Abstract
Cationic liposomes (CLs) have been regarded as the most promising gene delivery vectors for decades with the advantages of excellent biodegradability, biocompatibility, and high nucleic acid encapsulation efficiency. However, the clinical use of CLs in cancer gene therapy is limited because of many uncertain factors in vivo. Extracellular barriers such as opsonization, rapid clearance by the reticuloendothelial system and poor tumor penetration, and intracellular barriers, including endosomal/lysosomal entrapped network and restricted diffusion to the nucleus, make CLs not the ideal vector for transferring extrinsic genes in the body. However, the obstacles in achieving productive therapeutic effects of nucleic acids can be addressed by tailoring the properties of CLs, which are influenced by lipid compositions and surface modification. This review focuses on the physiological barriers of CLs against cancer gene therapy and the effects of lipid compositions on governing transfection efficiency, and it briefly discusses the impacts of particle size, membrane charge density, and surface modification on the fate of CLs in vivo, which may provide guidance for their preclinical studies.
Keywords: cationic liposomes, lipoplexes, gene transfer, extracellular barriers, intracellular barriers, lipid compositions, cancer gene therapy
Graphical Abstract
The concept of lipoplexes applied to cancer gene therapy has been popular for decades; however, no such medicine has been marketed. Deng and colleagues highlight the physiological barriers against the clinical success of the cationic liposomal gene delivery system in cancer therapy. They suggest that the rational design of clinically applicable lipoplexes requires designers to pay close attention to the barriers and the impact of physicochemical properties of cationic liposomes on the therapeutic outcome.
Main Text
Cationic liposomes (CLs) are near-spherical vesicles with a positive charge and are dominantly composed of cationic lipids that effectively condense nucleic acids1, 2, 3, 4 such as plasmid DNA (pDNA), messenger RNA (mRNA), or small interfering RNA (siRNA) via electrostatic interactions5 to form complexes called “lipoplexes.” Based on the fact that genes have been revealed to be involved in the development of cancer,6,7 CLs have been extensively studied in cancer gene therapy and have shown potential clinical success in different tumors (Table 1). In general, the selection of CLs as gene carriers is attributed to their permanent positive charge, which promotes their stronger interaction with negatively charged substances such as nucleic acid, cell membrane, and endosomal membrane. In addition, CLs can be adjusted to obtain appropriate physical and chemical properties, such as size and tumor targeting, so as to obtain good pharmacokinetic properties in vivo. However, despite the good performance showed in in vitro studies, cationic lipid-based nucleic acid therapies in clinical trials are less than satisfactory because of the complicated biological environment.8 Most clinical trials of cationic liposomal delivery systems for cancer gene therapy have been approved (Table 2), but most are still in phase I or even discontinued.
Table 1.
The Preclinical Studies of Cancer Gene Therapy Involving Lipoplexes
| Name | Drug | Carrier Components | Diameter (nm) | Disease | References |
|---|---|---|---|---|---|
| T7-LPC | EGFR siRNA | DOTAP/DOPE/Chol, 1:1:1 n/n, 5% T7-PEG-DSPE | 83 | glioma | 185 |
| PEGylated DC-Chol/DOPE cationic liposomes | KSP siRNA | DC-Chol/DOPE/mPEG-2000-DSPE, 195:195:1 n/n | 102 | ovarian cancer | 47 |
| DLPP | IL-22BP mRNA | DOTAP/Chol, 1:1 n/n | 157 | colon cancer | 186 |
| pegSA lipoplexes | BMP-9 pDNA | fSA/HSPC/DOPE/DSPE-mPEG-2000/Chol | <100 | osteoporosis | 187 |
| FRα-targeted liposomes | matrix protein pDNA | DOTAP/Chol/mPEG-suc-Chol/F-PEG-suc-Chol, 50:45:4.75:0.25, n/n | 200 | ovarian cancer | 188 |
| HA lipoplexes | CD44 siRNA | HA-DOPE/DE, 1:1 w/w | 230 | lung cancer | 189 |
| 2X3-DOPE/FC liposomes | MDR1 siRNA | 2X3/DOPE, 1:2 n/n | 60 | squamous carcinoma | 171 |
| HA-P-LP | shRNA mRIP3-pDNA | DOTAP/DOPE/PEG-DSPE/Chol | 290 | colon cancer | 158 |
| CL-siSOX2 | SOX2 siRNA | DOTAP/DPPC/DSPE-mPEG-2000, 2:3:2 n/n | 93 | lung cancer | 190 |
| DACC | CD31 siRNA | AtuFECT01/Chol/mPEG-2000-DSPE, 70:29:1 n/n | ~70 | lung cancer | 191 |
| Liposome-siRNA nanocomplex | STAT3 siRNA, curcumin | DOTAP/DOPE/sodium cholate/C6 ceramide, 50:30:10:10 w/w | 157 | skin cancer | 192 |
EGFR, epidermal growth factor receptor; Chol, cholesterol; DSPE, 1,2-distearoyl-sn-glycero-3-phosphorylethanolamine; KSP, kinesin spindle protein; IL-22BP, interleukin-22 binding protein; pegSA, PEGylated stearyl amine; BMP-9, bone morphogenetic protein-9; FRα, folate receptor α; suc, succinyl; HA, hyaluronic acid; DE, [2-(2-3-didodecyloxypropyl)hydroxyethyl]ammonium bromide; 2X3, 1,26-bis(cholest-5-en-3β-yloxycarbonylamino)-7,11,16,20-tetraazahexacosan tetrahydrochloride; FC liposomes, folate-containing lipoconjugate liposomes; SOX2, SRY HMG-box 2; AtuFECT01, β-l-arginyl-2,3-l-diaminopropionic acid-N-palmityl-N-oleyl-amide trihydrochloride; DLPP, DOTAP liposome-protamine complex; CD31, platelet endothelial cell adhesion molecule-1; STAT3, signal transducer and activator of transcription 3; n/n, molar ratio; w/w, weight ratio.
Table 2.
Cationic Liposomal Delivery System for Cancer Gene Therapy in Clinical Trials
| Name | Drug | Carrier Components | Administration Route | Condition | ClinicalTrials.gov Identifier (Phase) | Sponsor and/or Affiliations | First Posted |
|---|---|---|---|---|---|---|---|
| Atu027 | PKN3 siRNA | AtuFECT01-DPhyPE/DSPE-PEG-2000 | i.v. | pancreatic cancer | NCT01808638 (I/II) | Silence Therapeutics | 2013 |
| SGT-94 | RB94 pDNA | DOTAP/DOPE | i.v. | solid tumors | NCT01517464 (I) | Synergene Therapeutics | 2012 |
| SGT-53 | HWTp53 pDNA, PD1 antibody | DOTAP/DOPE | i.v. | glioblastoma, solid tumors, pancreatic cancer | NCT02340156 (II) | Synergene Therapeutics | 2015 |
| NCT02340117 (II) | |||||||
| Lipo-MERIT | (NY-ESO-1, MAGE-A3, tyrosinase and TPTE) RNAs | DOTMA/DOPE | i.v. | stage IIIB–IV melanoma | NCT02410733 (I) | Biopharmaceutical New Technologies | 2015 |
| Tusc2-nanoparticles | Tusc2 pDNA | DOTAP/Chol | i.v. | lung cancer | NCT01455389 (I/II) | MD Anderson Cancer Center | 2011 |
| Liposomal-DNA complexes | interleukin-2 pDNA | DOTMA/Chol | i.t. | head and neck cancer | NCT00006033 (II) | H. Lee Moffitt Cancer Center and Research Institute | 2004 |
| DC-Chol liposomes | EGFR antisense DNA | DC-Chol | i.t. | head and neck cancer | NCT00009841 (I) | University of Pittsburgh | 2004 |
| pbi-shRNA STMN1 lipoplexes | bi-shRNA-stathmin 1 pDNA | DOTAP/Chol | i.t. | solid tumors | NCT01505153 (I) | Gradalis | 2012 |
| IGTM-101 | (HSTK, cIFNβ, hIL-2, hGM-CSF) pDNA | DMRIE/DOPE | intratumoral/peritumoral injection | melanoma | NCT03338777 (I) terminateda | Hospital Italiano de Buenos Aires | 2017 |
Source: https://clinicaltrials.gov. PKN3, protein kinase N3; DPhyPE, 1,2-diphytanoyl-sn-glycero-3-phosphoethanolamine; RB94, an N-terminal truncated retinoblastoma protein; HWTp53, human wild-type tumor protein 53; PD1, programmed cell death-1; NY-ESO-1, New York esophageal squamous cell carcinoma 1; MAGE-A3 melanoma antigen family A3; TPTE, transmembrane phosphatase with tensin homology; Tusc2, tumor suppressor candidate 2; STMN1, stathmin 1; DOTMA, N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride; HSTK, herpes simplex thymidine kinase; cIFNβ, canine interferon β; hIL-2, human interleukin-2; hGM-CSF, human granulocyte-macrophage colony-stimulating factor; DMRIE, 1,2-dimyristyl oxypropyl-3-dimethyl-hydroxyethylammonium bromide; i.v., intravenous; i.t., intratumoral.
Failure to achieve primary objective, terminated at 2020.
The efficiency of CLs in gene delivery is affected by many factors in vivo and may not closely correspond to the studies in vitro.9,10 First, CLs can elicit opsonization after intravenous administration.11 CLs pick up plasma protein such as serum albumin, complements, immunoglobulins (Igs), and apolipoproteins to form a corona layer on their surface, providing them with a totally new biological identity that will dramatically alter their fates in vivo.12,13 Meanwhile, the reticuloendothelial system (RES) will first play the role of eliminating aliens to remove CLs from the blood circulation, and the inappropriate size and excessive positive charge of CLs accelerate this process.10,14,15 In addition, even if they manage to escape the RES trap and extravasate from vasculatures to the tumor tissues, particle characteristics determine whether they can successfully penetrate from the complex tumor environment into tumor cells, followed by effective ingestion via efficient internalization. Besides, endosomal/lysosomal entrapment is another obstacle for CLs to deliver genes to cytoplasm,16, 17, 18 which is greatly affected by the fusogenic capability of CLs.19 Furthermore, the reticular cytoskeletal network and the macromolecular crowding in the cytoplasm are not conducive to the transport of lipoplexes or DNA to the nucleus.20,21 Therefore, even though CLs have been considered to be the most promising nanocarriers for cancer gene therapy, gene delivery to target sites is bumpy, because CLs, as invaders, face a series of events such as opsonization, rapid clearance by the RES, poor tumor penetration, cellular uptake, and lysosomal degradation, resulting in therapeutic failure in the body.
The intracorporal fates of CLs are not only affected by the physiological environment but also by the physiochemical properties of CLs, while the latter are primarily determined by lipid compositions.8,22, 23, 24 CLs are usually composed of the cationic lipids and neutral helper lipids such as cholesterol, dioleoyl phosphatidylethanolamine (DOPE), and polyethylene glycol (PEG)-lipid, among others. Notably, the molecular structure of cationic lipid and the ability of neutral helper lipid to trigger fusion are of great importance to govern transfection efficiency (TE).9,25, 26, 27 It has been reported that TE is highly correlated with nanoparticle size and surface charge, both of which are affected by lipid composition.28,29 Additionally, the active targeting and appropriate characteristics of CLs can also be obtained by simply tailoring the lipid compositions, which are crucial to the outperformance of CLs in the body. Therefore, the rational design of CLs requires a full understanding of the relationship between lipid composition and therapeutic outcome.
The clinical research of lipoplex-based therapeutic agents in cancer treatment has made slow progress in the last two decades due to the complicated environment and poor therapeutic outcome in the human body. Therefore, understanding the factors affecting CL-based gene therapy is crucial for the design of efficient and clinically applicable gene delivery vectors.
The Extracellular Barriers
The Protein Corona Alters the Fate of CLs In Vivo
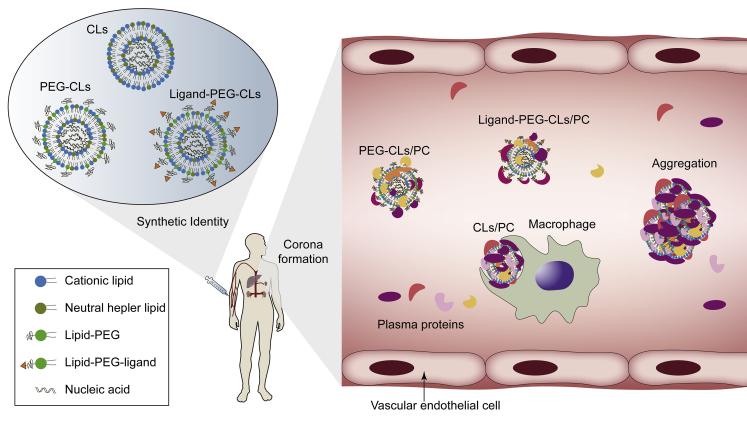
Once administered intravenously, the plasma proteins will adsorb to CLs or lipoplexes and eventually form a so called “protein corona” (PC) at their surface that can specifically bind to phagocytes (Figure 1).30,31 The absorption of complement protein activates the complement system,32 while Ig promotes the opsonization of CLs,33 with both resulting in rapid clearance of CLs. However, based on the immunogenicity of CLs, some researchers have exploited this property of liposomes to target immune cells such as tumor-associated macrophages and dendritic cells.34,35 In antitumor immunotherapy or vaccination, CLs can also serve as an attractive adjuvant36 in addition to its carrier function, because CLs themselves can elicit the release of cytokine and the activation of natural killer (NK) cells, resulting in a greatly enhanced pro-inflammatory response around the tumor tissue.37,38
Figure 1.
Schematic Representation of the Effects of PC on CLs
The decoration of PC obscures the surface charge and targeting ligands of CLs, increases their size, and leads to aggregation and off-target. Most of them are quickly cleared by macrophages. PEG-CLs reduce the adsorption of proteins to some extent.
Despite this, the impacts of PC on CLs are sometimes fatal, such as aggregation, charge neutrality, and size enlargement, leading to their instability and drug release before cell uptake.39,40 Meanwhile, the active moieties of ligand-modified CLs may also be shielded after opsonization.41 Several recent findings have confirmed that the decoration of PC on nanocarriers could alter their internalization mechanism and hence intracellular trafficking fate.12,39,42 All of these changes will produce different clinical effects than expected, which is one of the reasons for the little clinical progress of nanocarriers in recent decades. Importantly, however, note that the isolated serum or plasma is not completely consistent with the actual physiological environment, which means that in vitro experiments on liposomes cannot be fully used to predict their behavioral changes in vivo.43
Many efforts have been made to avoid the negative effects of corona, among which PEGylation44,45 is more effective and commonly used. Attaching PEG chains creates a shielding layer at the surface of CLs and hence the formation of steric barriers and the reduction of exposed charges,46 resulting in less formation of PC and extension of circulation time in the body.47 However, the stealth effect of PEGylated CLs is not conducive to cellular uptake and endosomal escape.48,49 By adjusting the PEG chain length and PEG density, the effects of anti-opsonization and inhibition of lipid-plasma membrane interactions caused by “PEG dilemma” can be balanced.49,50 Alternatively, the addition of a targeting ligand and a pH-sensitive fusogenic peptide,51 as well as introducing cleavable PEG-lipids to the liposomal formulation, not only helps PEGylated CLs52,53 to maintain their stealth role in blood circulation, but promotes penetration into target cells and subsequent endosomal escape. However, similar immune response problems can occur with repeated intravenous administration of PEGylated liposomes. A brief report by McSweeney et al.54 disclosed that PEG-modified therapeutics can induce specific PEG-binding antibodies, leading to rapid elimination of drugs and a significantly increased risk of serious adverse events.
The protein abundance of corona is affected by particle size and charge, which vary with lipid compositions.41,55,56 Ren et al.57 investigated whether liposomes with a slight negative charge might enhance their blood circulation time due to reduced combinations with plasma proteins and blood cells compared to CLs. However, after extensive experiments on the effects of lipid compositions on corona protein components, it has been discovered that not all of the formation of PC has an adverse effect on CLs. The adsorbed proteins can be functionally divided into opsonins and dysopsonins, while the later may give CLs a desirable biological identity that prolongs their blood circulation time or enhances accumulation in tumors.58 Caracciolo et al.59 found that 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP)-rich liposomes preferred vitronectin, whose receptor is highly expressed in tumor cells, while dimethylaminoethane-carbamoyl (DC)-cholesterol promoted the binding of Ig and complement proteins. Nevertheless, substitution of the cationic lipid DOTAP with neutral lipids such as DOPE decreases the net charge of the lipid vesicle, making it less attractive for vitronectin, fibrinogen, and other negatively charged proteins,60 but more attractive for dysopsonins such as apolipoproteins and serum albumin, which preferentially adhere to the DOPE molecules.61,62 In recent years, protein adsorption has been used to induce the formation of functional PCs to target tumor cells.11,56,63,64 These important findings suggest that researchers should take PC into account and design rationally “targeted” CLs (simply via optimizing the lipid compositions) so as to take advantage of the physiological environment as much as possible.11,65
The Insufficient EPR Effect and Poor Tumor Penetration
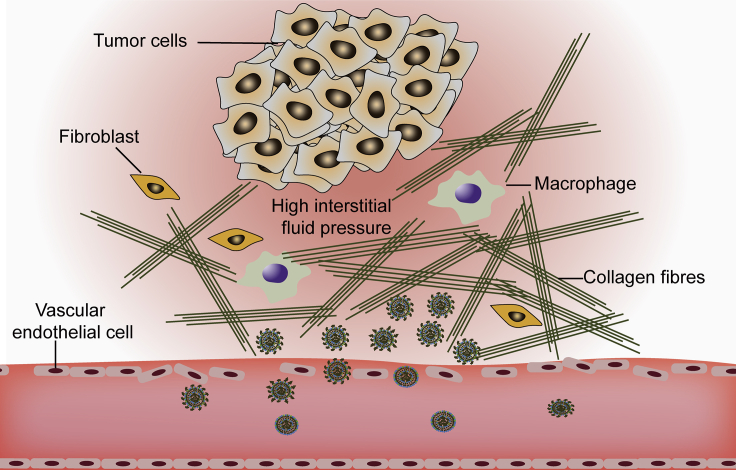
The advantage of CLs in the treatment of cancer, as with other nanomedicine, is that they can passively target tumor tissues because of the enhanced permeability and retention (EPR) effect, which is caused by leakiness of the neovasculature and inefficient lymphatic drainage in tumors.66,67 Rapid tumor growth leads to angiogenesis with pore diameter ranging from 100 to 800 nm,68 which allows molecules or particles larger than 40 kDa to extravasate from blood vessels69 and accumulate in tumor tissues, providing a huge opportunity for preferential tumor accumulation of nanomedicines. Therefore, there is an understanding that by simply optimizing the particle size (ranging from 100 to 200 nm, with 100 nm being commonly used70), effective passive tumor targeting can be designed based on the EPR effect. The EPR effect is not limited to tumors, as Ren et al.57 discovered that the optimal particle size for liposomes in rheumatoid arthritis targeting therapy was 100 nm, while larger (200 and 350 nm) and smaller (70 nm) liposomes had shorter circulation times due to their easier recognition by the RES and filtration by kidney, respectively. However, the cutoff sizes of capillary pores or optimal sizes for EPR-based drug delivery system are in conflict in several articles,68,71, 72, 73, 74, 75, 76 and the invalid delivery of nanocarriers into tumors exhibits an insufficient EPR effect due to the heterogeneity of tumor.67,77 In fact, the EPR effect is a phenomenon that is unique to cancer patients and relies on blood vessels with defective architecture.78 More specifically, the gaps between endothelial cells in tumor blood vessels and the vascular density as well as the blood supply are key determinants for EPR-mediated tumor targeting.79 The convection of lipoplexes is blocked by the increased interstitial fluid pressure (IFP), which is induced by abnormal vasculature, ineffective lymphatic drainage, interstitial fibrosis, and stromal matrix contraction (Figure 2).80, 81, 82 Differences in tumor size and vascular density of different stages, accompanied by a special tumor microenvironment, such as an abnormal tumor vascular system, dense extracellular matrix (ECM), and high IFP, make the EPR effect alone unsatisfactory for all solid tumors.69,83,84 Furthermore, abnormal ECM, together with the increased IFP, makes lipoplexes almost motionless in the periphery of the tumor.85
Figure 2.
Illustration of Tumor Microenvironment
Vascular abnormalities and lack of lymphatic drainage allow CLs to accumulate in tumor, but dense extracellular matrix and increased interstitial fluid pressure prevent the particles from penetrating deeper into the tumor.
Particle size is the most important factor that influences the elimination, tumor penetration, and retention of nanomedicine due to the inconsistent cutoff sizes in different sites, including kidney (<10–15 nm),86 liver (50–180 nm),8 leaky vasculature (>200 nm), and ECM (<20 nm),87 among others. Particles that are too small (<10 nm) or slightly larger (>50 nm) are not suitable for the retention and penetration in tumors;88,89 therefore, designing nanocarriers with intelligent tunable sizes is the most controllable way to balance the size-related accumulation and penetration abilities.90 For example, near-infrared light triggered size-tunable liposomes not only extended the blood circulation time, but also promoted their penetration into deep tumor after the size decreased from large (~162 nm) to small (~8.6 nm).91 Importantly, note that the actual size of effective penetration in a tumor varies with different delivery systems, tumor types, and individuals.84 More uplifting news is that transcytosis of nanomedicine with a positive charge and certain ligand modification has been shown to mediate the active intra-tumor penetration without the need to overcome the hindrance of ECM.92 Transcellular transport occurs in any nanoparticles modified with peptides containing C-terminal arginine that bind to neuropilin-1 or neuropilin-2 on the tumor plasma membrane.84 This is very exciting news for scientists to design a highly effective tumor-permeable gene delivery system, because it allows nanomedicines to target the vascular endothelial cells and trigger adsorption or receptor-mediated endocytosis for exocytosis to the adjacent cell on the other side without size limitation.85
Another promising but more traditional strategy for enhancing tumor penetration is a prior administration of liposomes encapsulating chemotherapy drugs to deplete the dense ECM, and then followed by liposomes loaded with genetic drugs.93 For example, Chen et al.94 successfully delivered drug-loaded liposomes into the stromal abundant pancreatic ductal adenocarcinoma due to the stromal depletion that was achieved by pretreatment with nitric oxide donor S-nitroso-N-acetylpenicillamine-loaded liposomes.
Intracellular Barriers
Cellular Entry
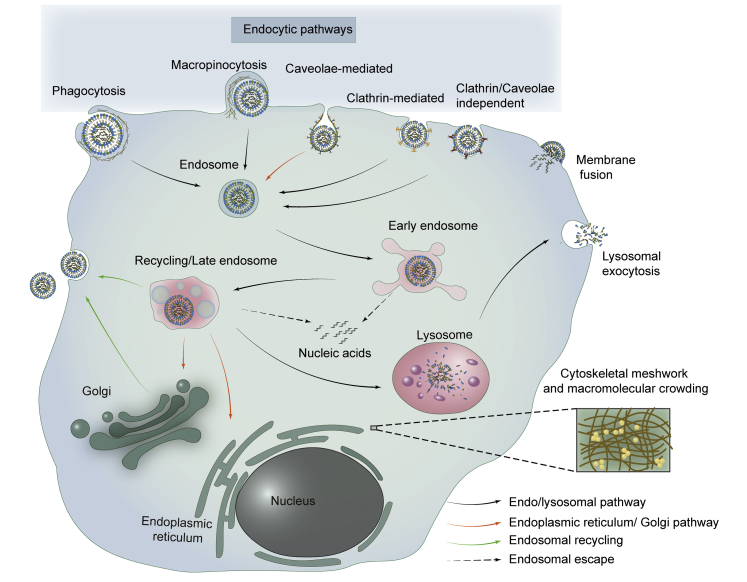
When CLs surf onto the surface of the cell membrane, cellular uptake may occur through endocytosis or membrane fusion (Figure 3). In contrast, membrane fusion occurs almost exclusively in fusogenic CLs,95,96 which directly translocate their cargo into the cytoplasm,97,98 resulting in efficient drug delivery to the cytoplasm without endosomal entrapment and metabolic degradation.99
Figure 3.
Schematic Diagram of Lipoplexes Entering and Trafficking Within Cells: Entry Pathways and Intracellular Barriers
Lipoplexes enter into cells via endocytosis or membrane fusion. Endocytic pathways include clathrin-mediated endocytosis, caveolae-mediated endocytosis, phagocytosis, micropinocytosis, and clathrin/caveolae-independent endocytosis. CLs must escape the endosomal entrapment before being transported to the lysosome except for membrane fusion and caveolae-mediated endocytosis. Furthermore, the mesh-like cytoskeletal network and macromolecular crowding in cytoplasm impede the diffusion of lipoplexes to the nucleus.
CLs that exist in a lamellar phase are known to be internalized by cells mainly through endocytosis,100 which can be divided into clathrin-mediated endocytosis (CME), caveolae-mediated endocytosis (CavME), phagocytosis, macropinocytosis, and clathrin/caveolae-independent endocytosis.101, 102, 103 Among the several endocytic pathways, CME, CavME, and macropinocytosis are more common in the cellular uptake of lipoplexes in ordinary cells (normal or cancerous cells of various tissues other than phagocytes). In particular, phagocytosis, which professional phagocytic cells use to internalize micro-size particles (such as dead cells or pathogens), is closely related to the intracorporal fate of CLs after opsonization in blood, as mentioned previously. Phagocytic cells, such as macrophages, neutrophils, and dendritic cells, recognize CLs by binding the specific composition of PC and, once ingested, the phagocytes digest them completely through lysosomal degradation.32 For instance, Erni et al.104 tried to deliver pDNA to phagocytic professional antigen-presenting cells by cationic solid lipid microparticles (cSLMs) in vitro. However, despite the efficient phagocytosis of cSLMs, TE was not detected in macrophage cell lines but in non-phagocytic cells.
There is a growing awareness that endocytic pathways are closely related to the intracellular fate of cargo and the ease of drug release,101,105 which involve lysosomal degradation. Lysosomes are present in all mammalian cells except mature erythrocytes, but they are most abundant in phagocytes (macrophages and neutrophils)106 compared with other cell types. Significantly, it has been reported that tumor cells have an increased content of lysosomes due to their rapid proliferation and metabolization,107 which generally leads to drug resistance or inefficacy, especially for weak-base chemotherapeutic agents.108 Lysosomal activity, a wide range of biological process involved in digestive function and host defense, relates to the degradation of substances from endocytosis or autophagy.109 Cell type110,111 and physicochemical properties of CLs, including size, charge, lipid composition, surface modification, are important factors in endocytosis.56,101 When considering the effect of particle characteristics on endocytosis, the most striking factor is usually the particle size, as clathrin-coated pit and caveolae are size-limited invaginations.90,101 However, the exact size of different endocytic pathways needed remains to be determined. For example, Inoh et al.112 investigated the effects of lipoplex sizes on the endocytosis pathway in bone marrow-derived dendritic cells, and they surprisingly found that CavME preferred to internalize smaller-sized lipoplexes (around 270 nm), while CME and macropinocytosis preferred to take up the larger ones (around 500 nm). However, the results of Bae et al.113 showed that cholesterol ester liposomes and DOTAP liposomes of different sizes were all internalized in COS-7 cells mainly via the CME pathway. Accordingly, the selection of liposome size for endocytosis does not seem obvious. As research continues, size-dependent internalization is not widely accepted, because even monodispersed liposomes can be internalized simultaneously by the same cell in multiple endocytic pathways.114 Kim et al.115 observed that particle size and charge had little effect on TE throughout all used cell lines, and it was most dependent on cell lines, and then the lipid compositions. In other words, it is apt to be cell line-dependent rather than the particle size or charge that influences the cellular endocytosis.
Endosomal Escape and Drug Release
Regardless of the route of endocytosis, CLs or lipoplexes are enveloped by endosomes and undergo maturation with the gradual acidification of the lumen, i.e., from early endosomes to recycling/multivesicular late endosomes, during which the wrapped CLs are sorted and directed to their destination (i.e., the plasma membrane, endoplasmic reticulum, trans-Golgi network, or lysosomes).102 However, not every endocytic pathway leads to a productive release of cargo, and the key to their effectiveness of the cytosolic delivery is to avoid the endosomal/lysosomal pathway.116,117 Unfortunately, the late endosomes prefer to transport their preys to lysosomes for further degradation, except CavME, which has been shown to bypass trafficking into lysosomes, resulting in higher TE (Figure 3).118,119 Although endosomal vesicles formed by macropinocytosis are inherently leaky, leading to only a small portion of drug leakage into cytosol without destabilizing the endosomal membrane, they are doomed to destined to the lysosomes by the microtubule network.111,120 Thus, if endocytosing is not via CavME, it is necessary for CLs to disrupt the endosomal membrane quickly so as to release their genetic cargos or incomplete lipoplexes to the cytoplasm before degradation by hydrolases in lysosomes.121,122
Endosomal escape, which relies on membrane fusion, is an indispensable step for lipoplexes trapped in endosomes to acquire satisfactory nucleic acid therapeutic outcome, as it is generally accompanied by drug release.123, 124, 125 The positive charge of the lipoplex attracts the anionic lipids of the endosomal membrane to flip-flop from the outer face to the inner face and diffuse into the lipoplex, forming a neutral ion pair with cationic lipids and resulting in displacement of DNA from the lipoplex as well as endosomal disruption.124 During the same time, the nucleic acid can be released into the cytoplasm when the total cationic charge of the lipoplex is completely neutralized by the endosomal membrane.126 However, effective DNA dissociation from the carrier is not easy: the thermodynamic stability and membrane charge density (σM, average charge/unit area of membrane) of lipoplexes strongly affect their cargo release.127,128 The thermodynamic stability of lipoplexes is greatly influenced by their phase structure; that is, the lamellar phase is considered to be more thermodynamically stable than the hexagonal phase, which requires lower energy to trigger membrane fusion.125 In addition, the lamellar complex needs complete fragmentation to release DNA, while the hexagonal complex can release DNA even if it is not completely disintegrated.127 Therefore, an excellent gene delivery vector should be capable of phase transition to meet the requirements of effective drug delivery and drug dissociation. Besides the capability of phase transition, the σM of lipoplexes is also critical to the endosomal escape and DNA dissociation. Although initial conditions for the escape of lipoplexes from endosome require liposomes with high σM, an excessive positive charge may lead to incomplete dissociation of complexes,128 because of the incomplete fusion that occurs only in the external membrane of the lipoplex. Therefore, the positive charge of lipoplexes requires a compromise between endosomal escape and complex dissociation.
Migration from Cytoplasm to Nucleus
Endosomal escape and DNA dissociation from lipoplexes are not necessarily a guarantee of successful transfection except for RNA-based cargo; the final obstacle is entry into the nucleus from cytoplasm. The cytoplasm is a mesh-like cytoskeletal network and macromolecular crowding in which only particles with a diameter less than 50 nm can diffuse freely, which inevitably increase the steric hindrance and random collision for the diffusion of lipoplexes or dissociative DNA (Figure 3).20,21,129 An earlier study by Lukacs et al.130 found that only small DNA fragments (<250 bp) diffused rapidly to the nucleus by Brownian motion after microinjection into the cytoplasm, and the movement of the larger counterpart (>2,000 bp) was almost retarded.129 Besides size-dependent geometrical constraints, non-specific interaction between nanocarriers and intracellular constituents, such as vesicles, organelles, and internal membranes, is another important factor affecting cytoplasmic diffusion.20
Except for cells that are undergoing mitosis or division where their nuclear envelopes are temporarily disassembled,131, 132, 133 nano-size lipoplexes do not appear to be able to freely diffuse into the peripheral nucleus through the cytoskeleton meshwork, let alone into the nucleus through the size-limited nuclear pore complexes.134,135 Apparently, it cannot be true since transfections do work, and there must be other means involving lipoplexes actively trafficking to the nucleus.132 Several studies have found that the microtubule network mediates the directed transport of lipoplexes in the cytoplasm to the nucleus, which contributes to the high TE.131,136, 137, 138 Conversely, Hasegawa et al.139 found that the microtubule network actively transported lipoplexes to the lysosomes rather than to the nucleus.140 However, Brownian diffusion of lipoplexes was still observed even when the microtubule pathway was disrupted, suggesting the existence of random Brownian motion of lipoplexes in cytosol.140,141 Cardarelli et al.142 demonstrated that, contrary to the DOTAP-based lipoplexes, Lipofectamine mostly adopted random Brownian diffusion to acquire optimal transfection. But now, there is a question of why Lipofectamine reagents are different from their counterparts (DOTAP-based lipoplexes) in the motion mode of cytoplasmic trafficking,142 since the diameters of particles of the former are relatively larger, typically more than 300 nm,143 whose movement should have been immensely restricted by the cytoskeletal network. The ability of lipoplexes to migrate within cells remains controversial and needs to be clarified. In any case, it is clear that exogenous DNA dissociated from a nanocarrier is unlikely to overcome the steric hindrance to get into the nucleus, because cells might not process the dissociative DNA in the same manner as lipoplexes.144 In conclusion, releasing DNA into the cytoplasm does not seem to make transfection successful, as transfection can be obtained only when cytoplasmic DNA enters into nucleus or dissociation occurs in the nucleus.145 Thus, the unpacking of the lipoplexes is critical for the release of the DNA therapeutic, and the location where it is released is also critical. It is best to dissociate in or around the nucleus. If the complex is disassembled far from the nucleus, it is difficult for DNA to migrate into the nucleus, and, alternatively, it may be engulfed by lysosomes again.
Factors Affecting the Therapeutic Efficacy
Lipid Composition
Cationic lipids are usually composed of a hydrophobic lipid anchor group, a linker arm, and a positively charged head group,9 forming the unique structure of a cationic lipid. The molecular structures of cationic lipids are crucial to cell uptake, endosomal escape, and cytotoxicity.18,146, 147, 148, 149 Kolašinac et al.95 reported that an inverted conical shape and an aromatic molecule in cationic lipid are indispensable to the fusion induction. Bruininks et al.16 elucidated the molecular effects of lipid tail saturation on lipid fusion and TE and suggested that cationic lipid tail saturation is a necessary condition for these processes. Additionally, the type of cationic lipid of lipoplexes greatly affects their biodistribution and therapeutic efficacy in the body after intravenous administration.16 Hattori et al.9 reported that the cationic lipids with a different amine head group, linker arm, and the length of alkyl chains strongly influenced siRNA biodistribution after injection of cationic lipoplexes.
Fusogenic lipid, DOPE, is a neutral helper lipid that is commonly added to cationic liposomal formulation for promoting membrane fusion-mediated endosomal escape.95,150, 151, 152, 153, 154 Meanwhile, as mentioned above, lipoplexes could also enter into the cells via membrane fusion-mediated internalization, which favors the phosphoethanolamine (PE) lipid-enriched lipoplexes.95 However, the amount of DOPE added to the liposome is controversial and most likely related to the structure and σM of the cationic lipids.2,115 For instance, one study showed that DOPE-dominated lipoplexes (e.g., DOTAP/DOPE molar ratios at 1:3) existed in a hexagonal phase completely that would destabilize lipoplexes and easily release their cargo before uptake,115 which was different from the transfection results shown in another study in which the optimum formulations for CLs containing lysine-derived cationic lipid preferred 80% of DOPE content.2
However, despite its excellent performance in in vitro studies, DOPE is unsuitable for improving gene delivery in vivo, whereas cholesterol-rich CLs work better than their DOPE counterpart to deliver genes in the body with prolonged blood circulation time.148 Similar to DOPE, cholesterol-rich lipoplexes could partially induce fusion-driven cellular uptake and facilitate endosomal destabilization.99 Cholesterol has a much smaller hydration repulsion layer than does 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), and thus the substitution of DOPC by cholesterol facilitates the fusion of CLs with the endosomal/lysosomal membrane, resulting in strong enhancement of TE.155
Experiments by Hattori et al.9 found that cationic lipids with longer alkyl chain length or added cholesterol to liposomal formulation could stabilize the structure of lipoplexes in blood circulation and hence enhance accumulation in the lungs. However, the addition of cholesterol did not always yield consistent results for TE in a variety of lipoplexes, as the work by Hattori et al.156 showed that the positive impact of cholesterol on TE only occurred in CLs containing cationic lipids with short and/or unsaturated alkyl chains. Another similar study by Abumanhal-Masarweh et al.157 investigated the effects of acyl chain length and saturation and the addition of cholesterol on cellular uptake in 4T1 cells. They found that liposomes with longer acyl chains and monounsaturated phospholipid performed much better than did shorter tails (i.e., 18:0 > 16:0 > 14:0) and saturated phospholipid (i.e., 18:1 > 18:0) on cellular uptake. Moreover, the effects of cholesterol on cellular uptake in 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC, 14:0, melting temperature [Tm] = 24°C) and hydrogenated soybean phosphatidylcholine (HSPC, 18:0, Tm = 52°C) were quite different. The addition of cholesterol transformed the lipid bilayer of DMPC from a liquid-disordered phase to a liquid-ordered phase that facilitated cellular endocytosis, while in HSPC liposomes, a solid-ordered phase and the most favorable phase for cellular uptake, transformed to a liquid-ordered phase, which weakened the cellular uptake. It is well known that cholesterol is added to make the membrane hardened and structurally stable; however, its impact on cellular uptake is lipid-dependent, and researchers should take the Tm of lipids into account.
Size and Surface Properties of CLs
Lipid composition and complexation with pDNA could influence the size of CLs and zeta potential and hence biodistribution and cellular entry.158, 159, 160 Particles smaller than 5 or 50 nm are easily excreted by urine or lodged in the liver, respectively.90 However, particles larger than 50 nm are difficult to penetrate deep into the tumor and thus reduce cellular uptake.90 Therefore, the size-turnable delivery system has a broad application prospect in cancer treatment. Although the size-dependent internalization mechanism prevails,70,90 the effect of particle size on the mechanism of cellular uptake has not been clearly elucidated, which varies with cell types and lipid compositions.115 Nevertheless, the correlation between size and TE is still evident.28 Muripiti et al.161 disclosed that TE varied with the change of charge ratios, and charge ratios at 4:1 obtained the highest TE due to their least size of ~200 nm and optimal zeta potential. Sakai-Kato et al.162 found that in a certain range (100–200 nm), the TE of lipoplexes was enhanced with the increase of particle size, which was due to the strong electrostatic interaction between CL and plasma membrane. Instead, however, Bruininks et al.16 found that fusion efficiency decreased as the size of lipoplexes increased because larger lipoplexes were more stable than their smaller counterparts during the process of endosomal membrane fusion.
Besides size, surface charge of the liposome can also affect its behavior in the body.163 Permanently charged cationic lipids are not conducive to good pharmacokinetics. Specifically, excessive positive charges are easily recognized by the immune system for accelerated removal, and aggregation occurs when electrostatic repulsion is too low, leading to accumulation in “first pass” organs.46 Unlike the uncertainty of the influence of particle size on TE, the impact of particle σM on TE is obvious. High σM of lipoplexes increases the structural stability of lipid/DNA complexes and the electrostatic interaction with biological membranes, but at the same time, their cytotoxicity and difficulty of intracellular complex dissociation also increase.128 The addition of PEG-lipid and neutral helper lipid into CLs alters their σM,164 resulting in a relative stable structure and PC reduction that extend blood circulation time of CLs,60,165 but poor adhesion to vessel walls and tumor penetration.166,167
Nowadays, it is a trend for ligand-receptor targeting in nanocarriers, because many specific receptors are more highly expressed in tumor than in normal tissues.70,168 Targeting ligands such as folate,169, 170, 171, 172 transferrin,173,174 and the cyclic Arg-Gly-Asp (cRGD) peptides175,176 are exploited to enhance tumor-targeted delivery, making it more effectively and rapidly internalized via receptor-mediated endocytosis of target cells. In recent years, dual-targeted nanomedicines are expected to overcome the uncontrollable and fluctuating targeting efficiency of single-ligand targeting, resulting in better cell selectivity and gene therapy.177,178 These surface modifications can work in different parts, such as plasma membrane,173,179 endosomal compartment,27,180 or even the nuclear envelope,181,182 making CLs versatile.
Conclusions
CL is a promising nanocarrier for gene delivery thanks to its large-scale preparation, biodegradability, and biocompatibility. The positive charge of CLs contributes to their interaction with cells and endosomal escape, but also inevitably increases the immunogenicity and toxicity of CLs in the body, leading to rapid clearance by the RES. In contrast, Onpattro (patisiran), the US Food and Drug Administration (FDA) first approved RNAi drug, used lipid nanoparticles as delivery vectors, suggesting that low zeta potential is a necessary condition for obtaining good pharmacokinetics.183 The successful clinical translation of Onpattro depends on its ionizable property (i.e., nearly neutral in blood but positively charged in endosomal compartment) and defined size (50 nm)184 by incorporating PEG-lipids, which indicates that the positive charge of CLs should be compromised between high TE and good pharmacokinetics. The relative traditional CLs, which have been favored by many researchers, are now greatly challenged. They should ensure high TE and at the same time obtain good pharmacokinetic properties to meet the clinical needs. Therefore, the clinical success of lipoplex-based cancer therapy requires designers to pay close attention to the barriers of lipoplexes in gene delivery and the impact of physicochemical properties of CLs on the therapeutic outcome.
Author Contributions
C.L. conducted the writing and literature review of the entire article; L.Z. and N.D. conducted the modification and proofreading of the entire article; and W.Z., X.C., R.G., and H.S. conducted the conception of the whole review and the final proofreading.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81972705).
References
- 1.Parashar D., Rajendran V., Shukla R., Sistla R. Lipid-based nanocarriers for delivery of small interfering RNA for therapeutic use. Eur. J. Pharm. Sci. 2020;142:105159–105173. doi: 10.1016/j.ejps.2019.105159. [DOI] [PubMed] [Google Scholar]
- 2.Martínez-Negro M., Sánchez-Arribas N., Guerrero-Martínez A., Moyá M.L., Tros de Ilarduya C., Mendicuti F., Aicart E., Junquera E. A non-viral plasmid DNA delivery system consisting on a lysine-derived cationic lipid mixed with a fusogenic lipid. Pharmaceutics. 2019;11:632–648. doi: 10.3390/pharmaceutics11120632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blakney A.K., McKay P.F., Yus B.I., Aldon Y., Shattock R.J. Inside out: optimization of lipid nanoparticle formulations for exterior complexation and in vivo delivery of saRNA. Gene Ther. 2019;26:363–372. doi: 10.1038/s41434-019-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Y.-G., Liu F.-L., Lu F.-H., Alam U., Tang Q., Huang J.-W., Lu Z.-L. [12]aneN3-based single aliphatic chain modified cationic lipids as gene delivery vectors. Tetrahedron. 2019;75:658–664. [Google Scholar]
- 5.Monpara J., Velga D., Verma T., Gupta S., Vavia P. Cationic cholesterol derivative efficiently delivers the genes: in silico and in vitro studies. Drug Deliv. Transl. Res. 2019;9:106–122. doi: 10.1007/s13346-018-0571-z. [DOI] [PubMed] [Google Scholar]
- 6.Wu B., Zhang L., Yu Y., Lu T., Zhang Y., Zhu W., Song Q., Lv C., Guo J., Tian Y., Deng N. miR-6086 inhibits ovarian cancer angiogenesis by downregulating the OC2/VEGFA/EGFL6 axis. Cell Death Dis. 2020;11:345. doi: 10.1038/s41419-020-2501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu T., Wu B., Yu Y., Zhu W., Zhang S., Zhang Y., Guo J., Deng N. Blockade of ONECUT2 expression in ovarian cancer inhibited tumor cell proliferation, migration, invasion and angiogenesis. Cancer Sci. 2018;109:2221–2234. doi: 10.1111/cas.13633. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Buck J., Grossen P., Cullis P.R., Huwyler J., Witzigmann D. Lipid-based DNA therapeutics: hallmarks of non-viral gene delivery. ACS Nano. 2019;13:3754–3782. doi: 10.1021/acsnano.8b07858. [DOI] [PubMed] [Google Scholar]
- 9.Hattori Y., Nakamura M., Takeuchi N., Tamaki K., Shimizu S., Yoshiike Y., Taguchi M., Ohno H., Ozaki K.I., Onishi H. Effect of cationic lipid in cationic liposomes on siRNA delivery into the lung by intravenous injection of cationic lipoplex. J. Drug Target. 2019;27:217–227. doi: 10.1080/1061186X.2018.1502775. [DOI] [PubMed] [Google Scholar]
- 10.Sercombe L., Veerati T., Moheimani F., Wu S.Y., Sood A.K., Hua S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015;6:286–299. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Digiacomo L., Pozzi D., Palchetti S., Zingoni A., Caracciolo G. Impact of the protein corona on nanomaterial immune response and targeting ability. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020;12:e1615. doi: 10.1002/wnan.1615. [DOI] [PubMed] [Google Scholar]
- 12.Francia V., Yang K., Deville S., Reker-Smit C., Nelissen I., Salvati A. Corona composition can affect the mechanisms cells use to internalize nanoparticles. ACS Nano. 2019;13:11107–11121. doi: 10.1021/acsnano.9b03824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Digiacomo L., Giulimondi F., Mahmoudi M., Caracciolo G. Effect of molecular crowding on the biological identity of liposomes: an overlooked factor at the bio-nano interface. Nanoscale Adv. 2019;1:2518–2522. doi: 10.1039/c9na00195f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vakili-Ghartavol R., Jaafari M.R., Nikpoor A.R., Rezayat S.M. Docetaxel delivery using folate-targeted liposomes: in vitro and in vivo studies. Nanomed. J. 2020;7:108–114. [Google Scholar]
- 15.Zahednezhad F., Saadat M., Valizadeh H., Zakeri-Milani P., Baradaran B. Liposome and immune system interplay: challenges and potentials. J. Control. Release. 2019;305:194–209. doi: 10.1016/j.jconrel.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 16.Bruininks B.M.H., Souza P.C.T., Ingolfsson H., Marrink S.-J.J. A molecular view on the escape of lipoplexed DNA from the endosome. eLife. 2020;9:52012–52028. doi: 10.7554/eLife.52012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bus T., Traeger A., Schubert U.S. The great escape: how cationic polyplexes overcome the endosomal barrier. J. Mater. Chem. B Mater. Biol. Med. 2018;6:6904–6918. doi: 10.1039/c8tb00967h. [DOI] [PubMed] [Google Scholar]
- 18.Wang B., Zhao R.M., Zhang J., Liu Y.H., Huang Z., Yu Q.Y., Yu X.Q. Rigid aromatic linking moiety in cationic lipids for enhanced gene transfection efficiency. Eur. J. Med. Chem. 2017;136:585–595. doi: 10.1016/j.ejmech.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 19.Bae Y.U., Huh J.W., Kim B.K., Park H.Y., Seu Y.B., Doh K.O. Enhancement of liposome mediated gene transfer by adding cholesterol and cholesterol modulating drugs. Biochim. Biophys. Acta. 2016;1858:3017–3023. doi: 10.1016/j.bbamem.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Etoc F., Balloul E., Vicario C., Normanno D., Liße D., Sittner A., Piehler J., Dahan M., Coppey M. Non-specific interactions govern cytosolic diffusion of nanosized objects in mammalian cells. Nat. Mater. 2018;17:740–746. doi: 10.1038/s41563-018-0120-7. [DOI] [PubMed] [Google Scholar]
- 21.Grady M.E., Parrish E., Caporizzo M.A., Seeger S.C., Composto R.J., Eckmann D.M. Intracellular nanoparticle dynamics affected by cytoskeletal integrity. Soft Matter. 2017;13:1873–1880. doi: 10.1039/c6sm02464e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foteini P., Pippa N., Naziris N., Demetzos C. Physicochemical study of the protein-liposome interactions: influence of liposome composition and concentration on protein binding. J. Liposome Res. 2019;29:313–321. doi: 10.1080/08982104.2018.1468774. [DOI] [PubMed] [Google Scholar]
- 23.Li T., He J., Horvath G., Próchnicki T., Latz E., Takeoka S. Lysine-containing cationic liposomes activate the NLRP3 inflammasome: Effect of a spacer between the head group and the hydrophobic moieties of the lipids. Nanomedicine (Lond.) 2018;14:279–288. doi: 10.1016/j.nano.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Xia Y., Tian J., Chen X. Effect of surface properties on liposomal siRNA delivery. Biomaterials. 2016;79:56–68. doi: 10.1016/j.biomaterials.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato Y., Hashiba K., Sasaki K., Maeki M., Tokeshi M., Harashima H. Understanding structure-activity relationships of pH-sensitive cationic lipids facilitates the rational identification of promising lipid nanoparticles for delivering siRNAs in vivo. J. Control. Release. 2019;295:140–152. doi: 10.1016/j.jconrel.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Cheng X., Lee R.J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 2016;99(Pt A):129–137. doi: 10.1016/j.addr.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Santiwarangkool S., Akita H., Nakatani T., Kusumoto K., Kimura H., Suzuki M., Nishimura M., Sato Y., Harashima H. PEGylation of the GALA peptide enhances the lung-targeting activity of nanocarriers that contain encapsulated siRNA. J. Pharm. Sci. 2017;106:2420–2427. doi: 10.1016/j.xphs.2017.04.075. [DOI] [PubMed] [Google Scholar]
- 28.Wu Y., Xiong Y., Wang L., Zhou Q., Li L., Levkin P.A., Davidson G., Gao L., Deng W. Development of new self-assembled cationic amino liposomes for efficient gene delivery. Biomater. Sci. 2020;8:3021–3025. doi: 10.1039/d0bm00331j. [DOI] [PubMed] [Google Scholar]
- 29.Wu Y., Li L., Chen Q., Su Y., Levkin P.A., Davidson G. Single-tailed lipidoids enhance the transfection activity of their double-tailed counterparts. ACS Comb. Sci. 2016;18:43–50. doi: 10.1021/acscombsci.5b00117. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Wu J.L.Y., Lazarovits J., Chan W.C.W. An analysis of the binding function and structural organization of the protein corona. J. Am. Chem. Soc. 2020;142:8827–8836. doi: 10.1021/jacs.0c01853. [DOI] [PubMed] [Google Scholar]
- 31.Xiao W., Gao H. The impact of protein corona on the behavior and targeting capability of nanoparticle-based delivery system. Int. J. Pharm. 2018;552:328–339. doi: 10.1016/j.ijpharm.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Capriotti A.L., Caracciolo G., Caruso G., Foglia P., Pozzi D., Samperi R., Laganà A. DNA affects the composition of lipoplex protein corona: a proteomics approach. Proteomics. 2011;11:3349–3358. doi: 10.1002/pmic.201000803. [DOI] [PubMed] [Google Scholar]
- 33.Szebeni J., Moghimi S.M. Liposome triggering of innate immune responses: a perspective on benefits and adverse reactions. J. Liposome Res. 2009;19:85–90. doi: 10.1080/08982100902792855. [DOI] [PubMed] [Google Scholar]
- 34.Roces C.B., Khadke S., Christensen D., Perrie Y. Scale-independent microfluidic production of cationic liposomal adjuvants and development of enhanced lymphatic targeting strategies. Mol. Pharm. 2019;16:4372–4386. doi: 10.1021/acs.molpharmaceut.9b00730. [DOI] [PubMed] [Google Scholar]
- 35.Uemura Y., Naoi T., Kanai Y., Kobayashi K. The efficiency of lipid nanoparticles with an original cationic lipid as a siRNA delivery system for macrophages and dendritic cells. Pharm. Dev. Technol. 2019;24:263–268. doi: 10.1080/10837450.2018.1469149. [DOI] [PubMed] [Google Scholar]
- 36.Li S., Yang Y., Lin X., Li Z., Ma G., Su Z., Zhang S. Biocompatible cationic solid lipid nanoparticles as adjuvants effectively improve humoral and T cell immune response of foot and mouth disease vaccines. Vaccine. 2020;38:2478–2486. doi: 10.1016/j.vaccine.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Audouy S.A.L., de Leij L.F.M.H., Hoekstra D., Molema G. In vivo characteristics of cationic liposomes as delivery vectors for gene therapy. Pharm. Res. 2002;19:1599–1605. doi: 10.1023/a:1020989709019. [DOI] [PubMed] [Google Scholar]
- 38.De Serrano L.O., Burkhart D.J. Liposomal vaccine formulations as prophylactic agents: design considerations for modern vaccines. J. Nanobiotechnology. 2017;15:83–105. doi: 10.1186/s12951-017-0319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Digiacomo L., Cardarelli F., Pozzi D., Palchetti S., Digman M.A., Gratton E., Capriotti A.L., Mahmoudi M., Caracciolo G. An apolipoprotein-enriched biomolecular corona switches the cellular uptake mechanism and trafficking pathway of lipid nanoparticles. Nanoscale. 2017;9:17254–17262. doi: 10.1039/c7nr06437c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Digiacomo L., Pozzi D., Amenitsch H., Caracciolo G. Impact of the biomolecular corona on the structure of PEGylated liposomes. Biomater. Sci. 2017;5:1884–1888. doi: 10.1039/c7bm00387k. [DOI] [PubMed] [Google Scholar]
- 41.Mahmoudi M., Bertrand N., Zope H., Farokhzad O.C. Emerging understanding of the protein corona at the nano-bio interfaces. Nano Today. 2016;11:817–832. [Google Scholar]
- 42.Palchetti S., Digiacomo L., Giulimondi F., Pozzi D., Peruzzi G., Ferri G., Amenitsch H., Cardarelli F., Mahmoudi M., Caracciolo G. A mechanistic explanation of the inhibitory role of the protein corona on liposomal gene expression. Biochim. Biophys. Acta Biomembr. 2020;1862:183159–183171. doi: 10.1016/j.bbamem.2019.183159. [DOI] [PubMed] [Google Scholar]
- 43.Walkey C.D., Chan W.C. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012;41:2780–2799. doi: 10.1039/c1cs15233e. [DOI] [PubMed] [Google Scholar]
- 44.Seraj S., Lee J., Ahn H.J. Systemic delivery of Eg5 shRNA-expressing plasmids using PEGylated DC-Chol/DOPE cationic liposome: long-term silencing and anticancer effects in vivo. Biochem. Pharmacol. 2019;166:192–202. doi: 10.1016/j.bcp.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 45.Sasayama Y., Hasegawa M., Taguchi E., Kubota K., Kuboyama T., Naoi T., Yabuuchi H., Shimai N., Asano M., Tokunaga A. In vivo activation of PEGylated long circulating lipid nanoparticle to achieve efficient siRNA delivery and target gene knock down in solid tumors. J. Control. Release. 2019;311–312:245–256. doi: 10.1016/j.jconrel.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Majzoub R.N., Chan C.-L., Ewert K.K., Silva B.F.B., Liang K.S., Jacovetty E.L., Carragher B., Potter C.S., Safinya C.R. Uptake and transfection efficiency of PEGylated cationic liposome-DNA complexes with and without RGD-tagging. Biomaterials. 2014;35:4996–5005. doi: 10.1016/j.biomaterials.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J., Ahn H.J. PEGylated DC-Chol/DOPE cationic liposomes containing KSP siRNA as a systemic siRNA delivery carrier for ovarian cancer therapy. Biochem. Biophys. Res. Commun. 2018;503:1716–1722. doi: 10.1016/j.bbrc.2018.07.104. [DOI] [PubMed] [Google Scholar]
- 48.Zhen S., Li X. Liposomal delivery of CRISPR/Cas9. Cancer Gene Ther. 2019 doi: 10.1038/s41417-019-0141-7. Published online November 2, 2019. [DOI] [PubMed] [Google Scholar]
- 49.Pozzi D., Colapicchioni V., Caracciolo G., Piovesana S., Capriotti A.L., Palchetti S., De Grossi S., Riccioli A., Amenitsch H., Laganà A. Effect of polyethyleneglycol (PEG) chain length on the bio-nano-interactions between PEGylated lipid nanoparticles and biological fluids: from nanostructure to uptake in cancer cells. Nanoscale. 2014;6:2782–2792. doi: 10.1039/c3nr05559k. [DOI] [PubMed] [Google Scholar]
- 50.Zhou Z., Liu X., Zhu D., Wang Y., Zhang Z., Zhou X., Qiu N., Chen X., Shen Y. Nonviral cancer gene therapy: delivery cascade and vector nanoproperty integration. Adv. Drug Deliv. Rev. 2017;115:115–154. doi: 10.1016/j.addr.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 51.Khalil I.A., Harashima H. An efficient PEGylated gene delivery system with improved targeting: synergism between octaarginine and a fusogenic peptide. Int. J. Pharm. 2018;538:179–187. doi: 10.1016/j.ijpharm.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Fang Y., Xue J., Gao S., Lu A., Yang D., Jiang H., He Y., Shi K. Cleavable PEGylation: a strategy for overcoming the “PEG dilemma” in efficient drug delivery. Drug Deliv. 2017;24(Suppl 1):22–32. doi: 10.1080/10717544.2017.1388451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao C., Deng H., Xu J., Li S., Zhong L., Shao L., Wu Y., Liang X.J. “Sheddable” PEG-lipid to balance the contradiction of PEGylation between long circulation and poor uptake. Nanoscale. 2016;8:10832–10842. doi: 10.1039/c6nr02174c. [DOI] [PubMed] [Google Scholar]
- 54.McSweeney M.D., Versfeld Z.C., Carpenter D.M., Lai S.K. Physician awareness of immune responses to polyethylene glycol-drug conjugates. Clin. Transl. Sci. 2018;11:162–165. doi: 10.1111/cts.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giselbrecht J., Wiedemann S., Reddy Pinnapireddy S., Goergen N., Loppnow H., Sedding D., Erdmann F., Bakowsky U., Hause G., Lúcio M. Nucleic acid carrier composed of a branched fatty acid lysine conjugate-Interaction studies with blood components. Colloids Surf. B Biointerfaces. 2019;184:110547–110561. doi: 10.1016/j.colsurfb.2019.110547. [DOI] [PubMed] [Google Scholar]
- 56.Francia V., Montizaan D., Salvati A. Interactions at the cell membrane and pathways of internalization of nano-sized materials for nanomedicine. Beilstein J. Nanotechnol. 2020;11:338–353. doi: 10.3762/bjnano.11.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren H., He Y., Liang J., Cheng Z., Zhang M., Zhu Y., Hong C., Qin J., Xu X., Wang J. Role of liposome size, surface charge, and PEGylation on rheumatoid arthritis targeting therapy. ACS Appl. Mater. Interfaces. 2019;11:20304–20315. doi: 10.1021/acsami.8b22693. [DOI] [PubMed] [Google Scholar]
- 58.Gao H., He Q. The interaction of nanoparticles with plasma proteins and the consequent influence on nanoparticles behavior. Expert Opin. Drug Deliv. 2014;11:409–420. doi: 10.1517/17425247.2014.877442. [DOI] [PubMed] [Google Scholar]
- 59.Caracciolo G., Cardarelli F., Pozzi D., Salomone F., Maccari G., Bardi G., Capriotti A.L., Cavaliere C., Papi M., Laganà A. Selective targeting capability acquired with a protein corona adsorbed on the surface of 1,2-dioleoyl-3-trimethylammonium propane/DNA nanoparticles. ACS Appl. Mater. Interfaces. 2013;5:13171–13179. doi: 10.1021/am404171h. [DOI] [PubMed] [Google Scholar]
- 60.Caracciolo G., Pozzi D., Capriotti A.L., Cavaliere C., Lagana A. Effect of DOPE and cholesterol on the protein adsorption onto lipid nanoparticles. J. Nanopart. Res. 2013;15:1498. [Google Scholar]
- 61.Caracciolo G. Liposome-protein corona in a physiological environment: challenges and opportunities for targeted delivery of nanomedicines. Nanomedicine (Lond.) 2015;11:543–557. doi: 10.1016/j.nano.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Caracciolo G., Pozzi D., Capriotti A.L., Cavaliere C., Piovesana S., Amenitsch H., Laganà A. Lipid composition: a “key factor” for the rational manipulation of the liposome-protein corona by liposome design. RSC Adv. 2015;5:5967–5975. [Google Scholar]
- 63.Caracciolo G. Clinically approved liposomal nanomedicines: lessons learned from the biomolecular corona. Nanoscale. 2018;10:4167–4172. doi: 10.1039/c7nr07450f. [DOI] [PubMed] [Google Scholar]
- 64.Chen D., Parayath N., Ganesh S., Wang W., Amiji M. The role of apolipoprotein- and vitronectin-enriched protein corona on lipid nanoparticles for in vivo targeted delivery and transfection of oligonucleotides in murine tumor models. Nanoscale. 2019;11:18806–18824. doi: 10.1039/c9nr05788a. [DOI] [PubMed] [Google Scholar]
- 65.Caracciolo G., Farokhzad O.C., Mahmoudi M. Biological identity of nanoparticles in vivo: clinical implications of the protein corona. Trends Biotechnol. 2017;35:257–264. doi: 10.1016/j.tibtech.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 66.Park J., Choi Y., Chang H., Um W., Ryu J.H., Kwon I.C. Alliance with EPR effect: combined strategies to improve the EPR effect in the tumor microenvironment. Theranostics. 2019;9:8073–8090. doi: 10.7150/thno.37198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maeda H., Matsumura Y. EPR effect based drug design and clinical outlook for enhanced cancer chemotherapy. Adv. Drug Deliv. Rev. 2011;63:129–130. doi: 10.1016/j.addr.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 68.Sapra P., Allen T.M. Ligand-targeted liposomal anticancer drugs. Prog. Lipid Res. 2003;42:439–462. doi: 10.1016/s0163-7827(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 69.Maeda H. Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J. Control. Release. 2012;164:138–144. doi: 10.1016/j.jconrel.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 70.Noble G.T., Stefanick J.F., Ashley J.D., Kiziltepe T., Bilgicer B. Ligand-targeted liposome design: challenges and fundamental considerations. Trends Biotechnol. 2014;32:32–45. doi: 10.1016/j.tibtech.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 71.Danaei M., Dehghankhold M., Ataei S., Hasanzadeh Davarani F., Javanmard R., Dokhani A., Khorasani S., Mozafari M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10:57–74. doi: 10.3390/pharmaceutics10020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ernsting M.J., Murakami M., Roy A., Li S.D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control. Release. 2013;172:782–794. doi: 10.1016/j.jconrel.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuan F., Dellian M., Fukumura D., Leunig M., Berk D.A., Torchilin V.P., Jain R.K. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55:3752–3756. [PubMed] [Google Scholar]
- 74.Hobbs S.K., Monsky W.L., Yuan F., Roberts W.G., Griffith L., Torchilin V.P., Jain R.K. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kang H., Rho S., Stiles W.R., Hu S., Baek Y., Hwang D.W., Kashiwagi S., Kim M.S., Choi H.S. Size-dependent EPR effect of polymeric nanoparticles on tumor targeting. Adv. Healthc. Mater. 2020;9:e1901223. doi: 10.1002/adhm.201901223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu R., Hu C., Yang Y., Zhang J., Gao H. Theranostic nanoparticles with tumor-specific enzyme-triggered size reduction and drug release to perform photothermal therapy for breast cancer treatment. Acta Pharm. Sin. B. 2019;9:410–420. doi: 10.1016/j.apsb.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ojha T., Pathak V., Shi Y., Hennink W.E., Moonen C.T.W., Storm G., Kiessling F., Lammers T. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv. Drug Deliv. Rev. 2017;119:44–60. doi: 10.1016/j.addr.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fang J., Nakamura H., Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 79.Sriraman S.K., Aryasomayajula B., Torchilin V.P. Barriers to drug delivery in solid tumors. Tissue Barriers. 2014;2:e29528. doi: 10.4161/tisb.29528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khawar I.A., Kim J.H., Kuh H.J. Improving drug delivery to solid tumors: priming the tumor microenvironment. J. Control. Release. 2015;201:78–89. doi: 10.1016/j.jconrel.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 81.Fukumura D., Jain R.K. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc. Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heldin C.H., Rubin K., Pietras K., Ostman A. High interstitial fluid pressure—an obstacle in cancer therapy. Nat. Rev. Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 83.Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015;91:3–6. doi: 10.1016/j.addr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 84.Ding J., Chen J., Gao L., Jiang Z., Zhang Y., Li M., Xiao Q., Lee S.S., Chen X. Engineered nanomedicines with enhanced tumor penetration. Nano Today. 2019;29:100800. [Google Scholar]
- 85.Zhou Q., Dong C., Fan W., Jiang H., Xiang J., Qiu N., Piao Y., Xie T., Luo Y., Li Z. Tumor extravasation and infiltration as barriers of nanomedicine for high efficacy: the current status and transcytosis strategy. Biomaterials. 2020;240:119902–119910. doi: 10.1016/j.biomaterials.2020.119902. [DOI] [PubMed] [Google Scholar]
- 86.Choi C.H., Zuckerman J.E., Webster P., Davis M.E. Targeting kidney mesangium by nanoparticles of defined size. Proc. Natl. Acad. Sci. USA. 2011;108:6656–6661. doi: 10.1073/pnas.1103573108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang L., Yang X., Yin Q., Cai K., Wang H., Chaudhury I., Yao C., Zhou Q., Kwon M., Hartman J.A. Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. USA. 2014;111:15344–15349. doi: 10.1073/pnas.1411499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang J., Wang X., Wen J., Su X., Weng L., Wang C., Tian Y., Zhang Y., Tao J., Xu P. Size effect of mesoporous organosilica nanoparticles on tumor penetration and accumulation. Biomater. Sci. 2019;7:4790–4799. doi: 10.1039/c9bm01164a. [DOI] [PubMed] [Google Scholar]
- 89.Tang L., Gabrielson N.P., Uckun F.M., Fan T.M., Cheng J. Size-dependent tumor penetration and in vivo efficacy of monodisperse drug-silica nanoconjugates. Mol. Pharm. 2013;10:883–892. doi: 10.1021/mp300684a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yu W., Liu R., Zhou Y., Gao H. Size-tunable strategies for a tumor targeted drug delivery system. ACS Cent. Sci. 2020;6:100–116. doi: 10.1021/acscentsci.9b01139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiong X., Xu Z., Huang H., Wang Y., Zhao J., Guo X., Zhou S. A NIR light triggered disintegratable nanoplatform for enhanced penetration and chemotherapy in deep tumor tissues. Biomaterials. 2020;245:119840–119852. doi: 10.1016/j.biomaterials.2020.119840. [DOI] [PubMed] [Google Scholar]
- 92.Liu Y., Huo Y., Yao L., Xu Y., Meng F., Li H., Sun K., Zhou G., Kohane D.S., Tao K. Transcytosis of nanomedicine for tumor penetration. Nano Lett. 2019;19:8010–8020. doi: 10.1021/acs.nanolett.9b03211. [DOI] [PubMed] [Google Scholar]
- 93.Yu Q., Qiu Y., Chen X., Wang X., Mei L., Wu H., Liu K., Liu Y., Li M., Zhang Z., He Q. Chemotherapy priming of the pancreatic tumor microenvironment promotes delivery and anti-metastasis efficacy of intravenous low-molecular-weight heparin-coated lipid-siRNA complex. Theranostics. 2019;9:355–368. doi: 10.7150/thno.29137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen X., Jia F., Li Y., Deng Y., Huang Y., Liu W., Jin Q., Ji J. Nitric oxide-induced stromal depletion for improved nanoparticle penetration in pancreatic cancer treatment. Biomaterials. 2020;246:119999–120010. doi: 10.1016/j.biomaterials.2020.119999. [DOI] [PubMed] [Google Scholar]
- 95.Kolašinac R., Jaksch S., Dreissen G., Braeutigam A., Merkel R., Csiszár A. Influence of environmental conditions on the fusion of cationic liposomes with living mammalian cells. Nanomaterials (Basel) 2019;9:1025–1041. doi: 10.3390/nano9071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kube S., Hersch N., Naumovska E., Gensch T., Hendriks J., Franzen A., Landvogt L., Siebrasse J.P., Kubitscheck U., Hoffmann B. Fusogenic liposomes as nanocarriers for the delivery of intracellular proteins. Langmuir. 2017;33:1051–1059. doi: 10.1021/acs.langmuir.6b04304. [DOI] [PubMed] [Google Scholar]
- 97.Hoffmann M., Hersch N., Merkel R., Csiszar A., Hoffmann B. Changing the way of entrance: highly efficient transfer of mRNA and siRNA via fusogenic nano-carriers. J. Biomed. Nanotechnol. 2019;15:170–183. doi: 10.1166/jbn.2019.2663. [DOI] [PubMed] [Google Scholar]
- 98.Csiszár A., Hersch N., Dieluweit S., Biehl R., Merkel R., Hoffmann B. Novel fusogenic liposomes for fluorescent cell labeling and membrane modification. Bioconjug. Chem. 2010;21:537–543. doi: 10.1021/bc900470y. [DOI] [PubMed] [Google Scholar]
- 99.Pozzi D., Marchini C., Cardarelli F., Amenitsch H., Garulli C., Bifone A., Caracciolo G. Transfection efficiency boost of cholesterol-containing lipoplexes. Biochim. Biophys. Acta. 2012;1818:2335–2343. doi: 10.1016/j.bbamem.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 100.Hui S.W., Langner M., Zhao Y.L., Ross P., Hurley E., Chan K. The role of helper lipids in cationic liposome-mediated gene transfer. Biophys. J. 1996;71:590–599. doi: 10.1016/S0006-3495(96)79309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Behzadi S., Serpooshan V., Tao W., Hamaly M.A., Alkawareek M.Y., Dreaden E.C., Brown D., Alkilany A.M., Farokhzad O.C., Mahmoudi M. Cellular uptake of nanoparticles: journey inside the cell. Chem. Soc. Rev. 2017;46:4218–4244. doi: 10.1039/c6cs00636a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Elkin S.R., Lakoduk A.M., Schmid S.L. Endocytic pathways and endosomal trafficking: a primer. Wien. Med. Wochenschr. 2016;166:196–204. doi: 10.1007/s10354-016-0432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang S., Gao H., Bao G. Physical principles of nanoparticle cellular endocytosis. ACS Nano. 2015;9:8655–8671. doi: 10.1021/acsnano.5b03184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Erni C., Suard C., Freitas S., Dreher D., Merkle H.P., Walter E. Evaluation of cationic solid lipid microparticles as synthetic carriers for the targeted delivery of macromolecules to phagocytic antigen-presenting cells. Biomaterials. 2002;23:4667–4676. doi: 10.1016/s0142-9612(02)00216-8. [DOI] [PubMed] [Google Scholar]
- 105.Juliano R.L. Intracellular trafficking and endosomal release of oligonucleotides: what we know and what we don’t. Nucleic Acid Ther. 2018;28:166–177. doi: 10.1089/nat.2018.0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gautier E.L., Yvan-Charvet L. Understanding macrophage diversity at the ontogenic and transcriptomic levels. Immunol. Rev. 2014;262:85–95. doi: 10.1111/imr.12231. [DOI] [PubMed] [Google Scholar]
- 107.Dielschneider R.F., Henson E.S., Gibson S.B. Lysosomes as oxidative targets for cancer therapy. Oxid. Med. Cell. Longev. 2017;2017:3749157–3749164. doi: 10.1155/2017/3749157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhitomirsky B., Assaraf Y.G. Lysosomes as mediators of drug resistance in cancer. Drug Resist. Updat. 2016;24:23–33. doi: 10.1016/j.drup.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 109.Kirkegaard T., Jäättelä M. Lysosomal involvement in cell death and cancer. Biochim. Biophys. Acta. 2009;1793:746–754. doi: 10.1016/j.bbamcr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 110.Vocelle D., Chan C., Walton S.P. Endocytosis controls siRNA efficiency: implications for siRNA delivery vehicle design and cell-specific targeting. Nucleic Acid Ther. 2020;30:22–32. doi: 10.1089/nat.2019.0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang X.X., Allen P.G., Grinstaff M. Macropinocytosis is the major pathway responsible for DNA transfection in CHO cells by a charge-reversal amphiphile. Mol. Pharm. 2011;8:758–766. doi: 10.1021/mp100366h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Inoh Y., Nagai M., Matsushita K., Nakanishi M., Furuno T. Gene transfection efficiency into dendritic cells is influenced by the size of cationic liposomes/DNA complexes. Eur. J. Pharm. Sci. 2017;102:230–236. doi: 10.1016/j.ejps.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 113.Bae Y.U., Kim B.K., Park J.W., Seu Y.B., Doh K.O. Endocytic pathway and resistance to cholesterol depletion of cholesterol derived cationic lipids for gene delivery. Mol. Pharm. 2012;9:3579–3585. doi: 10.1021/mp300458h. [DOI] [PubMed] [Google Scholar]
- 114.Lin Z., Bao M., Yu Z., Xue L., Ju C., Zhang C. The development of tertiary amine cationic lipids for safe and efficient siRNA delivery. Biomater. Sci. 2019;7:2777–2792. doi: 10.1039/c9bm00494g. [DOI] [PubMed] [Google Scholar]
- 115.Kim B.K., Hwang G.B., Seu Y.B., Choi J.S., Jin K.S., Doh K.O. DOTAP/DOPE ratio and cell type determine transfection efficiency with DOTAP-liposomes. Biochim. Biophys. Acta. 2015;1848(10 Pt A):1996–2001. doi: 10.1016/j.bbamem.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 116.Desai A.S., Hunter M.R., Kapustin A.N. Using macropinocytosis for intracellular delivery of therapeutic nucleic acids to tumour cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019;374:20180156. doi: 10.1098/rstb.2018.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rehman Zu., Zuhorn I.S., Hoekstra D. How cationic lipids transfer nucleic acids into cells and across cellular membranes: recent advances. J. Control. Release. 2013;166:46–56. doi: 10.1016/j.jconrel.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 118.Yuan X., Qin B., Yin H., Shi Y., Jiang M., Luo L., Luo Z., Zhang J., Li X., Zhu C. Virus-like nonvirus cationic liposome for efficient gene delivery via endoplasmic reticulum pathway. ACS Cent. Sci. 2020;6:174–188. doi: 10.1021/acscentsci.9b01052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kiss A.L., Botos E. Endocytosis via caveolae: alternative pathway with distinct cellular compartments to avoid lysosomal degradation? J. Cell. Mol. Med. 2009;13:1228–1237. doi: 10.1111/j.1582-4934.2009.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wadia J.S., Stan R.V., Dowdy S.F. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat. Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 121.Sayers E.J., Peel S.E., Schantz A., England R.M., Beano M., Bates S.M., Desai A.S., Puri S., Ashford M.B., Jones A.T. Endocytic profiling of cancer cell models reveals critical factors influencing LNP-mediated mRNA delivery and protein expression. Mol. Ther. 2019;27:1950–1962. doi: 10.1016/j.ymthe.2019.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rejman J., Oberle V., Zuhorn I.S., Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zelphati O., Szoka F.C., Jr. Mechanism of oligonucleotide release from cationic liposomes. Proc. Natl. Acad. Sci. USA. 1996;93:11493–11498. doi: 10.1073/pnas.93.21.11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu Y., Szoka F.C., Jr. Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry. 1996;35:5616–5623. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- 125.Avital Y.Y., Grønbech-Jensen N., Farago O. The thermodynamics of endosomal escape and DNA release from lipoplexes. Phys. Chem. Chem. Phys. 2016;18:2591–2596. doi: 10.1039/c5cp05778g. [DOI] [PubMed] [Google Scholar]
- 126.Caracciolo G., Pozzi D., Amenitsch H., Caminiti R. Interaction of lipoplexes with anionic lipids resulting in DNA release is a two-stage process. Langmuir. 2007;23:8713–8717. doi: 10.1021/la7017665. [DOI] [PubMed] [Google Scholar]
- 127.Pozzi D., Caracciolo G., Caminiti R., De Sanctis S.C., Amenitsch H., Marchini C., Montani M., Amici A. Toward the rational design of lipid gene vectors: shape coupling between lipoplex and anionic cellular lipids controls the phase evolution of lipoplexes and the efficiency of DNA release. ACS Appl. Mater. Interfaces. 2009;1:2237–2249. doi: 10.1021/am900406b. [DOI] [PubMed] [Google Scholar]
- 128.Ahmad A., Evans H.M., Ewert K., George C.X., Samuel C.E., Safinya C.R. New multivalent cationic lipids reveal bell curve for transfection efficiency versus membrane charge density: lipid-DNA complexes for gene delivery. J. Gene Med. 2005;7:739–748. doi: 10.1002/jgm.717. [DOI] [PubMed] [Google Scholar]
- 129.Dauty E., Verkman A.S. Actin cytoskeleton as the principal determinant of size-dependent DNA mobility in cytoplasm: a new barrier for non-viral gene delivery. J. Biol. Chem. 2005;280:7823–7828. doi: 10.1074/jbc.M412374200. [DOI] [PubMed] [Google Scholar]
- 130.Lukacs G.L., Haggie P., Seksek O., Lechardeur D., Freedman N., Verkman A.S. Size-dependent DNA mobility in cytoplasm and nucleus. J. Biol. Chem. 2000;275:1625–1629. doi: 10.1074/jbc.275.3.1625. [DOI] [PubMed] [Google Scholar]
- 131.Bai H., Lester G.M.S., Petishnok L.C., Dean D.A. Cytoplasmic transport and nuclear import of plasmid DNA. Biosci. Rep. 2017;37:20160616–20160632. doi: 10.1042/BSR20160616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vaughan E.E., DeGiulio J.V., Dean D.A. Intracellular trafficking of plasmids for gene therapy: mechanisms of cytoplasmic movement and nuclear import. Curr. Gene Ther. 2006;6:671–681. doi: 10.2174/156652306779010688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tseng W.C., Haselton F.R., Giorgio T.D. Mitosis enhances transgene expression of plasmid delivered by cationic liposomes. Biochim. Biophys. Acta. 1999;1445:53–64. doi: 10.1016/s0167-4781(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 134.Panté N., Kann M. Nuclear pore complex is able to transport macromolecules with diameters of about 39 nm. Mol. Biol. Cell. 2002;13:425–434. doi: 10.1091/mbc.01-06-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eguchi A., Furusawa H., Yamamoto A., Akuta T., Hasegawa M., Okahata Y., Nakanishi M. Optimization of nuclear localization signal for nuclear transport of DNA-encapsulating particles. J. Control. Release. 2005;104:507–519. doi: 10.1016/j.jconrel.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 136.Farina F., Pierobon P., Delevoye C., Monnet J., Dingli F., Loew D., Quanz M., Dutreix M., Cappello G. Kinesin KIFC1 actively transports bare double-stranded DNA. Nucleic Acids Res. 2013;41:4926–4937. doi: 10.1093/nar/gkt204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Coppola S., Estrada L.C., Digman M.A., Pozzi D., Cardarelli F., Gratton E., Caracciolo G. Intracellular trafficking of cationic liposome-DNA complexes in living cells. Soft Matter. 2012;8:7919–7927. doi: 10.1039/C2SM25532D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vaughan E.E., Dean D.A. Intracellular trafficking of plasmids during transfection is mediated by microtubules. Mol. Ther. 2006;13:422–428. doi: 10.1016/j.ymthe.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hasegawa S., Hirashima N., Nakanishi M. Microtubule involvement in the intracellular dynamics for gene transfection mediated by cationic liposomes. Gene Ther. 2001;8:1669–1673. doi: 10.1038/sj.gt.3301573. [DOI] [PubMed] [Google Scholar]
- 140.Coppola S., Cardarelli F., Pozzi D., Estrada L.C., Digman M.A., Gratton E., Bifone A., Marianecci C., Caracciolo G. The role of cytoskeleton networks on lipid-mediated delivery of DNA. Ther. Deliv. 2013;4:191–202. doi: 10.4155/tde.12.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pozzi D., Marchini C., Cardarelli F., Rossetta A., Colapicchioni V., Amici A., Montani M., Motta S., Brocca P., Cantù L., Caracciolo G. Mechanistic understanding of gene delivery mediated by highly efficient multicomponent envelope-type nanoparticle systems. Mol. Pharm. 2013;10:4654–4665. doi: 10.1021/mp400470p. [DOI] [PubMed] [Google Scholar]
- 142.Cardarelli F., Digiacomo L., Marchini C., Amici A., Salomone F., Fiume G., Rossetta A., Gratton E., Pozzi D., Caracciolo G. The intracellular trafficking mechanism of Lipofectamine-based transfection reagents and its implication for gene delivery. Sci. Rep. 2016;6:25879–25886. doi: 10.1038/srep25879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang L., Wang P., Feng Q., Wang N., Chen Z., Huang Y., Zheng W., Jiang X. Lipid nanoparticle-mediated efficient delivery of CRISPR/Cas9 for tumor therapy. NPG Asia Mater. 2017;9:e441. [Google Scholar]
- 144.Mieruszynski S., Digman M.A., Gratton E., Jones M.R. Characterization of exogenous DNA mobility in live cells through fluctuation correlation spectroscopy. Sci. Rep. 2015;5:13848–13857. doi: 10.1038/srep13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cornelis S., Vandenbranden M., Ruysschaert J.M., Elouahabi A. Role of intracellular cationic liposome-DNA complex dissociation in transfection mediated by cationic lipids. DNA Cell Biol. 2002;21:91–97. doi: 10.1089/104454902753604961. [DOI] [PubMed] [Google Scholar]
- 146.Zhi D., Bai Y., Yang J., Cui S., Zhao Y., Chen H., Zhang S. A review on cationic lipids with different linkers for gene delivery. Adv. Colloid Interface Sci. 2018;253:117–140. doi: 10.1016/j.cis.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 147.Jubeli E., Maginty A.B., Khalique N.A., Raju L., Nicholson D.G., Larsen H., Pungente M.D., Goldring W.P. Cationic lipids bearing succinic-based, acyclic and macrocyclic hydrophobic domains: synthetic studies and in vitro gene transfer. Eur. J. Med. Chem. 2017;125:225–232. doi: 10.1016/j.ejmech.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 148.Mukherjee D., Singh P., Rakshit T., Puthiya-Purayil T.P., Vemula P.K., Sengupta J., Das R., Pal S.K. Deciphering the response of asymmetry in the hydrophobic chains of novel cationic lipids towards biological function. Phys. Chem. Chem. Phys. 2020;22:1738–1746. doi: 10.1039/c9cp05405g. [DOI] [PubMed] [Google Scholar]
- 149.Rachamalla H.K., Mondal S.K., Deshpande S.S., Sridharan K., Javaji K., Jaggarapu M.M.C.S., Jinka S., Bollu V., Misra S., Banerjee R. Efficient anti-tumor nano-lipoplexes with unsaturated or saturated lipid induce differential genotoxic effects in mice. Nanotoxicology. 2019;13:1161–1175. doi: 10.1080/17435390.2019.1643049. [DOI] [PubMed] [Google Scholar]
- 150.Di Santo R., Quagliarini E., Palchetti S., Pozzi D., Palmieri V., Perini G., Papi M., Anna Laura Capriotti A.L., Laganà A., Caracciolo G. Microfluidic-generated lipid-graphene oxide nanoparticles for gene delivery. Appl. Phys. Lett. 2019;114:233701. [Google Scholar]
- 151.Martínez-Negro M., Guerrero-Martínez A., García-Río L., Domènech Ò., Aicart E., Tros de Ilarduya C., Junquera E. Multidisciplinary approach to the transfection of plasmid DNA by a nonviral nanocarrier based on a gemini-bolaamphiphilic hybrid lipid. ACS Omega. 2018;3:208–217. doi: 10.1021/acsomega.7b01657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yadav M.R., Kumar M., Murumkar P.R., Hazari P.P., Mishra A.K. Gemini amphiphile-based lipoplexes for efficient gene delivery: synthesis, formulation development, characterization, gene transfection, and biodistribution studies. ACS Omega. 2018;3:11802–11816. doi: 10.1021/acsomega.8b01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pozzi D., Cardarelli F., Salomone F., Marchini C., Amenitsch H., Barbera G.L., Caracciolo G. Role of cholesterol on the transfection barriers of cationic lipid/DNA complexes. Appl. Phys. Lett. 2014;105:073701. [Google Scholar]
- 154.Midoux P., Pichon C., Yaouanc J.-J., Jaffrès P.-A. Chemical vectors for gene delivery: a current review on polymers, peptides and lipids containing histidine or imidazole as nucleic acids carriers. Br. J. Pharmacol. 2009;157:166–178. doi: 10.1111/j.1476-5381.2009.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zidovska A., Evans H.M., Ahmad A., Ewert K.K., Safinya C.R. The role of cholesterol and structurally related molecules in enhancing transfection of cationic liposome-DNA complexes. J. Phys. Chem. B. 2009;113:5208–5216. doi: 10.1021/jp809000e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hattori Y., Tamaki K., Ozaki K.-i., Kawano K., Onishi H. Optimized combination of cationic lipids and neutral helper lipids in cationic liposomes for siRNA delivery into the lung by intravenous injection of siRNA lipoplexes. J. Drug Deliv. Sci. Technol. 2019;52:1042–1050. [Google Scholar]
- 157.Abumanhal-Masarweh H., da Silva D., Poley M., Zinger A., Goldman E., Krinsky N., Kleiner R., Shenbach G., Schroeder J.E., Shklover J. Tailoring the lipid composition of nanoparticles modulates their cellular uptake and affects the viability of triple negative breast cancer cells. J. Control. Release. 2019;307:331–341. doi: 10.1016/j.jconrel.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang L., Liu S., Liu H., Yang C., Jiang A., Wei H., Sun D., Cai Z., Zheng Y. Versatile cationic liposomes for RIP3 overexpression in colon cancer therapy and RIP3 downregulation in acute pancreatitis therapy. J. Drug Target. 2020;28:627–642. doi: 10.1080/1061186X.2019.1708370. [DOI] [PubMed] [Google Scholar]
- 159.Lou G., Anderluzzi G., Woods S., Roberts C.W., Perrie Y. A novel microfluidic-based approach to formulate size-tuneable large unilamellar cationic liposomes: Formulation, cellular uptake and biodistribution investigations. Eur. J. Pharm. Biopharm. 2019;143:51–60. doi: 10.1016/j.ejpb.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 160.Joshi S., Cooke J.R., Chan D.K., Ellis J.A., Hossain S.S., Singh-Moon R.P., Wang M., Bigio I.J., Bruce J.N., Straubinger R.M. Liposome size and charge optimization for intraarterial delivery to gliomas. Drug Deliv. Transl. Res. 2016;6:225–233. doi: 10.1007/s13346-016-0294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Muripiti V., Brijesh L., Rachamalla H.K., Marepally S.K., Banerjee R., Patri S.V. α-Tocopherol-ascorbic acid hybrid antioxidant based cationic amphiphile for gene delivery: design, synthesis and transfection. Bioorg. Chem. 2019;82:178–191. doi: 10.1016/j.bioorg.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 162.Sakai-Kato K., Yoshida K., Izutsu K.I. Effect of surface charge on the size-dependent cellular internalization of liposomes. Chem. Phys. Lipids. 2019;224:104726–104732. doi: 10.1016/j.chemphyslip.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 163.Zhao Z., Ukidve A., Krishnan V., Mitragotri S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv. Drug Deliv. Rev. 2019;143:3–21. doi: 10.1016/j.addr.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 164.Hosseini E.S., Nikkhah M., Hosseinkhani S. Cholesterol-rich lipid-mediated nanoparticles boost of transfection efficiency, utilized for gene editing by CRISPR-Cas9. Int. J. Nanomedicine. 2019;14:4353–4366. doi: 10.2147/IJN.S199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Semple S.C., Chonn A., Cullis P.R. Influence of cholesterol on the association of plasma proteins with liposomes. Biochemistry. 1996;35:2521–2525. doi: 10.1021/bi950414i. [DOI] [PubMed] [Google Scholar]
- 166.Wang H.-X., Zuo Z.-Q., Du J.-Z., Wang Y.-C., Sun R., Cao Z.-T., Ye X.-D., Wang J.-L., Leong K.W., Wang J. Surface charge critically affects tumor penetration and therapeutic efficacy of cancer nanomedicines. Nano Today. 2016;11:133–144. [Google Scholar]
- 167.Bugno J., Hsu H.J., Pearson R.M., Noh H., Hong S. Size and surface charge of engineered poly(amidoamine) dendrimers modulate tumor accumulation and penetration: a model study using multicellular tumor spheroids. Mol. Pharm. 2016;13:2155–2163. doi: 10.1021/acs.molpharmaceut.5b00946. [DOI] [PubMed] [Google Scholar]
- 168.Li Y., Lee R.J., Yu K., Bi Y., Qi Y., Sun Y., Li Y., Xie J., Teng L. Delivery of siRNA using lipid nanoparticles modified with cell penetrating peptide. ACS Appl. Mater. Interfaces. 2016;8:26613–26621. doi: 10.1021/acsami.6b09991. [DOI] [PubMed] [Google Scholar]
- 169.Tie Y., Zheng H., He Z., Yang J., Shao B., Liu L., Luo M., Yuan X., Liu Y., Zhang X. Targeting folate receptor β positive tumor-associated macrophages in lung cancer with a folate-modified liposomal complex. Signal Transduct. Target. Ther. 2020;5:6–20. doi: 10.1038/s41392-020-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hattori Y., Shimizu S., Ozaki K.I., Onishi H. Effect of cationic lipid type in folate-PEG-modified cationic liposomes on folate receptor-mediated siRNA transfection in tumor cells. Pharmaceutics. 2019;11:181–202. doi: 10.3390/pharmaceutics11040181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kabilova T.O., Shmendel E.V., Gladkikh D.V., Chernolovskaya E.L., Markov O.V., Morozova N.G., Maslov M.A., Zenkova M.A. Targeted delivery of nucleic acids into xenograft tumors mediated by novel folate-equipped liposomes. Eur. J. Pharm. Biopharm. 2018;123:59–70. doi: 10.1016/j.ejpb.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 172.Lee D.J., Kessel E., Lehto T., Liu X., Yoshinaga N., Padari K., Chen Y.C., Kempter S., Uchida S., Rädler J.O. Systemic delivery of folate-PEG siRNA lipopolyplexes with enhanced intracellular stability for in vivo gene silencing in leukemia. Bioconjug. Chem. 2017;28:2393–2409. doi: 10.1021/acs.bioconjchem.7b00383. [DOI] [PubMed] [Google Scholar]
- 173.Yang D., Yang X., Lee R.J., Liu S., Xie J. Liposomes incorporating transferrin and stearic acid-modified octa-arginine for siRNA delivery. Anticancer Res. 2017;37:1759–1764. doi: 10.21873/anticanres.11508. [DOI] [PubMed] [Google Scholar]
- 174.Yuan Y., Zhang L., Cao H., Yang Y., Zheng Y., Yang X.J. A Polyethylenimine-containing and transferrin-conjugated lipid nanoparticle system for antisense oligonucleotide delivery to AML. BioMed Res. Int. 2016;2016:1287128–1287135. doi: 10.1155/2016/1287128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang Y., Li S., Zhou X., Sun J., Fan X., Guan Z., Zhang L., Yang Z. Construction of a targeting nanoparticle of 3′,3″-bis-peptide-siRNA conjugate/mixed lipid with postinserted DSPE-PEG2000-cRGD. Mol. Pharm. 2019;16:4920–4928. doi: 10.1021/acs.molpharmaceut.9b00800. [DOI] [PubMed] [Google Scholar]
- 176.Fu S., Xu X., Ma Y., Zhang S., Zhang S. RGD peptide-based non-viral gene delivery vectors targeting integrin αvβ3 for cancer therapy. J. Drug Target. 2019;27:1–11. doi: 10.1080/1061186X.2018.1455841. [DOI] [PubMed] [Google Scholar]
- 177.Zhu Y., Feijen J., Zhong Z. Dual-targeted nanomedicines for enhanced tumor treatment. Nano Today. 2018;18:65–85. [Google Scholar]
- 178.Yang Y., Zhao Z., Xie C., Zhao Y. Dual-targeting liposome modified by glutamic hexapeptide and folic acid for bone metastatic breast cancer. Chem. Phys. Lipids. 2020;228:104882. doi: 10.1016/j.chemphyslip.2020.104882. [DOI] [PubMed] [Google Scholar]
- 179.Fisher R.K., Mattern-Schain S.I., Best M.D., Kirkpatrick S.S., Freeman M.B., Grandas O.H., Mountain D.J.H. Improving the efficacy of liposome-mediated vascular gene therapy via lipid surface modifications. J. Surg. Res. 2017;219:136–144. doi: 10.1016/j.jss.2017.05.111. [DOI] [PubMed] [Google Scholar]
- 180.Feng Y., Hu H., Liang S., Wang D. Preparation of gene drug delivery systems of cationic peptide lipid with 0G-PAMAM as hydrophilic end and its biological properties evaluation. Chem. Phys. Lipids. 2019;224:104685. doi: 10.1016/j.chemphyslip.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 181.Midoux P., Pigeon L., Goncalves C., Pichon C. Peptides mediating DNA transport on microtubules and their impact on non-viral gene transfer efficiency. Biosci. Rep. 2017;37 doi: 10.1042/BSR20170995. BSR20170995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang L., Shan X., Meng X., Gu T., Lu Q., Zhang J., Chen J., Jiang Q., Ning X. The first integrins β3-mediated cellular and nuclear targeting therapeutics for prostate cancer. Biomaterials. 2019;223:119471. doi: 10.1016/j.biomaterials.2019.119471. [DOI] [PubMed] [Google Scholar]
- 183.Akinc A., Maier M.A., Manoharan M., Fitzgerald K., Jayaraman M., Barros S., Ansell S., Du X., Hope M.J., Madden T.D. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019;14:1084–1087. doi: 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- 184.Kulkarni J.A., Witzigmann D., Chen S., Cullis P.R., van der Meel R. Lipid nanoparticle technology for clinical translation of siRNA therapeutics. Acc. Chem. Res. 2019;52:2435–2444. doi: 10.1021/acs.accounts.9b00368. [DOI] [PubMed] [Google Scholar]
- 185.Wei L., Guo X.Y., Yang T., Yu M.Z., Chen D.W., Wang J.C. Brain tumor-targeted therapy by systemic delivery of siRNA with transferrin receptor-mediated core-shell nanoparticles. Int. J. Pharm. 2016;510:394–405. doi: 10.1016/j.ijpharm.2016.06.127. [DOI] [PubMed] [Google Scholar]
- 186.Zhang R., Men K., Zhang X., Huang R., Tian Y., Zhou B., Yu C., Wang Y., Ji X., Hu Q., Yang L. Delivery of a modified mRNA encoding IL-22 binding protein (IL-22BP) for colon cancer gene therapy. J. Biomed. Nanotechnol. 2018;14:1239–1251. doi: 10.1166/jbn.2018.2577. [DOI] [PubMed] [Google Scholar]
- 187.Vhora I., Lalani R., Bhatt P., Patil S., Patel H., Patel V., Misra A. Colloidally stable small unilamellar stearyl amine lipoplexes for effective BMP-9 gene delivery to stem cells for osteogenic differentiation. AAPS PharmSciTech. 2018;19:3550–3560. doi: 10.1208/s12249-018-1161-6. [DOI] [PubMed] [Google Scholar]
- 188.He Z.Y., Deng F., Wei X.W., Ma C.C., Luo M., Zhang P., Sang Y.X., Liang X., Liu L., Qin H.X. Ovarian cancer treatment with a tumor-targeting and gene expression-controllable lipoplex. Sci. Rep. 2016;6:23764. doi: 10.1038/srep23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Leite Nascimento T., Hillaireau H., Vergnaud J., Rivano M., Deloménie C., Courilleau D., Arpicco S., Suk J.S., Hanes J., Fattal E. Hyaluronic acid-conjugated lipoplexes for targeted delivery of siRNA in a murine metastatic lung cancer model. Int. J. Pharm. 2016;514:103–111. doi: 10.1016/j.ijpharm.2016.06.125. [DOI] [PubMed] [Google Scholar]
- 190.Andey T., Bora-Singhal N., Chellappan S.P., Singh M. Cationic lipoplexes for treatment of cancer stem cell-derived murine lung tumors. Nanomedicine (Lond.) 2019;18:31–43. doi: 10.1016/j.nano.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fehring V., Schaeper U., Ahrens K., Santel A., Keil O., Eisermann M., Giese K., Kaufmann J. Delivery of therapeutic siRNA to the lung endothelium via novel lipoplex formulation DACC. Mol. Ther. 2014;22:811–820. doi: 10.1038/mt.2013.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jose A., Labala S., Venuganti V.V. Co-delivery of curcumin and STAT3 siRNA using deformable cationic liposomes to treat skin cancer. J. Drug Target. 2017;25:330–341. doi: 10.1080/1061186X.2016.1258567. [DOI] [PubMed] [Google Scholar]