Highlights
-
•
Titanium wear particles were identified in peri-implantitis tissues.
-
•
Overexpressed cytokine levels for RANKL and high levels of the other inflammatory cytokines (TGF-β1, IL-33 and CD68) were observed.
-
•
A causal relationship between the presence of particles and peri-implantitis cannot be confirmed.
Keywords: Peri-implantitis, Dental implants, Microscopy, Scanning electron microscopy, Corrosion, Foreign body reaction
Abstract
Objective
To investigate the presence of titanium particles in peri-implant tissues in cases diagnosed with peri-implantitis, and to identify immunological reactions that these particles may elicit.
Methods
Ten peri-implant tissue biopsies of patients diagnosed clinically and radiographically with peri-implantitis were obtained from the archives of Oral Pathology Centre, University of Otago. The inclusion criteria involves: bleeding on probing, ≥6 mm probing depth and ≥3 mm radiographic bone loss around the dental implant. Peri-implant tissue samples were evaluated using scanning electron microscopy-energy dispersive x-ray spectroscopy (SEM-EDS) to identify of sites with/without titanium particles. Antibodies against human transforming growth factor beta 1 (TGF-β1), receptor activator of nuclear factor kappa-B ligand (RANKL), interleukin 33 (IL-33) and cluster of differentiation 68 (CD68) were used to stain the specimens. ImageJ software was used to standardise the sampling area, compare and characterise the inflammatory infiltrate in tissues with/without titanium particles. Inflammatory cytokines positivity was assessed using the immunoreactive scores (IRSs).
Results
Light microscopy and SEM-EDS analysis identified titanium wear particles in 90% of the tissue samples, associated with a mixed chronic inflammatory infiltrate. Quantification analysis of RANKL revealed significantly higher IRS and intensity scores (p < 0.05) in areas containing titanium. High intensity, proportion and IRSs of TGF-β1 and IL-33 were observed in areas with titanium. CD68 had higher IRSs in the absence of titanium particles.
Conclusions
Significant overexpression of the cytokine RANKL was observed, with a trend for over-expression of IL-33 and TGF-B1 in areas with titanium. Further studies with large sample size and appropriate control group for quantification analysis is needed to confirm the role of titanium particles in initiating bone loss.
1. Introduction
Implant complications remain a notable concern with 1–5% of dental implants failing within 5–7 years (Pjetursson et al., 2012). Despite the inconsistencies in case definitions of peri-implantitis, disease prevalence varies between 5 and 56% of patients (Derks et al., 2016, Zitzmann and Berglundh, 2008). Biofilm accumulation is considered to be a key causal factor in peri-implantitis. The association between peri-implant disease and its microbial aetiology is a consequence of the lipopolysaccharide (LPS) component of bacterial cell walls, which activates neutrophils, macrophages, T cells and B cells and sets up, through a cascade of cytokine release and osteoclast differentiation, tissue reactions in the soft tissue and bone (Fernandes and Gomes, 2016, Taira et al., 2010). Some cytokines have been over-expressed in peri-implant crevicular fluid in diseased implant sites (Erhan and Tolga, 2016).
Recent research has focused on whether titanium particles can instigate foreign body reactions via the activation of pro-inflammatory cytokines, resulting in peri-implant bone and soft tissue loss (Alrabeah et al., 2017, Murray et al., 2013). Titanium particles can enter peri-implant tissues due to corrosion in the oral environment, micro-movements between the implant components, instrumentation of the implant surface or implant surgery. Corrosion is defined as the “the release of metal ions from metal surfaces as a result of a thermodynamically driven electrochemical process” (Tawse-Smith et al., 2017). The oral cavity provides a corrosive medium for dental implants which is further enhanced by inflammation, the presence of acidic bacteria, fluorides and dietary factors, all of which can contribute to disruption of the titanium oxide layer of the implant (Alrabeah et al., 2017, Tawse-Smith et al., 2017). The association between corrosion and osteolysis has been consistently demonstrated in the orthopaedic literature and the formation of particulate wear has been found to contribute to aseptic loosening of orthopaedic implants and peri-implant tissue inflammatory changes (Geetha et al., 2009, Olmedo et al., 2012).
Implant surface decontamination may be carried out through a combination of mechanical, chemical and photodynamic therapy (Meyle, 2012). In human and animal studies, the use of metallic instrumentation to remove biofilm deposits has been shown to reduce the inflammatory response and progression of bone loss in peri-implant tissues (Valderrama and Wilson, 2014). However, surface instrumentation may result in the formation of particulate wear (Valderrama and Wilson, 2014) which is enhanced by the low wear resistance of titanium and surface treatments, such as surface roughening and oxidization (Taira et al., 2010).
Furthermore, titanium particles in peri-implant tissues may cause a foreign body reaction which is determined by both the quantity and physicochemical properties of the metallic particles in addition to the host response. Proinflammatory and anti-inflammatory cytokines and chemokines have been proposed in influencing the progression of, and immunity to, peri-implantitis. Imbalances in the level of cytokine release may inhibit the resolution of inflammation, or contribute to alveolar bone loss (Fernandes and Gomes, 2016).
This descriptive study aims to investigate the presence of titanium particles in the peri-implant tissues in cases diagnosed with peri-implantitis, and to identify immunological effects within the same sample for sites with/without titanium particles.
2. Materials and Methods
2.1. Sample selection
Ethics approval was granted by the Otago Human Ethics Committee (Reference number: H16/074). The Ngāi Tahu Maori Research Consultation Committee was contacted for advice. This experimental pilot study analysed formalin-fixed paraffin-embedded (FFPE) peri-implant tissue biopsies from ten patients diagnosed clinically and radiographically with peri-implantitis between 2011 and 2016. The samples (obtained from the archives of the Oral Pathology Centre, Faculty of Dentistry, University of Otago) were diagnosed with inflamed mucosa consistent with peri-implantitis, and excised by an experienced periodontist ATS (Fig. 1). The inclusion criteria involved the following clinical/radiographic parameters: bleeding upon probing, probing depths of ≥6 mm and radiographic bone loss of ≥3 mm around the dental implant. Immunocompromised patients and patients with immune-mediated inflammatory diseases were excluded from the study.
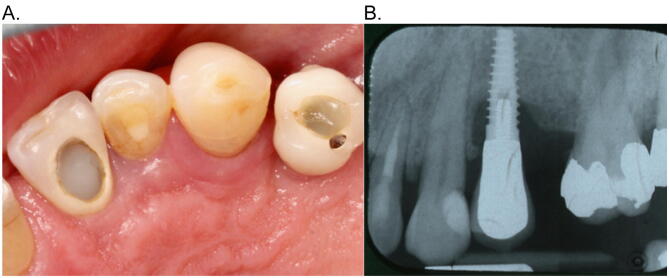
Fig. 1.
A: Occlusal view of upper left quadrant showing single implant crown – site #24 – with inflamed peri-implant tissues. B: Periapical radiograph showing saucer shaped peri-implant bone loss.
Sections were cut at 3–5 μm from the selected FFPE blocks, processed routinely and stained with haematoxylin and eosin (H&E).
2.2. Particle identification
2.2.1. Light microscopy
A camera attached to a light microscope (BX53 Olympus, Olympus Inc, Japan) was utilised to take photos of the tissue on each H&E slide at 2x magnification. These images were then used to create a mapping system via the use of a grid in ImageJ (US National Institutes of Health, Bethesda, Maryland, USA). The map dimensions consisted of six 1 mm × 1 mm squares labelled A-E on the side and 1–6 above. The tissue sections were then scanned visually at 10x magnification under the light microscope for any black particles that were consistent with titanium (Fig. 2). Once test sites (with black particles) and control sites (without particles) within the sample were identified, the location was transcribed onto the maps and photomicrographs were taken at 20x and 40x magnification. This mapping system allowed foreign material to be located for elemental testing. Three most representative areas positively confirmed in which titanium was present, and three areas with no titanium for each slide (as confirmed by scanning electron microscopy (SEM) were selected for evaluation.
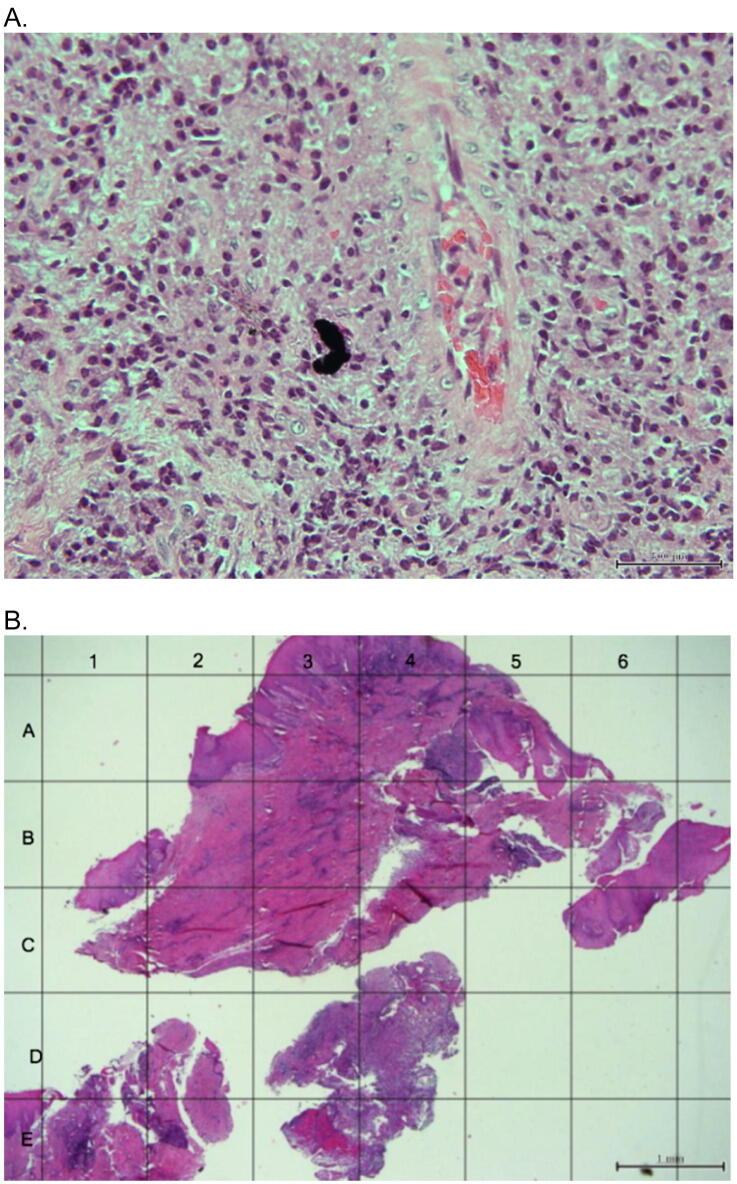
Fig. 2.
A: Tissue sections from a biopsy of the clinical case illustrated in Fig. 1, showing fragments of dark foreign material scattered within the connective tissue with a mixture of an acute and chronic inflammatory infiltrate (20× magnification; H&E staining). B: Mapping system.
2.2.2. SEM – EDS
SEM and energy dispersive x-ray spectroscopy (EDS) images were acquired using the Zeiss VP FEG SEM machine (Carl Zeiss Inc, Oberkocken, Germany). This was conducted to confirm and record the presence of titanium in pre-selected regions within the tissues (Fig. 3). Images of the slides on the SEM computer screen were matched to the corresponding regions on each slide in the SEM machine to allow slide orientation. This allowed accurate mapping of the titanium particles despite the high magnification of the SEM machine.
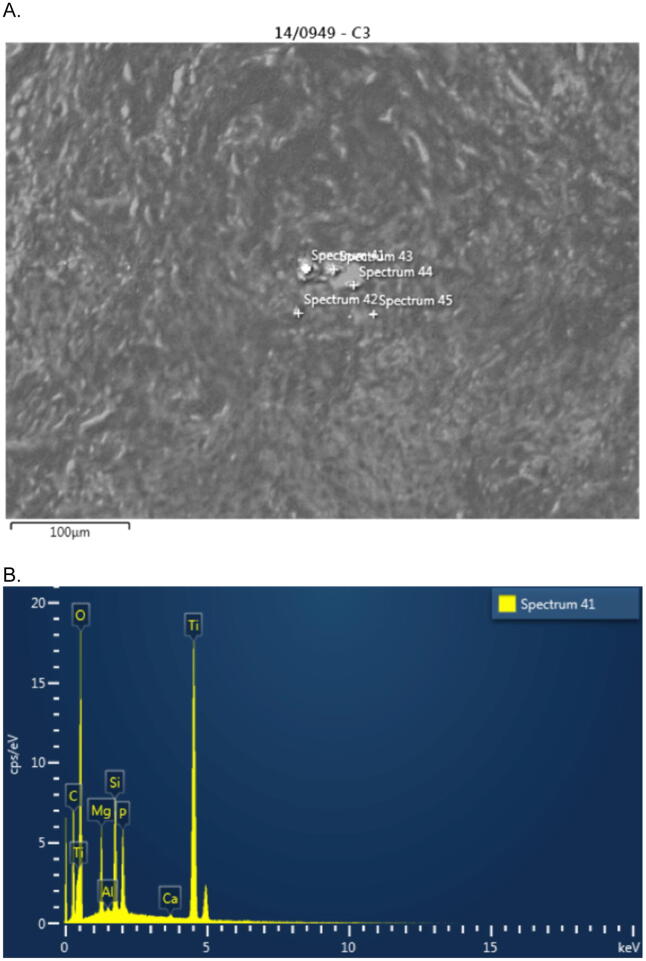
Fig. 3.
A: SEM analysis revealing different size particles in the tissue biopsy. B: EDS analysis confirming the presence of titanium particles in the biopsy.
2.3. Immunohistochemistry analysis
To prepare for staining with the four antibodies, a further four 3–5 μm sections were cut from the selected FFPE tissue blocks. Antibodies against human transforming growth factor beta 1 (TGF-β1), receptor activator of nuclear factor kappa-B ligand (RANKL), interleukin 33 (IL-33) and cluster of differentiation 68 (CD68) were used to stain the specimens with a matched immunoglobulin G (IgG) isotype control antibody (Fig. 4). Preliminary optimisation was carried out for each antibody with a positive control tissue using the Ventana autostainer (Ventana® Benchmark XT, Roche Inc., USA). The concentration ranges suggested by the manufacturer were utilised as a guide when choosing dilution factors. A dilution factor of 1:100 was selected for the antibodies TGF-β1 and CD68, while 1:50 was selected for RANKL and IL-33 after a range of dilutions were tested. The Ventana standard IHC protocol (CC1) was used for all four antibodies. Counterstaining was performed using Gill’s haematoxylin (GHS1128, Sigma-Aldrich, USA) and slides were rinsed in a container with cold running water.
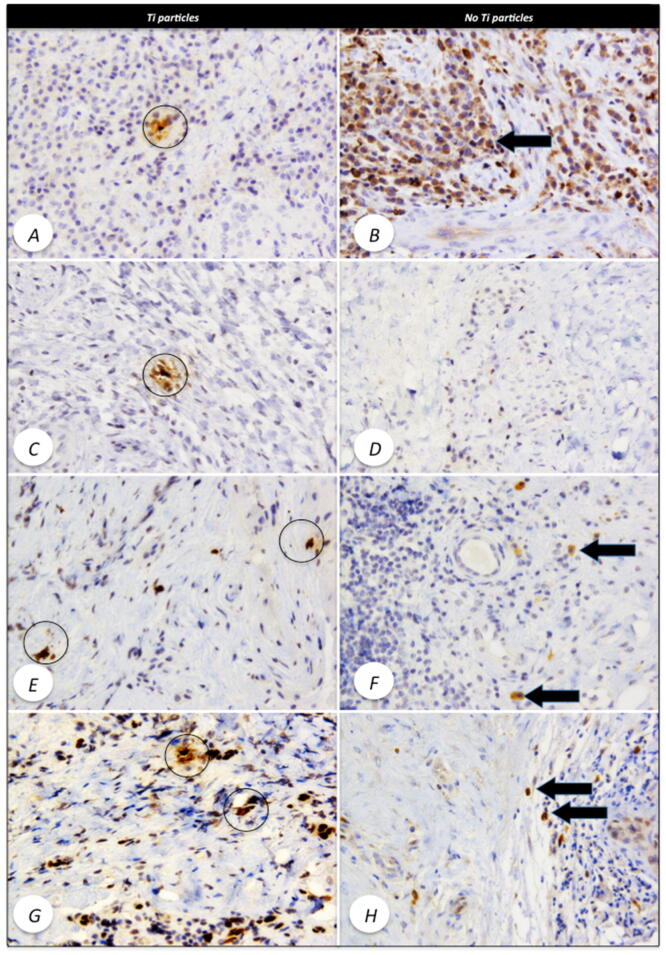
Fig. 4.
A: IHC with anti-CD68 antibody in tissues containing titanium particles (highlighted), only inflammatory cells around the particles are positive. B: IHC with anti-CD68 antibody in tissues without titanium particles. Arrow shows inflammatory cells with intense CD68 positivity. C: IHC with anti-TGF-β antibody in tissues containing titanium particles (highlighted), only inflammatory cells around the particles are positive. D: IHC with anti-CD68 antibody in tissues without titanium particles and minimal inflammatory cells positivity. E: IHC with anti-RANKL antibody in tissues containing titanium particles (highlighted), only inflammatory cells around the particles are positive. F: IHC with anti-RANKL antibody in tissues without titanium particles, certain inflammatory cells, morphologically identified as macrophages show intense positivity (arrow). G: IHC with anti-IL33 antibody in tissues containing titanium particles (highlighted), most inflammatory cells around the particles are positive. H: IHC with anti-IL33 antibody in tissues without Ti particles, some inflammatory cells, show intense positivity (arrow). Images are at 200× magnification.
2.4. Quantification analysis
The stained slides were viewed using light microscopy (BX50 Olympus, Japan) and photomicrographs were taken of all four antibodies at 200x magnification of three regions where titanium was present, and three areas with no titanium for each slide (as confirmed by SEM). For quantification, ImageJ plugin ‘IHC profiler’ was utilised to calculate the staining area (percentage) and intensity for the photomicrographs of the four antibodies. This method was selected because it considers both the percentage of IHC positivity and the staining intensity (Varghese et al., 2014). The ‘IHC profiler’ use colour deconvolution and pixel profiling to produce a score for images with IHC staining. Initially all the images were cropped by setting the scale and creating a centred 2000 µm squared grid on ImageJ. This system allows for calculation of an immunoreactive score (IRS), which is derived from multiplication of a proportion score (PS) and an intensity score (IS). To calculate the PS for each slide, a score was given between 0 and 4 depending on the percentage of positivity. To calculate the IS for each slide we compared the staining intensity to that of the positive control. A score of 0–3 was given depending on the intensity with zero representing no intensity and three, high intensity. The IS was standardised with two examiners (HH and ZB) until 95% reliability agreement was reached. The numeric values corresponding to the different results given by ImageJ for the percentage positivity, PS and IS are shown in Table 1.
Table 1.
Numeric ImageJ values corresponding to defined score categories.
| Percentage of positivity | Proportion score | Intensity score (IS) |
|---|---|---|
| 0% | 0 | Negative 0 |
| 1–10% | 1 | Low positive 1 |
| 11–50% | 2 | Positive 2 |
| 51–80% | 3 | High positive 3 |
| 81–100% | 4 |
2.4.1. Analysis of Acute/Chronic inflammatory cells
The numbers of acute and chronic inflammatory cells present in the H&E sections of areas with and without titanium were counted from the H&E stained slides using ImageJ. Photomicrographs were taken at 40x magnification of three areas containing titanium and three areas with no titanium for each of the samples. In order to have a standardised approach of differentiating acute cells from chronic cells, inter-observer correlation was calculated using Pearson correlation coefficient. The inter-observer correlation coefficient was 0.9 (P < 0.05), showing significant agreement between observers and therefore supported accurate analysis of the inflammatory infiltrate.
2.5. Statistical analysis
GraphPad Prism 7 (Graph Pad Software, La Jolla, California, USA) was utilised to calculate the mean, standard deviation, standard error of mean, and P-values. The P-value criteria chosen was two tailed, equal-variance. A P-value of less than 0.05 was considered to be statistically significant.
3. Results
3.1. Presence of titanium particles
Titanium particles were present in nine of the ten samples, as confirmed by SEM. In another sample titanium particles were present but were located outside of the mapped area. Therefore, two slides were excluded from the study, leaving eight remaining samples. The results depicting the percentages of positivity, PSs, ISs, and IRSs are shown in Table 2.
Table 2.
Percentage positivity, Proportion scores, Intensity scores and Immunoreactive scores for each of the antibodies.
| TGF-β1 |
RANKL |
IL-33 |
CD68 |
|||||
|---|---|---|---|---|---|---|---|---|
| No Ti | Ti | No Ti | Ti | No Ti | Ti | No Ti | Ti | |
| % of positivity | ||||||||
| Mean | 5.93 | 5.54 | 5.63 | 7.13 | 6.84 | 5.92 | 22.88 | 25.24 |
| Std. | 0.039 | 0.040 | 0.072 | 0.046 | 0.036 | 0.030 | 0.139 | 0.168 |
| Std. error | 0.158 | 0.170 | 0.303 | 0.171 | 0.137 | 0.125 | 0.292 | 0.334 |
| P-value | 0.741 | 0.413 | 0.360 | 0.615 | ||||
| Proportion score | ||||||||
| Mean | 1.136 | 1.182 | 1.091 | 1.190 | 1.182 | 1.182 | 1.818 | 1.818 |
| Std. | 0.351 | 0.395 | 0.294 | 0.402 | 0.390 | 0.395 | 0.501 | 0.588 |
| Std. error | 0.330 | 0.363 | 0.282 | 0.369 | 0.359 | 0.363 | 0.372 | 0.436 |
| P-value | 0.688 | 0.391 | 1.000 | 1.000 | ||||
| Intensity score | ||||||||
| Mean | 1.500 | 2.000 | 1.318 | *2.091 | 2.318 | 2.545 | 2.682 | 2.591 |
| Std. | 0.913 | 0.926 | 0.894 | 0.684 | 0.839 | 0.671 | 0.568 | 0.796 |
| Std. error | 1.570 | 2.079 | 1.394 | 2.529 | 2.531 | 3.107 | 3.559 | 2.903 |
| P-value | 0.078 | *0.002 | 0.327 | 0.665 | ||||
| IRS | ||||||||
| Mean | 1.773 | 2.455 | 1.591 | *2.545 | 2.818 | 3.091 | 5.091 | 5.000 |
| Std. | 1.378 | 1.654 | 1.593 | 1.405 | 1.563 | 1.540 | 2.000 | 2.370 |
| Std. error | 1.510 | 1.909 | 1.260 | 2.147 | 2.255 | 2.491 | 3.602 | 3.248 |
| P-value | 0.145 | *0.041 | 0.563 | 0.891 | ||||
Ti = titanium.
p < 0.05.
3.2. Quantification of inflammatory cytokines
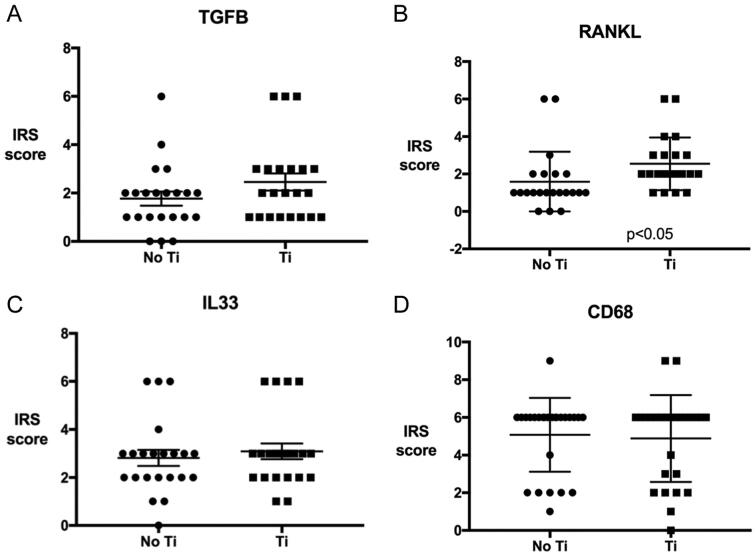
The quantification analysis of all four inflammatory cytokines revealed that the slides stained for RANKL showed a significantly higher IRSs and ISs (2.5 and 2.1, respectively) in the areas with titanium than without titanium (p < 0.05) (Fig. 5). In the slides stained for TGF-β1, the ISs, PSs and IRSs were higher in areas with titanium than areas without. However, these differences were not statistically significant. The ISs and IRSs were higher in the IL-33 slides for areas with titanium compared to those without titanium. The differences were again not statistically significant. For the slides stained for CD68, higher ISs and IRSs and lower percentages of positivity were observed in areas free of titanium particles compared to areas containing titanium. The differences were not statistically significant. The PSs were equal in both areas in the slides stained for IL-33 and CD68.
Fig. 5.
Cytokine IRS in areas with and without titanium particles: 5A: TGF β1; 5B: RANKL; 5C: IL33 and 5D: CD68.
3.3. Quantification of acute and chronic inflammatory cells
The majority of inflammatory cells in the peri-implant tissues were chronic inflammatory cells. The mean number of chronic cells in the areas with titanium (315.27 ± 187.48) was greater than in the regions with no titanium (177.27 ± 137.30). Most of the areas (regardless of their titanium status) contained fewer than 20 acute inflammatory cells, but the mean number of acute inflammatory cells (16.13 ± 8.09) was greater in areas with titanium compared to those without titanium (7.80 ± 7.78). The differences were statistically significant (p < 0.05).
4. Discussion
Titanium particles were present in 90% of the tissue samples signifying that titanium particles often dislodge from the implant surface. This has been confirmed in other dental implant studies (Flatebø et al., 2011, Tawse-Smith et al., 2012). Tawse-Smith et al. (2012) reported the presence of titanium particles in all peri-implantitis tissue samples using SEM and EDS and Flatebo et al. (2011) found titanium signals in the majority of samples via the use of Laser-Ablation-Inductively-Coupled-Plasma-Mass-Spectrometry. Although titanium particles were detected, it is difficult to confirm with this study where these particles originated from. The detachment of titanium particles may be caused by wear, surface instrumentation, corrosion or deposition from the implant body during implant placement.
Significant differences were observed in RANKL expression in the areas with titanium compared to areas without titanium particles. Since RANKL stimulates osteoclastic bone resorption and reduces the apoptosis of osteoclasts (Fernandes and Gomes, 2016), this may imply that more bone loss occurred in the areas with titanium particles. A number of studies have compared levels of soluble RANKL (sRANKL) in the crevicular fluid of patients with and without peri-implantitis, rather than comparing levels of RANKL in the peri-implant tissues (Erhan and Tolga, 2016, Duarte et al., 2009, Rakic et al., 2014). These studies have conflicting results with some reporting significantly higher amounts of sRANKL in samples with peri-implantitis compared to healthy implants, and other studies reporting higher amounts of sRANKL in healthy implant cases. One study found no correlation between adsorbed volume of sRANKL and clinical signs of probing depth, modified bleeding index, and modified plaque index. An increased ratio of osteoclastogenic RANKL to osteoprotegerin (OPG), an inhibitor of osteoclastogenesis, when titanium ions were present has also been noted (Fernandes and Gomes, 2016). Alrabeah et al. (2017) found that metal ions increased RANKL expression in a dose-dependent response.
For the other inflammatory cytokines, differences were not statistically significant between test and control sites, however, the presence of greater amount of TGF-β1 in areas with titanium suggests that increased bone loss may have been occurring in the areas with titanium particles. Increased levels of TGF-β1 has been found in peri-implantitis/periodontitis tissues compared to healthy tissues and greater TGF-β1 positivity in the epithelium surrounding failing implants compared to healthy implants (Cornelini et al., 2003, Steinsvoll et al., 1999). Gingival tissue with chronic marginal periodontitis contained 100 times more TGF-β1+ cells compared to healthy gingival tissue (Steinsvoll et al., 1999). To the best of our knowledge, the current study is the first to report increased levels of TGF-β1 in peri-implantitis tissues in areas proven to contain titanium particles. However, one in vitro study subjected MG-63 osteoblasts to commercially pure titanium and found that osteoblasts produced a large quantity of TGF-β1 (Goodman et al., 2006). Therefore, it is possible that TGF-β1 production may increase in response to titanium particles. Likewise, the orthopaedic literature showed that osteoblast stimulation in the presence of wear particles, increased the release of TGF-β (Goodman et al., 2006).
It is widely known that TGF-β1 is associated with wound healing. It increases the formation of blood vessels and collagen. As TGF-β1 plays a role in the middle phase of the wound healing pathway, both large and small quantities of TGF-β1 can be related to an impaired healing response (Schultze-Mosgau et al., 2005). TGF-β1 is also chemotactic for neutrophils, lymphocytes and mast cells and it can either stimulate proinflammatory cytokine production. or suppress humoral and cell mediated immunity and hence reduce proinflammatory cytokine stimulation. Therefore, we cannot conclude whether the increased levels of TGF-β1 in the areas containing titanium in this study were a sign of increased or decreased wound healing. It is possible that due to its functionality, TGF-β1 may play a preventive role in peri-implantitis by dampening the immune response and reducing tissue destruction (Cornelini et al., 2003).
The high ISs and IRSs for IL-33 in areas containing titanium particles may indicate increased cell signalling. One study found the expression of IL-33 was significantly higher in the crevicular fluid surrounding dental implants diagnosed with peri-implantitis compared to healthy peri-implant tissues (Severino et al., 2016). There are also an association between IL and 33 and fibrotic disorders which may indicate that a cytokine pathway stimulated by titanium particles may lead to the release of foreign body giant cells and collagen production (Christo et al., 2015).
The high ISs and IRSs for CD68 in areas with no titanium may be the result of variable cellular composition between the bone and peri-implant tissues. Several orthopaedic studies have reported an abundance of CD68+ macrophages in peri-prosthetic orthopaedic tissues containing wear debris (Yang et al., 2011, Zhang et al., 2009) and an increased number of CD68+ macrophages in aseptically loosened orthopaedic implants (Zhang and Revell, 1999). Yang and et al. (2011), analysed mice implanted with human periprosthetic tissue and reported CD68+ cells in much higher quantities when titanium particles were present compared to those free of titanium particles (Yang et al., 2011). This increase in CD68+ cells was thought to be associated with an increased number of particle-engulfed monocytes at sites containing titanium particles and wear debris.
The current study found a mixture of acute and chronic inflammatory cells in the peri-implantitis tissues. Similar findings were reported by (Wilson et al., 2015). The inflammatory infiltrate in the peri-implantitis tissues in this study was primarily chronic which is a common finding for peri-implantitis tissues (Wilson et al., 2015). More chronic inflammatory cells were found in the areas containing titanium suggesting that the presence of titanium particles initiated chronic inflammation in the tissues.
There is a strong correlation between overexpression of a cytokine or inflammatory marker and titanium particle size (Goodman et al., 2006). A particle size of 1.5–5 µm reduces osteoblast proliferation and survival and increases the expression of RANKL as well as other inflammatory markers such as colony stimulating factor 1 (CSF-1) (Goodman et al., 2006). Unlike large particles, particles of 1.5–5 µm can be phagocytosed and stimulate a biologic response. The particle size may influence the results of our study as some analysed regions may contain titanium particles of different sizes, thereby having reduced influence on expression of the investigated inflammatory markers. The effect of particles on osteoprogenitor cells is dose- and time-dependent with a greater effect in the presence of more particulate matter (Goodman et al., 2006).
The main strengths of this study included the ability to detect the presence of titanium using SEM and EDS technology, quantitatively measure the staining in peri-implant tissues and examine cytokine levels in response to titanium particles in tissues with peri-implantitis using the IHC profiler methodology. Our study is the first to use the IHC profiler to examine cytokine levels in response to titanium particles in tissues with peri-implantitis. The main limitations of this study were small sample size and lack of a control group that include healthy peri-implant tissues.
5. Conclusion
Significant overexpression of the cytokine RANKL was observed, with a trend for over-expression of IL-33 and TGF-B1 in areas with titanium. Further studies with large sample size and appropriate control group for quantification analysis is needed to confirm the role of titanium particles in initiating bone loss.
Declaration of Competing Interest
None.
Acknowledgements
The authors would like to thank Ms Liz Girvan and Dr Marianne Negrini for their guidance and support regarding the SEM and EDS Analyses.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Haizal Mohd Hussaini, Email: haizal.mh@otago.ac.nz.
Alison M. Rich, Email: alison.rich@otago.ac.nz.
Momen Atieh, Email: momen.atieh@mbru.ac.ae.
Andrew Tawse-Smith, Email: andrew.tawse-smith@otago.ac.nz.
References
- Alrabeah G.O., Brett P., Knowles J.C., Petridis H. The effect of metal ions released from different dental implant-abutment couples on osteoblast function and secretion of bone resorbing mediators. J. Dent. 2017;66:91–101. doi: 10.1016/j.jdent.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Christo S., Diener K., Bachhuka A., Vasilev K., Hayball J.D. Innate immunity and biomaterials at the nexus: friends or foes. Biomed. Res. Int. 2015 doi: 10.1155/2015/342304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelini R., Rubini C., Fioroni M., Favero G.A., Strocchi R., Piattelli A. Transforming growth factor-beta 1 expression in the peri-implant soft tissues of healthy and failing dental implants. J. Periodontol. 2003;74:446–450. doi: 10.1902/jop.2003.74.4.446. [DOI] [PubMed] [Google Scholar]
- Derks J., Schaller D., Håkansson J., Wennström J.L., Tomasi C., Berglundh T. Effectiveness of implant therapy analyzed in a Swedish population: prevalence of peri-implantitis. J. Dent. Res. 2016;95:43–49. doi: 10.1177/0022034515608832. [DOI] [PubMed] [Google Scholar]
- Duarte P.M., de Mendonca A.C., Maximo M.B., Santos V.R., Bastos M.F., Nociti F.H. Effect of anti-infective mechanical therapy on clinical parameters and cytokine levels in human peri-implant diseases. J. Periodontol. 2009;80:234–243. doi: 10.1902/jop.2009.070672. [DOI] [PubMed] [Google Scholar]
- Erhan D., Tolga F.T.j. Peri-implant crevicular fluid analysis, enzymes and biomarkers: a systemetic review. J. Oral Maxillofac. Res. 2016;7 doi: 10.5037/jomr.2016.7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes M.H., Gomes P.S. Bone cells dynamics during peri-implantitis: a theoretical analysis. J. Oral Maxillofac. Res. 2016;7 doi: 10.5037/jomr.2016.7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatebø R.S., Høl P.J., Leknes K.N., Kosler J., Lie S.A., Gjerdet N.R. Mapping of titanium particles in peri-implant oral mucosa by laser ablation inductively coupled plasma mass spectrometry and high-resolution optical darkfield microscopy. J. Oral Pathol. Med. 2011;40:412–420. doi: 10.1111/j.1600-0714.2010.00958.x. [DOI] [PubMed] [Google Scholar]
- Geetha M., Singh A.K., Asokamani R., Gogia A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants – a review. Prog. Mater. Sci. 2009;54:397–425. [Google Scholar]
- Goodman S.B., Ma T., Chiu R., Ramachandran R., Smith R.L. Effects of orthopaedic wear particles on osteoprogenitor cells. Biomaterials. 2006;27:6096–6101. doi: 10.1016/j.biomaterials.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Meyle J. Mechanical, chemical and laser treatments of the implant surface in the presence of marginal bone loss around implants. Eur. J. Oral Implantol. 2012;5:S71–S81. [PubMed] [Google Scholar]
- Murray C.M., Knight E.T., Russell A.A., Tawse-Smith A., Leichter J.W. Peri-implant disease: current understanding and future direction. New Zeal Dent J. 2013;109:55–62. [PubMed] [Google Scholar]
- Olmedo D.G., Paparella M.L., Spielberg M., Brandizzi D., Guglielmotti M.B., Cabrini R.L. Oral mucosa tissue response to titanium cover screws. J. Periodontol. 2012;83:973–980. doi: 10.1902/jop.2011.110392. [DOI] [PubMed] [Google Scholar]
- Pjetursson B.E., Thoma D., Jung R., Zwahlen M., Zembic A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clin. Oral Implants. 2012;23(S6):22–38. doi: 10.1111/j.1600-0501.2012.02546.x. [DOI] [PubMed] [Google Scholar]
- Rakic M., Struillou X., Petkovic-Curcin A., Matic S., Canullo L., Sanz M., Vojvodic D. Estimation of bone loss biomarkers as a diagnostic tool for peri-implantitis. J. Periodontol. 2014;85:1566–1574. doi: 10.1902/jop.2014.140069. [DOI] [PubMed] [Google Scholar]
- Schultze-Mosgau S., Blatz M.B., Wehrhan F., Schlegel K.A., Thorwart M., Holst S. Principles and mechanisms of peri-implant soft tissue healing. Quintessence Int. 2005;36:759–769. [PubMed] [Google Scholar]
- Severino V.O., Beghini M., de Araújo M.F., de Melo M.L.R., Miguel C.B., Rodrigues W.F., de Lima Pereira S.A. Expression of il-6, il-10, il-17 and il-33 in the peri-implant crevicular fluid of patients with peri-implant mucositis and peri-implantitis. Arch. Oral Biol. 2016;72:194–199. doi: 10.1016/j.archoralbio.2016.08.021. [DOI] [PubMed] [Google Scholar]
- Steinsvoll S., Halstensen T.S., Schenck K. Extensive expression of TGF-b1 in chronically-inflamed periodontal tissue. J. Clin. Periodontol. 1999;26:366–373. doi: 10.1034/j.1600-051x.1999.260606.x. [DOI] [PubMed] [Google Scholar]
- Taira M., Kagiya T., Harada H., Sasaki M., Kimura S., Narushima T., Nezu T., Araki Y. Microscopic observations and inflammatory cytokine productions of human macrophage phagocytising submicron titanium particles. J. Mater. Sci. – Mater. Med. 2010;21:267–275. doi: 10.1007/s10856-009-3834-x. [DOI] [PubMed] [Google Scholar]
- Tawse-Smith A., Ma S., Duncan W.J., Gray A., Reid M.R., Rich A.M. Implications of wear at the titanium-zirconia implant-abutment interface on the health of peri-implant tissues. Int. J. Oral Maxillofac. Implants. 2017;32:599–609. doi: 10.11607/jomi.5014. [DOI] [PubMed] [Google Scholar]
- Tawse-Smith A., Ma S., Siddiqi A., Duncan W.J., Girvan L., Hussaini H.M. Titanium particles in peri-implant tissues: Surface analysis and histologic response. Clin. Adv. Periodontics. 2012;2:232–238. [Google Scholar]
- Valderrama P., Wilson T.G. Detoxification of implant surfaces affected by peri-implant disease: an overview of surgical methods. Int. J. Dent. 2014;16:77–84. doi: 10.1155/2013/740680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese F.B.A., Malhotra R., De A. IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T.G., Valderrama P., Burbano M., Blansett J., Levine R., Kessler H., Rodrigues D.C. Foreign bodies associated with peri-implantitis human biopsies. J. Periodontol. 2015;86:9–15. doi: 10.1902/jop.2014.140363. [DOI] [PubMed] [Google Scholar]
- Yang S.-Y., Zhang K., Bai L., Song Z., Yu H., McQueen D.A., Wooley P.H. Polymethylmethacrylate and titanium alloy particles activate peripheral monocytes during periprosthetic inflammation and osteolysis. J. Orthop. Res. 2011;29:781–786. doi: 10.1002/jor.21287. [DOI] [PubMed] [Google Scholar]
- Zhang K., Jia T.-H., McQueen D., Gong W.-M., Markel D., Wooley P., Yang S.-Y. Circulating blood monocytes traffic to and participate in the periprosthetic tissue inflammation. Inflamm. Res. 2009;58:837–844. doi: 10.1007/s00011-009-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.S., Revell P.A. In situ localization of aptotic changes in the interface membrane of aseptically loosened orthopedic implants. J. Mater. Sci. – Mater. Med. 1999;10:879–883. doi: 10.1023/a:1008937419664. [DOI] [PubMed] [Google Scholar]
- Zitzmann N.U., Berglundh T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008;35:286–291. doi: 10.1111/j.1600-051X.2008.01274.x. [DOI] [PubMed] [Google Scholar]