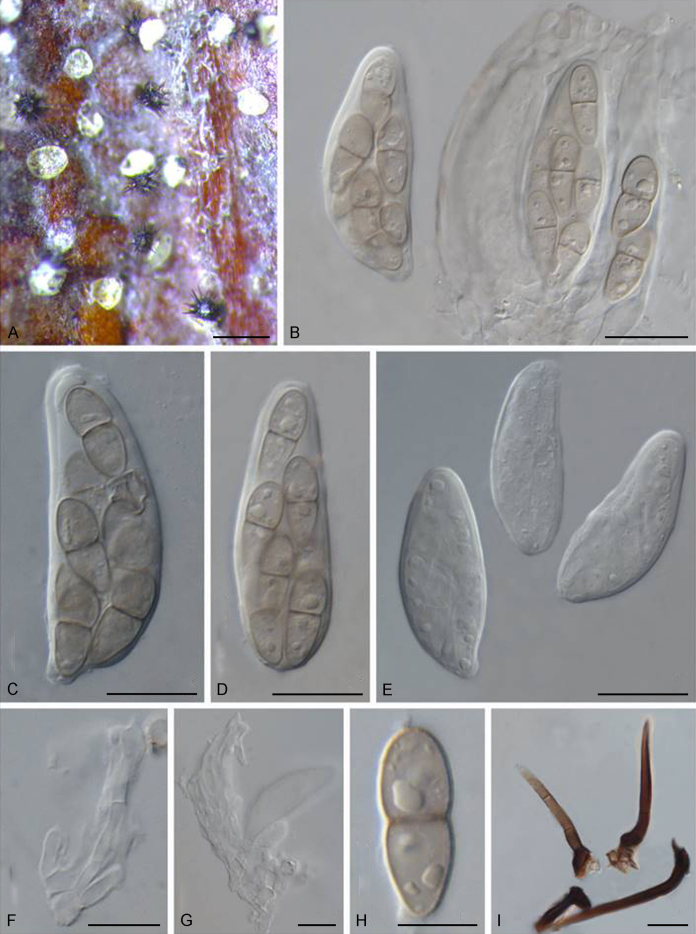
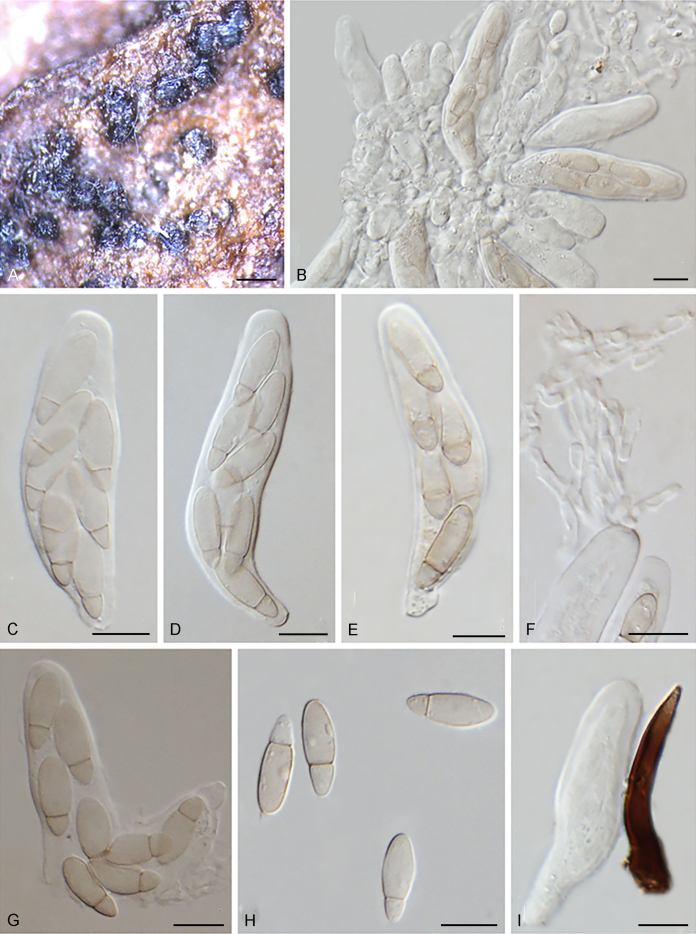
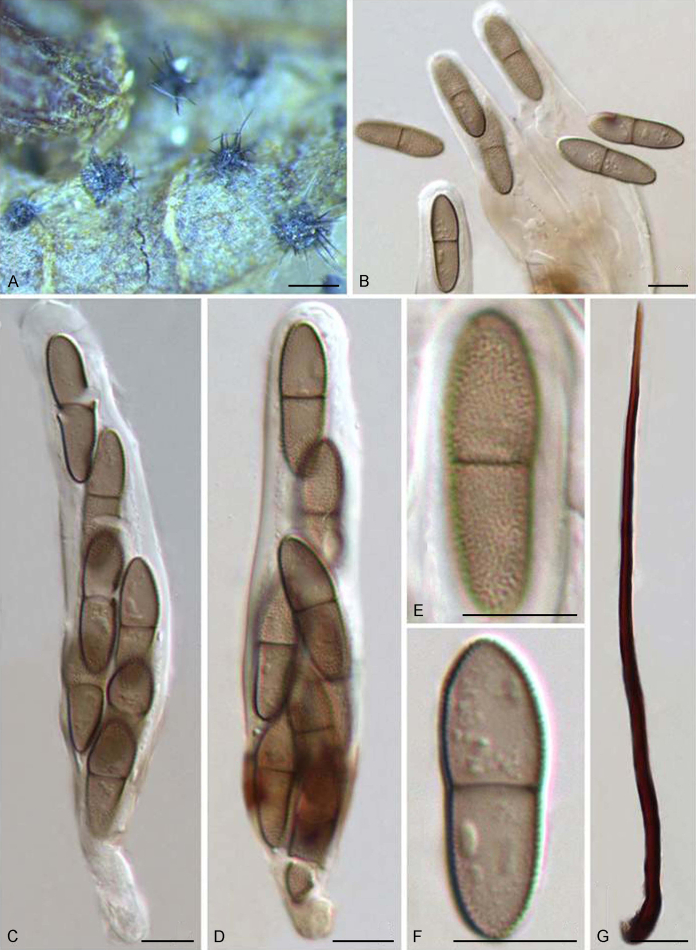
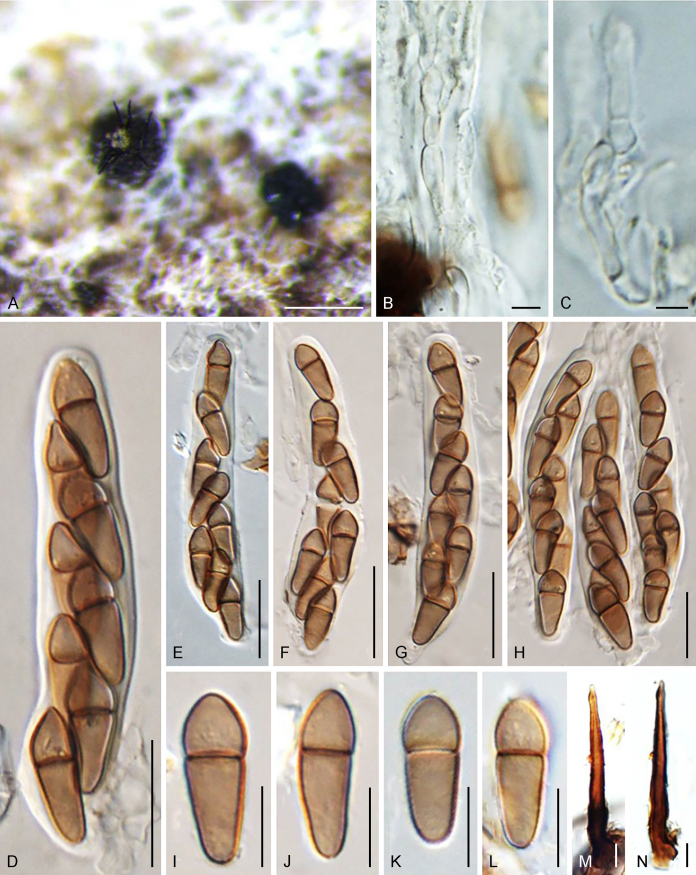
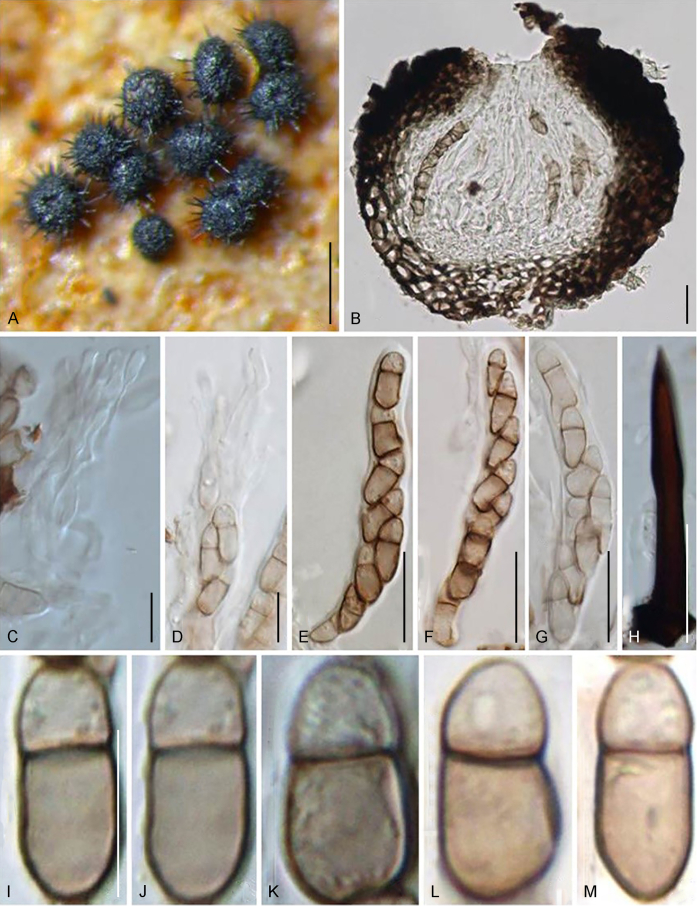
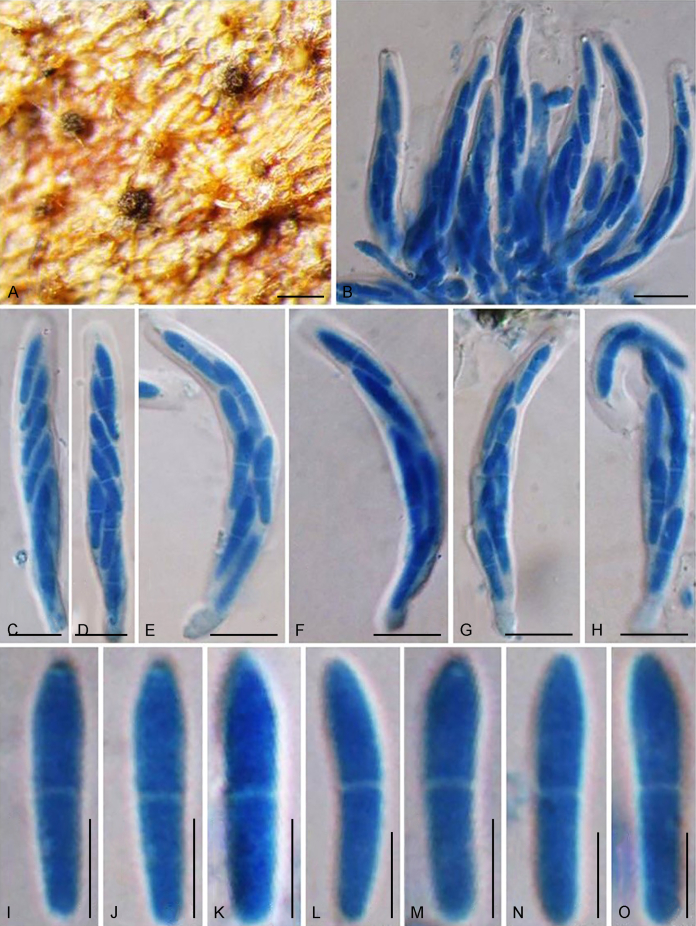
Abstract
Members of Venturiales (Dothideomycetes) are widely distributed, and comprise saprobes, as well as plant, human and animal pathogens. In spite of their economic importance, the general lack of cultures and DNA data has resulted in taxa being poorly resolved. In the present study five loci, ITS, LSU rDNA, tef1, tub2 and rpb2 are used for analysing 115 venturialean taxa representing 30 genera in three families in the current classification of Venturiales. Based on the multigene phylogenetic analysis, morphological and ecological characteristics, one new family, Cylindrosympodiaceae, and eight new genera are described, namely Bellamyces, Fagicola, Fraxinicola, Fuscohilum,Neofusicladium, Parafusicladium, Pinaceicola and Sterila. In addition, 12 species are described as new to science, and 41 new combinations are proposed. The taxonomic status of 153 species have been re-evaluated with 20 species excluded from Venturiales. Based on this revision of Venturiales, morphological characteristics such as conidial arrangement (solitary or in chains) or conidiogenesis (blastic-solitary, sympodial or annellidic), proved to be significant at generic level. Venturia as currently defined represents a generic complex. Furthermore, plant pathogens appear more terminal in phylogenetic analyses within Venturiaceae and Sympoventuriaceae, suggesting that the ancestral state of Venturiales is most likely saprobic.
Key words: Multigene analysis, New taxa, Scab disease, Systematics, Venturia
Taxonomical novelties: New family: Cylindrosympodiaceae Crous, M. Shen & Y. Zhang ter
New genera: Bellamyces Crous, Coppins & U. Braun; Fagicola Crous, M. Shen & Y. Zhang ter; Fraxinicola Crous, M. Shen & Y. Zhang ter; Fuscohilum Crous, M. Shen & Y. Zhang ter; Neofusicladium Crous, M. Shen & Y. Zhang ter; Parafusicladium Crous, M. Shen & Y. Zhang ter; Fuscohil`um Crous, M. Shen & Y. Zhang ter; Pinaceicola Crous, M. Shen & Y. Zhang ter; Sterila Crous, M. Shen & Y. Zhang ter
New species: Bellamyces quercus Crous, Coppins & U. Braun; Fraxinicola europaea Crous, M. Shen & Y. Zhang ter; Fraxinicola italica Crous, M. Shen & Y. Zhang ter; Neocoleroa cameroonensis Crous, M. Shen & Y. Zhang ter; Sterila eucalypti Crous, M. Shen & Y. Zhang ter; Tyrannosorus lichenicola Crous, M. Shen & Y. Zhang ter; Tyrannosorus pini-sylvestris Crous & R.K. Schumach.; Venturia albae Crous, M. Shen & Y. Zhang ter; Venturia australiana Crous, M. Shen & Y. Zhang ter; Venturia caesiae Crous, M. Shen & Y. Zhang ter; Venturia finlandica Crous, M. Shen & Y. Zhang ter; Venturia quebecensis Crous, M. Shen & Y. Zhang ter
New combinations: Fagicola fagi (Crous & de Hoog) Crous, M. Shen & Y. Zhang ter; Fraxinicola fraxini (Aderh.) Crous, M. Shen & Y. Zhang ter; Fraxinicola orni (M. Ibrahim et al.) Crous, M. Shen & Y. Zhang ter; Fuscohilum rhodensis (Crous & M.J. Wingf.) Crous, M. Shen & Y. Zhang ter, Fuscohilum siciliana (Koukol) Crous, M. Shen & Y. Zhang ter; Neofusicladium eucalypti (Crous & R.G. Shivas) Crous, M. Shen & Y. Zhang ter; Neofusicladium eucalypticola (Crous & M.J. Wingf.) Crous, M. Shen & Y. Zhang ter; Neofusicladium regnans (Crous) Crous, M. Shen & Y. Zhang ter; Niesslia iridicola (M.E. Barr) Crous, M. Shen & Y. Zhang ter; Niesslia parasitica (Ellis & Everh.) M. Shen & Y. Zhang ter; Niesslia vaccinii (Ellis & Everh.) Crous, M. Shen & Y. Zhang ter; Parafusicladium amoenum (R.F. Castañeda & Dugan) Crous, M. Shen & Y. Zhang ter; Parafusicladium intermedium (Crous & W.B. Kendr.) Crous, M. Shen & Y. Zhang ter; Parafusicladium paraamoenum (Crous et al.) Crous, M. Shen & Y. Zhang ter; Pinaceicola cordae (Koukol) Crous, M. Shen & Y. Zhang ter; Pinaceicola pini(Crous & de Hoog) Crous, M. Shen & Y. Zhang ter; Pseudosigmoidea excentrica (R.F. Castañeda et al.) Crous, M. Shen & Y. Zhang ter; Scolecobasidium aquaticum (Samerp. et al.) Crous, M. Shen & Y. Zhang ter; Scolecobasidium atlanticuum (A.M. Wellman) Crous, M. Shen & Y. Zhang ter; Scolecobasidium bacilliforme (Samerp. et al.) Crous, M. Shen & Y. Zhang ter; Scolecobasidium capsici (Crous & Cheew.) Crous, M. Shen & Y. Zhang ter; Scolecobasidium cordanae (Samerp. et al.) Crous, M. Shen & Y. Zhang ter; Scolecobasidium dracaenae (Crous) Crous, M. Shen & Y. Zhang ter; Scolecobasidium globale (Samerp. et al.) Crous, M. Shen & Y. Zhang ter; Scolecobasidium icarus (Samerp. et al.) Crous, M. Shen & Y. Zhang ter; Scolecobasidium macrozamiae (Crous & R.G. Shivas) Crous, M. Shen & Y. Zhang ter; Scolecobasidium minimum (Fassat.) Crous, M. Shen & Y. Zhang ter; Scolecobasidium musicola (Crous) Crous, M. Shen & Y. Zhang ter; Scolecobasidium olivaceum (A. Giraldo et al.) Crous, M. Shen & Y. Zhang ter; Scolecobasidium pandanicola (Crous & M.J. Wingf.) Crous, M. Shen & Y. Zhang ter; Scolecobasidium phaeophorum (Samerp. et al.) Crous, M. Shen & Y. Zhang ter; Scolecobasidium podocarpi (Crous) Crous, M. Shen & Y. Zhang ter; Scolecobasidium ramosum (A. Giraldo et al.) Crous, M. Shen & Y. Zhang ter; Scolecobasidium robustum (Samerp. et al.) Crous, M. Shen & Y. Zhang ter; Scolecobasidium sexuale (Samerp. et al.) Crous, M. Shen & Y. Zhang ter; Scolecobasidium verrucosum (Zachariah et al.) Crous, M. Shen & Y. Zhang ter; Sympoventuria africana (Crous) Crous, M. Shen & Y. Zhang ter; Tyrannosorus hanlinianus (U. Braun & Feiler) Crous, M. Shen & Y. Zhang ter; Tyrannosorus hystrioides (Dugan et al.) Crous, M. Shen & Y. Zhang ter; Venturia peltigericola (Crous & Diederich) Crous, M. Shen & Y. Zhang ter; Verruconis terricola (J. Ren et al.) Crous, M. Shen & Y. Zhang ter
Introduction
Venturiales represent an important order within Dothideomycetes (Ascomycota), members of which are widely distributed in temperate and tropical areas of the world, and have diverse lifestyles. Venturiales include plant pathogens causing leaf spots, necroses, scab diseases, leaf and fruit deformations, opportunistic neurotropic pathogens of aquatic animals or humans, and saprobes in soil or plant debris, with some even being thermophilic, living in hot springs (Barron and Busch, 1962, Sivanesan, 1977, Yarita et al., 2007, Yarita et al., 2010, Schoch et al., 2009a, Zhang et al., 2011, Giraldo et al., 2014, Samerpitak et al., 2014).
Members of Venturiaceae occupy about 80 % of the order, and represent the type family of Venturiales. Before the name “Venturiaceae” was introduced, genera of this family were assigned to various families, such as Venturia in Pleosporaceae, Coleroa in Trichosphaeriaceae, Gibbera in Cucurbitariaceae and Stigmatea in Stigmateaceae (Winter 1887). Petrak, 1924, Petrak, 1927, Petrak, 1947 compared the morphology of some genera, i.e., Antennularia, Coleroa, Eriosphaeria, Gibbera, Trichosphaeria and Venturia and proposed a possible relationship among them. Subsequently, the name Venturiaceae was introduced by Müller & von Arx (1950) to accommodate some morphologically comparable genera, such as Antennularia, Coleroa, Endostigme, Gibbera, Spilosticta, Stigmatea and Venturia, and the Venturiaceae was assigned to Pseudosphaeriales. von Arx (1952) redefined the morphological characteristics of Venturiaceae, and circumscribed it to include immersed, semi-immersed or superficial ascomata with or without setae, filiform pseudoparaphyses, clavate, obclavate, bitunicate, 8-spored (sometimes 4-spored) asci, hyaline, pale-olivaceous to brown, and 1-septate, often asymmetrical ascospores. Twelve genera were accepted in the family by von Arx (1952), which later increased to 25 (Müller & von Arx 1962), and eventually to 30 (Luttrell 1973). In further studies members of Venturiaceae of particular host genera or families were investigated (Menon, 1956, Müller, 1958). Nüesch (1960) studied five species of Venturia on Salix, while Bachmann (1963) reported five species of Venturia on Geraniaceae. Sivanesan (1977) studied the type or authentic materials of 58 venturiaceous species, of which 52 species were accepted within Venturia.
Barr (1979) validated the description of Venturiaceae with Venturia Sacc. (vs. Venturia De Not.) designated as the type genus, and accepted 12 genera, viz., Acantharia, Apiosporina, Coleroa, Gibbera, Metacoleroa, Phaeocryptopus, Platychora, Protoventuria, Pyrenobotrys, Trichodothis, Venturia and Xenomeris. Venturiaceae was assigned to Pleosporales based on its “Pleospora type of centrum and bitunicate asci” (Barr, 1968, Barr, 1979). This proposal was supported by subsequent molecular phylogenetic studies (Kodsueb et al., 2006, Kruys et al., 2006, Winton et al., 2007, Zhang et al., 2009, Zhang et al., 2011). A phylogeny of concatenated SSU, LSU and mtSSU DNA sequences indicated that the Venturiaceae clustered outside of Pleosporales (Kruys et al. 2006), being closely related to Tubeufiaceae (Kodsueb et al. 2006). Winton et al. (2007) further demonstrated the polyphyletic status of Venturiaceae and pointed out that the core members of Venturiaceae are monophyletic, while their taxonomic placement was undetermined.
Based on morphological, ecological and multi-locus (SSU, LSU, tef1, rpb1, rpb2) phylogenetic investigations, Zhang et al. (2011) redefined the Venturiaceae as parasitic or saprobic, with immersed, semi-immersed or superficial, gregarious or scattered ascomata, with or without setae, narrow-cellular, evanescent pseudoparaphyses, bitunicate, obclavate, obpyriform asci, and hyaline, yellowish, pale olivaceous to brown, 1-septate, mostly asymmetrical ascospores. Eight genera were accepted within Venturiaceae, viz., Acantharia, Apiosporina, Caproventuria, Coleroa, Dibotryon, Metacoleroa, Pseudoparodiella and Venturia (Zhang et al. 2011).
Asexual morphs of Venturiales include Fusicladium, Pollaccia, Spilocaea and Pseudocladosporium, of which Fusicladium is the most common. Fusicladium was introduced by Bonorden (1851) based on Fusicladium virescens, which is parasitic on pear. Subsequently, F. virescens was treated as a synonym of the older name F. pyrorum (Saccardo, 1886, Lindau, 1907, Viennot-Bourgin and Fernier, 1950, Tai, 1979, Sivanesan, 1984, von Arx, 1987). Lindau (1907) and Ferraris (1912) redefined Fusicladium s. lat. to include conidiogenous cells with sympodial and percurrent proliferation, including pollaccia- and spilocaea-like members. Baldacci & Ciferri (1937) separated Pollaccia from Fusicladium, and resurrected the name Pollaccia. Viennot-Bourgin (1949) accepted Fusicladium s. str., which includes species with percurrently proliferating conidiogenous cells, and those with sympodial conidiogenous cells were assigned to a new genus Megacladosporium. Megacladosporium, however, was invalid as it lacked a generic type. Hughes (1953) circumscribed Fusicladium s. str. as having sympodially proliferating conidiogenous cells and somewhat denticle-like conidiogenous loci and assigned the species with obvious percurrently proliferating conidiogenous cells to Spilocaea. Schubert et al. (2003) accepted Fusicladium s. lat., with Pollaccia and Spilocaea as synonyms.
Phylogenetic analyses of ITS and LSU sequences indicated that species of Pollaccia and Spilocaea were intermingled among Fusicladium species, and Pollaccia, Spilocaea as well as Pseudocladosporium were considered as synonyms of Fusicladium (Beck et al., 2005, Crous et al., 2007b). Crous et al. (2007b) indicated that the arrangement of the conidiophores (solitary, fasciculate or sporodochial), the proliferation of conidiogenous cells (sympodial, percurrent) and shape, size as well as formation of conidia (solitary, catenate) had little taxonomic value at generic level. However, a DNA phylogeny based on five loci, namely SSU, LSU, rpb1, rpb2 and tef1, supported a narrower circumscription of Venturia, which included only a small number of species closely related to the generic type of Venturia (V. inaequalis) (Zhang et al. 2011). Thus Pollaccia, Pseudocladosporium and Spilocaea were again treated as separate genera (Zhang et al. 2011).
Based on an ecological, morphological and molecular phylogenetic analysis, a second family, Sympoventuriaceae, was introduced to accommodate Sympoventuria, Veronaeopsis and fusicladium-like species (Zhang et al. 2011). Scolecobasidium, a soil-borne genus, was described based on two species, i.e., S. terreum (type species) and S. constrictum (Abbott 1927). Subsequently, more soil-borne or saprotrophic species were described within Scolecobasidium (Barron and Busch, 1962, Roy et al., 1962). Ochroconis was separated from Scolecobasidium based on its ellipsoidal, clavate or fusiform conidia, in contrast to the trilobate conidia of Scolecobasidium (De Hoog & von Arx 1974). This proposal was not supported by subsequent molecular phylogenetic analyses, in which members of Scolecobasidium and Ochroconis clustered in a single clade (Hao et al., 2013, Ren et al., 2013). Verruconis was introduced as a thermophilic genus, which includes V. gallopava, an opportunistic neurotropic pathogen, and its sibling, V. calidifluminalis (Samerpitak et al. 2014). Neocoleroa metrosideri was described as a pathogen causing leaf spots on Metrosideros excelsa, which was widespread in M. excelsa forests in northern New Zealand (Johnston & Park 2016). Although DNA sequences were not available for the type species of Neocoleroa, N. sibirica, the comparable morphological characteristics with N. metrosideri argued for their congeneric status (Barr, 1987, Johnston and Park, 2016). Clavatispora was introduced as monotypic genus within Sympoventuriaceae represented by Clavatispora thailandica, which is characterised by its muriformly septate ascospores (Boonmee et al. 2014). A further asexual genus, Yunnanomyces, was introduced to accommodate Y. pandanicola, with globose to broadly oval, yellow-brown, muriformly septate conidia (Tibpromma et al. 2018). Pseudosigmoidea was separated from Sigmoidea based on its enteroblastic conidia and phialidic conidiogenesis (Ando & Nakamura 2000), and Sympodiella was emended to include a repetophragma-like synasexual morph within Sympoventuriaceae (Crous et al. 2019a).
Numerous strains belonging to Venturiales were examined in the present study, including the established genera Clavatispora, Ochroconis, Scolecobasidium, Sympodiella, Sympoventuria, Veronaeopsis, Verruconis and Yunnanomyces. The primary objectives were: 1) to delineate the phylogenetic lineages, families and generic boundaries; 2) and to designate appropriate types to stabilise the application of names. To address these issues, we performed multi-locus phylogenetic analyses based on ITS, LSU rDNA, tef1, tub2 and rpb2 DNA sequence data.
Materials and methods
Isolates
Cultures were obtained from the culture collection (CBS) of the Westerdijk Fungal Biodiversity Institute (WI), Utrecht, the Netherlands, and the working collection of Pedro Crous (CPC) housed at the WI, and the Chinese General Microbiological Culture Collection Center (CGMCC) (Table 1). Isolates were subcultured onto fresh malt extract agar (MEA), oatmeal agar (OA), potato dextrose agar (PDA) and synthetic nutrient-poor agar (SNA) (Crous et al. 2019b) and incubated at 25 °C under continuous near-ultraviolet light to induce sporulation.
Table 1.
Collection details and GenBank accession number of isolatea belonging to species treated in this study.
| Taxa | Culture accession number(s)1 | Host, substrate | Country | Collector and collection date | GenBank accession numbers2 |
||||
|---|---|---|---|---|---|---|---|---|---|
| ITS | LSU | rpb2 | tef1 | tub2 | |||||
| Cylindrosympodiaceae | |||||||||
| Cylindrosympodium lauri | CBS 240.95T | Laurus sp., leaf litter | Spain | R.F. Castañeda, 4 Jan. 1995 | EU035414 | EU035414 | – | – | – |
| C. variabile | CBS 563.82T | Pinus sp., decaying needle | Netherlands | G.S. de Hoog, 5 Sep. 1982 | C. variabile | KX228353 | – | – | – |
| Pseudoanungitea syzygii | CBS 520.93T | Syzygium cordatum, leaf litter | South Africa | W.J. Swart, Mar. 1993 | MH107911 | MH107957 | – | – | – |
| P. vaccinii | CBS 143164T | Vaccinium myrtillus, stem | Germany | R.K. Schumacher, 16 Jan. 2016 | MK810899 | MK810786 | MK887794 | MK888724 | MK926466 |
| CPC 30523 | Vaccinium myrtillus, stem | Germany | R.K. Schumacher, 16 Jan. 2017 | MK810900 | MK810787 | MK887795 | MK888725 | MK926467 | |
| P. variabilis | CBS 132716T | Dead wood | Spain | M. Hernández-Restrepo, J. Mena & J. Guarro, May 2011 | KY853424 | KY853484 | – | – | – |
| Septonema crispulum | CBS 735.96T | Pinus pinea, needle litter | Italy | D. Lunghini, – | MH862607 | MH874232 | – | – | – |
| Sympodiella acicola | CBS 425.76 | Pinus sylvestris, decaying needle | Netherlands | W. Gams, Mar. 1976 | KY853467 | KY853529 | – | – | – |
| CBS 487.82 | Pinus sylvestris, needle | Netherlands | – | KY853468 | KY853530 | – | – | – | |
| S. goidanichii | CBS 987.70 | Betula sp., old leaf litter | UK | – | MH860019 | MH871803 | – | – | – |
| CBS 136.58T | Fagus sylvatica, cupule | Italy | – | MH857722 | MH869262 | – | – | – | |
| Tothia fuscella | CBS 130266 | Teucrium chamaedrys | Austria | H. Voglmayr, 15 Sep. 2010 | MH865619 | MH877042 | – | – | – |
| WU 31396T | Teucrium chamaedrys, stalks | Austria | W. Jaklitsch, 3 Jul. 2010 | JF927787 | JF927787 | – | – | – | |
| T. spartii | MFLUCC 14-0615T | Spartium junceum, living and dead branches | Italy | E. Camporesi, 17 Mar. 2012 | NR132917 | KR025865 | – | – | – |
| Sympoventuriaceae | |||||||||
| Bellamyces quercus | CBS 46217T = CPC 28858 | Lecanora chlarotera on Quercus trunks | UK | B.J. Coppins, 24 Aug. 2015 | MK810901 | MK810788 | MK887796 | MK888726 | – |
| Echinocatena arthrinioides | CBS 144202 | Acacia crassicarpa | Malaysia | M.J. Wingfield, 1 Jul. 2015 | MH107890 | MH107937 | – | – | – |
| Fuscohilum rhodensis | CBS 121641T | Ceratonia siliqua, branches | Greece | P.W. Crous & M.J. Wingfield, 1 Jun. 2006 | MK810909 | MK810796 | MK887802 | MK888733 | MK926471 |
| F. siciliana | CBS 105.85T | Chamaerops humilis | Italy | W. Gams, Nov. 1984 | MK810910 | MK810797 | MN091924 | MK888734 | MK926472 |
| Neocoleroa metrosideri | ICMP 21139T | Metrosideros excelsa | New Zealand | P.R. Johnston, 6 Oct. 2015 | KU131678 | KU131677 | – | – | – |
| Nc. cameroonensis | CBS 129041T | Crematogaster sp. (ant) carton on Barteria nigritana | Cameroon | R. Blatrix, 19 Dec. 2009 | MK810902 | MK810789 | MK887797 | MK888727 | MN078219 |
| Neofusicladium eucalypti | CBS 128216T | Eucalyptus regnans, leaf litter | Australia | P.W. Crous & R.G. Shivas, 12 Jul. 2009 | MK810903 | MK810790 | MK887798 | MK888728 | MK926468 |
| Nf. eucalypticola | CBS 141301T | Eucalyptus robusta, leaf litter | France | P.W. Crous & M.J. Wingfield, 8 Mar. 2015 | MK810904 | MK810791 | MK887799 | MK888729 | – |
| CBS 143427 | Eucalyptus dunnii, leaves | Australia | A.J. Carnegie, 20 Jan. 2016 | MK810905 | MK810792 | – | – | – | |
| Nf. regnans | CBS 143411T | Eucalyptus regnans, leaves | Australia | P.W Crous, 30 Nov. 2016 | MG386066 | MG386119 | – | – | MG386169 |
| Parafusicladium amoenum | CBS 254.95T | Eucalyptus sp., fallen leaves | Cuba | R.F. Castañeda, 2 Nov. 1994 | MK810906 | MK810793 | – | MK888730 | MK926469 |
| Pa. intermedium | CBS 110746T | Eucalyptus sp., leaf litter | Madagascar | P.W. Crous, 30 Apr. 1994 | MK810907 | MK810794 | MK887800 | MK888731 | MK926470 |
| Pa. paraamoenum | CBS 141322T | Eucalyptus regnans, leaf litter | Australia | P.W. Crous, J. Edwards & P.W.J. Taylor, 9 Nov. 2014 | MK810908 | MK810795 | MK887801 | MK888732 | – |
| Pinaceicola cordae | CBS 126959T | Pinus sylvestris, litter needles | Czech Republic | O. Koukol, 11 Dec. 2006 | MK810911 | MK810798 | – | MK888735 | MK926473 |
| CBS 675.82 | Pinus sylvestris, litter needles | Netherlands | G.S. de Hoog, 8 Nov. 1982 | MK810912 | MK810799 | – | MK888736 | MK926474 | |
| CBS 143494 | Pinus sylvestris, litter needles | Germany | R.K. Schumacher, 5 Feb. 2016 | MK810913 | MK810800 | – | MK888737 | MK926475 | |
| Pi. pini | CBS 462.82 | Pinus sp., litter needles | Netherlands | G.S. de Hoog, 12 Apr. 1982 | MK810914 | MK810801 | MK887803 | MK888738 | MK926476 |
| CBS 463.82T | Pinus sylvestris, litter needles | Netherlands | G.S. de Hoog, 12 Apr. 1982 | MK810915 | MK810802 | MK887804 | MK888739 | MK926477 | |
| Pseudosigmoidea excentrica | CBS 469.95T | Lauraceae, leaf litter | Cuba | R.F. Castañeda, 6 Aug. 1994 | HQ667543 | KF282669 | – | KF155975 | MK926478 |
| Ps. ibarakiensis | NBRC 107891T | Natural forest soil | Japan | –, 2008 | LC146758 | LC146759 | – | – | – |
| Scolecobasidium anellii | CBS 284.64T | Stalactite | Italy | A. Graniti, – | FR832477 | KF156138 | KF282684 | KF155995 | KF156184 |
| Sc. anomalum | CBS 131816T | Cave sediment | France | F. Bastian, – | HE575201 | KF156137 | HE575205 | KF155986 | KF156194 |
| Sc. aquaticum | CBS 140316T | Silicone seal in shower of fish-processing company | Germany | K. Gloyna, 28 Oct. 2014 | KX668258 | KX668259 | – | – | – |
| Sc. constrictum | CBS 211.53T | Soil | Canada: Ontario | R.G. Atkinson, 1952 | HQ667519 | KF282653 | KF282686 | KF156005 | KF156187 |
| Sc. cordanae | CBS 475.80T | Mauritia minor, leaf litter | Colombia | W. Gams & O. Vargas, 10 Dec. 1979 | KF156022 | KF156122 | KF282687 | KF155981 | – |
| Sc. dracaenae | CBS 141323T | Dracaena reflexa, leaf spots | USA | P.W Crous, Aug. 20113 | KX228283 | KX228334 | KX228370 | KX228377 | – |
| Sc. ellipsoideum | CBS 131796T | Soil | China | Hui-Mei Liu, – | MN077367 | – | KC337073 | – | – |
| Sc. gamsii | CBS 239.78T | Caryota plumosa, leaf | Sri Lanka | W. Gams, Jan. 1973 | KF156019 | KF156150 | – | KF155982 | KF156190 |
| Sc. globale | CBS 119644T | Indoor sample, house | Germany | –, 2002 | KF961086 | KF961097 | – | KF961075 | KF961065 |
| Sc. icarus | CBS 536.69T | Forest soil | Canada: Ontario | – | HQ667524 | KF156132 | – | – | KF156174 |
| Sc. lascauxense | CBS 131815T | Black stain on cave sediment | France | Fabiola Bastian, 26 Aug. 2008 | FR832474 | KF156136 | FR832481 | KF155994 | KF156183 |
| Sc. macrozamiae | CBS 137971T | Macrozamia, leaf litter | Australia | P.W. Crous & R.G. Shivas, 16 Jul. 2009 | KJ869123 | KJ869180 | – | – | – |
| Sc. minimum | CBS 510.71T | Gossypium arboreum, rhizosphere | Nigeria | M. Dransfield, – | HQ667522 | KF156134 | – | KF156007 | KF156172 |
| Sc. musae | CBS 729.95T | Regulator of diver | – | Streeklab voor Volksgezondheid Haarlem, – | KF156029 | KF156144 | KF282693 | KF155999 | KF156171 |
| Sc. musicola | CBS 144441T | Musa sp., leaf | Malaysia | P.W. Crous, 2010 | MH327824 | MH327860 | – | MH327887 | – |
| Sc. olivaceum | CBS 137170T | Man, bronchoalveolar lavage fluid | USA: Utah | D.A. Sutton, 2010 | LM644521 | LM644564 | – | – | LM644605 |
| Sc. pandanicola | CBS 140660T | Pandanus utilis, leaves | France | P.W Crous & M.J Wingfield, 6 Mar. 2014 | KT950850 | KT950864 | – | – | – |
| Sc. phaeophorum | CBS 206.96T | Leaf in coastal rain forest | Papua New Guinea | A. Aptroot & A. van Iperen, 1995 | KP798631 | KP798634 | KF282692 | KT272098 | KT272062 |
| Sc. podocarpi | CBS 143174T | Podocarpus grayae, leaves | Australia | P.W Crous, 25 Nov. 2016 | MG386032 | MG386085 | – | MG386162 | – |
| Sc. ramosum | UTHSC 12-1082T | Man, nail | USA: California | D.A. Sutton, 2012 | LM644524 | LM644524 | – | – | LM644608 |
| Sc. sexuale | CBS 135765T | Swabs (control in a laboratory providing medical supplies) | South Africa | E.J van der Linde, 2012 | KF156018 | KF156118 | – | KF155976 | KF156189 |
| Sc. terreum | CBS 203.27T | Soil | USA: Louisiana | E.V. Abbott, 1927 | HQ667544 | – | KF282698 | – | HQ877665 |
| Sc. tshawytschae | CBS 100438T | Fish | – | M.S. Doty | HQ667562 | KF156126 | KF282697 | KF155990 | KF156180 |
| Sc. verrucosum | CBS 383.81T | Soil | India | S. Zachariah, – | KF156015 | KF156129 | – | KT272099 | KF156185 |
| Sterila eucalypti | CPC 14942 | Eucalyptus sp. | Portugal | P.W. Crous, 24 Jan. 2008 | MK810916 | MK810803 | MK887805 | MK888740 | – |
| CPC 14943 | Eucalyptus sp. | Portugal | P.W. Crous, 24 Jan. 2008 | MK810917 | MK810804 | MK887806 | MK888741 | – | |
| CBS 144019T | Eucalyptus sp. | Portugal | P.W. Crous, 24 Jan. 2008 | MK810918 | MK810805 | MK887807 | MK888742 | – | |
| Sympoventuria africana | CBS 121639T | Eucalyptus sp., leaf litter | South Africa | P.W. Crous, 2006 | MK810919 | MK810806 | MK887808 | MK888743 | MK926479 |
| CBS 121640 | Eucalyptus sp., leaf litter | South Africa | P.W. Crous, 2006 | MK810920 | MK810807 | MK887809 | MK888744 | MK926480 | |
| Sy. capensis | CBS 120136T | Eucalyptus sp., leaf litter | South Africa | P.W. Crous, Jan. 2006 | MK810921 | MK810808 | MK887810 | MK888745 | MK926481 |
| CPC 12839 | Eucalyptus sp., leaf litter | South Africa | P.W. Crous, Jan. 2006 | MK810922 | MK810809 | MK887811 | MK888746 | MK926482 | |
| CPC 12840 | Eucalyptus sp., leaf litter | South Africa | P.W. Crous, Jan. 2006 | MK810923 | MK810810 | MK887812 | MK888747 | MK926483 | |
| Sy. melaleucae | CBS 143407T | Melaleuca sp., leaves | Australia | P.W Crous, 2 Dec. 2016 | MG386059 | MG386112 | – | – | MG386168 |
| Troposporella fumosa | CBS 351.94 | Plant litter | Italy | A. van Beverwijk, Sep. 1954 | MK810924 | MH874121 | – | – | – |
| T. monilipes | MUCL 19867 | – | Sweden | G.L. Hennebert, – | DQ351723 | AY856871 | – | – | – |
| T. olivaceum | CBS 728.83 | Dicksonia antarctica, dead petiole | Australia | W. Gams, Aug. 1983 | MH861681 | MH873393 | – | – | – |
| Veronaeopsis simplex | CBS 588.66T | Acacia karroo, leaf litter | South Africa | M.C. Papendorf, – | EU041820 | EU041877 | MN091925 | – | – |
| Verruconis calidifluminalis | CBS 125818T | Water of a hot stream | Japan | –, 1 Mar. 2004 | AB385698 | KF156108 | – | KF155959 | – |
| V. gallopava | CBS 118.91 | Man | USA: Georgia | A.A. Padhye, – | HQ667551 | KF282655 | KF282688 | JF440539 | HQ877643 |
| CBS 437.64T | Meleagris gallopavo (turkey), brain abscess | USA: South Carolina | W.B. Cooke, – | HQ667553 | KF282656 | KF282689 | KF155968 | KF156203 | |
| CBS 867.95 | Sputum from patient with angina and left ventricular heart dysfunction | USA: Maryland | A.A. Padhye, CDC, Atlanta, USA, – | HQ667561 | KF282657 | KF282690 | KF155972 | KF156213 | |
| V. panacis | CGMCC 3.18302T | Panax notoginseng, root | China: Yunnan province | Y. Zhang, 15 Oct. 2015 | MF536882 | MF536880 | – | MF536881 | MF536883 |
| V. terricola | CBS 131795T | Soil | China | Y.L. Zhang, Dec. 2009 | MK810925 | MK810811 | KC337072 | – | – |
| V. verruculosa | CBS 119775 | Hevea sp., root | Malaysia | – | KF156014 | KF282668 | – | KF155974 | KF156193 |
| Venturiaceae | |||||||||
| Apiosporina collinsii | CBS 118973 | Amelanchier alnifolia | Canada: Ontario | – | MK810926 | MK810812 | MK887813 | MK888748 | – |
| A. morbosa | dimosp | Prunus sp. | USA: Washington | – | – | EF114694 | – | – | – |
| Coleroa circinans | CBS 457.64 | Geranium rotundifolium | France | C. Bachmann, 26 Jun. 1961 | MK810931 | MK810817 | MK887818 | MK888753 | MN078220 |
| C. robertiani | CBS 458.64T | Geranium robertianum | Switzerland | C. Bachmann, 28 Sep. 1960 | MK810932 | MK810818 | MK887819 | MK888754 | MK926488 |
| Coleroa sp. 1 | CBS 372.53 | Acer pseudoplatanus | Switzerland | – | MK810927 | MK810813 | MK887814 | MK888749 | MK926484 |
| CBS 372.55 | Cephalaria alpina | Switzerland | – | MK810930 | MK810816 | MK887817 | MK888752 | MK926487 | |
| Coleroa sp. 2 | CBS 378.49 | Gentiana lutea | Switzerland | J.A. von Arx, 5 Jun. 1949 | MK810929 | MK810815 | MK887816 | MK888751 | MK926486 |
| Coleroa sp. 3 | CBS 370.55 | Anemone alpina | France | – | MK810928 | MK810814 | MK887815 | MK888750 | MK926485 |
| Cylindrosympodioides brabeji | CBS 141285T | Brabejum stellatifolium, leaf litter | South Africa | P.W. Crous & M.J. Wingfield, 17 Jan. 2015 | KX228256 | KX228308 | – | – | – |
| Fagicola fagi | CBS 621.84T | Fagus sylvatica, decaying leaves | Netherlands | G.S. de Hoog, 1 Oct. 1984 | MK810933 | MK810819 | MK887820 | MK888755 | MK926489 |
| Fraxinicola europaea | CBS 472.61T | Betula alba | Switzerland | E. Müller, 8 Apr. 1959 | MK810934 | MK810820 | MK887821 | MK888756 | MK926490 |
| CBS 477.61 | Populus tremula | France | – | MK810935 | MK810821 | MK887822 | MK888757 | MK926491 | |
| CBS 689.85 | Populus tremula, leaf litter | France | – | MK810936 | MK810822 | MK887823 | MK888758 | MK926492 | |
| CBS 377.53 | Epilobium montanum | France | – | MK810937 | MK810823 | MK887824 | MK888759 | MK926493 | |
| F. fraxini | CBS 130599T | Leaves of Protea sp., in assocation with Vizella interrupta | South Africa | P.W Crous, 5 May 2010 | MK810938 | MK810824 | MK887825 | MK888760 | MK926494 |
| CBS 140929 | Fraxinus ornus, leaf endophyte | Italy | M. Schlegel, – | MK810939 | MK810825 | MK887826 | MK888761 | MK926495 | |
| CBS 140930T | Fraxinus excelsior, leaf endophyte | Switzerland | M. Schlegel, – | MK810940 | MK810826 | MK887827 | MK888762 | MK926496 | |
| CBS 140935 | Fraxinus excelsior, leaf litter | Switzerland | M. Ibrahim, – | MK810941 | MK810827 | MK887828 | MK888763 | MK926497 | |
| CBS 374.55 | Fraxinus excelsior | Switzerland | E. Müller, 10 Jul. 1953 | MK810942 | MK810828 | MK887829 | MK888764 | MK926498 | |
| F. italica | CBS 140918T | Fraxinus ornus, leaf endophyte | Italy | M. Ibrahim, 5 Nov. 2013 | MK810943 | MK810829 | MK887830 | MK888765 | MK926499 |
| F. orni | CBS 140919 | Fraxinus ornus, leaf endophyte | Italy | M. Ibrahim, 5 Nov. 2013 | MK810944 | MK810830 | MK887831 | MK888766 | MK926500 |
| CBS 140920 | Fraxinus ornus, leaf endophyte | Italy | M. Ibrahim, 5 Nov. 2013 | MK810945 | MK810831 | MK887832 | MK888767 | MK926501 | |
| CBS 140921 | Fraxinus ornus, leaf endophyte | Italy | M. Ibrahim, 5 Nov. 2013 | MK810946 | MK810832 | MK887833 | MK888768 | MK926502 | |
| CBS 140922 | Fraxinus ornus, leaf endophyte | Switzerland | M. Ibrahim, 13 Nov. 2013 | MK810947 | MK810833 | MK887834 | MK888769 | MK926503 | |
| CBS 140924T | Fraxinus ornus, leaf litter | Switzerland | M. Schlegel, 4 May 2015 | MK810948 | MK810834 | MK887835 | MK888770 | MK926504 | |
| Gibbera conferta | CBS 191.53 | Vaccinium uliginosum | Switzerland | E. Müller, – | – | GU301814 | – | – | – |
| Helicoon myosuroides | CBS 743.96T | Betula pubescens, leaf | Austria | H. Voglmayr, 23 Oct. 1993 | MH862608 | MH874233 | – | – | – |
| Metacoleroa dickiei | medipc | Linnaea borealis | USA: Oregon | – | – | EF114695 | – | – | – |
| Protoventuria barriae | CBS 300.93 | Vaccinium macrocarpon | USA | L.M. Carris, – | MK810949 | JQ036232 | MK887836 | MK888771 | MK926505 |
| Tyrannosorus hystrioides | CBS 117727T | Prunus avium cv. Bing, Bing cherry fruit | USA | – | MK810950 | MK810835 | MK887837 | MK888772 | MK926506 |
| T. lichenicola | CBS 144018T | Letharia sp. | USA | A. Smith, 27 May 2013 | MK810953 | MK810838 | MK887840 | MK888775 | MK926509 |
| T. pini-sylvestris | CBS 143393T | Pinus sylvestris, needles | Germany | R.K. Schumacher, 5 Feb. 2016 | MK810952 | MK810837 | MK887839 | MK888774 | MK926508 |
| T. pinicola | CBS 124.88T | Pinus wood, from river | Pakistan | O. Petrini, – | MK810951 | MK810836 | MK887838 | MK888773 | MK926507 |
| Venturia albae | CBS 468.61 | Salix alba | Liechtenstein | J. Nüesch, 13 May 1958 | MK810954 | MK810839 | MK887841 | MK888776 | MK926510 |
| CBS 471.61T | Salix alba | Liechtenstein | – | MK810955 | MK810840 | MK887842 | MK888777 | MK926511 | |
| V. atriseda | CBS 371.55 | Gentiana punctata | Switzerland | – | EU035448 | – | – | – | KF808265 |
| V. aucupariae | CBS 365.35 | Sorbus aucuparia moravica | Germany | – | MK810956 | MK810841 | MK887843 | MK888778 | MK926512 |
| CBS 366.35 | Sorbus aucuparia moravica | Germany | – | MK810957 | MK810842 | MK887844 | MK888779 | MK926513 | |
| V. australiana | CBS 128286T | Leaf spot of unknown plant | Australia | – | MK810958 | MK810843 | – | – | MK926514 |
| V. caesiae | CBS 466.61T | Salix caesia | Switzerland | J. Nüesch, 2 Jul. 1959 | MK810959 | MK810844 | MK887845 | MK888780 | MK926515 |
| V. catenospora | CGMCC 3.18369 | Salix sp. | China | Y. Zhang & Y. Zhou, 22 Aug. 2014 | MK810960 | MK810845 | – | MK888781 | – |
| CBS 447.91T | Salix triandra, brown leaf spot | Germany | H. Butin, 7 Aug. 1990 | MK810961 | MK810846 | MK887846 | MK888782 | MK926516 | |
| CBS 469.61 | Salix caprea | Switzerland | J. Nüesch, 10 Jun. 1958 | MK810962 | MK810847 | MK887847 | MK888783 | MK926517 | |
| V. cerasi | CBS 160.55 | Prunus amygdalus, fruit | USA: California | – | MK810963 | MK810848 | MK887848 | MK888784 | MK926518 |
| CBS 444.54 | Prunus cerasus 'Schattenmorelle' | Germany | – | MK810964 | MK810849 | MK887849 | MK888785 | MK926519 | |
| CBS 497.62 | Prunus domestica subsp. syriaca 'Mirabelle' | Switzerland | – | MK810965 | MK810850 | – | MK888786 | MK926520 | |
| V. chinensis | CGMCC 3.17685T | Lonicera praeflorens | China | Y. Zhang & Y. Zhou, 26 Aug. 2014 | MK810966 | MK810851 | MK887850 | MK888787 | MK926521 |
| V. chlorospora | CBS 467.61 | Salix daphnoides | Switzerland | J. Nüesch, 2 Jul. 1959 | MK810967 | MK810852 | MK887851 | MK888788 | MK926522 |
| CBS 470.61 | Salix daphnoides | France | J. Nüesch, 25 Jun. 1958 | MK810968 | MK810853 | MK887852 | MK888789 | MK926523 | |
| V. convolvularum | CBS 112706T | Convolvulus arvensis, leaves | New Zealand | C.F. Hill, 7 Nov. 2000 | MK810969 | MK810854 | MK887853 | MK888790 | MK926524 |
| V. crataegi | CBS 367.35 | Sorbus aucuparia rossica | Germany | – | MK810970 | MK810855 | MK887854 | MK888791 | MK926525 |
| CBS 368.35 | Crataegus sp. | Germany | – | MK810971 | MK810856 | MK887855 | MK888792 | MK926526 | |
| CBS 369.35 | Crataegus sp. | Germany | – | MK810972 | MK810857 | MK887856 | MK888793 | MK926527 | |
| V. ditricha | CBS 115426 | Betula pubescens var. tortuosa | Finland | M. Helander, 1 Aug. 1992 | MK810973 | MK810858 | MK887857 | MK888794 | MK926528 |
| CBS 118894 | Betula pubescens var. tortuosa, leaves | Finland | M. Helander, – | MK810974 | MK810859 | MK887858 | MK888795 | MK926529 | |
| CBS 257.38 | Populus tremula | Italy | O. Servazzi, – | MK810975 | MK810860 | MK887859 | MK888796 | MK926530 | |
| V. finlandica | CBS 112703 | Betula pubescens var. tortuosa | Finland | M. Helander, 1 Jul. 1993 | MK810976 | MK810861 | – | MK888797 | MK926531 |
| CBS 115442T | Betula pubescens var. tortuosa | Finland | M. Helander, – | MK810977 | MK810862 | – | MK888798 | MK926532 | |
| V. fuliginosa | CGMCC 3.18370T | Salix capitata | China | Y. Zhang & Y. Zhou, 27 Aug. 2014 | MK810978 | MK810863 | MK887860 | MK888799 | MK926533 |
| V. helvetica | CBS 474.61 | Salix helvetica | Switzerland | J. Nüesch, 2 Jul. 1959 | MK810979 | MK810864 | MK887861 | MK888800 | MK926534 |
| CBS 475.61 | Salix helvetica | Switzerland | J. Nüesch, 1 Jul. 1959 | MK810980 | MK810865 | MK887862 | MK888801 | MK926535 | |
| V. inaequalis | CGMCC 3.18372 | Malus sp. | China | F. Ma, 27 Jul. 2015 | MK810981 | MK810866 | MK887863 | MK888802 | MK926536 |
| CBS 120625 | Apple (Malus x domestica) | South Africa | – | MK810982 | MK810867 | MK887864 | MK888803 | MK926537 | |
| CBS 120627T | Apple (Malus x domestica) | Sweden | – | MK810983 | MK810868 | MK887865 | MK888804 | MK926538 | |
| V. lonicerae | CBS 445.54 | Lonicera coerulea | Switzerland | – | MK810984 | MK810869 | MK887866 | MK888805 | MK926539 |
| V. mandshuricum | CBS 112235T | Populus simonii | China | –, 20 Apr. 1993 | MK810985 | MK810870 | MK887867 | MK888806 | MK926540 |
| V. martianoffiana | CGMCC 3.18375 | Populus sp. | China | Y. Zhang, 27 Aug. 2014 | MK810986 | MK810871 | MN091926 | MK888807 | MK926541 |
| CGMCC 3.18377 | Populus sp. | China | Y. Zhang, 4 Nov. 2015 | MK810987 | MK810872 | MK887868 | MK888808 | MK926542 | |
| V. minuta | CBS 478.61T | Salix nigricans | Switzerland | J. Nüesch, 20 May 1959 | MK810988 | MK810873 | – | MK888809 | MK926543 |
| CBS 479.61 | Salix cinerea | Switzerland | J. Nüesch, 20 May 1959 | MK810989 | MK810874 | – | MK888810 | MK926544 | |
| V. nashicola | CBS 793.84 | Pyrus serotina var. culta | Japan | – | MK810990 | MK810875 | MK887869 | MK888811 | MN078221 |
| CBS 794.84 | Pyrus serotina var. culta | Japan | – | MK810991 | MK810876 | MK887870 | MK888812 | MK926545 | |
| V. oleaginea | CBS 113427 | Olea europaea | New Zealand | – | MK810992 | MK810877 | MK887871 | – | MN078222 |
| CBS 113539 | – | Portugal | B. d'Oliveira, – | MK810993 | MK810878 | MK887872 | – | MN078223 | |
| CBS 120629 | Olea europaea | Morocco | – | MK810994 | MK810879 | MK887873 | – | MK926546 | |
| V. peltigericola | CBS 370.35 | Betula verrucosa | Germany | – | MK810995 | MK810880 | MK887874 | MK888813 | MK926547 |
| CBS 371.35 | Betula verrucosa | Germany | – | MK810996 | MK810881 | MK887875 | MK888814 | MN078224 | |
| CBS 128206T | Lichen on ground surface, Peltigera rufescens, along with Hawksworthiana peltigericola | Luxembourg | P. Diederich, May 2008 | HQ599579 | HQ599579 | – | – | – | |
| V. phaeosepta | CGMCC3.18373 | Populus sp. | China | Y. Zhang, 6 Aug. 2015 | MK810997 | MK810882 | MK887876 | MK888815 | MK926548 |
| CGMCC3.18371 | Populus sp. | China | Y. Zhang, 20 May 2014 | MK810998 | MK810883 | MK887877 | MK888816 | MK926549 | |
| CGMCC3.18368T | Populus sp. | China | Y. Zhang, 20 May 2014 | MK810999 | MK810884 | MK887878 | MK888817 | MK926550 | |
| V. polygoni-vivipari | CBS 114207 | Polygonum viviparum | Norway | K. & L. Holm, 12 Aug. 1988 | MK811003 | MK810888 | MK887882 | MK888821 | MK926554 |
| V. populina | CBS 256.38 | Populus canadensis | Italy | – | MK811004 | MK810889 | MK887883 | MK888822 | MK926555 |
| CBS 316.58 | Populus sp. | Italy | – | MK811005 | – | MK887884 | MK888823 | MK926556 | |
| V. pyrina | CBS 120825 | Pyrus communis | Brazil | MK811000 | MK810885 | MK887879 | MK888818 | MK926551 | |
| CBS 123189 | Pyrus communis | New Zealand | C.F. Hill, 20 Apr. 2008 | MK811001 | MK810886 | MK887880 | MK888819 | MK926552 | |
| CBS 379.35 | – | Germany | – | MK811002 | MK810887 | MK887881 | MK888820 | MK926553 | |
| V. quebecensis | CBS 695.85T | Populus tremuloides, leaf spot | Canada: Quebec | – | MK811006 | MK810890 | MK887885 | MK888824 | MK926557 |
| V. saliciperda | CBS 480.61T | Salix cordata | Switzerland | – | MK811007 | MK810891 | MK887886 | MK888825 | MK926558 |
| CBS 481.61 | Salix elegantissima | Switzerland | – | MK811008 | MK810892 | MK887887 | MK888826 | MK926559 | |
| V. tremulae | CBS 112625 | Populus tremula | France | –, 1 Sep. 1977 | MK811009 | MK810893 | MK887888 | MK888827 | MK926560 |
| CBS 694.85 | Populus alba, leaf spot | France | – | MK811010 | MK810894 | MK887889 | MK888828 | MK926561 | |
| CBS 692.85 | Populus tremula, leaf spot | France | – | MK811011 | MK810895 | MK887890 | MK888829 | MK926562 | |
| CBS 693.85 | Populus tremula, leaf spot | France | – | MK811012 | MK810896 | MK887891 | MK888830 | MK926563 | |
| V. viennotii | CBS 690.85 | Populus tremula, leaf litter | France | – | MK811013 | MK810897 | – | MK888831 | MK926564 |
| CBS 691.85 | Populus tremula, leaf litter | France | – | MK811014 | MK810898 | – | MK888832 | MK926565 | |
| Outgroup | |||||||||
| Microthyrium microscopicum | CBS 115976 | – | Netherlands | – | JGI project 1011369 | GU301846 | GU371734 | GU349042 | JGI project 1011369 |
CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; CGMCC: Chinese General Microbiological Culture Collection Center, Beijing, China; CPC: Culture collection of Pedro Crous, housed at Westerdijk Fungal Biodiversity Institute; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Ria, Thailand; MUCL: Université Catholique de Louvain, Louvain-la-Neuve, Belgium; PDD Herbarium of Plant Diseases Division; UTHSC: Fungus Testing Laboratory, Department of Pathology at the University of Texas Health Science Center, San Antonio, Texas, USA. A superscript T denotes cultures with a type status.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: partial 28S large subunit RNA gene; tef1: partial translation elongation factor 1-alpha gene; tub2: partial beta-tubulin gene; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene. Bold GenBank accession numbers represent sequences generated in this study; – indicates unavailable sequences or unknown collection data.
DNA extraction, amplification (PCR) and phylogeny
Total genomic DNA was extracted from fungal colonies using the FastDNA kit (MP Biomedicals, CA, USA), PrepMan Ultra sample preparation reagent (Applied Biosystems, Foster City, CA, USA) and the Wizard® Genomic DNA Purification Kit (Promega Corporation, WI, USA), following the manufacturer's protocols. The primer sets LR0R/LR5 and ITS5/ITS4 (Vilgalys and Hester, 1990, White et al., 1990), were used to amplify part of the nuclear rDNA LSU and ITS. The EF1-728F and EF-2 primers (Qiao et al. 2016) were used for the amplification of the partial tef1 (translation elongation factor 1-alpha) gene. The fRPB2-5F2 and fRPB2-7cR primers were used for the amplification of the partial rpb2 (DNA-directed RNA polymerase II second largest subunit) gene (Liu et al., 1999, Reeb et al., 2004). Several primer pairs including T1/Bt-2b, T1/Tub4Rd, and/or Bt-2a/Bt-2b were used to amplify the partial tub2 (Beta-tubulin) gene (Glass and Donaldson, 1995, Aveskamp et al., 2009, Guo et al., 2014). The amplification cycles were performed following Cano et al. (2004). PCR products were purified and sequenced with an Applied Biosystems 3730xl DNA Analyzer (Life Technologies, Carlsbad, CA, USA). The program SeqMan v. 7.0 (Lasergene, Madison, WI, USA) was used to obtain consensus sequences. The combined ITS, LSU, tef1, tub2 and rpb2 sequence dataset was used to infer the phylogenetic relationships among the new taxa and other reported taxa of Venturiales. Sequences generated were analysed with other sequences obtained from GenBank (Table 1). Phylogenetic trees were generated using Bayesian analyses performed with MrBayes v. 3.2.6 (Ronquist et al. 2012). MrModeltest v. 2.2 (Nylander 2004) was used to determine the best nucleotide substitution model settings for each data partition. The Markov Chain Monte Carlo (MCMC) analysis of four chains started in parallel from a random tree topology, the heat parameter was set at 0.15 and trees were saved every 100 generations until the average standard deviation of split frequencies reached 0.01 (stop value). Burn-in was set to 25 % after which the likelihood values were stationary. Obtained trees were viewed in FigTree v. 1.1.2 (Rambaut 2009) and subsequently printed with Geneious v. 11.0.3 (http://www.geneious.com, Kearse et al. 2012) and edited in Adobe ® Illustrator v. CC 2017. Posterior probability values (PP) were plotted on the branches.
Morphology
Specimens were loaned from the following herbaria: Herbarium Plant Pathology and Microbiology Herbarium (PPMH), Chinese Academy of Sciences (HMAS), Cornell University (CUP), New Zealand Fungarium (PDD), University of Michigan (MICH), The Royal Botanic Gardens, Kew (K), the New York Botanical Garden (NY), New York State Museum (NYS), Eidgenössische Technische Hochschule Zürich (ZT), Naturhistorisches Museum Wien (W), the Queensland Plant Pathology Herbarium (BRIP), the Chinese General Microbiological Culture Collection Center (CGMCC), and the Victorian Plant Pathogen Herbarium (VPRI). Attempts were made to trace and borrow type specimens of Venturia from herbaria worldwide, but only some of them could be obtained.
For sexual morphs, ascostromata and ascomata were examined under an Olympus SZ H10 dissecting microscope. Measurements and descriptions of sections of the ascomata, hamathecia, asci and ascospores were carried out after immersing ascomata in water, cotton blue, Melzer’s reagent or in 10 % lactic acid. Terminology follows Ulloa & Hanlin (2000). For asexual morphs, measurements and descriptions of microscopic structures including conidiophores, conidiogenous cells and conidia, were taken from specimens mounted in water or lactic acid. Photomicrographs were taken using differential interference contrast and phase contrast optics with a Zeiss Axio Imager M1 compound microscope (Zeiss, Oberkochen, Germany) and a DeltaPix Infinity X digital camera or a Nikon Eclipse Ni microscope, using a Nikon DS-U3 digital camera (Nikon, Tokyo, Japan) and NIS-Element imaging software v. 4.20.
Results
Phylogeny
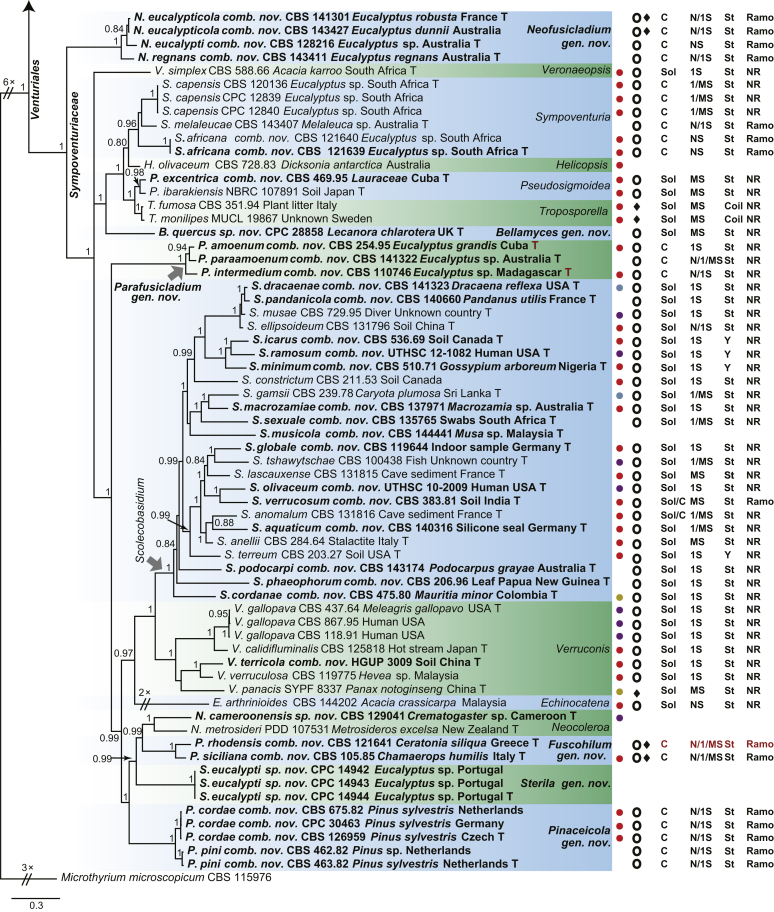
The concatenated DNA sequence dataset (ITS, LSU, tef1, tub2 and rpb2) used to infer delimitations at family and genus levels comprised 120 isolates (including outgroup sequences) of Venturiales and related fungi and the same concatenated alignment focused on Venturiaceae comprised 96 isolates (including outgroup sequences). The optimal substitution models recommended by MrModelTest and used in the Bayesian analyses. The number of generations ran and the number of trees from the two runs used to generate the 50 % consensus tree and posterior probabilities. The number of unique site patterns and the number of characters including alignment gaps used for each locus.
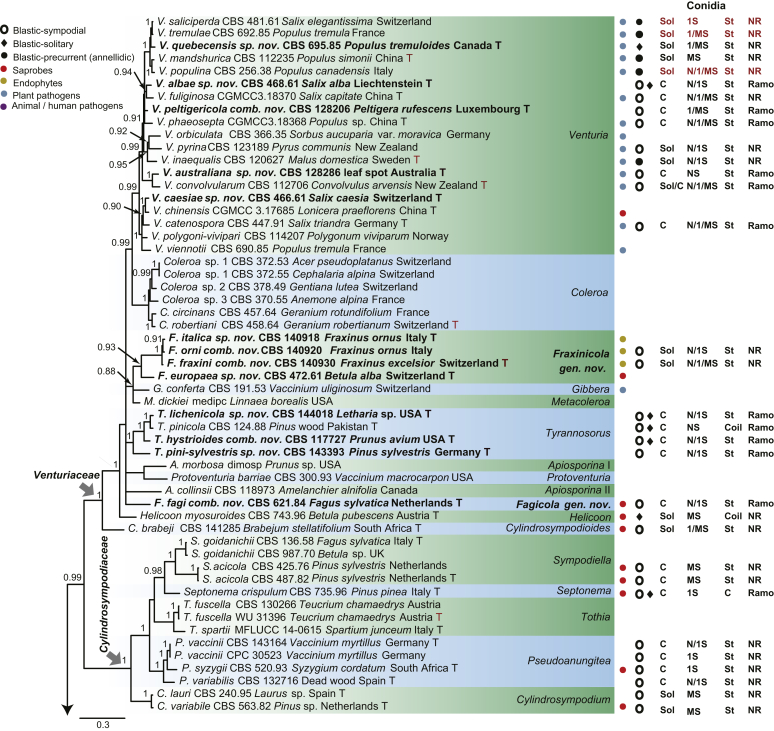
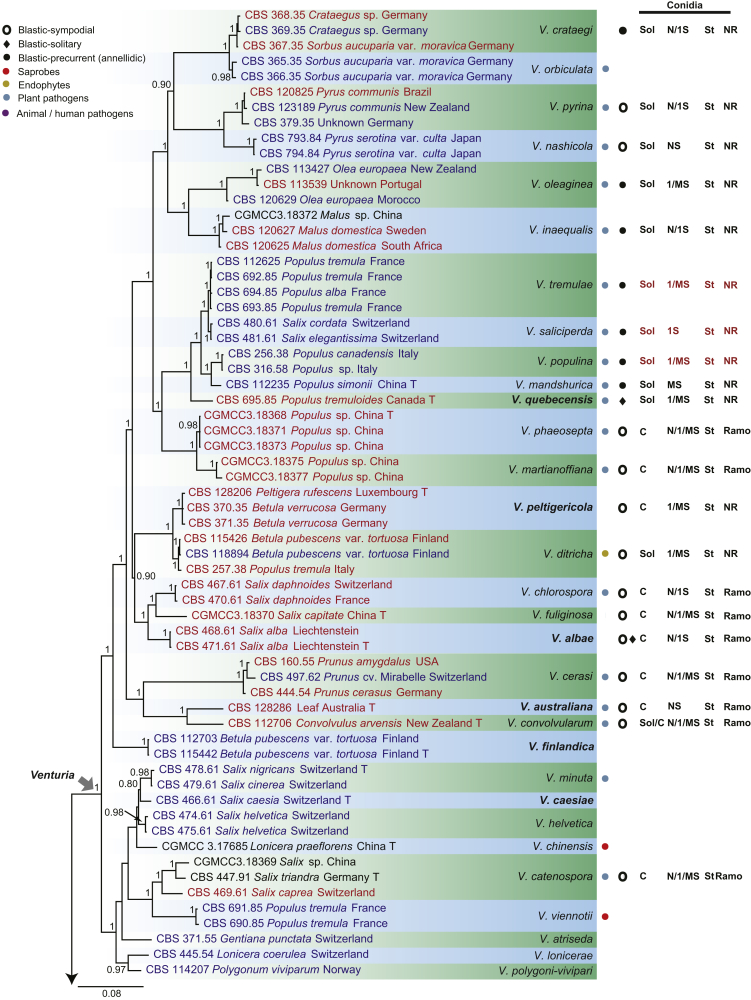
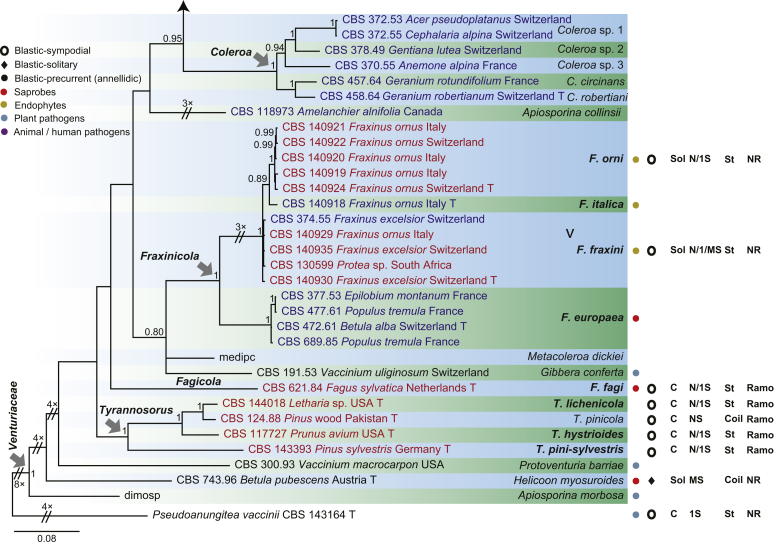
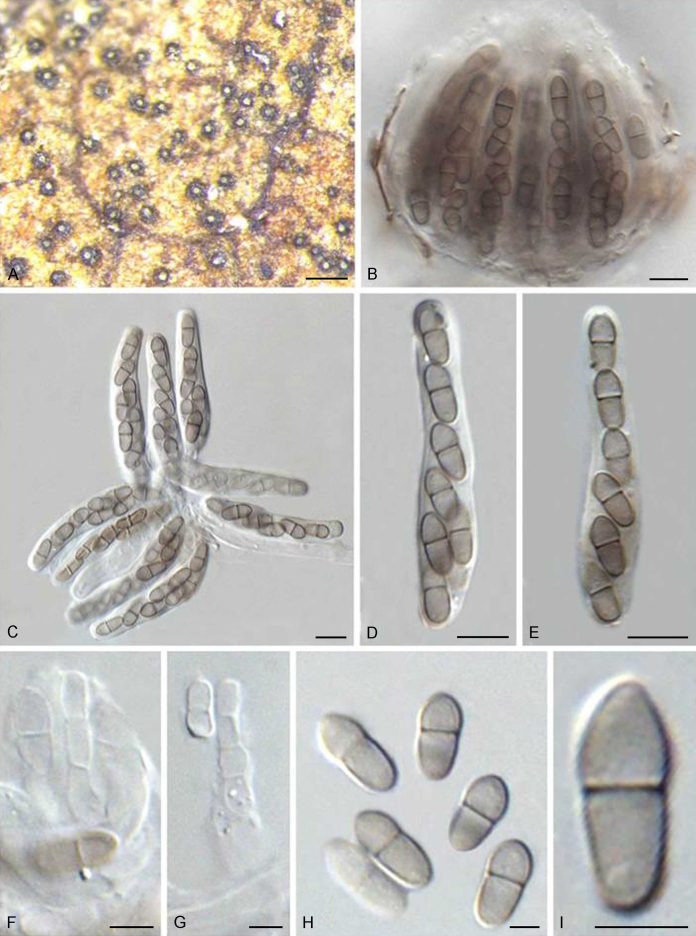
The phylogenetic tree distinguished three well-supported clades corresponding to the families Venturiaceae (PP = 1) and Sympoventuriaceae (PP = 1), as well as the new family Cylindrosympodiaceae (PP = 1) (Fig. 1). The Venturiaceae clade comprised 11 generic lineages, including two new genera, Fagicola and Fraxinicola (Fig 1, Fig 2). The fully supported clade of Venturia s. str. comprised 31 species including five newly described species, V. quebecensis, V. albae, V. australiana, V. caesiae and V. finlandica (Fig 1, Fig 2). The Coleroa clade (PP = 1) comprised five taxa, including C. circinans and C. robertiani, and three unidentified taxa (Fig 1, Fig 2). Fraxinicola, a newly described genus of Venturiaceae, comprised two new species, F. italica and F. europaea, as well as two new combinations F. orni and F. fraxini (Fig 1, Fig 2). Gibbera and Metacoleroa comprised one species each, namely G. conferta and M. dickiei, respectively (Fig 1, Fig 2). Tyrannosorus (PP = 1) comprised four species including two new species (T. lichenicola and T. pini-sylvestris) and one new combination (T. hystrioides) (Fig 1, Fig 2). Species of Apiosporina, A. morbosa and A. collinsii, did not cluster in a monophyletic clade, but were separated by Protoventuria barriae (Fig. 1). Helicoon myosuroides was basal in Venturiaceae, but its inclusion in the family was fully supported (Fig. 1).
Fig 1.
Consensus phylogram (50 % majority rule) of 691 952 trees resulting from a Bayesian analysis of the combined alignment of ITS, LSU, tef1, tub2 and rpb2 sequences of Venturiales. Bayesian posterior probabilities (PP) > 0.80 are shown at the nodes and the scale bar represents the expected changes per site. Some branches were shortened to facilitate layout. The tree was rooted with Microthyrium microscopicum (CBS 115976). Culture collection numbers, substrates and countries are indicated behind the species names. Those highlighted in bold are new taxa or new combinations proposed in this study, and type strains are marked with “T” (ex-type in black, ex-epitype in red). Relevant morphological characteristics plotted are abbreviated as follows: Sol – conidia solitary, C – conidia in chains, NS – aseptate conidia, 1S – 1-septate conidia, MS – multi-septate conidia (septa ≥ 2), St – straight or slightly curved conidia, Coil – coiled conidia, Y – Y-shaped conidia; Ramo – ramoconidia present, NR – ramoconidia not observed; ? – asexual morphology not available (either from references or from sporulation induced in this study); and morphological characters plotted in red means strains failed to sporulate in this study and plotted values are taken from the original description, observation of this study or related references. Other characteristics are explained in the legend.
Fig 2.
Consensus phylogram (50 % majority rule) of 42 902 trees resulting from a Bayesian analysis of the combined alignment of ITS, LSU, tef1, tub2 and rpb2 sequences of Venturiaceae. Bayesian posterior probabilities (PP) > 0.80 are shown at the nodes and the scale bar represents the expected changes per site. Some branches were shortened to facilitate layout. The tree was rooted with Pseudoanungitea vaccinii (CBS 143164). See title of Fig. 1 for an explanation of the characters plotted on the tree. Strains in red text sporulated in this study, while those in blue text failed to sporulate and those in black text were not studied.
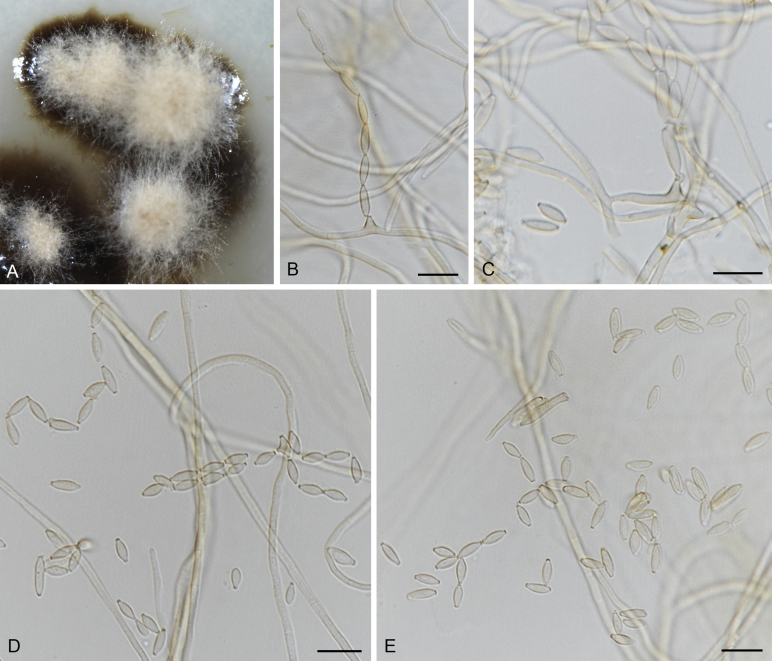
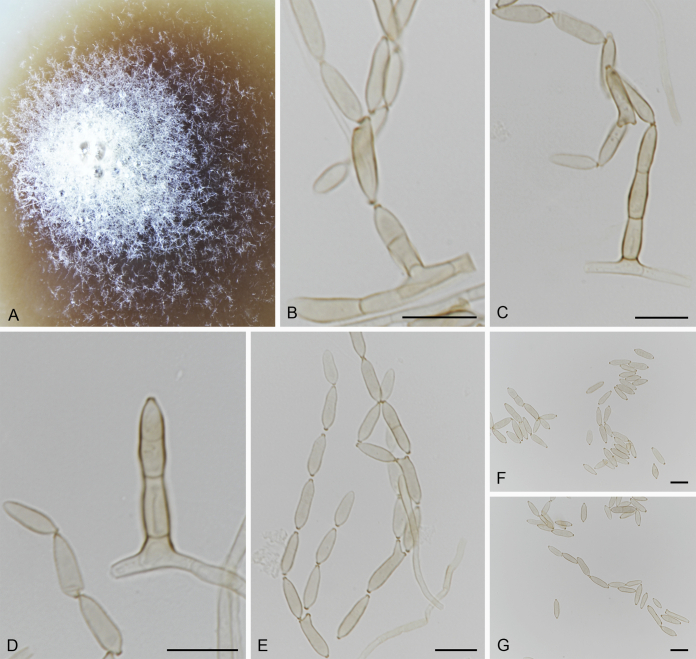
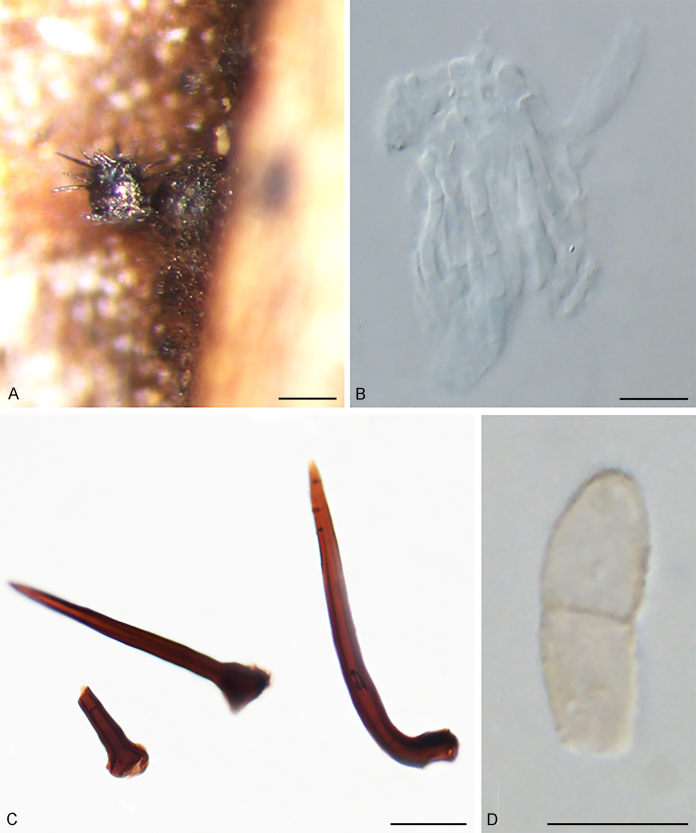
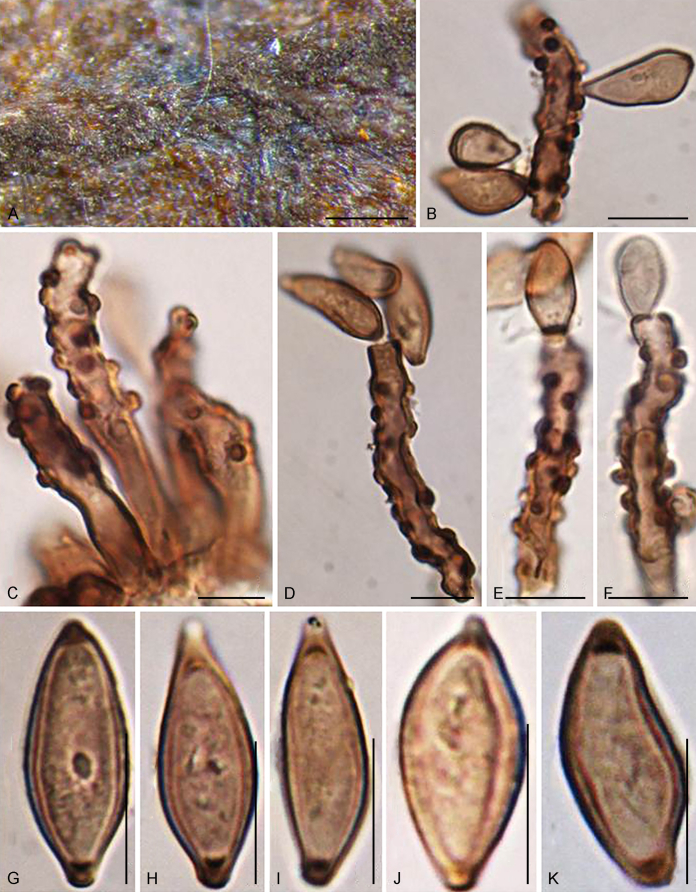
The Cylindrosympodiaceae clade (PP = 1), representing a new family of Venturiales, comprised four genera, namely Sympodiella (S. goidanichii and S. acicula), Tothia (T. fuscella and T. spartii), Pseudoanungitea (P. vaccinii, P. syzygii and P. variabilis), and Cylindrosympodium (C. lauri and C. variabile) (Fig. 1). Also included in this clade is Septonema crispulum, which is not congeneric with other Septonema species such as S. fasciculare and S. secedens (data not shown).
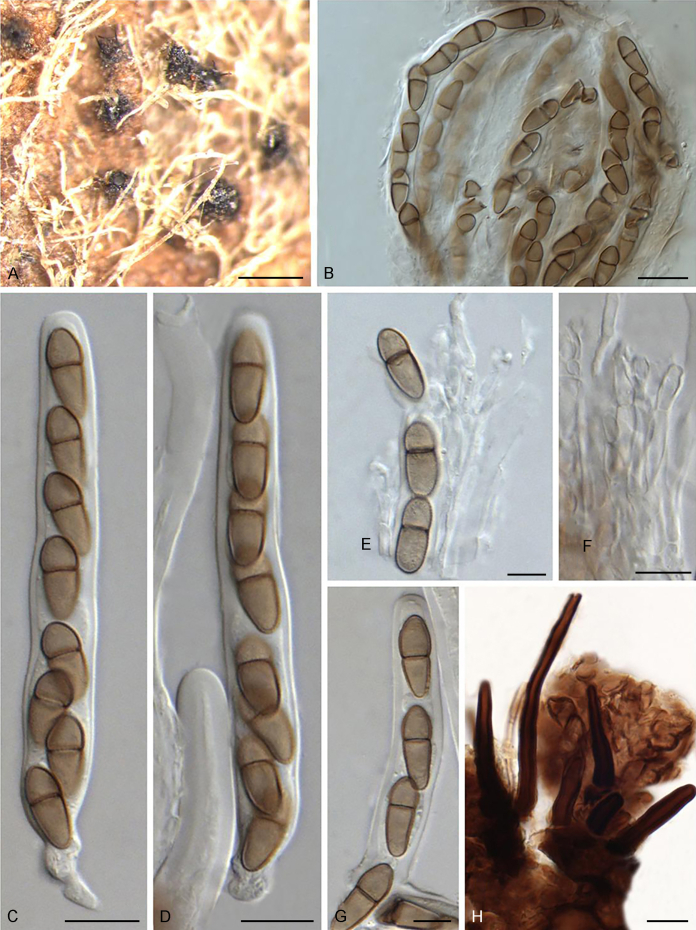
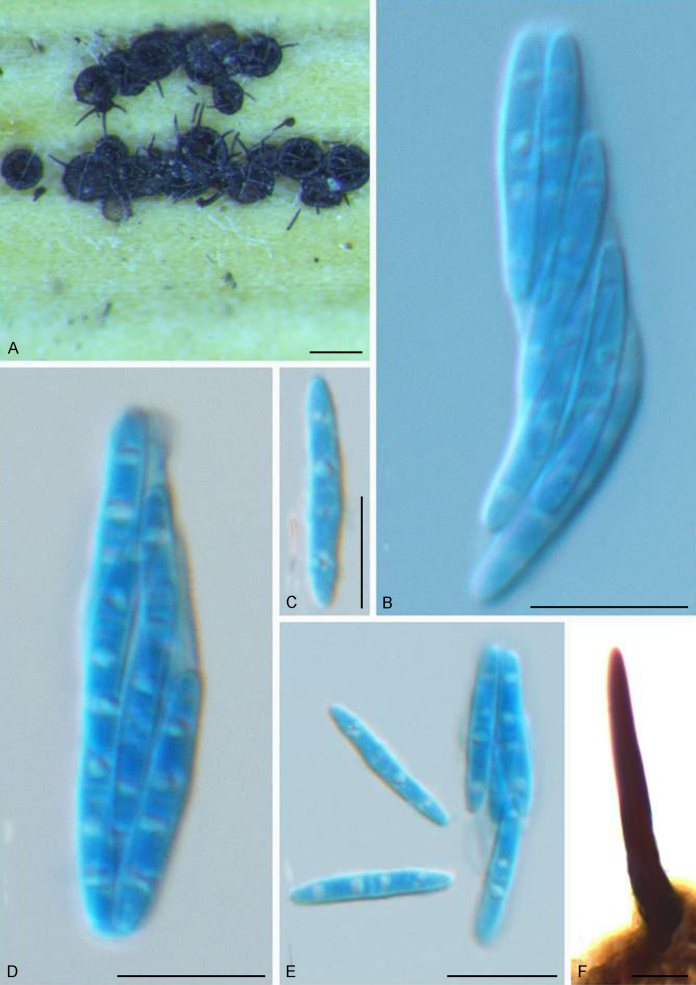
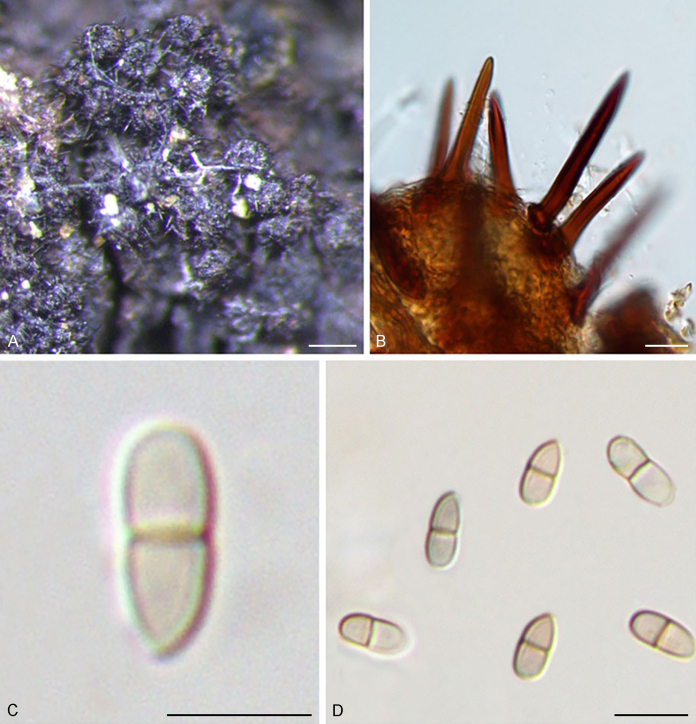
The Sympoventuriaceae (PP = 1; Fig. 1) comprised 14 genera, including six proposed here as new, viz., Neofusicladium, Parafusicladium, Bellamyces, Fuscohilum, Sterila and Pinaceicola (Fig. 1). The new genus Neofusicladium (PP = 1) (N. eucalypticola, N. eucalypti and N. regnans), is basal in Sympoventuriaceae (Fig. 1). The Sympoventuria clade (PP = 0.96) comprised S. capensis, S. melaleucae and S. africana. Troposporella is paraphyletic with the type species T. fumosa clustering with T. monilipes (PP = 1) and T. olivaceum forming a distinct lineage (Fig. 1). Pseudosigmoidea (P. excentrica and P. ibarakiensis) formed a well-supported lineage (PP = 0.98) (Fig. 1). Three species formerly of Fusicladium, namely F. amoenum, F. paraamoenum and F. intermedium, formed a well-supported clade (PP = 1; Fig. 1), and are allocated here to a new genus, Parafusicladium (as P. amoenum, P. intermedium and P. paraamoenum). Parafusicladium is basal to Echinocatena, Neocoleroa, Fuscohilum, Pinaceicola, Scolecobasidium, Sterila and Verruconis.
Scolecobasidium (PP = 1; Fig. 1) comprised 24 species, which chiefly clustered in two subclades, with one comprising S. dracaenae, S. pandanicola, S. musae, S. ellipsoideum, S. icarus, S. ramosum, S. minimum, S. constrictum, S. gamsii, S. macrozamiae, S. sexuale and S. musicola, and the other comprising S. globale, S. tshawytschae, S. lascauxense, S. olivaceum, S. verrucosum, S. anomalum, S. aquaticum, S. anellii and S. terreum (Fig. 1). Another three species, namely S. podocarpi, S. phaeophorum and S. cordanae were basal to other species of Scolecobasidium (Fig. 1). Members of Verruconis formed a fully supported clade (PP = 1; Fig. 1), which comprises V. gallopava, V. calidifluminalis, V. terricola, V. verruculosa and V. panacis (Fig. 1). Echinocatena, a monotypic genus represented by E. arthrinioides, was basal to the subclades comprising Scolecobasidium and Verruconis (Fig. 1). Neocoleroa included N. cameroonensis and N. metrosideri, which formed a robust clade with another three new genera, viz., Fuscohilum, Sterila and Pinaceicola (Fig. 1). These four genera formed a fully supported subclade which is sister to Scolecobasidium, Verruconis and Echinocatena (Fig. 1).
Taxonomy
Venturiales Y. Zhang ter et al., Fungal Diversity 51: 251. 2011.
Description and illustration: Habitat saprophytic, endophytic, parasitic on leaves or stems of plants, animals or human beings, rarely thermotrophic. Sexual morph: Ascomata immersed, erumpent to superficial, scattered or gregarious, globose, subglobose, mostly with setae around papilla or covering whole ascomata when superficial, ostiolate. Hamathecium of narrowly cellular pseudoparaphyses, mostly evanescent and rarely persistent when mature. Asci 8-spored, bitunicate, fissitunicate, usually obclavate, pedicel knob-like or lacking. Ascospores hyaline, light greenish olivaceous to brown, 1-septate, symmetrical, asymmetrical or apiosporous. Asexual morph: Mycelium consisting of branched, pale brown to medium brown, smooth, septate hyphae. Conidiophores solitary or loosely to densely fasciculate, arising from internal hyphae, or formed in sporodochia, arising from small to moderately large stromata, conidiophores often reduced to conidiogenous cells or composed of several cells, erect, cylindrical, pyriform, subclavate, narrowly obclavate, slightly to distinctly geniculate-sinuous, unbranched or occasionally branched, pale olivaceous to dark brown, tips sometimes paler, smooth to somewhat verruculose, sometimes only as short lateral conical prolongations of hyphae, occasionally irregular in shape. Conidiogenous cells integrated, terminal or intercalary or conidiophores reduced to conidiogenous cells, mono- to polyblastic, proliferation percurrent or sympodial; conidiogenous loci terminal or lateral, sometimes denticle-like, apex truncate to slightly convex, wall unthickened or almost so, sometimes slightly darkened-refractive. Conidia solitary or mostly catenate, in simple or branched chains, subcylindrical, ampulliform to fusoid-ellipsoid, acicular, straight, slightly curved or coiled, base truncate, septate or aseptate, subhyaline, pale to dark brown, but mostly olivaceous, sometimes constricted at septa, smooth to verruculose, ends pointed or rounded to truncate, hila truncate, thickened or not, occasionally darkened-refractive.
Type family: Venturiaceae E. Müll. & Arx ex M.E. Barr
Notes: Venturiales was introduced by Zhang et al. (2011) based on morphological and ecological characteristics, as well as DNA data. It comprised two families, viz., Venturiaceae (Venturia and its allied genera) and Sympoventuriaceae (Sympoventuria and its allied genera) (Zhang et al. 2011). A third familial lineage comprising Cylindrosympodium, Pseudoanungitea, Sympodiella and Tothia, is retrieved in the present phylogenetic analysis (Fig. 1). Thus, a new family, Cylindrosympodiaceae, is introduced here. Members of Venturiales could be saprophytic on woody substrates or in soil, endophytic, parasitic on leaves or stems of plants, animals or human beings. Some species of Verruconis are thermophilic, such as V. calidifluminalis and V. gallopava, both of which occur in hot springs (Samerpitak et al. 2014). Phylogenetically, Venturiales are closely related to Microthyriales, Natipusillales and Asterinales (Hyde et al. 2013).
Cylindrosympodiaceae Crous, M. Shen & Y. Zhang ter, fam. nov. MycoBank MB831510.
Mycelium consisting of branched, pale to medium brown, smooth, septate hyphae. Conidiophores solitary, erect, septate, subcylindrical to cylindrical, medium brown to brown, smooth, straight to flexuous, sometimes rejuvenating percurrently. Conidiogenous cells terminal or intercalary, subcylindrical to clavate, pale to medium brown, mono- or polyblastic, sometimes sympodial; conidiogenous loci sometimes arranged in a rachis, flat or prominent, thickened or unthickened, somewhat darkened and refractive. Conidia in chains or rarely solitary, subcylindrical, ampulliform to fusoid-ellipsoid, acicular, hyaline, pale to medium brown, smooth, prominently guttulate, septate or aseptate; hila truncate, sometimes darkened and refractive (adapted from De Hoog, 1985, Crous et al., 2007a, Crous et al., 2007b, Crous et al., 2018, Crous et al., 2019a).
Type genus: Cylindrosympodium W.B. Kendr. & R.F. Castañeda
Notes: Phylogenetically, Cylindrosympodium, Pseudoanungitea, Sympodiella and Tothia formed a fully supported clade (PP = 1), sister to the Venturiaceae (Fig. 1). Morphologically, the hyphomycetous asexual morph, blastic conidiogenesis, subcylindrical to clavate, pale to medium brown conidiogenous cells, as well as the solitary or concatenate, subcylindrical, ampulliform to fusoid-ellipsoid conidia point to Venturiales. Ecologically, members of Cylindrosympodium, Pseudoanungitea, Sympodiella and Tothia are mostly saprophytic on woody plant hosts, such as Pinaceae, Lauraceae, Myrtaceae or Ericaceae (Crous et al., 2007b, Crous et al., 2018, Crous et al., 2019a). Thus, a new family, Cylindrosympodiaceae, is proposed to accommodate these genera.
Cylindrosympodium W.B. Kendr. & R.F. Castañeda, Univ. Waterloo Biol. Ser. 32: 9. 1990.
Type species: Cylindrosympodium variabile (de Hoog) W.B. Kendr. & R.F. Castañeda
Notes: Cylindrosympodium was introduced based on Subulispora variabilis (as Cyl. variabile (Castañeda & Kendrick 1990). Subsequently, more species have been assigned to Cylindrosympodium (Marvanová and Laichmanová, 2007, Crous et al., 2007b, Paulus et al., 2003, Castañeda and Kendrick, 1991, Castañeda-Ruiz et al., 2012). Phylogenetically, Cylindrosympodium is basal to other genera of Cylindrosympodiaceae, while closely related to Pseudoanungitea. Morphologically, Cylindrosympodium can be readily distinguished from Pseudoanungitea by its conidia that are subhyaline to pale olivaceous, and the conidiogenous loci that are slightly darkened, but not refractive (De Hoog, 1985, Crous et al., 2007b).
Cylindrosympodium lauri Crous & R.F. Castañeda, Stud. Mycol. 58: 204. 2007.
Typus: Spain, Canary Islands, on leaf litter of Laurus sp. (Lauraceae), 4 Jan. 1995, R.F. Castañeda (holotype CBS H-19909, culture ex-type CBS 240.95).
Notes: Cylindrosympodium lauri introduced by Crous et al. (2007b) was isolated from leaf litter of Laurus sp. in Spain. It can be distinguished from Cyl. variabile (De Hoog 1985) by its longer conidiophores, subhyaline to pale olivaceous conidia, and the thin, slightly darkened but not refractive conidiogenous loci and hila (Crous et al. 2007b). Cylindrosympodium lauri is sister to C. variabile in Fig. 1.
Cylindrosympodium variabile (de Hoog) W.B. Kendr. & R.F. Castañeda, Univ. Waterloo Biol. Ser. 32: 10. 1990.
Basionym: Subulispora variabilis de Hoog, Stud. Mycol. 26: 56. 1985.
Typus: Netherlands, Utrecht Province, Baarn, De Vuursche, on rotten needle of Pinus sp. (Pinaceae), Sep. 1982, G.S. de Hoog (holotype CBS H-1634, culture ex-type CBS 563.82).
Notes: Ecologically, C. variabile has a broader host spectrum than C. lauri (Crous et al. 2007b). Phylogenetically, Cyl. variabile and Cyl. lauri form a fully supported clade representing the genus Cylindrosympodium (Fig. 1).
Pseudoanungitea Crous, Fungal Syst. Evol. 1: 199. 2018.
Type species: Pseudoanungitea syzygii (Crous et al.) Crous
Notes: Pseudoanungitea was separated from Anungitea based on its terminal and intercalary conidiogenous cells, and refractive, thickened conidiogenous loci that give rise to short conidial chains with somewhat darkened and refractive hila (Crous et al. 2018). So far three species, viz., P. syzygii, P. vaccinii and P. variabilis have been assigned Pseudoanungitea (Crous et al. 2018).
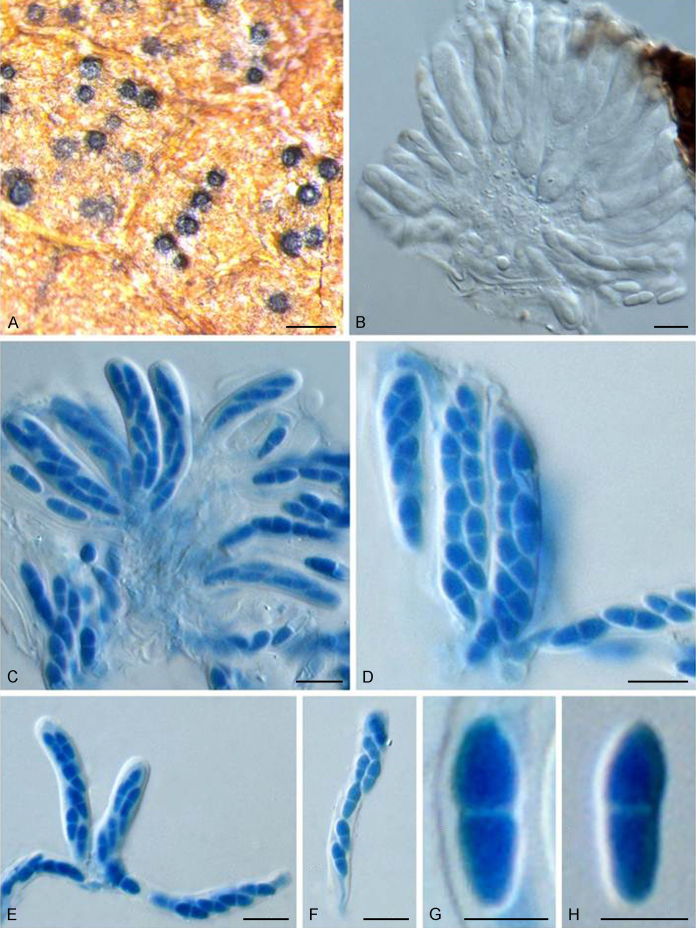
Pseudoanungitea syzygii (Crous et al.) Crous, Fungal Syst. Evol. 1: 199. 2018.
Basionym: Anungitea syzygii Crous et al., Canad. J. Bot. 73: 225. 1995.
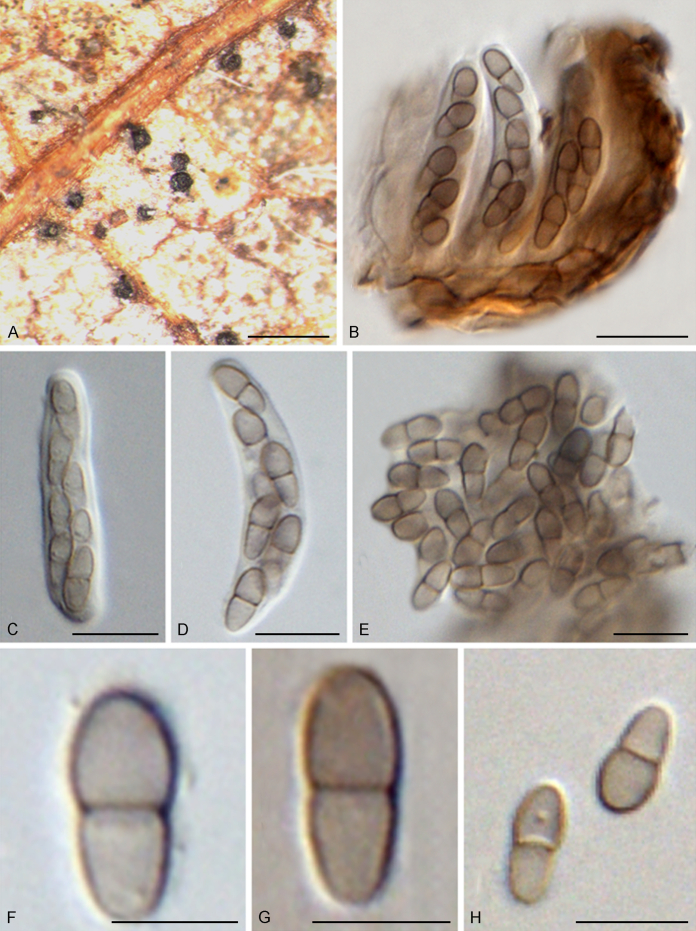
Typus: South Africa, Mpumalanga Province, Sabie, on leaf litter of Syzygium cordatum (Myrtaceae), Mar. 1993, W.J. Swart (holotype PREM 51687, culture ex-type CPC 578 = CBS 520.93).
Notes: Anungitea syzygii was originally described on leaf litter of Syzygium cordatum (South Africa), which was subsequently assigned to Pseudoanungitea (as P. syzygii) (Crous et al., 1995, Crous et al., 2018). Together with P. vaccinii and P. variabilis, this species formed a monophyletic clade representing the genus Pseudoanungitea (Fig. 1).
Pseudoanungitea vaccinii Crous & R.K. Schumach., Fungal Syst. Evol. 1: 199. 2018.
Typus: Germany, near Berlin, on stem of Vaccinium myrtillus (Ericaceae), 16 Jan. 2016, R.K. Schumacher (holotype CBS H-23422, culture ex-type CBS 143164 = CPC 30522).
Notes: Pseudoanungitea vaccinii was described from stems of Vaccinium myrtillus (Crous et al. 2018). Based on a multigene phylogenetic analysis, P. vaccinii was closely related to P. syzygii (Crous et al. 2018; Fig. 1 in present study). Morphologically, P. vaccinii can be distinguished from P. syzygii based on its conidial dimensions (Crous et al., 1995, Crous et al., 2018).
Pseudoanungitea variabilis Hern.-Restr., Fungal Syst. Evol. 1: 200. 2018.
Typus: Spain, Castilla la Mancha, Hayedo de la Tejera Negra Natural Park, on dead wood, May 2011, M. Hernández-Restrepo, J. Mena & J. Guarro (holotype CBS H-23494, culture ex-type CBS 132716).
Notes: Pseudoanungitea variabilis differs from other species of Pseudoanungitea in having dimorphic conidia, i.e., type 1 are fusoid-ellipsoid resembling those of P. syzygii and P. vaccinii, and type 2 are globose (Crous et al., 1995, Crous et al., 2018). It is basal in Pseudoanungitea in the present study (Fig. 1).
Septonema Corda, Icon. Fung. 1: 9. 1837.
Type species: Septonema secedens Corda
Notes: Septonema secedens is represented on GenBank by two cultures (both not ex-type): CBS 469.48 (GenBank MH856437 and MH867983 for ITS and LSU respectively) and CBS 174.74 (LSU GenBank MH878272). The former LSU sequence blasts with Alternaria/Stemphyllium while the latter is related to Septonema fasciculare strain CBS 127862 (GenBank MH876104; 898/916 (98 %) similar including 11 gaps) and Helicoon pluriseptatum strain CBS 812.68 (GenBank MH878409; 836/856 (98 %) similar including 9 gaps).
Septonema crispulum Lunghini & F. Toscano, Mycotaxon 63: 329. 1997.
Typus: Italy, on decaying needles of Pinus pinea (Pinaceae), 15 Nov. 1992, F. Toscano (holotype ROHB 187, culture ex-type CBS 735.96).
Notes: Septonema crispulum was introduced based on a taxon found on pine-needle litter in central Italy, which morphologically agrees with Septonema by having 1-septate and slightly thick-walled conidia (Lunghini & Toscano 1997). The LSU sequence of S. crispulum does not appear to be congeneric with the S. secedens strain CBS 174.74 LSU sequence (GenBank MH878272; 859/923 (93 %) similar including 24 gaps). We refrain from designating a new genus for S. crispulum (Fig. 1) pending recollection and molecular investigation of suitable authentic material of S. secedens.
Sympodiella W.B. Kendr., Trans. Brit. Mycol. Soc. 41: 519. 1958.
Type species: Sympodiella acicola W.B. Kendr.
Sympodiella acicola W.B. Kendr., Trans. Brit. Mycol. Soc. 41: 519. 1958. emend. Hern.-Restr. & Crous
Typus: UK, Cheshire, on Pinus sylvestris (Pinaceae), 1956, W.B. Kendrick (holotype IMI 69967). Netherlands, Baarn, De Vuursche, on P. sylvestris, 12 Apr. 1982, G.S. de Hoog (epitype CBS H-1620 MBT385535, ex-epitype culture CBS 487.82).
Note: This species is sister to S. goidanichii (Fig. 1).
Sympodiella goidanichii (Rambelli) Crous & Hern.-Restr., Fungal Syst. Evol. 3: 116. 2019.
Basionym: Ceratosporella goidanichii Rambelli, R.C. Secc. Atti Accad. Sci. Ist. Bologna, Cl. Sci. Fis., Rendiconti, Ser. 11, 5: 3. 1958.
Synonym: Repetophragma goidanichii (Rambelli) W.P. Wu, Fungal Diversity Res. Ser. 15: 80. 2005.
Typus: Italy, on capsule of Fagus sylvatica (Fagaceae), collection date unknown, A. Rambelli (culture ex-type CBS 136.58).
Notes: Ceratosporella goidanichii was first described from withered fruit of Fagus sylvatica. Subsequently, Ceratosporella goidanichii was assigned to Sympodiella as S. goidanichii based on its phylogenetic position (Crous et al. 2019a). In Fig. 1, S. goidanichii is sister to S. acicola.
Tothia Bat., Ann. Hist.-Nat. Mus. Natl. Hung. 52: 105. 1960.
Type species: Tothia fuscella (Sacc.) Bat.
Tothia fuscella (Sacc.) Bat., Ann. Hist.-Nat. Mus. Natl. Hung. 52: 106. 1960.
Basionym: Microthyrium fuscellum Sacc., Michelia 2 (no. 6): 57. 1880.
Typus: Hungary, on stems of Teucrium chamaedrys (Lamiaceae), further data not available (holotype URM 8210) (not seen). Austria, Kärnten, St. Margareten im Rosental, Aussicht, grid square 9452/3, on stalks of T. chamaedrys, soc. Ophiobolus erythrosporus, 3 July 2010, W. Jaklitsch (epitype WU31396, designated in Wu et al. 2011; ex-epitype culture TF1; iso-epitype IFRD8982) (not seen).
Note: This species is sister to T. spartii (Fig. 1).
Tothia spartii Qing Tian et al., Fungal Diversity 72: 159. 2015.
Typus: Italy, Province of Forlì-Cesena, Fiumicello, Premilcuore, on living and dead branches of Spartium junceum (Fabaceae), 17 Mar. 2012, E. Camporesi (holotype MFLU 14–0739, culture ex-type MFLUCC 14–0615) (not seen).
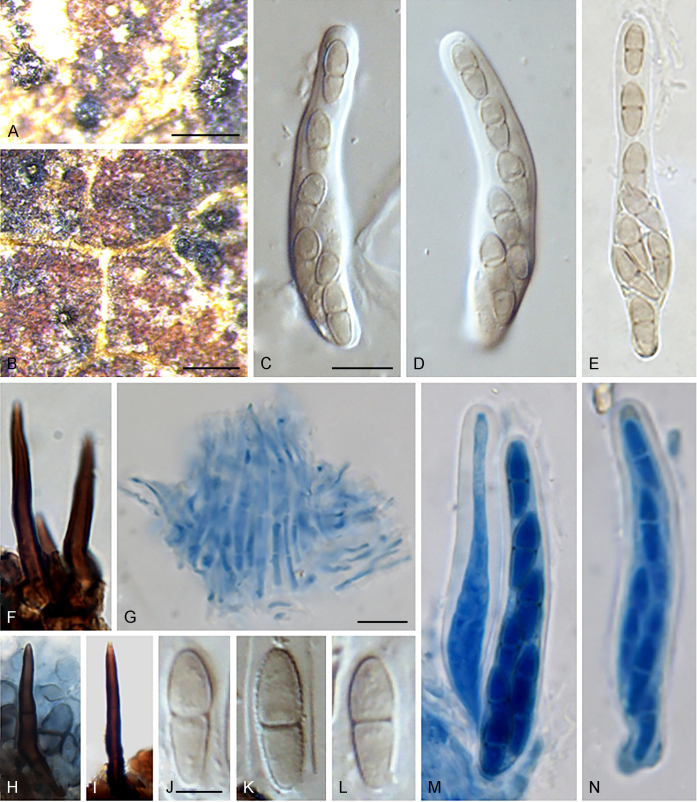
Notes: Despite the thyrothecial ascomata, the yellowish, greenish brown to brown, two-celled ascospores and obclavate asci of Tothia agree well with Venturiales (Zhang et al., 2011, Liu et al., 2015). Phylogenetically, Tothia nests in Cylindrosympodiaceae, and is sister to Sympodiella and Septonema (Fig. 1). So far two species, T. fuscella and T. spartii, were accommodated within Tothia (Wu et al., 2011, Liu et al., 2015). The ascospores of T. spartii are ellipsoid to fusiform with rounded ends, while the ascospores of T. fuscella are fusiform or oblong-ellipsoid with tapering ends (Wu et al., 2011, Liu et al., 2015).
Sympoventuriaceae Y. Zhang ter et al., Fungal Diversity 51: 255. 2011.
Habitat saprophytic, endophytic, parasitic on leaves or stems of plants, animals or humans, or as thermotrophic fungi living in hot springs. Sexual morph: Ascomata subglobose, immersed, black, papillate, ostiolate. Pseudoparaphyses hyaline, septate, constricted at septa, anastomosing, extending above the asci. Asci 8-spored, bitunicate, fissitunicate, subcylindrical, pedicellate. Ascospores hyaline, fusoid-ellipsoidal, constricted at median septum. Asexual morph: Mycelium consisting of smooth to finely roughened, pale to medium brown, branched, septate hyphae, sometimes forming hyphal coils. Conidiophores reduced to conidiogenous cells that are terminal or lateral on hyphae, or with basal supporting cell, solitary, erect, mono- to polyblastic, pale to dark brown, smooth, subcylindrical to doliiform, aseptate or septate, sometimes thick-walled, branched or rarely branched below, sometimes dimorphic; conidiogenous loci flat-tipped, somewhat darkened and thickened. Conidiogenous cells terminal or lateral, integrated, mono- or polyblastic and sympodial, subcylindrical or doliiform, pale to medium brown, smooth, proliferating sympodially; loci somewhat thickened and darkened, not refractive or sometimes slightly refractive. Ramoconidia present or not, brown, smooth, subcylindrical or fusoid-ellipsoid, aseptate or septate. Conidia solitary or occurring in branched or unbranched chains, pale brown to brown, smooth, subcylindrical to fusoid-ellipsoidal, aseptate or septate, straight, widest in middle to lower third, apex subobtuse, with or without transverse eusepta; hila truncate, sometimes thickened and darkened.
Type genus: Sympoventuria Crous & Seifert
Notes: The genus Sympoventuria is typified by S. capensis, which was originally collected on Eucalyptus leaf litter from the Western Cape Province of South Africa (Crous et al. 2007a). Sympoventuria was assigned to Venturiales based on its morphological and preliminary DNA data (Crous et al., 2007a, Crous et al., 2007b). Sympoventuriaceae was introduced to accommodate Sympoventuria (Zhang et al. 2011). It can be distinguished from other members of Venturiales by its saprophytic life style, presence of pseudoparaphyses, and hyaline, symmetrical ascospores (Zhang et al. 2011). Species of Sympoventuriaceae have mostly been collected from leaf litter, and some species have been reported from soil, hot springs, or even animals or humans (Crous et al., 2007a, Crous et al., 2007b, Zhang et al., 2011, Samerpitak et al., 2014). Based on a multigene phylogenetic analysis, morphological and ecological comparisons, eight genera have been included in Sympoventuriaceae, viz., Clavatispora, Ochroconis, Scolecobasidium, Sympodiella, Sympoventuria, Veronaeopsis, Verruconis and Yunnanomyces. Phylogenetically, Sympoventuriaceae forms a well-supported familial clade within Venturiales (Arzanlou et al., 2007, Crous et al., 2007a, Crous et al., 2007b, Zhang et al., 2011, Samerpitak et al., 2014, Johnston and Park, 2016).
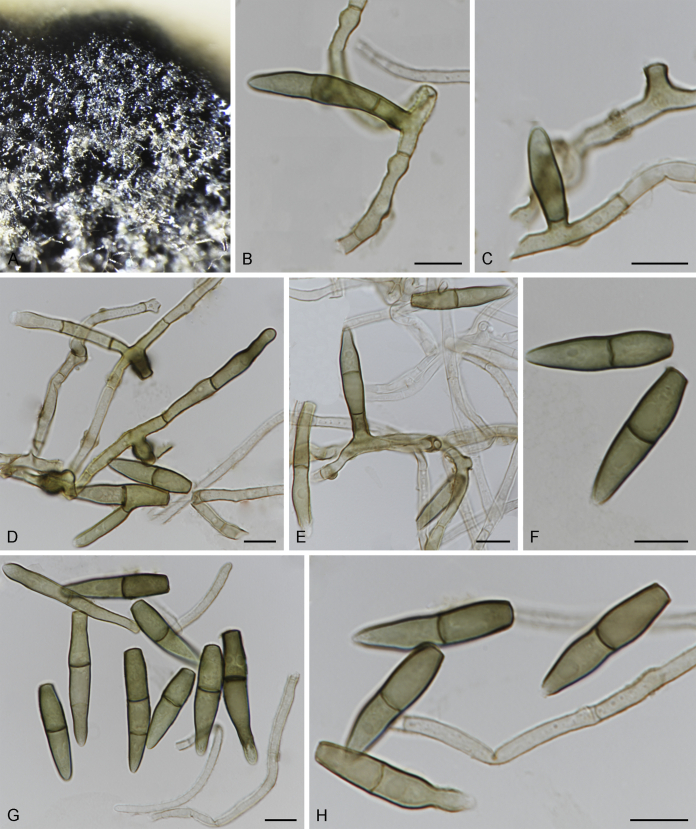
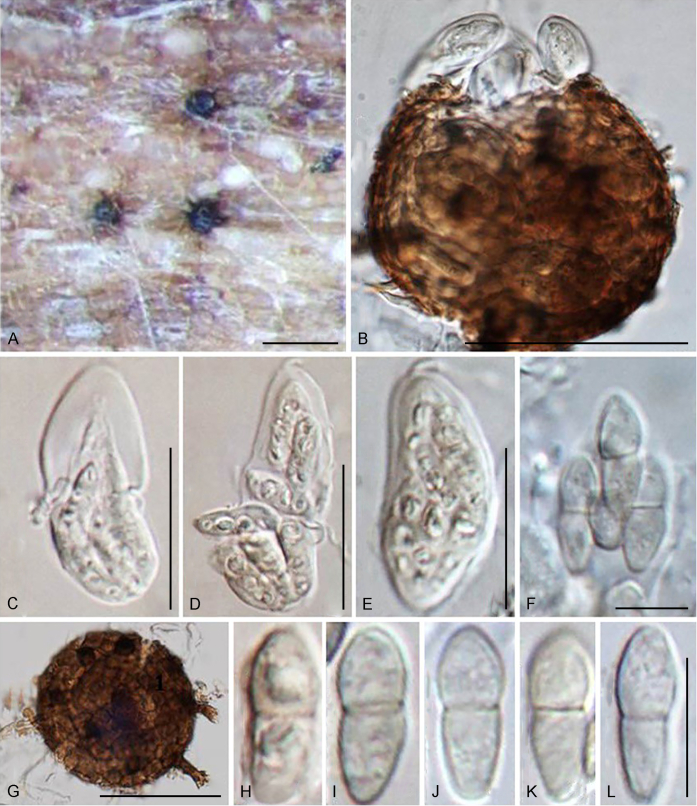
Bellamyces Crous, Coppins & U. Braun, gen. nov. MycoBank MB831519.
Etymology: Named after “Bella”, the beautiful dog that always accompanies Brian J. Coppins on his lichen excursions.
Mycelium consisting of branched, septate, medium brown, smooth hyphae. Conidiophores erect, brown, smooth, subcylindrical, straight to geniculate-sinuous, reduced to conidiogenous cells, or 0–1-septate. Conidiogenous cells terminal, subcylindrical, brown, smooth, proliferating sympodially and inconspicuously 1–2 times percurrently at apex. Conidia solitary, brown, smooth, subcylindrical, straight, widest in middle to lower third, apex subobtuse, transversely euseptate, rarely with 1–2 oblique septa; hila truncate, neither thickened, nor darkened.
Type species: Bellamyces quercus Crous, Coppins & U. Braun
Note: Phylogenetically, Bellamyces quercus clusters basal to Sympoventuria, Pseudosigmoidea and Troposporella (Fig. 1).
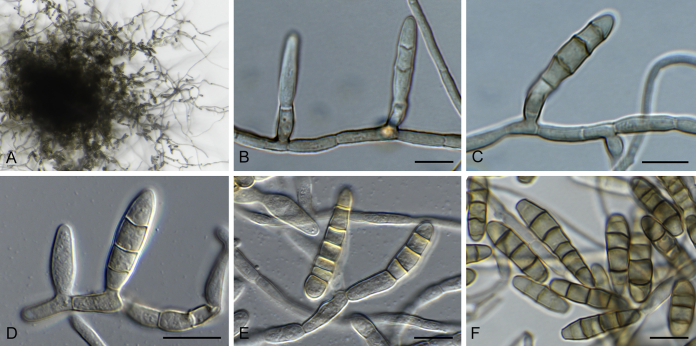
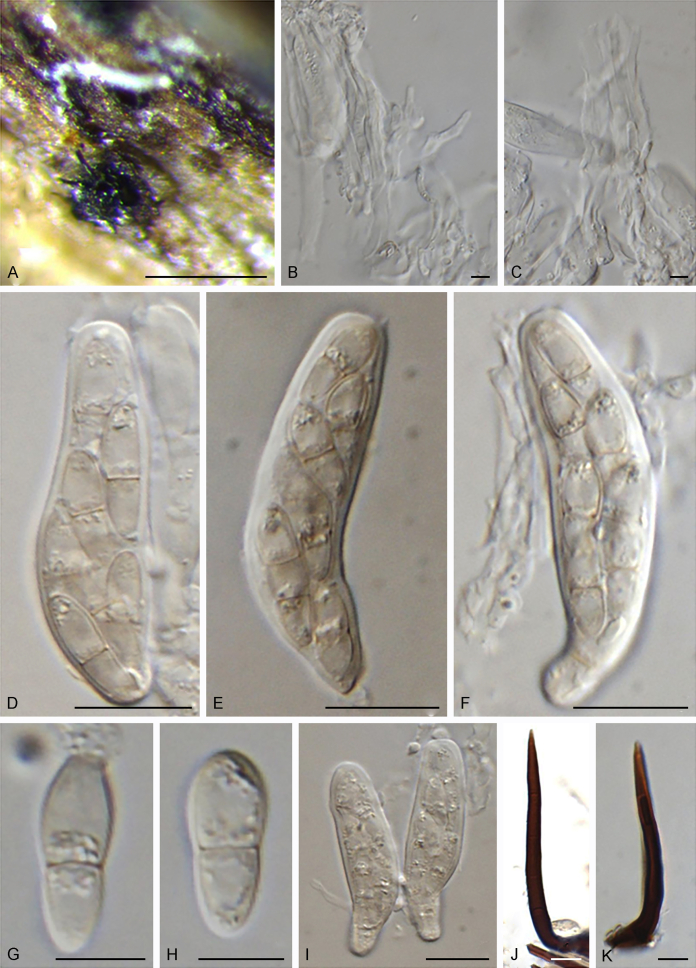
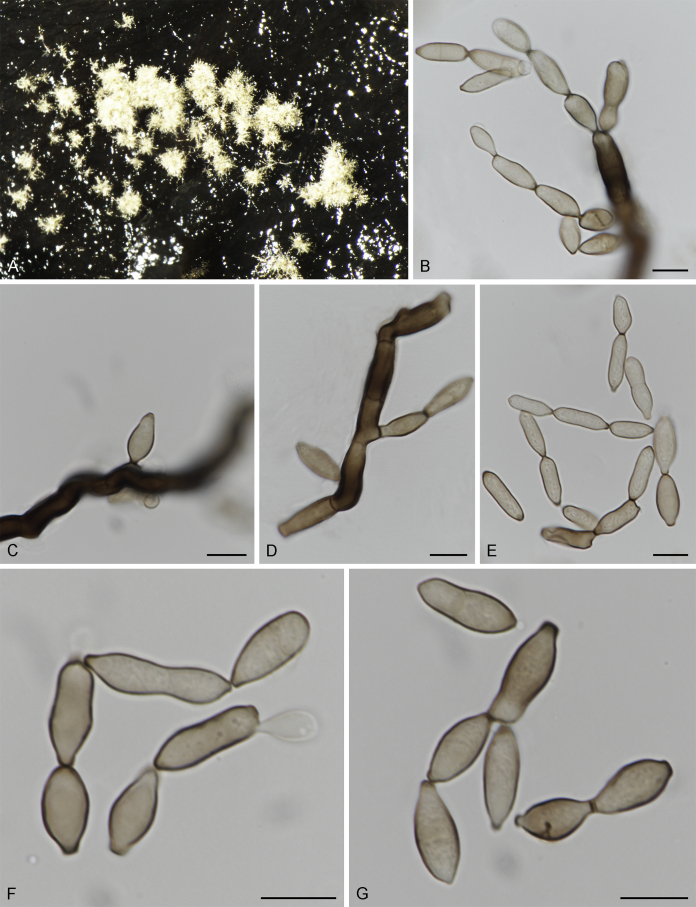
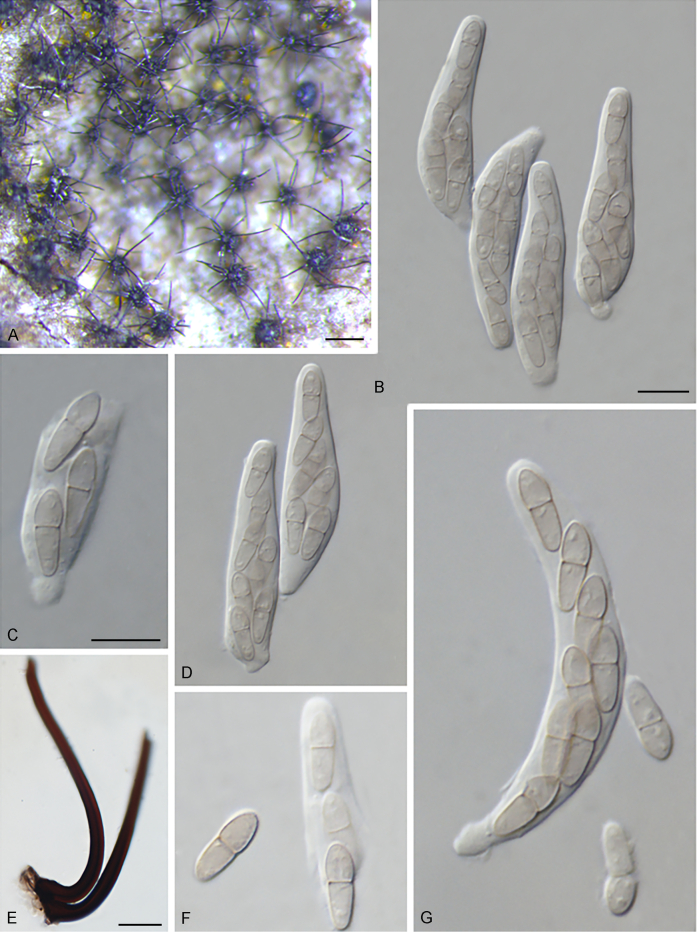
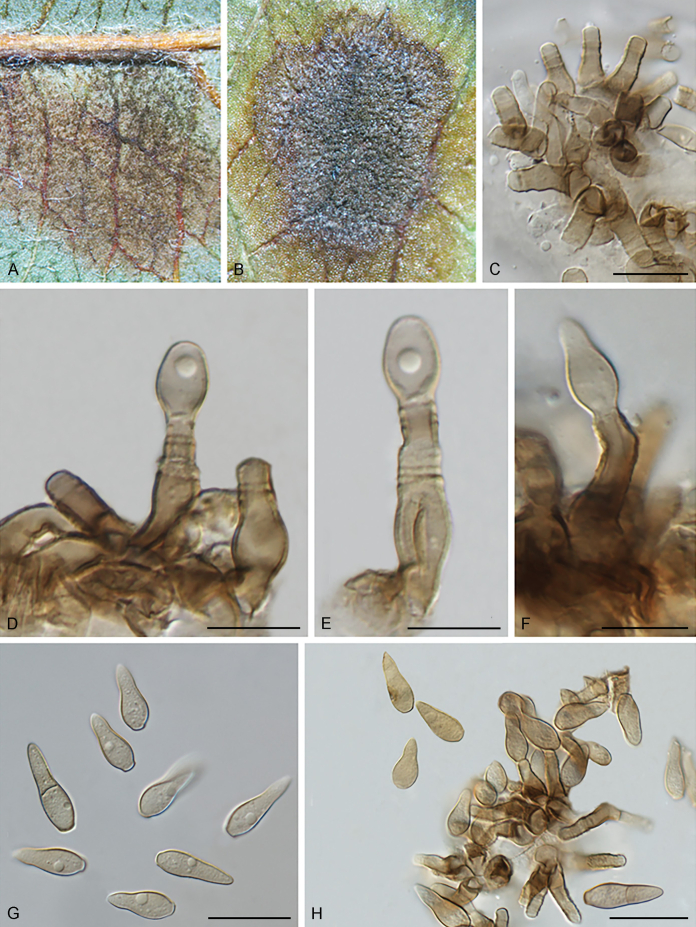
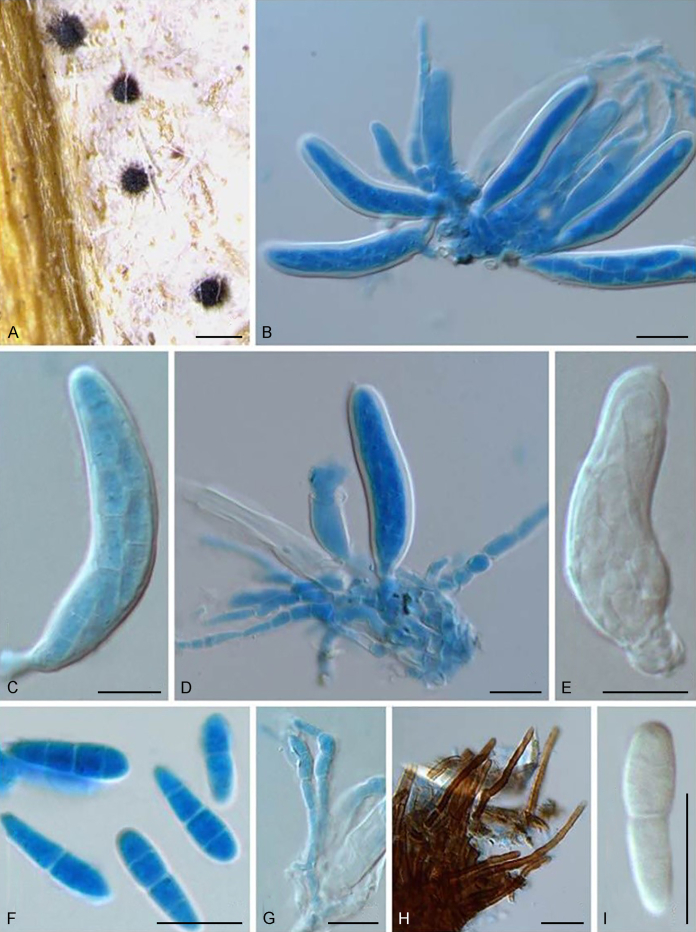
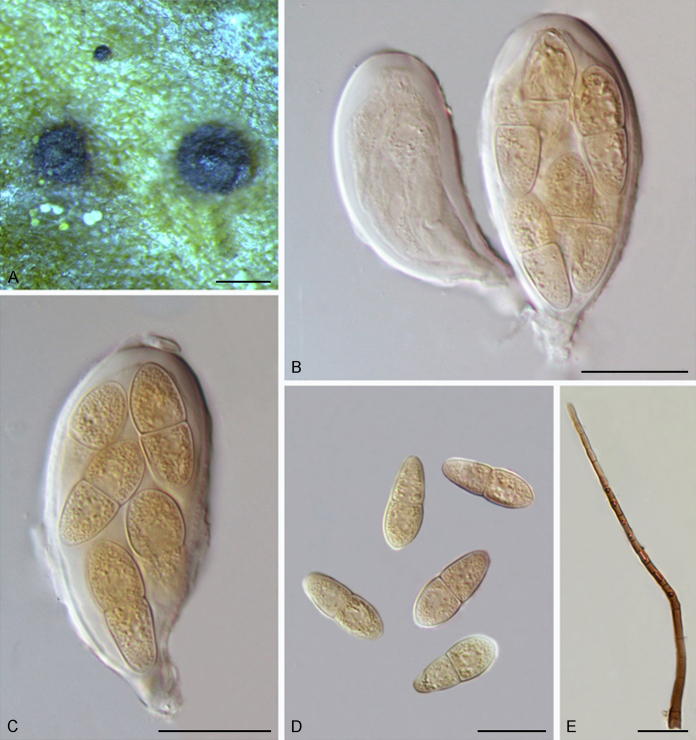
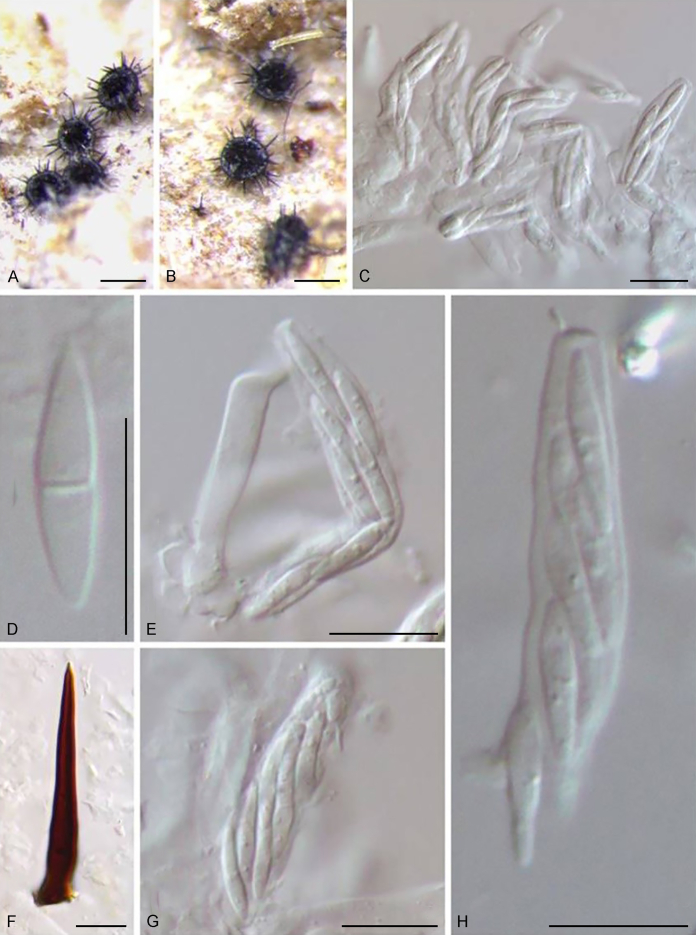
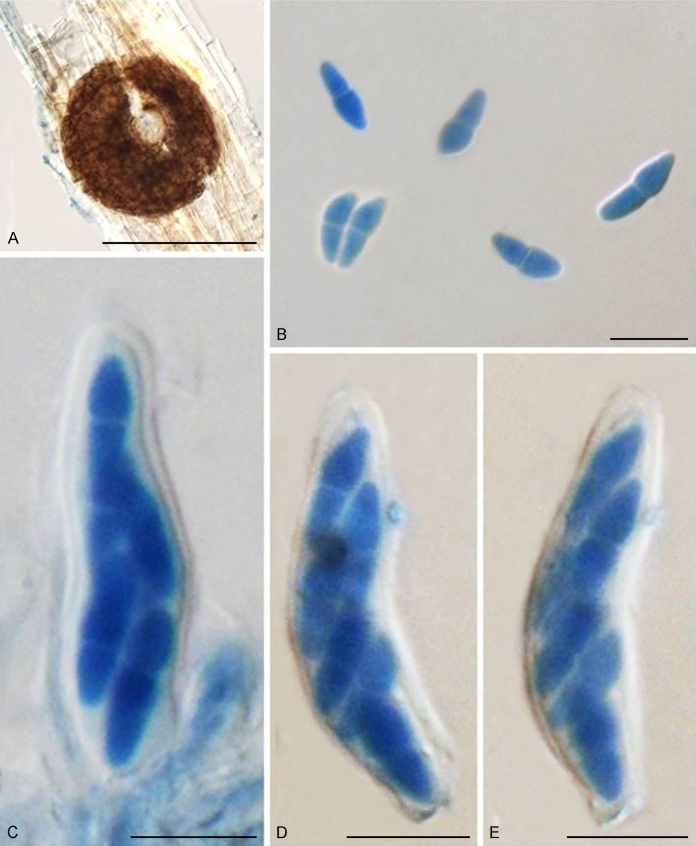
Bellamyces quercus Crous, Coppins & U. Braun, sp. nov. MycoBank MB831520. Fig. 3.
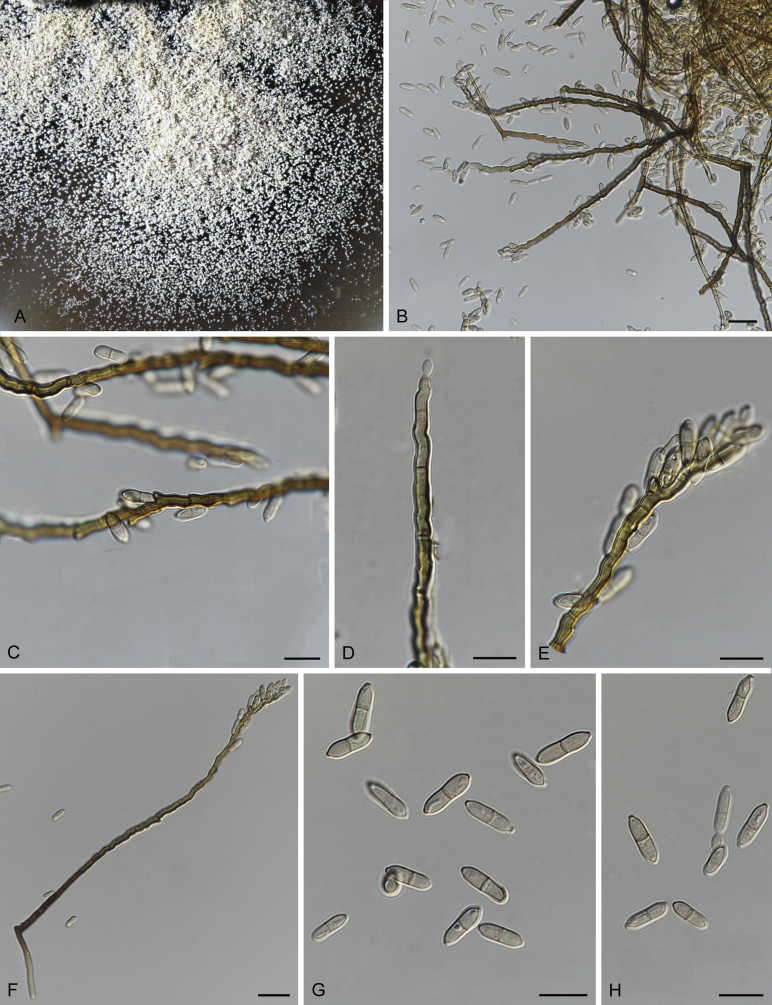
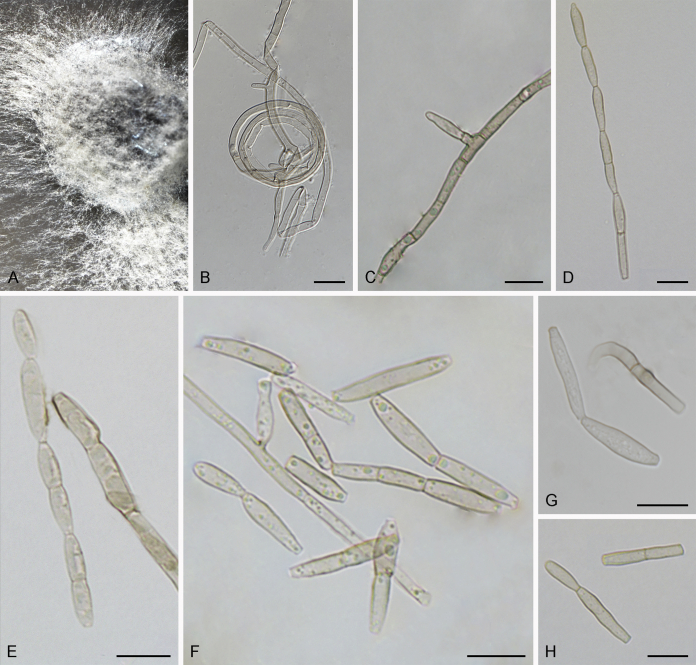
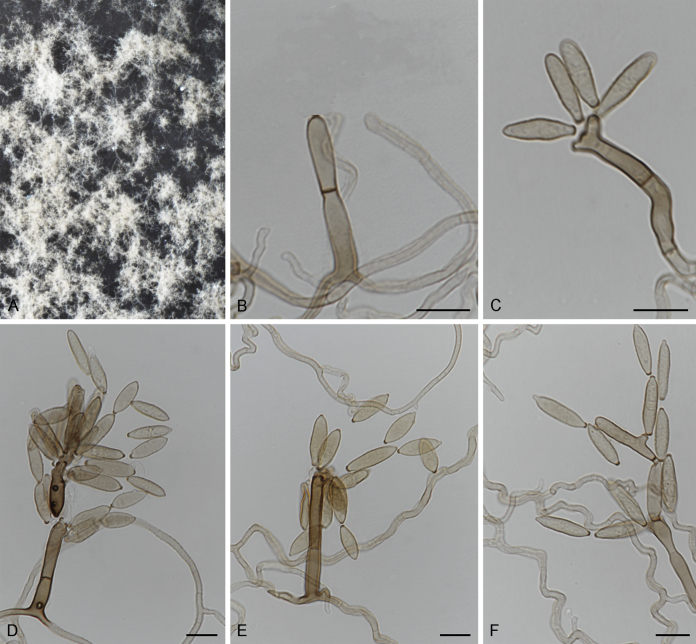
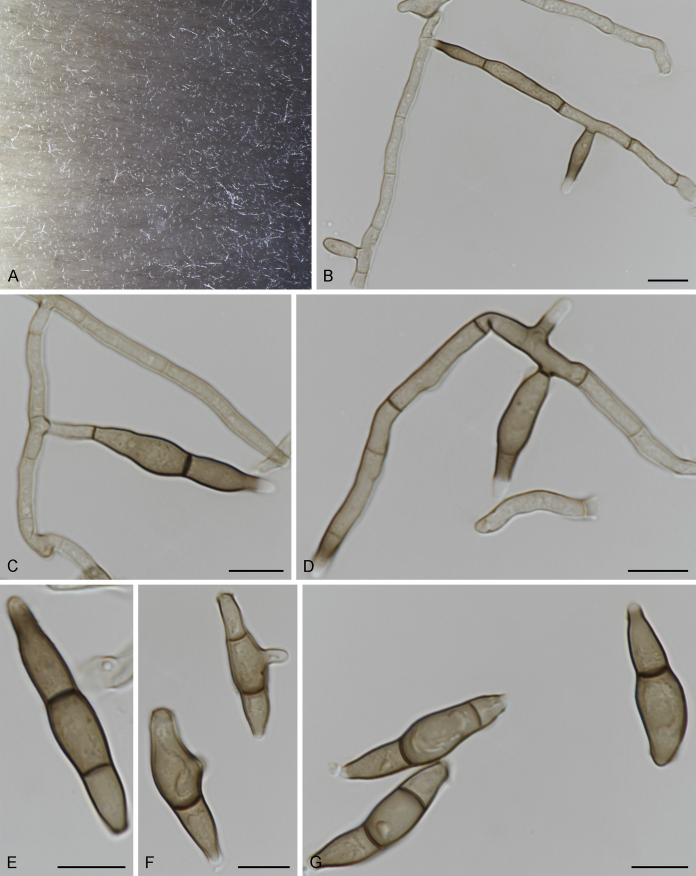
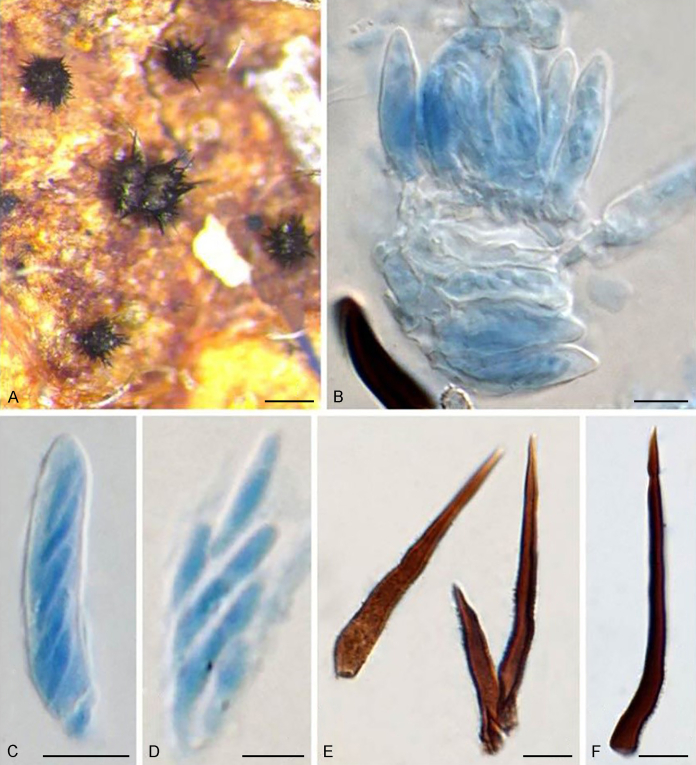
Fig. 3.
Bellamyces quercus (culture ex-type CPC 28858) asexual morph. A. Colony on OA. B–E. Conidiogenous cells producing conidia. F. Multi-septate conidia. Scale bars: B–F = 10 μm.
Etymology: The epithet refers to Quercus, the host genus on which apothecial discs of Lecanora chlarotera were collected.
Mycelium consisting of branched, septate, medium brown, smooth, 3–4 μm diam hyphae. Conidiophores erect, brown, smooth, subcylindrical, straight to geniculate-sinuous, reduced to conidiogenous cells, or 0–1-septate, unbranched, 2–10 × 4–6 μm. Conidiogenous cells terminal, subcylindrical, brown, smooth, proliferating sympodially and inconspicuously 1–2 times percurrently at apex, 2–5 × 4–5 μm. Conidia solitary, brown, smooth, subcylindrical, straight, widest in middle to lower third, apex subobtuse, transversely 3–8-euseptate, rarely with 1–2 oblique septa, (13–)18–22(–25) × (4–)5–6(–6.5) μm; hila truncate, neither thickened, nor darkened, 3–4 μm diam.
Culture characteristics: Colonies erumpent, with sparse aerial mycelium and smooth, lobate margin, reaching 7 mm diam after 1 wk at 25 °C. On MEA surface and reverse umber, on PDA surface umber, reverse chestnut, on OA surface bay with diffuse umber pigment.
Typus: UK, Scotland, VC 82, East Lothian, Spott, the Brunt, oak wood, S facing (former oak coppice), on apothecial discs of Lecanora chlarotera (Lecanoraceae) on Quercus (Fagaceae) trunks, 24 Aug. 2015, B.J. Coppins, Coppins no. 24965 = HPC 571 (holotype CBS H-23838, culture ex-type CBS 146217 = CPC 28858; isotype HAL 2918 F).
Notes: The conidia of Bellamyces are solitary, and transversely multiseptate, rarely oblique. Phylogenetically, it is not related to any other species known from sequence data (Fig. 1).
Echinocatena R. Campb. & B. Sutton, Trans. Brit. Mycol. Soc. 69: 126. 1977.
Type species: Echinocatena arthrinioides R. Campb. & B. Sutton
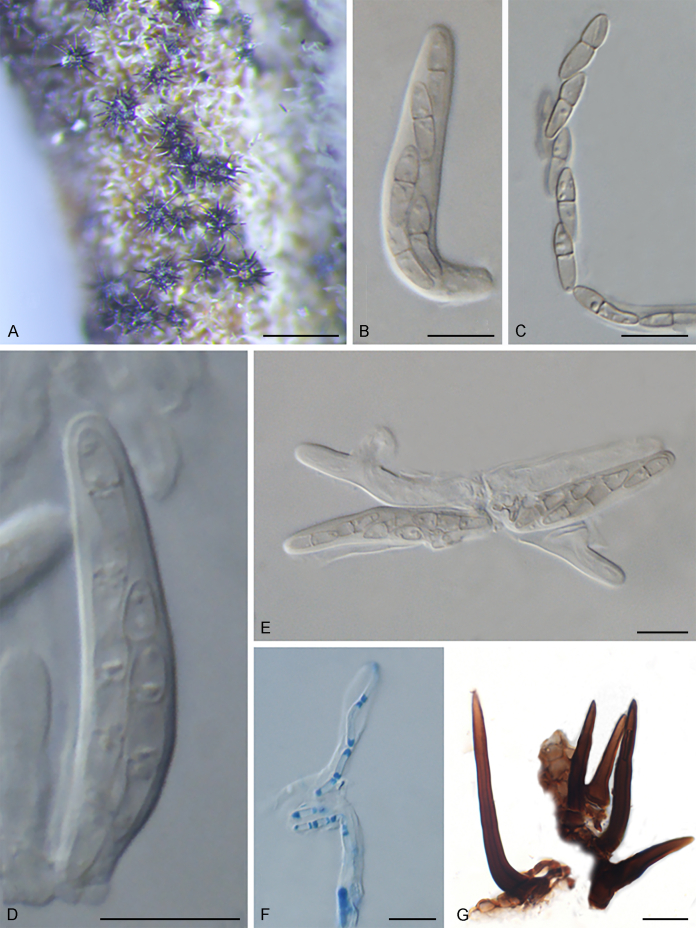
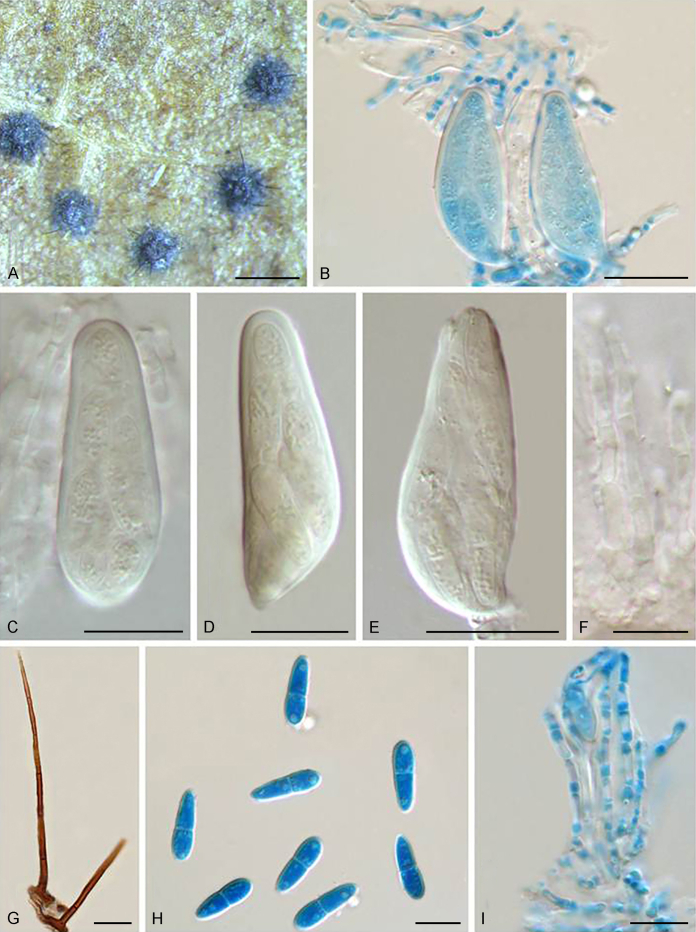
Notes: Echinocatena is a monotypic genus represented by E. arthrinioides, which was collected from leaf litter of an unknown plant in Rajasthan, India (Campbell & Sutton 1977). Morphologically, its straight to flexuous conidiophores and polyblastic conidiogenous cells are consistent with those of Venturiales. The spherical, aseptate conidia of E. arthrinioides, however, differ from other genera (Campbell and Sutton, 1977, Crous et al., 2018). Crous et al. (2018) retrieved an isolate from leaves of Acacia crassicarpa in Malaysia, which morphologically agrees well with Echinocatena arthrinioides, but has larger conidia [(4–)5–6(–7) μm vs. 3.5–4.5 μm]. Phylogenetically, Echinocatena clusters on a long branch basal to Scolecobasidium and Verruconis (Fig. 1).
Echinocatena arthrinioides R. Campb. & B. Sutton, Trans. Brit. Mycol. Soc. 69: 130. 1977.
Typus: India, Jodhpur, on decaying leaves of unknown plant, 25 Nov. 1975, K.S. Panwar (holotype IMI 199279).
Notes: Isolate CPC 28754 was identified as Echinocatena arthrinioides by Crous et al. (2018), which morphologically agrees well with the original description of Echinocatena arthrinioides (Campbell & Sutton 1977), but has slightly larger conidia (see comments above).
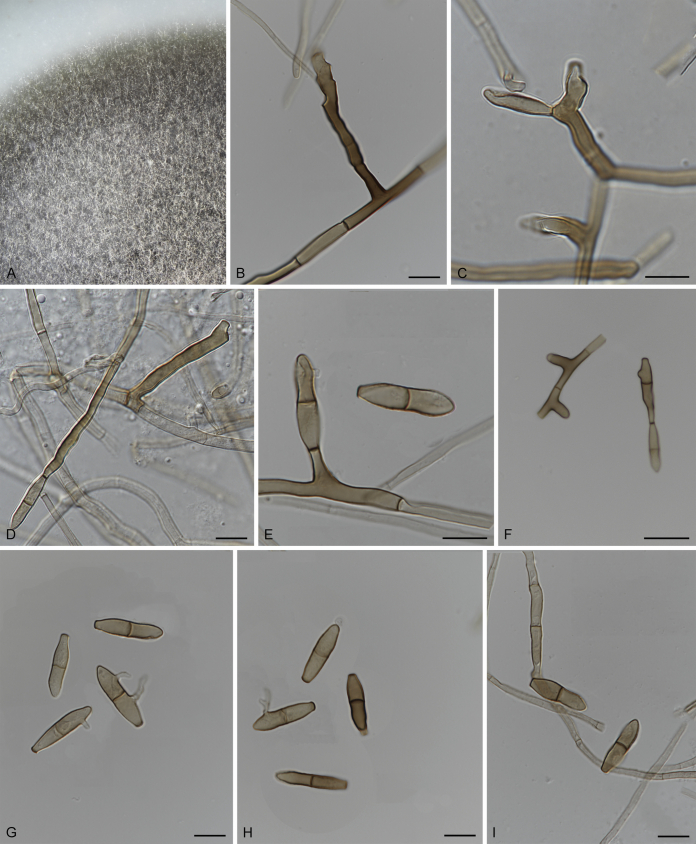
Fuscohilum Crous, M. Shen & Y. Zhang ter, gen. nov. MycoBank MB831514.
Etymology: The epithet refers to the thickened and darkened conidial hila.
Mycelium consisting of smooth to finely roughened, pale to medium brown, branched, septate hyphae, sometimes frequently forming hyphal coils. Conidiophores reduced to conidiogenous cells that are terminal or lateral on hyphae, medium brown, smooth, cylindrical or subcylindrical, erect to subdenticulate, or more distinct, mono- to polyblastic; conidiogenous loci flat-tipped, somewhat darkened and thickened, but not refractive. Ramoconidia present, aseptate or septate. Conidia formed in branched or unbranched chains, pale to medium brown, smooth, subcylindrical, 0–3-septate, slightly tapering towards the subtruncate ends, straight, but at times slightly curved; hila somewhat darkened and thickened, not refractive (adapted from Crous et al., 2007b, Koukol, 2010).
Type species: Fuscohilum rhodensis (Crous) Crous, M. Shen & Y. Zhang ter
Fuscohilum rhodensis (Crous & M.J. Wingf.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831553.
Basionym: Fusicladium rhodense Crous & M.J. Wingf., Stud. Mycol. 58: 212. 2007.
Description and illustration: Crous et al. (2007b).
Typus: Greece, Rhodos, on branches of Ceratonia siliqua (Fabaceae), 1 Jun. 2006, P.W. Crous & M.J. Wingfield (holotype CBS H-19910, culture ex-type CBS 121641 = CPC 13156).
Notes: Fusicladium rhodense was introduced by Crous et al. (2007b) having a pseudocladosporium-like morphology and conidial hila that are somewhat darkened and thickened. Phylogenetically, F. rhodense and F. sicilianum formed a separate generic clade within Sympoventuriaceae (Fig. 1). These two species were therefore assigned to a new genus, Fuscohilum.
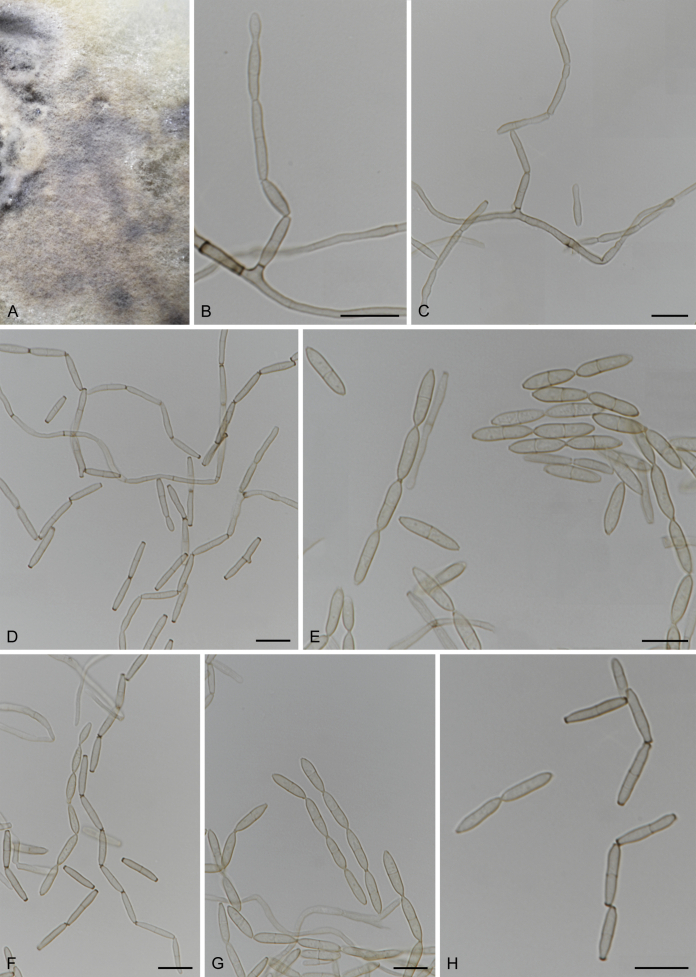
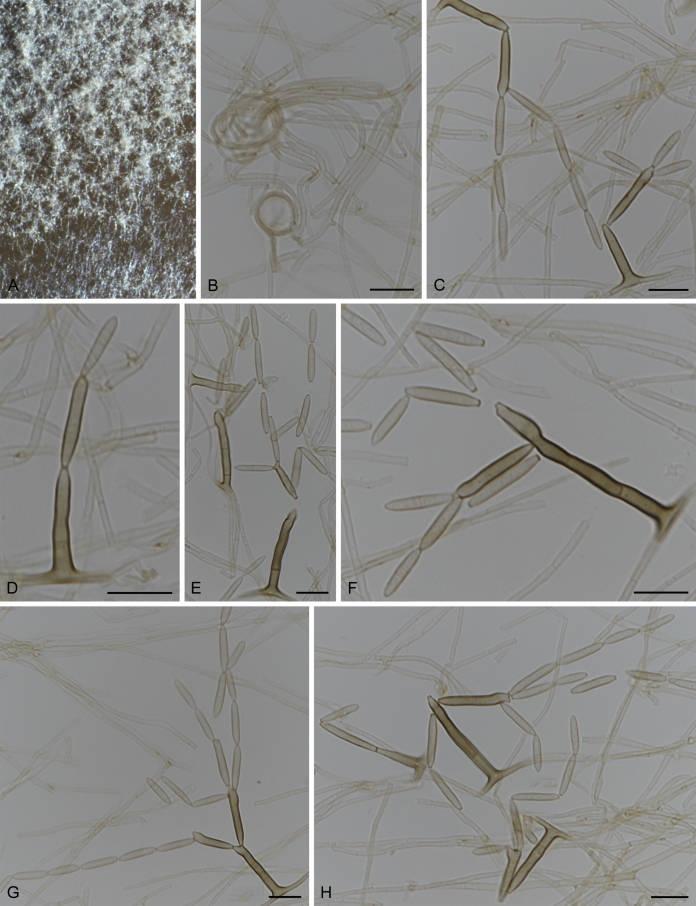
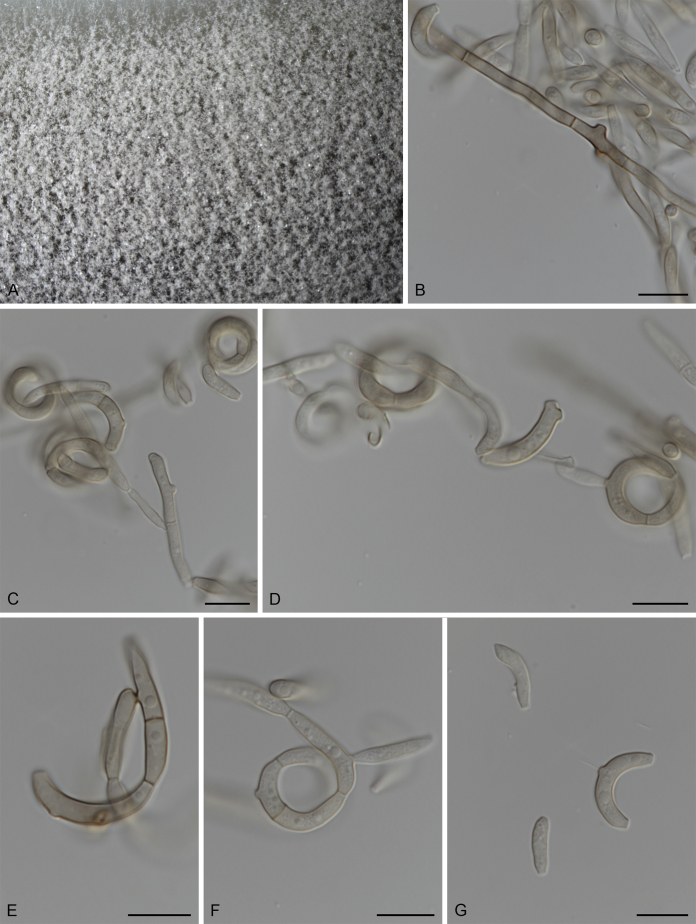
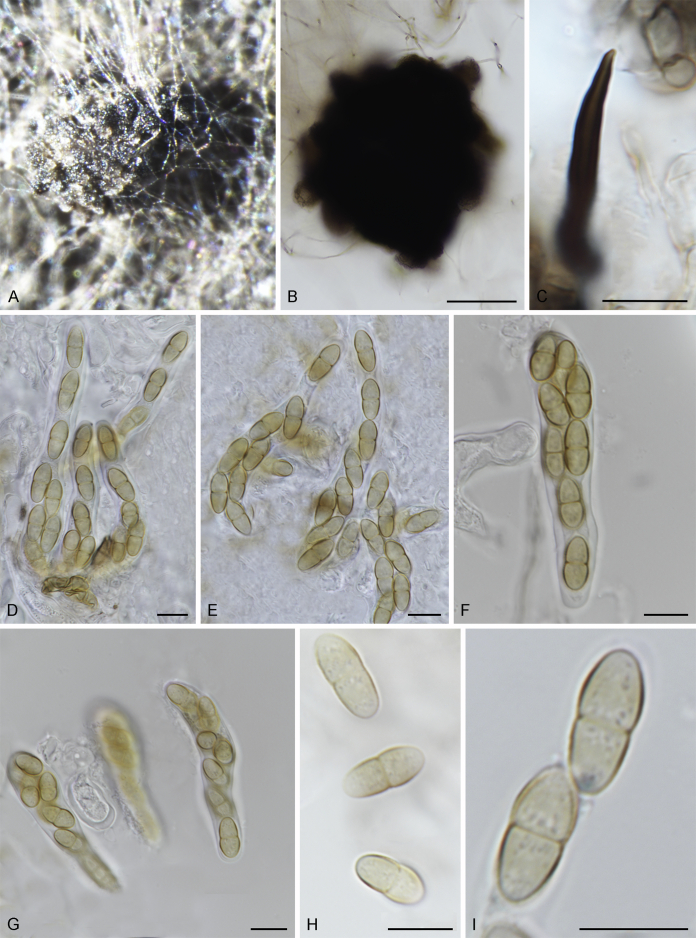
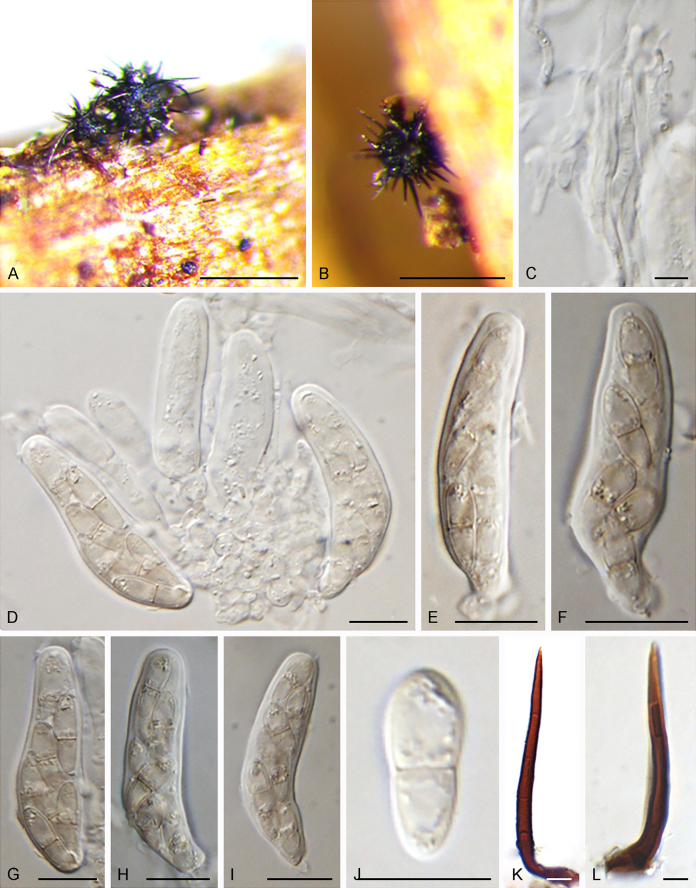
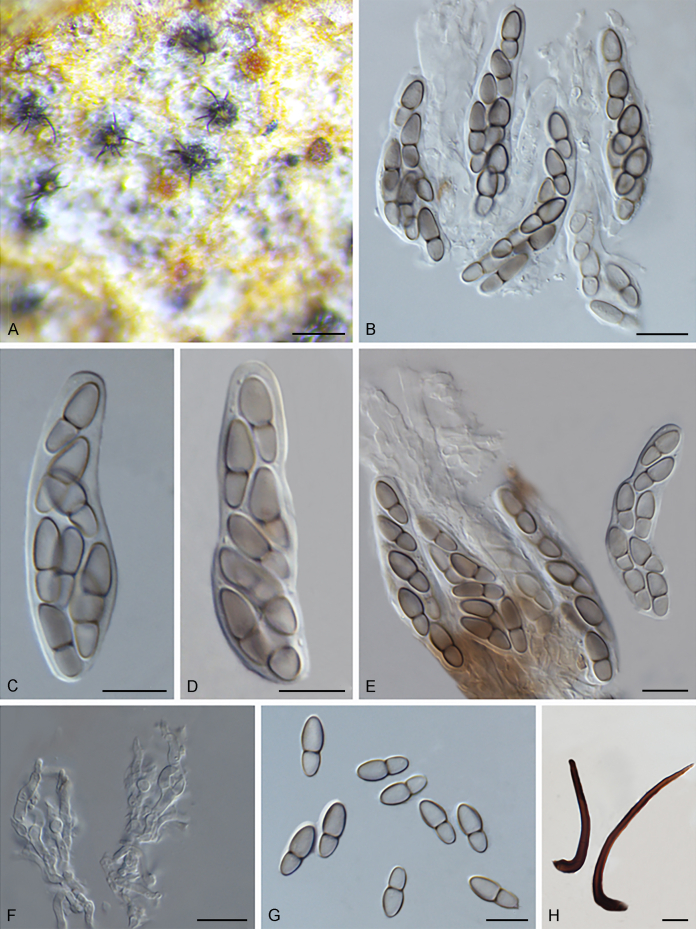
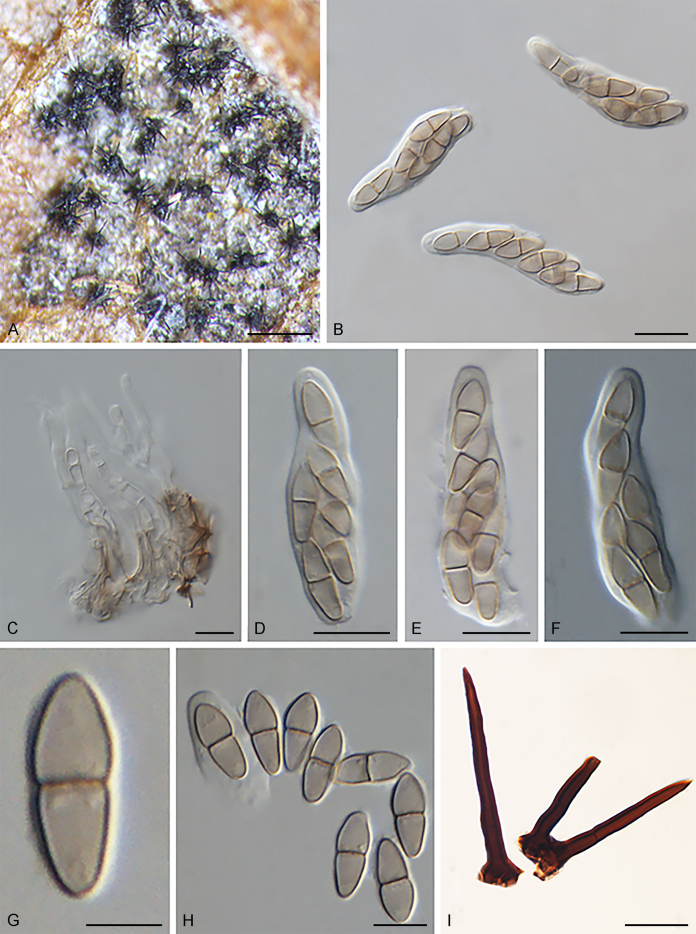
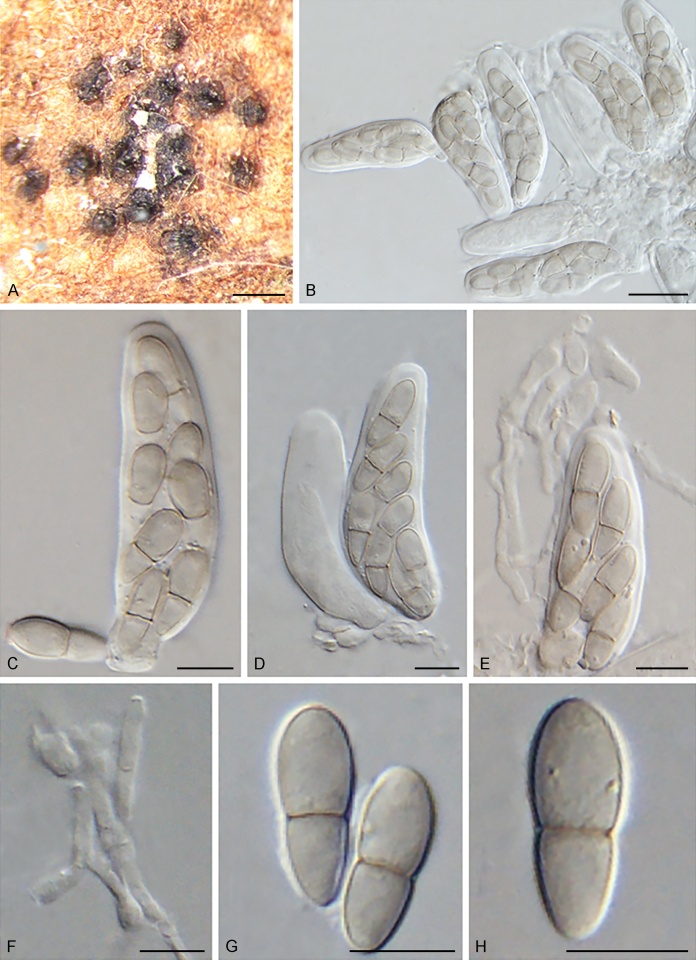
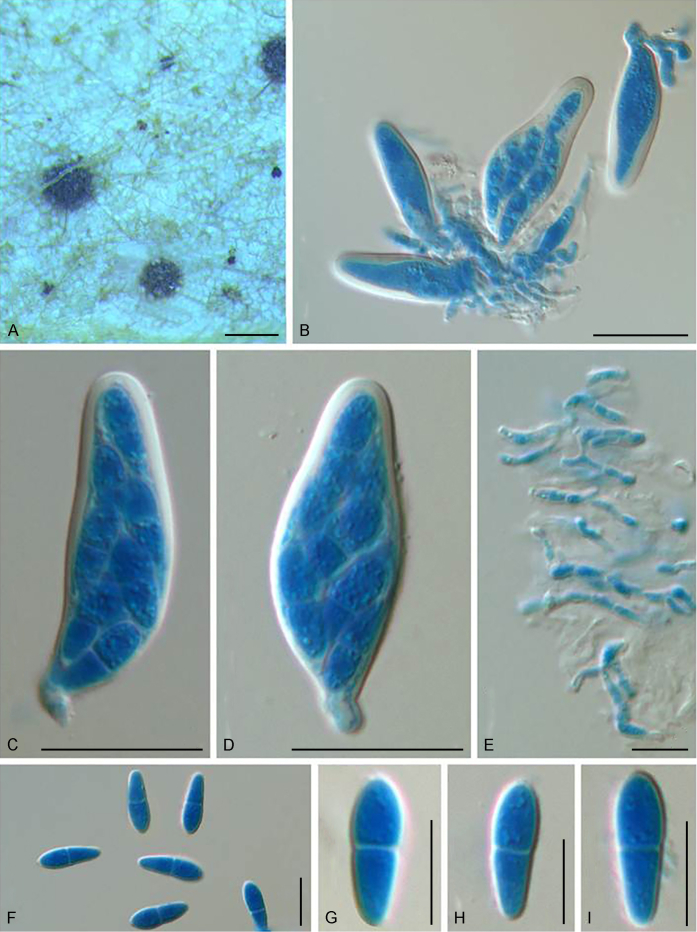
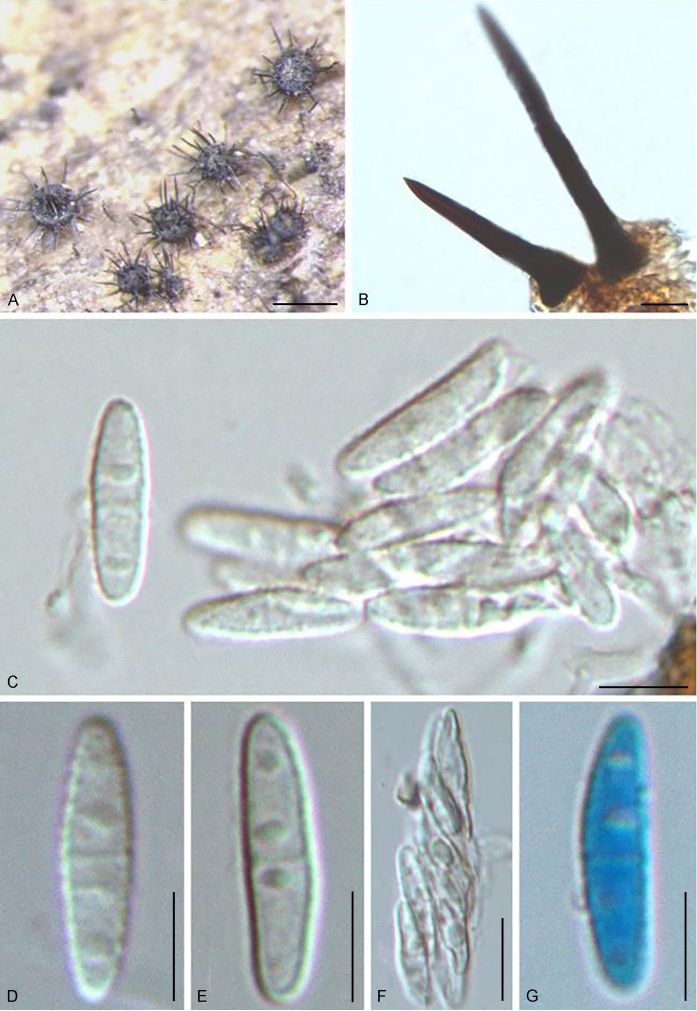
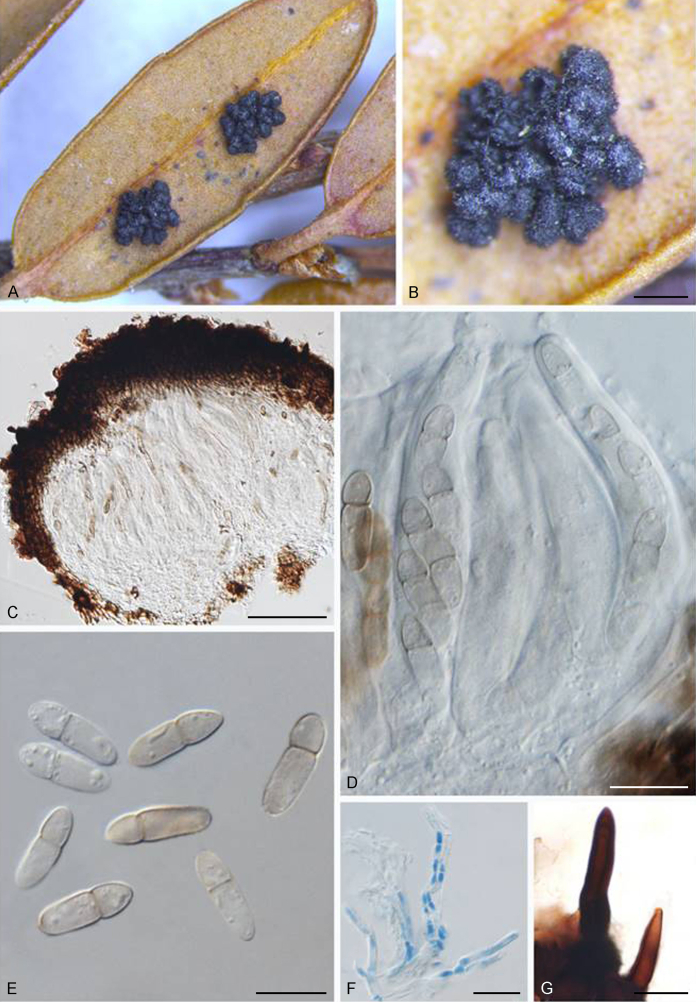
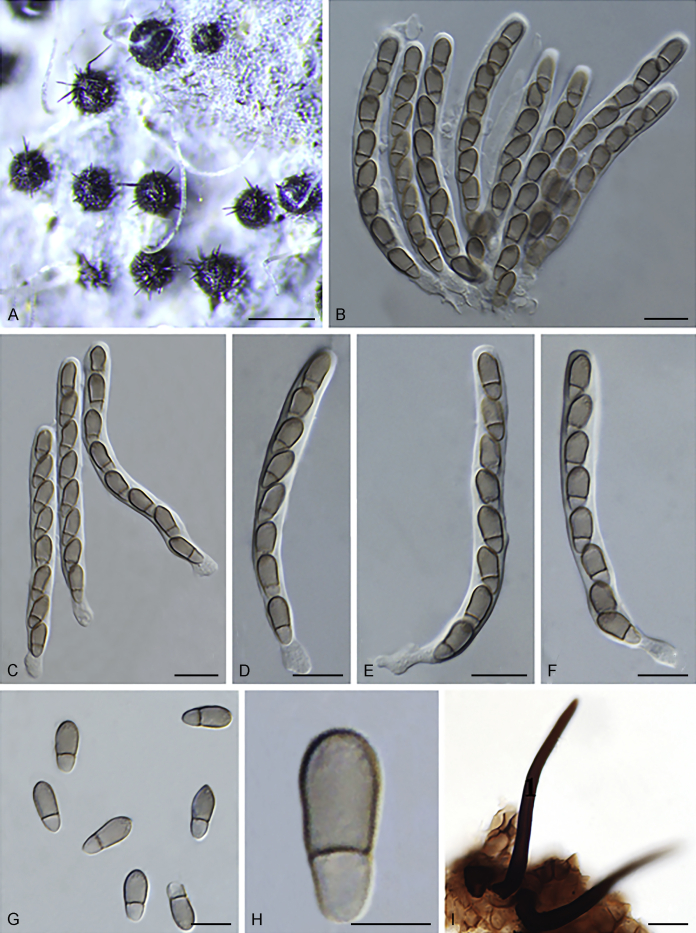
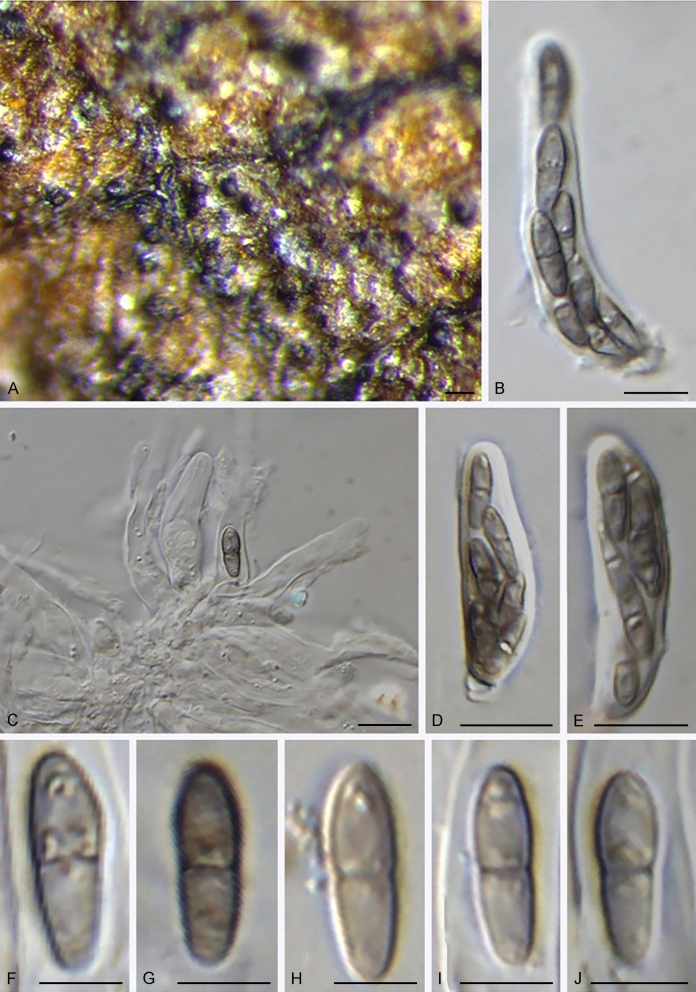
Fuscohilum siciliana (Koukol) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831554. Fig. 4.
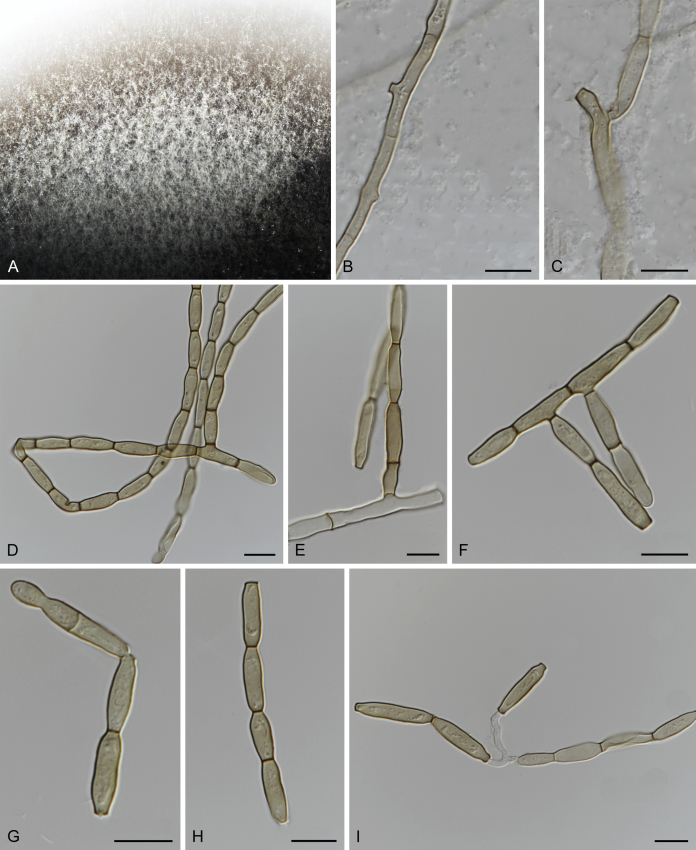
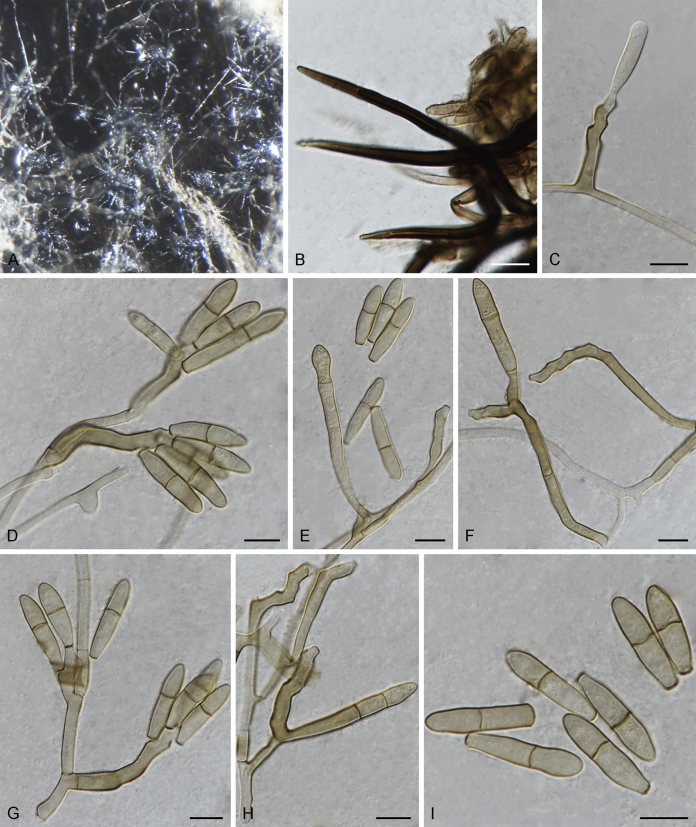
Fig. 4.
Fuscohilum siciliana (culture ex-type CBS 105.85) asexual morph. A. Colony on OA. B, C. Conidia arising from hyphae. D–H. Cylindrical and subcylindrical conidia in chains. Scale bars: B–H = 10 μm.
Basionym: Fusicladium sicilianum Koukol, Mycol. Progr. 9(3): 373. 2010.
Description and illustration: Koukol (2010).
Typus: Italy, Palermo, Botanic Garden, rotten plant of Chamaerops humilis (Arecaceae), Nov. 1984, W. Gams (holotype CBS H-3654, culture ex-type CBS 105.85).
Notes: According to the original description provided by Koukol (2010), the smaller-sized conidia [(8–)10–12(–18) × (1.5–)2–2.5(–3) μm vs. (8–)12–16(–20) × (2–)2.5–3(–4) μm] and the absence of hyphal coils of F. siciliana differs from those of F. rhodensis (Crous et al. 2007b). The two species are phylogenetically distinct (Fig. 1).
Helicopsis P. Karst., Rev. Mycol. (Toulouse) 11: 96. 1889.
Type species: Helicopsis olivacea P. Karst.
Helicopsisolivacea P. Karst. [as “olivaceus”], Rev. Mycol. (Toulouse) 11 (no. 42): 96. 1889.
Synonym: Helicopsis punctata Peck, Bull. New York St. Mus. 167: 26. 1913 [1912].
Troposporellaolivaceum (P. Karst.) C.K.M. Tsui & Berbee [as “olivaceum”], Mycoscience 51: 147. 2010.
Typus: Finland, near the village of Surikat, on the hymenium of Lyomyces roseus (Corticiaceae), Nov. 1886 (not seen).
Notes: Helicopsis was introduced as a monotypic genus, based on H. olivacea, and was assigned to Tubeufiaceae (Karsten 1888). Subsequently, a second species of Helicopsis, H. punctata, was described, which was treated as conspecific with H. olivacea (Peck, 1913, Tsui and Berbee, 2010). Based on the phylogenetic analysis of the small subunit (SSU) and internal transcribed spacers (ITS) rDNA sequences, H. olivacea was assigned to Troposporella as T. olivacea (Tsui & Berbee 2010). The phylogenetic analysis of Tsui & Berbee (2010) focused on class level (Dothideomycetes), and was too general to reflect a detailed classification of Helicopsis. This treatment is rejected in this study, as a strain representing Helicopsis olivacea clustered apart from the clade representing Troposporella (T. fumosa and T. monilipes) (Fig. 1).
Neocoleroa Petr., Hedwigia 74: 38. 1934.
Type species: Neocoleroa sibirica Petr.
Notes: Neocoleroa was introduced based on its lobed to dichotomously branched, blunt-tipped setae and persistent pseudoparaphyses, which was typified by N. sibirica (Petrak 1934). Morphologically, Neocoleroa is most comparable with Wentiomyces (Koorders 1907), and they both were assigned to Pseudoperisporiaceae (Dothideomycetes incertae sedis) (Barr, 1997, Kirk et al., 2008). Barr (1987) noted that some species of these two genera are morphologically similar to members of Venturiaceae. Neocoleroa metrosideri was reported from Metrosideros excelsa, and morphologically agrees with Sympoventuria in having broadly clavate to obclavate asci, hyaline, 1-septate ascospores and persistent pseudoparaphyses (Johnston & Park 2016). Phylogenetically, Neocoleroa metrosideri nested with a novel species N. cameroonensis described below, in Sympoventuriaceae, being sister to other genera of the family (Fig. 1). No DNA data are presently available for the generic type.
Neocoleroa metrosideri P.R. Johnst., Phytotaxa 253: 216. 2016.
Description and illustration: Johnston & Park (2016).
Typus: New Zealand, Auckland, Glen Innes, Auckland University Tamaki campus (S36.883037, E174.849881), on living leaves of Metrosideros excelsa (Myrtaceae), 6 Oct. 2015, P.R. Johnston (holotype PDD 107531, culture ex-type ICMP 21139) (not seen).
Notes: Neocoleroa metrosideri was introduced as the causal agent of leaf spots on Metrosideros excelsa in New Zealand (Johnston & Park 2016). This species is sister to N. cameroonensis (Fig. 1).
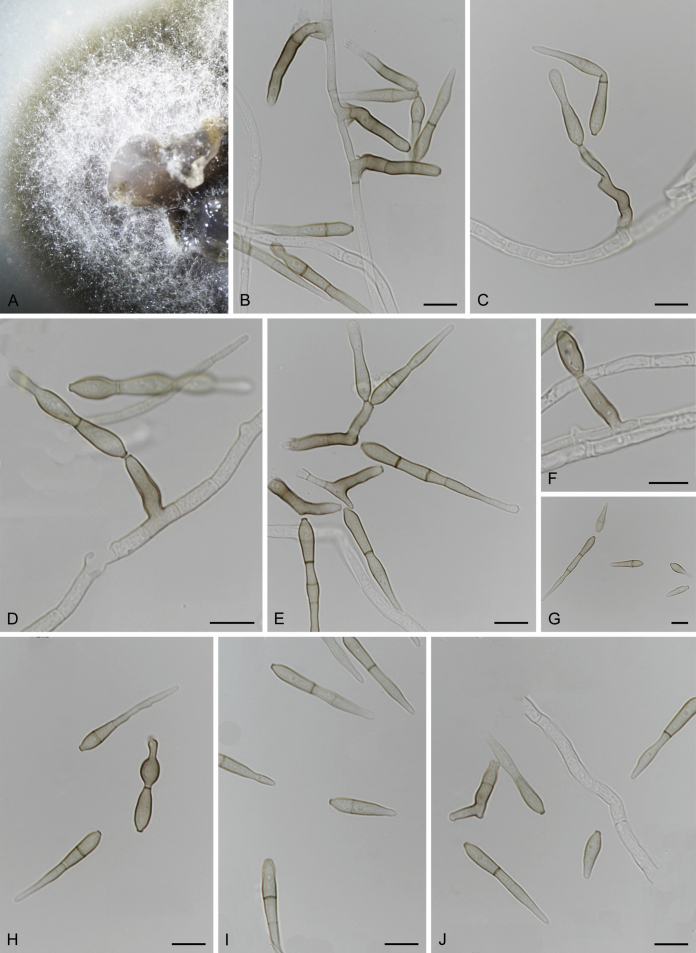
Neocoleroa cameroonensis Crous, M. Shen & Y. Zhang ter, sp. nov. MycoBank MB831521.
Etymology: Named after Cameroon, the country where this fungus was collected.
Cultures sterile. Neocoleroa cameroonensis (CBS 129041) differs from its closest phylogenetic neighbour N. metrosideri (PDD 107531) (Fig. 1) by unique fixed alleles in two loci based on alignments of the separate loci deposited in TreeBASE (S24573), by 56 bp in ITS (14 %) and 26 bp in LSU (3 %).
Culture characteristics: Colonies spreading, erumpent, with aerial mycelium and regular, smooth margins on OA, dark olivaceous brown (surface); reverse fuscous-black; on MEA dark brown (surface); reverse fuscous-black; on SNA dark brown (surface); reverse fuscous-black. Colonies reaching 8 mm diam after 2 wk on OA at 25 °C in the dark.
Typus: Cameroon, Londgi, Crematogaster sp. (ant) on Barteria nigritana (Passifloraceae), 19 Dec. 2009, R. Blatrix (holotype CBS H-23598, culture ex-type CBS 129041).
Notes: Based on the multigene phylogenetic analysis, Neocoleroa cameroonensis clusters together with the type specimen of N. metrosideri in Neocoleroa (Fig. 1). The species was isolated from a Crematogaster sp. (ant) but did not sporulate in culture on any of the media used here.
Neofusicladium Crous, M. Shen & Y. Zhang ter, gen. nov. MycoBank MB831512.
Etymology: Named after the genus Fusicladium, to which it is morphologically similar, and neo- to new.
Mycelium consisting of pale to medium brown, smooth, branched, septate hyphae. Conidiophores reduced to conidiogenous cells, or with basal supporting cell, solitary, erect, pale brown, smooth, subcylindrical to doliiform, sometimes dimorphic. Conidiogenous cells terminal or lateral, integrated, subcylindrical or doliiform, pale to medium brown, smooth, proliferating sympodial; conidiogenous loci somewhat thickened and darkened, not refractive. Ramoconidia brown, smooth, subcylindrical or fusoid-ellipsoid, aseptate or septate. Conidia mostly occurring in branched chains, pale brown, smooth, subcylindrical to fusoid-ellipsoidal, aseptate or septate, sometimes widest in middle, truncate at the ends; hila somewhat darkened and thickened, but not refractive (adapted from Crous et al., 2010, Crous et al., 2016, Crous et al., 2017).
Type species: Neofusicladium eucalypti (Crous & R.G. Shivas) Crous, M. Shen & Y. Zhang ter
Notes: So far, Neofusicladium comprises three species, viz., N. eucalypti, N. eucalypticola and N. regnans. All three Neofusicladium species were isolated from Eucalyptus leaves (Crous et al., 2010, Crous et al., 2016, Crous et al., 2017). The diagnostic characteristics of Neofusicladium includes sympodial conidiophores with somewhat thickened and darkened, non-refractive conidiogenous loci, mostly branched conidial chains, and the presence of ramoconidia (Crous et al., 2010, Crous et al., 2016, Crous et al., 2017). Phylogenetically, Neofusicladium is basal in Sympoventuriaceae and is introduced as a new genus (Fig. 1).
Neofusicladium eucalypti (Crous & R.G. Shivas) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831541. Fig. 5.
Fig. 5.
Neofusicladium eucalypti (culture ex-type CBS 128216) asexual morph. A. Colony on OA. B–D. Concatenated conidia arising from hypha. E. Pale brown, fusiform and aseptate conidia. Scale bars: B–E = 10 μm.
Basionym: Fusicladium eucalypti Crous & R.G. Shivas, Persoonia 25: 149. 2010.
Description and illustration: Crous et al. (2010).
Typus: Australia, Queensland, Brisbane, Mt. Coot-tha, Bardon Trail, on leaves of Eucalyptus sp. (Myrtaceae), 12 Jul. 2009, P.W. Crous & R.G. Shivas (holotype CBS H-20497, culture ex-type CBS 128216 = CPC 17324).
Notes: Neofusicladium eucalypti was first described (as Fusicladium eucalypti) from Eucalyptus leaves in Australia (Crous et al. 2010). Its dimorphic conidiophores can serve as a diagnostic character for this species (Crous et al. 2010). This species is sister to N. eucalypticola in Fig. 1.
Neofusicladium eucalypticola (Crous & M.J. Wingf.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831542. Fig. 6.
Fig. 6.
Neofusicladium eucalypticola (culture ex-type CBS 141301) asexual morph. A. Colony on OA. B–E. Concatenated conidia arising from hypha. F, G. Pale brown and aseptate or 1-septate conidia. Scale bars: B–G = 10 μm.
Basionym: Fusicladium eucalypticola Crous & M.J. Wingf., Persoonia 36: 369. 2016.
Description and illustration: Crous et al. (2016).
Typus: France, La Réunion, on leaves of Eucalyptus robusta (Myrtaceae), 8 Mar. 2015, P.W. Crous & M.J. Wingfield (holotype CBS H-22614, culture ex-type CBS 141301 = CPC 27238).
Notes: The broader conidia of N. eucalypticola [(2.5–)3(–4) μm] distinguish it from N. eucalypti [(2–)2.5(–3) μm)]. Furthermore, secondary ramoconidia of N. eucalypticola (15–20 × 3–5 μm) are larger than those of N. eucalypti (10–15 × 2–3 μm) and N. regnans (10–20 × 3–4 μm) (Crous et al., 2010, Crous et al., 2016, Crous et al., 2017). This species is sister to N. eucalypti in Fig. 1.
Neofusicladium regnans (Crous) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831543.
Basionym: Sympoventuria regnans Crous, Persoonia 39: 425. 2017.
Description and illustration: Crous et al. (2017).
Typus: Australia, Victoria, La Trobe State Forest, on leaves of Eucalyptus regnans (Myrtaceae), 30 Nov. 2016, P.W. Crous (holotype CBS H-23304, culture ex-type CBS 143411 = CPC 32720).
Notes: Neofusicladium regnans was first described (as Sympoventuria regnans) on leaves of Eucalyptus regnans collected in Victoria, Australia (Crous et al. 2017). The larger-sized conidia of N. regnans (8–20 × 2.5–3 μm) are easily distinguishable from those of N. eucalypti (7–10 × 2–3 μm) and N. eucalypticola (5–12 × 2.5–4 μm) (Crous et al., 2010, Crous et al., 2016, Crous et al., 2017). Neofusicladium regnans represents the most basal species in the Neofusicladium clade (Fig. 1).
Parafusicladium Crous, M. Shen & Y. Zhang ter, gen. nov. MycoBank MB831513.
Etymology: Named after Fusicladium, the morphologically most comparable genus. Para- means false.
Mycelium consisting of pale brown, smooth, branched, septate hyphae. Conidiophores erect, solitary, subcylindrical, brown to dark brown, septate, sometimes thick-walled, smooth, rarely branched below, sometimes dimorphic. Conidiogenous cells integrated, terminal, rarely lateral, brown, smooth, with several or numerous sympodial denticle-like loci, somewhat thickened and darkened, but not refractive. Conidia sometimes occurring in short chains, straight, cylindrical, subcylindrical, subhyaline to pale brown, smooth, guttulate, mostly 1-septate, ends obtusely rounded; hila somewhat thickened and darkened (adapted from Ho et al., 1999, Crous et al., 2007b, Crous et al., 2016).
Type species: Parafusicladium amoenum (R.F. Castañeda & Dugan) Crous, M. Shen & Y. Zhang ter
Notes: The sympodial conidiogenous cells and subcylindrical conidia with somewhat thickened and darkened hila of Parafusicladium point to Sympoventuriaceae. Based on a multigene phylogenetic analysis, it forms a subclade sibling to other genera of Sympoventuriaceae (Fig. 1). Parafusicladium is thus introduced here comprising three species, viz., P. amoenum, P. intermedium and P. paraamoenum.
Parafusicladium amoenum (R.F. Castañeda & Dugan) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831544. Fig. 7.
Fig. 7.
Parafusicladium amoenum (culture ex-type CBS 254.95) asexual morph. A. Colony on OA. B–F. Long conidiophores reduced to sympodial conidiogenous cells. G, H. Pale brown and 1-septate conidia. Scale bars: B–H = 10 μm.
Basionym: Anungitopsis amoena R.F. Castaneda & Dugan, Mycotaxon 72: 118. 1999.
Synonyms: Fusicladium amoenum (R.F. Castañeda & Dugan) Crous et al., Stud. Mycol. 58: 207. 2007.
Cladosporium amoenum R.F. Castañeda, BCCM MUCL Agro-industrial fungi-yeasts. 1998. Nom. inval., Art. 38.1(a) (Shenzhen).
Description and illustration: Untereiner et al., 1998, Ho et al., 1999, Crous et al., 2007b.
Typus: Cuba, Santiago de Cuba, La Gran Piedra, fallen leaves of Eucalyptus sp. (Myrtaceae), 2 Nov. 1994, R.F. Castañeda (Ho et al. 1999: 117, Fig 2, Fig. 3, holotype; epitype ATCC 200947 (designated in Ho et al. 1999), culture ex-epitype CBS 254.95 = ATCC 200947 = IMI 367525 = INIFAT C94/155 = MUCL 39143).
Notes: Cladosporium amoenum was first described from fallen leaves of Eucalyptus sp. collected in Cuba, which was, unfortunately, invalid because it lacked a Latin diagnosis (Untereiner et al. 1998). Ho et al. (1999) validated its name and assigned it to Anungitopsis (as A. amoena), which was subsequently assigned to Fusicladium (as F. amoenum) (Crous et al. 2007b). The colony of Fusicladium amoenum is pseudocladosporium-like, while the loci of the conidiogenous cells are neither prominently thickened, nor refractive (Ho et al., 1999, Crous et al., 2007a). Phylogenetically, Fusicladium amoenum clusters in Parafusicladium, sister to P. paraamoenum (Fig. 1).
Parafusicladium intermedium (Crous & W.B. Kendr.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831545. Fig. 8.
Fig. 8.
Parafusicladium intermedium (culture ex-epitype CBS 110746) asexual morph. A. Colony on OA. B–E. Brown conidiophores with sympodial conidiogenous loci. F–H. Subhyaline and cylindrical conidia. Scale bars: B–H = 10 μm.
Basionym: Anungitopsis intermedia Crous & W.B. Kendr., S. African J. Bot. 63: 286. 1997.
Synonym: Fusicladium intermedium (Crous & W.B. Kendr.) Crous, Stud. Mycol. 58: 209. 2007.
Descriptions and illustrations: Crous et al., 1997, Crous et al., 2007b.
Typus: Madagascar, Tamatave, leaf litter of Eucalyptus sp. (Myrtaceae), Apr. 1994, P.W. Crous (epitype CBS H-19918 (designated in Crous et al. 2007b), culture ex-epitype CBS 110746 = CPC 778 = IMI 362702). South Africa, Mpumalanga, from leaf litter of Eucalyptus sp., Oct. 1992, M.J. Wingfield (holotype PREM 51438).
Notes: Anungitopsis intermedia was described from leaf litter of Eucalyptus sp. in South Africa, and was subsequently assigned to Fusicladium (as F. intermedium) (Crous et al., 1997, Crous et al., 2007b). Morphologically, the conidiophores are dimorphic in culture, being either macronematous, anungitopsis-like, or micronematous, more pseudocladosporium-like (Crous et al., 1997, Crous et al., 2007b). This species is the most basal species in Parafusicladium (Fig. 1).
Parafusicladium paraamoenum (Crous et al.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831552. Fig. 9.
Fig. 9.
Parafusicladium paraamoenum (culture ex-type CBS 141322) asexual morph. A. Colony on OA. B. Hyphal coil. C–E. Conidia arising from sympodial conidiogenous cells. F. Pale brown, aseptate or 1-septate conidia. Scale bars: B–F = 10 μm.
Basionym: Fusicladium paraamoenum Crous et al., Persoonia 36: 377. 2016.
Description and illustration: Crous et al. (2016).
Typus: Australia, Victoria, Toolangi State Forest, on leaves of Eucalyptus regnans (Myrtaceae), 9 Nov. 2014, P.W. Crous, J. Edwards & P.W.J. Taylor (holotype CBS H-22618, culture ex-type CBS 141322 = CPC 25596).
Notes: Morphologically, P. paraamoenum is most comparable with P. amoenum, but has larger conidia [(13–)15–20(–28) × (3–)3.5(–4) μm vs. (6–)10.5–12.8(–17.3) × (1.5–)2.4–3(–3.8) μm] (Ho et al., 1999, Crous et al., 2016). They also differ in their dimorphic conidiophores (Crous et al. 2016). Phylogenetically, the two species are siblings (Fig. 1).
Pinaceicola Crous, M. Shen & Y. Zhang ter, gen. nov. MycoBank MB831515.
Etymology: The epithet refers to Pinaceae, the host family from which the genus was described.
Mycelium consisting of branched, septate, pale to medium brown, smooth hyphae. Conidiophores erect, pale to dark brown, subcylindrical, smooth, straight, reduced to conidiogenous cells, with one to several conidiogenous loci, subcylindrical to almost conical, widest at the base, tapering to a subtruncate or truncate apex; conidiogenous loci flat-tipped, somewhat darkened and thickened. Ramoconidia present, aseptate or septate. Conidia in branched or unbranched chains, pale to medium brown or pale olivaceous, smooth, narrowly ellipsoid, subcylindrical or fusoid, straight to slightly curved, 0–1-septate, mostly widest in the middle, tapering to subtruncate or truncate ends; hila somewhat darkened and thickened, not refractive (adapted from Crous et al., 2007b, Koukol, 2010).
Type species: Pinaceicola pini (Crous) Crous, M. Shen & Y. Zhang ter
Note: The two species of Pinaceicola presently recognised were both reported as saprobes on needles of Pinaceae (Crous et al., 2007b, Koukol, 2010).
Pinaceicola cordae (Koukol) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831555. Fig. 10.
Fig. 10.
Pinaceicola cordae (culture CBS 675.82) asexual morph. A. Colony on OA. B, C. Conidia arising from conidiogenous cells. D–F. Pale brown, aseptate or 1-septate conidia in branched chains. Scale bars: B–F = 10 μm.
Basionym: Fusicladium cordae Koukol, Mycol. Progr. 9(3): 371. 2010.
Description and illustration: Koukol (2010).
Typus: Czech Republic, Doubice, Tokáň, on needle litter of Pinus sylvestris (Pinaceae), 11 Dec. 2006, O. Koukol (holotype PRM 915688, culture ex-type CBS 126959 = CCF 3843).
Additional materials examined: Germany, on litter needles of Pinus sylvestris (Pinaceae), 5 Feb. 2016, R.K. Schumacher (culture CBS 143494 = CPC 30463; ibid., CPC 30466). Netherlands, Kootwijk, needles of P. sylvestris, 8 Nov. 1982, G.S. de Hoog (culture CBS 675.82).
Notes: Pinaceicola cordae was first described from the Czech Republic and the Netherlands (as Fusicladium cordae; Koukol 2010), and subsequently collected in Germany (present study). Pinaceicola cordae is thus far only known from needles of Pinus sylvestris. Together with Pinaceicola pini, P. cordae clusters in Sympoventuriaceae, and basal to Sterila, Fuscohilum and Neocoleroa (Fig. 1).
Pinaceicola pini (Crous & de Hoog) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831556. Fig. 11.
Fig. 11.
Pinaceicola pini (culture ex-type CBS 463.82) asexual morph. A. Colony on OA. B. Hyphal coil. C–H. Sympodial conidiogenous loci and concatenated conidia arising from conidiogenous cells. Scale bars: B–H = 10 μm.
Basionym: Fusicladium pini Crous & de Hoog, Stud. Mycol. 58: 210. 2007.
Synomym: Fusicladium ramoconidii Crous & de Hoog, Stud. Mycol. 58: 211. 2007.
Description and illustration: Crous et al. (2007b).
Typus:Netherlands, Baarn, De Vuursche, on litter needle of Pinus sp. (Pinaceae), 12 Apr. 1982, G.S. de Hoog (holotype CBS H-19908, culture ex-type CBS 463.82).
Additional material examined: Netherlands, Baarn, De Vuursche, on needle of Pinus sylvestris (Pinaceae), 12 Apr. 1982, G.S. de Hoog (dried culture CBS H-1610, ex-type culture of Fusicladium ramoconidii CBS 462.82).
Notes: Fusicladium pini and F. ramoconidii were introduced as different species based on differences in their ITS sequences (13 bp), and the absence of ramoconidia in F. pini (Crous et al. 2007b). However, the multigene data [Fig.1; ITS sequences (identity: 99 %), LSU sequences (identity: 100 %), tef1 sequences (identity: 99 %), tub2 sequences (identity: 99 %), rpb2 sequences (identity: 99 %)] suggest them to be conspecific belonging in the newly erected genus, Pinaceicola.
Pseudosigmoidea K. Ando & N. Nakam., J. Gen. Appl. Microbiol., Tokyo 46: 55. 2000.
Type species: Pseudosigmoidea cranei K. Ando & N. Nakam.
Pseudosigmoidea alnicola Crous & R.K. Schumach., Fungal Syst. Evol. 3: 109. 2019.
Description and illustration: Crous et al. (2019a).
Typus: Germany, near Berlin, leaf litter of Alnus glutinosa (Betulaceae), 3 May 2017, R.K. Schumacher, HPC 2100 (holotype CBS H-23826, culture ex-type CBS 145034 = CPC 33776).
Note: The phylogenetic position of this species is shown and discussed by Crous et al. (2019a).
Pseudosigmoidea cranei K. Ando & N. Nakam., J. Gen. Appl. Microbiol., Tokyo 46: 55. 2000.
Typus: USA, Maryland, Frederick County, Appalachian Trail, Bear Spring, from fresh water, collection date and collector unknown (holotype TNS F-100793, culture ex-type ATCC 16660) (not seen).
Note: This species is not known from molecular data, thus its phylogenetic position is unknown.
Pseudosigmoidea excentrica (R.F. Castañeda et al.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831557.
Basionym: Scolecobasidium excentricum R.F. Castañeda et al., Nova Hedwigia 64: 473. 1997.
Typus: Cuba, Santiago de Las Vegas, Ciudad de la Habana, Pinar del Rio, isolated from dead leaves of unidentified Lauraceae, 6. Aug. 1994, coll. R.F. Castaneda Ruiz (holotype CBS H-7739, isotype CBS H-6052, culture ex-type CBS 469.95 = INIFAT C94/202 = MUCL 39227).
Notes: Scolecobasidium excentricum was introduced based on its “eccentrically inflated” conidia, which are quite clearly illustrated (figs 1–3 in Castañeda-Ruis et al. 1997). In this study, the ex-type of Scolecobasidium excentricum (INIFAT C94/202 = CBS 469.95) was sequenced, showing it to cluster in Pseudosigmoidea (Fig. 1). Morphologically, Scolecobasidium excentricum has straight to flexuous conidiophores, and polyblastic or sympodial conidiogenous cells, which agree with Venturiales (Castañeda-Ruis et al. 1997).
Pseudosigmoidea ibarakiensis Diene & Narisawa, Microbes and Environm. 28: 384. 2013.
Typus: Japan, Ibaraki, obtained from natural forest soil, 2008, unknown collector (holotype NIAES H-20615, culture ex-type I.4-2-1 = NBRC 107891) (not seen).
Note: This species is sister to P. excentrica (Fig. 1).
Scolecobasidium E.V. Abbott, Mycologia 19: 30. 1927.
Synonym: Ochroconis de Hoog & Arx, Kavaka 1: 57. 1974 [1973].
Type species: Scolecobasidium terreum E.V. Abbott.
Notes: Scolecobasidium was introduced based on S. terreum and S. constrictum, with S. terreum designated as the generic type (Abbott 1927). The slow-growing, olivaceous colonies of these two species agree well with Venturiales. Morphologically, the diagnostic characteristics of Scolecobasidium includes that conidia are produced on “sterigmata” left as tubular appendages on conidiophores, and are produced singly (never in chains).
Although Barron & Busch (1962) considered species of Scolecobasidium with darker, unbranched conidia to be congeneric with S. terreum, this opinion was not shared by von Arx, and therefore De Hoog & von Arx (1974) introduced a separate genus, Ochroconis, typified by O. constricta, for species with ellipsoidal conidia. Ochroconis proved to be a rather common genus of saprotrophic soil hyphomycetes, some of which occasionally grow on plant litter, humans or fish (Samerpitak et al. 2014). Gams (2015) regarded Ochroconis as synonym of Scolecobasidium, which was supported by Seifert et al. (2011). Although the ex-type strains of both S. terreum (CBS 203.27) and O. constricta (CBS 202.27) are now sterile, together with other species of Ochroconis and Scolecobasidium, they nest in the clade of Scolecobasidium (Fig.1). Based on these results as well as their morphology, we therefore resurrect the older generic name Scolecobasidium, and reduce Ochroconis to synonymy with it.
Scolecobasidium aquaticum (Samerp. et al.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831640.
Basionym: Ochroconis aquatica Samerp. et al., Mycoscience 58: 292. 2017.
Typus: Germany, Mecklenburg-Vorpommern, isolated from silicone seal in shower of fish-processing company, 28 Oct. 2014, K. Gloyna (holotype CBS H-22391, culture ex-type CBS 140316).
Note: This species is sister to S. anomalum (Fig. 1).
Scolecobasidium atlanticum (A.M. Wellman) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831641.
Basionym: Ochroconis atlantica A.M. Wellman, Canad. J. Bot. 53: 1631. 1975.
Typus: Atlantic Ocean, 44.30″ N, 26.00″ W, on tar, cultured on Difco Marine 2216 agar, Jun. 1973, A.M. Wellman (holotype IMI 183133).
Scolecobasidium bacilliforme (Samerp. et al.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831642.
Basionym: Ochroconis bacilliformis Samerp. et al., Mycopathologia 180: 4. 2015.
Typus: Germany, Mülheim, from biofilm on stainless steel in drinking water, 1998, E. Göttlich (holotype CBS H-22032, culture ex-type CBS 100442 = M 37/2).
Scolecobasidium capsici (Crous & Cheew.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831643.
Basionym: Ochroconis capsici Crous & Cheew., Persoonia 37: 333. 2016.
Typus: Thailand, Chiang Rai, N19°48′01″ E99°41′27″, on Capsicum annuum (Solanaceae), 2013, R. Cheewangkoon (holotype CBS H-22883, culture ex-type CPC 28782 = CBS 142096).
Scolecobasidium cordanae (Samerp. et al.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831644.
Basionym: Ochroconis cordanae Samerp. et al., Fungal Diversity 65: 105. 2013 [2014].
Typus: Colombia, Villavicencio, from dead leaf, Dec. 1979, W. Gams (culture ex-type CBS 475.80).
Note: This species represents one of three basal lineages in the Scolecobasidium clade (Fig. 1).
Scolecobasidium dracaenae (Crous) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831645.
Basionym: Ochroconis dracaenae Crous, Persoonia 36: 379. 2016.
Typus: USA, Texas, Austin, on leaf spots of Dracaena reflexa (Asparagaceae), Aug. 2013, P.W. Crous (holotype CBS H-22619, culture ex-type CPC 26115 = CBS 141323).
Note: This species is sister to S. pandanicola (Fig. 1).
Scolecobasidium globale (Samerp. et al.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831646.
Basionym: Ochroconis globalis Samerp. et al., Mycol. Progr. 14 (no. 6): 3. 2015.
Typus: Germany, Düsseldorf, from indoor sample, dwelling house, 2002 (holotype CBS H-21940, culture ex-type CBS 119644).
Note: This species is sister to S. tshawytschae (Fig. 1).
Scolecobasidium icarus (Samerp. et al.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831647.
Basionym: Ochroconis icarus Samerp. et al., J. Clin. Microbiol. 52: 4195. 2014.
Typus: Canada, Ontario, from forest soil, 1969, G.L. Barron (holotype CBS H-21643, cultures ex-type CBS 536.69 = MUCL 15054 = OAC 10212).
Note: This species is sister to S. ramosum (Fig. 1).
Scolecobasidium macrozamiae (Crous & R.G. Shivas) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831648.
Basionym: Ochroconis macrozamiae Crous & R.G. Shivas, Persoonia 32: 205. 2014.
Typus: Australia, Queensland, Brisbane, Slaughter Falls, on Macrozamia (Zamiaceae) leaf litter, 16 Jul. 2009, P.W. Crous & R.G. Shivas (holotype CBS H-21682, culture ex-type CPC 17262 = CBS 137971).
Note: This species is sister to S. gamsii (Fig. 1).
Scolecobasidium minimum (Fassat.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831649.
Basionym: Humicola minima Fassat., Česká Mykol. 21: 87. 1967.
Synonym: Ochroconis minima (Fassat.) Samerp. & de Hoog, Fungal Diversity 65: 110. 2013 [2014].
Typus: Nigeria, Samaru, Zaria, from rhizosphere of Gossypium arboretum (Malvaceae), M. Dransfield (holotype PRC 981, ex-type CBS 510.71 = ATCC 22631 = IMI 082933).
Note: This species is sister to S. ramosum / icarus (Fig. 1).
Scolecobasidium musicola (Crous) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB832011.
Basionym: Ochroconis musicola Crous, Persoonia 40: 387. 2018.
Typus: Malaysia, leaves of Musa sp. (Musaceae), 2010, P.W. Crous (holotype CBS H-23562, culture ex-type CBS 144441).
Note: This species is sister to S. sexuale / macrozamiae / gamsii (Fig. 1).
Scolecobasidium olivaceum (A. Giraldo et al.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831652.
Basionym: Ochroconis olivacea A. Giraldo et al., J. Clin. Microbiol. 52: 4195. 2014.
Typus: USA, Utah, from bronchoalveolar lavage fluid, 2010, D.A. Sutton (holotype CBS H-21779, cultures ex-type CBS 137170 = FMR 12509 = UTHSC 10-2009).
Note: This species is sister to S. verrucosum (Fig. 1).
Scolecobasidium pandanicola (Crous & M.J. Wingf.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831653.
Basionym: Ochroconis pandanicola Crous & M.J. Wingf., Persoonia 35: 277. 2015.
Typus: France, La Réunion, S21°21′30.7″ E55°44′32.3″, Route Forestiere Mare Longue, on leaves of Pandanus utilis (Pandanaceae), 6 Mar. 2014, P.W. Crous & M.J. Wingfield (holotype CBS H-22397, culture ex-type CPC 26317 = CBS 140660).
Note: This species is sister to S. dracaenae (Fig. 1).
Scolecobasidium phaeophorum (Samerp. et al.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831654.
Basionym: Ochroconis phaeophora Samerp. et al., Mycopathologia 180: 4. 2015.
Typus: Papua New Guinea, Madang, Balek, from leaf in coastal rain forest, 1995, A. Aptroot & A. van Iperen (holotype CBS H-22033, culture ex-type CBS 206.96 = 36599/No. A 165).
Note: This species represents one of three basal lineages in the Scolecobasidium clade (Fig. 1).
Scolecobasidium podocarpi (Crous) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831655.
Basionym: Ochroconis podocarpi Crous, Persoonia 39: 361. 2017.
Typus: Australia, New South Wales, Australian Botanic Garden, Mount Annan, on leaves of Podocarpus grayae (Podocarpaceae), 25 Nov. 2016, P.W. Crous (holotype CBS H-23267, culture ex-type CPC 32829 = CBS 143174).
Note: This species represents one of three basal lineages in the Scolecobasidium clade (Fig. 1).
Scolecobasidium ramosum (A. Giraldo et al.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831656.
Basionym: Ochroconis ramosa A. Giraldo et al., J. Clin. Microbiol. 52: 4197. 2014.
Typus: USA, California, from human nail, 2012, D.A. Sutton (holotype CBS H-21780, culture ex-type CBS 137173 = FMR 12514 = UTHSC 12-1082).
Note: This species is sister to S. icarus (Fig. 1).
Scolecobasidium robustum (Samerp. et al.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831657.
Basionym: Ochroconis robusta Samerp. et al., Mycopathologia 180: 5. 2015.
Typus: Spain, from leaf litter of Quercus ilex (Fagaceae), 1996, R.F. Castañeda (holotype CBS H-22031, culture ex-type CBS 112.97 = INIFAT C96/119).
Scolecobasidium sexuale (Samerp. et al.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831658.
Basionym: Ochroconis sexualis Samerp. et al., Fungal Diversity 65: 114. 2013 [2014].
Typus: South Africa, Durban, obtained from quality control swabs in a laboratory providing medical supplies, collection date and collector unknown (culture ex-type PPRI 12991).
Note: This species is sister to S. macrozamiae / gamsii (Fig. 1).
Scolecobasidium verrucosum (Zachariah et al.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831659.
Basionym: Septonema verrucosum Zachariah et al. [as “verrucosa”], Mycologia 73: 208. 1981.
Synonym: Ochroconis verrucosa (Zachariah et al.) Samerp. & de Hoog, Fungal Diversity 65: 117. 2013 [2014].
Typus: India, Kerala, from soil, Jun. 1981, S. Zachariah (holotype CUCC F164, ex-type CBS 383.81 = IMI 211655).
Note: This species is sister to S. olivaceum (Fig. 1).
Sterila Crous, M. Shen & Y. Zhang ter, gen. nov. MycoBank MB831516.
Etymology: The epithet refers to the fact that colonies are sterile in culture.
Type species: Sterilaeucalypti Crous, M. Shen & Y. Zhang ter
Sterilaeucalypti Crous, M. Shen & Y. Zhang ter, sp. nov. MycoBank MB831522.
Etymology: The epithet refers to Eucalyptus, the host from which the fungus was isolated.
Cultures sterile. Sterila eucalypti differs from its closest phylogenetic neighbours Fuscohilum rhodensis and F. siciliana (Fig. 1) by unique fixed alleles in four loci based on alignments of the separate loci deposited in TreeBASE (S24573): Sterila eucalypti (CBS 144019) vs. Fuscohilum rhodensis (CPC13156) by 26 bp in ITS (10 %), 67 bp in LSU (8 %), 71 bp in rpb2 (22%), 112 bp in tef1 (24 %); S. eucalypti (CBS 144019) vs. P. siciliana (CBS 105.85) by 23 bp in ITS (9 %), 61 bp in LSU (8 %), 174 bp in rpb2 (22 %), 108 bp in tef1 (23 %).
Culture characteristics: Colonies spreading, erumpent, with sparse aerial mycelium and regular margins on OA, olivaceous brown (surface), margins dark olivaceous; reverse fuscous-black; on MEA pale grey (surface), margins brownish red; reverse fuscous-black; on SNA olivaceous brown (surface), margins pale olivaceous; reverse olivaceous to dark olivaceous. Colonies reaching 18 mm diam after 2 wk on OA at 25 °C in the dark.
Typus: Portugal, on Eucalyptus sp. (Myrtaceae), 24 Jan. 2008, P.W. Crous (holotype CBS H-23601, culture ex-type CBS 144019 = CPC 14944, CPC 14942, CPC 14943).
Notes: Sterila eucalypti was collected from leaves of a Eucalyptus sp. Unfortunately, it does not sporulate in culture. Although cultured from single ascospores, no ascomata could be located on the leaves of the fungarium specimen. According to multigene phylogenetic analysis, it forms a separate fully supported clade distinguishing it from other genera of Sympoventuriaceae (Fig. 1).
Sympoventuria Crous & Seifert, Fungal Diversity 25: 31. 2007.
Description and illustration: Crous et al. (2007a).
Type species: Sympoventuria capensis Crous & Seifert
Notes: The genus Sympoventuria was first introduced by Crous et al. (2007a) based on S. capensis, which was saprophytic on Eucalyptus leaves collected in South Africa. Although the small-sized immersed ascomata agree with Venturia, the persistent pseudoparaphyses, saprophytic lifestyle, hyaline, 1-septate, symmetric ascospores and subcylindrical asci of Sympoventuria capensis differ from those of Venturia s. str. (Sivanesan, 1977, Crous et al., 2007a, Zhang et al., 2011).
Sympoventuria africana (Crous) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831585.
Basionym: Fusicladium africanum Crous, Stud. Mycol. 58: 205. 2007.
Description and illustration: Crous et al. (2007b).
Typus: South Africa, Western Cape Province, Malmesbury, on leaf litter of Eucalyptus sp. (Myrtaceae), Jan. 2006, P.W. Crous (holotype CBS H-19904, culture ex-type CBS 121639 = CPC 12828, CBS 121640 = CPC 12829).
Notes: Both S. africana and S. capensis were collected from Eucalyptus leaf litter in South Africa (Crous et al., 2007a, Crous et al., 2007b). Morphologically, the fusiform conidia of S. africana can be distinguished from the cylindrical conidia of S. capensis. Sympoventuria africana represents the most basal species in the Sympoventuria clade (Fig. 1).
Sympoventuria capensis Crous & Seifert, Fungal Diversity 25: 32. 2007.
Description and illustration: Crous et al. (2007a).
Typus: South Africa, Western Cape Province, Malmesbury, on leaf litter of Eucalyptus sp. (Myrtaceae), Jan. 2006, P.W. Crous (holotype CBS H-19757, culture ex-type CPC 12838 = CBS 120136, CPC 12839, CPC 12840).
Notes: See the notes for Sympoventuria africana. This species is sister to S. melaleucae in Fig. 1.
Sympoventuria melaleucae Crous, Persoonia 39: 413. 2017.
Description and illustration: Crous et al. (2017).
Typus: Australia, Victoria, Royal Botanic Gardens Victoria, Melbourne Gardens, on leaves of Melaleuca sp. (Proteaceae), 2 Dec. 2016, P.W. Crous (holotype CBS H-23298, culture ex-type CBS 143407 = CPC 32576).
Notes: Sympoventuria melaleucae was introduced for a fungus which is occurring on leaves of Melaleuca sp. in Australia (Crous et al. 2017). Morphologically, S. melaleuca is distinct from S. capensis in that it has smaller conidia [(8–)11–17(–25) × 2–3 μm vs. up to (40–)55–65 × 4–5 μm], with fewer septa (0–1-septate vs. (1–)3(–5)-septate) (Crous et al., 2007a, Crous et al., 2007b). DNA sequence data also place S. melaleucae within Sympoventuria, sister to S. capensis (Fig. 1).
Troposporella P. Karst., Hedwigia 31: 299. 1892.
Type species: Troposporella fumosa P. Karst.
Troposporellafumosa P. Karst., Hedwigia 31: 299. 1892.
Typus: Finland, Mustiala, on the old bark of Populus tremula (Salicaceae), 13 Nov. 1892, P.A. Karsten 4286 (H6052538).
Note: Troposporellafumosa formed a robust clade with T. monilipes (Fig. 1). Linder (1929: 335) examined type material of T. fumosa deposited at H and provided a description based on this material.
Troposporellamonilipes (Ellis & L.N. Johnson) C.K.M. Tsui & Berbee, Mycoscience 51: 147. 2010.
Basionym: Helicoma monilipes Ellis & L.N. Johnson, Proc. Acad. Nat. Sci. Philadelphia 46: 376. 1894.
Typus: USA, Michigan, Ann Arbor, on decayed wood of Quercus (Fagaceae), Oct. 1893 (L.N. Johnson No. 666) (not seen).
Note: This species is sister to T. fumosa (Fig. 1).
Veronaeopsis Arzanlou & Crous, Stud. Mycol. 58: 91. 2007.
Type species: Veronaeopsis simplex (Papendorf) Arzanlou & Crous
Notes: Veronaeopsis was separated from Veronaea based on its shorter conidiophores, geniculate rachis and prominent conidiogenous loci (Papendorf, 1969, Arzanlou et al., 2007). Phylogenetically, Veronaeopsis simplex nests in the Sympoventuriaceae, and is sister to other genera of Sympoventuriaceae (Fig. 1).
Veronaeopsis simplex (Papendorf) Arzanlou & Crous, Stud. Mycol. 58: 91. 2007.
Basionym: Veronaea simplex Papendorf, Trans. Brit. Mycol. Soc. 52: 486. 1969.
Typus: South Africa, Potchefstroom, leaf-litter and top soil of a mixed Acacia karroo (= Vachellia karroo) (Fabaceae) community, Apr. 1966, J.W. du Toit (holotype PREM 43728, culture ex-type CBS 588.66).
Notes: According to Arzanlou et al. (2007), Veronaeopsis simplex is saprobic on leaf litter of V. karroo in South Africa, and distinct from species of other genera by having a well-developed rachis with densely aggregated conidiogenous loci.
Verruconis Samerp. et al., Fungal Diversity 65: 117. 2013 [2014].
Type species: Verruconis gallopava (W.B. Cooke) Samerp. & de Hoog
Notes: The genus Verruconis was separated from Scolecobasidium based on its ecological and physiological traits and morphological differences (Samerpitak et al. 2014). Presently there are seven species included in Verruconis (see below). Morphologically, the light to dark brown, verrucose to coarsely ornamented conidia of Verruconis are readily distinguishable from Scolecobasidium (Samerpitak et al., 2014, Zhang et al., 2018). Some species of Verruconis are thermophilic, such as V. calidifluminalis and V. gallopava, both of which originate from a hot spring (Samerpitak et al. 2014). Verruconis verruculosa and V. panacis originate from the soil environment, with V. verruculosa from grassland soil, and V. panacis from roots of Panax notoginseng (Roy et al., 1962, Samerpitak et al., 2014, Zhang et al., 2018). Verruconis hainanensis and V. pseudotricladiata were isolated from submerged dicotyledonous leaves in a stream of Hainan island, China (Qiao et al. 2019). Based on the morphological and phylogenetic characteristics, Scolecobasidium terricola was assigned to Verruconis (as V. terricola). Species of Verruconis form a separate clade basal to Scolecobasidium (Fig. 1).
Verruconis calidifluminalis (Yarita et al.) Samerp. & de Hoog, Fungal Diversity 65: 117. 2013 [2014].
Basionym: Ochroconis calidifluminalis Yarita et al., Mycopathologia 170: 29. 2010.
Typus: Japan, Hakone, Kanagawa Prefecture, from hot spring river water, Mar. 2004, K. Nishimura (holotype IFM 54738, not seen).
Notes: Ochroconis calidifluminalis was described by Yarita et al. (2010) from a hot spring in Japan, and was subsequently assigned to Verruconis (Samerpitak et al. 2014). Verruconis calidifluminalis was isolated concomitantly with V. gallopava as thermophilic fungi from a hot spring (Yarita et al., 2010, Samerpitak et al., 2014). Although V. calidifluminalis and V. gallopava are comparable in their ecology, morphology as well as culture characteristics, they are distinguishable by their pathogenic potential to vertebrates (Yarita et al., 2010, Samerpitak et al., 2014). Verruconis gallopava is a neurotropic invader in birds and also occurs in humans (Samerpitak et al. 2014). Verruconis calidifluminalis, however, has low virulence in mice (Samerpitak et al. 2014). In addition, DNA sequence data can also readily distinguish these two species (Fig. 1).
Verruconis gallopava (W.B. Cooke) Samerp. & de Hoog, Fungal Diversity 65: 117. 2013 [2014].
Basionym: Diplorhinotrichum gallopavum W.B. Cooke, Sabouraudia 3: 242. 1964.
Synonyms: Dactylaria gallopava (W.B. Cooke) G.C. Bhatt & W.B. Kendr., Canad. J. Bot. 46: 1257. 1968.
Ochroconis gallopava (W.B. Cooke) de Hoog, Fung. Path. Hum. Anim.: 181. 1983.
Dactylaria constricta var. gallopava (W.B. Cooke) Salkin & D.M. Dixon, Mycotaxon 29: 379. 1987.
Scolecobasidium gallopavum (W.B. Cooke) G.Y. Sun & Lu Hao, Mycol. Progr. 12(3): 492. 2012.
Typus: Turkey, isolated from the brain tissue of sick young Bos taurus, collection date and collector unknown (holotype CDC 45-492-62, culture ex-type CBS 437.64 = ATCC 16027 = CDC 45-492-62 = MUCL 6683 = IFM 52605) (not seen).
Notes: Verruconis gallopava is a widely distributed thermophile which was encountered in diverse types of hot environments, such as self-heated coal waste piles (Tansey & Brock 1973), hot springs (Tansey & Brock 1973, Weitzman et al., 1983, Yarita et al., 2007), warm effluents of a nuclear reaction station (Rippon et al. 1980), and broiler-house litter (Waldrip et al., 1974, Randall and Owen, 1981). Verruconis gallopava is a neurotropic invader in birds, chicken, humans, trumpeters and cats (Evans, 1971a, Evans, 1971b, Weitzman et al., 1983, Karesh et al., 1987, Horré and de Hoog, 1999, Redman et al., 1999, Yarita et al., 2007, Samerpitak et al., 2014). For differences from V. calidifluminalis see comments above.
Verruconis hainanensis Z.F. Yu & M. Qiao, MycoKeys 48: 47. 2019.
Typus: China, Hainan Province, Qixianling, 18°68′N, 109°69′E, 902 m alt., from leaves of an unidentified dicotyledonous plant submerged in a stream, 16 Jun. 2016, Z.F. Yu (holotype YMFT 1.04165; culture ex-type YMF 1.04165; isotype CGMCC 3.18974).
Verruconis panacis T. Zhang & Y. Zhang, Int. J. Syst. Evol. Microbiol. 68: 2502. 2018.
Typus: China, Yunnan Province, Wen-shan district, from the root of a 3-yr-old Panax notoginseng (Araliaceae), Oct. 2015, T. Zhang (holotype SYPFH 8337, culture ex-type CBS 142802 = CGMCC 3.18302 = SYPF 8337).
Notes: Verruconis panacis was introduced by Zhang et al. (2018), having been collected from Panax notoginseng roots in China. Morphologically, Verruconis panacis is distinguishable from other Verruconis species by its four-celled conidia (Zhang et al. 2018). This species is sister to V. verruculosa / terricola (Fig. 1).
Verruconis pseudotricladiata Z.F. Yu & M. Qiao, MycoKeys 48: 48. 2019.
Typus: China, Hainan Province, Diaoluo Mountain, 18°41′N, 109°41′E, 254 m alt., from leaves of an unidentified broad-leaf species submerged in a stream, 16 Jun. 2016, Z.F. Yu (holotype YMFT 1.04915; culture ex-type YMF 1.04915; isotype CGMCC 3.18939).
Verruconis terricola (J. Ren et al.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831671.
Basionym: Scolecobasidium terricola J. Ren et al., Mycoscience 54: 421. 2013.
Typus: China, Hainan Province, Wuzhi Mountain, isolated from soil, Dec. 2009, Y.-L. Zhang (holotype HGUPd3009, culture ex-type CBS 131795 = HGUP3009).
Notes: Scolecobasidium terricola was originally isolated from soil in a tropical region of China (Ren et al. 2013). Morphologically, the sympodial, holoblastic conidiogenous cells, solitary, ellipsoidal, 1-septate conidia with a sterigma left as a tubular appendage on the conidiogenous cell and the conidial base of conidia point to Scolecobasidium / Verruconis (Ren et al. 2013). Phylogenetically, Scolecobasidium terricola nests in Verruconis (Fig. 1), to which is assigned.
Verruconis verruculosa (R.Y. Roy et al.) Samerp. & de Hoog, Fungal Diversity 65: 120. 2013 [2014].
Basionym: Scolecobasidium verruculosum R.Y. Roy et al., Lloydia 25: 164. 1962.
Typus: India, Varanasi, grassland soil, collection date and collector unknown (holotype in Indian Type Culture Collection, IARI New Delhi) (not seen).
Notes: Verruconis verruculosa is a soil-borne fungus, which was saprophytic in grassland soil in India (Roy et al., 1962, Samerpitak et al., 2014). Morphologically, Verruconis verruculosa is distinguishable from other species of Verruconis by its oblong conidia with rounded ends and prominent spines (Samerpitak et al. 2014). Based on the multigene phylogenetic analysis, Verruconis verruculosa formed a single sister lineage distinguishing it from other species of Verruconis (Fig. 1).
Venturiaceae E. Müll. & Arx ex M.E. Barr, Mycologia 71: 947. 1979.
Habitat saprophytic, endophytic or parasitic on leaves or stems of dicotyledons, rarely on monocotyledons. Sexual morph: Ascomata immersed, erumpent to superficial, scattered or gregarious, sometimes composed of a well-developed subiculum, globose, subglobose, with or without setae around papilla, ostiolate. Hamathecium of narrowly cellular pseudoparaphyses, mostly evanescent. Asci 8-spored, bitunicate, fissitunicate, usually obclavate to obpyriform, rarely cylindrical, mostly apedicellate. Ascospores yellowish, light greenish olivaceous to brown, or hyaline, 1-septate, symmetrical, asymmetrical or apiosporous. Asexual morph: Mycelium consisting of pale to medium brown, smooth to finely verruculose, branched hyphae. Conidiophores singly or in clusters, sometime even in sporodochia, simple or branched. Conidiogenous cells integrated, terminal or sometimes intercalary, proliferating sympodially or percurrently, sometimes with conspicuous annellations. Conidia aseptate or euseptate, pigmented, solitary or in chains.
Type genus: Venturia Sacc.
Notes: Venturiaceae was first invalidly introduced by Müller & von Arx (1950), and von Arx (1952) provided a systematic key to genera of Venturiaceae. The familial type of Venturiaceae is Venturia Sacc. Barr (1979) validated the family, included 12 genera within Venturiaceae and provided a first detailed description with important diagnostic characters. Furthermore, Barr (1989) provided a key to North American genera and species. Lumbsch & Huhndorf (2010) assigned 27 genera to Venturiaceae. Venturiaceae sensu Zhang et al. (2011) comprises eight genera, viz. Acantharia, Apiosporina (including Dibotryon), Caproventuria, Coleroa, Pseudoparodiella, Metacoleroa, Tyrannosorus and Venturia with another seven genera ambiguously included without molecular data. Hyde et al. (2013) assigned 15 genera (including an ambiguous genus Spilodochium) to Venturiaceae. Based on morphological, ecological and molecular data, Caproventuria is treated as a synonym of Tyrannosorus in this study.
Acantharia Theiss. & Syd., Ann. Mycol. 16: 15. 1918.
Synonym: Zeuctomorpha Sivan. et al., In: Sivanesan, Bitunicate Ascomycetes and their Anamorphs: 572. 1984.
Type species: Acantharia echinata (Ellis & Everh.) Theiss. & Syd.
Note: Based on the morphological characteristics of the type species, e.g. its foliicolous habitat, superficial, setose ascomata, evanescent pseudoparaphyses, obclavate asci, and 1-septate, brown, constricted ascospores, Acantharia was assigned to Venturiaceae (Zhang et al. 2011). Molecular proof, however, is still needed to confirm its placement in Venturiaceae.
Apiosporina Höhn., Sitzungsber. Kaiserl. Akad. Wiss., Math.-Naturwiss. Cl., Abt. 1. 119: 439. 1910.
Synonym: Dibotryon Theiss. & Syd., Ann. Mycol. 13: 663. 1915.
Type species: Apiosporina collinsii (Schwein.) Höhn.
Notes: Based on the fusicladium-like asexual morph, morphological characteristics of the sexual morph, as well as the molecular phylogeny of A. collinsii and A. morbosa, Apiosporina was assigned to Venturiaceae (Zhang et al. 2011). Morphologically, the submedian ascospore septation of Apiosporina was the most striking characteristic of Apiosporina. The phylogenetic significance of ascospore septation (and position) still needs to be clarified.
Apiosporina collinsii (Schwein.) Höhn., Sitzungsber. Kaiserl. Akad. Wiss., Math.-Naturwiss. Cl., Abt. 1. 119: 439. 1910.
Basionym: Sphaeria collinsii Schwein., Trans. Amer. Philos. Soc., n.s. 4: 211. 1832 [1834].
Material examined: Canada, Ontario, Thunder Bay, Lakehead, Univ., Campus, on Amelanchier alnifolia (Rosaceae), 23 May 2005, L.J. Hutchinson (culture CBS 118973 = CPC 12229–12231).
Notes: Phylogenetically, Apiosporina collinsii (represented by CBS 118973), the generic type of Apiosporina, clustered apart from Apiosporina morbosa, and basal to most other members of Venturiaceae (Fig 1, Fig 2).
Apiosporina morbosa (Schwein.) Arx, Acta Bot. Neerl. 3: 86. 1954.
Basionym: Sphaeria morbosa Schwein., Schriften Naturf. Ges. Leipzig. 1: 40 [14 of repr.]. 1822.
Notes: Apiosporina morbosa is represented by strain “dimosp” from Prunus in USA in Fig. 1. Unfortunately, no ITS sequence is available for comparison to other A. morbosa ITS sequences on GenBank; the majority of which appears to be associated with Cladosporium (GenBank AF493982–AF493982, AY166451 and AY165751) or distantly with Periconia (GenBank MK575461). The generic status of Apiosporina morbosa cannot be confirmed until authentic isolates become available for further study.
Coleroa Rabenh., Klotzschii Herb. Viv. Mycol., Ed. 1, Cent. 15, no. 1456. 1850.
Description and illustration: Zhang et al. (2011).
Type species: Coleroa chaetomium (Kunze ex Fr.) Rabenh.
Notes: Based on the scattered, setose ascomata, deliquescing pseudoparaphyses, fusoid to obclavate asci and the 1-septate, constricted ascospores of C. chaetomium, Coleroa was assigned to Venturiaceae (Zhang et al. 2011). Two isolates, C. circinans and C. robertiani, nested in Venturiaceae in this study (Fig 1, Fig 2). Coleroa spp. 1–3 were all collected in Europe, and previously named as Venturia spp. Phylogenetically, they all nest in a clade with C. circinans and C. robertiani (Fig 1, Fig 2). They were thus treated as unnamed taxa within Coleroa. Both C. circinans and C. robertiani, however, were tentatively used to represent Coleroa. The phylogeny of Coleroa needs to be resolved once sequence data become available for the generic type.
Coleroa circinans (Fr.) G. Winter, Rabenh. Krypt.-Fl., Ed. 2, 1(2): 200. 1885.
Basionym: Perisporium circinans Fr., Syst. Mycol. 3: 252. 1829.
Material examined: France, Geranium rotundifolium (Geraniaceae), 26 Jun. 1961, C. Bachmann (ETH 2760, culture CBS 457.64).
Note: The species is sister to C. robertiani (Fig 1, Fig 2).
Coleroa robertiani (Fr.) E. Müll., Beitr. Kryptogamenfl. Schweiz. 11(2): 416. 1962.
Basionym: Dothidea robertiani Fr., Syst. mycol. 2(2): 564. 1823.
Typus: Switzerland, Oetliberg, Zürich-Witikon, on Geranium robertianum (Geraniaceae), 28 Sep. 1960, C. Bachmann (lectotype ETH 2757 designated here, MBT391368, epitype specimen designated here CBS 458.64, MBT391369, preserved as metabolically inactive culture, culture ex-epitype CBS 458.64).
Note: The species is sister to C. circinans (Fig 1, Fig 2).
Cylindrosympodioides Crous & M.J. Wingf., Persoonia 36: 336. 2016.
Description and illustration: Crous et al. (2016).
Type species: Cylindrosympodioides brabeji Crous & M.J. Wingf.
Notes: Cylindrosympodioides was first introduced based on C. brabeji, which shares a similar morphology with species of Cylindrosympodium in having solitary, septate, cylindrical to subacicular, hyaline conidia with truncate bases, somewhat darkened hila, and brown conidiogenous structures with sympodial proliferation (Crous et al. 2016). Cylindrosympodioides differs from Cylindrosympodium in that it has acicular conidia with slightly thickened hila, and a fusicladium-like synasexual morph, which has narrowly fusiform, 1-septate conidia and conidiophores reduced to conidiogenous cells. Cylindrosympodioides brabeji forms a distinct sister lineage basal in the Venturiaceae.
Cylindrosympodioides brabeji Crous & M.J. Wingf., Persoonia 36: 335. 2016.
Description and illustration: Crous et al. (2016).
Typus: South Africa, Western Cape Province, Franschhoek, on leaves of Brabejum stellatifolium (Proteaceae), 17 Jan. 2015, P.W. Crous & M.J. Wingfield (holotype CBS H-22594, culture ex-type CBS 141285 = CPC 25934).
Notes: Cylindrosympodioides is a monotypic genus represented by C. brabeji, which is an endophyte (presumed saprobe) on leaves of Brabejum stellatifolium (Crous et al. 2016). Phylogenetically (Fig. 1), Cylindrosympodioides brabeji is basal in Venturiaceae, sibling to Sympoventuriaceae and Cylindrosympodiaceae. The genus can be distinguished from Cylindrosympodium acicular conidia with slightly thickened hila, and a fusicladium-like synasexual morph, as well as conidiophores that are reduced to conidiogenous cells (Crous et al. 2016).
Fagicola Crous, M. Shen & Y. Zhang ter, gen. nov. MycoBank MB831517.
Etymology: Named after the host genus on which it occurs, Fagus; “-icola” means dweller, inhabiter.
Mycelium consisting of pale to medium brown, smooth to finely verruculose, branched hyphae. Conidiophores integrated, terminal on hyphae, aseptate or septate, mostly reduced to conidiogenous cells, also lateral, visible as small, protruding, denticle-like loci. Conidiogenous cells subcylindrical, pale to medium brown, smooth to finely verruculose, tapering to several apical indistinct loci. Ramoconidia present. Conidia pale brown, smooth, guttulate, subcylindrical to narrowly ellipsoid, occurring in simple or branched chains, aseptate or septate, tapering towards subtruncate ends; hila mostly inconspicuous, i.e., rarely thickened or darkened-refractive (adapted from Crous et al. 2007b).
Type species: Fagicola fagi (Crous & de Hoog) Crous, M. Shen & Y. Zhang ter
Fagicola fagi (Crous & de Hoog) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831586. Fig. 12.
Fig. 12.
Fagicola fagi (culture ex-type CBS 621.84) asexual morph. A. Colony on OA. B–E. Conidia in simple or branched chains arising from conidiogenous cells. F, G. Brown, aseptate or 1-septate conidia. Scale bars: B–G = 10 μm.
Basionym: Fusicladium fagi Crous & de Hoog, Stud. Mycol. 58: 209. 2007.
Description and illustration: Crous et al. (2007b).
Typus: Netherlands, Utrecht Province, Baarn, Maarschalksbosch, on decaying leaves of Fagus sylvatica (Fagaceae), 1 Oct. 1984, G.S. de Hoog (holotype CBS H-10366, culture ex-type CBS 621.84 = ATCC 200937).
Notes: Fagicola is proposed based on Fagicola fagi (as Fusicladium fagi), which is saprophytic on leaves of Fagus sylvatica collected in the Netherlands. A multigene phylogenetic analysis indicated that Fagicola fagi is sibling to other genera of Venturiacaeae (Fig 1, Fig 2).
Fraxinicola Crous, M. Shen & Y. Zhang ter, gen. nov. MycoBank MB831518.
Etymology: Named after the host genus on which it mostly occurs, Fraxinus. “-icola” means dweller, inhabiter.
In vivo: Ascomata scattered over the entire leaf surface, immersed, globose to subglobose, pseudoparaphysate, ostiolate, papillate, with or without setae. Peridium thin, composed of pigmented cells of textura angularis. Asci bitunicate, oblong to obclavate, with a short pedicel. Ascospores uniseriate, partially overlapping to biseriate, especially at the base, ellipsoidal, with broadly rounded ends, olivaceous pale brown, 1-septate, slightly constricted at the septum, the upper cells often shorter and wider than the lower ones, smooth-walled. Conidiophores fusicladium-like, arising in clusters (sporodochia) from erumpent subcuticular to intraepidermal, few-celled stromata, or from terminal or lateral hyphae in culture, erect, unbranched, geniculate, septate, dark brown, smooth, walls thickened. Sporodochia interconnected by subcuticular to intraepidermal mycelium of melanised, partly swollen short cells and intercellular chlamydospores. Conidiogenous cells terminal, geniculate, proliferation sympodial, with a few to numerous truncate loci, somewhat refractive or darkened. Conidia solitary, smooth, lanceolate but apical tip rounded, 0–2-septate, pale medium brown, with a truncate base which is often somewhat thickened (adapted from Aderhold, 1897, Crous et al., 2011, Ibrahim et al., 2016).
Type species: Fraxinicola fraxini (Aderh.) Crous, M. Shen & Y. Zhang ter
Fraxinicola europaea Crous, M. Shen & Y. Zhang ter, sp. nov. MycoBank MB831523.
Etymology: Named after the continent where it was collected. Europe.
Cultures sterile. Fraxinicola europaea differs from its closest phylogenetic neighbours, F. fraxini, F. italica and F. orni (Fig. 1) by unique fixed alleles in five loci based on alignments of the separate loci deposited in TreeBASE (S24573): Fraxinicola europaea (CBS 472.61) vs. F. fraxini (CBS 140930) by 34 bp in ITS (7 %), 7 bp in LSU (1 %), 95 bp in rpb2 (12 %), 59 bp in tef1 (17 %), 80 bp in tub2 (21 %); F. europaea (CBS 472.61) vs. F. italica (CBS 140918) by 34 bp in ITS (7 %), 8 bp in LSU (1 %), 94 bp in rpb2 (12 %); 60 bp in tef1 (18 %), 86 bp in tub2 (22 %); F. europaea (CBS 472.61) vs. F. orni (CBS 140920) by 35 bp in ITS (7 %), 9 bp in LSU (1 %), 98 bp in rpb2 (13 %), 62 bp in tef1 (18 %), 86 bp in tub2 (22 %).
Culture characteristics: Colonies spreading, erumpent, with aerial mycelium and regular and smooth margins on OA, grey to olivaceous brown (surface); reverse fuscous-black; on MEA grey to dark brown (surface); reverse fuscous-black; on SNA olivaceous (surface); reverse dark olivaceous. Colonies reaching 5 mm diam after 2 wk on OA at 25 °C in the dark.
Typus: Switzerland, Kt. Tessin, Gola di Lago, on Betula pubescens (Betulaceae), 8 Apr. 1959, E. Müller (holotype CBS-H 24308, culture ex-type CBS 472.61 = ETH 2839).
Additional materials examined: France, Hautes Alpes, Aiguilles, on dead leaf of Populus tremula (Salicaceae), 28 Jun. 1958, E. Müller (ETH 2831, culture CBS 477.61); Hautes Alpes, Monetier, on P. tremula, Jun. 1981, M. Morelet (culture CBS 689.85); Alpes Maritimes, Tende, on Epilobium montanum (Onagraceae), 24 Aug. 1953, E. Müller (culture CBS 377.53).
Notes: Fraxinicola europaea does not sporulate in culture, and thus lacks a morphological description. According to the multigene phylogenetic analyses, it forms a separate lineage distinguishing it from other species of Fraxinicola (Fig 1, Fig 2).
Fraxinicola fraxini (Aderh.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831587. Fig. 13.
Fig. 13.
Fraxinicola fraxini (culture CBS 140930) asexual morph. A. Colony on OA. B–F. Sympodial conidiogenous cells producing conidia. G–J. Brown, 1–3-septate, tapering conidia. Scale bars: B–J = 10 μm.
Basionym: Venturia fraxini Aderh., Hedwigia 36: 83. 1897.
Synonyms: Fusicladium fraxini Aderh., Hedwigia 36: 74, 83. 1897.
Fusicladium proteae Crous, Persoonia 27: 34. 2011.
See Schubert et al. (2003) for additional synonyms.
Typus: Parasitic on leaves of Fraxinus excelsior (Oleaceae) (lectotype designated here Aderhold 1897, plate IV, fig. 6, MBT391370). Switzerland, Spiez, on leaf of F. excelsior as endophyte, 31 Aug. 2013, M. Schlegel (epitype designated here, specimen CBS 140930, MBT391371, as metabolically inactive culture, ex-epitype culture CBS 140930).
Additional materials examined: Italy, Premia, on leaf of F. ornus (Oleaceae) as endophyte, 31 Aug. 2013, M. Schlegel (culture CBS 140929 = VE 2). South Africa, Western Cape Province, Hermanus, Fernkloof Nature Reserve, on leaves of Protea sp. (Proteaceae), 5 May 2010, P.W Crous (ex-type culture of F. proteae CBS 130599 = CPC 18282). Switzerland, Monte Caslano, on leaf litter of F. excelsior (Oleaceae), 5 Sep. 2013, M. Ibrahim (culture CBS 140935 = VE 12); Kt. Wallis, Brig, on F. excelsior, 10 Jul. 1953, E. Müller (culture CBS 374.55).
Notes: Venturia fraxini, the basionym of Fraxinicola fraxini, was reported as an endophyte of Fraxinus excelsior (Aderhold, 1897, Ibrahim et al., 2016). Morphologically, Fraxinicola fraxini (conidia fusoid to obclavate, 12–28 × 4–6(–7) μm, (0–)1(–3)-septate), is comparable with Fusicladium proteae (conidia obpyriform, unequally 1-septate, (13–)17–22(–30) × 4(–5) μm, Schubert et al., 2003, Crous et al., 2011). Phylogenetically, the type isolate of Fusicladium proteae (CBS 130599) forms a conspecific clade together with isolates identified as Fraxinicola fraxini (Fig. 2). Thus, we assigned Fusicladium proteae to synonymy with Fraxinicola fraxini. Fusicladium proteae was reported as a causal agent on leaf spots on Protea sp. in South Africa (Crous et al. 2011). Fraxinicola fraxini is sister to F. orni / italica in Fig 1, Fig 2.
Fraxinicola italica Crous, M. Shen & Y. Zhang ter, sp. nov. MycoBank MB831524.
Etymology: Named after Italy, where this species was collected.
Cultures sterile. Fraxinicola italica (CBS 140918) differs from its closest phylogenetic neighbour F. orni (CBS 140920) (Fig. 1) by unique fixed alleles in five loci based on alignments of the separate loci deposited in TreeBASE (S24573), by 11 bp in ITS (2 %), 3 bp in LSU (1 %), 6 bp in rpb2 (1 %), 10 bp in tef1 (2 %), 9 bp in tub2 (2 %).
Culture characteristics: Colonies spreading, erumpent, with moderate aerial mycelium and regular, smooth margins on OA, dark brown (surface); reverse fuscous-black; on MEA dark brown (surface); reverse fuscous-black; on SNA dark brown (surface); reverse fuscous-black. Colonies reaching 12 mm diam after 2 wk on OA at 25 °C in the dark.
Typus: Italy, Lago di Ledro, on leaf of Fraxinus ornus (Oleaceae) as endophyte, 5 Sep. 2013, M. Ibrahim (holotype specimen and culture ex-type CBS 140918 preserved as metabolically inactive culture).
Notes: Fraxinicola italica does not sporulate in culture. The species is sister to F. orni (Fig 1, Fig 2).
Fraxinicola orni (M. Ibrahim et al.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831588. Fig. 14.
Fig. 14.
Fraxinicola orni (culture CBS 140920) asexual morph. A, B. Sporodochia produced on OA. C–E. Sympodial conidiogenous cells producing conidia. F, G. Medium brown, 1-septate and asymmetrical conidia. Scale bars: A, C = 20 μm; B, D–G = 10 μm.
Basionym: Venturia orni M. Ibrahim et al., Mycol. Progr. 15 (29): 6. 2016.
Description and illustration: Ibrahim et al. (2016).
Typus: Switzerland, on leaf litter of Fraxinus ornus (Oleaceae), 4 May 2015, M. Schlegel (holotype ZT Myc 55333, culture ex-type CBS 140924 = VO 10).
Additional materials examined: Italy, Lago di Ledro, on leaf of Fraxinus ornus (Oleaceae) as endophyte, 5 Nov. 2013, M. Ibrahim (ZT Myc 55330, cultures CBS 140919 = VO 4; ZT Myc 55331, CBS 140920 = VO 5; CBS 140921 = VO 6). Switzerland, on leaf of F. ornus as endophyte, 13 Nov. 2013, M. Ibrahim (culture CBS 140922 = VO 8).
Notes: Fraxinicola orni is based on Venturia orni, which was described as saprobic on Fraxinus ornus (Ibrahim et al. 2016). Morphologically, Venturia orni can be distinguished from V. fraxini by the absence of setae (Aderhold, 1897, Ibrahim et al., 2016). The multigene phylogenetic analyses indicated that Fraxinicola orni clustered in Fraxinicola and is sister to other species, viz., F. italica, F. fraxini, and F. europaea (Fig 1, Fig 2).
Gibbera Fr., Syst. Orb. Veg. 1: 110. 1825.
Type species: Gibbera vaccinii (Sowerby) Fr.
Notes: Due to the lack of molecular data for the generic type species, the phylogenetic status of Gibbera remains unresolved. Currently, molecular data is available for G. conferta (Fig 1, Fig 2) and G. rosae (LSU GenBank JQ036234). However, a blast search using the G. rosae sequence shows it to be allied to Cadophora (data not shown).
Gibbera kalmiae (Peck) M.E. Barr, Canad. J. Bot. 39: 315. 1961. Fig. 15.
Fig. 15.
Gibbera kalmiae (holotype NYSf1621) sexual morph. A. Ascomata scattered on the host surface. B, D, E. Clavate, asci. C. Released, pale brown, 1-septate ascospores. F. Evanesent, cellular pseudoparaphyses. G. Setae. Scale bars: A = 200 μm; B–G = 10 μm.
Basionym: Venturia kalmiae Peck, Rep. (Annual) New York State Mus. Nat. Hist. 28: 82. 1876.
Ascomata epiphyllous, 43–71 μm diam, scattered or gregarious, initially immersed, becoming erumpent, globose to subglobose, wall black, with a conspicuously papillate ostiole, surrounded by setae. Setae dark brown, 28–65 × 5–7 μm, setae wall 1–2 μm thick, base swollen, up to 14 μm diam. Peridium 1-layered, composed of 1–2 rows of pigmented cells of textura angularis, cells 4–6 × 6–12 μm, cell wall 0.5–1 μm thick. Pseudoparaphyses rare, 1.5–3 μm wide, hyaline, septate, persistent. Asci 36–50 × 6–8 μm (av. 41.8 × 7.5 μm, n = 20), numerous, 8-spored, bitunicate, fissitunicate, broadly cylindrical to somewhat obclavate, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 9–12.5 × 2.5–4 μm (av. 10.8 × 3.2 μm, n = 20), narrowly fusiform, hyaline when young, becoming pale brown when mature, overlapping to biseriate, 1-septate, the upper cells wider and shorter than the lower ones (length ratio: 5:7–10:11), smooth-walled. Asexual morph unknown.
Typus: USA, New York, Oswego City, on the leaves of Kalmia glauca (Ericaceae), Jul. 1874, C.H. Peck (holotype NYSf1621).
Notes: The scattered ascomata, numerous asci, hyaline to pale brown ascospores as well as the persistent pseudoparaphyses of Venturia kalmiae point to Leptosphaeriaceae. Barr (1961) reported Venturia kalmiae as having a thin hypostroma and assigned it to Gibbera. The generic type of Gibbera, G. vaccinii, lacks molecular data, and thus the taxonomic status remains unresolved. No sequence data is currently available for G. kalmiae.
Helicoon Morgan (as “Helicoön”), J. Cincinnati Soc. Nat. Hist. 15: 49. 1892.
Type species: Helicoon sessile Morgan
Notes: There are currently two accessions listed under the name H. sessile on GenBank (accessions ITS: U72605 and SSU-ITS-LSU: KY659207). The former accession number is allied to Sarocladium whereas the latter accession number is allied to Orbilia. The phylogenetic status of this genus remains to be resolved.
Helicoon myosuroides Voglmayr, Mycol. Res. 101: 337. 1997.
Typus: Austria, Upper Austria, Hausruckviertel, distr. Vöcklabruck, comm. Tiefgraben, wooded raised bog ‘Wiehlmoos’ at Neuhäusl at the NW side of the Mondseeberg, 790 m s. m., 23 Oct. 1993, coll. H. Voglmayr (holotype WU, culture ex-type CBS 743.96).
Notes: Morphologically, Helicoon was introduced based on solitary, non-proliferating, barrel-shaped conidia borne on distinct conidiophores (Morgan, 1892, Goos et al., 1986, Goh and Hyde, 1996), and was regarded as synonym of Orbilia. Helicoon myosuroides was described from bark and leaves of Betula spp., but also from leaves of Fagus sylvatica in Austria, which is characterised by its percurrent, septate conidiophores and dark fuscous to blackish brown colonies (Goh & Hyde 1996), which point to Venturiales. Phylogenetically, H. myosuroides nests in the Venturiaceae, and is sibling to other genera, such as Apiosporina, Tyrannosorus, Gibbera, Metacoleroa, Protoventuria, Fraxinicola, Coleroa and Venturia (Fig 1, Fig 2).
Metacoleroa Petr., Ann. Mycol. 25: 332. 1927.
Type species: Metacoleroa dickiei (Berk. & Broome) Petr.
Metacoleroa dickiei (Berk. & Broome) Petr. (as “dieckiei”), Ann. Mycol. 25: 332. 1927.
Basionym: Sphaeria dickiei Berk. & Broome, Ann. Mag. Nat. Hist., ser. 2(9): 317. 1852.
Notes: Metacoleroa is a monotypic genus based on M. dickiei. Metacoleroa was assigned to Venturiaceae based on superficial ascomata and ascospores with a median or submedian septum (Zhang et al. 2011). The identification of isolate “medipc” from Linnaea borealis in Oregon, USA (Winton et al. 2007) used in our phylogenetic analyses (Fig 1, Fig 2) remains unconfirmed.
Pseudoparodiella F. Stevens, Illinois Biol. Monogr. 11(2): 166. 1927.
Type species: Pseudoparodiella vernoniae F. Stevens.
Pseudoparodiella vernoniae F. Stevens, Illinois Biol. Monogr. 11(2): 166. 1927.
Synonym: Spilodochium vernoniae Syd., Ann. Mycol. 25(1/2): 158. 1927.
Typus: Costa Rica, Peralta, on leaves of Vernonia canescens (Asteraceae), 12 Jul. 1923, F.L. Stevens 352 (holotype K(M) 154549).
Notes: Pseudoparodiella is a monotypic genus, based on P. vernoniae (Stevens 1927). Pseudoparodiella was assigned to Venturiaceae due to its small-sized ascomata produced on leaves of dicotyledons, rare pseudoparaphyses, obclavate asci, and 1-septate, olivaceous brown ascospores (Zhang et al. 2011). Spilodochium vernoniae is the asexual morph of P. vernoniae (Sivanesan 1986). No molecular data is available for P. vernoniae, and its phylogenetic status remains undetermined.
Tyrannosorus Unter. & Malloch, Mycol. Res. 99: 910. 1995.
Synonyms: Caproventuria U. Braun, Monogr. Cercosporella, Ramularia Allied Genera (Phytopath. Hyphom.) 2: 396. 1998.
Pseudocladosporium U. Braun, Monogr. Cercosporella, Ramularia Allied Genera (Phytopath. Hyphom.) 2: 392. 1998.
Type species: Tyrannosorus pinicola (Petrini & P.J. Fisher) Unter. & Malloch
Notes: Tyrannosorus was introduced based on its saprophytic lifestyle, immersed to erumpent ascomata, lacking a subcuticular stroma, which agrees with the diagnostic characteristics of Caproventuria (Untereiner and Straus, 1995, Zhang et al., 2011). Phylogenetically, Caproventuria nests in the well-supported Tyrannosorus clade (data not shown). The genus Tyrannosorus is older than Caproventuria, and has priority.
Tyrannosorus hanlinianus (U. Braun & Feiler) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831589.
Basionym: Capronia hanliniana U. Braun & Feiler, Microbiol. Res. 150: 90. 1995.
Synonyms: Venturia hanliniana (U. Braun & Feiler) Unter., Mycologia 89: 129. 1997.
Caproventuria hanliniana (U. Braun & Feiler) U. Braun, Monogr. Cercosporella, Ramularia Allied Genera (Phytopath. Hyphom.) 2: 396. 1998.
Cladophialophora brevicatenata U. Braun & Feiler, Microbiol. Res. 150: 84. 1995.
Pseudocladosporiumbrevicatenatum (U. Braun & Feiler) U. Braun, Monogr. Cercosporella, Ramularia Allied Genera (Phytopath. Hyphom.) 2: 393. 1998.
Fusicladium brevicatenatum (U. Braun & Feiler) Crous et al., Stud. Mycol. 58: 212. 2007.
Typus: Germany, Mecklenburg, Bornhof, 1994, U. Feiler (holotype pseudothecia formed in pure culture (strain 7623), dried culture on SNA (HAL 1579 F) (not seen).
Notes: The congeneric status of Caproventuria hanliniana and C. hystrioides (current name Tyrannosorus hystrioides) could be confirmed based on their similarity in morphology and DNA sequence data (data not shown).
Tyrannosorus hystrioides (Dugan et al.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831590. Fig. 16.
Fig. 16.
Tyrannosorus hystrioides (culture ex-type CBS 117727) asexual morph. A. Colony on OA. B. Hyphal coil. C–F. Concatenated conidia arising from conidiogenous cells. G, H. Aseptate or 1-septate conidia. Scale bars: B–H = 10 μm.
Basionym: Capronia hystrioides Dugan et al., Mycologia 87: 713. 1995.
Synonym: Venturia hystrioides (Dugan et al.) Crous & U. Braun, Stud. Mycol. 58: 212. 2007.
Description and illustration: (Dugan et al. 1995, Crous et al. 2007b, this study).
Typus:USA, Washington, Wenatchee, isolated from cherry fruit, 7 Jul. 1992, F.M. Dugan & R.G. Roberts (holotype ST10-7, permanent slide WSP 69609, culture ex-type CBS 117727, ATCC 96019).
Note: This species is sister to T. lichenicola / pinicola (Fig 1, Fig 2).
Tyrannosorus lichenicola Crous, M. Shen &Y. Zhang ter, sp. nov. MycoBank MB831525. Fig. 17.
Fig. 17.
Tyrannosorus lichenicola (culture ex-type CBS 144018) asexual morph A. Colony on OA. B. Hyphal coil. C. Conidium arising from conidiogenous locus. D–H. Aseptate or 1-septate conidia in chains. Scale bars: B–H = 10 μm.
Etymology: The epithet refers to the lichen, Letharia (Parmeliaceae), the host from which the fungus was collected.
In vitro on OA: Mycelium branched or unbranched, 2–3 μm wide, septate, not constricted or rarely constricted at septa, hyaline to pale brown, verrucose or smooth, straight or flexuous, walls unthickened, and not darkened, frequently with hyphal coils. Conidiophores arising from hyphae, often as short lateral conical prolongations of hyphae, reduced to conidiogenous cells. Conidiogenous cells erect or geniculate-sinuous, 8–20.5 × 2.5–4 μm, monoblastic or polyblastic, pale brown to brown, subcylindrical; loci truncate, 1.5–2 μm wide, usually not thickened, somewhat darkened. Ramoconidia 14–31.5 × 2.5–6.5 μm, 0–1-septate, often not constricted at the septum, pale brown, with a truncate base, cylindrical, subcylindrical, occasionally broadly fusiform, usually with several denticle-like apical loci. Conidia catenate, mostly formed in unbranched chains, straight or slightly curved, cylindrical, subcylindrical, oblong, occasionally broadly fusiform, 11.5–23 × 3–4 μm, subhyaline to pale brown, 0–1-septate, septum median, usually constricted at the septum, smooth, walls slightly thickened, but not darkened-refractive, tapering towards both ends; hila truncate, 1–2 μm wide, sometimes slightly thickened and darkened.
Culture characteristics: Colonies spreading, somewhat erumpent, with sparse aerial mycelium and regular margins on OA, white-grey (surface), margins fuscous-black; reverse fuscous-black; on MEA greyish (surface), margins fuscous-black; reverse fuscous-black; on SNA olivaceous (surface); reverse dark olivaceous. Colonies reaching 28 mm diam after 2 wk on OA at 25 °C in the dark; colonies fertile.
Typus: USA, on Letharia sp. (Parmeliaceae), 27 May 2013, A. Smith (holotype CBS H-23600, culture ex-type CBS 144018 = CPC 25106).
Notes: Tyrannosorus lichenicola was isolated from a Letharia sp. Morphologically, the monoblastic conidiogenous cells, catenate conidia, and the slow-growing, olivaceous colonies point to Venturiaceae. In particular, the occasional microcyclic conidia of Tyrannosorus lichenicola differs from other reported members of Venturiaceae. Phylogenetically, Tyrannosorus lichenicola nests in the Tyrannosorus clade, closely related to T. pinicola (Fig 1, Fig 2).
Tyrannosorus pinicola (Petrini & P.J. Fisher) Unter. & Malloch, Mycol. Res. 99: 910. 1995. Fig. 18.
Fig. 18.
Tyrannosorus pinicola (culture ex-type CBS 124.88) asexual morph. A. Colony on OA. B. Conidiogenous loci on hypha. C–F. Conidia in chains forming three-dimensional helix. G. Straight or curved conidia. Scale bars: B–G = 10 μm.
Basionym: Capronia pinicola Petrini & P.J. Fisher, Trans. Brit. Mycol. Soc. 88: 68. 1987.
Synonym: Helicodendron pinicola E. Müll. et al., ex Voglmayr & P.J. Fisher, Mycol. Res. 101: 1124. 1997.
Typus: Pakistan, on wood of Pinus sp. (Pinaceae), O. Petrini (holotype IMI 308599, culture ex-type CBS 124.88).
Note: This species is sister to T. lichenicola (Fig 1, Fig 2).
Tyrannosorus pini-sylvestris Crous & R.K. Schumach., sp. nov. MycoBank MB831526. Fig. 19.
Fig. 19.
Tyrannosorus pini-sylvestris (culture ex-type CBS 143393) asexual morph. A. Colony on OA. B–E. Conidia in penicillate-like chains. Scale bars: B applies to B, C, E = 10 μm; G = 10 μm.
Etymology: The epithet refers to Pinus sylvestris, the host species from which this fungus was isolated.
Mycelium consisting of pale brown, smooth, septate, branched, 2–2.5 μm wide hyphae. Conidiophores 0–2-septate, mostly reduced to conidiogenous cells on creeping hyphae, lateral or terminal, subcylindrical, erect, 5–20 × 2–3 μm. Conidiogenous cells integrated, 5–10 × 2–3 μm, sympodial, loci truncate, 0.5–1 μm wide, mostly somewhat darkened. Ramoconidia occurring. Conidia in penicillate heads of branched chains, subcylindrical, pale brown, smooth, guttulate, 0–1-septate, (7–)9–11(–13) × 2(–3) μm; loci truncate, somewhat darkened, 0.5 μm diam.
Culture characteristics: Colonies spreading, erumpent, with moderate aerial mycelium and smooth, lobate margins. On MEA and PDA grey olivaceous (surface); reverse iron-grey; on OA iron-grey (surface). Colonies reaching 15 mm diam after 2 wk at 25 °C; colonies fertile.
Typus: Germany, Berlin, on needles of Pinus sylvestris (Pinaceae), 5 Feb. 2016, R.K. Schumacher (holotype CBS H-23839, cultures ex-type CBS 143393 = CPC 30464, CPC 30461).
Notes: The sympodial conidiogenous cells of T. pini-sylvestris agree with Venturiaceae. Tyrannosorus pini-sylvestris can be distinguished from T. hystrioides by its penicillate heads formed by conidia occurring in branched chains. Phylogenetically, T. pini-sylvestris nests in the Tyrannosorus clade, and is basal to other species of Tyrannosorus, such as T. hystrioides, T. lichenicola and T. pinicola (Fig 1, Fig 2).
Venturia Sacc., Syll. Fung. 1: 586. 1882.
Synonyms: Spilocaea Fr., Novit. fl. svec. 5(cont.): 79. 1819.
Cycloconium Castagne, Cat. Pl. Mars.: 220. 1845.
Fusicladium Bonord., Handb. Mykol.: 80. 1851.
Napicladium Thüm., Hedwigia 14: 4. 1875.
Basiascum Cavara [as ‘Basiaschum’], Atti Ist. bot. R. Univ. Pavia, 2 Sér. 1: 433. 1888.
Pollaccia E. Bald. & Cif., Atti Ist. Bot. ‘Giovanni Briosi’, ser. 4, 10: 71. 1937.
Mostly parasitic on leaves, fruits or twigs, causing leaf spots, scab diseases, necroses or deformations. Sexual morph: Ascomata erumpent, semi-erumpent or superficial, rarely immersed, scattered or gregarious, often with papillate ostiole, mostly with setae (except species with immersed ascomata). Paraphyses narrowly cellular, hyaline, mostly evanescent in mature ascomata. Asci 8-spored, bitunicate, broadly cylindrical to obclavate, usually lacking a pedicel. Ascospores pale olivaceous brown to olivaceous brown, 1-septate, usually asymmetrical. Asexual morph: Colonies punctiform, scattered, caespitose or dendritic, olivaceous, olivaceous brown, dingy grey to blackish. Stromata lacking to well-developed, pseudostromatic, composed of rounded to isodiametric swollen hyphal cells, pigmented, wall often somewhat thickened. Mycelium consisting of unbranched or sparingly branched, septate, not constricted at septa, subhyaline, pale brown to brown, smooth hyphae. Conidiophores solitary or sparsely gregarious, arising from internal or external hyphae or stromata, conidiophores often reduced to conidiogenous cells or composed of several cells, erect, cylindrical, pyriform, subclavate, narrowly obclavate, slightly to distinctly geniculate-sinuous, unbranched or occasionally branched, pale olivaceous to dark brown, tips sometimes paler, smooth to somewhat verruculose, sometimes only as short lateral conical prolongations of hyphae, occasionally irregular in shape. Conidiogenous cells integrated, terminal or intercalary or conidiophores reduced to conidiogenous cells, monoblastic to polyblastic, proliferation percurrent or sympodial; conidiogenous loci terminal or lateral, sometimes denticle-like, apex truncate to slightly convex, wall unthickened or almost so, sometimes slightly darkened-refractive. Conidia solitary or catenate, sometimes in simple or branched chains, ellipsoid, obovoid, fusiform, obclavate to subcylindrical, straight to slightly curved, septate or aseptate, subhyaline, pale to dark brown, but mostly olivaceous, sometimes constricted at the septa, smooth to verruculose, ends pointed or rounded to truncate, hila thickened or not, occasionally darkened-refractive.
Type species: Venturia inaequalis (Cooke) G. Winter
Notes: Venturia was first described by De Notaris (1844) to accommodate V. rosae and V. dianthi with no type designated. Subsequently, Cesati & De Notaris (1863) described a further two species, V. dickiei and V. eres. Saccardo (1882) emended the description of Venturia, excluding both V. rosae and V. dianthi, while accepting V. dickiei and V. eres. Venturia Sacc. was widely accepted, and was neotypified with V. inaequalis (Korf, 1956, Sivanesan, 1977).
Venturia includes economically important plant pathogens, such as apple scab caused by V. inaequalis and pear scab caused by V. pyrina (Sivanesan 1977). In the monograph by Sivanesan (1977), herbarium specimens of 65 species were studied including type materials of 31 species. Sivanesan (1977) recognised 52 species and transferred five species to other genera, i.e., V. caulicola and V. himalayensis to Coleroa, V. enteleae and V. rhois to Mycosphaerella and V. microspora to Niesslia. Barr (1968) studied specimens of 35 venturiaceous species from North America, of which 12 species were based on type materials.
Morphologically, the circumscription of Venturia s. str. has been summarised as follows: 1) ascomata, erumpent, semi-erumpent, or superficial, rarely immersed, scattered or gregarious, often with papillate ostiole, mostly with setae; 2) hamathecium narrowly cellular, hyaline, usually evanescent in mature ascomata; 3) asci 8-spored, bitunicate, broadly cylindrical to obclavate, usually lacking a pedicel; 4) ascospores pale greenish olivaceous to dark brown, 1-septate, usually asymmetrical (Zhang et al., 2016a, Zhang et al., 2016b). Based on the circumscription above, type specimens of six species of Venturia, V. centaureae, V. chrysanthemi, V. corni, V. helvetica, V. muelleri and V. rhamni were revised, redescribed and illustrated, and V. corni was excluded from Venturia (Zhang et al., 2016a, Zhang et al., 2016b). Fungarium specimens of 59 species of Venturia are described and illustrated in the present study, with 22 species being excluded from Venturia (see below). The asexual morphs of Venturia have been assigned to Fusicladium, Spilocaea or Pollaccia, while the names in Venturia are more widely known than those in Fusicladium, Spilocaea or Pollaccia. Thus, Venturia was recommended for protection over Fusicladium and Pollaccia (Rossman et al. 2015).
Venturia acerina Plakidas ex M.E. Barr, Canad. J. Bot. 46: 814. 1968. Fig. 20.
Fig. 20.
Venturia acerina (holotype CUP 029477) sexual morph. A, B. Ascomata scattered on the host surface. C–E, M, N. Cylindrical asci. F, H, I. Setae. G. Evanescent pseudoparaphyses. J–L. Ascospores. Scale bars: A, B = 200 μm; C applies to C–F, M, N = 10 μm; G applies to G–I = 10 μm; J applies to J–L = 5 μm.
Synonyms: Venturia acerina Plakidas, Mycologia 34: 34. 1942. Nom. inval., Art. 39.1 (Shenzhen).
Cladosporiumhumile Davis, Trans. Wisconsin Acad. Sci. 19(2): 702. 1919.
Fusicladium humile (Davis) K. Schub. & U. Braun, I.M.I. Descript. Fungi Bact. 152, No. 1520. 2002.
Ascomata hypophyllous, 50–120 μm diam, scattered, solitary or gregarious, initially immersed, becoming erumpent, globose to subglobose, wall black, with a conspicuous papillate ostiole, surrounded by the setae. Setae dark brown, 37–52 × 4–5 μm, 0–2-septate, setae wall 1–1.5 μm thick, up to 6–7 μm wide at the base. Peridium 7–14 μm wide, 1–2 layered, composed of 1–2 rows of pigmented cells of textura angularis, cells 8–9 × 5–7 μm, cell wall 0.8–1 μm thick. Pseudoparaphyses rare, 2–3 μm wide, hyaline, septate, evanescent when mature. Asci 46–76 × 8–12 μm (av. 59.4 × 9.9 μm, n = 20), 8-spored, bitunicate, fissitunicate, obclavate, pedicel lacking, with an inconspicuous ocular chamber. Ascospores 9–17 × 3–6 μm (av. 13.8 × 4.5 μm, n = 14), oblong, ellipsoid, yellow or pale brown, uniseriate at the top and bi- to triseriate at the base, 1-septate, constricted at the septum, the upper cells shorter and wider than the lower ones (length ratio: 3:5–1:1), smooth-walled. Asexual morph: see Schubert et al. (2003: 57–58, fig. 26).
Typus: USA, New York, Tompkins Co., Ithaca, on overwintered leaves of Acer rubrum (Aceraceae), 16 May 1941, A.G. Plakidas (holotype CUP-029477).
Notes: The asexual morph of V. acerina is F. humile, which causes leaf spot of maple in the USA, and overwinters with ascospores and conidia (Sivanesan 1977). Venturia aceris, another venturiaceous species occurring on Acer sp., is distinguishable from V. acerina by the absence of setae as well as the lower positioned ascospore septum.
Venturia aesculi (Syd.) Sivan., Biblioth. Mycol. 59: 31. 1977. Fig. 21.
Fig. 21.
Venturia aesculi (isotype K(M) 189168) sexual morph. A. Ascomata on the host surface. B. Section of an ascoma (showing the asci). C, D. Broadly cylindrical asci. E–H. Released, olivaceous brown ascospores. Scale bars: A = 300 μm; B–E, H = 10 μm; F, G = 5 μm.
Basionym: Spilosticta aesculi Syd., Ann. Mycol. 27: 118. 1929.
Ascomata hypophyllous, (44–)58–118 μm diam, gregarious or scattered, initially immersed, becoming erumpent, globose to subglobose, wall black, with a conspicuous papillate ostiole. Setae not observed. Peridium 4–6.5 μm wide, 1-layered, composed of 1–2 rows of pigmented cells of textura angularis, cells 5–13 × 6–15 μm, cell wall 0.5–2 μm thick, thickened towards the ostiole. Pseudoparaphyses not observed. Asci 28–40 × 7–8 μm (av. 33.1 × 7.6 μm, n = 20), 8-spored, bitunicate, fissitunicate, broadly cylindrical, each with an inconspicuous ocular chamber. Ascospores 8–9.5 × 3–4(–4.5) μm (av. 8.7 × 3.5 μm, n = 20), fusiform to broadly fusiform, olivaceous brown, overlapping to biseriate near the base, 1-septate, slightly constricted at the septum, the upper cells somewhat shorter than the lower ones (length ratio: 4:5–1:1), smooth to slightly verruculose. Asexual morph unknown.
Typus: Germany, on overwintered leaves of Aesculus hippocastanum (Hippocastanaceae), 18 May 1924, P. Vogel (isotype K(M) 189168). Syd., Mycoth. Germ. 2339, lectotype S-F6679 designated here, MBT391372, isolectotypes, e.g., BPI 613455, CUP, K(M) 189168, PDD 42226, PH 44201, WIS-F-83314.
Venturia alaskensis M.E. Barr, Canad. J. Bot. 46: 821. 1968. Fig. 22.
Fig. 22.
Venturia alaskensis (type NY 00914426) sexual morph. A. Ascomata scattered on the host surface. B, C. Evanescent pseudoparaphyses. D–F, I. Subcylindrical asci. G, H. Released, pale brown, 1-septate ascospores. J, K. Dark brown setae. Scale bars: A = 200 μm; B–C = 5 μm; D–F, I = 20 μm; G, H, J, K = 10 μm.
Ascomata 80–100 × 100–120 μm, initially immersed, becoming erumpent to semi-immersed, gregarious or scattered, globose or subglobose, wall black, with a conspicuous papillate ostiole, surrounded by setae. Setae dark brown, 46–103 × 5–7 μm, aseptate, setae wall 1 μm thick, wider at the base. Peridium 7–14 μm wide, 1-layered, composed of 1–2 rows of pigmented cells of textura angularis, cells 7–11 × 6–9 μm, cell wall 0.8–1 μm thick. Pseudoparaphyses rare, 2–3 μm wide, hyaline, septate, evanescent when mature. Asci 50–75 × 13–29 μm (av. 63.2 × 16.2 μm, n = 20), 8-spored, bitunicate, fissitunicate, broadly obclavate to broadly fusiform, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 15–23 × 7–9 μm (av. 20.6 × 7.9 μm, n = 20), ellipsoid, pale brown, overlapping to biseriate, especially at the base, 1-septate, constricted at the septum, the upper cells wider than the lower ones (length ratio: 5:4–8:3), smooth-walled. Asexual morph unknown.
Typus: USA, Alaska, Yukon-Koyukuk. Porcupine Dome, about 12 miles from Miller House, alt. 1 466 m (4 810 ft.), on overwintered leaves of Geum sericeum (Rosaceae), 12 Jul. 1937, E.H. Scamman (holotype NY 00914426, isotype NY 00914427).
Notes: Morphologically, Venturia alaskensis is most comparable with V. atriseda, while the wider ascospores of V. alaskensis (7–9 μm) are distinguishable from V. atriseda [5–6(–7.5) μm] (Barr 1968, see page 36). Although the stromatic network of V. asteromorpha is comparable with V. alaskensis, the ascospores of V. asteromorpha are smaller with a beaked apex as well as its ascomata lacking setae, which differs from V. alaskensis (Barr 1968).
Venturia albae Crous, M. Shen & Y. Zhang ter, sp. nov. MycoBank MB831527. Fig. 23, Fig. 24.
Fig. 23.
Venturia albae (culture ex-type CBS 471.61) sexual morph. A, B. Ascomata on OA. C. Dark brown seta. D–G. Cylindrical, narrowly cylindrical or obclavate asci. H, I. Released, yellowish to brown ascospores. Scale bars: B = 100 μm; C–I = 10 μm.
Fig. 24.
Venturia albae (culture ex-type CBS 471.61) asexual morph. A. Colony on OA. B. Hyphal coil. C, D. Conidiophores with conidiogenous loci. E. Conidiogenous cell giving rise to conidia. F, G. Conidia in chains. H, I. Ramoconidium and conidia. Scale bars: B–I = 10 μm.
Etymology: The epithet refers to epithet of Salix alba, the host from which the species was first reported.
In vitro on OA: Sexual morph: Ascomata initially immersed, becoming erumpent to semi-immersed, gregarious or scattered, black, 198–404 μm diam, subglobose to globose, with a conspicuous papillate ostiole, surrounded by dark brown setae; setae aseptate, 26.5–27.5 × 3–3.5 μm, up to 4 μm wide at the base, wall black. Peridium 1-layered, composed of 2–4 rows of thin-walled, dark brown pigmented cells of textura angularis, cells 4.5–8 × 6.5–9.5 μm. Pseudoparaphyses evanescent when mature, hyaline. Asci 43–87.5 × 8.5–14 μm (av. 64 × 11 μm, n = 15), 8-spored, bitunicate, fissitunicate, broadly cylindrical, to obclavate, pedicel lacking, with an inconspicuous ocular chamber. Ascospores 10.5–14 × 3.5–5.5 μm (av. 12.5 × 5 μm, n = 30), ellipsoid to broadly fusiform, 1-septate, constricted at the septum, yellowish to brown, obliquely uniseriate or partially overlapping to biseriate, especially at the base, rounded at the both ends, the upper cells longer and wider than the lower ones (length ratio: 1:1–5:4), smooth-walled. Asexual morph: Mycelium unbranched or sparingly branched, 2.5–4.5 μm wide, septate, pale brown, smooth or occasionally verrucose, walls not thickened and darkened, frequently with hyphal coils. Conidiophores laterally or terminally arising from hypha, conidiophores reduced to conidiogenous cells, sometimes only as short lateral conical prolongations of hyphae. Conidiogenous cells erect, 12.5–29 × 2.5–4.5 μm, monoblastic or rarely sympodial, brown to medium brown, smooth, walls unthickened, cylindrical or subcylindrical, with a single conidiogenous locus; conidiogenous loci truncate, 2–3 μm wide, mostly unthickened, not darkened. Ramoconidia present, 21.5–27.5 × 4.5–6 μm, aseptate, occasionally 1-septate, not constricted at the septa, brown to medium brown, truncate at the ends, subcylindrical or broadly cylindrical, with 1–3 denticle-like apical loci, rarely thickened or slightly darkened. Conidia catenate, formed in unbranched or loosely branched chains, straight, narrowly cylindrical, 19.5–24.5 × 3.5–6 μm, mostly aseptate, rarely 1-septate, septum median, not constricted at the septa, pale brown to brown, smooth, walls not thickened, tapering towards the ends; hila truncate, 1.5–2 μm wide, slightly thickened, and somewhat darkened-refractive.
Culture characteristics: Colonies spreading, somewhat erumpent, with moderate to sparse aerial mycelium and regular margin on OA, uneven, greyish sepia (surface); reverse fuscous-black; on MEA spreading, smooth, greyish green (surface), margins pale grey to whitish; on SNA spreading, smooth, greyish sepia (surface). Colonies reaching 13 mm diam after 3 wk at 25 °C in the dark.
Typus: Liechtenstein, Bendern, on Salix alba (Salicaceae), 22 May 1959, E. Müller (holotype CBS H-23603, culture ex-type CBS 471.61).
Additional material examined: Liechtenstein, Schneckeneule, on Salix alba (Salicaceae), 13 May 1958, J. Nüesch (ETH 2821, culture CBS 468.61).
Notes: CBS 468.61 and CBS 471.61 were collected by J. Nüesch and E. Müller from Liechtenstein in 1958 and 1959 respectively, and were identified as Venturia chlorospora based on the shape and dimensions of their ascospores (Barr 1968). Ascospores of Venturia chlorospora were reported as 20–25 × 5–8 (Barr 1968), which is much larger than CBS 468.61 and CBS 471.61 (10.5–14 × 3.5–5.5 μm). In addition, the ascospore septum of V. chlorospora is in or near the upper third, while the ascospores of CBS 468.61 and CBS 471.61 have a median septum. Phylogenetically, CBS 468.61 and CBS 471.61 are sibling to other species of Venturia, and closely related to V. fuliginosa and V. chlorospora (Fig 1, Fig 2). Thus, a new species, V. albae, is introduced to accommodate CBS 468.61 and CBS 471.61.
Venturia antherici Hollós, Ann. Hist.-Nat. Mus. Natl. Hung. 8: 9. 1910. Fig. 25.
Fig. 25.
Venturia antherici (NY) sexual morph. A. Ascomata on the host surface. B, F. Broadly cylindrical asci. C, E. Released, broadly cylindrical, pale brown ascospores. D. Dark brown setae. Scale bars: A = 200 μm; B, D, F = 20 μm; C, E = 10 μm.
Synonyms: Venturia allii Ade & Rehm, Hedwigia 64: 292. 1923.
Spilosticta adeana Petr., Kryptogamenfl. Forsch. Bayer. Bot. Ges. Erforsch Heim. Flora 2: 173. 1931.
Ascomata epiphyllous, 70–170 μm diam, gregarious or solitary, initially immersed, becoming erumpent or subsuperficial, globose, wall black, with a conspicuous papillate ostiole, surrounded by setae. Setae dark brown, up to 200 μm long, 5–7 μm wide, septate, base wider, up to 11 μm diam. Peridium 11–16 μm wide, 1-layered, composed of several rows of pigmented cells of textura angularis, cells 8–12 μm wide, cell wall 0.5–1 μm thick, the inner wall thinner than the outer one. Pseudoparaphyses 2–3 μm wide, hyaline, septate. Asci 64–93 × 12–16 μm (av. 76.8 × 14 μm, n = 20), 8-spored, bitunicate, fissitunicate, broadly cylindrical, with a short, knob-like pedicel or pedicel lacking, with an inconspicuous ocular chamber. Ascospores 16.5–24 × 5–6.5 μm (av. 19.1 × 5.8 μm, n = 20), broadly cylindrical to clavate, pale olivaceous brown, overlapping to biseriate, with a submedian septum, constricted at the septum, the upper cells longer than the lower ones (length ratio: 5:4–2:1), smooth-walled. Asexual morph unknown.
Additional materials examined: Switzerland, on the leaves of Allium victorialis (Liliaceae), 20 Jul. 1955, E. Müller (NY); on the leaves of Polygonatum officinale (Liliaceae), 23 May 1954, E. Müller (NY).
Notes: Ascospore septa of V. antherici are mostly submedian (Müller, 1958, Barr, 1968). Venturia antherici was reported as a common species associated with some genera of Liliaceae in Europe, such as Allium, Polygonatum, Lloydia and Anthericum (Barr 1968).
Venturia asperata Samuels & Sivan., New Zealand J. Bot. 13: 646. 1975. Fig. 26, Fig. 27.
Fig. 26.
Venturia asperata (holotype PDD 31846) sexual morph. A. Ascomata scattered on the host surface. B–E. Broadly cylindrical to somewhat obclavate asci. F, G. Ellipsoid, pale brown ascospores. H. Dark brown setae. Scale bars: A = 200 μm; B–F, H = 10 μm; G = 5 μm.
Fig. 27.
Venturia asperata (holotype PDD 31846) asexual morph. A. Colony growing on OA. B–H. Conidiophores reduced to conidiogenous cells. I, J. Fusiform conidia. K. Subcylindrical ramoconidium. L. Germinating conidia. Scale bars: B–L = 5 μm.
Synonym: Fusicladium asperatum K. Schub. & U. Braun, Schlechtendalia 9: 18. 2003.
Ascomata amphigenous, 70–130 μm diam, solitary, scattered or in small groups of 2 to 3, initially immersed, becoming erumpent or subsuperficial, globose, wall black, with a conspicuous papillate ostiole, surrounded by setae. Setae dark brown, 18–45 × 4–6.5 μm, septate, setae wall 0.5–2 μm thick. Peridium 10–14 μm wide, 1-layered, composed of 3 rows of pigmented cells of textura angularis, cells 7–10 μm wide, cell wall 0.8–1.5 μm thick. Pseudoparaphyses rare, hyaline, evanescent when mature. Asci 47–61 × (8.5–)10–11 μm (av. 54.3 × 10.4 μm, n = 20), 8-spored, bitunicate, fissitunicate, narrowly obclavate to broadly cylindrical, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 11.5–15 × 4–6 μm (av. 12.9 × 5.3 μm, n = 20), ellipsoid, yellow to pale brown, uniseriate at the apex and biseriate at the base, 1-septate, mostly submedian, constricted at the septum, the upper cells somewhat longer than the lower ones (length ratio: 1:1–7:5), smooth-walled. Asexual morph: Mycelium 2–3 μm wide, branched or rarely branched, septate, not constricted at septa, subhyaline to pale brown, smooth, wall unthickened or slightly thickened. Conidiophores laterally arising from hyphae, erect, straight or somewhat flexuous, sometimes geniculate, unbranched, (6–)12–75 × (2.5–)3–4.5 μm, septate or aseptate, pale to medium brown, smooth, wall slightly thickened, sometimes only as short lateral conical hyphae, occasionally irregular in shape. Conidiogenous cells integrated, terminal, or conidiophores reduced to conidiogenous cells, 6–29 μm long, sometimes geniculate, proliferation sympodial, with several denticle-like loci, broadly truncate, 1.5–2(–2.5) μm wide, unthickened, somewhat darkened-refractive. Ramoconidia present, 20–28 × 5 μm, 0–1-septate, medium brown, broadly truncate base, with several loci at the apex, 3–4 μm wide. Conidia catenate, formed in unbranched or loosely branched chains, straight or slightly curved, cells sometimes irregularly swollen, 13–35 × 3.5–5.5(–6) μm, subcylindrical, fusiform, occasionally obpyriform, 0–3-septate, occasionally slightly constricted at the septum, few very large conidia with 5 septa, up to 75 μm long, 4.5–6 μm wide, subhyaline to pale brown, smooth, wall slightly thickened, slightly attenuated towards apex and base; hila broadly truncate, 1–2 μm wide, not or only slightly thickened, slightly darkened-refractive; microcyclic conidiogenesis present.
Typus: New Zealand, Auckland Prov., Waitemut Co., Oratia, P.D.D Research Orchard, on leaves of Malus sylvestris (Rosaceae), Aug. 1973, P.J. Brook, G.J. Samuels & M.A. Manning (holotype PDD 32263, isotypes IMI 186580, NY 00914428; holotype of Fusicladium asperatum PDD 31846).
Notes: Both V. asperata and V. inaequalis occur on Malus spp. Morphologically, the upper cells of ascospores of V. asperata are longer than the lower cells, distinguishing it from the shorter upper cells of ascospores of V. inaequalis. Furthermore, their asexual morphs differ in the sometimes concatenated conidia of V. asperata (vs. the not concatenated conidia of V. inaequalis), as does the position of the septum in their ascospores.
Venturia atriseda Rehm, Hedwigia 21(6): 84. 1882. Fig. 28.
Fig. 28.
Venturia atriseda (isotype K(M) 189232) sexual morph. A. Ascomata scattered on the host surface. B. Dark brown setae. C–E, H. Broadly cylindrical to somewhat obclavate asci. F, G. Released, fusiform, pale brown ascospores. Scale bars: A = 200 μm; B, F, G = 10 μm; C–E, H = 20 μm.
Synonyms: Eriosphaeria atriseda (Rehm) Rehm, in Jaap, Ann. Mycol. 5: 253. 1907. Nom. illeg., Art. 53.1, non E. atgriseda (Feltgen) Sacc. & D. Sacc., 1905.
Spilosticta atriseda (Rehm) Petr., Ann. Mycol. 25: 209. 1927.
Ascomata occur on stems of Gentiana lutea, 80–166 × 60–120 μm diam, scattered or solitary, initially immersed, becoming erumpent, globose to conical, wall black, with a conspicuously papillate ostiole, surrounded by setae. Setae dark brown, 34–93 × 4–8 μm, septate, wall 1.5–2 μm thick. Peridium 11–14 μm wide, 1-layered, composed of 2–3 rows of pigmented cells of textura angularis, cells 4–4.5 μm wide, cell wall 0.5–1.2 μm thick, gradually thickened towards the ostiole. Pseudoparaphyses 2–3 μm wide, hyaline, filiform. Asci 42–74 × 14–21 μm (av. 55.8 × 16.8 μm, n = 20), 8-spored, bitunicate, fissitunicate, broadly cylindrical to broadly obclavate, with a short, knob-like pedicel or pedicel lacking, with an inconspicuous ocular chamber. Ascospores 18–22 × 5–6(–7.5) μm (av. 19.5 × 5.7 μm, n = 20), fusiform to narrowly fusiform, pale brown to brown, obliquely uniseriate to biseriate at the top, triseriate near the base, 1-septate, apiosporous, with one septum at or nearly a third from the base, constricted or not at the septum, the upper cells longer and wider than the lower ones (length ratio: 11:9–25:14), smooth-walled. Asexual morph unknown.
Typus: Germany, Bavaria, Benediktenwand, Hausstatt Mountain, on dried stems of Gentiana lutea (Gentianaceae), Jul. 1881, Arnold (lectotype K(M) 189232 designated here, MBT391373).
Additional material examined: Switzerland, Kt. St. Gallen, Speergebiet, on Gentiana punctata (Gentianaceae), E. Müller, 2 Jul. 1953 (culture CBS 371.55).
Notes: Isolate CBS 371.55 was sterile, and its identity could not be confirmed. This isolate forms a distinct lineage in the Venturia clade (Fig. 2).
Venturiaaustraliana Crous, M. Shen & Y. Zhang ter, sp. nov. MycoBank MB831537. Fig. 29.
Fig. 29.
Venturiaaustraliana (culture ex-type CBS 128286) asexual morph. A. Colony on OA. B–D. Conidiogenous cells giving rise to conidia. E–G. Ramoconidia and conidia in chains. Scale bars: B–G = 10 μm.
Etymology: The epithet refers to Australia, the country where the species was collected.
Sexual morph unknown. In vitro on OA: Mycelium branched or sparingly unbranched, 2–3 μm wide, septate, not constricted at septa, hyaline to pale brown, smooth, straight to somewhat flexuous, walls not thickened and darkened. Conidiophores arising from wider and darker hyphae, erect or geniculate-sinuous, unbranched, 17.5–55 × 4–7.5 μm, sometimes up to 155.5 μm long, septate, not constricted at the septa, medium to dark brown, smooth, walls somewhat thickened or darkened, cylindrical or subcylindrical. Conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, cylindrical or subcylindrical, erect or sometimes geniculate, medium to dark brown, often becoming hyaline towards the apex, (6.5–)13–39.5 × 4.5–8 μm, proliferating sympodially or monoblastic, with a single or more conidiogenous loci, loci narrowly truncate, 1–3.5 μm wide, thickened and somewhat darkened. Ramoconidia present, 12–20 × 4–6.5 μm, 0(–1)-septate, occasionally constricted at the septa, pale to medium brown, sometimes dark brown, subcylindrical, broadly fusiform, or somewhat irregular, with 1–3 denticle-like apical loci. Conidia catenate, usually formed in branched chains, straight or occasionally slightly curved, cylindrical, subcylindrical, fusiform, broadly fusiform or somewhat irregular, 12–21 × 4–5.5 μm, pale to medium brown, smooth, mostly aseptate, rarely 1–2-septate, septum median, rarely constricted, tapered towards the ends, truncate at the base; hila 1–2 μm wide, slightly thickened, and somewhat darkened; conidia often germinating.
Culture characteristics: Colonies spreading, somewhat erumpent, with moderate to sparse aerial mycelium and smooth margins on OA; fuscous-black (surface); reverse fuscous-black; on SNA olivaceous (surface); reverse olivaceous. Colonies reaching 21 mm diam on OA after 2 wk at 25 °C in the dark; colonies fertile.
Typus: Australia, Victoria, Berwick, on leaf spot of Convolvulus cneorum (Convolvulaceae), 23 Jun. 2010 (holotype CBS H-23595, culture ex-type CBS 128286 = VPRI 41762).
Notes: Isolate CBS 128286 was originally identified as Fusicladium convolvularum, while the conidial dimensions are smaller than those of F. convolvularum (Schubert et al. 2003). Although F. convolvularum (as V. convolvularum) clusters sister to CBS 128286 (Fig 1, Fig 2; ITS identity 96 %, LSU identity 99 %, tub2 identity 93 %), the two species are distinct.
Venturia bistortae (Syd.) Sivan., Biblioth. Mycol. 59: 42. 1977. Fig. 30.
Fig. 30.
Venturia bistortae (isotype K(M) 189233) sexual morph. A. Ascomata scattered on the host surface. B–E. Broadly obclavate asci. F–I. Released, pale brown ascospores. Scale bars: A = 200 μm; B–E = 20 μm; F–I = 10 μm.
Basionym: Spilosticta bistortae Syd., Ann. Mycol. 21: 172. 1923.
Ascomata forming subcircular brown to black spots on leaf surfaces, 60–120 μm diam, solitary or rarely gregarious, initially immersed, becoming erumpent, globose or subglobose; wall black, with a conspicuous papillate ostiole, surrounded by setae. Setae dark brown, up to 60 μm long, base 2–3 μm wide. Peridium 10 μm wide, 1-layered, composed of 2–3 rows of pigmented cells of textura angularis, cells 8–10 μm wide, cell wall 0.8–1 μm thick, thicker near the apex. Pseudoparaphyses rare, evanescent when mature. Asci 38–60 × 12–19 μm (av. 52 × 15.9 μm, n = 20), 8-spored, bitunicate, fissitunicate, broadly cylindrical, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 15–19 × 5–7 μm (av. 17 × 5.6 μm, n = 20), broadly clavate, pale brown, overlapping to biseriate near the base, 1-septate, slightly constricted at the septum, the upper cells shorter and wider than the lower ones (length ratio: 3:5–1:1), smooth-walled. Asexual morph: unknown.
Typus: Germany, Westphalia, Ginsberger Heide, on leaves of Polygonum bistorta (Polygonaceae), 26 Jun. 1921, A. Ludwig (lectotype S-F6682 designated here, MBT391374, isolectotypes Syd., Mycoth. Germ. 1911, e.g., BPI 613462, CUP, PDD 42615, PH 44202).
Notes: Although this taxon conforms to Venturia in general morphology, the subcircular leaf spots, and pale brown ascospores of V. bistortae disagree with Venturia s. str. Its phylogenetic position remains to be resolved.
Venturia caesiae Crous, M. Shen & Y. Zhang ter, sp. nov. MycoBank MB831538.
Etymology: The epithet refers to the host species from which this fungus was collected, Salix caesia.
Cultures sterile. Venturia caesiae (CBS 466.61) differs from its closest phylogenetic neighbour V. minuta (CBS 479.61; Fig. 2) by unique fixed alleles in two loci based on alignments of the separate loci deposited in TreeBASE (S24582), by 13 bp in tef1 (4 %) and 14 bp in tub2 (3 %).
Culture characteristics: Colonies spreading, erumpent, with aerial mycelium and regular, smooth margins on OA, brown to dark olivaceous brown (surface); reverse fuscous-black; on MEA grey to olivaceous brown (surface); reverse fuscous-black; on SNA olivaceous brown (surface); reverse dark olivaceous brown. Colonies reaching 26 mm diam after 2 wk on OA at 25 °C in the dark.
Typus: Switzerland, on Salix caesia (Salicaceae), 2 Jul. 1959, J. Nüesch (holotype CBS 466.61, preserved as metabolically inactive culture, culture ex-type CBS 466.61).
Notes: CBS 466.61 was isolated from Salix caesia by E. Müller, and named Venturia chlorospora. According to the multigene phylogenetic analysis (Fig. 2), CBS 466.61 forms a separate lineage distinguishing it from closely related species (i.e., V. minuta, V. helvetica and V. chinensis) as well as isolates of V. chlorospora. Thus, a new name, V. caesiae, is proposed here. Venturia caesiae is sister to V. chinensis in Fig. 1.
Venturia canadensis M.E. Barr, Canad. J. Bot. 46: 818. 1968. Fig. 31.
Fig. 31.
Venturia canadensis (type NY 00914436) sexual morph. A, B. Ascomata scattered on the host surface. C. Evanescent pseudoparaphyses. D–I. Broadly cylindrical to somewhat obclavate asci. J. Released, fusiform, pale brown ascospores. K, L. Dark brown setae. Scale bars: A, B = 200 μm; C–L = 10 μm.
Ascomata 80–125 μm diam, solitary, initially immersed, becoming erumpent, to nearly superficial, globose to subglobose, black, with a conspicuously papillate ostiole, surrounded by setae. Setae dark brown, 30–100 × 4–7 μm, base up to 10 μm wide. Peridium 6–8 μm wide, 1-layered, composed of two rows of pale brown thick-walled cells of textura angularis, cells 4–6 μm wide, cell wall 0.8–1 μm thick, thickened along the apex. Pseudoparaphyses rare, evanescent when mature. Asci 41–60 × 11–13 μm (av. 49.6 × 12.4 μm, n = 20), 8-spored, bitunicate, fissitunicate, broadly cylindrical to somewhat obclavate, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 14–16(–19) × 5–6 μm (av. 15.5 × 5.4 μm, n = 20), fusiform, broadly fusiform, pale brown, bi- to triseriate near the base, 1-septate, with one septum in the lower half, constricted or not at the septum, the upper cells wider than the lower ones (length ratio: 8:7–11:8), smooth-walled. Asexual morph unknown.
Typus: Canada, Quebec, Gaspé Prov. Park, Mont Albert, on leaves and stalks of Rumex acetosella (Polygonaceae), 10 Jul. 1957, H.E. & M.E. Bigelow (holotype NY 00914436, isotype NY 00914437).
Note: Venturia caulicola is another venturiacous species occurring on Rumex sp., which was assigned to Coleroa based on its superficial ascomata (as Coleroa caulicola, Sivanesan 1977).
Venturia carpophila E.E. Fisher, Trans. Brit. Mycol. Soc. 44: 339. 1961. Nom. cons. prop. (Rossman et al. 2018). Fig. 32.
Fig. 32.
Venturia carpophila (isotype K(M) 189234 and PDD 32688) sexual morph. A. Ascomata scattered on the host surface. B–F. Clavate or cylindrical asci. G–I. Clavate, pale brown ascospores. J. Evanescent pseudoparaphyses. Scale bars: A = 200 μm; B–F, J = 20 μm; G–I = 10 μm.
Synonyms: Cladosporium carpophilum Thüm., Oesterr. Bot. Z. 27: 12. 1877.
Fusicladium carpophilum (Thüm.) Oudem., Verh. Kon. Akad. Wetensch., Tweede Sect.: 388. 1900.
Fusicladium pruni Ducomet, Thèse Fac. Sci. Paris: 137. 1907.
Fusicladium amygdali Ducomet, Ann. École Natl. Agric. Rennes 4: 11. 1911.
Megacladosporium carpophilum (Thüm.) Vienn.-Bourg., Les Champignons Parasites Pl. Cult. 1: 489. 1949.
Fusicladosporium carpophilum (Thüm.) Partr. & Morgan-Jones, Mycotaxon 85: 362. 2003.
Ascomata 100–160 μm diam, scattered or solitary, initially immersed, becoming erumpent, globose to subglobose, wall black, with a conspicuously papillate ostiole, surrounded by dark brown setae or setae not observed. Peridium 15–20 μm wide, 1-layered, composed of 2–3 rows of pale brown thick-walled cells of textura angularis, cells 4–11 μm wide, cell wall 1–1.2 μm thick. Pseudoparaphyses septate, hyaline, evanescent when mature. Asci 47–68 × 9–11 μm (av. 57.5 × 10.4 μm, n = 20), 8-spored (rarely 4-spored), bitunicate, fissitunicate, clavate, broadly cylindrical, with a short, knob-like pedicel, with an inconspicuous ocular chamber. Ascospores 12–16 × 4–6 μm (av. 13.9 × 5.1 μm, n = 20), clavate, pale brown, biseriate, rarely uniseriate, 1-septate, constricted at the septum, the upper cells much wider than the lower ones, smooth-walled. Asexual morph, see Schubert et al. (2003: 27–30, figs 7 and 8).
Typus: Australia, Victoria, on dead leaves of Prunus armeniaca (Rosaceae), 29 Aug. 1955, E.E. Fisher (holotype VPRI No. 984, isotypes K(M) 189234, PDD 32688, VPRI No. 983).
Notes: The diagnostic characteristics of Venturia carpophila are its asymmetrical ascospores (the upper cells much wider than the lower ones), and conspicuous constriction at the septum. Venturia carpophila causes freckle disease of apricots, plum and almonds and scab of peaches (Sivanesan 1977).
Venturia cassandrae Peck, Rep. (Annual) New York State Mus. Nat. Hist. 38: 104. 1885. Fig. 33.
Fig. 33.
Venturia cassandrae (holotype NYSf672) sexual morph. A. Ascomata scattered on the host surface. B–E. Broadly obclavate asci. F, G. Evanescent pseudoparaphyses. H. Released, pale brown ascospores. I. Setae. Scale bars: A = 200 μm; B–E, I = 20 μm; F–H = 10 μm.
Synonym: Gibbera cassandrae (Peck) M.E. Barr, Canad. J. Bot. 39: 313. 1961.
Ascomata scattered on the grey brown spots, 50–90 μm diam, globose to conical, wall black, with a conspicuous papillate ostiole, erumpent to nearly superficial, surrounded by setae. Setae dark brown, 43–75 × 5–7 μm, wall 1–2 μm thick, base swollen, up to 10–12 μm, septate. Peridium 1-layered, composed of two rows of pigmented cells of textura angularis, cells 4–8 μm wide, cell wall 1–1.5 μm thick. Pseudoparaphyses 2–4 μm wide, hyaline, septate, evanescent when mature. Asci 57–65 × 20–25 μm (av. 60.6 × 22.7 μm, n = 20), 8-spored, bitunicate, fissitunicate, oblong, pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 21–22(–24) × 7–10 μm (av. 21.5 × 8.6 μm, n = 20), fusiform to broadly fusiform, pale brown, with rounded or somewhat tapered ends, triseriate especially in the middle of the ascus, 1-septate, slightly constricted at the septum, the upper cell sometimes shorter and wider than the lower one (length ratio: 9:13–1:1), smooth-walled. Asexual morph unknown.
Typus: USA, New York, Fulton County, Caroga, on the leaves of Chamaedaphne calyculata (Ericaceae), Jul. 1884, C.H. Peck (holotype NYSf672).
Notes: Barr (1961) assigned V. cassandrae to Gibbera as G. cassandrae based on the erumpent ascomata and thin hypostroma. Morphologically, the nearly superficial, scattered ascomata with setae; narrowly cellular, hyaline, evanescent pseudoparaphyses; 8-spored, bitunicate, broadly obclavate asci lacking pedicels, as well as pale olivaceous, 1-septate ascospores of V. cassandrae fit Venturia s. str. very well. Thus, the name V. cassandrae is retained here.
Venturia catenospora (Butin) Rossman & Crous, IMA Fungus 6: 520. 2015.
Basionym: Pollaccia catenospora Butin, Mycol. Res. 96: 658. 1992.
Synonym: Fusicladium catenosporum (Butin) Ritschel & U. Braun, Schlechtendalia 9: 30. 2003.
Description and illustration: Schubert et al. (2003: 30–31, fig. 9).
Typus: Germany, Berlin, Eberswalde-Finow, on the leaf spot of Salix triandra (Salicaceae), 7 Aug. 1990, H. Butin (holotype IMI 349857, culture ex-type CBS 447.91).
Additional materials examined: China, Heilongjiang Province, on Salix sp. (Salicaceae), 22 Aug. 2014, Y. Zhang & Y. Zhou (culture CGMCC 3.18369 = BJFCC 140822-1). Switzerland, on S. caprea (Salicaceae), 10 Jun. 1958, J. Nüesch (culture CBS 469.61).
Note: The species is sister to V. chinensis / caesiae (Fig. 1) and to V. viennotii (Fig. 2).
Venturia cephalariae (Auersw.) Kalchbr. & Cooke, Grevillea 9(no. 49): 31. 1880. Fig. 34.
Fig. 34.
Venturia cephalariae (type K(M) 189236) sexual morph. A. Ascomata scattered on the host surface. B–E. Broadly cylindrical asci. F. Evanescent pseudoparaphyses. G, H. Released, pale brown, 1-septate, apiosporous ascospores. I. Dark brown setae. Scale bars: A = 200 μm; B–I = 10 μm.
Basionym: Sphaerella cephalariae Auersw., in Gonnermann & Rabenhorst, Mycol. Eur. 5–6: 14. 1869.
Synonyms: Laestadia cephalariae (Auersw.) Sacc., Syll. Fung. 1: 425. 1882.
Carlia cephalariae (Auersw.) Kuntze, Revis. Gen. Pl. 2: 846. 1891.
Guignardia cephalariae (Auersw.) F. Stevens, Trans. Illinois State Acad. Sci. 10: 184. 1917.
Ascomata hypophyllous, associated with leaf spots, 70–130 μm diam, gregarious or scattered, initially immersed, becoming erumpent, globose, wall black, with a conspicuously papillate ostiole, surrounded by setae. Setae dark brown, 27–43 × 5–8 μm, base swollen, base up to 10 μm wide, wall 1–2 μm thick, septate. Peridium 7–20 μm wide, 1-layered, composed of 2–3 rows of brown pigmented cells of textura angularis, cells 5–12 μm wide. Pseudoparaphyses 2–4 μm wide, swollen at the apex, septate, hyaline, evanescent when mature. Asci 55–74 × 13–17 μm, 8-spored, bitunicate, fissitunicate, broadly cylindrical to somewhat obclavate, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 14–19(–24) × 5–7 μm (av. 16.4 × 6 μm, n = 20), ellipsoid, pale brown, bi- to triseriate, 1-septate, apiosporous, with the septum in the lower third of ascospores, slightly constricted at the septum, the upper cells wider and longer than the lower ones [length ratio: 21:11–3:1(–19:5)], smooth-walled. Asexual morph unknown.
Material examined: South Africa, Cape Province, on leaves of Cephalaria attenuata (Calyceraceae), P. MacOwan 1338 (ex herb. M.C. Cooke, K(M) 189236).
Note: Colonies of V. cephalariae produced a sexual morph in malt agar media (Sivanesan 1977).
Venturia cerasi Aderh., Landw. Jahrb. 29: 541. 1900. Fig. 35.
Fig. 35.
Venturiacerasi (CBS 160.55) asexual morph. A. Colony on OA. B–F. Conidiogenous cells giving rise to conidia. Scale bars: B–F = 10 μm.
Synonym: Fusicladium cerasi (Rabenh.) Erikss., Meddeland. Kongl. Lantbruksakad. Exp.-fält 1: 73. 1885.
Sexual morph: see Sivanesan (1977: 51). In vitro on OA: Mycelium unbranched or only sparingly branched, 1.5–2.5 μm wide, septate, not constricted at septa, hyaline to pale brown, smooth, flexuous or straight, walls not thickened or almost so. Conidiophores solitary, arising laterally from hyphae, erect, straight to somewhat flexuous, sometimes geniculate, unbranched, 30.5–69.5 × 3–5 μm, aseptate or septate, pale brown or pale medium brown, sometimes paler towards the apex, smooth, walls somewhat thickened, sometimes only as short lateral conical prolongations of hyphae, subcylindrical. Conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, sometimes geniculate, 17–24 × 3–4 μm, proliferating sympodially, pale brown or pale medium brown, with several conidiogenous loci, crowded at the apex, loci denticulate, 0.5–1.5 μm wide, not thickened or sometimes slightly darkened-refractive. Ramoconidia present, 12–22 × 3–6 μm, (0)–1-septate, not constricted at the septa, pale to medium brown, oval or broadly cylindrical, with a truncate base, usually with two loci. Conidia catenate, formed in unbranched or loosely branched chains, straight to rarely curved, narrowly or broadly fusiform, subcylindrical or ellipsoid, 11.5–24.5 × 2.5–5 μm, 0–1(–2)-septate, not constricted at the septa, pale brown, smooth, walls slightly thickened, attenuated towards apex and base; hila truncate, 0.5–1.5 μm wide, not thickened or only slightly thickened, somewhat darkened-refractive.
Culture characteristics: Colonies spreading, somewhat erumpent, with moderately sparse aerial mycelium and regular margins on OA, uneven, greyish (surface), margins greyish sepia; reverse fuscous-black. Colonies reaching 44 mm diam after 1.5 wk at 25 °C in the dark; colonies fertile.
Typus: Germany, Borussia, on fruits of Prunus cerasus (Rosaceae) (lectotype designated in Schubert et al. 2003: 33: Braun (l.c.: Pl. 1, B, 1–2); Aschersleben, on Prunus cerasus, 17 Sep. 1954, H. Schweizer (epitype specimen designated here CBS 444.54, MBT391375, preserved as metabolically inactive culture, ex-epitype culture CBS 444.54).
Additional materials examined: Switzerland, Zollikon, Kt. Zürich, on Prunus mirabelle (Rosaceae), 10 Aug. 1961, E. Müller (ETH 4568, culture CBS 497.62). USA, California, on the fruit of Prunus amygdalus (Rosaceae) (specimen CBS H-23596, culture CBS 160.55 = ATCC 12062 = MUCL 10087).
Notes: The ITS sequence identity among CBS 160.55, CBS 497.62 (sterile) and CBS 444.54 are 99 %, thus they are conspecific. None of the isolates, however, are ex-type. Morphologically, CBS 160.55 is most comparable with CBS 444.54 in its conidial size (11.5–24.5 × 2.5–5 vs. 20–24 × 3.5–4.5) and sympodially proliferating conidiogenous cells, which agree with the description of the asexual morph of V. cerasi (Fusicladium cerasi) provided by Schubert et al. (2003). Thus, we apply the name V. cerasi to this clade (Fig. 2).
Venturia chamaemori (P. Karst.) Arx, Acta Bot. Neerl. 6: 340. 1957. Fig. 36.
Fig. 36.
Venturia chamaemori (isotype K(M) 189238) sexual morph. A. Ascomata scattered on the host surface. B. Evanescent pseudoparaphyses. C. Dark brown setae. D. Pale brown ascospores. Scale bars: A = 100 μm; B, D = 10 μm; C = 20 μm.
Basionym: Sphaerella chamaemori P. Karst., Fungi Fenn. Exs., Fasc. 9: no. 899. 1869.
Synonym: Mycosphaerella chamaemori (P. Karst.) Lindau, in Engler & Prantl, Nat. Pflanzenf. Teil 1, 1(1): 424. 1897.
Ascomata amphigenous, 100–160 × 90–120 μm diam, scattered, solitary, initially immersed, becoming erumpent, globose to conical, wall black, with a conspicuously papillate ostiole, surrounded by dark brown setae. Setae 40–86 × 4–8 μm, base swollen, up to 14 μm diam, setae wall 1–1.5 μm thick, septate. Peridium 13–19 μm wide, 1-layered, composed of three rows of brown pigmented cells of textura angularis, cells 4–9 μm diam. Pseudoparaphyses 2–4 μm wide, septate, hyaline. Asci 50–80 × 10–26 μm, 8-spored, bitunicate, fissitunicate, cylindrical, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 11–15 × 4–7 μm (av. 12.7 × 5.3 μm, n = 20), ellipsoid, olivaceous, obliquely uniseriate and partially overlapping to biseriate, medianly 1-septate, slightly constricted at the septum, the upper cells sometimes longer and wider than the lower ones (length ratio: 1:1–7:6), smooth-walled. Asexual morph unknown.
Typus: Finland, Etelä-Häme, Mustalia, on leaves of Rubus chamaemorus (Rosaceae), P.A. Karsten [Fungi Fenn. Exs. no. 899, syntypes H 6052060, K(M) 189238].
Note: The specimen is depauperate, and information about asci refers to von Arx (1957) and Sivanesan (1977).
Venturia chinensis Y. Zhang ter & J.Q. Zhang, Saudi J. Biol. Sci. 23: 594. 2015 [2016].
Description and illustration: Zhang et al., 2016a, Zhang et al., 2016b.
Typus: China, Heilongjiang province, Yichun, Wuyiling district, Wuyiling forestry station, on leaves of Lonicera praeflorens (Caprifoliaceae), 26 Aug. 2014, Y. Zhang & Y. Zhou (holotype HAMS 246485, culture ex-type CBS 142240 = CGMCC 3.17685 = BJFC 140826-17).
Note: The species is sister to V. caesiae (Fig. 1) and to V. helvetica / caesiae / minuta (Fig. 2).
Venturia chlorospora (Ces.) P. Karst., Bidr. Känn. Finl. Nat. Folk 23: 189. 1873. Fig. 37.
Fig. 37.
Venturia chlorospora (culture CBS 467.61) asexual morph. A. Colony on OA. B, C. Hypha with conidiogenous loci. D. Long, branched conidial chains. E, F. Conidiophores giving rise to concatenate conidia. G, H. Ramoconidium and conidia in chains. I. Germinating conidia. Scale bars: B–I = 10 μm.
Basionym: Sphaeriachlorospora Ces., Erb. Critt. Ital., Ser. 1, Fasc. 2: no. 296, 1859 [Ces., in Rabenh., Fungi Eur. Exs. (Klotzschii Herb. Viv. Mycol. Continuatio, Ed. Nova, Ser. Sec.), Cent. 1: no. 48, 1859; Bot. Zeitung 17: 296, 1859; Flora 42: 434, 1859].
Synonyms: Endostigme chlorospora (Ces.) Syd., Ann. Mycol. 21(3/4): 173. 1923.
Venturia chlorospora var. canescens P. Karst., Bidrag Kännedom Finlands Natur. Folk 23: 190. 1873.
Venturia chlorospora var. salicis-vitellinae Sacc., Syll. Fung. 1: 587. 1882.
Sexual morph: see Nüesch (1960: 342–344). In vitro on OA: Mycelium branched, 1.5–3.5 μm wide, hyaline to pale brown, smooth or verrucose, straight or somewhat flexuous, septate, often not constricted at the septa, walls not darkened or thickened, with hyphal coils. Conidiophores arising as short branches from subhyaline to pale brown hyphae, reduced to conidiogenous cells. Conidiogenous cells terminal or intercalary, erect, 10–20 × 3–7 μm, monoblastic, pale brown to brown, walls not thickened nor darkened, subcylindrical, with single loci, 1.5–3.5 μm wide, not thickened nor darkened-refractive. Ramoconidia present, 13–36.5 × 2.5–6.5 μm, aseptate, sometimes 1-septate, usually not constricted at septa, pale brown to brown, truncate at the both ends, occasionally rounded towards the apex, cylindrical to subcylindrical, smooth or somewhat verrucose, straight or flexuous, with one to two denticle-like loci; loci broadly truncate, 1.5–2.5 μm wide, slightly thickened or somewhat darkened. Conidia catenate, mostly formed in long, branched chains, cylindrical to subcylindrical, 11–29 × 3–7 μm, (0–)1-septate, constricted at the septa, brown, verrucose to smooth, straight or occasionally geniculate, walls slightly thickened and darkened, truncate at both ends, sometimes rounded towards the apex; hila truncate, 1.5–3 μm wide, slightly thickened and somewhat darkened.
Culture characteristics: Colonies spreading, somewhat erumpent, with moderate sparse aerial mycelium and regular margins on OA, uneven, greyish sepia (surface); reverse fuscous-black; on MEA spreading, smooth, greyish green (surface), margins pale grey to whitish; on SNA spreading, smooth, greyish sepia (surface); reverse fuscous-black. Colonies reaching 32 mm diam on OA after 3 wk at 25 °C in the dark; colonies fertile.
Typus: Italy, Vercellis, on Salix (triandrta, alba) (Salixaceae), 1858, V. de Cesati [Erb. Critt. Ital. 296 and Rabenh., Fungi Eur. Exs. 48, syntypes, e.g. B, CUP, HAL, S].
Additional materials examined: France, Aiguilles, Val Queyras, Hautes Alpes, on Salix daphnoides (Salixaceae), 25 Jun. 1958, J. Nüesch (ETH 2828, culture CBS 470.61). Switzerland, La Punt, Kt. Graubünden, on S. daphnoides, 2 Jul. 1959, J. Nüesch (CBS H-23602, culture CBS 467.61, ETH 2504).
Notes: CBS 467.61 and CBS 470.61 were collected by Nüesch in Europe in 1958 and 1959 respectively. The conidia of CBS 467.61 agree morphologically with V. chlorospora (20–25 × 5–8 μm) (Nüesch, 1960, Sivanesan, 1977). This species is sister to V. fuliginosa (Fig. 2). The nomenclature of Sphaeria chlorospora has recently been discussed in detail by Braun & Bensch (2019: 3), including copy of the label of the original description and illustration in Rabenh., Fungi. Eur. Exs. 48.
Venturia convolvularum (Ondřej) Rossman & Crous, IMA Fungus 6: 520. 2015.
Basionym: Fusicladium convolvularum Ondřej, Ceská Mykol. 25: 171. 1971.
Description and illustration: Schubert et al., 2003, Crous et al., 2007b.
Typus: Czech Republic, Libina, okraj pole pod nadrazim (okr. Sumperk), on Convolvulus arvensis (Convolvulaceae), 7 Sep. 1970, Ondřej (holotype BRA) (not seen). New Zealand, on the leaves of C. arvensis, 7 Nov. 2000, C.F. Hill (epitype CBS H-19911 designated in Crous et al. (2007b), culture ex-epitype CBS 112706 = CPC 3884 = LYN 136 = IMI 383037).
Note: The species is sister to V. australiana (Fig 1, Fig 2).
Venturia crataegi Aderh., Ber. Deutsch. Bot. Ges. 20: 200. 1902. Fig. 38, Fig. 39.
Fig. 38.
Venturia crataegi (MICH 15147) sexual morph. A. Ascomata scattered on the host surface. B, C. Squash mounts with a large number of asci (C in cotton blue). D–F. Cylindrical asci (in cotton blue). G, H. Released, colourless to pale brown ascospores (in cotton blue). Scale bars: A = 100 μm; B–F = 10 μm; G, H = 5 μm.
Fig. 39.
Venturia crataegi (culture CBS 367.35) asexual morph. A. Colonies on OA. B, C. Conidiophores giving rise to conidia. D. Conidiogenous cells with sympodial proliferation. E–G. Germinating conidia. Scale bars: B–G = 10 μm.
Synonyms: Fusicladium crataegi Aderh., Ber. Deutsch. Bot. Ges. 20: 200. 1902.
Endostigme crataegi (Aderh.) Syd., Ann. Mycol. 21(3/4): 173. 1923.
Megacladosporium crataegi (Aderh.) Vienn.-Bourg., Les Champignons Parasites Pl. Cult. 1: 539. 1949.
Ascomata amphigenous, 80–140 μm diam, scattered, initially immersed, becoming erumpent, globose to subglobose, wall black, with a conspicuously papillate ostiole, surrounded by setae. Setae dark brown, 32–56 × 4–5 μm, setae wall 1–1.5 μm thick. Peridium 9–14 μm wide, 1-layered, composed of 1–3 rows of brown pigmented cells of textura angularis, cells 5–10 × 4–10 μm, cell wall 0.5–1.2 μm thick. Pseudoparaphyses numerous, filamentous, hyaline, evanescent when mature. Asci 45–55 × 8–11 μm, 8-spored, bitunicate, fissitunicate, broadly cylindrical, with a short, knob-like pedicel, each with an inconspicuous ocular chamber. Ascospores 10–15 × 4–6 μm (av. 12.8 × 5.2 μm, n = 20), colourless to pale brown, fusiform, with narrowly to broadly rounded ends, biseriate, 1-septate, slightly constricted at the septum, the upper cells mostly shorter and wider than the lower ones (length ratio: 4:7–3:4), smooth-walled.
In vitro on OA: Mycelium unbranched, 1.5–3 μm wide, septate, not constricted at septa, subhyaline, smooth, straight, walls neither thickened nor darkened. Conidiophores laterally arising from hyphae, erect, sometimes geniculate, unbranched, up to 135 μm long, (0–)7-septate, medium to dark brown, smooth, subcylindrical. Conidiogenous cells integrated, terminal, erect, 14.5–58.5(–98) × 2–3.5 μm, medium to dark brown, sometimes becoming hyaline towards the apex, proliferating sympodially, with several truncate loci, 1–1.5 μm wide, slightly thickened and darkened. Conidia solitary, straight, narrowly fusiform, 15–24.5 × 3–5 μm, often medianly 1-septate, sometimes 2-septate, not or slightly constricted at the septum, mostly widest in the middle or lower third, pale brown to brown, smooth, becoming tapered to both ends, walls not thickened, inconspicuous to somewhat darkened; hila truncate, 1–1.5 μm wide, slightly thickened or darkened, but not refractive.
Culture characteristics: Colonies spreading, somewhat erumpent, with sparse aerial mycelium and regular margins on OA, uneven, greyish (surface), margins dark brown; reverse fuscous-black. Colonies reaching 16 mm diam after 2 wk at 25 °C in the dark; colonies fertile.
Typus (Fusicladium crataegi): Germany, Thuringia, Steiger, on Crataegus laevigata (Rosaceae) (C. oxyacantha auct.), 15 Mar. 1902, H. Diedicke [Syd., Mycoth. Germ. 45] (HBG, lectotype, designated in Schubert et al. 2003: 37). Isolectotypes: B, BPI 423805, ILL 6195, IND-F-3788, JE, LE, MICH 15608, PH 5573, S-F-45734, WIS-F70209.
Additional materials examined: Germany, Müncheberg, on Sorbus aucuparia (Rosaceae) (CBS H-23599, culture CBS 367.35); on Crataegus sp. (Rosaceae) (cultures CBS 368.35, CBS 369.35).
Notes: CBS 367.35 and CBS 368.35 / CBS 369.35 were from different host genera, but all from members of Rosaceae collected in Germany. Morphologically, all of these isolates agree with the description of V. crataegi provided by Schubert et al. (2003). Thus, we identify the clade as V. crataegi. This species is sister to V. orbiculata (Fig. 2).
Venturia curviseta Peck, Rep. (Annual) New York State Mus. Nat. Hist. 35: 145. 1884 [1882]. Fig. 40.
Fig. 40.
Venturia curviseta (holotype NYSf 925) sexual morph. A. Ascoma scattered on the host surface. B, D, G. Broadly clavate to somewhat obclavate asci. C, F. Pale brown ascospores. E. Dark brown setae. Scale bars: A = 100 μm; B applies to B, D = 10 μm; C applies to C, F, G = 10 μm; E = 10 μm.
Synonym: Antennularia curviseta (Peck) M.E. Barr, Canad. J. Bot. 46: 848. 1968.
Ascomata hypophyllous, 50–100 μm diam, 60–93 μm high, gregarious or scattered, becoming superficial, globose to subglobose, wall black, with a conspicuous papillate ostiole, surrounded by long setae. Setae dark brown, 120–160 × 4–6 μm, setae wall 1–2 μm, base swollen, up to 7–9 μm diam, aseptate. Peridium 8–14 μm wide, 1-layered, composed of 1–2 rows of pigmented cells of textura angularis, cells 5–10 × 4–9 μm, cell wall 0.5–1 μm thick. Pseudoparaphyses 2–3 μm wide, hyaline, septate, evanescent when mature. Asci 40–65 × 9–13 μm (av. 52.5 × 10.7 μm, n = 20), 8-spored, bitunicate, fissitunicate, clavate, with a short, knob-like pedicel, each with an inconspicuous ocular chamber. Ascospores 11–15 × 4–5 μm (av. 12.9 × 4.3 μm, n = 20), cylindrical to oblong, pale olivaceous brown, with narrowly rounded ends, overlapping to biseriate at the base, 1-septate, the upper cells somewhat shorter than the lower ones (length ratio: 5:7–1:1), smooth-walled. Asexual morph unknown.
Typus: USA, New York, Albany, on fallen leaves of Nemopanthes mucronata (Aquifoliaceae), Jun. 1881, C.H. Peck (holotype NYSf 925).
Notes: Venturia curviseta was assigned to Antennularia based on its long, reflexed setae surrounding the apex of ascostromata, and ascospores being close to medianly septate (Barr 1968). The small-sized ascomata with setae, evanescent pseudoparaphyses, pale olivaceous brown, 1-septate ascospores agree with Venturia s. str.
Venturia ditricha (Fr.) P. Karst., Bidr. Känn. Finl. Nat. Folk 23: 188. 1873. Fig. 41.
Fig. 41.
Venturia ditricha (culture CBS 115426) sexual / asexual morph. A. Ascomata scattered on OA. B. Dark brown setae on ascoma. C–H. Sympodial conidiogenous cells giving rise to conidia. I. Fusiform to subcylindrical conidia. Scale bars: B–I = 10 μm.
Basionym: Sphaeria ditricha Fr., Syst. Mycol. 2(2): 515. 1823.
Synonyms: Exosporium ditrichum (Fr.) Link, Sp. Pl., Ed. 4, 6: 123. 1825.
Vermicularia ditricha (Fr.) Schwein. [as ‘ditricham’], Trans. Amer. Philos. Soc., n.s. 4: 228. 1832.
Sphaerella ditricha (Fr.) Auersw., Rabenh., Fungi Eur. Exs. (Klotzschii Herb. Viv. Mycol. Continuatio, Ed. Nova, Ser. Sec.), Cent. 10: no. 943, 1866 [Bot. Zeitung 24: 300, 1866; Hedwigia 5: 191, 1866].
Fusicladium betulae Aderh., Centralbl. Bakteriol., 2. Abth., 2: 57. 1896.
In vitro on OA: Sexual morph: Ascomata solitary, scattered, initially immersed or slightly erumpent, becoming superficial, dark black, globose, wall black, the ascomata covered with setae. Setae dark brown, 4–4.5 × 62–76 μm, aseptate, wall black. Asci not observed. Asexual morph: Mycelium branched or unbranched, 2–3.5 μm wide, septate, not constricted at septa, hyaline to pale brown, smooth, straight, walls thickened or darkened. Conidiophores laterally or terminally arising from darker hyphae, erect or geniculate-sinuous, sometimes branched, subcylindrical, 40–60.5(–72.5) × 3.5–5 μm, mainly aseptate or 1-septate, not constricted at septa, medium brown, smooth, walls somewhat thickened and slightly darkened-refractive. Conidiogenous cells terminal, integrated, geniculate or sometimes erect, subcylindrical, 25.5–46.5 × 4–4.5 μm, polyblastic, proliferating sympodially, brown to medium brown, with several loci, mostly truncate, 2–2.5 μm wide, not thickened, inconspicuous to somewhat darkened, but not refractive. Conidia solitary, mostly straight to rarely curved, fusiform, subcylindrical, 19–29.5 × 4.5–6.5 μm, mainly 1-septate, septum median or somewhat in the upper half, rarely 2(–3)-septate, often not constricted at septa, yellowish to brown, smooth, becoming rounded towards the apex, truncate at the base, hila flattened, 1.5–3 μm wide, slightly thickened and somewhat darkened.
Culture characteristics: Colonies spreading, somewhat erumpent, with moderate sparse aerial mycelium and regular margins on OA, greyish (surface), margins olivaceous; reverse fuscous-dark. Colonies reaching 12 mm diam after 2 mo at 25 °C in the dark, colonies fertile.
Typus: Sweden, on deciduous leaves of Betula (Betulaceae) [Fries, Scleromyc. Suec. 54, syntypes, e.g., B, UPS].
Additional materials examined: Finland, Kevo, on Betula pubescens var. tortuosa (Betulaceae), 1 Aug. 1992, M. Helander (dry culture CBS H-23625, cultures CBS 115426, CBS 118894). Italy, on Populus tremula (Salixaceae), collection date and collector unknown, isol. O. Servazzi (culture CBS 257.38).
Notes: Fusicladium betulae is the asexual morph of V. ditricha. CBS 115426 was morphologically comparable with the description provided by Sivanesan (1977). Schubert et al. (2003: 20–22, fig. 3) provided a detailed description of the asexual morph in vivo and neotypified the name F. betulae. This species is sister to V. peltigericola (Fig. 2).
Venturia elegantula Rehm, Hedwigia 24(6): 241. 1885. Fig. 42.
Fig. 42.
Venturia elegantula (type NY 00914439) sexual morph. A. Globose ascomata scattered on the host surface. B, E, F. Released, ellipsoid, olivaceous or brown ascospores. C, D. Somewhat obclavate asci with short pedicels. G. Long, dark brown seta. Scale bars: A = 200 μm; B–G = 10 μm.
Synonym: Gibbera elegantula (Rehm) Petr., Sydowia 1: 200. 1947.
Ascomata epiphyllous, 100–184 μm diam, scattered, initially immersed, becoming erumpent, globose, wall black, with a conspicuously papillate ostiole, surrounded by tapered setae. Setae dark brown, up to 200 μm long, 4–5 μm wide, aseptate. Pseudoparaphyses not observed. Asci 96–116 × 18–22 μm (av. 107.6 × 20.3 μm, n = 20), 8-spored, bitunicate, fissitunicate, clavate, with a short, knob-like pedicel, each with an inconspicuous ocular chamber. Ascospores 22–26 × 7–8 μm (av. 24.1 × 7.8 μm, n = 20), ellipsoid, olivaceous brown, overlapping to biseriate, 1-septate, the upper cells shorter and wider than the lower ones (length ratio: 11:13–1:1), wall verruculose. Asexual morph unknown.
Typus: Italy, Tyrol, Gampenhofe, Sulden, Ortler, on rotting leaves of Vaccinium myrtillus (Ericaceae), Jul. 1884, Rehm [Rehm, Ascomyc. 841, syntypes, e.g., B, S-F11555, 11556, also NY 914439 and PH 44546].
Venturia fagi M.E. Barr, Canad. J. Bot. 46: 816. 1968. Fig. 43.
Fig. 43.
Venturia fagi (holotype NY 00914440) sexual morph. A. Ascomata scattered on the host surface. B–F. Broadly cylindrical to subcylindrical asci with short, knob-like pedicels. G, K. Dark brown setae. H, I. Evanescent pseudoparaphyses and immature asci. J. Colourless ascospores. Scale bars: A = 100 μm; B–G, K = 10 μm; H–J = 5 μm.
Ascomata hypophyllous, 40–110 μm diam, scattered, initially immersed, becoming erumpent, globose, wall black, with a conspicuously papillate ostiole, surrounded by setae. Setae dark brown, 40–56 × 6–8 μm, setae wall 1–2 μm thick, swollen at the base, base up to 11 μm wide, septate. Peridium 1-layered, composed of 2 rows of pigmented cells of textura angularis, cells 6–9 × 5–6 μm, cell wall 1–1.5 μm thick. Pseudoparaphyses rare, 2–2.5 μm wide, hyphal, hyaline, septate, evanescent when mature. Asci 39–50 × 8–12 μm (av. 46.2 × 9.6 μm, n = 20), 8-spored, bitunicate, fissitunicate, broadly cylindrical to subcylindrical, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 11–13(–15) × 3–4 μm (av. 12.2 × 3.8 μm, n = 20), ellipsoid, hyaline, biseriate, 1-septate, constricted at the septum, the upper cells often shorter and broader than the lower ones (length ratio: 5:6–1:1), smooth-walled. Asexual morph unknown.
Typus: USA, Maine, near Baxter State Park, Abol Field, on overwintered leaves of Fagus grandifolia (Fagaceae), 18 Jul. 1962, M.E. Barr & H.E. Bigelow (holotype NY 00914440, isotype NY 00914441).
Notes: Barr (1968) described the ascospores of Venturia fagi as green, olivaceous or brown. No mature ascomata were observed in this study, and the ascospores were mostly hyaline.
Venturiafinlandica Crous, M. Shen & Y. Zhang ter, sp. nov. MycoBank MB831539.
Etymology: The epithet refers to Finland, the country where these isolates were collected.
Cultures sterile. Venturia finlandica (CBS 115442) differs from its closest phylogenetic neighbour V. minuta (CBS 479.61) (Fig. 2) by unique fixed alleles in four loci based on alignments of the separate loci deposited in TreeBASE (S24582), by 23 bp in ITS (5 %), 7 bp in LSU (1 %), 40 bp in tef1 (8 %), 31 bp in tub (14 %).
Culture characteristics: Colonies spreading, erumpent, with aerial mycelium and regular and smooth margins on OA, dark olivaceous brown (surface); reverse dark olivaceous brown to fuscous-black; on MEA dark brown (surface); reverse fuscous-black; on SNA olivaceous brown (surface); reverse dark olivaceous brown. Colonies reaching 29 mm diam after 2 wk on OA at 25 °C in the dark.
Typus: Finland, on Betula pubescens var. pumila (Betulaceae), collection date unknown, M. Helander (holotype CBS H-23733, culture ex-type CBS 115442 = CPC 3864).
Additional material examined: Finland, on Betula pubescens var. pumila (Betulaceae), 1 Jul. 1993, M. Helander (culture CBS 112703 = CPC 3865).
Notes: Venturia finlandica was collected from Betula pubescens var. pumila. According to multigene phylogenetic analysis, it forms a separate single clade distinguishing it from closely related species (Fig. 2). Venturia ditricha (CBS 115426), another Venturia species known from Betula, differs from V. finlandica (CBS 115442) by unique fixed alleles in four loci based on alignments of the separate loci deposited in TreeBASE (S24582), by 19 bp in ITS (4 %), 4 bp in LSU (1 %), 38 bp in tef1 (8 %), 17 bp in tub (10 %).
Venturia frangulae Krieger, Ann. Mycol. 7(6): 542. 1909. Fig. 44.
Fig. 44.
Venturia frangulae (syntype MICH 15149) sexual morph. A. Ascomata scattered on the host surface. B–F. Asci and immature asci. G. Dark brown seta. H. Released, pale brown ascospores. I. Evanescent pseudoparaphyses and with mature and immature asci. Scale bars: A = 200 μm; B = 20 μm; C–I = 10 μm.
Ascomata hypophyllous, 43–85 μm diam, gregarious, scattered or solitary, initially immersed, becoming erumpent, globose, wall black, with a conspicuously papillate ostiole, surrounded by setae. Setae dark brown, 24–50 × 4–7 μm, setae wall 1–1.5 μm thick, swollen at the base, base up to 14 μm wide, septate. Peridium 9–12 μm wide, 1-layered, composed of 1–2 rows of pigmented cells of textura angularis, cells 4–13 × 3–12 μm, cell wall 0.5–1 μm thick. Pseudoparaphyses rare, evanescent when mature. Asci 30–46(–62) × 8–12 μm (av. 38.5 × 9.8 μm, n = 20), 8-spored, bitunicate, fissitunicate, broadly clavate to obclavate, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 10.5–13.5 × 4–6 μm (av. 12.2 × 5.1 μm, n = 20), fusiform to broadly fusiform, pale brown, biseriate, 1-septate, with the septum in the upper half, slightly constricted at the septum, with a narrowly rounded to somewhat pointed ends, the upper cells shorter and wider than the lower ones (length ratio: 6:7–1:1), smooth-walled. Asexual morph unknown.
Typus: Germany, Königstein, on fallen leaves of Frangula alnus (Rhamnaceae), 14 Apr. 1898, W. Krieger (Krieger, Fungi Saxon. Exs. 2068, syntypes MICH 15149, K(M) 189239).
Venturia fuliginosa Y. Zhang ter & J.Q. Zhang, Mycosphere 7: 1295. 2016.
Description and illustration: Shen et al. (2016).
Typus: China, Heilongjiang Province, Yichun City, Fenglin Nature Reserve, on leaves of Salix capitata (Salicaceae), 27 Aug. 2014, Y. Zhang & Y.P. Zhou (holotype HMAS 247007, culture ex-type CGMCC 3.18370 = CBS 142241 = BJFCCC 140827-14).
Note: The species is sister to V. albae (Fig. 1) and V. chlorospora (Fig. 2).
Venturia gaultheriae Ellis & Everh., J. Mycol. 1: 153, 1885. Fig. 45.
Fig. 45.
Gibbera gaultheriae (isotype MICH 15150) sexual morph. A. Ascomata scattered on the host surface. B. Section of an ascoma. C–E. Pale brown, broadly cylindrical to somewhat obclavate asci. F. Brown seta. G–I. Released, fusiform, pale brown ascospores. Scale bars: A = 100 μm; B = 20 μm; C–F = 10 μm; G–I = 5 μm.
Synonym: Gibbera gaultheriae (Ellis & Everh.) M.E. Barr, Canad. J. Bot. 46: 841, 1968.
Protoventuria gaultheriae (Ellis & Everh.) M.E. Barr, Sydowia 41: 37, 1989.
Ascomata epiphyllous, 40–80 μm diam, scattered or solitary, becoming superficial, globose, wall black, with a conspicuously papillate ostiole, surrounded by setae. Setae dark brown, 20–50 × 5–6 μm, setae wall 1–1.5 μm thick, base swollen, up to 7–8 μm thick, septate. Peridium 7–11 μm wide, 1-layered, composed of 1–2 rows of pigmented cells of textura angularis, cells 5–10 × 3–9 μm, cell wall 0.5–1 μm thick. Pseudoparaphyses not observed. Asci 31–45 × 9–11 μm (av. 57.6 × 14.8 μm, n = 10), 8-spored, bitunicate, fissitunicate, broadly clavate or broadly obclavate, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 12–14 × 4–4.5 μm (av. 18.6 × 6.6 μm, n = 10), fusiform, pale brown, overlapping to biseriate, especially at the base, 1-septate, slightly constricted at the septum, with narrowly to broadly rounded ends, the upper cells often shorter and wider than the lower ones (length ratio: 3:4–1:1), smooth-walled. Asexual morph unknown.
Typus: USA, New Jersey, Gloucester, on leaves of Gaultheria procumbens (Ericaceae), Jul. 1884, Ellis & Everhart (holotype NY 00938215 (not seen), isotype MICH 15150).
Venturia helvetica Nüesch, Phytopathol. Z. 39: 346. 1960.
Description and illustration: Zhang et al., 2016a, Zhang et al., 2016b.
Typus: Switzerland, Kt. Graubünden, on decaying leaves of Salix helvetica (Salicaceae), Jul. 1959 (holotype Reinkultur Stamm ETH Nr. 2571).
Additional materials examined: Switzerland Kt. Graubünden, Albulapasshöhe, on Salix helvetica (Salicaceae), 2 Jul. 1959, J. Nüesch (ETH 2571, IMI 163990, culture CBS 474.61); Val Tuors, on S. helvetica, 1 Jul. 1959, J. Nüesch (ETH 2587, culture CBS 475.61).
Note: The species is sister to V. caesiae / minuta (Fig. 2).
Venturia inaequalis (Cooke) G. Winter, Mycoth. Univ., Cent. 3: no. 261. 1875. Fig. 46, Fig. 47.
Fig. 46.
Venturia inaequalis (NY 00914442) sexual morph. A. Ascomata scattered on the host surface. B, C. Evanescent pseudoparaphyses. D–H. Broadly cylindrical to somewhat obclavate asci. I–L. Olivaceous brown, asymmetrical ascospores. M, N. Dark brown setae. Scale bars: A = 200 μm; B–N = 10 μm.
Fig. 47.
Venturia inaequalis (CGMCC 3.18372) asexual morph. A, B. Leaf spots caused by Venturia inaequalis (from herbarium specimen). C. Conidiophores. D–F. Annellidic conidiogenous cells giving rise to conidia. G, H. Released subpyriform conidia. Scale bars: C, G, H = 20 μm; D–F = 10 μm.
Basionym: Sphaerella inaequalis Cooke, J. Bot. (London) 4: 248. 1866. Nom. cons. prop. (Rossman et al. 2018).
Synonyms: Endostigme inaequalis (Cooke) Syd., Ann. Mycol. 21(3/4): 171. 1923.
Spilosticta inaequalis (Cooke) Petr., Ann. Mycol. 38(2/4): 193. 1940.
Didymosphaeria inaequalis (Cooke) Niessl, Fungi Eur. Exsicc.: no. 2663. 1881.
Spilocaea pomi Fr., Novit. Fl. Svec. 5 (cont.): 79. 1819 (Nom. sanct., Fr., Syst. mycol. 3: 504. 1832).
Fusicladium pomi (Fr.) Lind, Dan. Fung.: 521. 1913.
Sphaeria cinerascens Fuckel, Fungi Rhen. Exs., Fasc. 9: no. 824, 1863. Nom. illeg., Art. 53.1, non Schwein., 1832.
Cladosporium dendriticum Wallr., Fl. Crypt. Germ. 2: 169. 1833.
Fusicladium dendriticum (Wallr.) Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 357. 1870.
Passalora dendritica (Wallr.) Sacc., Michelia 1(no. 2): 265. 1878.
Sphaeria cinerascens Fuckel, Fungi Rhen. Exs., Fasc. 9: no. 824. 1863. Nom. illeg., Art. 53.1.
Sphaerella cinerascens Rabenh. (as “(Fuckel) Rabenh.”), Fungi Eur. Exs. (Klotzschii Herb. Viv. Mycol. Continuatio, Ed. Nova, Ser. Sec.), Cent. 9: no. 845, 1865 [Bot. Zeitung 23: 288, 1865].
Mycosphaerella cinerascens (Rabenh.) Vestergr., Bot. Not.: 267. 1897.
Venturia inaequalis var. cinerascens (Rabenh.) Aderh., Hedwigia 36(2): 82. 1897.
Endostigme cinerascens (Rabenh.) Jørst., Nytt Mag. Naturvidensk 84: 252. 1945.
Spilosticta cinerascens (Rabenh.) Petr., Sydowia 1(4–6): 197. 1947.
Fusicladium dendriticum var. opuli Thüm., Fungi Austr., Cent. 11, no. 1091 (1873). Nom. inval., Art. 38.1(a) (Shenzhen).
Napicladium soraueri Thüm., Mycoth. Univ., Cent. I, no. 91. 1875.
Fusicladium dendriticum var. soraueri (Thüm.) Sacc., Syll. Fung. 4: 346. 1886.
Fusicladium pyrorum var. amelanchieris Sacc., Syll. Fung. 4: 346. 1886.
Fusicladium dendriticum f. microsperma Roum., Fungi Sel. Exs., Cent. 61, no. 5592. 1891.
Fusicladium dendriticum var. eriobotryae Scalia, Boll. Accad. Gioenia Sci. Nat. Catania 70: 5. 1901.
Leaf spots amphigenous, subcircular to somewhat irregular, initially pale olivaceous brown, later black-grey, with pale brown halo, 5–10 mm diam. Ascomata amphigenous, 120–225 μm diam, scattered, initially immersed, becoming erumpent, globose, wall black, with papillate ostiole, with or without setae. Setae dark brown, 28–65 × 5–7 μm, setae wall 1–2 μm thick, base swollen, up to 14 μm wide. Peridium 10–18 μm wide, 1-layered, composed of 3–4 rows of pigmented cells of textura angularis, cells 5–10 μm wide, cell wall 0.5–1.5 μm thick. Pseudoparaphyses 2–4 μm wide, septate, hyaline, evanescent when mature. Asci 56–79 × 11–15 μm (av. 63.4 × 12.3 μm, n = 20), 8-spored, bitunicate, fissitunicate, broadly cylindrical, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 11.5–16 × 5–7 μm (av. 14.2 × 6.2 μm, n = 20), fusiform to broadly clavate, olivaceous brown, uniseriate to partially overlapping at the top, biseriate near the base, 1-septate, with septum in the upper third, the upper cell tapers toward the apex, the lower cell with a broadly to narrowly rounded base, the upper cells shorter than the lower cells (length ratio: 3:5–7:8), smooth-walled. Asexual morph: Mycelium amphigenous, gregarious, olivaceous brown to black, mostly subcircular. Stromata variable in size, pale olivaceous to brown, angular or circular, composed of thin-walled parenchyma cell, cells 4–7 μm diam. Conidiophores reduced to conidiogenous cells. Conidiogenous cells solitary or sparsely gregarious, arising from stromata or hyphae, straight or flexuous, unbranched, 16–35 × 5–6 μm, 0(–1)-septate, pale to medium brown, verruculose, wall slightly thickened, base swollen. Conidia solitary, subpyriform to obclavate, 18–29 × 6–8 μm, pale brown to brown, 0(–1)-septate, not constricted at the septum, becoming tapered towards the apex, base truncate, 4–5 μm wide, slightly refractive, not darkened.
Typus: UK, England, Surrey, Shere, on Sorbus aria (Rosaceae), Apr. 1866, Herb. Cooke (lectotype of Sphaerella inaequalis K(M) 237177, designated by Rossman et al. 2018; isolectotypes of Sphaerella inaequalis BPI 798917, K(M) Nos. 237173, 237174, 237175, 237176 & 237178). Sweden, Malus domestica (Rosaceae) (epitype specimen designated here as metabolically inactive culture, CBS 120627, MBT391376, culture ex-epitype CBS 120627).
Additional materials examined: China, Jilin, Yushu, on leaves of Malus sp. (Rosaceae), 27 Jul. 2015, D. Ma (culture CGMCC 3.18372 = BJFCC 150727-1). South Africa, on Malus domestica (Rosaceae), collection date and collector unknown (cultures CBS 120625 = SA 14, CBS 120627 = SU 05 AL GAL3). Switzerland, on leaves of Cotoneaster integerrima (Rosaceae), 11 Jun. 1953, collector unknown (type of Venturia inaequalis f. sp. cotoneasteris ZT 57113). Czech Republic, Brünn, falling leaves of Sorbus torminalis (Rosaceae), June of unknown year, Niessl (isotypes of Sphaerella inaequalis NY 00914442, NY 00914443, NY 00914444).
Note: The species is sister to V. orbiculata and V. pyrina (Fig. 1) and to V. oleaginea (Fig. 2).
Venturia lonicerae Sacc., Syll. Fung. 1: 589. 1882.
Synonym: Sphaeria lonicerae Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 111. 1870. Nom. illeg., Art. 53.1, non Sowderby 1803. Nom. illeg., Art. 53.1, non Sphaeria lonicerae Sowerby 1803.
Description and illustration: Sivanesan (1977).
Typus: Germany, Hessen, Oestrich, on Lonicera xylosteum (Caprifoliaceae) [Fuckel, Fungi Rhen. Exs. 1688, syntypes, e.g., B, BPI 613122, HAL, S-F90852, 908554) (not seen).
Material examined: Switzerland, Kt. Wallis, Grächen, on Lonicera coerulea (Caprifoliaceae), 1 Jun. 1953, E. Müller (IMI 163997, culture CBS 445.54).
Notes: Isolate CBS 445.54 proved to be sterile in culture. This species is sister to V. polygoni-vivipari (Fig. 2).
Venturia maculans Peck, Rep. (Annual) New York State Mus. Nat. Hist. 28: 81. 1876 [1875]. Fig. 48.
Fig. 48.
Venturia maculans (holotype NYSf1816) sexual morph. A. Ascomata scattered on the host surface. B–E. Broadly subcylindrical to obclavate asci. F. Evanescent pseudoparaphyses. G. Released, brown ascospores. H. Dark brown setae. Scale bars: A = 200 μm; B–H = 10 μm.
Ascomata hypophyllous, 86–145 μm diam, scattered or solitary, initially immersed, becoming erumpent, globose to subglobose, wall black, with a conspicuous papillate ostiole, surrounded by setae. Setae dark brown, 54–90 × 5–6 μm, setae wall 1–1.5 μm thick, 0(–1)-septate, base swollen, up to 9 μm wide. Peridium 15–28 μm thick, 1-layered, composed of 2–3 rows of pigmented cells of textura angularis, cells 6–13 × 5–10 μm, cell wall 1–1.2 μm thick. Pseudoparaphyses rare, 2–3 μm wide, septate, hyaline, evanescent when mature. Asci 43–62 × 11–16 μm (av. 54.1 × 12.8 μm, n = 20), 8-spored, bitunicate, fissitunicate, broadly cylindrical to obclavate, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 12–16 × 4–6.5 μm (av. 14.2 × 5.4 μm, n = 20), fusiform, olivaceous brown, overlapping to biseriate near the base, 1-septate, deeply constricted at the septum, the upper cells longer and wider than the lower ones (length ratio: 13:11–9:5), smooth-walled. Asexual morph unknown.
Typus: USA, New York, Albany, Karner, on fallen leaves of Betula populifolia (Betulaceae), Jun. 1870?, C.H. Peck (holotype NYSf1816).
Venturia maculiformis (Desm.) G. Winter [as “maculaeformis”], Rabenh. Krypt. -Fl., Ed. 2, 1(2): 435. 1885. Fig. 49.
Fig. 49.
Venturia maculiformis (HMAS 243785) sexual morph. A. Ascomata scattered on the host surface. B–I. Oblong to obclavate asci. J–O. Released, pale brown to olivaceous brown, 1-septate ascospores. Scale bars: A = 200 μm; B–I = 20 μm; J–O = 10 μm.
Basionym: Dothidea maculiformis Desm. [as “maculaeformis”], Ann. Sci. Nat., Bot. 3, 8: 176. 1847.
Synonyms: Stigmatea maculiformis (Desm.) Fr., Summa Veg. Scand. 2: 421. 1849. Nom. illeg., Art. 53.1.
Spilosticta maculiformis (Desm.) Petr. [as “maculaeformis”], Hedwigia 65: 241. 1925.
Sphaeria microspila Berk. & Broome, Ann. Mag. Nat. Hist., ser. 3, 7: 455. 1861.
Laestadia epilobiana Sacc., Syll. Fung. 1: 429. 1882.
Ascomata hypophyllous, 60–150 μm diam, solitary, scattered or in small groups of 2–3, initially immersed, becoming erumpent, globose to subglobose, wall black, with a conspicuous papillate ostiole. Setae not observed. Peridium 8–12 μm thick, 1-layered, composed of (1–)2–3 rows of pigmented cells of textura angularis, cells 4–8 μm wide, cell wall 0.8–1.5 μm thick. Pseudoparaphyses rare, evanescent when mature. Asci 30–50 × 9–12 μm (av. 48.5 × 10.5 μm, n = 20), 8-spored, bitunicate, fissitunicate, oblong to somewhat obclavate, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 9–14 × 3–5 μm (av. 12.8 × 3.8 μm, n = 20), pale olivaceous brown, overlapping to biseriate near the base, oblong to fusiform, with broadly to narrowly rounded ends, 1-septate, slightly constricted at the septum, the upper cells somewhat longer and wider than the lower ones, smooth-walled. Asexual morph unknown.
Typus: France, on autumn leaves of Epilobium montanum (Onagraceae) (not seen).
Material examined: UK, England, Surrey, on ?Epilobium montanum (Onagraceae), 13 Jul. 2003, B.M. Spooner (HMAS 243785).
Notes: Venturia maculiformis is morphologically comparable with V. muelleri, V. rumicis and V. rhamni, which have semi-immersed ascomata lacking setae, and ascospores with a septum in the lower half. The small-sized ascomata (40–60 μm) of V. muelleri (Zhang et al., 2016a, Zhang et al., 2016b), however, are distinguishable from the other three species. The asci and ascospores of V. rumicis are longer and wider than V. maculiformis, and asci of V. rhamni are also longer than those of V. maculiformis (Zhang et al., 2016a, Zhang et al., 2016b).
Venturia mandshurica M. Morelet [as “mandshuria”], Ann. Soc. Sci. Nat. Archéol. Toulon Var. 45(3): 219. 1993.
Synonyms: Pollaccia mandshurica M. Morelet, Ann. Soc. Sci. Nat. Archéol. Toulon Var. 45: 218. 1993.
Fusicladium mandshuricum (M. Morelet) Ritschel & U. Braun, Schlechtendalia 9: 62. 2003.
Description and illustration: Schubert et al. (2003).
Typus: China, Liaoning, on leaves and branches of Populus simonii × Populus nigra (Salicaceae), 17 Jun. 1992, M. Morelet (holotype, Laboratoire de Pathologie Forestière de Nancy, PC (PFN 1466)) (not seen); on P. simonii, 20 Apr. 1993 (epitype CBS H-19912, designated in Crous et al. 2007b, culture ex-epitype CBS 112235 = CPC 3639 = MPFN 307) (examined).
Note: The species is sister to V. populina (Fig 1, Fig 2).
Venturia martianoffiana (Thüm.) Y. Zhang ter & J.Q. Zhang, Stud. Mycol. 86: 205. 2017. Fig. 50, Fig. 51.
Fig. 50.
Venturia martianoffiana (BJFU 150828-1) asexual morph. A, B. Leaf spots caused by V. martianoffiana (from herbarium specimen). C–F, H. Sympodial conidiophores and conidiogenous cells. G. Fusiform, non-septate or 1-septate conidia. I. Conidia in chains. Scale bars: C–I = 10 μm.
Fig. 51.
Venturia martianoffiana (culture CGMCC 3.18375) asexual morph. A. Colony growing on MEA. B. Hyphae and conidial chains. C–G. Conidiophores with conidiogenous loci. H. Geniculate-sinuous hyphae. I, L, M. Conidiophore with branching conidial chains. J. Ramoconidia and conidia. K. Conidiophores reduced to conidiogenous cells. Scale bars: B–E, H, J, K = 20 μm; F, G, I, L, M = 10 μm.
Basionym: Cladosporium martianoffianum Thüm., Bull. Soc. Imp. Naturalistes Moscou 55: 74. 1880.
Synonyms: Fusicladium martianoffianum (Thüm.) K. Schub. & U. Braun, I.M.I. Descript. Fungi Bact. 152 (nos 1511–1520): [10]. 2002.
Fusicladium martianoffianum var. sinensis M. Morelet, Bull. Mens. Soc. Linn. Lyon 75: 179. 2006.
Sexual morph: Unknown. Asexual morph: Leaf spots amphigenous, subcircular to angular, 1–9 mm wide, often confluent, diffuse, numerous, dark brown to black, with an irregular margin. Colonies amphigenous, caespitose, olivaceus dark brown to blackish. Mycelium mainly subcuticular. Stromata variable in size, composed of pale olivaceous to brown, angular to rounded, thick-walled, pseudoparenchymatous cells, 4–8 μm diam. Conidiophores solitary or loosely fasciculate, arising from stromata or from hyphae, erect, straight, sometimes flexuous at the apex, unbranched or apically branched, 17–30 × 5–7 μm, 0–1-septate, pale to medium brown, smooth, with somewhat thickened walls. Conidiogenous cells integrated, terminal or intercalary, or conidiophores reduced to conidiogenous cells, 14–27 × 4.5–6 μm, with a single or several denticle-like conidiogenous loci, proliferation sympodial, loci unthickened, not or only somewhat darkened-refractive, 1.5–2.5 μm wide. Conidia in simple or branched chains, 15–31 × 4.5–6 μm, pyriform, ellipsoid, subcylindrical, fusiform, pale brown, 0–1(–3)-septate, smooth, attenuated towards apex and base, apex mostly truncate, occasionally rounded or pointed, base truncate; hila 1.5–3 μm wide, not thickened, but somewhat darkened-refractive. In culture on MEA: Mycelium unbranched or only sparingly branched, 2–5 μm wide, septate, not constricted at septa, subhyaline to pale brown, smooth, walls unthickened or almost so. Conidiophores laterally arising from hyphae, erect, straight to somewhat flexuous, sometimes geniculate, unbranched, 21–65 × 4–6 μm, aseptate or septate, pale or medium brown, smooth, walls somewhat thickened, sometimes only as short lateral conical prolongations of hyphae, occasionally irregular in shape. Conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, sometimes geniculate, 21–36 μm long, proliferation sympodial, with 1–4 denticle-like loci, broadly truncate, 1.5–2(–2.5) μm wide, unthickened, somewhat refractive or darkened. Ramoconidia present, 18–26 × 4–5 μm, 0–2-septate, pale to medium brown, with a broadly truncate base, 3–4 μm wide, usually with 2–3 denticle-like apical loci. Conidia catenate, formed in unbranched or loosely branched chains, straight to sometimes curved, cells sometimes irregularly swollen, fusiform, subcylindrical, sometimes obpyriform, 17–29 × 4–6 μm, pale to medium brown, smooth, 0(–2)-septate, walls slightly thickened, sometimes attenuated towards apex and base; hila broadly truncate, 2–2.2 μm wide, not or only slightly thickened, somewhat darkened-refractive; microcyclic conidiogenesis present.
Culture characteristics: Colonies erumpent, spreading, with abundant aerial mycelium and feathery to smooth margins on PDA; grey olivaceous (surface), reverse dark olivaceous. Colonies reaching 14 mm diam after 1 mo at 25 °C in the dark; colonies fertile.
Typus: Russia, Sibiria, Minussinsk, near river Jenissei, on living leaves of Populus laurifolia (Salicaceae), Aug. 1879, N. Martianoff (M: lectotype designated in Schubert et al. 2003: 64; isolectotypes, Thüm., Mycoth. Univ. 2067, BPI 427257, 427258, HAL, ILL 77739) (not seen).
Materials examined: China, Shanxi, Yangling, on leaves of Populus sp. (Salicaceae), 4 Nov. 2015, Y.F. Zhang (culture CGMCC 3.18377 = BJFC 150904-1); Liaoning, Dengta city, Wan Bao Qiao, Bridge Street Road Village, on leaves of Populus sp., 27 Aug. 2014, Y.F. Zhang (BJFU 150828-1, culture CGMCC 3.18375 = BJFC 150828-1).
Note: This species is sister to V. phaeosepta (Fig. 2).
Venturia minuta M.E. Barr, Canad. J. Bot. 46: 815. 1968.
Replaced synonym: Venturia microspora Nüesch, Phytopathol. Z. 39: 347. 1960. Nom. illeg., Art. 53.1, non Venturia microspora Speg. 1887.
Description and illustration: Barr (1968).
Typus: Switzerland, Aareaue bei Rubigen, on decaying leaves of Salix nigricans (Salicaceae), Kt. Bern, 30 May 1959, collector unknown (holotype ETH Nr. M 523, culture ex-type ETH 523 = IMI 163991 = CBS 478.61).
Additional material examined: Switzerland, on Salix cinerea (Salicaceae), 20 May 1959, J. Nüesch (culture ETH 525 = CBS 479.61).
Notes: Venturia minuta was introduced to replace the illegitimate V. microspora. Unfortunately, both isolates representing V. minuta (CBS 478.61, CBS 479.61) proved to be sterile in culture. Venturia minuta is sibling to V. caesiae (Fig. 2).
Venturia nashicola S. Tanaka & S. Yamam., Ann. Phytopathol. Soc. Japan 29: 136. 1964.
Synonym: Fusicladium nashicola K. Schub. & U. Braun, Schlechtendalia 9: 65. 2003.
Description and illustration: Schubert et al. (2003), Tanaka & Yamamoto (1964).
Typus (of Fusicladium nashicola): Japan, Tsukuba, Ibaraki, Orchard of the National Institute of Agro-Environmental Sciences, on leaves of Pyrus pyrifolia (Rosaceae), 18 Aug. 2000, H. Ishii (holotype HAL 1749 F).
Additional materials examined: Japan, on Pyrus serotina var. culta (Rosaceae), collection date and collector unknown (cultures CBS 793.84, CBS 794.84).
Notes: Unfortunately, both isolates (CBS 793.84, CBS 794.84) proved to be sterile in culture. This species is sister to V. pyrina (Fig. 2).
Venturia oleaginea (Castagne) Rossman & Crous, IMA Fungus 6: 520. 2015. Fig. 52.
Fig. 52.
Venturia oleaginea (culture CBS 113539) asexual morph. A. Colony on OA. B, C, E. Conidiogenous cells giving rise to conidia. D. Hypha with conidiogenous loci and conidia. F–H. Released conidia. Scale bars: B–H = 10 μm.
Basionym: Cycloconium oleagineum Castagne, [as “oleaginum”], Cat. Pl. Marseille: 220. 1845.
Synonyms: Spilocaea oleaginea (Castagne) S. Hughes, Canad. J. Bot. 31: 564. 1953.
Fusicladium oleagineum (Castagne) Ritschel & U. Braun, Schlechtendalia 9: 70. 2003.
Sexual morph: unknown. In vitro on OA: Mycelium unbranched or only sparingly branched, 2–3.5 μm wide, septate, not constricted at septa, subhyaline or pale brown, smooth or occasionally verrucose, straight, walls somewhat thickened or darkened. Conidiophores reduced to conidiogenous cells, sometimes only as short lateral conical prolongations of hyphae. Conidiogenous cells arising terminally or laterally from darker hyphal cells, subcylindrical, straight, 19–35.5 × 3–4.5 μm, monoblastic, medium to dark brown, walls somewhat thickened and darkened, often with 1–2 conidiogenous loci, broadly truncate, 2–3.5 μm wide, somewhat thickened and darkened. Conidia solitary, straight or occasionally slightly curved, subcylindrical, fusiform, 24.5–44 × 6–8 μm, 1-septate, septum median or somewhat in the lower half, sometimes 2-septate, not constricted or rarely constricted at the septa, medium to dark brown, dark olivaceous, smooth, walls slightly thickened and darkened, becoming attenuated and hyaline towards the apex, broadly truncate at the base; hila 3.5–5.5 μm wide, somewhat thickened and darkened. Description and illustration in vivo, see Schubert et al. (2003: 70, 71, fig. 35).
Culture characteristics: Colonies spreading, smooth, somewhat erumpent, with moderate to sparse aerial mycelium and margins irregular on OA, uneven, dark brown to fuscous-black (surface), margins greyish white; reverse fuscous-black. Colonies reaching 12 mm diam after 2 mo at 25 °C in the dark; colonies fertile.
Typus: France, Marseille, on leaves of Olea europaea (Oleaceae), Castagne (lectotype STR, designated by Schubert et al. 2003: 70; isolectotypes M and IMI 69757, slide).
Additional materials examined: Morocco, on Olea europaea (Oleaceae), collection date and collector unknown (culture CBS 120629). New Zealand, Parkhurst, Northland, on O. europaea, Jun. 2003, C.F. Hill (culture CBS 113427). Portugal, unknown substratum, 1982, Branquinho d'Oliveira (dried culture CBS H-23606, culture CBS 113539 = UPSC 1329).
Notes: Isolate CBS 113539 was collected from Portugal on an unknown host, but is morphologically comparable with Fusicladium oleagineum (as Venturia oleaginea) according to Schubert et al. (2003). In addition, CBS 113427 (New Zealand) and CBS 120629 (Morocco) are both from Olea europaea, which is the same host as in the original description of V. oleaginea from France. This species is sister to V. inaequalis (Fig. 2).
Venturia orbicula (Schwein.) Cooke & Peck, Rep. (Annual) New York State Mus. Nat. Hist. 25: 105. 1873. [1872]. Fig. 53.
Fig. 53.
Venturia orbicula (lectotype NYSf 2176) sexual morph. A. Ascomata scattered on the host surface. B, D–F. Oblong to somewhat obclavate asci. C. Evanescent pseudoparaphyses. G, H. Released, fusiform, 1-septate ascospores. I. Dark brown setae. Scale bars: A = 200 μm; B–I = 10 μm.
Basionym: Sphaeria orbicula Schwein., Trans. Amer. Philos. Soc., n.s. 4: 224. 1832. [1834].
Ascomata hypophyllous, 42–70 μm diam, gregarious or scattered on circular spots, superficial, globose to subglobose, wall black, with a conspicuous papillate ostiole, surrounded by setae. Setae dark brown, 38–69 × 5–7 μm, wall 1–1.5 μm wide at the base, septate. Peridium 1-layered, composed of one row of pigmented cells of textura angularis, cells 5–12 × 4–8 μm, cell wall 1 μm thick. Pseudoparaphyses 2–4 μm wide, septate, hyaline. Asci 33–45 × 10–12 μm (av. 38.2 × 10.6 μm, n = 10), 8-spored, bitunicate, fissitunicate, broadly cylindrical to somewhat obclavate, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 11–13 × 4–6 μm (av. 11.8 × 5 μm, n = 20), fusiform to narrowly fusiform, pale olivaceous brown to brown, obliquely overlapping to biseriate near the base, 1-septate, constricted at the septum, the upper cells shorter and wider than the lower ones (length ratio: 6:5–1:2), smooth-walled. Asexual morph unknown.
Typus: USA, Rensselaer, Lake Sander, on the fallen leaves of Quercus montana (as Quercus monticola, Fagaceae), Jun. of unknown year, C.H. Peck (lectotype NYS-F- 002176).
Venturia orbiculata (Desm.) U. Braun, Schlechtendalia 36: 66 (2019).
Synonyms: Cladosporium orbiculatum Desm., Ann. Sci. Nat., Bot., Sér. 3, 11: 275, 1849.
Fusicladium orbiculatum (Desm.) Thüm., Fungi Austr., Cent. VIII, no. 774.1873.
Passalora dendritica var. orbiculata (Desm.) Berk., in Sacc., Mycoth. Ven., Cent. XII, no. 1246. 1876 [Michelia 1: 265, 1878].
Fusicladium dendriticum var. orbiculatum (Desm.) Sacc., Syll. Fung. 4: 345,.1886.
Misapplied name: Venturia aucupariae (Plowr.) Rostr. [as “(Lasch)”], Plantepatologi: 466, 1902.
Materials examined: Germany, Müncheberg, on Sorbus aucuparia var. moravica (Rosaceae), collection date and collector unknown, isol. Jul. 1935, M. Schmidt (cultures CBS 365.35, CBS 366.35).
Notes: Isolates CBS 365.35 and CBS 366.35 were sterile. The species is sister to V. pyrina (Fig. 1) and to V. crataegi (Fig. 2).
Venturia peltigericola (Crous & Diederich) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831592. Fig. 54.
Fig. 54.
Venturia peltigericola (culture CBS 370.35) asexual morph. A. Colony on OA. B–D. Sympodial conidiogenous loci. E. Conidiogenous cell giving rise to conidia. F. Conidia in chain. G–I. Conidia and germinating conidia. Scale bars: B–E, G–I = 10 μm; F = 20 μm.
Basionym: Fusicladiumpeltigericola Crous & Diederich, Persoonia 25: 129. 2010.
In vitro on OA: Mycelium mostly branched, 2–3.5 μm wide, septate, unconstricted at septa, pale brown to brown, smooth, walls unthickened, sometimes hyphal cells swollen. Conidiophores laterally arising from hyphae, solitary, erect, straight to geniculous-sinuous, subcylindrical, 23–75 × 3.5–5 μm, unbranched, 1–3-septate, unconstricted at septa, brown to medium brown, smooth, walls somewhat thickened but not darkened-refractive. Conidiogenous cells terminal, integrated, geniculate-sinuous or straight, subcylindrical, 14–45.5 × 2.5–4 μm, polyblastic, proliferating sympodially, brown to medium brown, smooth; conidiogenous loci flattened, 2.5–3 μm wide, inconspicuous to somewhat darkened, but not refractive, not appearing thickened. Ramoconidia present, subcylindrical, 18.5–21.5 × 4.5–5 μm, medianly 1-euseptate, relatively thick-walled, medium brown, finely verruculose, basal hilum flattened, somewhat darkened, 2–2.5 μm wide, with one to several sympodial, apical loci; frequently with a lateral branch up to 20 μm long, 3–4 μm wide. Conidia catenate, proliferating in sympodially to form short chains of conidia, straight or slightly curved, subcylindrical, 16.5–24.5 × 4–5.5 μm, (0–)1-euseptate, septum mostly in the upper third of the conidium, rarely 2-septate, medium brown to brown, smooth, finely verruculose, apex obtusely rounded or flattened; hila flattened, 1.5–3.5 μm wide, somewhat darkened, but not thickened.
Culture characteristics: Colonies spreading, smooth, somewhat erumpent, with aerial mycelium and regular margins on OA, fuscous-black (surface); reverse fuscous-black. Colonies reaching 25 mm diam on OA after 2 wk at 25 °C in the dark; colonies fertile.
Typus: Luxembourg, on Peltigera rufescens (Peltigeraceae), May 2008, P. Diederich (holotype CBS-H 20487, culture ex-type CBS 128206 = CPC 15252).
Additional materials examined: Germany, Müncheberg, on Betula verrucosa (Betulaceae), Jul. 1935, M. Schmidt (cultures CBS 370.35, CBS 371.35).
Notes: Isolates CBS 370.35 and CBS 371.35 are in the same clade (Fig. 2) as the ex-type isolate of Fusicladium peltigericola (CBS 128206, Crous et al. 2010), but occur on different hosts. These isolates, however, appear to represent a morphologically distinct species as F. peltigericola has larger conidia. Additional loci will have to be sequenced to resolve this issue. Venturia peltigericola is sister to V. ditricha (Fig. 2) and not sister to a specific species in Fig. 1.
Venturia phaeosepta Y. Zhang ter & J.Q. Zhang, Stud. Mycol. 86: 205. 2017.
Description and illustration: Marin-Felix et al. (2017).
Typus: China, Henan province, Puyang City Academy Experimental Farm, on leaves of Populus × euramericana (Salicaceae) cv. 74/76 (sect. Aigeiros), 20 May 2015, W. He (holotype HMAS 246998, culture ex-type CGMCC3.18368); Y.F. Zhang, 20 Jun. 2015 (paratype, HMAS 246999, CGMCC3.18371); 6 Aug. 2015 (paratype, HMAS 247000, CGMCC3.18373); 7 Aug. 2015 (paratype, HMAS 247002, CGMCC3.18374); 8 Aug. 2015 (paratype, HMAS 247001, CGMCC3.18375); Shanxi, Yangling, on leaves of Populus sp. (sects. Aigeiros), 4 Sep. 2015, Y.F. Zhang (paratype, HMAS 247004, CGMCC3.18378); ibid. (paratype, HMAS 247005, CGMCC3.18379).
Note: Venturia phaeosepta is sibling to V. martianoffiana (Fig. 2).
Venturia polygoni-vivipari Arx, Sydowia 4: 391. 1950.
Description and illustration: von Arx (1950).
Typus: Switzerland, Kt. Valais, Val de Bagnes, Corbassieres, on leaves of Polygonum viviparum (Polygonaceae), 19 Jul. 1948, H. Kobel (ETH) (not seen).
Material examined: Norway, on Polygonum viviparum (Polygonaceae), 12 Aug. 1988, K. & L. Holm (culture CBS 114207 = UPSC 2754).
Notes: Unfortunately, isolate CBS 114207 was sterile. The species is related to V. viennotii (Fig. 1) and V. lonicerae (Fig. 2).
Venturia populina (Vuill.) Fabric., Jahresber. Neuerungen Pflanzenkrankh. 5: 282. 1902.
Basionym: Didymosphaeria populina Vuill., in: Duchartre, Compt. Rend. Hebd. Séances Acad. Sci. 108: 634. 1889.
Synonyms: Fusicladium radiosum [(Lib.) Lind] var. balsamiferae Davis, Trans. Wisconsin Acad. Sci. 20: 402. 1922.
Pollaccia elegans Servazzi, Boll. Lab. Sperim. Osserv. Fitopatol. 15(3–4): 64. 1939.
Pollaccia balsamiferae (Davis) M. Morelet, Bull. Soc. Sci. Nat. Archéol. Toulon Var 4: 3. 1972.
Fusicladium elegans (Servazzi) Ritschel & U. Braun, Schlechtendalia 9: 43. 2003.
Description and illustration: Sivanesan, 1977, Schubert et al., 2003.
Typus: France, Loir-et-Cher, Montdoubleau, on young branches of Populus (Salicaceae), Apr. 1889, Prillieux s.n. (lectotype, PC) (not seen).
Materials examined: Italy, on Populus canadensis (Salicaceae), collection date and collector unknown, isol. O. Servazzi (culture CBS 256.38 = IMI 163996); on Populus sp., collection date and collector unknown, isol. R. Ciferri (culture CBS 316.58).
Notes: Unfortunately, isolates CBS 256.38 and CBS 316.58 proved to be sterile. The species is sister to V. mandshurica (Fig 1, Fig 2).
Venturia pyrina Aderh., Landw. Jahrb. 25: 875. 1896. Nom. cons. prop. (Rossman et al. 2018). Fig. 55.
Fig. 55.
Venturia pyrina (HMAS 03905) asexual morph. A. Dense fascicle of conidiophores on the host surface. B–F. Solitary or fasciculate conidiophores with sympodial conidiogenous loci. G–K. Fusiform to broadly fusiform conidia. Scale bars: A = 200 μm; B–F = 20 μm; G–K = 5 μm.
Synonyms: Helminthosporium pyrorum Lib. (p.p.), Pl. Crypt. Arduenna, Fasc. 2, 188. 1832.
Arthrinium pyrinum Wallr., Fl. Crypt. Germ. 2: 163. 1833.
Fusidium pyrinum Corda, Icon. Fung. 1: 3. 1837.
Fusicladium virescens Bonord., Handb. Mykol.: 80. 1851.
Fusicladium fuscescens Rabenh., Bot. Zeitung (Berlin) 15: 430. 1857.
Cladosporium polymorphum Peyl, Lotos 15: 18. 1865.
Passalora pomi G.H. Otth, Mitth. Naturf. Ges. Bern 1868: 66. 1868.
Cercospora porrigo Speg., Anales Mus. Nac. Buenos Aires. II. 3: 341. 1899.
Acrotheca dearnessiana Sacc., Ann. Mycol. 10: 314. 1912.
Endostigme pyrina (Aderh.) Syd., Ann. Mycol. 21: 173. 1923.
Colonies amphigenous, dark brown to olivaceous brown. Stroma well developed on fruit, leaves, rarely on young twigs and buds, sometimes composed of only a few cells. Conidiophores erect, solitary or fasciculate unbranched, usually short, up to 90 μm long, 5–7 μm wide, dark brown to olivaceous brown, mostly aseptate. Conidiogenous cells integrated, terminal, proliferation sympodially, with several conspicuous loci. Conidia solitary, 16–24.5 × 6–9 μm, broadly fusiform, sometimes irregular, pale to olivaceous brown, smooth, wrinkled or verruculose, 0(–1)-septate, not constricted at the septum, pointed at the apex, truncate at the base; hila narrowly truncate, somewhat thickened and darkened. Description in vivo and illustration, see Schubert et al. (2003: 82–85, fig. 41).
Typus: lectotype [icon in] Landw. Jahrb. 25: 878, t. 31, fig. 1–11. 1896 (designated in Rossman et al. 2018).
Materials examined: Brazil, on Pyrus communis (Rosaceae), collection date and collector unknown (culture CBS 120825 = BR 04 PC S2.2). China, on leaves of Pyrus sp., Aug. 1942, F. Dai (HMAS 03923); idem., 1 Sep. 1942, F. Dai (HMAS 03905); Shandong, Wendeng, on leaves of Pyrus sp., 4 Aug. 2014, J. Zhang & Y. Liu (culture BJFCC 140804-2). Germany, unknown host, Jul. 1935, M. Schmidt (culture CBS 379.35). New Zealand, on P. communis, 20 Apr. 2008, C.F. Hill (culture CBS 123189 = CPC 15384).
Notes: The species is sister to V. orbiculata (Fig. 1) and to V. nashicola (Fig. 2). Fusicladium virescens, the type species of Fusicladium, was reported on apple leaves (or on pear leaves., see Schubert et al. 2003: 3–4), and was reduced to a synonym of F. pyrorum based on morphological features of the conidiophores given in the original description (Bonorden, 1851, Saccardo, 1897, Lindau, 1907).
Venturia quebecensis Crous, M. Shen & Y. Zhang ter, sp. nov. MycoBank MB831540. Fig. 56.
Fig. 56.
Venturia quebecensis (ex-type culture CBS 695.85) asexual morph. A. Colony on OA. B–D. Conidiogenous cells giving rise to conidia. E–G. Conidia or germinating conidia. Scale bars: B–G = 10 μm.
Etymology: The epithet refers to Quebec, the province where this isolate was collected.
Sexual morph unknown. In vitro on OA: Mycelium branched or unbranched, pale to medium brown, 2.5–3.5 μm wide, septate, not constricted at septa, smooth or occasionally verrucose, straight, wall not thickened. Conidiophores laterally or terminally arising from hyphae, reduced to conidiogenous cells. Conidiogenous cells integrated, terminal, erect or geniculate-sinuous, 6–24 × 3–6 μm, antenna- or hyphopodium-like, phialidic, collarette sometimes present, pale to medium brown, subcylindrical, smooth, walls somewhat thickened, with a single locus, 2.5–4 μm wide, slightly thickened or darkened, sometimes only as short lateral conical prolongations of hyphae. Conidia solitary, straight or slightly curved, fusiform, sometimes obpyriform, 25–38.5 × 5.5–8 μm, 1–2(–3)-septate, usually constricted at the septa, medium to dark brown, smooth, walls somewhat thickened or darkened, often widest in the middle or just below, becoming tapered and hyaline towards the apex, attenuated or rounded towards the base; hila truncate, 2–3.5 μm wide, somewhat thickened and darkened.
Culture characteristics: Colonies spreading, somewhat erumpent, smooth, with sparse aerial mycelium and regular margins on OA, uneven, greyish sepia (surface), becoming paler towards margins; reverse fuscous-black. Colonies reaching 65 mm diam after 6.5 wk at 25 °C in the dark; colonies fertile.
Typus: Canada, Quebec, Portneuf, on the spotted leaf of Populus tremuloides (Salicaceae), 18 Jun. 1979, M. Morelet (holotype CBS H-23604, culture ex-type CBS 695.85).
Notes: Isolate CBS 695.85 was collected on Populus tremuloides in Quebec by M. Morelet, and was identified as Venturia tremulae. However, the 2-septate conidia of CBS 695.85 are distinct from those of V. tremulae (Schubert et al. 2003). Phylogenetically, this isolate also does not cluster with strains of V. tremulae and therefore a novel species is introduced to accommodate it (Fig 1, Fig 2).
Venturia rumicis (Desm.) G. Winter, Rabenh. Krypt.-Fl., Ed. 2, 1(2): 435. 1885. Fig. 57.
Fig. 57.
Venturia rumicis (? type K(M) 189242) sexual morph. A. Ascomata scattered on the host surface. B–D. Broadly clavate to somewhat obclavate asci. E. Obclavate ascus and evanescent pseudoparaphyses. F. Evanescent pseudoparaphyses. G, H. Released, pale brown 1-septate ascospores. Scale bars: A = 200 μm; B–H = 10 μm.
Basionym: Sphaeria rumicis Desm., Ann. Sci. Nat., Bot. ser. 2, 19: 361. 1843.
Synonyms: Sphaerella rumicis (Desm.) Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 103. 1870.
Stigmatea rumicis (Desm.) J. Schröt., Kryptogamenfl. Schlesien 3.2: 332. 1894.
Ascospora rumicis (Desm.) Kuntze, Rev. Gen. Plant 3: 444. 1898.
Spilosticta rumicis (Desm.) Syd., Ann. Mycol. 21: 171. 1923.
Mycosphaerella rumicis (Desm.) Grove, J. Bot. (London) 71: 253. 1933.
Mycosphaerella rumicis f. caulicola Grove, J. Bot. (London) 71: 253. 1933.
Mycosphaerella stromatoidea Dearn., Mycologia 18: 245. 1926.
Ascomata epiphyllous, 60–170 μm diam, gregarious or scattered, initially immersed, becoming erumpent, globose to subglobose, wall black, with a conspicuous papillate ostiole. Setae not observed. Peridium 10–25 μm wide, 1-layered, composed of 2–3 rows of pigmented cells of textura angularis, cells 8–9 × 4–8 μm, cell wall 0.8–1.2 μm thick. Pseudoparaphyses 2–4 μm wide, rare, evanescent when mature, septate, hyaline. Asci 39–67 × 13–19 μm (av. 53.6 × 15.8 μm, n = 20), 8-spored, bitunicate, fissitunicate, broadly cylindrical to somewhat obclavate, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 14–19 × 5.5–7 μm (av. 16.1 × 6.5 μm, n = 20), fusiform, hyaline to pale olivaceous brown, obliquely uni- or triseriate near the base, 1-septate, constricted at the septum, the upper cells wider and longer than the lower ones (length ratio: 9:8–3:2), smooth-walled. Asexual morph unknown.
Typus: France, on overwintered leaves of Rumex sp. (Polygonaceae), J.B.H.J. Desmazières (? type K(M) 189242).
Additional material examined: USA, California, on overwintered leaves of Rumex occidentalis (Polygonaceae), 26 May 1930, L. Bonar (HMAS 49551).
Notes: Although both Venturia rumicis and V. canadensis occur on Rumex spp., the immersed ascomata and larger-sized ascospores of V. rumicis are distinguishable from those of V. canadensis. Neither of these species are currently known from molecular data.
Venturia saliciperda J. Nüesch, Phytopathol. Z. 39: 349. 1960.
Synonyms: Septogloeum saliciperdum Allesch. & Tubeuf, Fungi Bavar. Exsicc.: no. 485. 1895.
Fusicladium saliciperdum (Allesch. & Tubeuf) Lind, Ann. Mycol. 3: 430. 1905.
Pollaccia saliciperda (Allesch. & Tubeuf) Arx, Tijdschr. Plantenziekten 63: 233. 1957.
Description and illustration: Nüesch (1960) and Schubert et al. (2003).
Typus: Switzerland, Katzensee bei Zürich, Kt. Zürich, Salix cordata (Salicaceae), 24 Sep. 1958, collector unknown (holotype ETH Nr. 2836, culture ex-type CBS 480.61 = ETH 2836).
Additional materials examined: France, on Populus tremula (Salicaceae), 1 Sep. 1977, collector unknown (culture CBS 112625 = CPC 3638 = MPFN 349 = STE-U 3638). Switzerland, on Salix elegantissima (Salicaceae), collection date and collector unknown, isol. E. Müller, 24 Sep. 1958 (culture CBS 481.61 = ETH 2837).
Notes: Unfortunately, these isolates proved to be sterile in culture. Venturia saliciperda is sister to V. tremulae (Fig 1, Fig 2). Venturia saliciperda is exclusively associated with Salix spp., while V. tremulae associates with Populus spp.
Venturia syringae (Syd.) M.E. Barr, Canad. J. Bot. 46: 815. 1968. Fig. 58.
Fig. 58.
Venturia syringae (isotype MICH 139624) sexual morph. A. Ascomata scattered on the host surface. B. Section of an ascoma. C–E. Cylindrical to somewhat obclavate asci. F, G. Evanescent pseudoparaphyses. H, I. Released, olivaceous brown ascospores. Scale bars: A = 200 μm; B–G = 10 μm; H–I = 5 μm.
Basionym: Phaeosphaerella syringae Syd., Ann. Mycol. 21(1–2): 145. 1923.
Synonyms: Spilosticta syringae (Syd.) Petr., Hedwigia 65: 241. 1925.
Fusicladium diedickeanum U. Braun, Nova Hedwigia 55(1–2): 211. 1992.
Sexual morph in vitro: Ascomata 65–90 μm diam, hypophyllous, solitary, gregarious or scattered, initially immersed, becoming erumpent, globose to subglobose, wall black, with conspicuous papillate ostiole. Setae not observed. Peridium 5–10 μm wide, 1-layered, composed of 1–2 rows of pigmented cells of textura angularis, cells 6–14 × 5–11 μm, cell wall 0.5–1.2 μm thick. Pseudoparaphyses 2–4 μm wide, rare, evanescent when mature, septate, hyaline. Asci 40–55 × 10–12 μm (av. 48.8 × 10.6 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 9–12.5 × 4–6 μm (av. 10.6 × 4.5 μm, n = 20), oblong to broadly clavate, olivaceous brown, obliquely uniseriate to biseriate near the base, 1-septate, slightly constricted at the septum, the upper cells shorter and wider than the lower ones (length ratio: 2:3–1:1), smooth-walled. Asecual moprh in vivo: see Schubert et al. (2003: 40, 41, fig. 16).
Typus: Germany, Leutenthal, near Kleinbrembach, Hopfenberg at Buttelstedt, on the rotting leaves of Syringa vulgaris (Oleaceae), May 1921 (syntype MICH 139624). Topotype material (from 1923/24): Syd., Mycoth. Germ. 2116, e.g., B, BPI 618036, 618038, MICH 139623, PH 315989, WIS-F75798.
Venturia tomentosae R. Menon, Phytopathol. Z. 27: 132. 1956. Fig. 59.
Fig. 59.
Venturia tomentosae (holotype ZT 57089) sexual morph. A. Ascostromata scattered on the host surface. B. Section of an ascostroma. C, D. Cylindrical asci with short pedicels. E, G. Released, medium brown, asymmetrical ascospores. F. Evanescent pseudoparaphyses. H. Dark brown setae. Scale bars: A = 200 μm; B–H = 10 μm.
Ascomata hypophyllous, 100–215 μm diam, scattered, solitary, initially immersed, becoming erumpent, globose to subglobose, wall black, with a conspicuously papillate ostiole, sparsely surrounded by setae. Setae dark brown, 28–85 × 5–7 μm, setae wall 1–2 μm thick, aseptate. Peridium 20–28 μm wide, 1-layered, composed of (3–)4–5 rows of pigmented cells of textura angularis, cells 6–14 × 5–15 μm, cell wall 1–1.5 μm thick. Pseudoparaphyses rare, 2–4 μm wide, evanescent when mature, septate, hyaline. Asci 90–139 × 7–15 μm (av. 112.7 × 11.8 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 15–20 × 7–8 μm (av. 18.2 × 7.5 μm, n = 20), ellipsoid to somewhat clavate, olivaceous brown, obliquely uniseriate, 1-septate, slightly constricted at the septum, the upper cells shorter and wider than the lower ones (length ratio: 1:2–7:8), smooth-walled. Asexual morph unknown.
Typus: Switzerland, on leaves of Cotoneaster tomentosa (Rosaceae), 28 Sep. 1937, A. Volkart (holotype ZT 57089).
Venturia radiosa (Lib.) Ferd. & C.A. Jørg., Skovtraeernes Sygdomme 1: 125. 1938.
Basionym: Oidium radiosum Lib., Pl. Crypt. Ard. Fasc. 3, no. 285. 1834.
Synonyms: Venturia tremulae Aderh., Hedwigia 36: 81. 1897.
Fusicladium radiosum (Lib.) Lind, Ann. Mycol. 3: 430. 1905.
Pollaccia radiosa (Lib.) E. Bald. & Cif., in E. Bald., Atti Ist. Bot. “Giovanni Briosi” 10: 61. 1937.
Venturia tremulae var. populi-albae M. Morelet, Cryptog. Mycol. 6: 112. 1985.
Venturia tremulae var. grandidentatae M. Morelet, Cryptog. Mycol. 6: 113. 1985.
Description and illustration: Schubert et al. (2003).
Typus: Belgium, Belgian Ardennes, on Populus tremula (Salicaceae), 1834, Libert (lectotype BR, selected by Morelet 1985; isolectotypes: Lib., Pl. crypt. ard., Fasc. 3, 285) (not seen).
Materials examined: France, on spotted leaf of Populus alba (Salicaceae) (culture CBS 694.85); on spotted leaf of P. tremula (cultures CBS 692.85, CBS 693.85).
Notes: Unfortunately, these isolates proved to be sterile. The species is sister to V. saliciperda (Fig 1, Fig 2).
Venturia viennotii M. Morelet, Trav. Dédiés à Georges Viennot-Bourgin (Paris): 261. 1977.
Synonym: Venturia viennotii var. levispora M. Morelet, Cryptog. Mycol. 6: 107. 1985.
Description and illustration: Morelet (1985).
Typus: France, Velaine-sous-Amance (Meurthe-et-Moselle), on Populus tremula (Salicaceae), Jun. 1972, M. Morelet (holotype PFN 813).
Additional materials examined: France, on dead leaves of Populus tremula (Salicaceae), collection date and collector unknown, isol. M. Morelet, May 1979 (cultures CBS 690.85, CBS 691.85).
Notes: Unfortunately, these isolates proved to be sterile but they were deposited by the original author of the species and collected from the same host and region as the holotype and could therefore be regarded as authentic for the species. The species is related to V. polygoni-vivipari (Fig. 1) and V. catenospora (Fig. 2).
Species excluded from Venturiales
Acanthostigma saccardioides (Ellis & G. Martin) Sacc., Syll. Fung. 9: 854. 1891. Fig. 60.
Fig. 60.
Acanthostigma saccardioides (holotype NY 00938225) sexual morph. A. Ascostromata scattered on the host surface. B, D. Immature asci in evanescent pseudoparaphyses. C, E. Cylindrical to broadly obclavate asci. F, I. Released ascospores with one to three septa. G. Evanescent pseudoparaphyses. H. Dark brown setae. Scale bars: A = 200 μm; B–I = 10 μm.
Basionym: Venturia saccardioides Ellis & G. Martin, Amer. Naturalist 18: 60. 1884.
Ascomata hypophyllous, 130–190 μm diam, scattered, becoming superficial, globose to subglobose, with setae. Setae brown, up to 100 μm long, 4–5 μm wide, setae wall 0.5–1 μm thick, septate. Pseudoparaphyses rare, 2–3.5 μm wide, hyaline, septate, branched. Asci 38–56 × 9–11 μm (av. 45.1 × 9.4 μm, n = 20), 8-spored, bitunicate, fissitunicate, broadly cylindrical to clavate, with a short, knob-like pedicel, each with an inconspicuous ocular chamber. Ascospores 12–15 × 3–4 μm (av. 13.8 × 3.7 μm, n = 20), obclavate, hyaline, obliquely biseriate, 3-septate, slightly constricted at the median septum, smooth-walled.
Typus: USA, Florida, Clay Co., Green Cove Springs, on underside of leaf of Magnolia glauca (Magnoliaceae), Mar. 1883, G. Martin (holotype NY 00938226, isotype NY 00938225).
Notes: The larger-sized ascomata, and hyaline, 3-septate ascospores of Venturia saccardioides are distinguishable from Venturia s. str.
Chaetothyrina applanata (Ellis & G. Martin) M.E. Barr, Mycotaxon 46: 73. 1993. Fig. 61.
Fig. 61.
Chaetothyrina applanata (type NY 00938204) sexual morph. A. Ascomata scattered on the host surface. B–D. Broadly obclavate asci. E. Evanescent pseudoparaphyses. F–I. Released, hyaline ascospores with one septum. Scale bars: A = 200 μm; B–D = 20 μm; E–I = 10 μm.
Basionym: Venturia applanata Ellis & G. Martin, Amer. Naturalist 18: 69. 1884.
Ascomata hypophyllous, 115–220 μm diam, scattered or solitary, superficial, globose to subglobose, dark brown at the apex, hyaline at the base, with setae. Setae dark brown, up to 100 μm long, 3.5–5.5 μm wide. Pseudoparaphyses 2–3 μm wide, narrowly cellular, septate, hyaline. Asci 31–45 × 11–16 μm (av. 38.5 × 13.1 μm, n = 20), 8-spored, bitunicate, fissitunicate, obclavate, each with an inconspicuous ocular chamber. Ascospores 12–15 × 4–5 μm (av. 13.6 × 4.6 μm, n = 20), overlapping to triseriate, especially near the base, broadly clavate, hyaline, 1-septate, the upper cells shorter and wider than the lower ones (length ratio: 5.5:7–1:1), smooth-walled.
Typus: USA, Florida, on underside of living leaves of Magnolia glauca (Magnoliaceae), Mar. 1883, G. Martin s.n. (holotype NY 00938204).
Notes: The hyaline, clavate ascospores and persistent pseudoparaphyses of Venturia applanata differ from Venturia s. str.
Chaetothyrina asterinoides (Ellis & G. Martin) M.E. Barr, Mycotaxon 29: 504. 1987. Fig. 62.
Fig. 62.
Chaetothyrina asterinoides (holotype NY 00938205) sexual morph. A. Ascomata scattered on the host surface. B–E. Broadly cylindrical to somewhat obclavate asci. F, I. Evanescent pseudoparaphyses. G. Dark brown setae. H. Hyaline, 1-septate ascospores. Scale bars: A = 200 μm; B–E, G = 20 μm; F, H, I = 10 μm.
Basionym: Venturia asterinoides Ellis & G. Martin, in Ellis N. Amer. Pyren.: 138. 1892.
Sexual morph: Ascomata superficial, 100–140 μm diam, gregarious, subglobose, wall black, with a conspicuous papillate ostiole, surrounded by slender setae. Setae dark brown, up to 130 μm long, 4–4.5 μm wide, wall 0.5–1.2 μm thick. Peridium thin, composed of brown pigmented cells of textura angularis. Pseudoparaphyses 1.5–2 μm wide, numerous, septate, hyaline, branched. Asci 36–47 × 13–18 μm (av. 43.6 × 15.7 μm, n = 20), 8-spored, bitunicate, fissitunicate, broadly obclavate, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 16–18.5 × 4.5–5.5 μm (av. 17.2 × 5.1 μm, n = 20), obliquely uni- to triseriate near the base, broadly clavate, hyaline, 1-septate, the upper cells shorter and wider than the lower ones (length ratio: 7:10–1:1), smooth-walled. Asexual morph: unknown.
Typus: USA, Florida, on leaves of Quercus laurifolia (Fagaceae), Mar. 1883, G.W. Martin s.n. (holotype NY 00938205).
Notes: The hyaline and clavate ascospores and persistent pseudoparaphyses of Venturia asterinoides differ from Venturia s. str. Barr (1987) suggested that Venturia asterinoides is morphologically comparable to Chaetothyrina applanata, although they differ in their hosts and dimensions of asci and ascospores.
Dimeriella sacchari (Breda de Haan) Hansf. ex E.V. Abbott, Sugar Cane Dis. World, II: 43. 1964. Fig. 63.
Fig. 63.
Dimeriella sacchari (HMAS 11669) sexual morph. A. Ascomata scattered on the host surface. B. Ascoma and asci. C–E. Ellipsoid, subhyaline asci. F, H–L. Fusiform, 1-septate ascospores. G. Globose ascoma. Scale bars: A = 100 μm; C–E = 20 μm; B, G = 50 μm; F = 10 μm; L applies to H–L = 10 μm.
Basionym: Coleroa sacchari Breda de Haan, Meded. Proefstat. Suikerriet W.-Java Kagok-Tegal. 33: 22. 1892.
Synonym: Venturia sacchari (Breda de Haan) Sacc., Syll. Fung. 11: 306. 1895.
Ascomata epiphyllous, 30–60 μm diam, scattered or solitary, becoming superficial, globose to subglobose, with a conspicuously papillate ostiole. Peridium thin, 1-layered, composed of 2–3 rows of pigmented cells of textura angularis, cells 6–8 μm wide, cell wall 0.8–1.2 μm thick. Pseudoparaphyses rare, evanescent when mature. Asci 31–45 × 7–12 μm (av. 38.6 × 10 μm, n = 20), 8-spored, bitunicate, fissitunicate, ellipsoid, with a short, knob-like pedicel, each with an inconspicuous ocular chamber. Ascospores 11–15 × 4–6 μm (av. 12.8 × 5.2 μm, n = 20), ellipsoid, subhyaline, bi- to triseriate, 1-septate, constricted at the septum, with broadly rounded ends, the upper cells shorter and wider than the lower ones, smooth-walled. Asexual morph: unknown.
Material examined: China, Taipei, Ilan County, on the leaves of Saccharum officinarum (Poaceae), 28 Nov. 1925, K. Sawada (HMAS 11669).
Note: The ellipsoid asci and hyaline ascospores of Venturia sacchari differ from Venturia s. str.
Johansonia formosa (Ellis & G.W. Martin) M.E. Barr, Mycotaxon 46: 65. 1993. Fig. 64.
Fig. 64.
Johansonia formosa (holotype NY 00938214) sexual morph. A. Ascomata scattered on the host surface. B, C. Broadly clavate asci. D. Pale brown ascospores. E. Dark brown seta. Scale bars: A = 200 μm; B–E = 20 μm.
Basionym: Venturia formosa Ellis & G.W. Martin, in Ellis, N. Amer. Pyren.: 139. 1892.
Ascomata hypophyllous, 115–260 μm diam, scattered or solitary, becoming superficial, discoid, wall black, surrounded by slight setae. Setae dark brown, septate, 150–180 × 6 μm, setae wall 0.5–1 μm thick, base swollen, up to 11 μm. Pseudoparaphyses rare, 3 μm wide, hyaline, septate. Asci 50–69 × 23–33 μm (av. 63.1 × 29.8 μm, n = 10), 8-spored, bitunicate, fissitunicate, ellipsoid, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 23–29 × 10.5–13 μm (av. 26.0 × 11.5 μm, n = 10), ellipsoid to somewhat clavate, pale brown, bi- or triseriate, 1-septate, slightly constricted at the septum, base narrowly rounded to tapered, the upper cells shorter and wider than the lower ones (length ratio: 21:25–1:1), smooth-walled. Asexual morph: unknown.
Typus: USA, Florida, clay companies, Green Bay Spa, on the leaves of Olea americana (Oleaceae), 15 Apr. 1885, G. Martin s.n. (holotype NY 00938214).
Note: The larger-sized ascomata and ellipsoid asci differ from Venturia s. str.
Nematostoma occidentale (Ellis & Everh.) M.E. Barr, Mycol. Res. 46: 860. 1968. Fig. 65.
Fig. 65.
Nematostoma occidentale (lectotype NYSf 2176) sexual morph. A. Ascomata scattered on the host surface. B–D. Clavate asci. E. Pale brown, 3-septate ascospores. F. Evanescent pseudoparaphyses. G. Dark brown setae. Scale bars: A = 200 μm; B–G = 10 μm.
Basionym: Venturia occidentalis Ellis & Everh., J. Mycol. 2: 43. 1886.
Ascomata hypophyllous, 120–284 μm diam, scattered or solitary, becoming erumpent or superficial, globose to subglobose, wall black, with a conspicuously papillate ostiole, surrounded by setae. Setae dark brown, up to 300 μm long, 5–7.5 μm wide, setae wall 2–3 μm thick, base swollen, septate. Pseudoparaphyses 1.5–2.5 μm wide, hyaline, septate, branched. Asci 70–90 × 9–11 μm (av. 78.7 × 10.2 μm, n = 10), 8-spored, bitunicate, fissitunicate, cylindrical or clavate, with a short pedicel, each with an inconspicuous ocular chamber. Ascospores 20–27 × 4–6 μm (av. 22.6 × 4.6 μm, n = 20), narrowly cylindrical to fusiform, pale brown, overlapping to biseriate near the top, 3-septate, constricted at the median septum, smooth-walled. Asexual morph: unknown.
Typus: USA, Illinois, Urbana, on leaves of Cirsium discolor (Compositae), 23 Oct. 1885, C.A. Hart 6597 (syntype NY 00938217); idem., 3 Nov. 1885, C.A. Hart 6607 (syntype NY 00938218).
Note: The 3-septate and cylindrical ascospores of Nematostoma occidentale differ from Venturia s. str.
Niesslia erysiphoides (Ellis & Everh.) M.E. Barr [as “erysipheoides”], Mycotaxon 46: 50. 1993. Fig. 66.
Fig. 66.
Niesslia erysiphoides (holotype NY 00938212) sexual morph. A. Gregarious ascomata on the host surface. B, D. Subcylindrical to somewhat obclavate asci (in cotton blue). C, E. Hyaline, narrowly fusiform ascospores (in cotton blue). F. Dark brown seta. Scale bars: A = 100 μm; B–F = 10 μm.
Basionym: Venturia erysiphoides Ellis & Everh., J. Mycol. 3: 128. 1887.
Ascomata on stems or leaf sheaths, up to 100 μm diam, in small groups, becoming superficial, globose, collapsing into a cup-shape when dry, wall black, with a conspicuous ostiole, covered with setae. Setae dark brown, 40–70 × 5–7 μm, wall 2 μm thick, aseptate. Pseudoparaphyses not observed. Asci 34–49 × 6–8 μm (av. 41.5 × 7 μm, n = 20), 8-spored, oblong, without pedicel. Ascospores 16–20 × 2.5–3 μm (av. 18.1 × 2.8 μm, n = 20), narrowly fusiform, hyaline, bi- to triseriate, 1-septate, with a median septum. Asexual morph: unknown.
Typus: USA, Louisiana, on stems or leaf sheaths of Panicum curtisii (Poaceae), 24 Feb. 1887, A.B. Langlois 1023 (holotype NY 00938213, isotype NY 00938212).
Notes: The slender, hyaline and symmetrical ascospores and the absence of pseudoparaphyses of Niesslia erysiphoides differs from Venturia s. str. The ascospores of N. erysiphoides are larger than other species of Niesslia reported in South America (Barr 1993).
Niesslia iridicola (M.E. Barr) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831595. Fig. 67.
Fig. 67.
Niesslia iridicola (holotype NY 00914445) sexual morph. A. Ascomata scattered on the host surface. B, C. Broadly obclavate asci. D. Evanescent pseudoparaphyses. E. Dark brown setae. F. Immature asci and hyaline, 1-septate ascospores. Scale bars: A = 200 μm; B–F = 10 μm.
Basionym: Venturia iridicola M.E. Barr, Sydowia 41: 27. 1989.
Ascomata on leaves or stems, 70–85 × 71–100 μm diam, solitary, scattered or in small groups of 2–3, initially immersed, becoming erumpent, globose to subglobose, wall black, apex erumpent, with the ostiole surrounded by setae. Setae dark brown, 30–44 × 4–6 μm, 0–1-septate, swollen at the base, up to 9 μm wide. Peridium 1-layered, composed of 1–2 rows of pigmented cells of textura angularis, cells 4–7 × 5–10 μm, cell wall 1 μm thick. Pseudoparaphyses 2–3 μm wide, hyaline, septate, branched, persistent. Asci 42–50(–67) × 10–13 μm (av. 47.9 × 11.9 μm, n = 20), 8-spored, broadly cylindrical to obclavate, each with an inconspicuous ocular chamber. Ascospores 15–18 × 4–5 μm (av. 16.9 × 4.7 μm, n = 20), narrowly fusiform, hyaline, obliquely overlapping to biseriate, 1-septate, the upper cells wider than the lower ones, smooth-walled. Asexual morph: unknown.
Typus: Canada, Newfoundland and Labrador, Blanc Sablon, on leaves and stems of Iris sp. (Iridaceae), 19 Jul. 1957, R.T. Wilce 158 (holotype NY 00914445).
Notes: The hyaline, symmetric ascospores, persistent pseudoparaphyses as well as its monocotyledon host disagrees with Venturia s. str. The ascomata of V. iridicola are tiny, superficial, dark brown and covered with shiny, typical spines, tending to collapse into a cup-like shape when mature and dry. The asci are broadly cylindrical to obclavate and ascospores are narrowly fusiform, hyaline, 1-septate. All these characteristics point to Niesslia (Gams et al. 2019).
Niesslia parasitica (Ellis & Everh.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831594. Fig. 68.
Fig. 68.
Niesslia parasitica (holotype NY 00938219) sexual morph. A. Gregarious ascomata on the host surface. B. Dark brown setae on the surface of ascoma. C, D. Released, pale brown ascospores. Scale bars: A = 100 μm; B–D = 10 μm.
Basionym: Venturia parasitica Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia 42: 233. 1890.
Ascomata on bark surface, 90–100 μm diam, gregarious, globose to subglobose, with an ostiole, surrounded with setae. Setae dark brown, 30–44 × 4–5 μm, setae wall 1–1.5 μm thick, aseptate, base swollen. Pseudoparaphyses not observed. Asci 30 × 5 μm (Ellis & Everhart 1890), 8-spored, oblong to cylindrical. Ascospores 6–8 × 2–3 μm (av. 7.3 × 2.7 μm, n = 20), fusiform to broadly fusiform, pale brown, 1-septate, constricted at median septum. Asexual morph: unknown.
Typus: USA, Louisiana, near San Martinsville, on the bark of Magnolia sp. (Magnoliaceae), 21 Jan. 1889, A.B. Langlois 1781 (holotype NY 00938219).
Notes: The gregarious ascomata and the absence of pseudoparaphyses disagree with Venturia s. str. Because of the poor quality of the specimen, no asci were observed, the description of which was taken from Ellis & Everhart (1890). The tiny, superficial, dark brown ascomata covered with shiny and typical spines, that tend to collapse into a cup-like shape when dry point to Niesslia (Gams et al. 2019). Gams et al. (2019) treated Venturia parasitica as synonym of Niesslia pulchriseta, while the broader ascospores with constricted septa of Venturia parasitica are readily distinguished from those of Niesslia pulchriseta.
Niesslia sabalicola (Ellis & Everh.) W. Gams, Mycol. Progr. 18(1-2): 62. 2019. Fig. 69.
Fig. 69.
Niesslia sabalicola (holotype NY 00938225) sexual morph. A. Ascomata scattered on the host surface. B. Dark brown setae. C–G. Released, hyaline, 1-septate ascospores (G in cotton blue). Scale bars: A = 200 μm; B–G = 10 μm.
Basionym: Venturia sabalicola Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia 42: 233. 1890.
Ascomata epiphyllous, 125–141 μm diam, scattered or subgregarious, erumpent to superficial, globose to subglobose, collapsing into a cup-like shape when dry, surrounded with setae. Setae dark brown, 50–90 × 6–8 μm, aseptate. Pseudoparaphyses not observed. Asci 38–56 × 9–11 μm (av. 45.1 × 9.4 μm, n = 20), 8-spored, oblong to broadly clavate. Ascospores 10–15 × 2.5–3 μm (av. 12.6 × 2.9 μm, n = 20), narrowly fusiform, hyaline, 1-septate, with a median septum, slightly constricted or not at the septum, smooth-walled. Asexual morph unknown.
Typus: USA, Louisiana, Bayou Chene, on dead leaves of Sabal palmetto (Arecaceae), 25 Oct. 1888, A.B. Langlois 1546 (holotype NY 00938203).
Notes: The symmetrical, 1-septate, hyaline ascospores, and absence of pseudoparaphyses of V. sabalicola disagree with Venturia s. str. The tiny, superficial, dark brown ascomata covered with shiny spines, and the ascomata that tend to collapse into a cup-like shape when dry, as well as the oblong to broadly clavate asci and narrowly fusiform, hyaline, 1-septate ascospores are reminiscent of Niesslia (Gams et al. 2019). Because of the poor quality of the specimen, most information was adapted from Ellis & Everhart (1890).
Niesslia vaccinii (Ellis & Everh.) Crous, M. Shen & Y. Zhang ter, comb. nov. MycoBank MB831593. Fig. 70.
Fig. 70.
Niesslia vaccinii (holotype NY 00938227) sexual morph. A, B. Ascomata scattered on the host surface. C, E, G, H. Lanceolate asci. D. Hyaline, fusiform ascospore. F. Dark brown seta. Scale bars: A, B = 200 μm; C–H = 10 μm.
Basionym: Venturia vaccinii Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia 46: 325. 1894.
Ascomata hypophyllous, 60–115 μm diam, solitary, scattered, rarely in small groups, erumpent to superficial, globose to subglobose, collapse into a cup-like shape when dry, wall black, surrounded with setae. Setae dark brown, 37–65 × 5–7 μm, setae wall 1–2 μm thick, base swollen, aseptate. Pseudoparaphyses not observed. Asci 30–37 × 6–7 μm (av. 34.1 × 6.3 μm, n = 20), 8-spored, fusiform. Ascospores 10–12.7 × 2–2.5 μm (av. 11.3 × 2.1 μm, n = 20), narrowly fusiform, hyaline, bi- to triseriate, 1-septate, symmetrical, slightly constricted at the septum, smooth-walled. Asexual morph unknown.
Typus: USA, Washington, Seattle, on dead leaves of Vaccinium ovatum (Ericaceae), 16 Dec. 1893, C.V. Piper No. 225 (holotype NY 00938227).
Notes: The tiny, superficial, dark brown ascomata covered with shiny spines, which tend to collapse into a cup-like shape when dry, as well as the fusiform asci and hyaline, 1-septate, symmetric narrowly fusiform ascospores point to Niesslia (Gams et al. 2019). Gams et al. (2019) assigned Venturia vaccinii to synonymy with Niesslia exilis. However, the larger-sized asci and ascospores of Venturia vaccinii could be readily distinguished from those of Niesslia exilis (30–37 × 6–7 μm vs. 40–50 × 4–5 μm and 10–12.7 × 2–2.5 μm vs. (6–)7–8.5(–11) × 1.5–2.0 μm).
Phomatosporopsis sphaerelloidea (Höhn.) Petr., Ann. Mycol. 25: 249. 1927. Fig. 71.
Fig. 71.
Phomatosporopsis sphaerelloidea (type W 0553) sexual morph. A. Ascoma on host surface. B. Released, hyaline, 1-septate ascospores (in cotton blue). C–E. Broadly clavate to somewhat obclavate asci (in cotton blue). Scale bars: A = 100 μm; B–E = 10 μm.
Basionym: Venturia sphaerelloidea Höhn., Sitzungsber. Kaiserl. Akad. Wiss., Math.-Na. Cl., Abt. 1. 118: 1203. 1909.
Ascomata hypophyllous, 60–130 μm diam, solitary or scattered, becoming semi-immersed, globose to subglobose, wall black, with a conspicuously papillate ostiole. Setae not observed. Peridium thin, 1-layered, composed of 1–2 rows of pigmented cells of textura angularis, cells 5–12 × 5–11 μm, cell wall 1 μm thick. Pseudoparaphyses rare, 2–3 μm wide, hyaline, septate. Asci 30–36 × 8–10 μm (av. 32.8 × 9 μm, n = 20), 8-spored, bitunicate, fissitunicate, broadly clavate or somewhat obclavate. Ascospores 8–12 × 3–5 μm (av. 10.4 × 3.2 μm, n = 20), fusiform, hyaline, obliquely biseriate, 1-septate, with a median septum, slightly constricted at the septum, the upper cells longer and wider than the lower ones (length ratio: 1:1–3:2), smooth-walled. Asexual morph unknown.
Typus: Austria, Niederösterreich, on branches of Impatiens nolitangere (Balsaminaceae), 12 Jul. 1908, P. Strasser (holotype W 0553).
Notes: The ascomata lack setae and the ascospores are hyaline, which differ from Venturia s. str., and are reminiscent of Mycosphaerellaceae.
Pyrenobotrys compacta (Peck) B. Erikss., Svensk Bot. Tidskr. 68: 224. 1974. Fig. 72.
Fig. 72.
Pyrenobotrys compacta (holotype NYSf 826) sexual morph. A, B. Gregarious ascomata on the host surface. C. Section of an ascoma. D. Squash mount with a large number of asci. E. Released, hyaline to pale brown, asymmetrical ascospores. F. Evanescent pseudoparaphyses (in cotton blue). G. Dark brown setae. Scale bars: B = 200 μm; C = 50 μm; D–G = 10 μm.
Basionym: Venturia compacta Peck, Annual Rep. New York St. Mus. Nat. Hist. 25: 106. 1873 [1872].
Ascomata hypophyllous, 143–200 μm diam, gregarious, superficial, globose to subglobose, wall black, with short, spiny, dark brown setae, setae 19–35 × 6–7 μm, wall 1–2.5 μm thick. Peridium 50 μm wide, 1-layered, composed of several rows of pigmented cells of textura angularis. Pseudoparaphyses 2–4 μm wide, hyaline, septate. Asci 59–66 × 10–11 μm (av. 62.6 × 8.8 μm, n = 10), 8-spored, bitunicate, fissitunicate, cylindrical to clavate, each with an inconspicuous ocular chamber. Ascospores 14–20 × 4–6 μm (av. 16.5 × 5.3 μm, n = 20), broadly cylindrical, hyaline to pale brown, with broadly rounded ends, overlapping to biseriate near the base, 1-septate, with the septum in the upper third, slightly constricted at the septum, the upper cells shorter and wider than the lower ones (length ratio: 1:3–2:3), smooth-walled. Asexual morph unknown.
Typus: USA, New York, Rensselaer, Sandlake, on fallen leaves of Vaccinium macrocarpum (Ericaceae), Jun. 1871, C.H. Peck (holotype NYSf 826).
Note: The large-sized, gregarious ascomata, cylindrical asci and broadly cylindrical ascospores disagree with Venturia s. str.
Venturia clintonii Peck, Annual Rep. New York St. Mus. Nat. Hist. 28: 82. 1876. Fig. 73.
Fig. 73.
Venturia clintonii (holotype NYSf794) sexual morph. A. Ascomata scattered on the host surface. B–F. Narrowly cylindrical asci with short pedicels. G, H. Olivaceous brown, asymmetrical ascospores. I. Dark brown setae. Scale bars: A = 200 μm; B–I = 10 μm.
Ascomata hypophyllous, 100–170 μm diam, scattered, or solitary, erumpent to nearly superficial, globose to subglobose, wall black, with a conspicuous papillate ostiole, surrounded by setae. Setae dark brown, 60–115 × 6–7 μm, wall 1–1.5 μm thick, base swollen, up to 8–12 μm, septate. Peridium 10–23 μm wide, 1-layered, composed of 2–3 rows of pigmented cells of textura angularis, cells 7–113 × 6–11 μm, cell wall 0.5–1 μm thick. Pseudoparaphyses rare, evanescent when mature. Asci 68–87 × 6–8 μm (av. 75.9 × 6.9 μm, n = 20), 8-spored, bitunicate, fissitunicate, narrowly cylindrical, with a short, furcate pedicel, each with an inconspicuous ocular chamber. Ascospores 10–11 × 4–5 μm (av. 10.4 × 4.8 μm, n = 20), broadly clavate, olivaceous brown, with narrowly rounded ends, obliquely uniseriate, 1-septate, apiosporous, septum in the lower third, the upper cells much longer and wider than the lower ones (length ratio: 7:4–8:3), smooth-walled.
Typus: USA, New York, Lake Erie, on fallen leaves of Cornus circinata (Cornaceae), May 1874, G.W. Clinton (holotype NYSf794).
Note: The numerous, cylindrical asci of Venturia clintonii have furcate pedicles, which disagree with Venturia s. str. Its phylogenetic position remains unclear (no molecular data).
Venturia musae Sawada, Special Publ. Coll. Agric., Natl. Taiwan Univ. 8: 73. 1959. Nom. inval., Art. 39.1 (Shenzhen). Fig. 74.
Fig. 74.
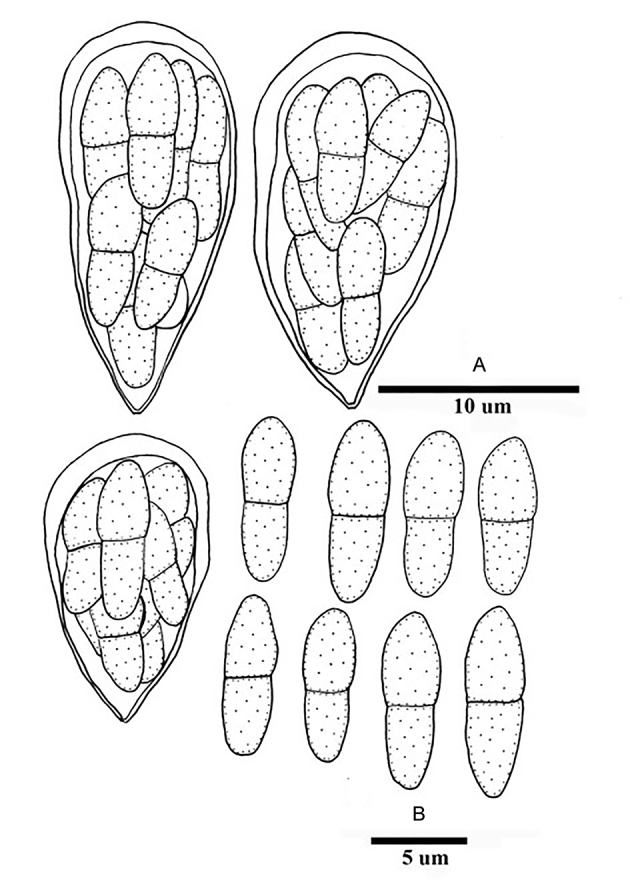
Venturia musae (based on the type PPMH) sexual morph. A. Obovoid asci. B. Hyaline to pale brown ascospores. Scale bars: A = 10 μm; B = 5 μm.
Leaf spots 2–8 mm diam, scattered, diamond, grey or sometimes pale grey in the medium, margin dark brown. Ascomata 35–40 μm diam, 28–41 μm high, scattered or solitary, initially immersed to erumpent, becoming superficial, globose to subglobose, wall black, with a conspicuously papillate ostiole, ostiole 10–12 μm diam, surrounded with setae. Setae dark brown, up to 20 μm long, setae wall 1 μm thick, 1(–2)-septate. Peridium 1-layered, composed of (1–)2–3 rows of pigmented cells of textura angularis, cells 4–6 μm wide, cell wall 0.8–1 μm thick. Pseudoparaphyses not observed. Asci 13–19 × 7–8 μm (av. 15.6 × 7.4), 8-spored, bitunicate, fissitunicate, obovoid, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 8–10 × 3–3.5 μm (av. 9.4 × 3.4), ellipsoid, hyaline to pale brown, irregularly triseriate or more, medianly 1-septate. Asexual morph unknown.
Typus: China, Taipei Wooden Gate, on the leaves of Musa cavendishii (Musaceae), 25 Apr. 1916, Saburo Fuji (type PPMH).
Note: The small ascomata, symmetrical ascospores and the complete absence of pseudoparaphyses of V. musae disagree with Venturia s. str.
Venturia nebulosa Ellis & Everh., J. Mycol. 8: 66. 1902. Fig. 75.
Fig. 75.
Venturia nebulosa (holotype NY 00938216) sexual morph. A. Ascomata scattered on the host surface. B. A crushed ascostroma with setae. C. Dark brown seta. D, F, G. Oblong asci (D and G in cotton blue). E. Section of the peridium. H. Hyaline, 1-septate ascospores (in cotton blue). I. Evanescent pseudoparaphyses (in cotton blue). Scale bars: A = 200 μm; B = 50 μm; C–I = 10 μm.
Ascomata epiphyllous, 70–115 μm diam, gregarious or solitary, becoming superficial, subglobose, wall black, with a conspicuously papillate ostiole, surrounded with setae. Setae dark brown, 30–40 × 4–5 μm, setae wall 1–1.2 μm thick, base swollen, septate. Peridium 1-layered, composed of one row of pigmented cells of textura angularis, cells 4–8 μm diam, cell wall 1 μm thick. Pseudoparaphyses 1.5–3 μm wide, hyaline, branched, septate, persistent. Asci 30–50 × 13–15 μm (av. 39.5 × 14.2 μm, n = 10), 8-spored, bitunicate, fissitunicate, narrowly oblong, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 15–18 × 5–6 μm (av. 16.2 × 5.7 μm, n = 20), fusiform to narrowly fusiform, hyaline, overlapping to triseriate, constricted at the median septum, the upper cells wider than the lower ones, smooth-walled. Asexual morph unknown.
Typus: USA, Alabama, on overwintered leaves of Eragrostis sp. (Gramineae), Nov. 1901, G.W. Carver 613 (holotype NY 00938216).
Note: The narrowly oblong asci, persistent pseudoparaphyses and the hyaline ascospores of Venturia nebulosa disagree with Venturia s. str., while point to Lasiostemma. Its taxonomic status cannot be determined yet.
Venturia pezizoidea Sacc. & Ellis, Michelia 2: 567. 1882. Fig. 76.
Fig. 76.
Venturia pezizoidea (syntype MICH 15151) sexual morph. A. Ascomata scattered on the host surface. B, C. Broadly cylindrical asci (in cotton blue). D. Subcylindrical, hyaline ascospores (in cotton blue). E, F. Dark brown setae. Scale bars: A = 100 μm; B–F = 10 μm.
Ascomata hypophyllous, 60–115 μm diam, solitary or scattered, erumpent to superficial, globose to subglobose, covered with setae. Setae dark brown, 34–75 × 4–7 μm, setae wall 1–1.2 μm thick, base swollen, aseptate. Peridium thin, composed of pale to brown cells of textura angularis. Pseudoparaphyses not observed. Asci 32–35 × 7–8 μm (av. 32.5 × 7.3 μm, n = 20), 8-spored, broadly cylindrical to clavate. Ascospores 8–10 × 1.5–2 μm (av. 9.3 × 1.7 μm, n = 20), subcylindrical, hyaline, obliquely uniseriate, aseptate. Asexual morph unknown.
Typus: USA, New Jersey, Newfield, on fallen leaves of Andromeda racemosa (Ericaceae) (syntypes NY 00938220, 00938221, 00938222, 00938223, 00938224; MICH 15151).
Note: The hyaline, aseptate ascospores of Venturia pezizoidea are readily distinguishable from Venturia s. str. Its taxonomic status cannot be determined yet.
Venturia pruni M.E. Barr, Canad. J. Bot. 46: 816. 1968. Fig. 77.
Fig. 77.
Venturia pruni (holotype NY 00914448) sexual morph. A. Ascomata densely scattered on the host surface. B, D, E. Broadly cylindrical to somewhat obclavate asci. C. Squash mount with several immature asci. F–J. Olivaceous to medium olivaceous, 1-septate ascospores. Scale bars: A = 100 μm; B–J = 10 μm.
Ascomata epiphyllous, 55–75 μm diam, gregarious, scattered, initially immersed, becoming erumpent, globose to subglobose, wall black, with a conspicuously papillate ostiole. Setae not observed. Peridium 4–6 μm wide, 1-layered, composed of pigmented cells of textura angularis, cells up to 6 μm wide, cell wall 0.8–1.2 μm thick. Pseudoparaphyses not observed. Asci 25–41 × 7–9 μm (av. 32 × 8.2 μm, n = 20), 8-spored, bitunicate, fissitunicate, broadly cylindrical, broadly clavate or somewhat obclavate, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 10.5–12 × 3–4 μm (av. 11.3 × 3.4 μm, n = 20), fusiform to narrowly fusiform, olivaceous brown, obliquely uniseriate to biseriate near the base, 1-septate, with a slightly constricted median septum, the upper cells somewhat shorter than the lower ones (length ratio: 5:6–1:1), smooth-walled. Asexual morph unknown. Asexual morph unknown.
Typus: Canada, Quebec, on leaves of Prunus pennsylvanica (Rosaceae), 6 Jul. 1957, M.E. Barr & H.E. Bigelow (holotype NY 00914448, isotype NY 00914449).
Note: The gregarious, immersed ascomata as well as the absence of pseudoparaphyses of V. pruni disagree with Venturia s. str.
Venturia pulchella Cooke & Peck, in Peck, Annual Rep. New York St. Mus. Nat. Hist. 25: 106. 1873 [1872]. Fig. 78.
Fig. 78.
Venturia pulchella (HMAS 43696) sexual morph. A. Ascomata densely scattered on the host surface. B. Section of an ascoma, the peridium of which comprises a few layers of textura angularis. C, D. Evanescent pseudoparaphyses. E–G. Broadly cylindrical to somewhat obclavate asci. H. Seta. I–M. Pale brown to olivaceous brown, 1-septate, asymmetrical ascospores. Scale bars: A = 200 μm; B = 20 μm; C–H = 10 μm; I applies to I–M = 10 μm.
Synonym: Gibbera pulchella (Cooke & Peck) Petr., Sydowia 1: 200. 1947.
Ascomata epiphyllous, 100–180 μm diam, 100–140 μm high, gregarious, scattered or solitary, superficial, globose to subglobose, wall black, rough, covered with setae. Setae dark brown, 31–61 × 6–9 μm, base swollen, up to 10–15 μm, setae wall 1.2–1.8 μm. Peridium 18–24 μm wide, thicker near the apex (38–45 μm wide), 2-layered, outer wall composed of thickened cells of textura angularis, cells 6–13 μm diam, cell wall 1–3 μm thick; inner wall composed of thin-walled textura angularis. Pseudoparaphyses dense, 2–4 μm wide, hyaline, septate, constricted at the septum, apex swollen. Asci 60–93 × 8–13 μm (av. 76.5 × 10 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical to somewhat obclavate. Ascospores 11–14 × 4–5 μm (av. 13 × 6 μm, n = 20), ellipsoid, pale brown, obliquely uniseriate or partly overlapping to biseriate near the base, 1-septate, slightly constricted at the septum, with broadly rounded ends, the upper cells shorter than the lower ones (length ratio: 9:17–3:4), smooth-walled. Asexual morph unknown.
Typus:USA, New York, Albany, Center, C.H. Peck (isotype NYSf2478).
Additional materials examined: Canada, Lake Ontario, on leaves of Chamaedaphne calyculata (Ericaceae), 3 Jul. 1935, J.W. Groves (HMAS 03160). USA, New Hampshire, on leaves of C. calyculata, 18 May 1908, W.G. Farlow (HMAS 43696).
Note: The superficial ascomata, dense paraphysoids with swollen pigmented tips that are closely agglutinated, forming a heavy epithecium above the asci, point to Patellariaceae (Patellariales).
Venturia rhois Sawada, Special Publ. Coll. Agric., Natl. Taiwan Univ. 8: 73. 1959. Nom. inval., Art. 39.1 (Shenzhen). Fig. 79.
Fig. 79.
Venturia rhois (type HMAS 11670) sexual morph. A. Ascomata scattered on the host surface. B–H. Cylindrical to subclavate asci (in cotton blue). I–O. Hyaline, 1-septate ascospores (in cotton blue). Scale bars: A = 100 μm; B–H = 10 μm; I–O = 5 μm.
Ascomata amphigenous, 60–80 μm diam, solitary or scattered, initially immersed, becoming erumpent, globose to subglobose, wall black, with a conspicuous papillate ostiole. Setae not observed. Peridium 1-layered, composed of (1–)2–3 rows of pigmented cells of textura angularis, cells 8–12 μm diam, cell wall 0.8–1.5 μm thick. Pseudoparaphyses not observed. Asci 29–66 × 7–10 μm (av. 42.2 × 8.4 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical, with a short, knob-like pedicel or pedicel lacking, each with an inconspicuous ocular chamber. Ascospores 11–17 × 2.5–4 μm (av. 13.5 × 2.8 μm, n = 20), cylindrical, hyaline, obliquely uniseriate or partly overlapping to biseriate, 1-septate, with narrowly rounded ends, the upper cells slightly wider than the lower ones, smooth-walled. Asexual morph unknown.
Typus: China, Taichung City, on overwintered leaves of Rhus javanicus (Anacardiaceae), 23 Aug. 1944, K. Sawada (holotype HMAS 11670).
Notes: Venturia rhois was described by Sawada (1959) without a Latin diagnosis, rendering it invalid. Its hyaline, 1-septate ascospores and absence of paraphyses point to Mycosphaerellaceae.
Discussion
A total of 30 genera are treated in the Venturiales, of which eight are newly described. For 19 of these genera, the phylogenetic status has been confirmed via DNA data of the type species, i.e., Bellamyces, Cylindrosympodioides, Cylindrosympodium, Fagicola, Fraxinicola, Neofusicladium, Parafusicladium, Fuscohilum, Pinaceicola, Pseudoanungitea, Scolecobasidium, Sterila, Sympodiella, Sympoventuria, Tothia, Tyrannosorus, Venturia s. str., Veronaeopsis and Verruconis. Although more than 20 genera have previously been linked to Venturiales (Hyde et al., 2013, Wijayawardene et al., 2014, 2017), these proposed classifications were mostly devoid of DNA data.
Based on the multilocus datasets generated in the present study, three families are now recognised within Venturiales, i.e., Cylindrosympodiaceae, Sympoventuriaceae and Venturiaceae. The Cylindrosympodiaceae includes the genera Cylindrosympodium, Pseudoanungitea, Septonema, Sympodiella and Tothia. Morphologically, the hyphomycetous asexual morph, sympodial conidiogenesis, solitary as well as concatenate, subcylindrical, ampulliform to fusoid-ellipsoid conidia point to Venturiales. Although the lifestyles of only a few members of Cylindrosympodiaceae were clarified, where known, they are saprophytic (Fig. 1). The host range of genera of Cylindrosympodiaceae is rather wide, with Sympodiella occurring on members of Pinus (Pinaceae), Betula (Betulaceae) or Fagus (Fagaceae), and Cylindrosympodium on Laurus (Lauraceae) or Pinus (Pinaceae). Geographically, almost all of the known species of Cylindrosympodiaceae are from Europe, which could be due to limited sampling on other continents.
The Venturiaceae is the largest family within Venturiales, comprising 11 genera, of which two, Fraxinicola and Fagicola, are newly described. Venturia, the largest genus within the Venturiaceae, had a rather confused history. Venturia De Not. was introduced to accommodate V. rosea and V. dianthi (De Notaris 1844). Subsequently, Cesati & De Notaris (1863) described two additional species, i.e., V. dickiei and V. eres. Saccardo (1882) emended the description of Venturia De Not., excluded both V. rosea and V. dianthi, while accepting V. dickiei and V. eres. Venturia Sacc. was widely accepted, and was neotypified by V. inaequalis (Korf, 1956, Sivanesan, 1977). The circumscription of Venturia had been modified several times (Saccardo 1883, Sydow 1932, Korf, 1956, Müller and Menon, 1956, Sivanesan, 1977). Based on morphology, ecological characteristics and DNA sequence comparisons, Zhang et al. (2011) proposed a narrower concept for Venturia, comprising plant parasitic species closely related to the generic type, V. inaequalis. By comparing morphological characteristics and related DNA sequence data, Venturia was re-defined as follows: 1) ascomata immersed, semi-immersed or superficial, scattered or gregarious, often papillate and ostiolate with setae (except for members with immersed ascomata); 2) hamathecium narrowly cellular, hyaline, evanescent in mature ascomata; 3) asci 8-spored, bitunicate, fissitunicate, broadly cylindrical to obclavate, usually lacking a pedicel; 4) ascospores pale olivaceous to brown, 1-septate, usually asymmetrical (Zhang et al., 2016a, Zhang et al., 2016b). This generic circumscription of Venturia was followed in the present study. Of the 59 specimens of species loaned from herbaria, 37 (59 %) were accepted within Venturia, while other species were reallocated to Gibbera, Niesslia, or the Mycosphaerellaceae.
The Sympoventuriaceae was introduced based on a well-supported subclade comprising Sympoventuria, Veronaeopsis simplex and fusicladium-like species (Zhang et al. 2011). Subsequently, more genera have been accepted in the family, such as Ochroconis, Scolecobasidium and Verruconis (Machouart et al., 2014, Samerpitak et al., 2014). Scolecobasidium, the largest genus within the Sympoventuriaceae, was described based on two species, S. terreum and S. constrictum, which are characterised by rust-brown to olivaceous colonies producing small, brownish conidiophores bearing small numbers of dark, septate, rough-walled, rhexolytic conidia (Abbott, 1927, Ellis, 1976). Scolecobasidium terreum was designated as the generic type, which has Y-shaped and yellowish conidia (Abbott 1927). More species with unbranched and darker conidia were described within Scolecobasidium (Matsushima 1975), which led to the introduction of another genus, Ochroconis (de Hoog & von Arx 1974). Ochroconis, typified by O. constricta, has sympodial conidiogenesis and unbranched, subspherical to cylindrical or clavate, melanised conidia. The number of species in the generic complex has increased significantly over the years (De Hoog, 1985, Samerpitak et al., 2014, Samerpitak et al., 2017). Ochroconis is a rather common genus of saprotrophic soil hyphomycetes, some of which are parasitic on humans, fish or other animals (Samerpitak et al. 2017). The type strains of both S. terreum (CBS 203.27) and O. constricta (CBS 202.27), unfortunately, are now sterile (Horré et al. 1999, Gams 2015). Based on the single-locus analyses of nuSSU, nuLSU, ITS, ACT1, TUB2, and tef1-a, Samerpitak et al. (2014) indicated that Ochroconis and Scolecobasidium clustered together, while Scolecobasidium was considered as doubtful because of the type material was “ambiguous”. This proposal, however, was not recognised by some researchers (Seifert et al., 2011, Gams, 2015). Although the ex-type strain of S. terreum is sterile, there are many reliably named cultures of S. terreum globally, which clearly define the identity of this characteristic fungus (Gams 2015), which clusters with species accommodated in Ochroconis. Based on the principle of priority, Scolecobasidium was thus chosen over Ochroconis in the present study. Furthermore, six new genera were introduced within Sympoventuriaceae. The multilocus phylogenetic analyses indicated that Scolecobasidium and its closely related neighbours belong to the family Sympoventuriaceae in the order Venturiales (Machouart et al. 2014, this study).
The morphological characteristics of sexual morphs within Venturiales are rather conservative. Due to the overlapping morphological characteristics of sexual morphs among venturiaceous species, the asexual morph proved to be more reliable for species identification (Schubert et al. 2003). The morphology of the conidial apparatus, including conidiophores, conidiogenous cells and conidia has been widely used in the traditional taxonomy of Venturiales (Sivanesan, 1977, Schubert et al., 2003, Crous et al., 2007b). Of all the features plotted in Fig 1, Fig 2, conidial arrangement (solitary or in chains), proved to be informative at the generic level (except in Venturia s. str.). The mode of conidiogenesis, i.e., sympodial proliferation (Fusicladium), monoblastic, determinate to percurrent proliferation (Pollaccia, with few rather inconspicuous annellations) and percurrent proliferation with conspicuous annellations (Spilocaea) showed little significance at generic level classification. This view was also supported by Schubert et al. (2003) for Venturia, and for various genera in Mycosphaerellaceae (Videira et al. 2017).
The Venturia clade presently includes isolates from various host families such as Betulaceae, Caprifoliaceae, Convolvulaceae, Gentianaceae, Oleaceae, Polygonaceae, Rosaceae, Salicaceae as well as lichens. The tendency of host-shift speciation between hosts and Venturia species had been documented by Schnabel et al. (1999) and Schubert et al. (2003). In this study, some well-circumscribed genera, such as Fraxinicola, Neofusicladium, Parafusicladium, Sympoventuria and Tyrannosorus showed a stronger host generic specialization. In contrast, the current Venturia s. str. clade seems not well resolved, as it contains taxa associated with various host genera or families.
The ancestral state of Venturiales is most likely saprobic, and plant pathogens appear to be a new evolutionary state, as has been reported for Capnodiales (Abdollahzadeh et al. 2020) and Dothideomycetes in general (Haridas et al. 2020). Members of plant pathogens have arisen from saprotrophic members in both Venturiaceae and Sympoventuriaceae, clustering terminal in the phylogenetic trees (Fig 1, Fig 2). Similar results have been reported for the majority of lineages in the larger context of Ascomycota (Schoch et al., 2009a, Schoch et al., 2009b), or at ordinal level, such as Pleosporales and Capnodiales (Crous et al. 2009, Zhang et al. 2009). The most interesting is that saprotrophic fungal ancestors had repeatedly lost their plant cell wall degradation enzymes and obtained effector-like secreted proteins to fit a plant-fungal associated lifestyle (as ectomycorrhizas, ECM) (Kohler et al., 2015, Martin et al., 2016). Thus, the saprotrophic lifestyle seems ancestral at ordinal level (Venturiales), as well as at class level (Dothideomycetes) (Haridas et al. 2020).
Although the present study has clarified our understanding of families and genera in Venturiales, future studies will undoubtedly add many more genera and species to this order, given its wide ecological and geographic distribution.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (General Programs, 31971658, 31770015, 31370063) and NSFC Projects of International Cooperation and Exchanges (3155461143028). We are grateful to the Directors and Curators of the following herbaria for loan of specimens in their keeping: BRIP, CGMCC, CUP, HMAS, K, MICH, NY, NYS, PDD, PPMH, VPRI, W and ZT. We are also grateful to Arien van Iperen, Diana Vos-Kleyn, Yda Vlug (cultures), Mieke Starink-Willemse (DNA isolation, amplification and sequencing), and Marjan Vermaas (photographic plates) for their technical assistance.
Footnotes
Peer review under responsibility of Westerdijk Fungal Biodiversity Institute.
References
- Abbott E.V. Scolecobasidium, a new genus of soil fungi. Mycologia. 1927;19:29–31. [Google Scholar]
- Abdollahzadeh J., Groenewald J.Z., Coetzee M.P.A., et al. Evolution of lifestyles in Capnodiales. Studies in Mycology. 2020 doi: 10.1016/j.simyco.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderhold R. Revision der Species Venturia chlorospora, inaequalis und ditricha autorum. Hedwigia. 1897;36:67–83. [Google Scholar]
- Ando K., Nakamura N. Pseudosigmoidea: a new genus for a hyphomycete (ATCC 16660) formerly identified as Sigmoidea prolifera. Journal of General and Applied Microbiology. 2000;46:51–57. doi: 10.2323/jgam.46.51. [DOI] [PubMed] [Google Scholar]
- Arzanlou M., Groenewald J.Z., Gams W., et al. Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology. 2007;58:57–93. doi: 10.3114/sim.2007.58.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveskamp M.M., Verkley G.J.M., de Gruyter J., et al. DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia. 2009;101:363–382. doi: 10.3852/08-199. [DOI] [PubMed] [Google Scholar]
- Bachmann C. Untersuchungen an Geraniaceen bewohnenden Venturiaceen. Journal of Phytopathology. 1963;47:197–206. [Google Scholar]
- Baldacci E., Ciferri R. vol. 10. 1937. Un nuovo genere di micete parassita del pioppo Pollaccia radiosa (Lib.) Baldacci e Ciferri, Revisione dei G. Stigmella e Stigmina. I. Pollaccia radiosa (Lib.) Baldacci e Ciferri; pp. 55–72. (Atti dell'Istituto Botanico della Università e Laboratorio Crittogamico di Pavia). [Google Scholar]
- Barr M.E. Northern Pyrenomycetes: II. Gaspesian park. Canadian Journal of Botany. 1961;39:307–325. [Google Scholar]
- Barr M.E. The Venturiaceae in North America. Canadian Journal of Botany. 1968;46:799–864. [Google Scholar]
- Barr M.E. A classification of Loculoascomycetes. Mycologia. 1979;71:935–957. [Google Scholar]
- Barr M.E. University of Massachusetts; Amherst: 1987. Prodromus to class Loculoascomycetes. [Google Scholar]
- Barr M.E. The Venturiaceae in North America: revisions and additions. Sydowia. 1989;41:25–40. [Google Scholar]
- Barr M.E. Redisposition of some taxa described by. J.B. Ellis. Mycotaxon. 1993;46:45–76. [Google Scholar]
- Barr M.E. Notes on some ‘Dimeriaceous’ fungi. Mycotaxon. 1997;64:149–171. [Google Scholar]
- Barron G.L., Busch L.V. Studies on the soil hyphomycete Scolecobasidium. Canadian Journal of Botany. 1962;40:77–84. [Google Scholar]
- Beck A., Ritschel A., Schubert K., et al. Phylogenetic relationships of the anamorphic genus Fusicladium s. lat. as inferred by ITS nrDNA data. Mycological Progress. 2005;4:111–116. [Google Scholar]
- Bonorden H.F. Schweizerbart'sche Verlagshandlung; Stuttgart: 1851. Handbuch der allgemeinen Mykologie. [Google Scholar]
- Boonmee S., Bhat J.D., Maharachchikumbura S.S.N., et al. Clavatispora thailandica gen. et sp. nov., a novel taxon of Venturiales (Dothideomycetes) from Thailand. Phytotaxa. 2014;176:092–101. [Google Scholar]
- Braun U., Bensch K. Annotated list of taxonomic novelties published in “Fungi Europaei Exsiccati, Klotzschii Herbarium Vivum Mycologicum Continuato, Editio Nova, Series Secunda” Cent. 1 to 26 issued by G. L. Rabenhorst between 1859 and 1881 (first part – Cent. 1 to 10) Schlechtendalia. 2019;36:1–60. [Google Scholar]
- Campbell R., Sutton B.C. Conidial ontogeny in Echinocatena arthrinioides gen. et sp. nov. (Deuteromycotina: Hyphomycetes) Transactions of the British Mycological Society. 1977;69:125–131. [Google Scholar]
- Cano J., Guarro J., Gene J. Molecular and morphological identification of Colletotrichum species of clinical interest. Journal of Clinical Microbiology. 2004;42:2450–2454. doi: 10.1128/JCM.42.6.2450-2454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañeda-Ruiz R.F., Kendrick B. vol. 32. University of Waterloo Biology Series; 1990. pp. 1–53. (Conidial fungi from Cuba: I). [Google Scholar]
- Castañeda-Ruiz R.F., Kendrick B. vol. 35. University of Waterloo Biology Series; 1991. pp. 1–132. (Ninety-nine conidial fungi from Cuba and three from Canada). [Google Scholar]
- Castañeda-Ruiz R.F., Gams W., Saikawa M. Three new conidial fungi (Hyphomycetes) from Cuba. Nova Hedwigia. 1997;64:473–483. [Google Scholar]
- Castañeda-Ruiz R.F., Heredia G.A., Arias R.M.M., et al. Two new fungi from Mexico: Anaseptoidium gen. nov. and Cylindrosympodium sosae sp. nov. Mycotaxon. 2012;119:141–148. [Google Scholar]
- Cesati V., De Notaris G. Schema di classificazione degli sferiacei italici aschigeri. Commentario della Società Crittogamologica Italiana. 1863;1:177–240. [Google Scholar]
- Crous P.W., Groenewald J.Z., Shivas R.G. Fusicladium eucalypti. Fungal Planet 64. Persoonia. 2010;25:148–149. [Google Scholar]
- Crous P.W., Kendrick W.B., Alfenas A.C. New species of hyphomycetes associated with Eucalyptus. South African Journal of Botany. 1997;63:286–290. [Google Scholar]
- Crous P.W., Mohammed C., Glen M., et al. Eucalyptus microfungi known from culture. 3. Eucasphaeria and Sympoventuria genera nova, and new species of Furcaspora, Harknessia, Heteroconium and Phacidiella. Fungal Diversity. 2007;25:19–36. [Google Scholar]
- Crous P.W., Schubert K., Braun U., et al. Opportunistic, human-pathogenic species in the Herpotrichiellaceae are phenotypically similar to saprobic or phytopathogenic species in the Venturiaceae. Studies in Mycology. 2007;58:185–217. doi: 10.3114/sim.2007.58.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Schoch C.L., Hyde K.D., et al. Phylogenetic lineages in the Capnodiales. Studies in Mycology. 2009;64:17–47. doi: 10.3114/sim.2009.64.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Schumacher R.K., Akulov A., et al. New and Interesting Fungi. 2. Fungal Systematics and Evolution. 2019;3:57–134. doi: 10.3114/fuse.2019.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Schumacher R.K., Wingfield M.J., et al. New and Interesting Fungi. 1. Fungal Systematics and Evolution. 2018;1:169–215. doi: 10.3114/fuse.2018.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Summerell B.A., Swart L., et al. Fungal pathogens of Proteaceae. Persoonia. 2011;27:20–45. doi: 10.3767/003158511X606239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Verkley G.J.M., Groenewald J.Z., et al., editors. Westerdijk Laboratory manual series 1: fungal biodiversity. Westerdijk Fungal Biodiversity Institute; Utrecht, the Netherlands: 2019. [Google Scholar]
- Crous P.W., Wingfield M.J., Burgess T.I., et al. Fungal planet description sheets: 625–715. Persoonia. 2017;39:270–467. doi: 10.3767/persoonia.2017.39.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Kendrick W.B. Foliicolous dematiaceous hyphomycetes from Syzygium cordatum. Canadian Journal of Botany. 1995;73:224–234. [Google Scholar]
- Crous P.W., Wingfield M.J., Richardson D.M., et al. Fungal planet description sheets: 400–468. Persoonia. 2016;36:316–458. doi: 10.3767/003158516X692185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hoog G.S. Taxonomy of the Dactylaria complex, IV. Dactylaria, Neta, Subulispora and Scolecobasidium. Studies in Mycology. 1985;26:1–60. [Google Scholar]
- De Hoog G.S., von Arx J.A. [“1973”], Revision of Scolecobasidium and Pleurophragmium. Kavaka. 1974;1:55–60. [Google Scholar]
- De Notaris G. Cenno sμlla tribu de'pirenomiceti sferiacei e descrizione di alcuni nuovi generi. Nuovo Giornale Botanico Italiano. 1844;1:322–335. [Google Scholar]
- Dugan F.M., Roberts R.G., Hanlin R.T. New and rare fungi from cherry fruits. Mycologia. 1995;87:713–718. [Google Scholar]
- Ellis M.B. Commonwealth Mycological Institute; Kew, England: 1976. More Dematiaceous Hyphomycetes. [Google Scholar]
- Ellis J.B., Everhart B.M. New species of fungi from various localities. Proceedings of the Academy of Natural Sciences of Philadelphia. 1890;42:219–249. [Google Scholar]
- Evans H.C. Thermophilous fungi of coal spoil tips. I. Taxonomy. Transactions of the British Mycological Society. 1971;57:241–254. [Google Scholar]
- Evans H.C. Thermophilous fungi of coal spoil tips. II. Occurrence, distribution and temperature relationships. Transactions of the British Mycological Society. 1971;57:255–266. [Google Scholar]
- Ferraris T. Hyphales, Dematiaceae. Flora Italica Cryptogama Fungi. 1912;1:195–534. [Google Scholar]
- Gams W. An ex-type culture cannot always tell the ultimate truth. IMA Fungus. 2015;6:69. [Google Scholar]
- Gams W., Stielow B., Gräfenhan T., et al. The ascomycete genus Niesslia and associated monocillium-like anamorphs. Mycological Progress. 2019;18:1–72. [Google Scholar]
- Giraldo A., Sutton D.A., Samerpitak K., et al. Occurrence of Ochroconis and Verruconis species in clinical specimens from the United States. Journal of Clinical Microbiology. 2014;52:4189–4201. doi: 10.1128/JCM.02027-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass N.L., Donaldson G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied & Environmental Microbiology. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh T.K., Hyde K.D. Helicoon gigantisporum sp. nov. and an amended key to the genus. Mycological Research. 1996;100:1485–1488. [Google Scholar]
- Goos R.D., Abdullah S.K., Fisher P.J., et al. The anamorph genus Helicoon. Transactions of the British Mycological Society. 1986;87:115–122. [Google Scholar]
- Guo M., Pan Y.M., Dai Y.L., et al. First report of brown blight disease caused by Colletotrichum gloeosporioides on Camellia sinensis in Anhui Province, China. Plant Disease. 2014;98:284. doi: 10.1094/PDIS-08-13-0896-PDN. [DOI] [PubMed] [Google Scholar]
- Hao L., Chen Ch, Zhang R., et al. A new species of Scolecobasidium associated with the sooty blotch and flyspeck complex on banana from China. Mycological Progress. 2013;12:489–495. [Google Scholar]
- Haridas S., Albert R., Binder M., et al. 101 Dothideomycetes genomes: a test case for predicting lifestyles and emergence of pathogens. Studies in Mycology. 2020;96:141–153. doi: 10.1016/j.simyco.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M.H.-M., Castañeda R.F., Dugan F.M., et al. Cladosporium and Cladophialophora in culture: descriptions and an expanded key. Mycotaxon. 1999;72:115–157. [Google Scholar]
- Horré R., de Hoog G.S. Primary cerebral infections by melanised fungi: a review. Studies in Mycology. 1999;43:176–193. [Google Scholar]
- Hughes S.J. Some foliicolous hyphomycetes. Canadian Journal of Botany. 1953;31:560–576. [Google Scholar]
- Hyde K.D., Jones E.B.G., Liu J.K., et al. Families of Dothideomycetes. Fungal Diversity. 2013;63:1–313. [Google Scholar]
- Ibrahim M., Schlegel M., Sieber T.N. Venturia orni sp. nov. a species distinct from Venturia fraxini, living in the leaves of Fraxinus ornus. Mycological Progress. 2016;15:1–12. [Google Scholar]
- Johnston P.R., Park D. Neocoleroa metrosideri sp. nov. (Sympoventuriaceae, Venturiales) Phytotaxa. 2016;253:214–218. [Google Scholar]
- Karesh W.B., Russell R., Gribble D. Dactylaria gallopava encephalitis in two grey-winged trumpeters (Psophia crepitans) Avian Diseases. 1987;31:685–688. [PubMed] [Google Scholar]
- Karsten P.A. Diagnoses fungorum nonnullorum novorum, in Fennia detectorum. Revue Mycologique Toulouse. 1888;10:73–75. [Google Scholar]
- Kearse M., Moir R., Wilson A., et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk P.M., Cannon P.F., Minter D.W., et al. CAB International; Wallingford: 2008. Ainsworth & Bisby’s dictionary of the fungi. [Google Scholar]
- Kodsueb R., Jeewon R., Vijaykrishna D., et al. Systematic revision of Tubeufiaceae based on morphological and molecular data. Fungal Diversity. 2006;21:105–130. [Google Scholar]
- Kohler A., Kuo A., Nagy L.G., et al. Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nature Genetics. 2015;47:410–415. doi: 10.1038/ng.3223. [DOI] [PubMed] [Google Scholar]
- Koorders S.H. Botanische Untersuchungen. Verhandelingen Koninklijke Nederlandse Akademie van Wetenschappen Afdeling Natuurkunde. 1907;13(2):1–264. [Google Scholar]
- Korf R.P. Nomenclatural Notes. I. Misuse of Neotypes for Venturia and Phaeosphaerella. Mycologia. 1956;48:591–595. [Google Scholar]
- Koukol O. Revision of “Septonema ochraceum” revealed three new species of Venturiaceae and Herpotrichiellaceae. Mycological Progress. 2010;9:369–378. [Google Scholar]
- Kruys A., Eriksson O.E., Wedin M. Phylogenetic relationships of coprophilous Pleosporales (Dothideomycetes, Ascomycota), and the classification of some bitunicate taxa of unknown position. Mycological Research. 2006;110:527–536. doi: 10.1016/j.mycres.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Lindau G. In: Kryptogamen-Flora von Deutschland, Österreich und der Schweiz. Rabenhorst L., editor. 1907. Fungi imperfecti: Hyphomycetes. (1 Pilze, VIII Abt). [Google Scholar]
- Linder D.H. A monograph of the helicosporous fungi imperfecti. Annals of the Missouri Botanical Garden. 1929;16:227–388. [Google Scholar]
- Liu J.K., Kevin D.H., Gareth Jones E.B., et al. Fungal diversity notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Diversity. 2015;72:1–197. [Google Scholar]
- Liu Y.J., Whelen S., Hall B.D. Phylogenetic relationships among ascomycetes: evidence from an RNA Polymerase II subunit. Molecular Biology and Evolution. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- Lumbsch H.T., Huhndorf S.M. Myconet Volume 14. Part One. Outline of Ascomycota (2009). Part Two. Notes on Ascomycete Systematics. Nos. 4751–5113. Fieldiana Life and Earth Sciences. 2010;1:1–64. [Google Scholar]
- Lunghini D., Toscano F. Studies on Mediterranean hyphomycetes. VII. Septonema crispulum anam.-sp. nov. Mycotaxon. 1997;63:329–334. [Google Scholar]
- Luttrell E.S. In: The fungi, an advanced treatise, a taxonomic review with keys: ascomycetes and fungi imperfecti. Ainsworth G.C., Sparrow F.K., Sussman A.S., editors. Academic; New York: 1973. Loculoascomycetes. [Google Scholar]
- Machouart M., Samerpitak K., de Hoog G.S., et al. A multigene phylogeny reveals that Ochroconis belongs to the family Sympoventuriaceae (Venturiales, Dothideomycetes) Fungal Diversity. 2014;65:77–88. [Google Scholar]
- Marin-Felix Y., Groenewald J.Z., Cai L., et al. Genera of phytopathogenic fungi: GOPHY 1. Studies in Mycology. 2017;86:99–216. doi: 10.1016/j.simyco.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F., Kohler A., Murat C., et al. Unearthing the roots of ectomycorrhizal symbioses. Nature Reviews Microbiology. 2016;14:760–773. doi: 10.1038/nrmicro.2016.149. [DOI] [PubMed] [Google Scholar]
- Marvanová L., Laichmanová M. Subulispora biappendiculata, anamorph sp. nov. from Borneo (Malaysia) and a review of the genus. Fungal Diversity. 2007;26:241–256. [Google Scholar]
- Matsushima T. 1975. Icones microfungorum: a Matsushima lectorum. Kobe, Japan. [Google Scholar]
- Menon R. Studies on Venturiaceae on Rosaceous plants. Phytopathologische Zeitschrift. 1956;27:117–146. [Google Scholar]
- Morelet M. Les Venturia des peupliers de la section Leuce. I. Taxonomie. Cryptogamie Mycologie. 1985;6:101–117. [Google Scholar]
- Morgan A.P. North American helicosporae. Cincinnati Society of Natural History Journal. 1892;15:39–52. [Google Scholar]
- Müller E. Systematische Bemerkungen über einige Venturia-Arten. Sydowia. 1958;11:79–92. [Google Scholar]
- Müller E., Menon R. Uber Venturia rosae de Not. und die Nomenklatur der Gattung Venturia de Not. Phytopathologische Zeitschrift. 1956;25:190–195. [Google Scholar]
- Müller E., von Arx J.A. Einige Aspekte zur Systematik pseudosphaerialer Ascomyceten. Berichte der Schweizerischen Botanischen Gesellschaft. 1950;60:329–397. [Google Scholar]
- Müller E., von Arx J.A. Die Gattungen der didymosporen Pyrenomyceten. Beiträge zur Kryptogamenflora der Schweiz. 1962;11:1–922. [Google Scholar]
- Nüesch J. Beitrag zur Kenntnis der weidenbewohnenden Venturiaceae. Phytopathologische Zeitschrift. 1960;39:329–360. [Google Scholar]
- Nylander J.A.A. Evolutionary Biology Centre, Uppsala University; 2004. MrModeltest v2. Program distributed by the author. [Google Scholar]
- Papendorf M.C. New South African soil fungi. Transactions of the British Mycological Society. 1969;52:483–489. [Google Scholar]
- Paulus B.C., Gadek P.A., Hyde K.D. Cylindrosympodium cryptocaryae sp. nov. (anamorphic fungi), with keys to the described species and to similar genera. Australian Systematic Botany. 2003;16:577–580. [Google Scholar]
- Peck C.H. Report of the State Botanist. 1912. Bulletin of the New York State Museum. 1913;167:1–137. [Google Scholar]
- Petrak F. Mykologische Notizen. VII. Annales Mycologici. 1924;22:1–182. [Google Scholar]
- Petrak F. Mykologische Notizen. IX. Annales Mycologici. 1927;25:193–343. [Google Scholar]
- Petrak F. Mykologische Beiträge zur Flora von Sibirien. Hedwigia. 1934;74:30–78. [Google Scholar]
- Petrak F. Über Gibbera Fr. und verwandte Gattungen. Sydowia. 1947;1:169–201. [Google Scholar]
- Qiao M., Tian W.G., Castañeda-Ruiz R.F., Xu J.P., Yu Z.F. Two new species of Verruconis from Hainan, China. Mycokeys. 2019;48:41–53. doi: 10.3897/mycokeys.48.32147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao T.M., Zhang J., Li S.J., et al. Development of nested PCR, multiplex PCR, and loop-mediated isothermal amplification assays for rapid detection of Cylindrocladium scoparium on Eucalyptus. Plant Pathology Journal. 2016;32:414–422. doi: 10.5423/PPJ.OA.03.2016.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A. 2009. FigTree v. 1.3.1.http://tree.bio.ed.ac.uk/software/ Computer program and documentation distributed by the author at. [Google Scholar]
- Randall C.J., Owen D.W. Encephalitis in broiler chickens caused by a hyphomycete resembling Dactylaria gallopava. Avian Pathology. 1981;10:31–41. doi: 10.1080/03079458108418456. [DOI] [PubMed] [Google Scholar]
- Reeb V., Lutzoni F., Roux C. Contribution of RPB2 to multilocus phylogenetic studies of the euascomycetes (Pezizomycotina, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Molecular Phylogenetics and Evolution. 2004;32:1036–1060. doi: 10.1016/j.ympev.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Redman R.S., Litvenseva A., Sheehan K.B., et al. Fungi from geothermal soil in Yellowstone National Park. Applied and Environmental Microbiology. 1999;65:5193–5197. doi: 10.1128/aem.65.12.5193-5197.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J., Jie C.Y., Zhou Q.X., et al. Molecular and morphological data reveal two new species of Scolecobasidium. Mycoscience. 2013;54:420–425. [Google Scholar]
- Rippon J.W., Gerhold R., Heath M. Thermophilic and thermotolerant fungi isolated from the thermal effluent of nuclear power generating reactors: dispersal of human opportunistic and veterinary pathogenic fungi. Mycopathologia. 1980;70:169–179. doi: 10.1007/BF00443028. [DOI] [PubMed] [Google Scholar]
- Ronquist F., Teslenko M., Van der Mark P., et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman A.Y., Castlebury L.A., Aguirre-Hudson B., et al. 2647–2651) Proposals to conserve the names Venturia acerina against Cladosporium humile; Venturia borealis against Torula maculicola; Venturia carpophila against Fusicladium amygdali and Cladosporium americanum; Sphaerella inaequalis (Venturia inaequalis) against Spilocaea pomi, Fumago mali, Actinonema crataegi, Cladosporium dendriticum, Asteroma mali, and Scolicotrichum venosum; and Venturia pyrina against Helminthosporium pyrorum, Fusicladium virescens, F. fuscescens, Cladosporium polymorphum and Passalora pomi (Ascomycota: Dothideomycetes) Taxon. 2018;67:1209–1211. [Google Scholar]
- Rossman A.Y., Crous P.W., Hyde K.D., et al. Recommended names for pleomorphic genera in Dothideomycetes. IMA Fungus. 2015;6:507–523. doi: 10.5598/imafungus.2015.06.02.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R.Y., Dwivedi R.S., Mishra R.R. Two new species of Scolecobasidium from soil. Lloydia. 1962;25:164–166. [Google Scholar]
- Saccardo P.A. Sumptibus auctoris; Patavii: 1882. Sylloge fungorum omnium hucusque cognitorum. [Google Scholar]
- Saccardo P.A. vol. II. Sumptibus auctoris; Patavii: 1883. (Sylloge Fungorum). [Google Scholar]
- Saccardo P.A. Sumptibus auctoris; Patavii: 1886. Sylloge Fungorum Omnium Hucusque Cognitorum Pavia. [Google Scholar]
- Saccardo P.A. vol. XII. Borntraeger; Berlin: 1897. (Sylloge Fungorum). [Google Scholar]
- Samerpitak K., Gloyna K., Gerrits van den Ende A.H.G., et al. A novel species of the oligotrophic genus Ochroconis colonizing indoor wet cells. Mycoscience. 2017;58:290–296. [Google Scholar]
- Samerpitak K., Linde E.V.D., Choi H.J., et al. Taxonomy of Ochroconis, genus including opportunistic pathogens on humans and animals. Fungal Diversity. 2014;65:89–126. [Google Scholar]
- Sawada K. Vol. 8. Special Publication College of Agriculture National Taiwan University; 1959. Descriptive catalogue of Taiwan (Formosan) fungi. Part XI; pp. 1–268. [Google Scholar]
- Schnabel G., Schnabel E.L., Jones A.L. Characterization of ribosomal DNA from Venturia inaequalis and its phylogenetic relationship to rDNA from other tree-fruit Venturia species. Phytopathology. 1999;89:100–108. doi: 10.1094/PHYTO.1999.89.1.100. [DOI] [PubMed] [Google Scholar]
- Schoch C.L., Crous P.W., Groenewald J.Z., et al. A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology. 2009;64:1–15. doi: 10.3114/sim.2009.64.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch C.L., Sung G.H., Lopez-Giraldez F., et al. The Ascomycota tree of life: a phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Systematic Biology. 2009;58:224–239. doi: 10.1093/sysbio/syp020. [DOI] [PubMed] [Google Scholar]
- Schubert K., Ritschel A., Braun U. A monograph of Fusicladium s. lat. (Hyphomycetes) Schlechtendalia. 2003;9:1–132. [Google Scholar]
- Seifert K.A., Morgan-Jones G., Gams W., et al. CBS-KNAW Fungal Biodiversity Centre; Utrecht, the Netherlands: 2011. The genera of Hyphomycetes. [Google Scholar]
- Shen M., Zhang J.Q., Zhang Y. Venturia species form sooty mold-like colonies on leaves of Salix: introducing Venturia fuliginosa sp. nov. Mycosphere. 2016;7:1292–1300. [Google Scholar]
- Sivanesan A. Lubrecht & Cramer Ltd; Vaduz: 1977. The taxonomy and pathology of Venturia species. [Google Scholar]
- Sivanesan A. J. Cramer; Vaduz: 1984. The bitunicate ascomycetes and their anamorphs. [Google Scholar]
- Sivanesan A. The Pseudoparodiella teleomorph of Spilodochium vernoniae. Transactions of the British Mycological Society. 1986;86:187–190. [Google Scholar]
- Stevens F.L. Fungi from Costa Rica and Panama. Illinois Biology Monographs. 1927;11:157–255. [Google Scholar]
- Tai F.L. Science Press, Academia Sinica; Peking China: 1979. Sylloge Fungorum Sinicorum. [Google Scholar]
- Tanaka S., Yamamoto S. Studies on pear scab. II. Taxonomy of the causal fungus of Japanese pear scab. Annals of the Phytopathological Society of Japan. 1964;29:128–136. [Google Scholar]
- Tibpromma S., Hyde K.D., Mckenzie E.H.C., et al. Fungal diversity notes 840–928: micro-fungi associated with Pandanaceae. Fungal Diversity. 2018;92:1–160. [Google Scholar]
- Tsui C.K.M., Berbee M.L. Transfer of two Helicoma species to Troposporella based on molecular and morphological data. Mycoscience. 2010;51:144–148. [Google Scholar]
- Ulloa M., Hanlin R.T. American Phytopathological Society Press; St Paul, Minnesota: 2000. Illustrated dictionary of mycology. [Google Scholar]
- Untereiner W., Bonjean B., Decock C., et al. Belgian Office for Scientific, Technical and Cultural Affairs; Brussels: 1998. BCCM MUCL agro-industrial fungi-yeasts. [Google Scholar]
- Untereiner W.A., Straus N.D. A molecular-morphotaxonomic approach to the systematics of the Herpotrichiellaceae and allied black yeasts. Mycological Research. 1995;99:897–913. [Google Scholar]
- Videira S.I.R., Groenewald J.Z., Nakashima C., et al. Mycosphaerellaceae – chaos or clarity? Studies in Mycology. 2017;87:257–421. doi: 10.1016/j.simyco.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viennot-Bourgin G. Les champignons parasites des plantes cultivées. 1949;1:1–755. [Google Scholar]
- Viennot-Bourgin G., Fernier H. Polymorphisme du Genre Cladosporium. Revue Internationale de Botanique Appliquée et d'agriculture Tropicale, 30e année. Bulletin. 1950;331–332:297–306. [Google Scholar]
- Vilgalys R., Hester M. Rapid genetic identification and mapping enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Arx J.A. Einige neue Ascomyceten aus der Schweiz. Sydowia. 1950;4:389–398. [Google Scholar]
- von Arx J.A. Studies on Venturia and related genera. Tijdschrift Over Plantenziekten. 1952;58:260–266. [Google Scholar]
- von Arx J.A. Mycosphaerella joerstadii auf Rubus chamaemorus. Acta Botanica Neerlandica. 1957;6:337–340. [Google Scholar]
- von Arx J.A. J. Cramer; Berlin: 1987. Plant pathogenic fungi. [Google Scholar]
- Waldrip D.W., Padhye A.A., Ajello L., et al. Isolation of Dactylaria gallopava from broiler-house litter. Avian Diseases. 1974;18:445–451. [PubMed] [Google Scholar]
- Weitzman I., Rosenthal S.A., Shupack J.L. A comparison between Dactylaria gallopava and Scolecobasidium humicola: first report of an infection in tortoise caused by S. humicola. Sabouraudia. 1983;23:287–293. [PubMed] [Google Scholar]
- White T.J., Bruns T., Lee S., et al. In: PCR protocols: a guide to methods and applications. Innis M.A., Gelfand D.H., Sninsky J.J., et al., editors. Academic Press; New York, USA: 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics; pp. 315–322. [Google Scholar]
- Wijayawardene N.W., Crous P.W., Kirk P.M., et al. Naming and outline of Dothideomycetes. Fungal Diversity. 2014;69:1–55. doi: 10.1007/s13225-014-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijayawardene N.N., Hyde K.D., Rajeshkumar K.C., et al. Notes for genera – Ascomycota. Fungal Diversity. 2017;86:1–594. [Google Scholar]
- Winter G. Pilze: Ascomyceten. Rabenhorst's Kryptogamen Flora von Deutschland, Oesterreich und der Schweiz, 1, Abt. 1887;2:1–928. [Google Scholar]
- Winton L.M., Stone J.K., Hansen E.M., et al. The systematic position of Phaeocryptopus gaeumannii. Mycologia. 2007;99:240–252. doi: 10.3852/mycologia.99.2.240. [DOI] [PubMed] [Google Scholar]
- Wu H.X., Schoch C.L., Boonmee S., et al. A reappraisal of Microthyriaceae. Fungal Diversity. 2011;51:189–248. doi: 10.1007/s13225-011-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarita K., Sano A., Murata Y., et al. Pathogenicity of Ochroconis gallopava isolated from hot spring in Japan and a review of published reports. Mycopathologia. 2007;164:135–147. doi: 10.1007/s11046-007-9034-7. [DOI] [PubMed] [Google Scholar]
- Yarita K., Sano A., Samerpitak K., et al. Ochroconis calidifluminalis, a sibling of the neurotropic pathogen O. gallopava, isolated from hot spring. Mycopathologia. 2010;170:21–30. doi: 10.1007/s11046-010-9292-7. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Crous P.W., Schoch C.L., et al. A molecular, morphological and ecological re-appraisal of Venturiales – a new order of Dothideomycetes. Fungal Diversity. 2011;51:249–277. doi: 10.1007/s13225-011-0141-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.Q., Dou Z.P., Zhou Y.P., et al. Venturia chinensis sp. nov. a new venturialean ascomycete from Khingan Mountains. Saudi Journal of Biological Sciences. 2016;23:592–597. doi: 10.1016/j.sjbs.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Schoch C.L., Fournier J., et al. Multi-locus phylogeny of Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Studies in Mycology. 2009;64:85–102. doi: 10.3114/sim.2009.64.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.Y., Yu Y., Zhang M.Y., et al. Verruconis panacis sp. nov. an endophyte isolated from Panax notoginseng. International Journal of Systematic and Evolutionary Microbiology. 2018;68:2499–2503. doi: 10.1099/ijsem.0.002862. [DOI] [PubMed] [Google Scholar]
- Zhang J.Q., Zhou Y.P., Dou Z.P., et al. Type studies of six Venturia species. Nova Hedwigia. 2016;102:173–184. [Google Scholar]