Abstract
Animal venoms are an almost inexhaustible source for promising molecules with biological activity and the venom of Phoneutria nigriventer spider is a good example of this. Among several other toxins obtained from this venom, PnTx4(6–1), also called δ-Ctenitoxin-Pn1a, was isolated and initially described as an insect toxin that binds to the site 3 of sodium channels in cockroach nerve cord synaptosomes (Periplaneta americana) and slows down sodium current inactivation in isolated axons of this animal. This toxin did not cause any apparent toxicity to mice when intracerebroventricularly injected (30 μg). Subsequently, it was demonstrated that PnTx4(6–1) has an antinociceptive effect in three different pain models: inflammatory, induced by carrageenan; nociceptive, induced by prostaglandin E2 and neuropathic, induced by sciatic nerve constriction. Using diverse antagonists from receptors, it was shown that the cannabinoid system, via the CB1 receptor, and the opioid system, through the μ and δ receptors, are both involved in the antinociceptive effect of PnTx4(6–1). In the present work, it was synthesized a peptide, named PnAn13, based on the amino acid sequence of PnTx4(6–1) in order to try to reproduce or increase the analgesic effect of the toxin. As it was seen for the toxin, PnAn13 had antinociceptive activity, when intrathecally injected, and this effect involved the cannabinoid and opioid systems. In addition, when it was evaluated the peripheral effect of PnAn13, via intraplantar administration, this peptide was able to reverse the hyperalgesic threshold, evoked by prostaglandin E2. Therefore, using different pharmacological tools, it was shown the participation of cannabinoid and opioid systems in this effect.
Keywords: Antinociception, PnAn13, PnTx4(6–1), δ-Ctenitoxin-Pn1a, Opioid system, Cannabinoid system
Highlights
-
•
A synthetic peptide PnAn13, reproduced the antinociceptive effects of the PnTx4(6-1) (δ-Ctenitoxin-Pn1a) toxin.
-
•
PnAn13 showed a clear analgesic effect in the nociceptive in vivo rat pain model, both centrally and peripherally.
-
•
The antinociceptive effect of PnAn13 involves cannabinoid and opioid systems.
1. Introduction
The molecules present in animal venoms have been under constant evolutionary pressure and have become very specifics for their molecular targets. In this way, these venoms turn into an inexhaustible source to prospect molecules with biological activity. The venom of Phoneutria nigriventer spider is a great example of this reality. Since the beginning of studies with Professor Carlos Diniz in the’90s, until now, 30 years later, 41 neurotoxins have been identified from the crude venom of P. nigriventer (Herzig et al., 2011, Pineda et al., 2017, De Lima et al., 2015, Peigneur et al., 2018). This spider occurs mainly in South America, it is extremely aggressive and actively hunts its prey and, its success as a predator is directly related to the high toxicity of its venom (Simó and Brescovit, 2001).
In addition to the studies on bioactive molecules isolated directly from the P. nigriventer venom, some researchers have focused on the task to create new molecules inspired by the native ones, reproducing or enhancing their biological effects. An example is the recombinant PnTx4(5-5) (Γ-ctenitoxin-Pn1a), successfully expressed in a heterologous system, this toxin modified mammalian sodium channels like the native molecule. Moreover, the rPnTx4(5-5) showed a typical α-toxin effect on site 3 in insect sodium channel, blocking the channel fast inactivation kinetics and increasing the peak of the current (Paiva et al., 2016). Another example is the peptide PnPP-19, designed from P. nivriventer toxin PnTx2-6 (δ-ctenitoxin-Pn2a). This peptide potentiates erection in rats and mice, like the native toxin, although it seems by a different mechanism and, according to the authors, PnPP-19 potentiates erection through an increase in NO/cGMP production (Silva et al., 2015). Surprisingly, this same peptide, unlike the native toxin PnTx2-6 used as its template, also induces both, peripheral and central antinociception. This effect has shown to involve the activation of opioid and cannabinoid receptors, along with the activation of the NO/cGMP/KATP pathway (Freitas et al., 2016, Freitas et al., 2017; Pacheco et al., 2016).
Regarding the potential antinociceptive effect of the toxins present in P. nigriventer venom, we highlight the toxins belonging to the inset-toxic family. PnTx4(5-5) (Γ-ctenitoxin-Pn1a) reversibly inhibited the current of N-methyl-D-aspartate (NMDA), a subtype of ionotropic glutamate receptors, in rat hippocampal neurons (de Figueiredo et al., 2001). This toxin was able to reduce the hyperalgesia, induced by prostaglandin E2 (PGE2) in the rat paw when systemically administered (Oliveira et al., 2019). Concerning the local effect, PnTx4(5-5) promoted a peripheral and dose-dependent antinociceptive effect on hyperalgesia induced by carrageenan and PGE2 (Oliveira et al., 2019). In previous work, it was shown that PnTx4(6–1), also named δ-Ctenitoxin-Pn1a (King et al., 2008), initially characterized as an insect-toxin (Figueiredo et al., 1995), induced a clear analgesic effect in inflammatory, neuropathic and nociceptive in vivo pain models. This analgesic effect, in the nociceptive pain model, seems to involve CB1 cannabinoid and μ and δ-opioid receptors (Emerich et al., 2016). This insect-toxin is extremely toxic to house flies and cockroachs and did not cause any apparent toxicity to mice when intracerebroventricularly injected (30 μg) (Figueiredo et al., 1995). PnTx4(6–1) also binds to site 3 of sodium channels in cockroach nerve cord synaptosomes (Periplaneta americana), however, it does not affect the currents in rat skeletal muscle (rNav1.4/rSKM1) or brain (rNav1.2/rBIIA) (de Lima et al., 2002). However, it does not exclude the possible action of this toxin on others not yet tested sub-types of sodium channels, as it was observed to PnTx4(5-5) that, although did not show apparent toxicity when intracerebroventricularly injected in mice, showed to be active in different sodium channels sub-types (Paiva et al., 2016).
One of the biggest problems faced in the study of toxins derived from arthropods is the small amount of toxin obtained after purification of the crude venom. The pure PnTx4(6–1) toxin, for example, corresponds to only 0.65% of the total venom applied at the beginning of the purification process (Figueiredo et al. 1995). Given that, a smaller and easily synthesized peptide, which reproduces the effects of the native toxin, show to be much more advantageous in allowing both the synthesis of large quantities and the deepening of studies related to its activity.
Undoubtedly, everyone in life has gone through some situations that caused considerable physical pain. In The International Association for the Study of Pain described the pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage”. Besides, it is known that the ability to feel pain was also progressively selected, as it contributes to the physical integrity of our bodies (Cox et al., 2006). However, pathologies related to painful disorders have been increasing and the research for molecules capable to treat these cases is extremely necessary. So, in this work, based on the amino acid sequence of PnTx4(6–1), it was proposed a peptide with 13 amino acid residues, named PnAn13, trying to reproduce or even potentialize the analgesic effect of the toxin.
2. Material and methods
2.1. Animals
Male Wistar Rats, weighing 180–200 g, were kept in a home cage environment, with free access to water and food, in a temperature-controlled room at 24 ± 2 °C, and a 12–12 h light-dark cycle (lights on at 6:00 a.m.). Animals were habituated with the experimental room for one day before testing. All experiments were performed according to the current guidelines for the care of laboratory animals and ethical guidelines for investigations of experimental pain in conscious animals (Zimmermann, 1983), and were approved by the Ethics Committee on Animal Experimentation of the Federal University of Minas Gerais (protocol number: 102/2012).
2.2. Design, synthesis and Purification of PnAn13
In order to obtain more informatios about PnTx4(6–1) theoretical three-dimensional structure, we used the epitope prediction tool, available on the website “Immune Epitope Database and Analysis Resource” (www.iedb.org), to identify the most exposed residues on PnTx4(6–1) amino acid sequence. We obtained that amino acid residues 24 to 30 were considered the most exposed. We also compared the amino acid sequence of PnTx4(6–1) and PnTx4(5-5) (Γ-ctenitoxin-Pn1a), another Phoneutria nigriventer antinociceptive toxin from the same venom (Oliveira et al., 2019) and one of the most conserved region in these sequences are the residues 21 to 33 (Fig. 1). In this similar region the cysteines residues 23 and 29 were replaced by serine residues peptide to facilitate both manual synthesis and subsequent studies of peptide conformation, allowing the formation of only one disulfide bond.
Fig. 1.
Comparison of the amino acid sequences of PnTx4(6–1), PnTx4(5-5) and PnAn13. (*) Conserved amino acids. Obtained and modified from Clustal Omega.
The peptide PnAn13 (H-CDSYWSKSSKCRE-NH2), was synthesized by stepwise solid-phase using the N-9-fluorenylmethyloxycarbonyl (Fmoc) strategy (Chan and White, 2000) on a Rink-amide resin (0.68 mmol.g-1). The couplings were performed with 1,3-diiso-propylcarbodiimide (DIC), dichloromethane in dimethylformamide (DMF) for 3–4 h. Fmoc deprotection steps were carried out with 4-methylpiperidine in DMF (1:4; by volume) (20 min, twice). The cleavage step and the side chain deprotection were performed with trifluoroacetic acid (TFA)/thioanisole/water/1,2-ethanedithiol/triisopropylsilane, (86.5/5.0/5.0/2.5/1.0, by volume) at room temperature during 3 h. The final product was precipitated with cold diisopropyl ether and lyophilized. The crude synthetic products were purified by RP-HPLC, using a semi-preparative Discovery® BIO Wide Pore C8 column (Supelco analytical, United States), previously equilibrated with 0.1% aqueous TFA (solvent A). The elution was performed with a stepped gradient of 0.1% TFA in acetonitrile (solvent B) (0–15% of solvent B in 4 min; 15–27% of solvent B in 27 min; 27–100% of solvent B, in 4 min). The flow was 5.0 mL min−1 and detection at 214 nm the fractions were manually collected, and the pure peptide was lyophilized and stored at – 20 °C. PnAn13 was named in this way to represent Phoneutria nigriventer (Pn), antinociception (An) and the number of amino acid residues composing the molecule (13).
2.3. Mass spectrometry analysis
The quality of peptide synthesis and purification were evaluated by MALDI-TOF/TOF mass spectrometry analyses carried out on an AutoFlex III instrument (Bruker Daltonics, Billerica, MA, United States). The samples were co-crystallized with a supersaturated α-ciano-4-hydroxycinnamic solution (1:1, by volume) on MTP AnchorChip 400/384 or 600/384 plates (Bruker Daltonics, Billerica, MA, United States). The instrument was operated in positive reflector mode and the results were analyzed on FlexAnalysis 3.1 (Bruker Daltonics, Billerica, MA, United States).
2.4. Drugs
The following drugs and chemicals were used: Prostaglandin E2 (PGE2, Enzo Life Sciences, USA), Naloxone (Sigma, USA), MAFP (acid (5Z,8Z,11Z,14Z)-eicosatetraenil- fosfonofluorídrico metil ester) (Tocrisolve, EUA), AM251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; Tocris, USA), AM630 (6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl(4-ethoxyphenyl) methanone; Tocris), VDM11 [(5Z,8Z,11Z,14Z)-N-(4- Hidroxi-2- metilfenil)-5,8,11,14-eicosatetraenamida] (Tocrisolve, EUA). These drugs were dissolved as follows: PGE2 (2% ethanol in saline); AM251 and AM630 (10% DMSO in saline); MAFP (3% ethanol in saline); VDM11 (10% Trocrisolve in saline); PnAn13 and Naloxone (saline).
2.5. Drug treatments
PnAn13 (0.25-20 μg/site), AM251 (80–320 μg/site), AM630 (100 μg/site) and Naloxone (100 μg/site) were injected intrathecally (it.) (Mestre et al., 1994) in a volume of 20 μL/site per rat, and for intraplantar route (ipl.) PnAn13 (2.5-20 μg/site), AM251 (80–320 μg/site), AM630 (100 μg/site), Naloxone (100 μg/site), MAFP (0.5 μg/site) and VDM11 (2.5 μg/site) were administered in a volume of 50 μL. Prostaglandin E2 (2 μg/site) was injected intraplantarlly (ipl.) in a volume of 100 μL.
2.6. Prostaglandin E2-Induced nociceptive hyperalgesia
Rats received 100 μL intradermal injection of PGE2 (0.02 mg/mL, stored in ethanol, diluted in isotonic saline at the moment of the experiment) into the right hind paw. PnAn13 (0.25, 0.5, 1, 2 and 20 μg/site) was intrathecally administered 2 h and 55 min after PGE2 injection, the same condition performed for the toxin PnTx4(6–1) in previous work (Emerich et al., 2016). Peripherally, to establish the dose-response curve, the peptide was injected into the paw 3 h after PGE2 injection, in the doses of 2.5, 5, 10 and 20 μg/site. The nociceptive threshold was evaluated 5 min after the PnAn13 administration and every 10 min. To test the antagonists, PnAn13 (10 μg/site) was intraplantarlly or intrathecally administered 2 h and 55 min after PGE2 injection. The doses and times of administration of each antagonist considered the peak of action of each one, were indicated in the legend of the corresponding results.
2.7. Nociceptive test
The nociceptive threshold was measured according to the rat paw pressure test (Randall and Selitto, 1957) using an analgesimeter (Ugo Basile, Varese, Italy) with a cone-shaped paw presser with a rounded tip, which applies a linearly increasing force to the rat's hind paw. The nociceptive threshold was determined as being the weight (g) required to elicit a nociceptive response, in this case, paw flexion. A cutoff value of 300 g was applied to minimize damage to the rat paws. The nociceptive threshold was measured in the right paw (except in the systemic effect exclusion test, where both paws were measured) and determined as the average of three consecutive trials recorded. All the experiments were performed in a blind way, where one person did know the treatment while the other one did not.
2.8. Statistical analysis
Data were analyzed for statistical significance by one-way ANOVA analysis of variance followed by Bonferroni's test. The minimum level of significance considered was p < 0.05. All graphics and analyses were performed using Prisma 5.0 (GraphPad Software, Inc.).
3. Results
3.1. Desing, Synthesis and Purification of PnAn13
When compared both antinociceptive toxins PnTx4(6–1) and PnTx4(5-5) (Fig. 1) it is possible to note that the residues 21 to 33 are very similar in the two molecules. Furthermore, using an epitope prediction tool, we identify that the most exposed residues of PnTx4(6–1) amino acid sequence are 24–30. Considering these data, we chose the sequence between residues 21 to 33 to compose the peptide. In order to facilitate both manual synthesis and subsequent studies of peptide conformation we replaced the cysteines residues 23 and 29 for serine residues as shown below.
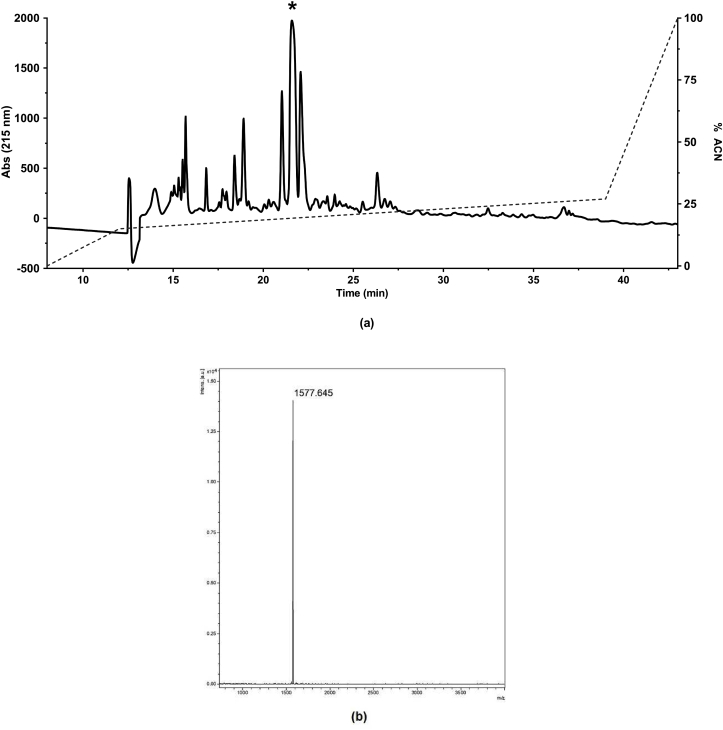
The synthetic pure products were assessed by RP-HPLC (Fig. 2a). The chromatogram exhibited the compounds present in the synthetic product, the major and well-defined peak (*), corresponds to the peptide PnAn13, the manual collection allowed to obtain PnAn13 with high purity. The peak was collected and analyzed by MALDI-TOF-MS and data showed the expected molecular weight for the peptide, 1577.6 Da (Fig. 2b).
Fig. 2.
Purification of PnAn13 synthetic peptide (a). The crudely synthesized peptide was purified by RP-HPLC, using a semi-preparative Discovery® BIO Wide Pore C8 column (Supelco analytical, United States) with a stepped gradient of 0.1% TFA in acetonitrile. The flow was 5.0 mL min−1 and detection at 214 nm the fractions were manually collected. Mass spectrometer spectra of PnAn13 (b). The molecular weight was 1577.6 Da.
3.2. Central antinociceptive effect of PnAn13
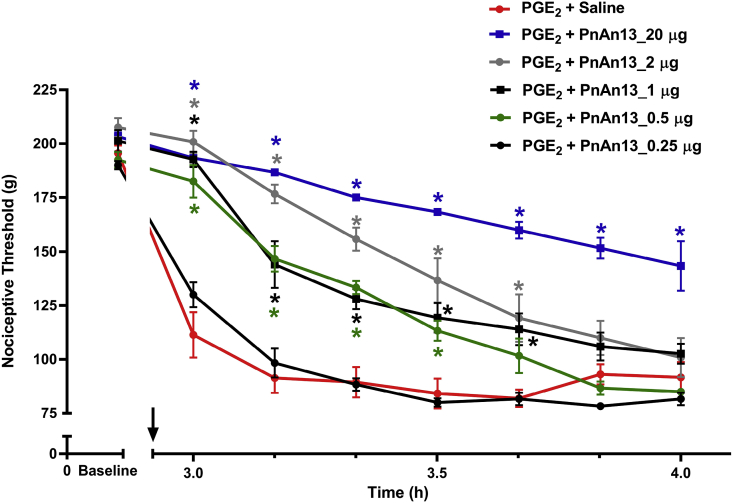
To verify the possible antinociceptive effect of PnAn13, the peptide was tested against PGE2 hyperalgesia, and it was observed that the peptide increased, in a dose-dependent manner, the nociceptive threshold of rats. The highest dose tested (20 μg) induced a significant antinociception, which persisted for all the time of experiment, 1 h. This effect was less durable for the other tested doses (2, 1, and 0.5 μg). The lowest dose tested (0.25 μg) did not show any antinociceptive effect (Fig. 3).
Fig. 3.
PnAn13 central antinociceptive effect following prostaglandin E2-induced hyperalgesia. Prostaglandin E2 (PGE2) (2 μg/paw) was injected intraplantarlly. Rats received 0.25, 0.5, 1, 2 and 20 μg (0.15, 0.3, 0.6, 1.2 and 12 nmol) of PnAn13 or saline (control) intrathecally, 2 h and 55 min after PGE2 injection (indicating by the arrow). Nociceptive threshold was measured every 10 min, starting 5 min after peptide or saline injections. Each symbol represents the MEAN ± SEM. n = 4 rats per group. Data were analyzed using ANOVA, and Bonferroni post-test. p < 0.05 compared to PGE2 + Saline (*).
3.2.1. Involvement of cannabinoid and opioid systems in central PnAn13 antinociception
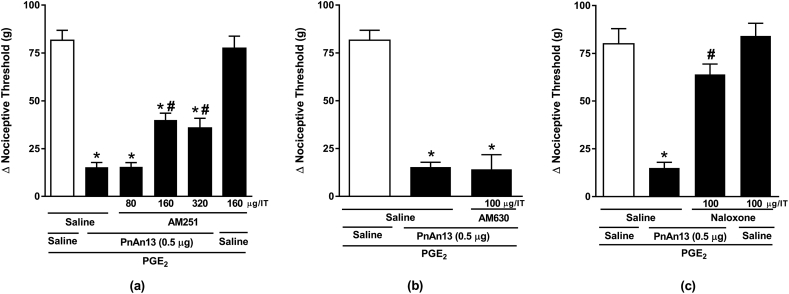
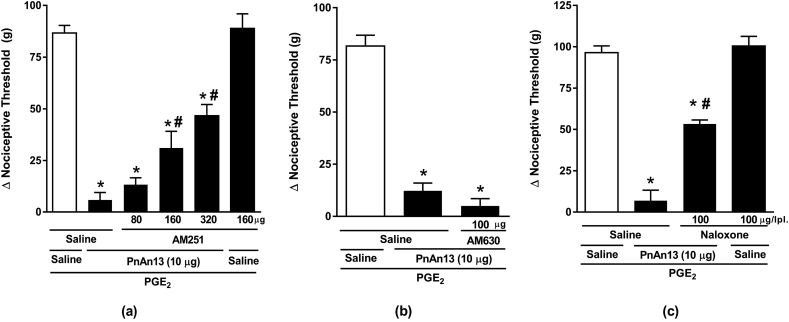
The mechanism underlying PnAn13 central effect on the hyperalgesia induced by PGE2 was investigated and the involvement of the cannabinoid and opioid systems was assessed. In order to explore the participation of the cannabinoid system, animals were treated with AM251 (80, 160 and 320 μg), a selective CB1 receptor antagonist, or AM630 (100 μg) (Romero et al., 2013), a selective CB2 receptor antagonist, both intrathecally administered 10 min prior to peptide. We observed that AM251 reduced, in a dose-dependent manner, the antinociceptive effect of PnAn13 (Fig. 4a), on the other hand, 100 μg of AM630 showed no significant effect on PnAn13 antinociceptive effect (Fig. 4b).
Fig. 4.
Effect of cannabinoid and opioid antagonists on PnAn13 antinociception following prostaglandin E2-induced hyperalgesia. Rats received AM251 (a) or AM630 (b) intrathecally 2 h and 45 min after prostaglandin E2 (PGE2) injection (2 μg/paw). PnAn13 (0.5 μg/0.3 nmol) or saline (control) were intrathecally injected 10 min after the antagonists. Rats received Naloxone (c) intrathecally 2 h and 25 min after prostaglandin E2 (PGE2) injection (2 μg/paw). PnAn13 (0.5 μg) or saline (control) were intrathecally injected 30 min after the antagonists. The nociceptive threshold was measured 5 min after peptide or saline injection. Vertical bars represent MEAN ± SEM. n = 4 rats per group. Data were analyzed using ANOVA and Bonferroni post-test. p < 0.05 compared to PGE2 + Saline (*) or PGE2 + PnAn13 + Saline (#).
Animals were treated with opioid receptor antagonists, in order to explore the involvement of the opioid system. As a result, we showed that the non-selective opioid antagonist, Naloxone in a dose of 100 μg, completely reversed the antinociceptive effect of PnAn13 (Fig. 4c).
3.3. Peripheral antinociceptive effect of PnAn13
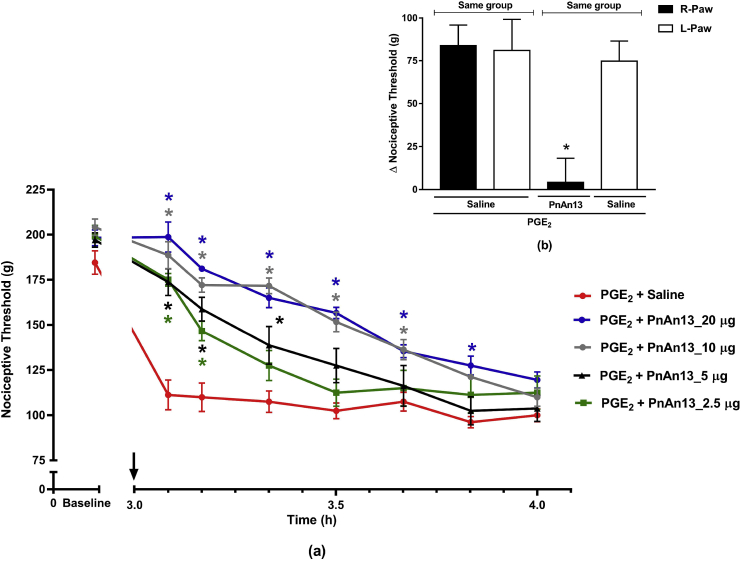
In order to explore the peripheral antinociceptive effect of the peptide, PnAn13 was administered intraplantarlly (2.5, 5, 10 and 20 μg) into the animals paw 3 h after PGE2 injection. It was found that PnAn13 had an analgesic effect in a dose-dependent manner (Fig. 5a). All tested doses promoted a fast antinociception 5 min after administration. It is possible to observe that the higher doses promoted longer-lasting antinociception when compared to the lower doses. The dose of 20 μg had an effect lasting 50 min while the dose of 2.5 μg had an effect that lasts for only 10 min. The effect of all the doses decreasead along the time.
Fig. 5.
PnAn13 peripheral antinociceptive effect following prostaglandin E2-induced hyperalgesia. Prostaglandin E2 (PGE2) (2 μg/paw) was injected intraplantarlly (a). Rats received 2.5, 5, 10 and 20 μg (1.5, 3, 6, 12 nmol) of PnAn13 or saline (control) intraplantarlly, 3 h after PGE2 injection (indicating by the arrow). Nociceptive threshold was measured every 10 min, starting 5 min after peptide or saline injections. p < 0.05 compared to PGE2 + Saline (*). Exclusion of systemic antinociceptive effect of PnAn13 at a dose of 20 μg (12 nmol) (b). Peptide or saline were given into right paw (R-paw) and saline was given into the left paw (L-paw) 3 h after administration PGE2 injection (2 μg/paw) in both hind paws of the animals. p < 0.05 compared to PGE2 + Saline – R-paw (*). Each symbol represents MEAN ± SEM. n = 4 rats per group. Data were analyzed using ANOVA, and Bonferroni post-test.
Further assay confirmed the peripheral antinociceptive effect of PnAn13 (20 μg/paw), since its effect was restricted to the peptide-treated paw, in our case the right paw. No change in the nociceptive threshold was observed in the contralateral paw (Fig. 5b).
3.3.1. Involvement of opioid and cannabinoid systems in peripheral PnAn13 antinociception
Once we saw the involvement of the opioid system in the central antinociceptive effect of PnAn13, it was used the same antagonist to test the participation of this system in the peripheral effect of the peptide. It was observed that the non-specific opioid antagonist Naloxone, in a dose of 100 μg, partially prevents the peripheral effect of PnAn13 (Fig. 6c).
Fig. 6.
Effect of cannabinoid and opioid antagonists on PnAn13 antinociception following prostaglandin E2-induced hyperalgesia. Rats received AM251 (a) or AM630 (b) intraplantarlly 2 h and 45 min after prostaglandin E2 (PGE2) injection (2 μg/paw). PnAn13 (10 μg/6 nmol) or saline (control) were intraplantar injected 10 min after the antagonists. Rats received Naloxone (c) intraplantarlly 2 h and 25 min after prostaglandin E2 (PGE2) injection (2 μg/paw). PnAn13 (10 μg/6 nmol) or saline (control) were intraplantar injected 30 min after the antagonist. The nociceptive threshold was measured 5 min after peptide or saline injection. Vertical bars represent MEAN ± SEM. n = 4 rats per group. Data were analyzed using ANOVA and Bonferroni post-test. p < 0.05 compared to PGE2 + Saline (*) or PGE2 + PnAn13 + Saline (#).
In the same way, it was investigated the participation of the cannabinoid system in the PnAn13 peripheral effect, using the selective antagonists AM251 and AM630. AM251, but not AM630, was able to partially antagonize the analgesic effect of the peptide, indicating the participation of the CB1 cannabinoid receptor in the PnAn13 effect (Fig. 6a and b).
Because PnAn13 effect was antagonized by CB1 antagonist, it was used MAFP, an inhibitor of the fatty acid amide hydrolase (FAAH), the major anandamide metabolizing enzyme, and the anandamide uptake inhibitor VDM11 to confirm the potentiation of PnAn13 effect on the nociceptive pathway. Both MAFP (0.5 μg) (Fig. 7a) and VDM11 (2.5 μg) (Fig. 7b) enhanced the antinociception induced by a low dose of PnAn13 (2.5 μg per paw). MAFP and VDM11 given alone did not induce any effect.
Fig. 7.
Potentiation on PnAn13 antinociception by the FAAH inhibitor - MAFP and anandamide uptake inhibitor -VDM11. Rats received The MAFP (0.5 μg/paw) and VDM11 (2.5 μg/paw) intraplantarlly 2 h and 45 min after prostaglandin E2 (PGE2) injection (2 μg/paw). PnAn13 (2.5 μg/1.5 nmol) was injected at 2 h and 55 min after local administration of PGE2 (2 μg/paw). Nociceptive threshold was measured 5 min after toxin or saline injection. Vertical bars represent MEAN ± SEM. n = 4 rats per group. Data were analyzed using ANOVA and Bonferroni post-test. p < 0.05 compared to PGE2 + Saline (*) or PGE2 + PnAn13 + Saline (#).
4. Discussion
Peptides with biological activity, based on toxins isolated from animal venoms, used as templates, can be a promising strategy to obtain new drugs. It is the case of our work. Inspired in the toxin PnTx4(6–1) (δ-Ctenitoxin-Pn1a) it was designed the peptide PnAn13, which reproduces similar central antinociceptive effect of the toxin in the nociceptive pain model, involving both cannabinoid and opioid systems (Emerich et al., 2016). Comparing the dose-response curves of PnTx4(6–1) (Emerich et al., 2016, Fig. 3) and PnAn13 (Fig. 3, in this work) there is a remarkable similarity of the antinociceptive effect in both cases. We highlight that PnAn13, when administered at the dose of 0.5 μg (0.3 nmol), had an antinociceptive effect for 30 min, the same duration observed for the equimolar dose of PnTx4(6–1) (Emerich et al., 2016).
The literature describes the presence of cannabinoid receptors in several sites of the central nervous system (Mackie, 2005). The injection of the CB1 receptor antagonist, but not CB2, partially antagonized the antinociceptive effect of PnAn13. Comparing to PnTx4(6–1) in both cases, the specific antagonist for the CB1 receptor, but not for CB2 receptors, antagonized the antinociceptive effect of the toxin and the peptide. With a careful look, it is possible to observe that the analgesic effect of PnTx4(6–1) is more susceptible to the antagonism of AM251, once the dose of 320 μg of AM251 completely antagonized the analgesia promoted by the toxin (Emerich et al., 2016, Fig. 5) but partially antagonized the effect of PnAn13 (Fig. 4, in this work). This could happen due to the greater involvement of different systems in this analgesic effect of the peptide.
Wittert et al., 1996, described the expression of opioid receptors on the central nervous system, therefore, it was used the non-specific opioid antagonist naloxone to analyze the participation of this system. It was observed, for both PnTx4(6–1) and PnAn13, that the non-specific opioid antagonist Naloxone, in a dose of 100 μg, completely antagonized the antinociceptive effect of both molecules, against the hyperalgesia promoted by PGE2 (Fig. 4). To determine the specific opioid receptors involved in the analgesic effect of PnAn13, was not a concern of this work, instead of that, we focused on exploring the peripheral effect of the peptide.
Pain treatment using intrathecal administration of peptides derived from animal toxins already occurs. The example is Ziconotide (Prialt®), a synthetic version of the peptide ω-conotoxin MVIIA, isolated from the venom of Conus magus, used to treat severe chronic pain, which has its analgesic effect related to a potent and selective blockade of N-type voltage-gated calcium channels (Miljanich, 2004). Because of its action on calcium channels in the central nervous system, Ziconotide has a limited therapeutic window; patients who received Ziconotide had a much higher risk of experiencing dizziness, confusion, ataxia, abnormal gait and memory impairment (Rauck et al., 2006). It is noted that the treatment via intrathecal administration may not be the best alternative to treat pain. In addition, it requires a special device to inject the drug into the spinal cord.
Therefore, to investigate the peripheral effect of PnAn13 it was performed a dose-response curve against the hyperalgesia promoted by PGE2. It was observed a fast antinociceptive effect 5 min after administration of all doses tested, restoring the nociceptive threshold of the animal when compared to the control, equally found in the central assay. It is interesting to observe the relationship between the dose and the time of the duration of antinociception when intraplantarlly injected, 20 μg of PnAn13 has 50 min of action, whereas, 2.5 μg has only 10 min of action (Fig. 5). The decreasing effect of the antinociception is also dependent on the doses of the peptide. The dose of 20 μg did not change the nociceptive threshold of the contralateral paw, showing the peripheral action of the peptide at the highest dose. The literature also shows the peripheral analgesic effect of different peptides from toxins (Freitas et al., 2016, Machado et al., 2014). The presence of proteases in the body is the major limitation to use peptides as a drug (Bruno et al., 2013), some strategies can be employed to avoid the degradation by endoproteases, like encapsulation in cyclodextrin, inclusion in liposomes, pegylation of the molecule, among others. These strategies could be employed to PnAn13 in an attempt to increase its time of action.
There is an intrinsic relationship between the cannabinoidergic and opioidergic pathways. The central and peripheral antinociception produced by morphine, for example, by a potent and selective agonism of μ-opioid receptors, has also been shown to involve the release of endogenous cannabinoids that, in turn, activate CB1-type receptors (Pacheco et al., 2008, Pacheco et al., 2009).
To investigate the participation of the opioid system in the peripheral antinociception of PnAn13, it was used the non-specific antagonist Naloxone, which partially antagonized the effect of the peptide, suggesting greater involvement of opioid system in its antinociception (Fig. 6). When analyzing the involvement of the cannabinoid system in this event, it was used four pharmacological tools: AM251, a selective antagonist for CB1 receptors; AM630, a selective antagonist for CB2 receptors; MAFP, an inhibitor of the fatty acid amide hydrolase (FAAH), the major anandamide metabolizing enzyme, and VDM11 an anandamide uptake inhibitor. These four experimental approaches show the participation of the cannabinoid system in peripheral PnAn13 antinociception in the nociceptive pain model induced by PGE2.
It was shown that AM251 antagonized the antinociceptive effect of PnAn13 in PGE2-induced hyperalgesia, whereas AM630 in a dose of 100 μg did not change PnAn13 analgesia (Fig. 6). Literature describes the presence of cannabinoid receptors in peripheral sensory neurons (Hohmann and Herkenham, 1999a, Price et al., 2003, Agarwal et al., 2007) and their axonal transport to the sensory nerve terminals (Hohmann and Herkenham, 1999b). In addition, the receptor expression and axonal transport appear to be increased in response to peripheral inflammation (Amaya et al., 2006). Furthermore, although studies report a higher presence of CB2 receptors instead of CB1 in immune cells (Pertwee and Ross, 2013), Jourdan et al. (2013) described that the CB1 receptor is highly expressed in macrophages. Mai et al. (2015), also have shown the ability of these cells to express CB1-type receptors. According to the literature, these immune cells have specific machinery able of synthesis, reuptake, and degrade endocannabinoids such as anandamide and palmitoylethanolamide (PEA) (Bisogno et al., 1997) corroborating the idea of the possible participation of the CB1 receptor in these cells. CB1 receptors are densely expressed in the superficial laminae of the dorsal horn of the spinal cord, in the dorsal root ganglion, in the peripheral terminals of the primary afferent neurons and in the descending pathway of pain (Stein, 2003). This wide distribution may be related to the antagonism of AM251 on the analgesic effect of PnAn13, both at the medullary and peripheral levels.
Using both, MAFP and VDM11 that promote bigger availability of the neurotransmitter anandamide, it was observed a potentiation of the antinociceptive effect of the peptide, showing that PnAn13, somehow, stimulates the endocannabinoids release, perhaps by a synergistic effect with these drugs. However, although the literature describes that the endocannabinoids are synthetized on demand in a presence of a stimulus (Guindon and Hohmann, 2009), the doses of MAFP and VDM11 used possibly were not enough to induce antinociception by themselves. Ferreira et al. (2018), used the same doses of MAFP and VDM11 (0.5 μg and 2.5 μg respectively) in rats, and have shown the same response found in this study. Even using a higher dose of these drugs (4 μg and 20 μg), our research group also demonstrated that MAFP and VDM11 were not able to induce antinociceptive response in presence of PGE2 or in an absence of hyperalgesic stimulus in rats (Freitas et al., 2016).
PnPP19, a peptide synthesized by our research group and derived from another P. nigriventer toxin PnTx2-6, showed antinociceptive activity against PGE2 hyperalgesia through activation of μ and δ opioid, and CB1 cannabinoid receptors. This peptide also seems to be able to induce, indirectly, antinociception through inhibition of a neuronal endopeptidase responsible for the cleavage of the endogenous opioid peptide encephalin (Freitas et al., 2016). Different from these findings, Crotalphine, a peptide derived from the Crotalus durissus terrificus venom, reduced PGE2-induced hyperalgesia through increased activation of both κ-opioid and CB2 cannabinoid receptors, being this effect mediated by dynorphin A (Machado et al., 2014). This result reinforces the interaction between cannabinoid and opioid systems, as observed in our work, and highlights the complexity of the nociceptive pathways.
5. Conclusions
In the present study, we reveal that PnAn13, a synthetic peptide based on the sequence of the toxin PnTx4(6–1) isolated from the venom of Phoneutria nigriventer spider, shows a clear analgesic effect in the nociceptive in vivo rat pain model, both centrally and peripherally. Moreover, it was shown the involvement of CB1 cannabinoid and opioid systems in this effect, although it is unclear if the receptor activation is directly or indirectly. Therefore, taken together, our results may contribute to the development of novel therapeutic agents for the management of pain, although studies are still necessary to better clarify the mechanisms involved in the PnAn13 effects.
Credit author statement
Maria Elena de Lima: Conceptualization, Supervision. Igor Dimitri G. Duarte: Supervision. Ricardo Andrez Machado de Avila: Conceptualization. Bruna Luiza Emerich: Investigation, Writing - original draft. Renata Cristina Mendes Ferreira: Investigation. Jarbas Magalhães Resende: Supervision. All authors: Writing - review & editing.
Author contributions
Maria Elena de Lima proposed the project and, together with Igor Dimitri G. Duarte, supervised the research and revised this paper. Ricardo Andrez Machado de Avila contributed with the sequence of the peptide. Bruna Luiza Emerich performed the nociceptive experiments and data analyses, besides writing the paper. Renata Cristina Mendes Ferreira also performed the nociceptive experiments. Jarbas Magalhães Resende supervised the peptide synthesis. All authors contributed extensively to the work presented in this paper.
Funding
This work was supported by the Brazilian agencies: Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Declaration of competing interest
The authors have no competing interests to declare.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank Dr. Carlos Delfin Chávez Olórtegui for opening his laboratory for our students to synthesize the peptides.
Contributor Information
Bruna Luiza Emerich, Email: brunaemerich@gmail.com.
Renata Cristina Mendes Ferreira, Email: recmferreira@gmail.com.
Ricardo Andrez Machado-de-Avila, Email: r_andrez@yahoo.com.br.
Jarbas Magalhães Resende, Email: jarbasufmg@hotmail.com.
Igor Dimitri G. Duarte, Email: dimitri@icb.ufmg.br.
Maria Elena de Lima, Email: mariaelena@santacasabh.org.br.
References
- Agarwal N., Pacher P., Tegeder I., Amaya F., Constantin C.E., Brenner G.J., Rubino T., Michalski C.W., Marsicano G., Monory K., Mackie K., Marian C., Batkai S., Parolaro D., Fischer M.J., Reeh P., Kunos G., Kress M., Lutz B., Woolf C.J., Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat. Neurosci. 2007;10:870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya F., Shimosato G., Kawasaki Y., Hashimoto S., Tanaka Y., Ji R.-R., Tanaka M. Induction of CB1 cannabinoid receptor by inflammation in primary afferent neurons facilitates antihyperalgesic effect of peripheral CB1 agonist. Pain. 2006;124:175–183. doi: 10.1016/j.pain.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Bisogno T., Maurelli S., Melck D., Petrocellis L. De, Marzo V.Di. Biosynthesis, uptake, and degradation of anandamide and palmitoylethanolamide in leukocytes. J. Biol. Chem. 1997;272:3315–3323. doi: 10.1074/jbc.272.6.3315. [DOI] [PubMed] [Google Scholar]
- Bruno B.J., Miller G.D., Lim C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013;4(11):1443–1467. doi: 10.4155/tde.13.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W.C., White P.D. Oxford University Press; 2000. Fmoc Solid Phase Peptide Synthesis: A Practical Approach. [Google Scholar]
- Cox J.J., Reimann F., Nicholas A.K., Thornton G., Roberts E., Springell K., Karbani G., Jafri H., Mannan J., Raashid Y., Al-Gazali L., Hamamy H., Valente E.M., Gorman S., Williams R., McHale D.P., Wood J.N., Gribble F.M., Woods C.G. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444:894–898. doi: 10.1038/nature05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Figueiredo S.G., De Lima M.E., Cordeiro M.N., Diniz C.R., Patten D., Halliwell R.F., Gilroy J., Richardson M. Purification and amino acid sequence of a highly insecticidal toxin from the venom of the Brazilian spider Phoneutria nigriventer which inhibits NMDA-evoked currents in rat hippocampal neurones. Toxicon. 2001;39(2–3):309–317. doi: 10.1016/s0041-0101(00)00129-x. [DOI] [PubMed] [Google Scholar]
- de Lima M.E., Stankiewicz M., Hamon A., de Figueiredo S.G., Cordeiro M.N., Diniz C.R., Martin-Eauclaire M.-F., Pelhate M. The toxin Tx4(6-1) from the spider Phoneutria nigriventer slows down NA+ current inactivation in insect CNS via binding to receptor site 3. J. Insect Physiol. 2002;48(1):53–61. doi: 10.1016/s0022-1910(01)00143-3. [DOI] [PubMed] [Google Scholar]
- De Lima M.E., Torres F.S., Magalhães B.L.E., Freitas A.C.N. Perspectivas inovadoras para o uso terapêutico de toxinas da aranha “armadeira” Phoneutria nigriventer (Keyserling, 1891) na dor e na disfunção erétil. In: Resende R.R., Soccol C.R., editors. Biotecnologia Aplicada à Saúde: Fundamentos e Aplicações. vol. 1. 2015. pp. 539–572. Blucher: São Paulo, Brazil. (In Portuguese) [Google Scholar]
- Emerich B.L., Ferreira R.C.M., Cordeiro M.N., Borges M.H., Pimenta A.M.C., Figueiredo S.G., Duarte I.D.G., de Lima M.E. δ-Ctenitoxin-Pn1a, a peptide from Phoneutria nigriventer spider venom, shows antinociceptive effect involving opioid and cannabinoid systems, in rats. Toxins. 2016;8(4):1–13. doi: 10.3390/toxins8040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R.C.M., Castor M.G.M., Piscitelli F., Di Marzo V., Duarte I.D.G., Romero T.R.L. The involvement of the endocannabinoid system in the peripheral antinociceptive action of ketamine. J. Pain. 2018;19(5):487–495. doi: 10.1016/j.jpain.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Figueiredo S.G., Garcia M.E., Valentim A.C., Cordeiro M.N., Ribeiro-Diniz C.R., Richardson M. Purification and amino acid sequence of the insecticidal neurotoxin Tx4(6-1) from the venom of the “armed” spider Phoneutria nigriventer (Keys) Toxicon. 1995;33(1):83–93. doi: 10.1016/0041-0101(94)00130-z. [DOI] [PubMed] [Google Scholar]
- Freitas A.C., Freitas A.C.N., Pacheco D.F., Machado M.F.M., Carmona A.K., Duarte I.D.G., Lima M.E. PnPP-19, a spider toxin peptide, induces peripheral antinociception through opioid and cannabinoid receptors and inhibition of neutral endopeptidase. Br. J. Pharmacol. 2016;173:1491–1501. doi: 10.1111/bph.13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas A.C., Silva G.C., Pacheco D.F., Pimenta A.M.C., Lemos V.S., Duarte I.D.G., de Lima M.E. The synthetic peptide PnPP-19 induces peripheral antinociception via activation of NO/cGMP/KATP pathway: role of eNOS and nNOS. Nitric Oxide. 2017;64:31–38. doi: 10.1016/j.niox.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Guindon J., Hohmann A.G. The endocannabinoid system and pain. CNS Neurol. Disord. - Drug Targets. 2009;8(6):403–421. doi: 10.2174/187152709789824660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig V., Wood D.L., Newell F., Chaumeil P.A., Kaas Q., Binford G.J., Nicholson G.M., Gorse D., King G.F. ArachnoServer 2.0, an updated online resource for spider toxin sequences and structures. Nucleic Acids Res. 2011:D653–D657. doi: 10.1093/nar/gkq1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann A.G., Herkenham M. Cannabinoid receptors undergo axonal flow in sensory nerves. Neuroscience. 1999;92:1171–1175. doi: 10.1016/s0306-4522(99)00220-1. [DOI] [PubMed] [Google Scholar]
- Hohmann A.G., Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience. 1999;90:923–931. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- Jourdan T., Godlewski G., Cinar R., Bertola A., Szanda G., Liu J., Tam J., Han T., Mukhopadhyay B., Skarulis M.C., Ju C., Aouadi M., Czech M.P., Kunos G. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med. Sep. 2013;19(9):1132–1140. doi: 10.1038/nm.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G.F., Gentz M.C., Escoubas P., Nicholson G.M. A rational nomenclature for naming peptide toxins from spiders and other venomous animals. Toxicon. 2008;52:264–276. doi: 10.1016/j.toxicon.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Machado F.C., Zambelli V.O., Fernandes A.C., Heimann A.S., Cury Y., Picolo G. Peripheral interactions between cannabinoid and opioid systems contribute to the antinociceptive effect of crotalphine. Br. J. Pharmacol. 2014;171:961–972. doi: 10.1111/bph.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb. Exp. Pharmacol. 2005:299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- Mai P., Yang L., Tian L., Wang L., Jia S., Zhang Y., Liu X., Yang L., Li L. Endocannabinoid system contributes to liver injury and inflammation by activation of bone marrow-derived monocytes/macrophages in a CB1-dependent manner. J. Immunol. 2015;195:3390–3401. doi: 10.4049/jimmunol.1403205. [DOI] [PubMed] [Google Scholar]
- Mestre C., Pélissier T., Fialip J., Wilcox G., Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J. Pharmacol. Toxicol. Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Miljanich G.P. Ziconotide: neuronal calcium channel blocker for treating severe chronic pain. Curr Med Chem, Schiphol (Netherlands) 2004:3029–3040. doi: 10.2174/0929867043363884. [DOI] [PubMed] [Google Scholar]
- Oliveira C.F.B., Alves D.P., Emerich B.L., Figueiredo S.G., Cordeiro M.N., Borges M.H., Richardson M., Pimenta A.M.C., Duarte I.D.G., de Lima M.E. Antinociceptive effect of PnTx4(5-5), a peptide from Phoneutria nigriventer spider venom, in rat models and the involvement of glutamatergic system. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019;25 doi: 10.1590/1678-9199-JVATITD-2019-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco D. da F., Freitas A.C.N., Pimenta A.M.C., Duarte I.D.G., de Lima M.E. A spider derived peptide, PnPP-19, induces central antinociception mediated by opioid and cannabinoid systems. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016;22:34. doi: 10.1186/s40409-016-0091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco D.F., Klein A., Perez A.C., Pacheco C.M. da F., de Francischi J.N., Reis G.M.L., Duarte I.D.G. Central antinociception induced by μ-opioid receptor agonist, but not κ- or δ-, is mediated by canabinoid CB1 receptor. Br. J. Pharmacol. 2009:225–231. doi: 10.1111/j.1476-5381.2009.00310.x. (Sep) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco D.F., Klein A., Perez A. de C., Pacheco C.M. da F., de Francischi J.N., Duarte D.G. The μ-opioid receptor agonist morphine, but not agonists at delta- or kappa opioid receptors, induces peripheral antinociception mediated by cannabinoid receptors. Br. J. Pharmacol. 2008:1143–1149. doi: 10.1038/bjp.2008.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva A.L.B., Matavel A., Peigneur S., Cordeiro M.N., Tytgat J., Diniz M.R.V., de Lima M.E. Differential effects of the recombinant toxin PnTx4(5-5) from the spider Phoneutria nigriventer on mammalian and insect sodium channels. Biochimie. 2016;121:326–335. doi: 10.1016/j.biochi.2015.12.019. [DOI] [PubMed] [Google Scholar]
- Peigneur S., de Lima M.E., Tytgat J. Phoneutria nigriventer venom: a pharmacological treasure. Toxicon. 2018;1(151):96–110. doi: 10.1016/j.toxicon.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Pertwee R.G., Ross R.A. Cannabinoid receptors and their ligands. Prostaglandins Leukot. Essent. Fatty Acids. 2013;66:101–121. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- Pineda S.S., Chaumeil P.A., Kunert A., Kaas Q., Thang M.W.C., Li L., Nuhn M., Herzig V., Saez N.J., Cristofori-Armstrong B., Anangi R., Senff S., Gorse D., King G.F. ArachnoServer 3.0: an online resource for automated discovery, analysis and annotation of spider toxins. Bioinformatics. 2017;34(6) doi: 10.1093/bioinformatics/btx661. [DOI] [PubMed] [Google Scholar]
- Price T.J., Helesic G., Parghi D., Hargreaves K.M., Flores C.M. The neuronal distribution of cannabinoid receptor type 1 in the trigeminal ganglion of the rat. Neuroscience. 2003;120:155–162. doi: 10.1016/S0306-4522(03)00333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L.O., Selitto J.J. A method for measurement of analgesic activity on inflamed tissues. Arch. Int. Pharmacody. 1957;111:409–419. [PubMed] [Google Scholar]
- Rauck R.L., Wallace M.S., Leong M.S., Minehart M., Webster L.R., Charapata S.G., Abraham, Buffington D.E., Ellis D., Kartzinel R. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J. Pain Symptom Manag. 2006;31:393–406. doi: 10.1016/j.jpainsymman.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Romero T.R., Resende L.C., Guzzo L.S., Duarte I.D. CB1 and CB2 cannabinoid receptor agonists induce peripheral antinociception by activation of the endogenous noradrenergic system. Anesth. Analg. 2013;116(2):463–472. doi: 10.1213/ANE.0b013e3182707859. [DOI] [PubMed] [Google Scholar]
- Silva C.N., Nunes K.P., Torres F.S., Cassoli J.S., Santos D.M., Almeida F.M., Matavel A., Cruz J.S., Santos-Miranda A., Nunes A.D.C., Castro C.H., Machado de Ávila R.A., Chávez-Olórtegui C.D., Láuar S.S., Felicori L., Resende J.M., Camargos E.R., Borges M.H., Cordeiro M.N., Peigneur S., Tytgat J., De lima M.E. PnPP-19, a synthetic and non toxic peptide designed from a P. nigriventer toxin, potentiates erectile function via NO/cGMP. J. Urol. 2015;194(5):1481–1490. doi: 10.1016/j.juro.2015.06.081. [DOI] [PubMed] [Google Scholar]
- Simó M., Brescovit A.D. Revision and cladistic analysis of the neotropical spider genus Phoneutria perty, 1833 (araneae, ctenidae), with notes on related cteninae. Bull. Br. Arachnol. Soc. 2001;12:67–82. [Google Scholar]
- Stein C. Opioid receptors on peripheral sensory neurons. Adv. Exp. Med. Biol. 2003;521:69–76. [PubMed] [Google Scholar]
- Wittert G., Hope P., Pyle D. Tissue distribution of opioid receptor gene expression in the rat. Biochem. Biophys. Res. Commun. 1996;218:877–881. doi: 10.1006/bbrc.1996.0156. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]