Abstract
Purpose
To report use of ultrasound biomicroscopy (UBM) and anterior segment ocular coherence tomography (AS-OCT) in a case of pseudophakic glaucoma in a patient with an anterior chamber intraocular lens.
Observations
UBM and AS-OCT were critical in determining a non-drug related etiology of angle closure. Images indicated anterior obstruction of the pupil secondary to anterior chamber intraocular lens, but also posterior obstruction of the pupil secondary to the anterior hyaloid face.
Conclusions
When evaluating a patient with suspected angle closure, it is important to perform a full ophthalmologic examination, including gonioscopy, as well as a thorough review of past medical history and medications so as not to miss systemic-related etiologies. Imaging with B-scan, UBM, and, more recently in the last decade, AS-OCT is a key component of evaluation of a patient in angle closure, especially one with a complex medical and ocular history.
Keywords: Pseudophakic glaucoma, Secondary angle closure, Pupillary block
1. Introduction
Secondary angle closure has many different etiologies, and the pathophysiology is often divided into a “pushing” and “pulling” mechanism.1 The “push” mechanism is described as a posterior force causing anterior displacement of the iris that blocks the trabecular meshwork and outflow pathway. This can be due to anterior displacement of the lens complex as seen in phacomorphic glaucoma, anterior rotation of the ciliary body in cilio-choroidal effusions, or an anatomical variation like plateau iris syndrome. Alternatively, the iris can be pulled forward by the formation of a neovascular membrane or fibrous tissue that closes the angle.
When a patient presents with an elevated intraocular pressure (IOP), one of the crucial parts of a comprehensive ophthalmic examination is performing gonioscopy to determine whether angle closure is present.2 However, this does not differentiate among the causes of angle closure, and other imaging modalities, namely B-scan ultrasonography, ultrasound biomicroscopy (UBM), and/or anterior segment ocular coherence tomography (AS-OCT) are necessary. Moreover, it is always essential to perform a thorough review of medications as drug-induced angle closure can easily be overlooked.3 B-scan and UBM visualize structures posterior to the iris to help determine the etiology of angle closure, like cilio-choroidal effusions, a large lens, or a posterior mass.2 AS-OCT can also be beneficial, as the high-resolution scans can show whether pupillary block is present.4
We report here a unique case of angle closure in a patient with major depressive disorder status post anterior chamber intraocular lens placement with a patent surgical iridectomy.
2. Case report
A 57-year-old male presented to an optometrist with distorted vision and light sensitivity in his left eye for ten days. His best corrected visual acuity was 20/25–2 in the right eye (OD) and 20/30–2 in the left eye (OS). Intraocular pressures (IOP) were 20 mm Hg OD and 35 mm Hg OS by Goldmann applanation tonometry. He was started on nightly latanoprost in the left eye and referred to the glaucoma clinic for evaluation.
The patient had a past ocular history significant for bilateral, spontaneous lens dislocations treated with lensectomies, surgical iridectomies, and anterior chamber intraocular lens (ACIOL) implants more than 10 years prior to presentation. He had no history of connective tissue disorders and was of normal stature and intelligence. His past medical history was remarkable for attention-deficit hyperactivity disorder and depression, well-controlled with aripiprazole 2 mg daily and duloxetine delayed release 30 mg BID. There was no family history of lens abnormalities or glaucoma.
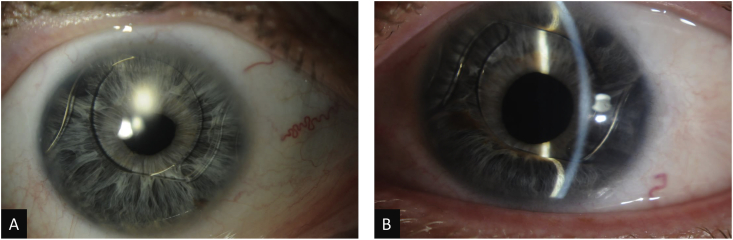
One week later, his best corrected visual acuity was 20/25–1 OD and 20/25 OS. IOPs were 13 mm Hg OD and 21 mm Hg OS by Goldmann applanation tonometry. His corneal pachymetry was 660 μm OD and 638 μm OS. Slit lamp examination of the right eye revealed a deep anterior chamber (AC), irregular pupil, patent surgical peripheral iridectomy (PI), and ACIOL. The left eye had a peripherally shallow AC superiorly and inferiorly, superior and inferior iridocorneal (IK) touch with forward bowing of the iris anterior to the ACIOL optic, irregular pupil, patent surgical PI (approximately 1.5mm in diameter) by retroillumination, and ACIOL (Fig. 1). Gonioscopy demonstrated open angles (Shaffer grade 4 in all quadrants) in the right eye and partially open angles (Shaffer grade 0 superiorly and inferiorly and grade 4 nasally and temporally) in the left eye. Indentation gonioscopy demonstrated appositional angle closure superiorly and inferiorly without peripheral anterior synechiae (PAS). A B-scan showed no obvious posterior abnormality to cause anterior bowing of the iris.
Fig. 1.
Slit lamp photographs of the right (A) and left (B) eyes.
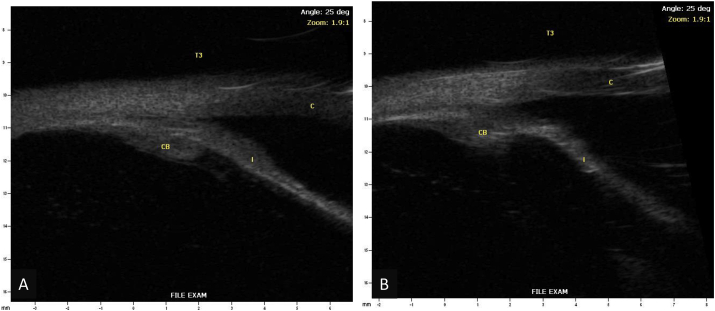
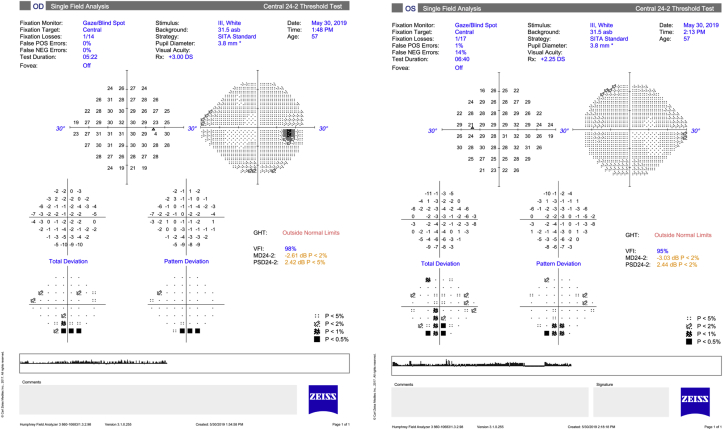
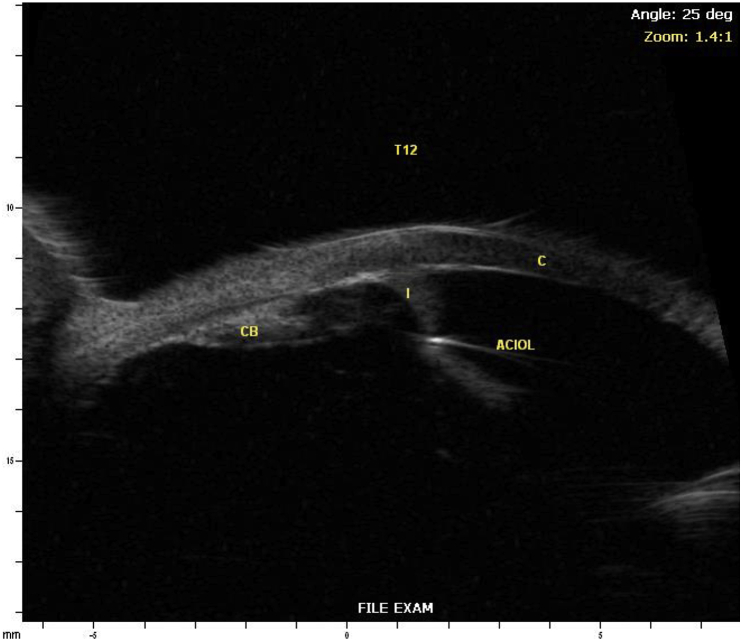
One month later, the patient's IOP OS was 32 mmHg despite taking latanoprost as prescribed and dorzolamide/timolol was added. The patient disclosed that he had started aripiprazole a few weeks prior to his vision changes. He was advised to discontinue aripiprazole to rule out the possibility of a medication-induced angle closure. A UBM showed an anteriorly located ciliary body with possible plateau iris configuration but no evidence of choroidal effusions (Fig. 2). Standard automated perimetry showed bilateral inferior arcuate defects at baseline (Fig. 3).
Fig. 2.
Ultrasound biomicroscopy of the right (A) and left (B) eyes (I – Iris, CB – Ciliary Body, C – Cornea).
Fig. 3.
Humphrey Visual Field 24-2 SITA Standard of the right and left eyes.
One week later after stopping aripiprazole, IOP OS was 13 mm Hg and slit lamp examination demonstrated an improvement in AC depth. Given the improvement in IOP and clinical appearance, dorzolamide/timolol was discontinued.
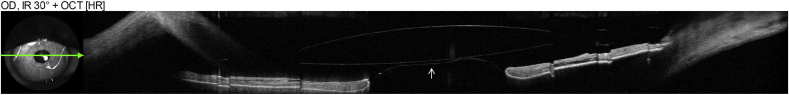
Six weeks later, the patient started lurasidone and brexpiprazole to better manage his depression. IOP OS was 30 mm Hg on latanoprost. Slit lamp examination showed recurrent AC shallowing and anterior bowing of the iris. An AS-OCT demonstrated pupillary block by the ACIOL anteriorly and the vitreous face posteriorly (Fig. 4). A repeat UBM demonstrated more severe anterior iris bowing and an absence of choroidal effusions (Fig. 5). The patient was advised to discontinue lurasidone and brexpiprazole as this class of medication could cause angle closure.
Fig. 4.
AS-OCT of the left eye. White arrow indicates posterior face of the ACIOL optic, red arrow indicates anterior vitreous face. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 5.
Repeat ultrasound biomicroscopy of the left eye (I – Iris, CB – Ciliary Body, C – Cornea).
Two weeks later, the IOP OS was 28 mm Hg on latanoprost. Slit lamp exam continued to show anterior iris bowing and closure of the superior and inferior angles despite discontinuing his psychiatric medications. Due to his ongoing need for psychoactive medications and recurrent episodes of angle closure (Fig. 5), management options (i.e. inferior laser peripheral iridotomy, YAG hyaloidotomy, vitrectomy) were discussed with the patient. The patient elected to undergo the more definitive treatment with a pars plana vitrectomy (PPV).
One month following PPV, the IOP OS was 23 mm Hg on dorzolamide/timolol. On slit lamp exam, the AC was deep with a flat iris. Gonioscopy showed open angles (Shaffer grade 4) with few intermittent peripheral anterior synechiae. To better treat his depression and after discussing the potential risks of recurrent angle closure, a plan was made to restart aripiprazole. On a 2-month follow-up visit, his IOP was 19 mm Hg on dorzolamide/timolol, and there was no evidence of recurrent angle closure on slit lamp examination.
3. Discussion
Here, we present an unusual case of pseudophakic glaucoma and angle closure in a patient with an ACIOL and antipsychotic medication use. Drug-induced angle closure has been reported with the use of antipsychotic medications, including aripiprazole.5 Often, stopping the offending drug can promptly reverse the angle closure and avoid an unnecessary laser peripheral iridotomy.3 In this case, medication discontinuation led to a transient improvement in ocular findings. Surgical intervention led to a definitive treatment that allowed resumption of aripiprazole without ocular sequelae. This likely indicated an anatomical rather than a drug-induced etiology. UBM scans performed after discontinuing antipsychotic medications further support an anatomical etiology as ciliary or choroidal effusions were not observed. While drug-induced angle closure was not the source of pathology in this case, it is important to assess all etiologies leading to angle closure and rule out the possibility of medication-induced angle closure by discontinuing culprit pharmaceuticals.
When evaluating causes of increased intraocular pressure, gonioscopy is a key diagnostic tool to directly visualize and diagnose angle closure.2 However, other imaging modalities, such as UBM and AS-OCT, are needed to help differentiate between causes of angle closure. UBM can help in visualizing posterior structures to rule out posterior masses, cilio-choroidal effusions, and anterior rotation of the ciliary body or rule in abnormal iris anatomy like plateau iris configuration.2
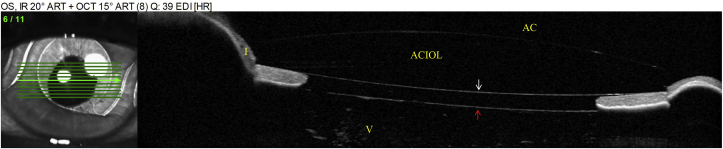
In our patient, AS-OCT revealed that the pathogenesis of angle closure was either due to pseudophakic pupillary block, pupillary block from the anterior vitreous face, or aqueous misdirection. Typically, the clinical exam in aqueous misdirection shows diffuse shallowing of the anterior chamber whereas pupillary block will result in iris bombe with a deep central anterior chamber.6 However, the ACIOL in our patient may have prevented central shallowing of the anterior chamber and complicated the differentiation between aqueous misdirection and pupillary block. Although an iridectomy was performed at the time of ACIOL placement, it is possible for the anterior vitreous to block the iridotomy, essentially rendering the alternative aqueous flow pathway non-functional and leading to angle closure. Of note, in the asymptomatic fellow eye, the anterior vitreous face was found to be anteriorly displaced at the posterior pupillary margin but the ACIOL did not appose the anterior pupillary margin, indicating unobstructed aqueous flow through the patent iridectomy (Fig. 6).
Fig. 6.
AS-OCT of the right eye. Arrow indicates anterior vitreous face.
Therapies for pseudophakic pupillary block glaucoma are similar to therapies for pupillary block in phakic patients. Laser peripheral iridotomy (LPI) is currently the standard initial treatment. If angle closure with elevated IOP persists, surgical intervention (i.e. trabeculectomy, glaucoma drainage device placement, or cyclophotocoagulation) may be required, especially in cases with diffuse PAS.7 In aqueous misdirection, IOP-lowering drops and cycloplegics are often given to help break the attack. Sources cite neodymium:yttrium–aluminum–garnet (Nd:YAG) laser anterior hyaloidotomy accompanied by posterior capsulotomy as first-line treatment; however, this has up to a 75% rate of recurrence.8 Recurrence can also occur following transcorneal needling through the iridotomy.9 We discussed the options of inferior LPI, YAG hyaloidotomy, needling, and PPV with the patient. The patient was less motivated to undergo LPI and YAG due to his concern for photopsias and the high rate of recurrence, respectively. Due to the few intermittent PAS and clinical status, filtering surgery was not necessary at this time. Our patient chose to undergo a more definitive and effective PPV, and the patient has not had a recurrence of angle closure post-operatively.
4. Conclusions
When evaluating a patient with suspected angle closure, it is important to perform a full ophthalmologic examination, including gonioscopy, as well as a thorough review of past medical history and medications so as not to miss systemic-related etiologies. Imaging with B-scan, UBM, and, more recently in the last decade, AS-OCT, is a key component in evaluating a patient presenting with angle closure.
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Funding sources
Mentoring for the Advancement of Physician Scientists, American Glaucoma Society (JLD).
K12 Career Development grant (5K12EY024225-04, National Eye Institute) (JLD).
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The authors have no conflicts of interest to disclose.
Acknowledgements
None.
References
- 1.European Glaucoma Society Terminology and guidelines for glaucoma, 4th edition - chapter 2: classification and terminology supported by the EGS foundation: Part 1: foreword; introduction; glossary; chapter 2 classification and terminology. Br J Ophthalmol. 2017;101(5):73–127. doi: 10.1136/bjophthalmol-2016-EGSguideline.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinreb R.N., Aung T., Medeiros F.A. The pathophysiology and treatment of glaucoma: a review. J Am Med Assoc. 2014;311(18):1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durai I., Mohan Dhavalikar M., Anand C.P., Ganesh V., Krishnadas R. Bilateral, simultaneous, acute angle closure glaucoma in pseudophakia induced by chlorthalidone. Case Rep Ophthalmol Med. 2016;2016:3713818. doi: 10.1155/2016/3713818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moghimi S., Chen R., Hamzeh N., Khatibi N., Lin S.C. Qualitative evaluation of anterior segment in angle closure disease using anterior segment optical coherence tomography. J Curr Ophthalmol. 2016;28(4):170–175. doi: 10.1016/j.joco.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen E., Farukhi S., Schmutz M., Mosaed S. Acute angle-closure glaucoma associated with aripiprazole in the setting of plateau iris configuration. J Glaucoma. 2018;27(2):40–43. doi: 10.1097/IJG.0000000000000836. [DOI] [PubMed] [Google Scholar]
- 6.Van Buskirk E.M. Pupillary block after intraocular lens implantation. Am J Ophthalmol. 1983;95(1):55–59. doi: 10.1016/0002-9394(83)90333-1. [DOI] [PubMed] [Google Scholar]
- 7.Lee L.C., Pasquale L.R. Surgical management of glaucoma in pseudophakic patients. Semin Ophthalmol. 2002;17(3–4):131–137. doi: 10.1076/soph.17.3.131.14778. [DOI] [PubMed] [Google Scholar]
- 8.Grzybowski A., Kanclerz P. Acute and chronic fluid misdirection syndrome: pathophysiology and treatment. Graefes Arch Clin Exp Ophthalmol. 2018;256:135–154. doi: 10.1007/s00417-017-3837-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandler P.A. A new operation for malignant glaucoma: a preliminary report. Trans Am Ophthalmol Soc. 1964;62:408–424. [PMC free article] [PubMed] [Google Scholar]