Abstract
Intracranial hemorrhagic metastases are a relatively common finding in patients with thyroid carcinoma. Consequently, more unusual vascular lesions may be overlooked in contemplating a differential diagnosis in this patient group. A 50-year-old female with previously treated papillary thyroid carcinoma presented to the emergency department following new onset seizures. Her work up revealed multiple intraparenchymal brain lesions, hyperdense on computed tomography and demonstrating susceptibility effect, T1 shortening and contrast enhancement on magnetic resonance imaging, suggestive of metastases. Subsequent studies revealed lesional architecture consistent with multiple cavernous malformations, made evident by resolution of edema and evolution of blood products. Clinicians should be aware of the possibility of unusual intracranial hemorrhagic lesions in oncology patients which may only become evident on serial imaging evaluation. Cavernous hemangioma has typical MRI characteristic features which includes “mulberry” appearance on T2-weighted and fluid attenuation inversion recovery images with varying internal signal intensity which indicates multiple stages of blood products within the cavernous hemangioma. The lesions commonly have a typical T2-weighted dark hemosiderin rim. Blood sensitive demonstrates prominent surrounding hypointensity representing blooming secondary to internal blood products and/or calcification, if present. Cavernous hemangioma may rarely demonstrate some degree of contrast enhancement. Perfusion imaging may show alteration in capillary permeability involving cavernous malformations which has been previously described in the literature.
Abbreviations: CT, computed tomography; FLAIR, fluid attenuation inversion recovery; GRE, gradient echo sequences; MRI, magnetic resonance imaging; RAI, radioactive iodine; SWI, susceptibility-weighted imaging
Keywords: Cavernous hemangioma, Papillary thyroid carcinoma, Intracranial hemorrhagic metastasis
Introduction
Cerebral cavernous venous malformations (or cavernous hemangioma) are common vascular malformations that are usually asymptomatic and incidentally found [1]. However, patients may present with seizures, headache or focal neurologic deficit in the relatively rare event of hemorrhage [1], [2], [3]. Most lesions are sporadic; however, if multiple lesions are present, this could indicate familial multiple cavernous malformation syndrome [4]. Eighty percent of cavernous hemangiomas are supratentorial [2]. Multiple cavernous hemangioma can also be seen following intracranial radiation therapy [5,6].
Cavernous hemangioma are unusually large collections of blood vessels without parenchyma interceding between the sinusoidal vessels [1], [2], [3]. These vascular channels have thin walls and have no smooth muscle support. They may also be associated with adjacent developmental venous anomalies [3].
Cavernous hemangioma have fairly characteristic imaging appearances, not typically causing a diagnostic dilemma [7,8]. However, within the context of a history of cancer with potential hemorrhagic brain metastases, neuroimagers need to be vigilant to ensure they contemplate this possibility, especially when the cavernous hemangioma have internal hemorrhage with edema, potentially obfuscating the typical lesional architecture the reader would likely anticipate [9].
In this case report, we describe a case of cavernous hemangioma, who was initially thought to have developed intracranial metastatic disease from primary papillary thyroid cancer.
Case report
A 50-year-old female with past medical history significant for papillary thyroid cancer (status postradioactive iodine ablation and resection 10 years prior to presentation), scleroderma, Raynaud's syndrome, polymyositis, and pericarditis presented to the emergency department following new onset seizures. She described her symptoms as right arm jerking, drooling, and encephalopathy.
On examination, she was afebrile and hemodynamically stable. Physical examination, including neurologic examination, on admission, was unremarkable. Initial laboratory testing included blood workup, urine drug screen, and urine analysis which were negative.
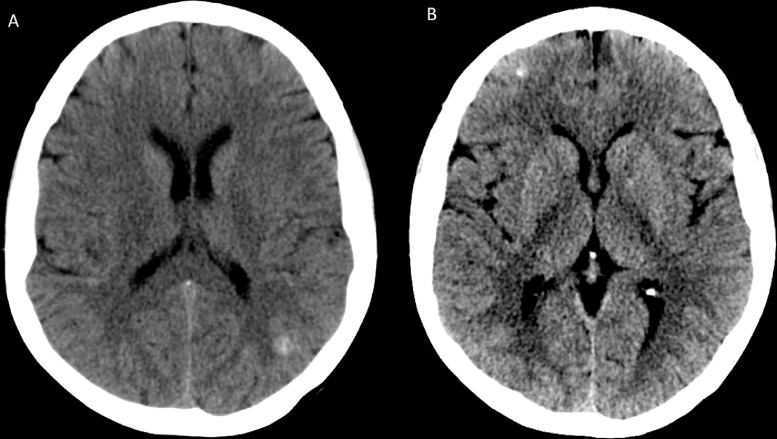
Noncontract computed tomography (CT) of the head (Fig. 1) demonstrated multiple supratentorial and infratentorial hyperdensities measuring up to 8 mm, which were concerning for possible hemorrhagic metastases in the setting of history of malignancy. The largest lesion was in the left parietal lobe and measured approximately 8 mm (Fig. 1A). Additional smaller hyperattenuating lesions were also seen in the right frontal lobe (Fig. 1B) as well as in the medial right cerebellar hemisphere and left frontal centrum semiovale.
Fig. 1.
Noncontrast computed tomography (CT) of the brain at the time of presentation. The noncontrast CT scan at the time of presentation demonstrated multiple supratentorial and infratentorial hyperdensities measuring up to 8 mm, which were concerning for possible hemorrhagic metastases in the setting of history of malignancy. The largest lesion was in the left parietal lobe and measured approximately 8 mm (A). Additional smaller hyperattenuating lesions were also seen in the right frontal lobe (B) as well as in the medial right cerebellar hemisphere and left frontal centrum semiovale (not shown on current figures).
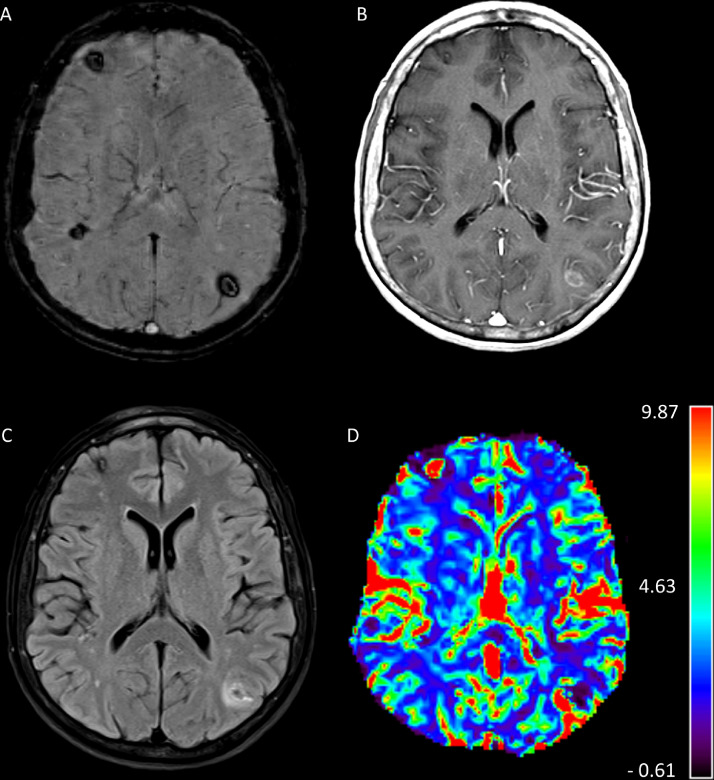
Magnetic resonance imaging (MRI) brain with and without contrast demonstrated numerous foci of signal dropout (hypointensity) within these lesions on susceptibility-weighted imaging (SWI), including in the cerebellar hemispheres and brainstem (Fig. 2A). Postcontrast imaging demonstrated peripheral contrast enhancement in some of these lesions, including a left parietal lesion (Fig. 2B) which had peripheral vasogenic edema (Fig. 2C), and internal hyperperfusion (Fig. 2D). These imaging findings, together with the patient's history of thyroid carcinoma, raised concern for possible multiple hemorrhagic brain metastases.
Fig. 2.
Magnetic resonance imaging (MRI) of brain with and without contrast at the time of presentation. Susceptibility-weighted imaging (SWI) demonstrated numerous foci of signal dropout (hypointensity) including in the cerebellar hemispheres and brainstem (A). Postcontrast T1-weighted imaging demonstrated peripheral contrast enhancement in some of these lesions, including a left parietal lobe lesion (B). T2-fluid attenuated inversion recovery (FLAIR) sequence demonstrated peripheral vasogenic edema (C) around the parietal lobe lesion. Cerebral blood volume (CBV) perfusion sequence demonstrated internal hyperperfusion (D). These imaging findings, together with the patient's history of thyroid carcinoma, raised concern for possible multiple hemorrhagic brain metastases.
Following which CT chest, abdomen and pelvis was performed with no evidence of tumor recurrence or further potential metastatic disease. Inpatient colonoscopy was negative for malignancy. The patient was initiated on Levetiracetam and improved clinically over the course of the hospital stay and was discharged. She reported no further seizures at the follow-up clinic visit in 2 weeks.
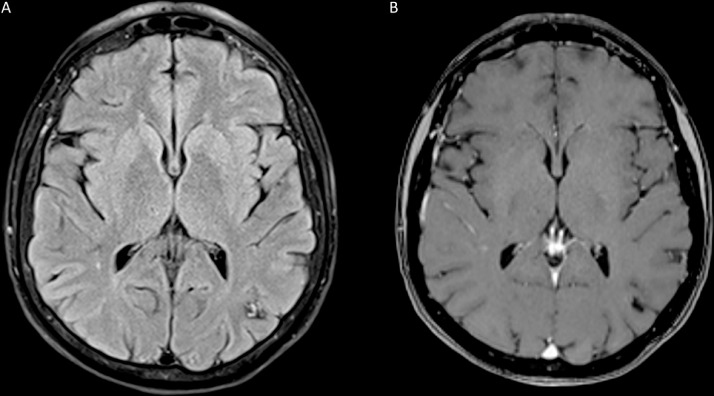
Three-month follow-up MRI of the brain demonstrated resolution of the previously visualized vasogenic edema surrounding some of these lesions, including the aforementioned left parietal lesion with a surrounding hemosiderin ring and heterogeneous internal signal now able to be visualized (Fig. 3A). Contrast enhancement (Fig. 3B) was also reduced. As such, the characteristic findings of multiple cavernous malformations became evident with the passage of time and resolution of blood products and vasogenic edema. She continues to be followed in clinic and reports no further episodes of seizures.
Fig. 3.
Magnetic resonance imaging (MRI) of brain with and without contrast at 3-month follow-up. Three-month follow-up MRI of the brain demonstrated resolution of the previously visualized vasogenic edema on T2-fluid attenuated inversion recovery (FLAIR) sequence surrounding some of these lesions, including the aforementioned left parietal lesion with a surrounding hemosiderin ring and heterogeneous internal signal now able to be visualized (A). T1-weighted postcontrast scan demonstrated decreased peripheral enhancement (B).
Discussion
Cerebral cavernous venous malformations are common vascular malformations that are usually asymptomatic and incidentally found [1,2]. However, patients may present with seizures, headache or focal neurologic deficit in the relatively rare event of hemorrhage [3]. Most lesions are sporadic; however, if multiple lesions are present, this could indicate familial multiple cavernous malformation syndrome [4,10]. Eighty percent of cavernous hemangioma are supratentorial [2]. Cavernous hemangioma of the brain and spinal cord can present in any age group, most commonly seen in third-sixth decades of life and has no gender predisposition. Reportedly, it is present in 0.5% of the general population [3]. Multiple cavernous hemangiomas can also be seen following intracranial radiation therapy [5,6].
Cavernous hemangiomas are unusually large collections of blood vessels without parenchyma interceding between the sinusoidal vessels. These vascular channels have thin walls and have no smooth muscle support. They may also be associated with adjacent developmental venous anomalies [1], [2], [3].
On CT, cavernous hemangiomas may not be visible. However, when seen, they appear as hyperdense lesions that may resemble hemorrhage. They may also exhibit small foci of calcification. If there has been recent hemorrhage, perilesional vasogenic edema may be evident [11].
MRI reveals a characteristic “mulberry” appearance on T2-weighted and fluid attenuation inversion recovery (FLAIR)-weighted images with varying internal signal intensity which indicates multiple stages of blood products within the cavernous hemangioma. They also will typically have a T2-weighted dark hemosiderin rim. Blood sensitive imaging (gradient echo [GRE] and SWI) demonstrates prominent surrounding hypointensity representing blooming secondary to internal blood products and/or calcification, if present. Cavernous hemangiomas may rarely demonstrate some degree of contrast enhancement. Perfusion imaging may show alteration in capillary permeability involving cavernous malformations which has been previously described in the literature. In addition, blood products surrounding the cavernous hemangiomas could also lead to misinterpretation of perfusion data due to susceptibility effect [5], [6], [7], [8], [9],11].
As seen in this case, cavernous hemangiomas can mimic intracranial hemorrhagic metastatic disease. The most common other primary tumors with hemorrhagic brain metastases include thyroid carcinoma, choriocarcinoma, renal cell carcinoma, melanoma, and bronchogenic carcinoma [12]. Other, infrequent causes of intracranial hemorrhagic metastases include hepatoblastoma and osteogenic sarcoma [12].
An unusual case of cavernous hemangioma mimicking brain metastases status post-thyroidectomy and RAI in a patient with thyroid carcinoma was reported by Bulzico et al. [9]. As mentioned above, cavernous hemangiomas rarely enhance and do not exhibit surrounding vasogenic edema unless recent hemorrhage is present. Perfusion imaging may also be helpful in differentiating metastases from cavernous hemangiomas as metastases are more likely to show evidence of increased blood volume. In our case, only 1 lesion in the left parietal lobe showed surrounding edema which likely represented recent hemorrhage. Brain metastases would typically be expected to demonstrate surrounding vasogenic edema unless very small in size. Additionally, the majority of the foci of signal dropout on blood sensitive imaging did not demonstrate enhancement, which is highly atypical for metastases [7], [8], [9].
Last, as described above, subsequent follow-up showed decreased associated enhancement, resolution of edema, and no new lesions, all findings that support the diagnosis of multiple cavernous hemangiomas rather than hemorrhagic metastases.
Other differential consideration would also include chronic hypertensive encephalopathy, amyloid angiopathy, diffuse axonal injury, cerebral vasculitis, hemorrhagic metastases, and Parry-Romberg syndrome [1]. In this case, the patient had no history of intracranial radiotherapy. Her history of multiple autoimmune disorders (Raynaud's, scleroderma and polymyositis) may potentially have contributed to the formation of multiple cerebral cavernous malformations. In addition, other possible etiologies include familial cerebral cavernous malformation syndrome which leads to the formation of multiple cavernous hemangiomas.
Cerebral amyloid angiopathy may also simulate multiple cavernous malformations. In contrast to multiple cavernous hemangiomas which are usually randomly distributed, the microhemorrhages in cerebral amyloid angiopathy tend to be more superficially located near the gray white junction. Patients with cerebral amyloid angiopathy also may have sequelae of superficial siderosis, indicative of chronic subarachnoid hemorrhage [8].
Intracranial hemorrhagic metastases are a relatively common finding in patients with thyroid carcinoma. Consequently, more unusual vascular lesions may be overlooked in contemplating a differential diagnosis in this patient group. We present an example of multiple cavernous malformations masquerading as brain metastases. It is important to differentiate cerebral cavernous malformations from hemorrhagic brain metastases as this will greatly affect the treatment and care of the patient. Clinicians should be aware of the possibility of unusual intracranial hemorrhagic lesions in oncology patients which may only become evident on serial imaging evaluation.
Acknowledgment
Institutional Review Board at West Virginia University authorized the publication of case report, under IRB protocol number: 2006046810.
Footnotes
Declaration of Competing Interest: Authors have no relevant disclosures.
Contributor Information
Parissa Feizi, Email: pfeizi@hsc.wvu.edu.
Dhairya A. Lakhani, Email: dhairya.lakhani@hsc.wvu.edu.
Abdul R. Tarabishy, Email: artarabishy@hsc.wvu.edu.
Gerard Deib, Email: gerard.deib@hsc.wvu.edu.
Shitiz Sriwastava, Email: shitiz.sriwastava@hsc.wvu.edu.
References
- 1.Bergui M., Bradac G.B. Uncommon symptomatic cerebral vascular malformations. Am J Neuroradiol. 1997;18(4):779–783. [PMC free article] [PubMed] [Google Scholar]
- 2.D'Angelo V.A., De Bonis C., Amoroso R., Cali A., D’Agruma L., Vito Guarnieri V. Supratentorial cerebral cavernous malformations: clinical, surgical, and genetic involvement. Neurosurg Focus. 2006;21(1):e9. doi: 10.3171/foc.2006.21.1.10. [DOI] [PubMed] [Google Scholar]
- 3.Cortés Vela J.J., Concepción Aramendía L., Ballenilla Marco F., Gallego Leónb J.I., González-Spínola San Gil I. Cerebral cavernous malformations: spectrum of neuroradiological findings. Radiologia. 2012;54(5):401–409. doi: 10.1016/j.rx.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Dziedzic T., Kunert P., Matyja E., Ziora-Jakutowicz K., Sidoti A., Marchel A. Familial cerebral cavernous malformation. Folia Neuropathol. 2012;50(2):152–158. [PubMed] [Google Scholar]
- 5.Jain R., Robertson P.L., Gandhi D., Gujar S.K., Muraszko K.M., Gebarski S. Radiation-induced cavernous hemangiomas of the brain. Am J Neuroradiol. 2005;26(5):1158–1162. [PMC free article] [PubMed] [Google Scholar]
- 6.Cutsforth-Gregory J.K., Lanzino G., Link M.J., Brown R.D., Flemming K.D. Characterization of radiation-induced cavernous malformations and comparison with a nonradiation cavernous malformation cohort. J Neurosurg. 2015;122(5):1214–1222. doi: 10.3171/2015.1.JNS141452. [DOI] [PubMed] [Google Scholar]
- 7.Vilanova J.C., Barceló J., Smirniotopoulos J.C., Pérez-Andrés R., Villalón M., Miró J., Martin F., Capellades J., Ros P.R. Hemangioma from head to toe: MR imaging with pathologic correlation. Radiographics. 2004;24(2):367–385. doi: 10.1148/rg.242035079. [DOI] [PubMed] [Google Scholar]
- 8.Pinker K., Stavrou I., Knosp E., Trattnig S. Are cerebral cavernous hemangiomas truly nonenhancing lesions and thereby distinguishable from arteriovenous malformations? MRI findings and histopathological correlation. Magn Reson Imaging. 2006;24(5):631–637. doi: 10.1016/j.mri.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 9.Daniel B., Fernanda V., Noronha Pessoa C.de, Cencita H., Corbo R. Cavernous angioma mimicking a differentiated thyroid carcinoma brain metastasis. Clin Nucl Med. 2011;36(1):62–63. doi: 10.1097/RLU.0b013e3181feefc2. [DOI] [PubMed] [Google Scholar]
- 10.Brunereau L., Labauge P., Tournier-Lasserve E., Laberge S., Levy C., Houtteville J.P. Familial form of intracranial cavernous angioma: MR imaging findings in 51 families. French Society of Neurosurgery. Radiology. 2000;214(1):209–216. doi: 10.1148/radiology.214.1.r00ja19209. [DOI] [PubMed] [Google Scholar]
- 11.Hegde A.N., Mohan S., Lim C.C. CNS cavernous haemangioma: ``popcorn'' in the brain and spinal cord. Clin Radiol. 2012;67(4):380–388. doi: 10.1016/j.crad.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Mandybur T.I. Intracranial hemorrhage caused by metastatic tumors. Neurology. 1977;27(7):650–655. doi: 10.1212/wnl.27.7.650. [DOI] [PubMed] [Google Scholar]