Abstract
Pretreatment nutritional and immunological status is useful for predicting survival outcomes for various types of malignant tumors. Our objective was to determine the impact of the pretreatment Onodera's prognostic nutritional index (OPNI) on outcomes of patients who underwent definitive chemoradiotherapy for advanced oral squamous cell carcinoma (OSCC). We reviewed 47 patients treated for OSCC with definitive chemoradiotherapy (CRT) at our institution between January 2004 and December 2011. We determined the OPNI according to the following formula: 10 × serum albumin (g/dL) + 0.005 × total lymphocyte count (per μL). We determined the optimum OPNI cut-off through a receiver operating characteristic analysis. We analyzed the associations between OPNI status and various clinicopathological features and evaluated the effects of OPNI on the prognosis. We examined the relationships between OPNI and systemic inflammatory response parameters and analyzed intratumoral CD8+ T cells and their correlation with OPNI. The optimum OPNI cut-off was 42.7. A Kaplan–Meier curve analysis revealed that low OPNI was significantly associated with poor overall survival and cause-specific survival. The multivariate analysis revealed that low OPNI was independently correlated with poor 5 year overall survival and cause-specific survival. OPNI was significantly correlated with systemic inflammatory response parameters. Intratumoral CD8+ T cell counts in primary tumors were significantly lower for low OPNI than for high OPNI. The present data demonstrate that pretreatment OPNI is a valuable independent prognostic indicator of overall and cause-specific survival in advanced OSCC following definitive CRT. OPNI might reflect the tumor immune microenvironment characterization in OSCC.
Abbreviations: CRT, chemoradiotherapy; CSS, cause-specific survival; HNSCC, head and neck squamous cell carcinoma; LMR, lymphocyte–monocyte ratio; NLR, neutrophil–lymphocyte ratio; OPNI, Onodera's prognostic nutritional index; OS, overall survival; OSCC, oral squamous cell carcinoma; PFS, progression-free survival; PLR, platelet–lymphocyte ratio; RECIST, Response Evaluation Criteria In Solid Tumors; ROC, receiver operating characteristic; SIR, systemic inflammatory response; TIL, tumor-infiltrating lymphocyte.
Keywords: Oral squamous cell carcinoma, Onodera's prognostic nutritional index, Chemoradiotherapy, prognosis, Tumor immune environment
Highlights
-
•
The optimum Onodera's prognostic nutritional index (OPNI) cut-off was 42.7 in OSCC patients who underwent definitive chemoradiotherapy
-
•
Low OPNI was significantly associated with poor overall survival and cause-specific survival
-
•
OPNI was significantly correlated with systemic inflammatory response parameters
-
•
High-OPNI group showed significantly higher intratumoral CD8+ T cell counts in primary tumors
Introduction
Oral squamous cell carcinoma (OSCC) is the most common malignant tumor in the oral cavity and can threaten quality of life and survival [1]. Advanced OSCC is typically treated with multimodal therapy including surgery, radiotherapy, and chemotherapy [2]. Definitive chemoradiotherapy (CRT) is one of the most effective treatment options for patients with unresectable tumors [2]. We recently reported that CRT with S-1, an oral fluoropyrimidine anticancer agent, is an acceptable treatment option for advanced OSCC when compared with standard CRT in terms of prognosis and safety [3]. To further improve patient outcomes, however, it is imperative to identify useful prognostic markers that can predict the efficacy and outcomes of CRT among patients with advanced OSCC.
The Onodera prognostic nutritional index (OPNI) is an indicator calculated from serum albumin and total lymphocyte counts in peripheral blood, which can be utilized to evaluate patients' nutritional and immune status [4]. Although OPNI was initially regarded as an indicator of postoperative complications in patients with gastrointestinal cancer [4], recent studies have demonstrated a relationship between OPNI and outcomes for patients with cancer [[5], [6], [7]]. The clinical significance of OPNI for surgical cases with head and neck squamous cell carcinoma (HNSCC) (including OSCC) has been reported by several authors [8,9]; however, the prognostic value of OPNI for patients with advanced OSCC who undergo definitive CRT has not been fully elucidated.
Increasing evidence has shown that cancer-related inflammation, in the form of local and systemic inflammatory response (SIR), is a key factor in disease progression and survival for several types of cancer [10]. Easily measurable parameters of SIR include the neutrophil–lymphocyte ratio (NLR), the platelet–lymphocyte ratio (PLR), and the lymphocyte–monocyte ratio (LMR) [11]. On the other hand, CD8+ cell infiltration in tumors plays an important role in the local antitumor immune response [12], and the status of tumor-infiltrating CD8+ cells is an important index of the immune response to cancer and has prognostic, pharmacodynamic, and predictive potential [13]. Although OPNI is considered to be closely related to SIR and tumor-infiltrating CD8+ cells in terms of the calculation method, there have been no reports analyzing these factors in detail in OSCC.
The aim of this study was to determine the impact of pretreatment OPNI on the outcomes of patients who have undergone CRT for advanced OSCC and elucidate the correlation between OPNI and the local and systemic inflammatory response, with a special focus on parameters related to SIR and tumor-infiltrating CD8+ cells.
Materials and methods
Patients and tissue specimens
The study included 47 patients with advanced OSCC treated with definitive CRT (total radiation dose of 60 or 70 Gy) at the Kumamoto University Hospital between January 2004 and December 2011. The patients were not initially treated surgically due to technically and/or medically unresectable disease. We excluded patients with factors that could affect the OPNI and SIR parameters, such as concurrent infection, chronic inflammatory disease, recent steroid therapy. The clinical stage (according to the Union for International Cancer Control and American Joint Committee on Cancer criteria) was identified at a meeting of oral surgeons, radiologists, and radiation oncologists who interpreted the imaging data. The radiological diagnosis of nodal involvement was based on widely accepted morphological criteria [14]. All tumors were staged according to the TNM classification of the Union for International Cancer Control (2002), and the degree of differentiation was determined based on the World Health Organization's classification. All patients underwent definitive CRT, with a total dose of 60–70 Gy delivered in 2 Gy fractions using two to four fields. S-1 (Taiho Pharmaceutical, Tokyo, Japan) was concurrently administered (80 mg/m2/day) for 14 consecutive days followed by a 1 week drug-free period or on the days of irradiation (65 mg/m2/day) [[4], [5], [6]]. Elective nodal irradiation included the tumor extension and levels I and II, even in cN0 necks; clinically positive as well as equivocal node levels were added in the fields. Thereafter, boost irradiation was delivered to the primary tumor and clinically positive nodes. The maximum dose to the spinal cord did not exceed 40 Gy during the two courses of CRT. This study was performed with the approval of the Ethics Committee of Kumamoto University (approval number 174 and RINRI1427) and in accordance with the Good Clinical Practice and the Declaration of Helsinki guidelines. The current study followed the guidelines of the Ethics Committee of Kumamoto University. As the present study is a retrospective analysis, individual written consent is not required; however, the opportunity to refuse participation is guaranteed in an opt-out format (RINRI1427).
Follow-up
Patients underwent hematologic tests and symptom assessments every 2 weeks. The presence of recurrence was determined by means of imaging modalities, including computed tomography, magnetic resonance imagining, ultrasound, and positron emission tomography–computed tomography. The patients underwent at least one type of imaging examination at 3–4 month intervals for the first 2 years and at 4–6 month intervals thereafter until 5 years after CRT.
Nutritional assessment
Serum albumin levels and total lymphocyte counts measured at pretreatment and post-treatment were used to calculate OPNI using the following equation: 10 × serum albumin (g/dL) + 0.005 × total lymphocyte count (per μL). Pretreatment OPNI was calculated using laboratory data before administering CRT, whereas post-treatment OPNI was calculated at 1–1.5 months after the end of treatment. We generated receiver operating characteristic (ROC) curves for the multiple logistic regression analysis, using 5 year overall survival (OS) as the endpoint, thereby determining an optimal OPNI cut-off. Patients were then assigned to either a high OPNI or a low OPNI group.
Assessment of SIR parameters
Before administering CRT, blood samples were collected for routine laboratory analysis of full blood count, neutrophil count, platelet count, monocyte count, and lymphocyte count. We determined NLR by dividing the absolute neutrophil count by the absolute lymphocyte count, the PLR by dividing the absolute platelet count by the absolute lymphocyte count, and the LMR by dividing the absolute lymphocyte count by the absolute monocyte count.
Double-immunohistochemical staining and histopathological evaluation
We utilized paraffin-embedded tumor tissue samples to analyze intratumoral CD8+ T cell tumor infiltration, along with two mouse monoclonal antibodies, CD8 (C8/144B; Dako, Glostrup, Denmark) and cytokeratin (AE1/AE3; Dako). Two observers blinded to all information regarding the samples evaluated the CD8+ cell infiltration and averaged the results. For double immunostaining, sections were first reacted with anti-CD8 antibodies and visualized using the DAB system (Nichirei, Tokyo, Japan). The resulting antibodies were washed in glycine buffer (pH 2.2); sections were then reacted with anticytokeratin antibody and visualized with HistoGreen solution (Linaris Biologische Produkte, Wertheim-Bettingen, Germany).
Statistical analysis
We utilized the chi-squared test to determine the association between pretreatment OPNI and the clinical and pathological variables. We defined OS, progression-free survival (PFS), and cause-specific survival (CSS) as the time from treatment initiation (CRT) to the date of death from any cause, the date of tumor recurrence and the date of death from OSCC, respectively. We utilized the Kaplan–Meier method to estimate the probability of OS, PFS, and CSS as a function of time and compared the statistical differences in survival for the patient groups using the log-rank test. We performed a multivariate survival analysis using the Cox regression model to study the effects of pretreatment OPNI on OS and CSS. We utilized scatter plots to observe the associations between pretreatment OPNI and the SIR parameters or tumor-infiltrating cells and investigated the relationships between these parameters with Pearson's correlation coefficient test. All p-values were based on two-tailed statistical analyses, and p-values of <0.05 were considered statistically significant (*p < .05 and ** p < .01). The statistical analyses were completed using JMP 9 software (SAS Institute Inc., Cary, NC).
Results
OPNI changes at pretreatment and post-treatment
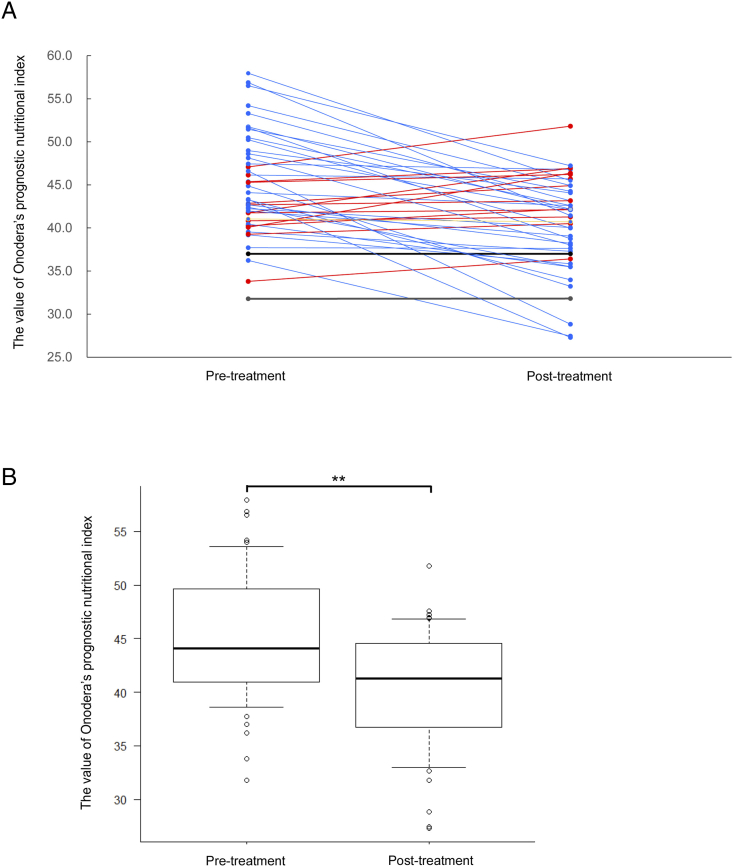
To elucidate OPNI changes in patients with OSCC who underwent CRT, pretreatment and post-treatment OPNI values were compared. As shown in Fig. 1, OPNI values decreased in 33 (70.2%), increased in 12 (25.5%), and were stable in two patients (4.3%; Fig. 1A). Therefore, the post-treatment OPNI values were significantly lower than the pretreatment ones (Fig. 1B, p < .01).
Fig. 1.
OPNI changes at pretreatment and post-treatment.
(A) OPNI changes at pretreatment and post-treatment in each case. The red line indicates “increased” OPNI at post-treatment. The blue line indicates “decreased” OPNI at post-treatment. The black line indicates “no change” at post-treatment.
(B) Box plot showing the OPNI at pretreatment and post-treatment. Differences in mean values between the groups were statistically analyzed using Mann–Whitney's U test. **, p < .01.
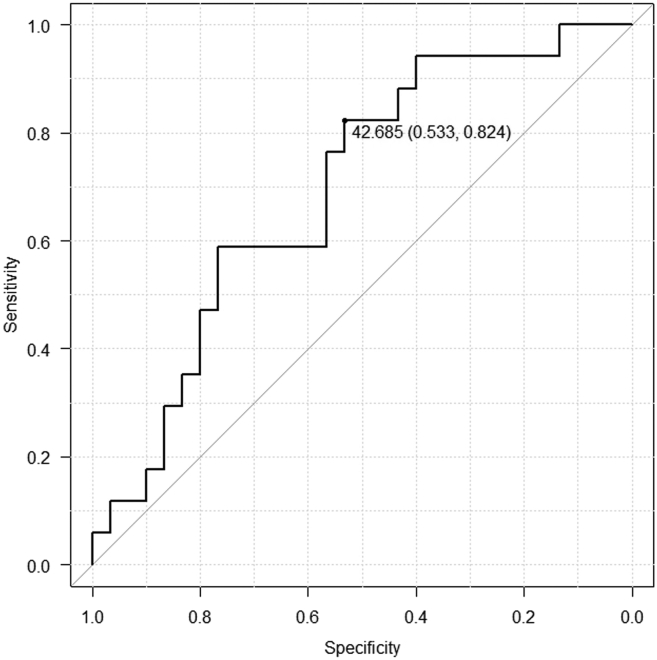
ROC curve
The mean OPNI was 45.2 (range, 31.8–58.0). To determine the OPNI cut-off for further study, we investigated the cut-off by using ROC. The OPNI ranged from 31.8 to 58.0 (mean of 44.1), and the area under the ROC curve in the multiple logistic regression analysis (with 5 year OS as the endpoint) was 0.692. For an OPNI of 42.685, the projected 5 year survival was optimal (sensitivity 0.533; specificity 0.824) (Fig. 1). This value was therefore adopted as the cut-off, stratifying the patients as low OPNI (OPNI ≤42.685) or high OPNI (OPNI >42.685). (See Fig 2.)
Fig. 2.
Receiver operating characteristic (ROC) curve analysis for the Onodera's prognostic nutritional index (OPNI).
In the ROC analysis, an OPNI cut-off was determined to predict overall patient survival.
Relationship between the pretreatment OPNI and clinicopathological characteristics
To determine the clinical significance of the pretreatment OPNI of patients with OSCC treated with 5-FU-based CRT, we examined the correlations between OPNI and the clinicopathological variables. Table 1 shows the distribution of the clinical background characteristics of the study patients divided into the two groups (low OPNI and high OPNI). There were no differences in OPNI according to age, sex, primary tumor site, T stage, N stage, clinical stage, differentiation, Worst pattern of invasion, or the Response Evaluation Criteria in Solid Tumors (RECIST).
Table 1.
Correlation between the OPNI status and clinicopathological factors in 47 patients with OSCC.
| Characteristics | Total | OPNI status |
p-Value | |
|---|---|---|---|---|
| Low |
High |
|||
| n (%) | n (%) | |||
| Age(years) | ||||
| Median | 79 | 72.8 | 76.9 | |
| Range | 45–90 | 45–86 | 53–90 | |
| ≤65 | 10 | 5 (50.0) | 5 (50.0) | 0.391 |
| >65 | 37 | 13 (35.1) | 24 (64.9) | |
| Gender | ||||
| Male | 23 | 9 (39.1) | 14 (60.9) | 0.908 |
| Female | 24 | 9 (37.5) | 15 (62.5) | |
| Primary site | ||||
| Tongue | 16 | 8 (50.0) | 8 (50.0) | 0.743 |
| Mandible | 12 | 3 (25.0) | 9 (75.0) | |
| Maxilla | 7 | 3 (42.9) | 4 (57.1) | |
| Oral floor | 4 | 2 (50.0) | 2 (50.0) | |
| Buccal mucosa | 4 | 1 (25.0) | 3 (75.0) | |
| Palate | 4 | 1 (25.0) | 3 (75.0) | |
| cT-stage | ||||
| T1, T2 | 6 | 3 (50.0) | 3 (50.0) | 0.808 |
| T3 | 13 | 5 (38.5) | 8 (61.5) | |
| T4 | 28 | 10 (35.7) | 18 (64.3) | |
| cN-stage | ||||
| N0 | 13 | 7 (53.8) | 6 (46.2) | 0.377 |
| N1, 2b | 20 | 6 (30.0) | 14 (70.0) | |
| N2c | 14 | 5 (35.7) | 9 (64.3) | |
| cStage | ||||
| III | 8 | 4 (50.0) | 4 (50.0) | 0.358 |
| IV | 39 | 14 (35.9) | 25 (64.1) | |
| Differentiation | ||||
| Poor, Moderate | 33 | 10 (30.3) | 23 (69.7) | 0.083 |
| Well | 14 | 8 (57.1) | 6 (42.9) | |
| WPOI | ||||
| 1, 2 | 7 | 2 (28.6) | 5 (71.4) | 0.757 |
| 3 | 36 | 12 (33.3) | 24 (66.7) | |
| 4,5 | 4 | 2 (50.0) | 2 (50.0) | |
| RECISTa | ||||
| NC | 14 | 4 (28.6) | 10 (71.4) | 0.319 |
| PR | 18 | 8 (44.4) | 10 (55.6) | |
| CR | 8 | 3 (37.5) | 5 (62.5) | |
Abbreviation: OSCC: Oral squamous cell carcinoma. OPNI: Onodera's prognostic nutritional index. WPOI: Worst pattern of invasion. RECIST: Response Evaluation Criteria in Solid Tumors. CR: Complete response. PR: Partial response. NC: No change.
Seven patients could not evaluate the response to CRT. Chi-square test was used to examine the relationships between OPNI status and clinicopathologic factors.
Relationship between the pretreatment OPNI and survival time
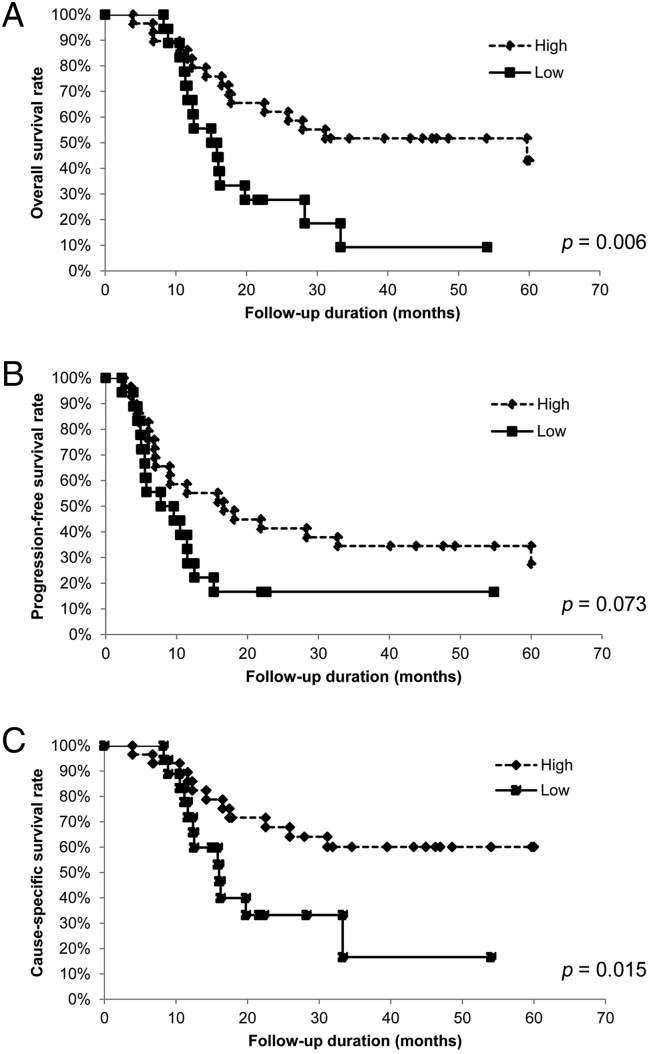
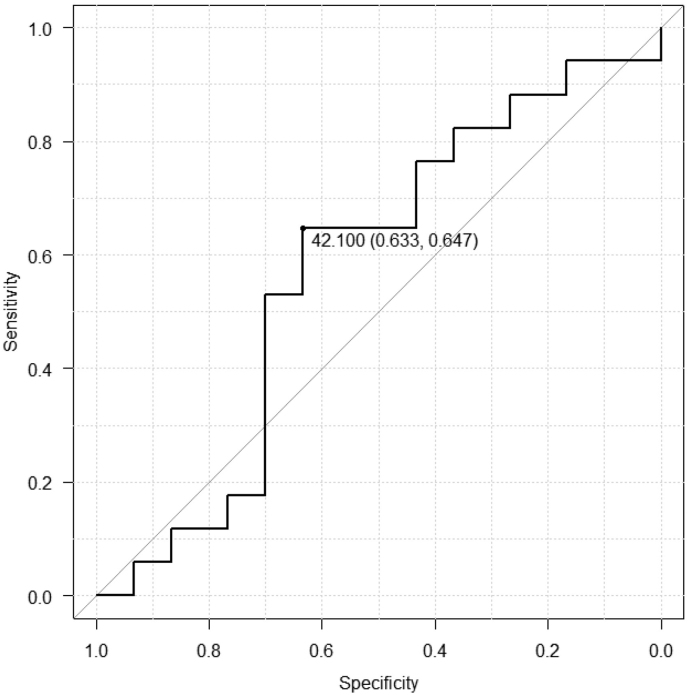
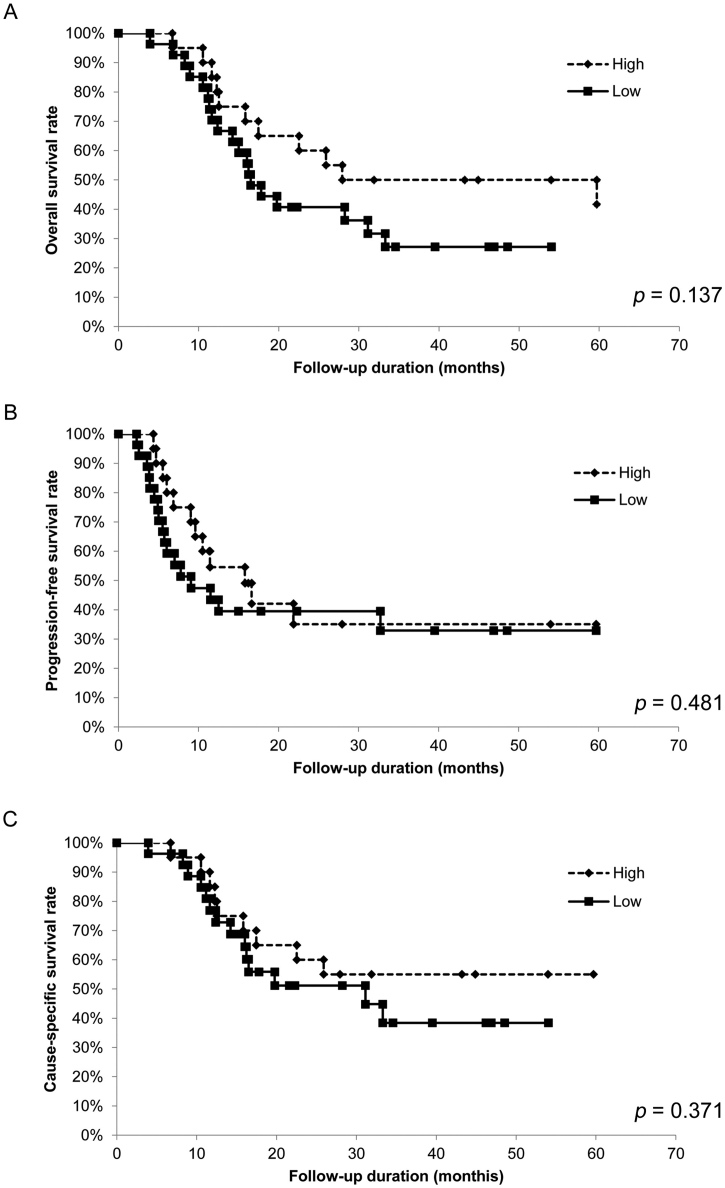
To assess the relationship between pretreatment OPNI and survival time, we analyzed the OS, PFS, and CSS of the 47 patients with OSCC using the Kaplan–Meier method. The 5-year OS rates were significantly lower in patients with low OPNI values than in those with high OPNI values (p = .006; Fig. 3A). The 5-year CSS rates were also significantly lower in patients with low OPNI values than in those with high OPNI values (p = .015; Fig. 3B). Although the 5-year PFS rate tended to be lower in patients with low OPNI values, the difference was not statistically significant (p = .073; Fig. 3C). Conversely, whether post-treatment OPNI values could be considered as a predictor of patient prognosis was also examined, no significant difference was observed (Supplementary Fig. S2, Supplementary Fig. S3). Collectively, our data indicated that pretreatment OPNI values could be a potential prognostic factor for patients with OSCC undergoing CRT.
Fig. 3.
Relationship between OPNI and survival for patients with oral squamous cell carcinoma.
In the Kaplan–Meier survival analysis of patients with oral squamous cell carcinoma (OSCC), the patients were divided into two groups based on OPNI (low and high).
(A) Overall survival (OS) of the 47 patients with OSCC based on OPNI. *, p < .05.
(B) Progression-free survival (PFS) of the 47 patients with OSCC based on OPNI. *, p < .05.
(B) Cause-specific survival (CSS) of the 47 patients with OSCC based on OPNI. *, p < .05.
Supplementary Fig. S2.
Receiver operating characteristic (ROC) curve analysis for the post-treatment Onodera's prognostic nutritional index (OPNI).
In the ROC analysis, an OPNI cut-off was determined to predict overall patient survival.
Supplementary Fig. S3.
Relationship between post-treatment OPNI and survival for patients with oral squamous cell carcinoma.
In the Kaplan–Meier survival analysis of patients with oral squamous cell carcinoma (OSCC), the patients were divided into two groups based on post-treatment OPNI (low and high).
(A) Overall survival (OS) of the 47 patients with OSCC based on OPNI.
(B) Progression-free survival (PFS) of the 47 patients with OSCC based on OPNI.
(B) Cause-specific survival (CSS) of the 47 patients with OSCC based on OPNI.
Univariate and multivariate analysis of prognostic factors
To determine the independent prognostic value of pretreatment OPNI for OS and CSS, we performed a univariate and multivariate analysis using a Cox proportional hazards regression model. After adjusting for age, sex, primary site, T stage, N stage, poorest pattern of invasion, and RECIST, the influence of OPNI on OS (hazard ratio, 3.567; 95% CI 1.527–8.617; p = .003) and CSS (hazard ratio, 23.752; 95% CI 1.468–9.965; p = .006) remained. RECIST was also a significant prognostic factor in OS (hazard ratio, 8.960; 95% CI 1.630–54.684; p = .011) and CSS (hazard ratio, 17.410; 95% CI 2.502–145.69; p = .004) (Table 2, Table 3).
Table 2.
The results of a univariate regression analysis for predicting the survival of 47 patients with OSCC.
| Variables | OS |
CSS |
PFS |
|||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p-Value | Hazard ratio (95% CI) | p-Value | Hazard ratio (95% CI) | p-Value | |
| Age, years | ||||||
| ≤65 | 1.534 (0.605–3.429) | 0.344 | 2.086 (0.800–4.903) | 0.982 | 1.014 (0.32–3.69) | 0.982 |
| >65 | ||||||
| Gender | ||||||
| Male | 1.113 (0.539–2.301) | 0.770 | 0.592 (0.17–1.82) | 0.364 | 0.592 (0.17–1.82) | 0.364 |
| Female | ||||||
| T stage | ||||||
| T1 | 0.777 (0.220–3.308) | 0.715 | 7.003 (1.84–32.5) | 0.004⁎⁎ | 7.003 (1.84–32.5) | 0.004⁎⁎ |
| T2 | ||||||
| T3 | ||||||
| T4 | ||||||
| N stage | ||||||
| N0 | 1.350 (0.533–3.621) | 0.532 | 5.844 (1.24–36.1) | 0.024⁎ | 5.844 (1.24–36.1) | 0.024⁎ |
| N1 | ||||||
| N2b | ||||||
| N2c | ||||||
| Primary site | ||||||
| Tongue | 0.108 (0.020–0.444) | 0.001⁎⁎ | 3.135 (0.61–17.4) | 0.169 | 3.135 (0.61–17.4) | 0.169 |
| Mandible | ||||||
| Maxilla | ||||||
| Oral floor | ||||||
| Buccal mucosa | ||||||
| Patate | ||||||
| WPOI | ||||||
| 1 | 0.608 (0.128–3.469) | 0.561 | 1.262 (0.36–4.08) | 0.707 | 1.262 (0.36–4.08) | 0.707 |
| 2 | ||||||
| 3 | ||||||
| 4 | ||||||
| RECIST | ||||||
| CR | 5.702 (1.329–27.079) | 0.019⁎ | 0.101 (0.02–0.38) | <0.001⁎⁎ | 0.101 (0.02–0.38) | <0.001⁎⁎ |
| PR | ||||||
| NC | ||||||
| OPNI status | ||||||
| High | 2.825 (1.327–6.060) | 0.007⁎⁎ | 3.308 (1.08–11.6) | 0.035⁎ | 3.308 (1.08–11.6) | 0.035⁎ |
| Low | ||||||
Abbreviation: OSCC: Oral squamous cell carcinoma. WPOI: Worst pattern of invasion. RECIST: Response Evaluation Criteria in Solid Tumors. CR: Complete response. PR: Partial response. NC: No change. OPNI: Onodera's prognostic nutritional index. CI: confidence interval. OS: overall survival. CSS: cause-specific survival. PFS: progression-free survival.
p < .05.
p < .01.
Table 3.
The results of a multivariate regression analysis for predicting the survival of 47 patients with OSCC.
| Variables | OS |
CSS |
||
|---|---|---|---|---|
| Multivariate analysis |
Multivariate analysis |
|||
| Hazard ratio (95% CI) | p-Value | Hazard ratio (95% CI) | p-Value | |
| Primary site | ||||
| Tongue, Mandible, Maxilla, | 0.184 (0.029–0.921) | 0.039⁎ | ||
| Oral floor, Baccul mucosa, Palate | ||||
| RECIST | ||||
| CR, PR, NC | 8.960 (1.630–54.684) | 0.011⁎ | 17.410 (2.502–145.690) | 0.004⁎⁎ |
| OPNI | ||||
| High, Low | 3.567 (1.527–8.617) | 0.003⁎⁎ | 3.752 (1.468–9.965) | 0.006⁎⁎ |
Abbreviation: OSCC: Oral squamous cell carcinoma. WPOI: Worst pattern of invasion. RECIST: Response Evaluation Criteria in Solid Tumors. CR: Complete response. PR: Partial response. NC: No change. OPNI: Onodera's prognostic nutrittional index. CI: confidence interval. OS: overall survival. CSS: cause-specific survival.
p < .05.
p < .01.
Relationship between the pretreatment OPNI and the SIR parameter
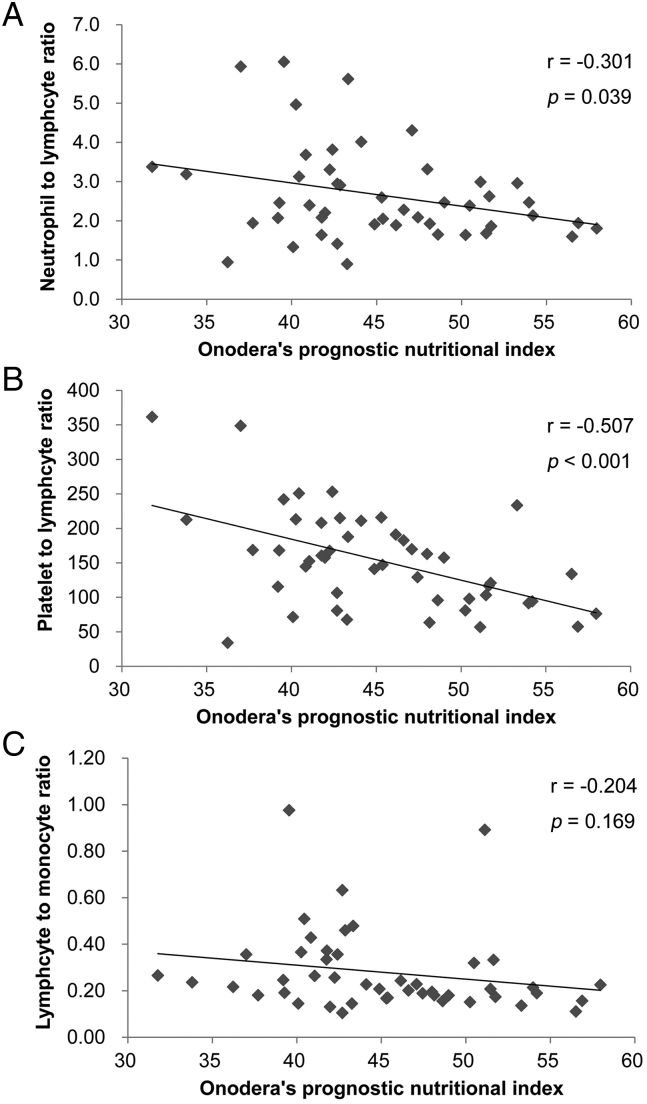
To determine the relationship between pretreatment OPNI and SIR parameters, we examined the correlations between OPNI and the major parameters of SIR: NLR, PLR, and LMR. In the Pearson correlation coefficient test, pretreatment OPNI was significantly associated with NLR (r = −0.301, p = .039; Fig. 4A) and PLR (r = −0.507, p < .001; Fig. 4B) but not with LMR (r = −0.204, p = .169; Fig. 4C).
Fig. 4.
Relationship between OPNI and systemic inflammatory response parameters in the 47 patients with OSCC.
Scatter plots of the OPNI and systemic inflammatory response indicators.
(A) Relationship between OPNI and neutrophil–lymphocyte ratio (NLR).
The x-axis indicates the OPNI, and the y-axis shows the NLR. The correlation was investigated using Pearson's correlation coefficient test. *, p < .05.
(B) Relationship between the OPNI and platelet–lymphocyte ratio (PLR).
The x-axis indicates the OPNI, and the y-axis shows the PLR. The correlation was investigated using Pearson's correlation coefficient test. *, p < .05.
(C) Relationship between the OPNI and lymphocyte–monocyte ratio (LMR).
The x-axis indicates the OPNI, and the y-axis shows the LMR. The correlation was investigated using Pearson's correlation coefficient test. *, p < .05.
Relationship between intratumoral CD8+ T cells and the pretreatment OPNI
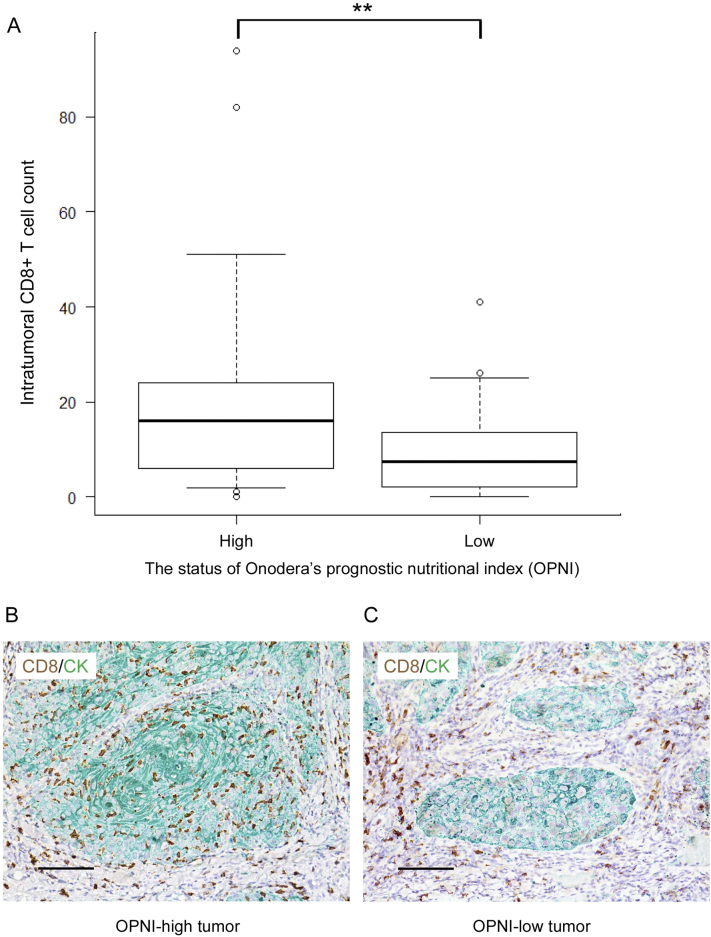
To explore the potential relationships between OPNI and tumor-infiltrating lymphocyte (TIL) counts in patients with OSCC, we measured the intratumoral CD8+ T cell count in pretreatment OSCC tissues using immunohistochemistry and examined the correlation between the TIL counts and OPNI. In the Pearson correlation coefficient test, TIL count was significantly associated with OPNI (r = 0.32, p = .028; Fig. 5A). As shown in Fig. 5B and C, TIL counts were higher in patients with high OPNI values than in those with low OPNI values.
Fig. 5.
Relationship between the OPNI and intratumoral CD8+ cell counts in the 47 patients with OSCC.
(A) Box plot showing the number of intratumoral CD8+ cells according to OPNI status. The differences in mean values between the two groups were statistically analyzed using the Mann–Whitney's U test. **, p < .01.
(B) Representative photographs of the results of double-immunohistochemical staining of CD8 (brown) and cytokeratin (green) in OPNI-high tumor. Original magnification: ×400, scale bar = 50 μm.
(C) Representative photographs of the results of double-immunohistochemical staining of CD8 (brown) and cytokeratin (green) in OPNI-low tumor. Original magnification: ×400, scale bar = 50 μm.
Discussion
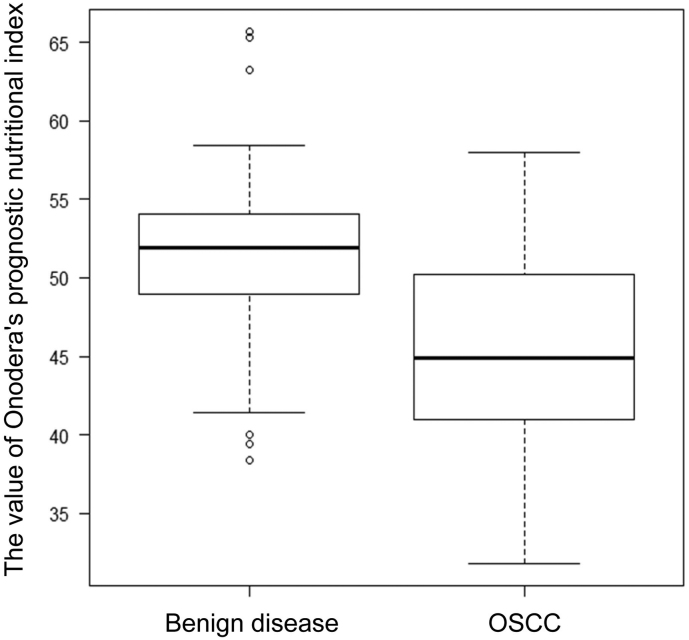
OPNI was originally intended for assessing the perioperative nutritional and immunological status and postoperative complications of patients with colorectal cancer [15]. OPNI changes during treatment are generally considered to decrease with nutritional status and laboratory data deterioration due to treatment. Recently, Arribas et al. [16] reported that OPNI changes were observed in almost all patients who received chemo- or bio-radiotherapy for HNSCC. The present data are in line with that of a previous report, suggesting the importance of monitoring the immuno-nutritional status during chemoradiotherapy in patients with OSCC. Originally, the optimal cut-off for OPNI was 40 for predicting a high risk of postoperative complications [15]. However, OPNI has been shown to be a prognostic marker in various malignancies, including colon [6], stomach [5], and pancreatic cancers [7]. Previous studies have generally set the cut-off OPNI for various types of malignancies at 45, because an OPNI <45 has been regarded as malnutrition and correlated to the risk of postoperative complications [15]. In surgical cases with head and neck cancer including OSCC, Wu et al. reported that the optimal OPNI cut-off was 47.4 [8]. In a large-scale prospective study, Bao et al. reported a cut-off of 49.3, which correlates with poor OS [9]. In the present study, the ROC analysis resulted in an OPNI cut-off of 42.7, which is relatively low compared with previous data. Generally, patients with advanced OSCC often experience odynophagia and dysphagia and can experience chronic fatigue, which increases the risk of malnutrition [17,18]. The present cohort consisted of patients with advanced disease treated with definitive CRT, for whom it was difficult to apply curative surgery because of their general condition and postoperative dysfunction. Our preliminary study showed that the mean OPNI for the patients with benign disease was 51.7 (Supplementary Fig. S1). Collectively, the differences in patient characteristics in the individual studies might have led to this discrepancy.
Supplementary Fig. S1.
Box plot of OPNI according to disease. Box plot showing the value of OPNI according to disease. Benign disease (n = 50): Median = 51.6, Standard deviation = 5.51. OSCC (n = 47): Median = 45.3, Standard deviation = 6.15. The differences in mean values between the two groups were statistically analyzed using the Mann-Whitney U test. **, p < .01
In the present study, we demonstrated that low OPNI was associated with poor OS and CSS and identified OPNI as an independent prognostic factor for patients with advanced OSCC who undergo definitive CRT. Recently, growing evidence has shown that low OPNI is related to poorer prognoses in various types of cancers [[5], [6], [7]]. In OSCC, Wu et al. evaluated the predictive performance of OPNI and reported that low preoperative OPNI was significantly related to a poor prognosis and serves as a novel prognostic biomarker [8]. Similarly, Bao et al. reported the predictive value of OPNI, along with other nutritional indicators such as body mass index, serum albumin, and the nutritional risk index [9]. In terms of radiotherapy and chemoradiotherapy, the clinical significance of OPNI for patients undergoing chemotherapy and surgical resection has been reported in esophagus, breast, urinary bladder, and cervical cancers [[19], [20], [21], [22]]. However, there have been few studies that have described the prognostic significance of OPNI for patients with head and neck cancer who undergo radiotherapy or chemoradiotherapy [23]. Although Bruixola et al. reported that OPNI is an independent prognostic factor in locoregionally advanced squamous cell head and neck cancer who undergo chemoradiotherapy following induction chemotherapy [24]; to the best of our knowledge, our study is the first to report the prognostic value of OPNI in patients with OSCC who undergo definitive CRT. The results of the present study are also in line with current evidence, suggesting that OPNI could be useful for guiding treatment decisions for patients with OSCC undergoing chemotherapy and/or radiotherapy.
Our present findings show that OPNI is correlated with inflammatory response parameters. Studies have shown that the local inflammatory response and SIR in tumors suppress the antitumor immunity and contribute to tumor progression [25,26]. The pre-existing state of the tumor microenvironment established by SIR might determine the response to anticancer therapy in several types of cancer [27]. Indeed, studies have reported that various SIR parameters are correlated with treatment response in various malignant tumors [[28], [29], [30]]. Our previous study on OSCC also demonstrated that the pretreatment NLR status was correlated to poor prognoses and the pathological response to preoperative CRT [31]. The present data and current evidence suggest that OPNI could reflect the SIR status, which affects the treatment response and prognosis for OSCC, as well as for other malignancies. As Bruixola et al. pointed out in their study [24], OPNI is considered to be more reproducible, inexpensive, and universally available compared with other inflammation-based biomarkers, which are susceptible to external factors such as comorbidity, medication, and infection. OPNI could be a robust biomarker, with good internal and external validity, thereby providing reliable information regarding host antitumor immunity.
We found that OPNI was significantly correlated with intratumoral CD8+ cell counts in primary tumors. Recently, several researchers have demonstrated the significance of TILs as a prognostic factor in malignant tumors such as breast, colon, esophagus, stomach, and head and neck cancers [[32], [33], [34], [35], [36], [37]]. Studies have reported that pretreatment intratumoral CD8+ cells in primary tumors have a favorable therapeutic effect in chemoradiotherapy, even in preoperative settings [38,39]. In HNSCC carcinoma, Balermpas et al. reported that CD8+ TILs have antitumor activity and a prognostic value for patients who undergo postoperative chemoradiotherapy [40]. Several reports have suggested that SIR reflects the local tumor immunity of patients with cancer [41,42]. In particular, various inflammatory cytokines from cancer cells activate the neutrophil proliferation and activity, suppress lymphocytes, and increase the degradation of proteins including albumin [43]. These phenomena are therefore considered to result in a decrease in the OPNI. Taken together, the previous data and the present findings indicate that OPNI could reflect the local antitumor immunity status and the SIR of patients with advanced OSCC. OPNI might also be a convenient marker of local tumor immunity prior to treatment and could be a useful indicator for treatment selection. To the best of our knowledge, our study is the first to elucidate the relationship between the SIR and local tumor immunity in patients with OSCC who undergo definitive chemoradiotherapy.
Tabachnyk et al. demonstrated that tumor-infiltrating CD8+ and granzyme B+ cytotoxic cell counts only slightly decreased compared with other tumor-infiltrating inflammatory cells after neoadjuvant chemoradiotherapy. The authors therefore suggested that CRT provides a favorable antitumor immune environment for patients with residual tumors in HNSCC carcinoma [44]. Recently, Lee et al. [45] reported that patients with high OPNI had a higher CR rate after the primary treatment for follicular lymphoma. In locally advanced esophageal cancer, pretreatment nutritional status was significantly associated with the clinical response and survival of patients who underwent definitive CRT [46]. Therefore, although OPNI was not significantly correlated with any clinicopathological factor, including RECIST, favorable local antitumor immunity at pretreatment could be further enhanced by CRT and could contribute to long-term tumor control, resulting in favorable prognoses. In addition, recent meta-analysis shows the favorable effect of nutritional intervention on the outcomes of chemo(radio)therapy [47]. From this perspective, nutrition interventions might not only reduce treatment complications but could also improve outcomes, as mentioned by Prieto et al. also in OSCC [48].
The present study has a several limitations. First, the number of cases was small, and more cases are needed to clarify the clinical significance of OPNI in curative chemoradiotherapy. Second, the present data were obtained from patients who were treated with 5-FU-based chemoradiotherapy. Further investigation is needed to determine whether our results can be applied to all patients with OSCC (e.g., those treated with cisplatin-based CRT). Despite these limitations, our findings suggest that OPNI is a useful prognostic marker for patients with OSCC who undergo CRT. OPNI could also reflect the antitumor immunity status of the local tumor, which can be measured easily and can be a useful indicator for determining treatment options and indications for nutritional interventions, thereby contributing to improved prognoses.
Conclusions
Pretreatment OPNI is a valuable independent prognostic indicator of OS and CSS for patients with advanced OSCC following definitive CRT. OPNI might also reflect the tumor immune microenvironment characterization in primary OSCC tumors.
The following are the supplementary data related to this article.
Funding
The author(s) declare the receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a Grant-in-Aid for Scientific Research (C) (18K09771) from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
CRediT authorship contribution statement
Ryoji Yoshida: Conceptualization, Writing - original draft. Shunsuke Gohara: Investigation, Formal analysis. Junki Sakata: Investigation, Formal analysis. Yuichiro Matsuoka: Formal analysis, Writing - original draft. Akiyuki Hirosue: Data curation, Validation. Kenta Kawahara: Validation, Writing - review & editing. Sho Kawaguchi: Investigation, Resources. Yuka Nagao: Investigation, Resources. Keisuke Yamana: Investigation, Resources. Masashi Nagata: Validation, Data curation. Daiki Fukuma: Data curation, Validation. Ryo Toya: Resources, Writing - review & editing. Ryuji Murakami: Resources, Writing - review & editing. Akimitsu Hiraki: Supervision, Resources. Masanori Shinohara: Project administration. Hideki Nakayama: Supervision, Resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for the English language review.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J. Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Gore S.M., Crombie A.K., Batstone M.D., Clark J.R. Concurrent chemoradiotherapy compared with surgery and adjuvant radiotherapy for oral cavity squamous cell carcinoma. Head & neck. 2015;37(4):518–523. doi: 10.1002/hed.23626. [DOI] [PubMed] [Google Scholar]
- 3.Murakami R., Semba A., Kawahara K., Matsuyama K., Hiraki A., Nagata M. Concurrent chemoradiotherapy with S-1 in patients with stage III-IV oral squamous cell carcinoma: A retrospective analysis of nodal classification based on the neck node level. Molecular and clinical oncology. 2017;7(1):140–144. doi: 10.3892/mco.2017.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onodera T., Goseki N., Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–1005. [PubMed] [Google Scholar]
- 5.Migita K., Takayama T., Saeki K., Matsumoto S., Wakatsuki K., Enomoto K. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann. Surg. Oncol. 2013;20(8):2647–2654. doi: 10.1245/s10434-013-2926-5. [DOI] [PubMed] [Google Scholar]
- 6.Mohri Y., Inoue Y., Tanaka K., Hiro J., Uchida K., Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J. Surg. 2013;37(11):2688–2692. doi: 10.1007/s00268-013-2156-9. [DOI] [PubMed] [Google Scholar]
- 7.Kanda M., Fujii T., Kodera Y., Nagai S., Takeda S., Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br. J. Surg. 2011;98(2):268–274. doi: 10.1002/bjs.7305. [DOI] [PubMed] [Google Scholar]
- 8.Wu X., Jiang Y., Ge H., Diao P., Wang D., Wang Y. Predictive value of prognostic nutritional index in patients with oral squamous cell carcinoma. Oral Dis. 2020;26(5):903–911. doi: 10.1111/odi.13318. [DOI] [PubMed] [Google Scholar]
- 9.Bao X., Liu F., Lin J., Chen Q., Chen L., Chen F. Nutritional assessment and prognosis of oral cancer patients: A large-scale prospective study. BMC Cancer. 2020;20(1):146. doi: 10.1186/s12885-020-6604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diakos C.I., Charles K.A., McMillan D.C., Clarke S.J. Cancer-related inflammation and treatment effectiveness. The Lancet Oncology. 2014;15(11):e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 11.Dolan R.D., Lim J., McSorley S.T., Horgan P.G., McMillan D.C. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: Systematic review and meta-analysis. Sci. Rep. 2017;7(1):16717. doi: 10.1038/s41598-017-16955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spranger S., Gajewski T.F. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat. Rev. Cancer. 2018;18(3):139–147. doi: 10.1038/nrc.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steele K.E., Tan T.H., Korn R., Dacosta K., Brown C., Kuziora M. Measuring multiple parameters of CD8+ tumor-infiltrating lymphocytes in human cancers by image analysis. Journal for immunotherapy of cancer. 2018;6(1):20. doi: 10.1186/s40425-018-0326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murakami R., Uozumi H., Hirai T., Nishimura R., Shiraishi S., Ota K. Impact of FDG-PET/CT imaging on nodal staging for head-and-neck squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:377–382. doi: 10.1016/j.ijrobp.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 15.Onodera T., Goseki N., Kosaki G. Prognostic nutrient index in gastrointestinal surgery of malnutrished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–1005. [PubMed] [Google Scholar]
- 16.Arribas L., Hurtos L., Taberna M., Peiro I., Vilajosana E., Lozano A. Nutritional changes in patients with locally advanced head and neck cancer during treatment. Oral Oncol. 2017;71:67–74. doi: 10.1016/j.oraloncology.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Righini C.A., Timi N., Junet P., Bertolo A., Reyt E., Atallah I. Assessment of nutritional status at the time of diagnosis in patients treated for head and neck cancer. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2013;130(1):8–14. doi: 10.1016/j.anorl.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Kono T., Sakamoto K., Shinden S., Ogawa K. Pre-therapeutic nutritional assessment for predicting severe adverse events in patients with head and neck cancer treated by radiotherapy. Clin. Nutr. 2017;36(6):1681–1685. doi: 10.1016/j.clnu.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Dai Y., Fu X., Li T., Yao Q., Su L., Su H. Long-term impact of prognostic nutritional index in cervical esophageal squamous cell carcinoma patients undergoing definitive radiotherapy. Annals of translational medicine. 2019;7(8):175. doi: 10.21037/atm.2019.03.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haraga J., Nakamura K., Omichi C., Nishida T., Haruma T., Kusumoto T. Pretreatment prognostic nutritional index is a significant predictor of prognosis in patients with cervical cancer treated with concurrent chemoradiotherapy. Molecular and clinical oncology. 2016;5(5):567–574. doi: 10.3892/mco.2016.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hua X., Long Z.Q., Huang X., Deng J.P., He Z.Y., Guo L. The value of prognostic nutritional index (PNI) in predicting survival and guiding radiotherapy of patients with T1-2N1 breast Cancer. Front. Oncol. 2019;9:1562. doi: 10.3389/fonc.2019.01562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stangl-Kremser J., D'Andrea D., Vartolomei M., Abufaraj M., Goldner G., Baltzer P. Prognostic value of nutritional indices and body composition parameters including sarcopenia in patients treated with radiotherapy for urothelial carcinoma of the bladder. Urol. Oncol. 2019;37(6):372–379. doi: 10.1016/j.urolonc.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Miao J., Xiao W., Wang L., Han F., Wu H., Deng X. The value of the prognostic nutritional index (PNI) in predicting outcomes and guiding the treatment strategy of nasopharyngeal carcinoma (NPC) patients receiving intensity-modulated radiotherapy (IMRT) with or without chemotherapy. J. Cancer Res. Clin. Oncol. 2017;143(7):1263–1273. doi: 10.1007/s00432-017-2360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruixola G., Caballero J., Papaccio F., Petrillo A., Iranzo A., Civera M. Prognostic nutritional index as an independent prognostic factor in locoregionally advanced squamous cell head and neck cancer. ESMO open. 2018;3(6) doi: 10.1136/esmoopen-2018-000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson P.A., Khatami M., Baglole C.J., Sun J., Harris S.A., Moon E.Y. Environmental immune disruptors, inflammation and cancer risk. Carcinogenesis. 2015;36(Suppl. 1):S232–S253. doi: 10.1093/carcin/bgv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 27.McMillan D.C. Systemic inflammation, nutritional status and survival in patients with cancer. Current opinion in clinical nutrition and metabolic care. 2009;12(3):223–226. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 28.Tong Y.S., Tan J., Zhou X.L., Song Y.Q., Song Y.J. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J. Transl. Med. 2017;15(1):221. doi: 10.1186/s12967-017-1326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirahara T., Arigami T., Yanagita S., Matsushita D., Uchikado Y., Kita Y. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer. 2019;19(1):672. doi: 10.1186/s12885-019-5903-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J.H., Song C., Kang S.B., Lee H.S., Lee K.W., Kim J.S. Predicting pathological complete regression with haematological markers during neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Anticancer Res. 2018;38(12):6905–6910. doi: 10.21873/anticanres.13067. [DOI] [PubMed] [Google Scholar]
- 31.Nakashima H., Matsuoka Y., Yoshida R., Nagata M., Hirosue A., Kawahara K. Pre-treatment neutrophil to lymphocyte ratio predicts the chemoradiotherapy outcome and survival in patients with oral squamous cell carcinoma: A retrospective study. BMC Cancer. 2016;16:41. doi: 10.1186/s12885-016-2079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J.S., Won H.S., Sun S., Hong J.H., Ko Y.H. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: A systematic review and meta-analysis. Medicine. 2018;97(32) doi: 10.1097/MD.0000000000011769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimizu S., Hiratsuka H., Koike K., Tsuchihashi K., Sonoda T., Ogi K. Tumor-infiltrating CD8(+) T-cell density is an independent prognostic marker for oral squamous cell carcinoma. Cancer medicine. 2019;8(1):80–93. doi: 10.1002/cam4.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang D., Liu Y., Wang H., Wang H., Song Q., Sujie A. Tumour infiltrating lymphocytes correlate with improved survival in patients with esophageal squamous cell carcinoma. Sci. Rep. 2017;7:44823. doi: 10.1038/srep44823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang C.Y., Chiang S.F., Ke T.W., Chen T.W., You Y.S., Chen W.T. Clinical significance of programmed death 1 ligand-1 (CD274/PD-L1) and intra-tumoral CD8+ T-cell infiltration in stage II-III colorectal cancer. Sci. Rep. 2018;8(1):15658. doi: 10.1038/s41598-018-33927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eriksen A.C., Sorensen F.B., Lindebjerg J., Hager H. dePont Christensen R, Kjaer-Frifeldt S, et al. the prognostic value of tumor-infiltrating lymphocytes in stage II colon cancer. A nationwide population-based study. Transl. Oncol. 2018;11(4):979–987. doi: 10.1016/j.tranon.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahmoud S.M., Paish E.C., Powe D.G., Macmillan R.D., Grainge M.J., Lee A.H. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. Journal of clinical oncology : official journal of the American society of clinical oncology. 2011;29(15):1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 38.Fassan M., Cavallin F., Guzzardo V., Kotsafti A., Scarpa M., Cagol M. PD-L1 expression, CD8+ and CD4+ lymphocyte rate are predictive of pathological complete response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic esophagus. Cancer medicine. 2019;8(13):6036–6048. doi: 10.1002/cam4.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsutani S., Shibutani M., Maeda K., Nagahara H., Fukuoka T., Nakao S. Significance of tumor-infiltrating lymphocytes before and after neoadjuvant therapy for rectal cancer. Cancer Sci. 2018;109(4):966–979. doi: 10.1111/cas.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balermpas P., Rodel F., Rodel C., Krause M., Linge A., Lohaus F. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: A multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG) Int. J. Cancer. 2016;138(1):171–181. doi: 10.1002/ijc.29683. [DOI] [PubMed] [Google Scholar]
- 41.Canna K., McArdle P.A., McMillan D.C., McNicol A.M., Smith G.W., McKee R.F. The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br. J. Cancer. 2005;92(4):651–654. doi: 10.1038/sj.bjc.6602419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guthrie G.J., Roxburgh C.S., Richards C.H., Horgan P.G., McMillan D.C. Circulating IL-6 concentrations link tumour necrosis and systemic and local inflammatory responses in patients undergoing resection for colorectal cancer. Br. J. Cancer. 2013;109(1):131–137. doi: 10.1038/bjc.2013.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coffelt S.B., Wellenstein M.D., de Visser K.E. Neutrophils in cancer: neutral no more. Nat. Rev. Cancer. 2016;16(7):431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 44.Tabachnyk M., Distel L.V., Buttner M., Grabenbauer G.G., Nkenke E., Fietkau R. Radiochemotherapy induces a favourable tumour infiltrating inflammatory cell profile in head and neck cancer. Oral Oncol. 2012;48(7):594–601. doi: 10.1016/j.oraloncology.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 45.Lee S.F., Ng T.Y. Wong, FCS. The Value of Prognostic Nutritional Index in Follicular LymphomaAmerican journal of clinical oncology. 2019;42(2):202–207. doi: 10.1097/COC.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 46.Song T., Wan Q., Yu W., Li J., Lu S., Xie C. Pretreatment nutritional risk scores and performance status are prognostic factors in esophageal cancer patients treated with definitive chemoradiotherapy. Oncotarget. 2017;8(58):98974–98984. doi: 10.18632/oncotarget.21940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de van der Schueren M.A.E., Laviano A., Blanchard H., Jourdan M., Arends J., Baracos V.E. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: Current evidence and guidance for design of future trials. Annals of oncology : official journal of the European Society for Medical Oncology. 2018;29(5):1141–1153. doi: 10.1093/annonc/mdy114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prieto I., Montemuino S., Luna J., de Torres M.V., Amaya E. The role of immunonutritional support in cancer treatment: Current evidence. Clin. Nutr. 2017;36(6):1457–1464. doi: 10.1016/j.clnu.2016.11.015. [DOI] [PubMed] [Google Scholar]