Abstract
Objectives
This study compared the effects of normal salivary pH, and acidic pH found in patients with poor oral hygiene, on the durability of aesthetic archwire coated with epoxy resin and polytetrafluoroethylene (PTFE).
Methods
The posterior parts of the archwires were sectioned into 20 mm segments (N = 102) and divided among six groups. Four groups were treated with different pH levels and two served as controls. The specimens were immersed in individual test tubes containing 10 ml of artificial saliva adjusted to a pH of 6.75 or 3.5. The tubes were sealed and stored in a 37 °C water bath for 28 days. After 28 days, the specimens were ligated to brackets embedded in an acrylic block and subjected to mechanical stress using an electronic toothbrush for 210 s. The specimens were photographed, and images were measured for coating loss using AutoCAD® software. Surface morphology was observed using a scanning electron microscope (SEM).
Results
Significant coating loss (p < 0.001) was found in the epoxy resin groups, regardless of pH value, but not in the PTFE groups. The acidic pH caused epoxy resin layer coating loss by twice as much as normal pH. SEM revealed existing manufacturing defects on the as-received epoxy resin coating, whereas the retrieved epoxy resin demonstrated rupture, roughness, and coating loss in multiple locations.
Significance
Epoxy resin coatings demonstrate poor durability in acidic environments. This condition is worsened by the existing manufacturing defects found on as-received archwires. Hence, archwires coated with epoxy resin are not recommended in patients with poor oral hygiene.
Keywords: Dental material, Epoxy resin, Polytetrafluoroethylene, Coating durability, Salivary pH, Coating material, Aesthetic archwire
Abbreviations: PTFE, polytetrafluoroethylene; SENiTi, superelastic nickel titanium; NaOH, sodium hydroxide; SEM, scanning electron microscope; ICC, intraclass correlation coefficient
1. Introduction
Fixed appliances are known to cause plaque accumulation and retention. Orthodontic patients with poor oral hygiene tend to have more acidic salivary pH as low as 3.5. Consequently, these patients can experience a higher release of nickel ions sufficient to cause allergic reactions (Kuhta et al., 2009). Acidic salivary pH can also cause increased surface roughness in certain orthodontic materials (Escobar et al., 2015).
A new generation of orthodontic archwires that are coated with tooth-coloured polymers such as polytetrafluoroethylene (PTFE), epoxy resin, parylene-polymer, or—less commonly—silver-palladium have become increasingly popular because these materials address several aesthetic concerns expressed by orthodontic patients. The coating processes take place using one of two methods: (1) through surface treatment of the core archwire to support coating adhesion, followed by spraying of the atomised particles using compressed air to coat the archwire, which is then placed in the kiln for heat treatment; or (2) mixing of epoxide using high-voltage electrostatic coating technology which is applied to the core archwire; an opposite charge is applied to the epoxy resin, followed by the atomised liquid epoxy particles, which are sprayed onto the wire and placed in the kiln for heat treatment (Kravitz, 2013).
Clinically, commercially-available coated archwires have demonstrated partial or full coating loss after as few as 21 to 28 days of intraoral exposure (Argalji et al., 2017, Ulhaq et al., 2017). These losses are noticeable and leave patients feeling dissatisfied (Bradley et al., 2013). Coating losses are also related to the depletion of nickel and titanium ions and the appearance of extra elements which occur due to interaction with saliva (Zegan et al., 2012). The coated archwires are routinely destroyed after three weeks of intraoral exposure as a result of oral enzymatic activity and chewing forces (Lim et al., 1994, Kusy, 2002).
These coated archwires are still marketed by the manufacturers and are used clinically by orthodontists, despite the unfavourable results reported from various studies with regards to coating durability and surface changes, as well as the lack of scientific information to provide a clear understanding of their mechanical properties and vital interactions (Elayyan et al., 2010). It is suggested that this phenomenon occurs because of an interaction that exists between the coated archwires and the surrounding oral environment such as salivary pH, enzymes, bacteria, and others. However, no studies have investigated the effects of salivary pH on coated archwires.
The purpose of this study was to compare the effect of normal and acidic salivary pH, found in patients with poor oral hygiene, on archwire coating durability. We also observed the surface morphology of the coated aesthetic archwires.
2. Materials and methods
The sample size was calculated using PS software (Vanderbilt University, Tennessee, USA) (Dupont and Plummer, 1997) based on a two-mean comparison formula. The power was set at 80% and the alpha (α) was set at 0.05. (Elayyan et al., 2008)Based on a standard deviation of 0.25, we required 17 archwire specimens in each group (da Silva et al., 2013). Therefore, a total of 102 archwire specimens (N = 102) were required for these study objectives.
2.1. Preparation of archwire specimen
Superelastic nickel-titanium (SE NiTi) archwires, 0.018 in. in diameter, which were readily coated with epoxy resin (OrthoForce® Ultraesthetic™, G&H Orthodontics®, Franklin, USA) and PTFE (Euroform™ Cosmetic, Ortho-Care LTD, Yorkshire, UK) were used. The archwires were measured using a digital calliper, and the posterior parts were sectioned into smaller, 20 mm specimens using orthodontic archwire cutter.
2.2. Preparation of treatment solution
Artificial saliva (Biotene®, GlaxoSmithKline, Victoria, Australia) with pH levels of 6.75 and 3.5 were prepared using 10 M sodium hydroxide (NaOH) and lactic acid (Sigma-Aldrich®, Kuala Lumpur, Malaysia), as described by Kuhta et al. (2009). The actual pH values were measured using a pH meter (827 pH Lab, Metrohm, Herisau, Switzerland).
2.3. Treatment of archwire specimen
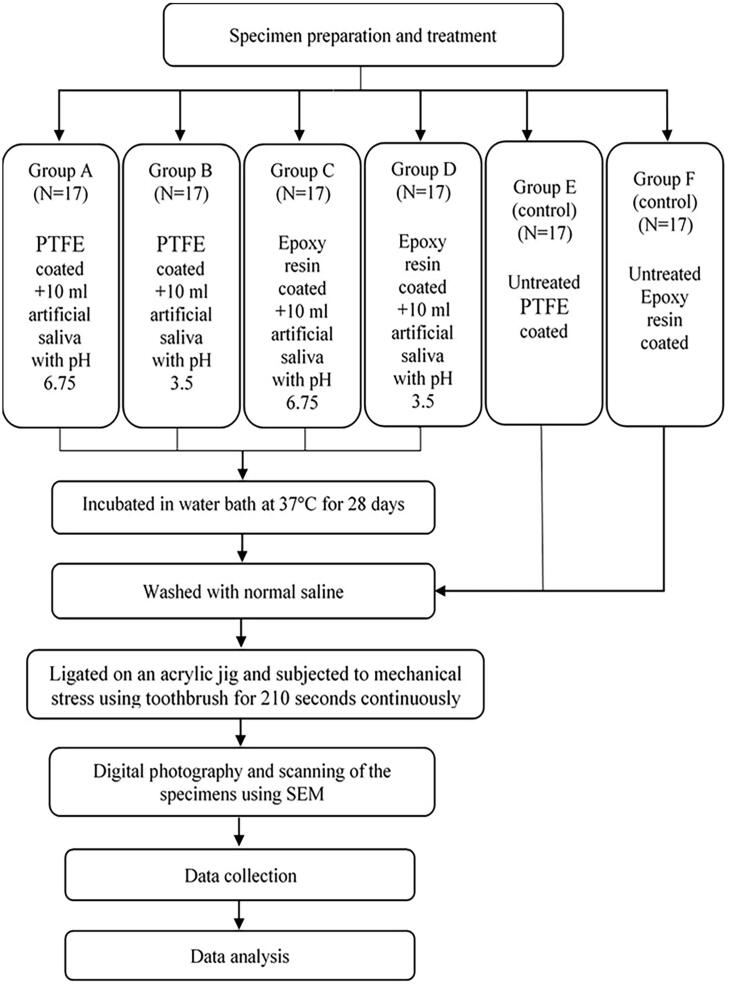
Seventeen archwire specimens of each type were immersed in individual test tubes containing 10 ml artificial saliva of pH 6.75 or 3.5. Each test tube was incubated in a water bath at 37 °C for 28 days. At the end of the incubation period, all archwire specimens were collected and washed with normal saline before being subjected to tooth brushing. The archwire specimens for the control groups were washed with normal saline and ligated to acrylic jigs to receive the mechanical stress from tooth brushing. The flow chart of the experiment is presented in Fig. 1.
Fig. 1.
Flow chart of the experiment.
2.4. Toothbrushing force
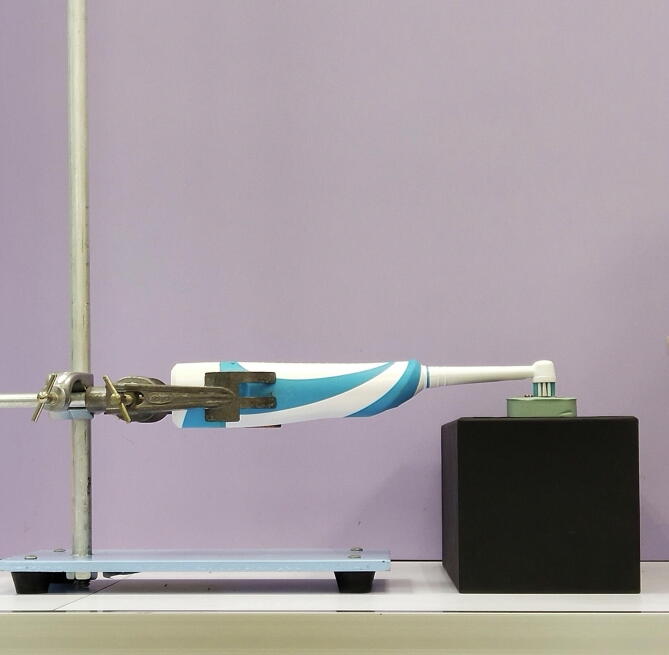
Prior to tooth brushing, an acrylic block of 25 mm width × 35 mm length × 10 mm thickness was prepared with two orthodontic brackets embedded on each end, at the distance of 10 mm (Fig. 2). Each specimen was tied to the orthodontic brackets embedded on an acrylic block using an elastomeric module. The experimental region was continuously subjected to mechanical stress from an electronic toothbrush for 210 s (Fig. 3). The tooth-brushing duration was determined based on the manufacturer's recommendation of 120 s per brushing, twice daily. For 28 days of the experiment, by assuming that the tooth brushing took place twice daily for 120 s per session, the total duration of brushing became 6720 s. The total duration of 6720 s was divided by 32 (considering all 32 permanent teeth have erupted) to obtain an estimated duration for a single tooth. This gave a total of 210 s of tooth brushing per tooth. As the distance of the experimental region was 10 mm, which represented an average width for a maxillary central incisor, 210 s of tooth brushing was deemed sufficient.
Fig. 2.
Archwire specimen ligated on brackets embedded in an acrylic block.
Fig. 3.
Toothbrushing setup used in this study.
2.5. Archwire photography
Each specimen was photographed using a digital single-lens reflex (DSLR) camera with a 60 mm macro lens using a standardised intraoral photography set up: aperture set at f/36, shutter speed at 1/200, and 0.1 m camera-to-specimen distance (set using the manual lens focus).
2.6. Reliability of measurements
The first examiner was trained and calibrated before the actual measurement took place. Ten archwire specimens were randomly selected. The measurement was repeated after a two-week interval under the same setup. This measurement was also compared to the measurement made by the second examiner.
2.7. Data collection
Photographed images were uploaded to Autodesk AutoCAD® software, version 2018 (Autodesk Inc, California, USA) and coating loss measurements were recorded as percentages. Scanning electron microscope (SEM) images were obtained at ×200 magnification.
2.8. Statistical analyses
Data analysis was carried out using IBM Statistical Package for the Social Sciences (SPSS) version 22.0 (IBM Corporation, New York, USA). The reliability of the measurements was calculated using the Intraclass Correlation Coefficient (ICC) test. The normality of the data distribution was calculated using the Shapiro-Wilk test, which showed that the data were not normally distributed (p < 0.001). Therefore, the Kruskal-Wallis test was used to compare the coating loss amongst the groups, followed by multiple pairwise comparisons using Dunn's test.
3. Results
3.1. Coating loss
The ICC test showed excellent agreement for both intra- and inter-examiner reliability (ICC = 0.993, 0.952). Comparison of coating loss in all groups revealed that the epoxy resin group treated with pH 3.5 showed the highest percentage of coating loss (48.1% ± 23.55). The Kruskal-Wallis test indicated that there was a statistically significant difference between the groups (p < 0.001) (Table 1).
Table 1.
The percentage of coating loss between six groups using Kruskal-Wallis test.
| Group (N = 17) | Mean ± SD | Median ± IQRa | 95% CI |
χ2 statistic (df)b | P valueb | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Epoxy resin pH 3.5 | 48.1 ± 23.55 | – | 36.0 | 60.2 | 75.38 (5) | <0.001 |
| Epoxy resin pH 6.75 | 31.1 ± 32.21 | 23.0 ± 62.62 | 14.6 | 47.7 | ||
| Epoxy resin (control) | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 | 0.0 | ||
| PTFE pH 3.5 | 0.2 ± 0.50 | 0.0 ± 0.00 | −0.1 | 0.4 | ||
| PTFE pH 6.75 | 0.5 ± 1.23 | 0.0 ± 0.00 | −0.1 | 1.1 | ||
| PTFE (control) | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 | 0.0 | ||
The distribution is skewed to the left.
Kruskal-Wallis test.
Multiple pairwise comparisons (Table 2) revealed that the epoxy resin group treated with pH 3.5 showed a statistically significant difference (p < 0.001) compared to other groups, except the same coating polymer treated with pH 6.75. The same result was observed in the epoxy resin group treated with pH 6.75, which showed a significant difference when compared to other groups. The raw data are presented in Table 3.
Table 2.
Post hoc comparison using Dunn’s test. Mean differences shown.
| Epoxy Resin pH 3.5 | Epoxy resin pH 6.75 | Epoxy resin (control) | PTFE pH 3.5 | PTFE pH 6.75 | PTFE (control) | |
|---|---|---|---|---|---|---|
| Epoxy resin pH 3.5 | – | 1.000 | <0.001* | <0.001* | <0.001* | <0.001* |
| Epoxy resin pH 6.75 | – | <0.001* | <0.001* | 0.001* | <0.001* | |
| Epoxy resin (control) | – | 1.000 | 1.000 | 1.000 | ||
| PTFE pH 3.5 | – | 1.000 | 1.000 | |||
| PTFE pH 6.75 | – | 1.000 | ||||
| PTFE (control) | – |
Indicates the mean difference is significant at 0.05 level. Groups in vertical column considered as significant groups.
Table 3.
Raw data showing the percentage of coating loss for each specimen.
| Specimen number | Percentage of coating loss |
|||||
|---|---|---|---|---|---|---|
| Epoxy resin pH 3.5 | Epoxy resin pH 6.75 | Epoxy resin (control) | PTFE pH 3.5 | PTFE pH 6.75 | PTFE (control) | |
| 1 | 59.27 | 6.19 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2 | 60.83 | 5.04 | 0.00 | 0.00 | 0.00 | 0.00 |
| 3 | 65.76 | 94.12 | 0.00 | 0.00 | 0.00 | 0.00 |
| 4 | 58.60 | 11.61 | 0.00 | 0.00 | 0.00 | 0.00 |
| 5 | 90.86 | 70.60 | 0.00 | 1.79 | 0.00 | 0.00 |
| 6 | 32.33 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 7 | 55.70 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 8 | 54.19 | 40.74 | 0.00 | 0.00 | 1.41 | 0.00 |
| 9 | 54.82 | 77.78 | 0.00 | 0.00 | 0.00 | 0.00 |
| 10 | 15.71 | 4.55 | 0.00 | 0.00 | 0.00 | 0.00 |
| 11 | 19.37 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 12 | 79.77 | 28.30 | 0.00 | 1.18 | 0.00 | 0.00 |
| 13 | 21.65 | 71.81 | 0.00 | 0.00 | 0.00 | 0.00 |
| 14 | 22.81 | 36.26 | 0.00 | 0.00 | 0.00 | 0.00 |
| 15 | 28.90 | 23.02 | 0.00 | 0.00 | 2.99 | 0.00 |
| 16 | 74.66 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 17 | 22.45 | 59.18 | 0.00 | 0.00 | 4.21 | 0.00 |
3.2. Surface morphology
The epoxy resin group treated with pH 3.5 showed remarkable coating loss while the group treated with pH 6.75 showed noticeable change to the surface morphology, as evidenced by the presence of cracks and ruptures (Fig. 4, Fig. 5). Localized abrasion caused by friction from the toothbrush was observed in the epoxy resin control group (Fig. 6). Also, Fig. 7 showed existing manufacturing defects found on the as-received archwire coated with epoxy resin.
Fig. 4.
Coating loss observed on the epoxy resin coating exposed to the acidic environment.
Fig. 5.
Presence of cracks and rupture on the epoxy resin coating exposed to a normal pH.
Fig. 6.
Localized abrasion caused by friction from the toothbrush was observed in the epoxy resin control group.
Fig. 7.
Existing manufacturing defects found on the as-received epoxy resin coating.
4. Discussion
Retained plaque around the fixed appliances is readily converted to acid by the intraoral bacteria. This acid production alters the normal salivary pH, exerting some negative effects on the properties and performance of orthodontic brackets and auxiliaries, including coated aesthetic archwires. Low pH exposure has been shown to cause surface corrosion on the NiTi-based archwires, regardless of their percentage composition and often exacerbated by the presence of fluoride (Perinetti et al., 2010, Močnik et al., 2017).
The epoxy resin coating was unstable in all groups, including control. This result was supported by previous studies (Iijima et al., 2011, Zegan et al., 2012, da Silva et al., 2013) which also reported coating instability on NiTi wires. This instability was manifest as coating loss, roughness, and tearing. There was also unwanted exposure of the core NiTi wire in multiple locations, which resulted from severe damage to the epoxy resin layer. Such exposures are clinically undesirable as they exposes patients to allergic reactions. In addition, metal ions released from orthodontic appliances could have carcinogenic, mutagenic, and cytotoxic effects (Kuhta et al., 2009). Besides, these exposures also cause undesirable aesthetic effects such as discolouration and ruptures that appear when it is used clinically (Ulhaq et al., 2017).
One study reported rupture and contraction on the epoxy layer after being subjected to a three-point bending test (Alavi and Hosseini, 2012). This is in line with a study conducted ex vivo, which found that the surfaces of NiTi archwires coated with epoxy resin showed roughness, changes in colour, and coating layer rupture with a loss of 25% of the coating layer along the retrieved specimens after four to six weeks in the mouth (Elayyan et al., 2008). The SEM images showed remarkable changes in the epoxy resin coating layer, which differed according to the type of treatment. The loss of the coating layer occurred at a greater rate in acidic, as opposed to alkaline, media. Also, the presence of cracks represented the deterioration of the external coating. Swelling and the emergence of bubbles were greater in the groups treated with pH 6.75. This is likely to occur because of the existing manufacturing defects spotted on the as-received archwire as confirmed by the SEM (Fig. 5). Kim and Johnson (1999), however, reported that the epoxy resin coated archwires exhibited low corrosion potential and recommended it to be used in patients with a nickel allergy.
Nonetheless, the result may not reflect the actual intraoral condition as the experiment was carried out using 0.9% sodium choride (NaCl) immersion solution with neutral pH 7.0. The existence of manufacturing defects within as-received polymer coated archwire surfaces [Esthetic Superelastic Titanium Memory Wire and EverWhite (American Ortho, Wisconsin, USA)] has also been reported previously (Rongo et al., 2013). Another as-received archwire [Imagination (Gestenco International, Gothenburg, Sweden)] reportedly exhibited manufacturing anomalies that manifest as deep dimples that reached the metal base and exposed the core archwire (Shamohammadi et al., 2019). However, the types of coating polymer of the three archwires above were not mentioned by the authors.
These microscopic holes found on the as-received archwire surface allow external factors to reach the interface between the coating and the core archwire. This subsequently causes decomposition of the binding factors between the two surfaces. This can be explained by a finding from a study which reported that the systematic destruction of the epoxy resin coating layer could be related to the hydrophilic property of resins (Kang et al., 2017). When the epoxy resin is immersed in a solution, it absorbs the water and begins to crack before losing the epoxy layer after bulging (Li and Xue, 2016). The joint interactions of water and corrosive liquids (corrosive liquids can burn and destroy body tissues on contact, including hydrochloric acid, sulphuric acid, ammonium hydroxide, and sodium hydroxide) expands the material and causes changes. The material is soft and smooth at first, but after a period of immersion, it becomes coarser, especially in solutions that contain NaOH, which cause the dissolution of the material. Meanwhile, acids enhance the occurrence of cracking (Amaro et al., 2013). Temperature is also a catalyst for these changes. Water absorption of the epoxy was reported to increase at 30 °C (Ahmad et al., 2010), causing faster deterioration of the surface of the archwire.
In contrast, the PTFE coating showed greater stability than epoxy resin coating in all groups due to its hydrophobic properties (Kravitz, 2013). Relative roughness was observed on the outer surface of the wire in the other treatments. This finding is supported by another study which reported that the PTFE coating had less impact on the mechanical properties of the archwire and was more stable than the epoxy resin and a silver and platinum coating polymer composite (Ryu et al., 2015). An electrochemical characteristic study of PTFE coated NiTi orthodontic wires also supported the finding that the PTFE coated archwires, which were immersed in artificial saliva under a low pH (pH 4), demonstrated lower corrosion rates and passive current densities than uncoated NiTi substrates because of electrolyte penetration in the pore of the PTFE deposits (Mareci et al., 2015). However, this is inconsistent with a previous study that reported that the PTFE coating had poor stability due to thinner coating layer of the as-received PTFE coated aesthetic archwires than what was reported by the manufacturer (Argalji et al., 2017). Another study also reported that PTFE coated archwires showed the highest surface roughness after 28 days of immersion in artificial saliva with pH 6.75 when compared to epoxy resin (Muayad and Ghaib, 2017). The interaction between PTFE and different acids varies in intensity and behaviour depending on the factors such the type of acid, the manufacturing process, and the thickness of the coating layer (Giorgini et al., 2016). Different manufacturing companies and pH solutions reportedly affect the corrosion rate, breakdown potential, and crevice‐corrosion susceptibility of NiTi archwires; however, these factors did not correspond with surface roughness and pre‐existing defects (Huang, 2005). Kim & Johnson (1999) also reported similar findings in which they recognized that the breakdown potential of nickel titanium alloy wires varied depending on the manufacturer. This suggests that manufacturer-specific variations in the composition of the coating polymers also influence the physical properties of coated archwires.
This deterioration and coating loss exposes the core metal wire, causing absorption of large amounts of hydrogen because the titanium attracts hydrogen. This results in a gradual change in mechanical properties (Yokoyama et al., 2003). Retrieved epoxy resin coated NiTi archwires that are used intraorally for four to six weeks have zero unloading forces, which are caused by the accumulation of damaged coating layer and increases the friction to that area, thereby prolonging treatment and delaying the tooth movement (Elayyan et al., 2008).
Because of observed effects between the coated wires and brackets, it is better to avoid the use of coated archwires during orthodontic treatment stages where sliding movement is important. The unwanted exposure of the core NiTi wire in multiple locations, which results from severe damage to the epoxy resin layer, is clinically undesirable as it exposes patients to allergic reactions. In addition, metal ions released from orthodontic appliances could have carcinogenic, mutagenic, and cytotoxic effects (Kuhta et al., 2009). These exposures also cause undesirable aesthetic effects such as discolouration and ruptures that appear when the archwires are used clinically (Ulhaq et al., 2017).
We did not study the mechanical properties of the archwires because of limitations in time. Another study challenge involved finding an advanced tooth brushing simulator which could be digitally controlled. This problem was rectified by using an electronic toothbrush, as described earlier. Further studies should examine factors that cause these changes in order to achieve a full understanding of the aetiology of this problem, as faced by orthodontists, manufacturers and patients alike.
5. Conclusion
The aesthetic archwires coated with the PTFE polymers demonstrated stable and durable coatings, whereas the aesthetic archwires coated with the epoxy resin showed undesirable surface changes under normal and acidic salivary pH. These surface changes were influenced by the presence of manufacturing defects found within the as-received archwires. Therefore, careful consideration should be given when selecting a patient’s optimal aesthetic archwire.
Declaration of Competing Interest
None.
Acknowledgements
We would like to acknowledge Madam Zuraizah Zulkifli for her help in the fabrication of the acrylic block and the other IPPT nurses who assisted in this study. This work was supported by a USM Short Term Grant (304/CIPPT/6315069).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Youssef Chikh Abdulkader, Email: josiph.syria@gmail.com.
Anis F. Kamaruddin, Email: anisfarhan@usm.my.
Rabiatul Basria S.M.N. Mydin, Email: rabiatulbasria@usm.my.
References
- Ahmad Z., Ansell M.P., Smedley D. Effect of nano-and micro-particle additions on moisture absorption in thixotropic room temperature cure epoxy-based adhesives for bonded-in timber connections. Int. J. Adhes. Adhes. 2010;30:448–455. [Google Scholar]
- Alavi S., Hosseini N. Load-deflection and surface properties of coated and conventional superelastic orthodontic archwires in conventional and metal-insert ceramic brackets. Dental Res. J. 2012;9:133. doi: 10.4103/1735-3327.95225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaro A., Reis P., Neto M., Louro C. Effects of alkaline and acid solutions on glass/epoxy composites. Polym. Degrad. Stab. 2013;98:853–862. [Google Scholar]
- Argalji N., Silva E.M.D., Cury-Saramago A., Mattos C.T. Characterization and coating stability evaluation of nickel-titanium orthodontic esthetic wires: an in vivo study. Braz. Dent. J. 2017:31. doi: 10.1590/1807-3107BOR-2017.vol31.0068. [DOI] [PubMed] [Google Scholar]
- Bradley T.G., Berzins D.W., Valeri N., Pruszynski J., Eliades T., Katsaros C. An investigation into the mechanical and aesthetic properties of new generation coated nickel-titanium wires in the as-received state and after clinical use. Eur. J. Orthod. 2013;36:290–296. doi: 10.1093/ejo/cjt048. [DOI] [PubMed] [Google Scholar]
- da Silva D.L., Mattos C.T., Simão R.A., De Oliveira Ruellas A.C. Coating stability and surface characteristics of esthetic orthodontic coated archwires. Angle Orthodontist. 2013;83:994–1001. doi: 10.2319/111112-866.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont W.D., Plummer W.J. PS power and sample size program available for free on the Internet. Control. Clin. Trials. 1997;18 274 274. [Google Scholar]
- Elayyan F., Silikas N., Bearn D. Ex vivo surface and mechanical properties of coated orthodontic archwires. Eur. J. Orthod. 2008;30:661–667. doi: 10.1093/ejo/cjn057. [DOI] [PubMed] [Google Scholar]
- Elayyan F., Silikas N., Bearn D. Mechanical properties of coated superelastic archwires in conventional and self-ligating orthodontic brackets. Am. J. Orthod. Dentofacial Orthop. 2010;137:213–217. doi: 10.1016/j.ajodo.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Escobar C.G.N., Domingues J.A., Gomes J.C., Cohelo U. Effects of diferent salivary ph on the surface and roughness of different orthodontic wires. J. Res. Dent. 2015;2:527–536. [Google Scholar]
- Giorgini L., Fragassa C., Zattini G., Pavlovic A. Acid aging effects on surfaces of PTFE gaskets investigated by fourier transform infrared spectroscopy. Tribol. Indus. 2016;38:286–296. [Google Scholar]
- Huang H.H. Surface characterizations and corrosion resistance of nickel–titanium orthodontic archwires in artificial saliva of various degrees of acidity. J. Biomed. Mater. Res. Part A: Off. J. Soc. Biomat., Japanese Soc. Biomater., Australian Soc. Biomater. Korean Soc. Biomater. 2005;74:629–639. doi: 10.1002/jbm.a.30340. [DOI] [PubMed] [Google Scholar]
- Iijima M., Muguruma T., Brantley W., Choe H.-C., Nakagaki S., Alapati S.B., Mizoguchi I. Effect of coating on properties of esthetic orthodontic nickel-titanium wires. Angle Orthod. 2011;82:319–325. doi: 10.2319/021511-112.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang W.-S., Rhee K.Y., Park S.-J. Influence of surface energetics of graphene oxide on fracture toughness of epoxy nanocomposites. Compos. B Eng. 2017;114:175–183. [Google Scholar]
- Kim H., Johnson J.W. Corrosion of stainless steel, nickel-titanium, coated nickel-titanium, and titanium orthodontic wires. Angle Orthod. 1999;69:39–44. doi: 10.1043/0003-3219(1999)069<0039:COSSNT>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- Kravitz, N.D. 2013. Aesthetic archwire [Online]. Available: http://www.orthodonticproductsonline.com/2013/05/aesthetic-archwires/ [Accessed].
- Kuhta M., Pavlin D., Slaj M., Varga S., Lapter-Varga M., Slaj M. Type of archwire and level of acidity: effects on the release of metal ions from orthodontic appliances. Angle Orthod. 2009;79:102–110. doi: 10.2319/083007-401.1. [DOI] [PubMed] [Google Scholar]
- Kusy R.P. Orthodontic biomaterials: from the past to the present. Angle Orthod. 2002;72:501–512. doi: 10.1043/0003-3219(2002)072<0501:OBFTPT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Li Y., Xue B. Hydrothermal ageing mechanisms of unidirectional flax fabric reinforced epoxy composites. Polym. Degrad. Stab. 2016;126:144–158. [Google Scholar]
- Lim K., Lew K., Toh S. Bending stiffness of two aesthetic orthodontic archwires: an in vitro comparative study. Clin. Mater. 1994;16:63–71. doi: 10.1016/0267-6605(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Mareci D., Earar K., Zetu I., Bolat G., Crimu C., Istrate B., Munteanu C., Matei M.N. Comparative electrochemical behaviour, of uncoated and coated Ni Ti, for dental orthodontic wires. Rev. Chim. 2015;52 [Google Scholar]
- Močnik P., Kosec T., Kovač J., Bizjak M. The effect of pH, fluoride and tribocorrosion on the surface properties of dental archwires. Mater. Sci. Eng., C. 2017;78:682–689. doi: 10.1016/j.msec.2017.04.050. [DOI] [PubMed] [Google Scholar]
- Muayad N., Ghaib N.H. The effect of artificial saliva on the surface roughness of different esthetic archwires (An in vitro study) J. Baghdad College Dent. 2017;29:106–112. [Google Scholar]
- Perinetti G., Contardo L., Ceschi M., Antoniolli F., Franchi L., Baccetti T., di Lenarda R. Surface corrosion and fracture resistance of two nickel–titanium-based archwires induced by fluoride, pH, and thermocycling. An in vitro comparative study. Eur. J. Orthod. 2010;34:1–9. doi: 10.1093/ejo/cjq093. [DOI] [PubMed] [Google Scholar]
- Rongo R., Ametrano G., Gloria A., Spagnuolo G., Galeotti A., Paduano S., Valletta R., D'Antò V. Effects of intraoral aging on surface properties of coated nickel-titanium archwires. Angle Orthod. 2013;84:665–672. doi: 10.2319/081213-593.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S.H., Lim B.S., Kwak E.J., Lee G.J., Choi S., Park K.H. Surface ultrastructure and mechanical properties of three different white-coated NiTi archwires. Scanning. 2015;37:414–421. doi: 10.1002/sca.21230. [DOI] [PubMed] [Google Scholar]
- Shamohammadi M., Hormozi E., Moradinezhad M., Moradi M., Skini M., Rakhshan V. Surface topography of plain nickel-titanium (NiTi), as-received aesthetic (coated) NiTi, and aesthetic NiTi archwires sterilized by autoclaving or glutaraldehyde immersion: a profilometry/SEM/AFM study. Int. Orthod. 2019;17:60–72. doi: 10.1016/j.ortho.2019.01.016. [DOI] [PubMed] [Google Scholar]
- Ulhaq A., Esmail Z., Kamaruddin A., Meadows S., Daus J., Vitale M., Perillo L., Sherriff M., Bister D. Alignment efficiency and esthetic performance of 4 coated nickel-titanium archwires in orthodontic patients over 8 weeks: a multicenter randomized clinical trial. Am. J. Orthod. Dentofacial Orthop. 2017;152:744–752. doi: 10.1016/j.ajodo.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Yokoyama K.I., Kaneko K., Moriyama K., Asaoka K., Sakai J.I., Nagumo M. Hydrogen embrittlement of Ni-Ti superelastic alloy in fluoride solution. J. Biomed. Mater. Res. A. 2003;65:182–187. doi: 10.1002/jbm.a.10457. [DOI] [PubMed] [Google Scholar]
- Zegan G., Sodor A., Munteanu C. Surface characteristics of retrieved coated and nickel-titanium orthodontic archwires. Rom. J. Morphol. Embryol. 2012;53:935–939. [PubMed] [Google Scholar]