums, and (iv) the infection frequency by IFAT of 61% showed high exposure to T. cruzi transmission. Similarly, the importance of D. marsupialis in the eco-epidemiology of Chagas disease has been recognized in Ecuador, where it has been described due to its synanthropic behavior and its role as a T. cruzi reservoir (Ocana-Mayorga et al., 2010).
Different infection levels in opossum have been reported in Colombia where R. pallescens and T. maculata are present, such as the Momposina depression zone in the Caribbean plains bioregion, where rates of infections were reported between 61.5% and 86.3% (Cantillo-Barraza et al., 2015; Cantillo-Barraza et al., 2014). While, in the Orinoco region, where the presence of wild R. prolixus has been observed, infection levels of this species reached 21% (Rendon et al., 2015). The prevalence levels reported here and the abundance of this species demonstrates the importance of opossums in an area with reports of triatomine species in the domiciliation process as P. geniculatus.
The epidemiological importance of common opossum in Chagas disease is highlighted for several reasons, including its ability to act as both a reservoir and a vector of T. cruzi (Deane et al., 1984; Urdaneta-Morales and Nironi, 1996). In Colombia, this mammal has been implicated in outbreaks resulting from oral transmission in different regions, suggesting they can contaminate human food sources via anal secretions (Ramirez et al., 2013a; Hernandez et al., 2016). The prevalence levels reported here and the abundance of this species in the peridomestic area represents a potential risk for oral transmission of Chagas disease for both humans and canines in the department of Antioquia. These results emphasize the need for continuous surveillance, including the organization and clearing of peridomestic areas to remove D. marsupialis.
In the present study, we did not capture triatomine bugs in the domestic or peridomestic habitats. Therefore, we hypothesized that the lack of previously reported P. geniculatus in these habitats could be related to the indiscriminate use of insecticides in illegal coca crops which have been predominant in the study area (Grijalva et al., 2010). However, in dogs and other carnivores mammals, other routes of transmission play one most important role than to vector transmission (Jansen and Roque, 2010). The ingestion of parasite-infected flesh or blood, besides the ingestion of infected food by triatomines or opossum feces, are important mechanisms for T. cruzi transmission in wild cycles (Chagas, 1909; Jansen and Roque, 2010). We recognize that the lack of sampling in extra-domestic areas as a consequence of the internal armed conflict is the first limitation of the present study. Thus, we considered it necessary to develop future research in this habitat during the post-conflict era to clarify the T. cruzi dynamics.
5. Conclusions
Here we presented an eco-epidemiological study in a limited area of the Andean region in Colombia with previous reports of P. geniculatus. Our results showed that domestics dogs and common opossum are synanthropic reservoirs by establishing a link between peridomestic and sylvatic transmission cycles. Entomological research and the evaluation of children under fifteen-years-old evidenced there is no risk of T. cruzi transmission in this area. Conversely, the parasitological, serological, and molecular evaluation of the infection of both species could be used to estimate the potential risk for a sylvatic T. cruzi transmission cycle in the peridomestic areas.
Acknowledgments
This study was carried out thanks to the agreement to the Antioquia University, specially of BCEI Group.
References
- Burgos J.M., Altcheh J., Bisio M., Duffy T., Valadares H.M., Seidenstein Direct molecular profiling of minicircle signatures and lineages of Trypanosoma cruzi bloodstream populations causing congenital Chagas disease. Int. J. Parasitol. 2007;37:1319–1327. doi: 10.1016/j.ijpara.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Caicedo-Garzon V., Salgado-Roa F.C., Sanchez-Herrera M., Hernandez C., Arias-Giraldo L.M. Genetic diversification of Panstrongylus geniculatus (Reduviidae: Triatominae) in northern South America. PLoS One. 2019;14 doi: 10.1371/journal.pone.0223963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo M. Reacciones serológicas y consecuencias sociales de los resultados positivos a la enfermedad de Chagas. Boletin de la Oficina Sanitaria Panamericana. 1972:576–581. [PubMed] [Google Scholar]
- Cantillo-Barraza O., Chaverra D., Marcet P., Arboleda-Sánchez S., Triana-Chávez O. Trypanosoma cruzi transmission in a Colombian Caribbean region suggests that secondary vectors play an important epidemiological role. Parasit. Vectors. 2014;7:381. doi: 10.1186/1756-3305-7-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantillo-Barraza O., Garces E., Gomez-Palacio A., Cortes L.A., Pereira A., Marcet P.L. Eco-epidemiological study of an endemic Chagas disease region in northern Colombia reveals the importance of Triatoma maculata (Hemiptera: Reduviidae), dogs and Didelphis marsupialis in Trypanosoma cruzi maintenance. Parasit. Vectors. 2015;8:482. doi: 10.1186/s13071-015-1100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagas C. Nova tripanozomiaze humana: estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem. Inst. Oswaldo Cruz. 1909;1:159–218. doi: 10.1590/S0074-02761909000200008. [DOI] [Google Scholar]
- Coura J.R., Vinas P.A., Junqueira A.C. Ecoepidemiology, short history and control of Chagas disease in the endemic countries and the new challenge for non-endemic countries. Mem. Inst. Oswaldo Cruz. 2014;109:856–862. doi: 10.1590/0074-0276140236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis-Robles R., Snowden K.F., Dominguez B., Dinges L., Rodgers S., Mays G. Epidemiology and molecular typing of Trypanosoma cruzi in naturally-infected hound dogs and associated triatomine vectors in Texas, USA. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005298. (https://doi.org/1:e0005298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane M.P., Lenzi H.L., Jansen A. Trypanosoma cruzi: vertebrate and invertebrate cycles in the same mammal host, the opossum Didelphis marsupialis. Mem. Inst. Oswaldo Cruz. 1984;79(4):513–515. doi: 10.1590/s0074-02761984000400021. [DOI] [PubMed] [Google Scholar]
- Enriquez G.F., Bua J., Orozco M.M., Wirth S., Schijman A.G., Gurtler R.E. High levels of Trypanosoma cruzi DNA determined by qPCR and infectiousness to Triatoma infestans support dogs and cats are major sources of parasites for domestic transmission. Infect. Genet. Evol. 2014;25:36–43. doi: 10.1016/j.meegid.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Grijalva M.J., Palomeque F.S., Villacis A.G., Black C.L., Arcos-Teran L. Absence of domestic triatomine colonies in an area of the coastal region of Ecuador where Chagas disease is endemic. Mem. Inst. Oswaldo Cruz. 2010;105:677–681. doi: 10.1590/S0074-02762010000500013. [DOI] [PubMed] [Google Scholar]
- Guhl F., Ramírez J.D. Retrospective molecular integrated epidemiology of Chagas disease in Colombia. Infect. Genet. Evol. 2013;20:148–154. doi: 10.1016/j.meegid.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Guhl F., Aguilera G., Pinto N., Vergara D. Updated geographical distribution and ecoepidemiology of the triatomine fauna (Reduviidae: Triatominae) in Colombia. Biomedica. 2007;27(Suppl. 1):143–162. [PubMed] [Google Scholar]
- Gurtler R.E., Cecere M.C., Canale D.M., Castanera M.B., Chuit R., Cohen J.E. Monitoring house reinfestation by vectors of Chagas disease: a comparative trial of detection methods during a four-year follow-up. Acta Trop. 1999;72:213–234. doi: 10.1016/S0001-706X(98)00096-5. [DOI] [PubMed] [Google Scholar]
- Gurtler R.E., Cecere M.C., Lauricella M.A., Cardinal M.V., Kitron U., Cohen J.E. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology. 2007;134(1):69–82. doi: 10.1017/s0031182006001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez C., Vera M.J., Cucunuba Z., Florez C., Cantillo O., Buitrago L.S. High-resolution molecular typing of Trypanosoma cruzi in 2 large outbreaks of acute Chagas disease in Colombia. J. Infect. Dis. 2016;214:1252–1255. doi: 10.1093/infdis/jiw360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera C., Guhl F., Falla A., Fajardo A., Montilla M., Vallejo G.A. Genetic Variability and phylogenetic relationships within Trypanosoma cruzi I isolated in Colombia based on miniexon gene sequences. J. Parasitol. Res. 2009 doi: 10.1155/2009/897364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimes-Duenez J., Triana-Chavez O., Cantillo-Barraza O., Hernandez C., Ramirez J.D., Gongora-Orjuela A. Molecular and serological detection of Trypanosoma cruzi in dogs (Canis lupus familiaris) suggests potential transmission risk in areas of recent acute Chagas disease outbreaks in Colombia. Prev. Vet. Med. 2017;141:1–6. doi: 10.1016/j.prevetmed.2017.03.009. [DOI] [PubMed] [Google Scholar]
- Jansen A.M., Roque A.L.R. Domestic and wild mammalian reservoirs. In: Telleria J., Tibayrenc M., editors. American Trypanosomiasis Chagas Disease: One Hundred Years of Research. 1 ed. Elsevier; London: 2010. pp. 249–276. [Google Scholar]
- Mesa-Arciniegas P., Parra-Henao G., Carrión-Bonifacio Á., Casas-Cruz A., Patiño-Cuellar A., Díaz-Rodríguez K. Trypanosoma cruzi infection in naturally infected dogs from an endemic region of Cundinamarca, Colombia. Vet. Parasitol. Reg. Stud. Rep. 2020;14:212–216. doi: 10.1016/j.vprsr.2018.11.006. [DOI] [PubMed] [Google Scholar]
- Miles M.A., Feliciangeli D., Rojas de Arias A. American trypanosomiasis (Chagas' disease) and the role of molecular epidemiology in guiding control strategies. BMJ. 2003;326:1444–1448. doi: 10.1136/bmj.326.7404.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser D.R., Kirchhoff L.V., Donelson J.E. Detection of Trypanosoma cruzi by DNA amplification using the polymerase chain reaction. J. Clin. Microbiol. 1989;27:1477–1482. doi: 10.1128/jcm.27.7.1477-1482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocana-Mayorga S., Llewellyn M.S., Costales J.A., Miles M.A., Grijalva M.J. Sex, subdivision, and domestic dispersal of Trypanosoma cruzi lineage I in southern Ecuador. PLoS Negl. Trop. Dis. 2010;4(12) doi: 10.1371/journal.pntd.0000915. e915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera M.J., Fory J.A., Porras J.F., Buitrago G. Prevalence of Chagas disease in Colombia: A systematic review and meta-analysis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0210156. e0210156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco M.M., Piccinali R.V., Mora M.S., Enriquez G.F., Cardinal M.V., Gurtler R.E. The role of sigmodontine rodents as sylvatic hosts of Trypanosoma cruzi in the Argentinean Chaco. Infect. Genet. Evol. 2014;22:12–22. doi: 10.1016/j.meegid.2013.12.020. [DOI] [PubMed] [Google Scholar]
- Pineda V., Saldaña A., Monfante I., Santamaría A., Gottdenker N.L., Yabsley M.J. Prevalence of trypanosome infections in dogs from Chagas disease endemic regions in Panama, Central America. Vet. Parasitol. 2011;178(3–4):360–363. doi: 10.1016/j.vetpar.2010.12.043. [DOI] [PubMed] [Google Scholar]
- Ramirez J.D., Guhl F., Messenger L.A., Lewis M.D., Montilla M., Cucunuba Z. Contemporary cryptic sexuality in Trypanosoma cruzi. Mol. Ecol. 2012;21:4216–4226. doi: 10.1111/j.1365-294X.2012.05699.x. [DOI] [PubMed] [Google Scholar]
- Ramirez J.D., Montilla M., Cucunuba Z.M., Florez A.C., Zambrano P., Guhl F. Molecular epidemiology of human oral Chagas disease outbreaks in Colombia. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002041. (e2041. 10.1371.https://doi.org//journal.pntd.0002041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J.D., Turriago B., Tapia-Calle G., Guhl F. Understanding the role of dogs (Canis lupus familiaris) in the transmission dynamics of Trypanosoma cruzi genotypes in Colombia. Vet. Parasitol. 2013;196:216–219. doi: 10.1016/j.vetpar.2012.12.054. [DOI] [PubMed] [Google Scholar]
- Rendon L.M., Guhl F., Cordovez J.M., Erazo D. New scenarios of Trypanosoma cruzi transmission in the Orinoco region of Colombia. Mem. Inst. Oswaldo Cruz. 2015;110:283–288. doi: 10.1590/0074-02760140403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha F.L., Roque A.L., Arrais R.C., Santos J.P., Lima Vdos S., Xavier S.C. Trypanosoma cruzi TcI and TcII transmission among wild carnivores, small mammals and dogs in a conservation unit and surrounding areas, Brazil. Parasitology. 2013;140:160–170. doi: 10.1017/S0031182012001539. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Monguí E., Cantillo-Barraza O., Prieto-Alvarado F.E., Cucunubá Z.M. Heterogeneity of Trypanosoma cruzi infection rates in vectors and animal reservoirs in Colombia: a systematic review and meta-analysis. Parasit. Vectors. 2019;12:308. doi: 10.1186/s13071-019-3541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque A.L., Xavier S.C., Gerhardt M., Silva M.F., Lima V.S., D’Andrea P.S. Trypanosoma cruzi among wild and domestic mammals in different areas of the Abaetetuba municipality (Para state, Brazil), an endemic Chagas disease transmission area. Vet. Parasitol. 2013;193:71–77. doi: 10.1016/j.vetpar.2012.11.028. [DOI] [PubMed] [Google Scholar]
- Rueda K., Trujillo J.E., Carranza J.C., Vallejo G.A. Transmisión oral de Trypanosoma cruzi: una nueva situación epidemiológica de la enfermedad de Chagas en Colombia y otros países suramericanos. Biomédica. 2014;34:631–641. doi: 10.7705/biomedica.v34i4.2204. [DOI] [PubMed] [Google Scholar]
- Tomasini N., Lauthier J.J., Monje M.M., Ragone P.G., D’Amato A.A., Pérez C. Interest and limitations of spliced leader Intergenic region sequences for analyzing Trypanosoma cruzi I phylogenetic diversity in the Argentinean Chaco. Infect. Genet. Evol. 2011;11:300–307. doi: 10.1016/j.meegid.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Tovar Acero C., Negrete Penata J., Gonzalez C., Leon C., Ortiz M., Chacon Pacheco J. New scenarios of Chagas disease transmission in northern Colombia. J. Parasitol. Res. 2017;3943215 doi: 10.1155/2017/3943215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turriago B.C., Vallejo G.A., Guhl F. Seroprevalencia de Trypanosoma cruzi en perros de dos áreas endémicas de Colombia. Rev. Med. 2008:11–18. [Google Scholar]
- Urdaneta-Morales S., Nironi I. Trypanosoma cruzi in the anal glands of urban opossums. I—Isolation and experimental infections. Mem. Inst. Oswaldo Cruz. 1996;91:399–403. doi: 10.1590/S0074-02761996000400002. [DOI] [PubMed] [Google Scholar]
- WHO Chagas disease (American trypanosomiasis) Epidemiology. 2019 https://www.who.int/chagas/en/ 24 May 2019. (accessed 16 March 2020) [Google Scholar]
- Xavier S.C., Roque A.L., Lima V.S., Monteiro K.J., Otaviano J.C., Ferreira da Silva L.F. Lower richness of small wild mammal species and chagas disease risk. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingales B., Andrade S.G., Briones M.R., Campbell D.A., Chiari E., Fernandes O. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem. Inst. Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/S0074-02762009000700021. [DOI] [PubMed] [Google Scholar]