Abstract
Anaplasma species are tick-borne pathogens that are obligatory intracellular of ruminants and other mammalians. In this investigation, we systematically reviewed the distribution of anaplasmosis among domestic ruminants in Iran. Five and four English and Persian databases were studied, respectively, based on keywords and throughout 17 years (2001–2017). Thirty-eight articles were included in this systematic review and meta-analysis. Totally, 5093 cattle, 1958 sheep, and 1232 goats corresponding to prevalence of Anaplasma infection from different areas of Iran were examined. The total prevalence of Anaplasma infection was estimated to be 34% (95% CI 27%, 41%) in domestic ruminants. Based on our data, Khozestan (54%) and Khorasan Razavi (46%) provinces were the most prevalent areas in Iran and Kerman (3%) and Hamedan (1%) provinces are the lowest. The highest prevalence of Anaplasma spp. infection was belonged to A. ovis (44%) and the lowest to A. phagocytophilum (1%) with a significant difference among them (p < .001). In addition, the most common diagnostic tests were PCR (54%), microscopy (35%) and ELISA (7%) assays. The high prevalence of ovine and bovine anaplasmosis in Iran, confirms the stability situations of animal anaplasmosis in the studied regions particularly northeastern and southwestern parts of the country. Our data offer valuable and encouraging information as regards the current situation of anaplasmosis in domestic livestock in Iran, which might be useful for active and passive surveillance and preventing plans.
Keywords: Anaplasma spp., Domestic ruminants, Systematic review, Iran
1. Introduction
Anaplasma belongs to the family of Anaplasmataceae (Order Rickettsiales), Gram-negative and infects red blood cells that vertebrates are its main hosts and reservoirs. Anaplasmosis is an important bacterial infection in human and animal health (Bah, 2016). It is mainly transmitted by a number of species of hard Ixodes ticks. According to the many previous reports, Ixodes, Dermacentor, Rhipicephalus and Amblyomma genera are the main species that transmit the Anaplasma spp. in different districts of the world (Rymaszewska and Grenda, 2008). To date, six Anaplasma spp. are recognized in domestic animals (Rymaszewska and Grenda, 2008). Five species of them include, A. marginale, A. centrale, A. phagocytophilium, A. bovis and A. ovis were identified in Iranian ruminants (Aktas et al., 2011). Anaplasmosis, causes important economic losses to animal breeders. Clinical manifestation such as anemia, fever, weight loss, breathlessness, jaundice, abortion and finally death are common in ruminants with anaplasmosis infections (Kaewmongkol et al., 2017). Diagnosis of anaplasmosis in animals is often based on microscopically examinations of thin blood smears with Giemsa staining. Also, several conventional diagnostic tools vary from low to high sensitivity, such as Enzyme-Linked Immunosorbent Assay (ELISA) and Polymerase Chain Reaction (PCR) techniques, were used for determining the prevalence and differentiating the Anaplasma spp. (Khaki et al., 2015; Rodriguez-Morales et al., 2019).
The current study is designed to review the studies that have been conducted on the prevalence of Anaplasma infections in domesticated ruminants from different parts of Iran. Despite different studies about Anaplasma spp. prevalence among domestic animals in Iran, there is not any comprehensive information. According to the effect of anaplasmosis on the economy and public health, more epidemiological studies are recommended. Based on our research, there is no documented review about the prevalence of anaplasmosis among livestock in Iran.
2. Materials and methods
2.1. Searching approach
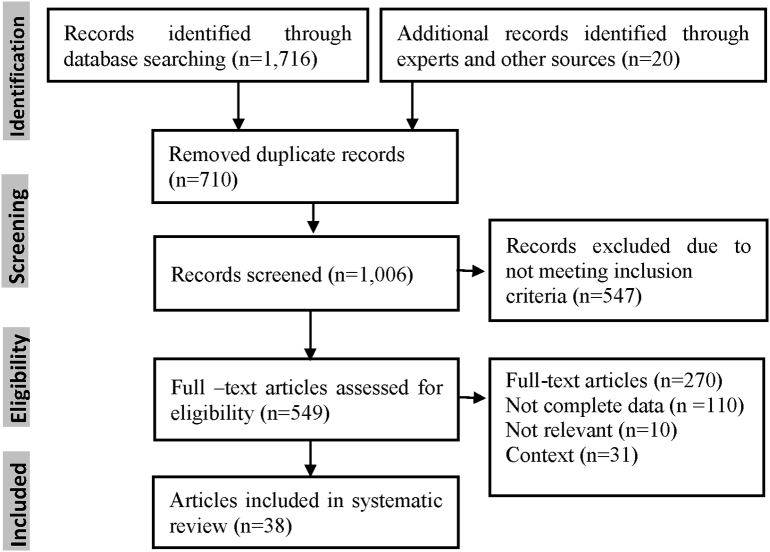
Nine most valuable databases in medicine and veterinary sciences in English and Persian languages, including, Science Direct, Scopus, Web of Science, PubMed, Medical Subject Headings (MeSH/mh), Google Scholar, Magiran, Barakatk (formerly Iranmedex), Elm net, and Scientific Information Database (SID), were selected between 2001 and 2017. To explore the articles, some key words such as: Anaplasma spp., anaplasmosis, Anaplasma phagocytophilum, Anaplasma marginale, Anaplasma ovis, Anaplasma bovis, Anaplasma centrale, livestock, domestic herbivores, cattle, sheep and goat and “Iran” alone or in combination were used. To avoid the risk of selection bias in this study, the inclusion criteria were clearly classified and studied. The stages of the study plan are briefly explained in Fig. 1.
Fig. 1.
PRISMA flowchart describing the study design process.
2.2. Paper selection
All studies were independently screened and eligibility was determined by two reviewers (MH and MS) with the agreement between reviewers of 94% using Kappa index and a third opinion (MF) resolved the disagreements.
2.3. Statistical analysis
The quality of meta-analysis was evaluated with STROBE scale. The score under 7.75 considered poor quality, between 7.76 and 15.5 low, between 15.6 and 23.5 moderate and more than 23.6 high quality (Von Elm et al., 2007). The mean of scores for the STROBE scale was obtained 19.43 which showed that the quality of these studies was moderate to high. The prevalence of Anaplasma spp. infection in each study was collected and according to binomial distribution, standard error () for each study was calculated and the inverse of SE for each study considered as the weight of that study. The effect size (ES) for each study and pooled outcome revealed as a forest plot [reported as ES with a 95% confidence interval (95%CI)].
Cochran's heterogeneity statistics based on chi-square test Q-test (p < .1 as heterogeneities) and the I-squared statistic were used to evaluate the percentage of variation through studies with the value of 25% (low), 50% (moderate), and 75% (high) of heterogeneity. The mean of scores for the STROBE scale was obtained 19.43 which showed that the quality of these studies was moderate to high.
At present heterogeneity, random effects model (DerSimonian and Kacker, 2007) and otherwise applied fixed effect model (Mantel Haenszel) were used to compute overall effect size. Subgroup analyses were performed to investigate potential sources of heterogeneity from different types of animal, Anaplasma spp., laboratory methods and study area. Egger's test was used to evaluate publication bias. All statistical analyses were done with the Statistical Software Package (Stata) version 11.1.
3. Results
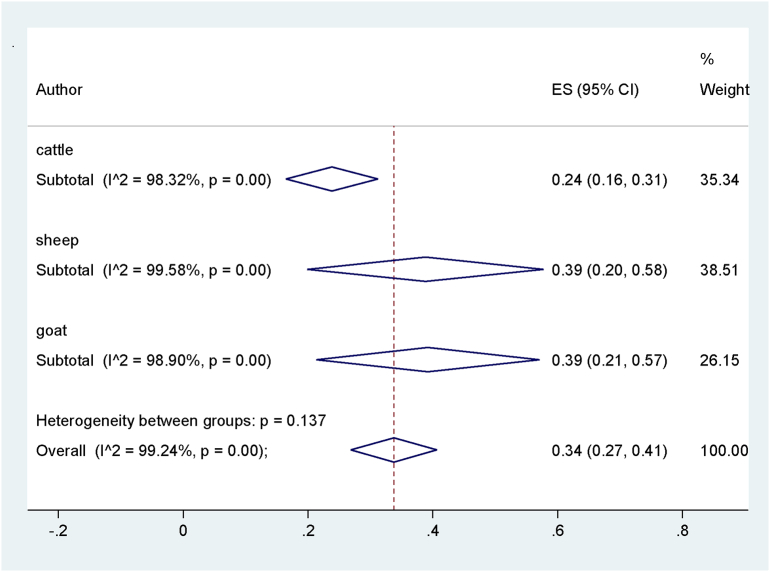
The process of study selection is shown in Fig. 1. Among all databases; a total of 38 articles published during 17 years (2001–2017) were selected to be included in this systematic review and meta-analysis (Table 1). All articles were cross-sectionally designated and evaluated the prevalence of Anaplasma infection in domestic herbivorous including cattle, sheep and goat in different districts of Iran. Totally, 5093 cattle, 1958 sheep, and 1232 goats were examined. The Overall prevalence of Anaplasma infection based on a random effect meta-analysis was estimated to be 34% (95% CI, 27–41%, I2 = 99.24%, p < .001), which indicated a substantial heterogeneity among studies. The results of subgroup analysis indicated the prevalence of Anaplasma infection among sheep 39.0% (95% CI, 20.0 _58.0%), cattle 24.0% (95% CI, 16.0 _ 31.0%) and goats 39.0% (95% CI, 21.0 _ 57.0%), which the differences was not statistically significant among them (p = .14) (Table. 2, Fig. 2).
Table 1.
Characteristics of studies included in systematic review and meta-analysis.
| Author | Year of publication | Type of animal | No. of examined | No. of positive | Anaplasma spp. | Laboratory method | Place |
|---|---|---|---|---|---|---|---|
| (Noaman and Kachouei, 2001) | 2001 | cattle | 3269 | 546 | A.marginale | Microscopic | Isfahan |
| (Razmi et al., 2006) | 2006 | cattle | 160 | 31 | A.marginale | Microscopic | Mashhad |
| 2006 | sheep | 391 | 314 | A.ovis | Microscopic | Mashhad | |
| 2006 | goat | 385 | 150 | A.ovis | Microscopic | Mashhad | |
| (Noaman and Shayan, 2009, Noaman et al., 2009) | 2009 | cattle | 150 | 2 | A.phagocytophilum | Nested-PCR | Isfahan |
| 2009 | cattle | 150 | 58 | A.marginale | PCR-RFLP | Isfahan | |
| 2009 | cattle | 150 | 75 | A.marginale | Microscopic | Isfahan | |
| (Ahmadi-Hamedani et al., 2009) | 2009 | goat | 193 | 123 | A.ovis | PCR-RFLP | Gonbad& Mashhad |
| 2009 | goat | 193 | 43 | Anaplasma spp. | Microscopic | Gonbad& Mashhad | |
| (Noaman and Shayan, 2010a, Noaman and Shayan, 2010b, Noaman and Shayan, 2010c) | 2010 | cattle | 150 | 58 | A.marginale | PCR-RFLP | Isfahan |
| (Noaman and Shayan, 2010a, Noaman and Shayan, 2010b, Noaman and Shayan, 2010c) | 2010 | cattle | 150 | 91 | Anaplasma spp. | Microscopic | Isfahan |
| (Noaman and Shayan, 2010a, Noaman and Shayan, 2010b, Noaman and Shayan, 2010c) | 2010 | cattle | 150 | 4 | A.ovis | Nested-PCR | Isfahan |
| (Ahmadi-hamedani et al., 2012) | 2011 | goat | 193 | 123 | A.ovis | PCR | Gonbad& Mashhad |
| 2011 | goat | 193 | 43 | Anaplasma spp. | Microscopic | Gonbad& Mashhad | |
| (Salehzadeh et al., 2011) | 2011 | cattle | 200 | 6 | A.marginale | Microscopic | kerman |
| (Jalali et al., 2013; Jalali et al., 2016) | 2012 | sheep | 119 | 40 | Anaplasma spp. | Microscopic | Ahvaz |
| 2012 | sheep | 119 | 104 | Anaplasma spp. | PCR | Ahvaz | |
| 2012 | sheep | 104 | 52 | A.marginale | PCR-RFLP | Ahvaz | |
| Noaman et al. | 2012 | sheep | 150 | 50 | A.ovis | PCR-RFLP | Isfahan |
| Noaman et al. | 2013 | cattle | 150 | 75 | A.marginale | Microscopic | Isfahan |
| Noaman et al. | 2013 | cattle | 150 | 10 | A.marginale | PCR-RFLP | Isfahan |
| Noaman et al. | 2013 | sheep | 150 | 50 | A.ovis | Microscopic | Isfahan |
| Noaman et al. | 2013 | sheep | 150 | 10 | A.ovis | PCR-RFLP | Isfahan |
| Ahmadi-Hamedani et al. | 2013 | goat | 84 | 47 | A.ovis | PCR | Gonbad& Mashhad |
| Hosseini-Vasoukolaei et al. | 2014 | cattle | 9 | 2 | Anaplasma spp. | PCR | Mazandaran |
| Hosseini-Vasoukolaei et al. | 2014 | sheep | 65 | 28 | Anaplasma spp. | PCR | Mazandaran |
| Hosseini-Vasoukolaei et al. | 2014 | goat | 4 | 1 | Anaplasma spp. | PCR | Mazandaran |
| Khaki et al. | 2015 | sheep | 109 | 35 | Anaplasma spp. | Microscopic | Ahvaz |
| Khaki et al. | 2015 | sheep | 109 | 94 | A.ovis | PCR-RFLP | Ahvaz |
| Khaki et al. | 2015 | sheep | 109 | 50 | A.ovis | PCR-RFLP | Ahvaz |
| Khezri et al. | 2015 | cattle | 105 | 8 | Anaplasma spp. | ELISA | Kurdestan |
| Khezri et al. | 2015 | sheep | 77 | 5 | Anaplasma spp. | ELISA | Kurdestan |
| Noaman et al. | 2016 | cattle | 100 | 0 | Anaplasma spp. | Nested-PCR | West Azarbaijan |
| Noaman et al. | 2016 | sheep | 100 | 5 | A.ovis | Nested-PCR | West Azarbaijan |
| Jalali et al. | 2016 | goat | 104 | 30 | A.ovis | Microscopic | Ahvaz |
| Jalali et al. | 2016 | goat | 104 | 68 | A.ovis | PCR-RFLP | Ahvaz |
| Yousefi et al. | 2017 | sheep | 206 | 1 | A.phagocytophilum | Nested-PCR | Hamedan |
| Yousefi et al. | 2017 | goat | 164 | 3 | A.phagocytophilum | Nested-PCR | Hamedan |
Table 2.
Subgroup meta-analysis of the prevalence of Anaplasma spp. according to the type of animal, Anaplasma spp., detection method and place.
| Characteristics | Factors | N | EF (95%CI) | I-square (%) | P-value |
|---|---|---|---|---|---|
| Type of animals | Cattle | 12 | 0.24(0.16, 0.31) | 98.0 | 0.14 |
| Sheep | 14 | 0.39(0.20, 0.58) | 99.0 | ||
| Goat | 10 | 0.39(0.21, 0.57) | 99.0 | ||
| Anaplasma spp. | marginale | 9 | 0.30(0.20, 0.39) | 97.9 | P < .001 |
| Ovis | 14 | 0.44(0.26, 0.61) | 99.3 | ||
| phagocytophilum | 3 | 0.01(0.0, 0.02) | 93.2 | ||
| Anaplasma spp. | 11 | 0.33(0.16, 0.51) | 98.3 | ||
| Method | Microscopy | 14 | 0.35(0.23, 0.47) | 99.1 | P < .001 |
| Nested-PCR | 5 | 0.02(0.001, 0.03) | 41.0 | ||
| PCR-RFLP | 10 | 0.43(0.25, 0.62) | 98.7 | ||
| PCR | 6 | 0.54(0.36, 0.72) | 93.63 | ||
| ELISA | 2 | 0.07(0.03, 0.11) | 99.2 | ||
| Place | Isfahan | 12 | 0.28(0.19, 0.36) | 98.2 | P < .001 |
| Mashhad | 3 | 0.46(0.10, 0.83) | 97.6 | ||
| Gonbad and Mashhad | 5 | 0.45(0.25, 0.66) | 97.6 | ||
| Kerman | 2 | 0.03(0.01, 0.06) | 90.3 | ||
| Ahvaz | 8 | 0.54(0.36, 0.72) | 97.5 | ||
| West Azerbaijan | 2 | 0.05(0.02, 0.11) | 91.8 | ||
| Mazandaran | 3 | 0.37(0.24, 0.50) | 84.2 | ||
| Kurdistan | 2 | 0.07(0.03, 0.11) | 89.7 | ||
| Hamedan | 2 | 0.01(0.001, 0.02) | 98.7 |
Fig. 2.
Pooled prevalence of Anaplasma spp. infection according to the type of animals.
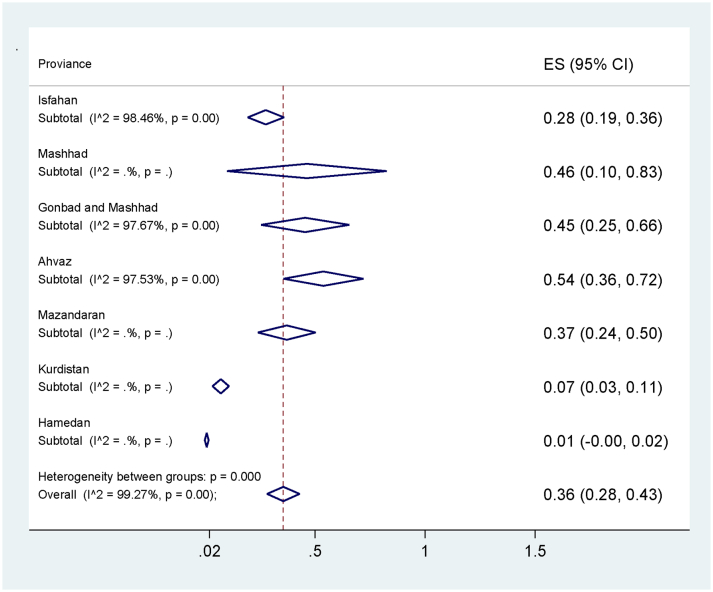
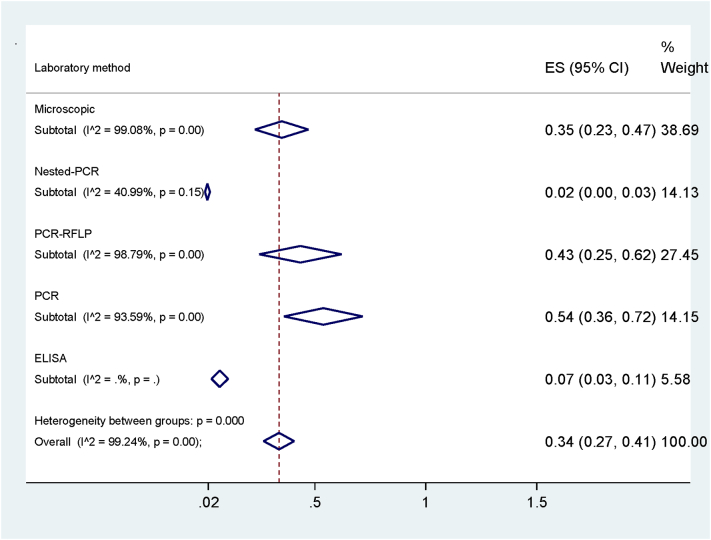
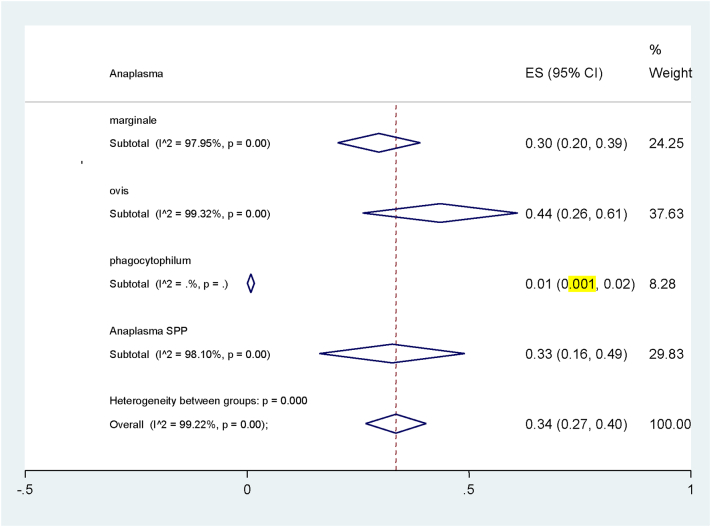
The maximum prevalence of Anaplasma spp. was in Ahvaz (54%, 95% CI: 36% -72%) and the minimum occurred in Hamedan (1%, 95% CI, 0.1% -72%) (Table 2, Fig. 3). Additionally, the most common diagnostic tests were PCR (54%), microscopy (35%) and ELISA (7%) assays (Fig. 4). In total, the highest prevalence of Anaplasma spp. infection was belonged to A. ovis (44%) and the lowest to A. phagocytophilum (1%) with a significant difference among them (p < .001) (Table 2, Fig. 5). For assessing publication bias of studies, we used Egger's test. The results showed that there was no essentially publication bias in included studies of this meta-analysis (t = −46, p = .651).
Fig. 3.
Pooled prevalence of Anaplasma spp. according to the place of study.
Fig. 4.
Pooled prevalence of Anaplasma spp. according to the laboratory methods.
Fig. 5.
Pooled prevalence of Anaplasma spp. according to the species of Anaplasma.
4. Discussion
Anaplasmosis is common among farm animals and, in Iran, overall prevalence of infection is 34%, and the prevalence in sheep, cattle and goat were 39, 24 and 39%, respectively (Fig. 2). In comparison to the other studies in neighboring countries, anaplasmosis in Iran is more prevalent than that found in Russia, Pakistan, Turkey and Iraq (Rar et al., 2010; Atif et al., 2012; Aktas et al., 2011; Ameen et al., 2012). The diseases is widely distributed throughout the world including tropical and sub-tropical areas of Asia, South, Central and North America, Europe, Africa and Australia with a prevalence ranging of 1 to 100% (Noaman et al., 2009; Sainz et al., 2015; Pokorn et al., 2016).
Around Iran; Middle East countries, including Jordan, Egypt, United Arab Emirates, Iraq, Qatar, Cyprus, and Israel are prevalent areas for Anaplasma spp. infections (Ameen et al., 2012; Jafarbekloo et al., 2014; Razzaq et al., 2015; Kaewmongkol et al., 2017). The prevalence of Anaplasma spp. infection among herbivores in different years in the same region of Russia, the northern Iranian neighbor, varies from 1% to 0.5% (Rar et al., 2010). In a similar study, in Pakistan, the southeast Iranian neighbor, 1050 blood samples from livestock farms were microscopically examined and revealed that 21.14% of samples were positive for blood parasites that Anaplasma with the prevalence of 5.81%, was the most prevalent hemoparasite (Atif et al., 2012).
Different countries from Africa, including Uganda, Kenya, Morocco, Ghana and Tanzania reported bovine anaplasmosis outbreaks during 2003 to 2019 (Bah, 2016; Sisson et al., 2017; Byaruhanga et al., 2018. Ringo et al., 2018). In a study conducted by Ait Lbacha et al., 71% (303/422) of small ruminants in Morocco were infected by Anaplasma spp. using PCR technique (Ait Lbacha et al., 2017).
Anaplasmosis in countries of Latin America and in Caribbean Islands exception of desert areas and certain mountain ranges is enzootic (Rodriguez-Morales et al., 2019). Human granulocytic anaplasmosis is uncommon in Europe, but it's the most prevalent tick-borne infection in animals (Sainz et al., 2015; Pokorn et al., 2016). It's reported that the prevalences of the Ixodes ricinus as vector have been increasing in most of the states of the USA (Ringo et al., 2018). In general, types of grazing system in the husbandry of the livestock, climate conditions of the area, flock size, strain of Anaplasma, abundance of the tick as vector are some variables that significantly affects on the prevalence of infection (Rosso et al., 2017). Despite the importance of the disease in the livestock industry, there are still several areas in Iran, which none study has been performed on the Anaplasma spp. infection among livestock and its vectors in those areas.
Anaplasmosis as one of the most important endemic disease in many regions of Iran and the prevalence of infection is seasonally different which, increasing in spring and summer in the northern (Mazandaran, Mashhad, Gonabad and West Azerbaijan), western (Hamedan, Ahvaz and Kurdistan) and central (Isfahan, Kerman) parts of the country (Razmi et al., 2006; Ahmadi-Hamedani et al., 2009; Ahmadi-hamedani et al., 2012; Ahmadi-hamedani et al., 2014; Hosseini-Vasoukolaei et al., 2014; Noaman and Bastani, 2016), (Jalali et al., 2013; Khaki et al., 2015; Jalali et al., 2016; Yousefi et al., 2017), (Noaman and Kachouei, 2001; Noaman and Shayan, 2009; Noaman et al., 2009; Noaman and Shayan, 2010a, Noaman and Shayan, 2010b, Noaman and Shayan, 2010c; Salehzadeh et al., 2011; Noaman, 2012; Noaman, 2013) (see Table 1). In Iran, because of the biodiversity of tick, variety of ecological and climate situations, anaplasmosis is highly prevalent. The domestic industry has a long history in Iran and cattle, sheep and goat are the most important livestock. Also, some livestock and products are exported to different parts of the world (Jalali et al., 2013; Razzaq et al., 2015). Ticks have key roles in the transmission of many infectious diseases such as viral, parasitic and also bacterial diseases. Ticks are usually presence in tropical and also subtropical regions. Nowadays, because of the alteration of the land use patterns, and changes in climate; the rate of tick-borne diseases have been significantly increased and spreading to new zones (Rodriguez-Morales et al., 2019). Based on many published reports, Ixodes, Dermacentor, Rhipicephalus and Amblyomma are the most important vectors for Anaplasma spp. in different districts of Iran (Noaman, 2012). Anaplasmosis can cause respiratory distress, enlarge the prescapular lymph nodes reduction of milk production, body weight, abortion and maybe death (Stuen et al., 2003).
In the current study, the pooled prevalence rate of infection is estimated to be 34% in Iran. Additionally, the prevalence rate of different geographical zones demonstrated that there are five zones in Iran with different prevalence rates: 54% in Ahvaz (Khozestan Province) (Jalali et al., 2013; Khaki et al., 2015), 46% in Mashhad (Khorasan Razavi Province) (Razmi et al., 2006), 45% in Gonabad and Mashhad (Khorasan Razavi Province) (Ahmadi-hamedani et al., 2012), 37% in Mazandaran Province (Hosseini-Vasoukolaei et al., 2014), 28% in Isfahan Province (Noaman and Shayan, 2010a, Noaman and Shayan, 2010b, Noaman and Shayan, 2010c) and 3% and 1% in Kerman and Hamedan provinces (Salehzadeh et al., 2011) (Fig. 3). Accordingly, the highest mean of prevalence rate of infection was in southwestern and northeastern parts of Iran (Fig. 3). In Turkey, Iranian western neighbor, Anaplasma spp. infection was reported that 9% (35/389) of bovine were positive using PCR method in 2011 (Aktas et al., 2011).
The western provinces of Iran such as West Azarbaijan and Kurdistan have similar weather, the height and environmental conditions to Turkey. Also in a similar study in Iraq, from 184 cattle, 44 sheep, 59 goats and 70 ibex, 4, 2, 3 and 1cases were positive for anaplasmosis, respectively (Ameen et al., 2012). Our findings are in agreement with different studies on sheep and goats in Pakistan in 2014 which showed 9 positive samples of 210 horses, with PCR -RFLP method, and the prevalence rate of 16% is recorded by PCR method (Razzaq et al., 2015). The prevalence of anaplasmosis in Pakistan, the southeast Iranian neighbor, is almost close to the prevalence of Khorasan Razavi, eastern Iran. In addition, in Borderline of Iran-Afghanistan, in June 2013 to May 2014, molecular studies were done on 53 samples, which the Anaplasma's DNA was found in 14 samples (26.4%) out of the 53 specimens. It is a concerning prevalence of anaplasmosis among animals in Afghanistan, as the eastern neighbor of Iran (Jafarbekloo et al., 2014).
In addition, positive rate of infection in studies using PCR-RFLP method (43%) was significantly higher than other laboratory tests (p < .001) (Fig. 4 and Table. 2). The highest infection was in Ahvaz (54%) (Jalali et al., 2013; Khaki et al., 2015; Jalali et al., 2016), and Mashhad (46%) (Razmi et al., 2006) and the lowest rate was in Hamedan (1%) (Yousefi et al., 2017) and Kerman (3%) (Salehzadeh et al., 2011), with a significant difference between them (Fig. 3 and Table. 2). The most prevalence of infection among Anaplasma spp. was belonged to A. ovis with 44% and A. marginale with 30% of infection rates and the lowest prevalence to A. phagocytophilum with 1% with significant differences among them (Fig. 4 and Table. 2). One of the most important causes of evaluation of the Anaplasma spp. infection is the pathogenicity of this parasite to humans and the likelihood of its transmission from animal to human. A. phagocytophilum is one of the most pathogenic species that is seriously posing a risk for humans, particularly pregnant women (Dhand et al., 2007).
5. Conclusion
The high occurrence of ovine and bovine anaplasmosis in Iran, confirms the stability situations of animal anaplasmosis in the studied regions, particularly northeastern and southwestern provinces of the country and may be a warning for animal welfare and health. In brief, our data offer valuable and encouraging information as regards the current situation of anaplasmosis in domestic livestock in Iran, which might be useful for active and passive surveillance and preventing plans. Further investigation and monitoring will be needed to expand the surveillance and control policies, such as vaccination and improvement the traditional diagnostic tools and assessment the pesticide resistance in ticks to reduce the mortality and morbidity of anaplasmosis among livestock and consequently decrease the risk of outbreaks and economic failure and public health hazard in Iran.
Declaration of Competing Interest
All authors declare that they have no conflicts of interest.
Acknowledgments
The authors wish to acknowledge all researchers that their publications were used in our review for hard work on anaplasmosis in the field. This study was funded by the Vice Chancellor of Research and Technology, Mazandaran University of Medical Sciences (grant number: 1219).
References
- Ahmadi-Hamedani M., Khaki Z., Rahbari S., Kazemi B., Bandehpour M. Molecular identification of anaplasmosis in goats using a new PCR-RFLP method. Iran. J. Vet. Res. 2009;10(4):367–372. [Google Scholar]
- Ahmadi-hamedani M., Khaki Z., Rahbari S., Ahmadi-hamedani M.A. Hematological profiles of goats naturally infected with Anaplasma ovis in north and Northeast Iran. Comp. Clin. Pathol. 2012;21(6):1179–1182. [Google Scholar]
- Ahmadi-hamedani M., Ahmadi-hamedani M., Fathi E., Sani R.N. Comparison of selected biochemical parameters between naturally infected and non-infected goats with Anaplasma ovis. Comp. Clin. Pathol. 2014;23(4):989–992. [Google Scholar]
- Ait Lbacha H., Alali S., Zouagui Z., El Mamoun L., Rhalem A., Petit E., Haddad N., Gandoin C., Boulouis H.J., Maillard R. High prevalence of Anaplasma spp. in small ruminants in Morocco. Transbound. Emerg. Dis. 2017;64(1):250–263. doi: 10.1111/tbed.12366. [DOI] [PubMed] [Google Scholar]
- Aktas M., Altay K., Dumanli N. Molecular detection and identification of Anaplasma and Ehrlichia species in cattle from Turkey. Ticks Tick-Borne Dis. 2011;2(1):62–65. doi: 10.1016/j.ttbdis.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Ameen K., Abdullah B., Abdul-Razaq R. Seroprevalence of Babesia bigemina and Anaplasma marginale in domestic animals in Erbil, Iraq. Iraqi J. Vet. Sci. 2012;26(Suppl. 3):109–114. [Google Scholar]
- Atif F., Khan M., Iqbal H., Roheen T. Prevalence of tick-borne diseases in Punjab (Pakistan) and hematological profile of Anaplasma marginale infection in indigenous and crossbred cattle. Pakistan J. Sci. 2012;64:11–15. [Google Scholar]
- Bah M. Vol. 2016. University of Ghana; 2016. Effects of arthropod Ectoparasite infestations on livestock productivity in three districts in southern Ghana.http://ugspace.ug.edu.gh/handle/123456789/22955 PhD diss. available on. [Google Scholar]
- Byaruhanga C., Collins N.E., Knobel D.L., Khumalo Z.T., Chaisi M.E., Oosthuizen M.C. Molecular detection and phylogenetic analysis of Anaplasma marginale and Anaplasma Centrale amongst transhumant cattle in North-Eastern Uganda. Ticks Tick-Borne Dis. 2018;9(3):580–588. doi: 10.1016/j.ttbdis.2018.01.012. [DOI] [PubMed] [Google Scholar]
- DerSimonian R., Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp. Clin. Trial. 2007;28(2):105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Dhand A., Nadelman R.B., Aguero-Rosenfeld M., Haddad F.A., Stokes D.P., Horowitz H.W. Human granulocytic anaplasmosis during pregnancy: case series and literature review. Clin. Infect. Dis. 2007;45(5):589–593. doi: 10.1086/520659. [DOI] [PubMed] [Google Scholar]
- Hosseini-Vasoukolaei N., Oshaghi M.A., Shayan P., Vatandoost H., Babamahmoudi F., Yaghoobi-Ershadi M.R., Telmadarraiy Z., Mohtarami F. Anaplasma infection in ticks, livestock and human in Ghaemshahr, Mazandaran Province, Iran. J. Arthropod. Borne Dis. 2014;8(2):204–211. [PMC free article] [PubMed] [Google Scholar]
- Jafarbekloo A., Bakhshi H., Faghihi F., Telmadarraiy Z., Khazeni A., Oshaghi M.A., Ramzgouyan M.R., Sedaghat M.M. Molecular detection of Anaplasma and Ehrlichia infection in ticks in borderline of Iran-Afghanistan. J. Biomed. Sci. Eng. 2014;7(11):919–926. [Google Scholar]
- Jalali S., Khaki Z., Kazemi B., Bandehpour M., Rahbari S., Razi Jalali M., Yasini S. Molecular detection and identification of Anaplasma species in sheep from Ahvaz, Iran. Iran. J. Vet. Res. 2013;14(1):50–56. [Google Scholar]
- Jalali S.M., Bahrami S., Rasooli A., Hasanvand S. Evaluation of oxidant/antioxidant status, trace mineral levels, and erythrocyte osmotic fragility in goats naturally infected with Anaplasma ovis. Trop. Anim. Health Prod. 2016;48(6):1175–1181. doi: 10.1007/s11250-016-1071-0. [DOI] [PubMed] [Google Scholar]
- Kaewmongkol G., Lukkana N., Yangtara S., Kaewmongkol S., Thengchaisri N., Sirinarumitr T., Jittapalapong S., Fenwick S.G. Association of Ehrlichia canis, Hemotropic mycoplasma spp. and Anaplasma platys and severe anemia in dogs in Thailand. Vet. Microbiol. 2017;201:195–200. doi: 10.1016/j.vetmic.2017.01.022. [DOI] [PubMed] [Google Scholar]
- Khaki Z., Jalali S.M., Kazemi B., Razi Jalali M., Yasini S.P. A study of hematological changes in sheep naturally infected with Anaplasma spp. and Theileria ovis: molecular diagnosis. Iran. J. Vet. Med. 2015;9(1):19–26. [Google Scholar]
- Noaman V. Identification of hard ticks collected from sheep naturally infected with Anaplasma ovis in Isfahan province, Central Iran. Comp. Clin. Pathol. 2012;21(3):367–369. [Google Scholar]
- Noaman V. Discrimination between Anaplasma marginale and Anaplasma ovis by PCR-RFLP. World Appl. Sci. J. 2013;21(2):190–195. [Google Scholar]
- Noaman V., Bastani D. Molecular study on infection rates of Anaplasma ovis and Anaplasma marginale in sheep and cattle in West-Azerbaijan province, Iran. Vet. Res. Forum. 2016;7(2):163–167. [PMC free article] [PubMed] [Google Scholar]
- Noaman V.S. Arabzadeh, Kachouei B. A study on anaplasmosis in cattle of Flavarjan city, Isfahan Province (1995–2000) Pajouhesh-va-Sazandegi. 2001;14(3):10–12. [Google Scholar]
- Noaman V., Shayan P. Molecular detection of Anaplasma phagocytophilum in carrier cattle of Iran-first documented report. Iran. J. Microbiol. 2009;1(2):37–42. [Google Scholar]
- Noaman V., Shayan P. Comparison of microscopy and PCR-RFLP for detection of Anaplasma marginale in carrier cattle. Iran. J. Microbiol. 2010;2(2):89–94. [PMC free article] [PubMed] [Google Scholar]
- Noaman V., Shayan P. Molecular detection of Anaplasma bovis in cattle from central part of Iran. Vet. Res. Forum. 2010;1:117–122. [Google Scholar]
- Noaman V., Shayan P. A new PCR-RFLP method for detection of Anaplasma marginale based on 16S rRNA. Vet. Res. Commun. 2010;34(1):43–50. doi: 10.1007/s11259-009-9331-3. [DOI] [PubMed] [Google Scholar]
- Noaman V., Shayan P., Amininia N. Molecular diagnostic of Anaplasma marginale in carrier cattle. Iran. J. Parasitol. 2009;4(1):26–33. [Google Scholar]
- Pokorn M., Županc T.A., Strle F. Pediatric human granulocytic anaplasmosis is rare in Europe. Pediatr. Infect. Dis. J. 2016;35(3):358–359. doi: 10.1097/INF.0000000000001004. [DOI] [PubMed] [Google Scholar]
- Rar V.A., Livanova N.N., Panov V.V., Doroschenko E.K., Pukhovskaya N.M., Vysochina N.P., Ivanov L.I. Genetic diversity of Anaplasma and Ehrlichia in the Asian part of Russia. Ticks Tick-Borne Dis. 2010;1(1):57–65. doi: 10.1016/j.ttbdis.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Razmi G.R., Dastjerdi K., Hossieni H., Naghibi A., Barati F., Aslani M. An epidemiological study on Anaplasma infection in cattle, sheep, and goats in Mashhad Suburb, Khorasan Province, Iran. Ann. N. Y. Acad. Sci. 2006;1078(1):479–481. doi: 10.1196/annals.1374.089. [DOI] [PubMed] [Google Scholar]
- Razzaq F., Khosa T., Ahmad S., Hussain M., Saeed Z., Khan M., Shaikh R., Ali M., Iqbal F. Prevalence of Anaplasma phagocytophilum in horses from Southern Punjab (Pakistan) Trop. Biomed. 2015;32:233–239. [PubMed] [Google Scholar]
- Ringo A.E., Moumouni P.F.A., Lee S.-H., Liu M., Khamis Y.H., Gao Y., Guo H., Zheng W., Efstratiou A., Galon E.M. Molecular detection and characterization of tick-borne protozoan and rickettsial pathogens isolated from cattle on Pemba Island, Tanzania. Ticks Tick-Borne Dis. 2018;9(6):1437–1445. doi: 10.1016/j.ttbdis.2018.06.014. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Morales A.J., Bonilla-Aldana D.K., Idarraga-Bedoya S.E., Garcia-Bustos J.J., Cardona-Ospina J.A., Faccini-Martínez Á.A. Epidemiology of zoonotic tick-borne diseases in Latin America: Are we just seeing the tip of the iceberg? F1000Research. 2019;7:1988. doi: 10.12688/f1000research.17649.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso F., Tagliapietra V., Baráková I., Derdáková M., Konečný A., Hauffe H.C., Rizzoli A. Prevalence and genetic variability of Anaplasma phagocytophilum in wild rodents from the Italian alps. Parasit. Vectors. 2017;10(1):293. doi: 10.1186/s13071-017-2221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymaszewska A., Grenda S. Bacteria of the genus Anaplasma–characteristics of Anaplasma and their vectors: a review. Vet. Med. 2008;53(11):573–584. [Google Scholar]
- Sainz Á., Roura X., Miró G., Estrada-Peña A., Kohn B., Harrus S., Solano-Gallego L. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit. Vectors. 2015;8(1):75. doi: 10.1186/s13071-015-0649-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehzadeh S., Fathi S., Dehaghi M.M., Asl E.N., Nezhad H.A. Survey of Theileria annulata and Anaplasma marginale in cattle in Kerman area, southeast of Iran. Scientia Parasitologica. 2011;12(2):61–66. [Google Scholar]
- Sisson D., Hufschmid J., Jolles A., Beechler B., Jabbar A. Molecular characterization of anaplasma species from african buffalo (Syncerus caffer) in kruger national park, South Africa. Ticks Tick-Borne Dis. 2017;8(3):400–406. doi: 10.1016/j.ttbdis.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Stuen S., Bergström K., Petrovec M., Van de Pol I., Schouls L.M. Differences in clinical manifestations and hematological and serological responses after experimental infection with genetic variants of Anaplasma phagocytophilum in sheep. Clin. Diagn. Lab. Immunol. 2003;10(4):692–695. doi: 10.1128/CDLI.10.4.692-695.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann. Intern. Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- Yousefi A., Rahbari S., Shayan P., Sadeghi-dehkordi Z., Bahonar A. Molecular evidence of Anaplasma phagocytophilum: an emerging tick-borne pathogen in domesticated small ruminant of Iran; first report. Comp. Clin. Pathol. 2017;26(3):637–642. [Google Scholar]