Abstract
Etiology-specific onabotulinumtoxinA utilization to manage spasticity is largely unknown. In this 1-year interim analysis, we evaluated real-world onabotulinumtoxinA utilization and effectiveness across several etiologies from the Adult Spasticity International Registry (ASPIRE) study. ASPIRE is a multicenter, prospective, observational registry (NCT01930786) examining stroke, multiple sclerosis [MS], cerebral palsy [CP], traumatic brain injury [TBI], and spinal cord injury [SCI] patients with spasticity treated with onabotulinumtoxinA at the clinician's discretion. Assessments included onabotulinumtoxinA utilization (each session), clinician (subsequent session)/patient (5±1 weeks post-treatment) satisfaction, and the Disability Assessment Scale (DAS; subsequent session). 730 patients received ≥1 onabotulinumtoxinA treatment, with 37% naïve to botulinum toxin(s) for spasticity. The most common etiology was stroke (n=411, 56%), followed by MS (N=119, 16%), CP (N=77, 11%), TBI (N=45, 6%), and SCI (N=42, 6%). The total body mean cumulative dose (±SD) of onabotulinumtoxinA per session ranged from 296 U (±145) in CP to 406 U (±152) in TBI. The most commonly treated upper limb presentations were clenched fist (stroke, MS, and SCI), flexed wrist (CP), and flexed elbow (TBI). Equinovarus foot was the most commonly treated lower limb presentation in all etiologies. Stroke patients showed improved DAS scores for nearly all subscales in both limbs, indicative of improved global function. All etiologies showed improved lower limb mobility DAS scores. Across all sessions, clinicians (range: 87.4% [SCI]-94.2% [CP]) and patients (range: 67.6% [TBI]-89.7% [SCI]) reported extreme satisfaction/satisfaction that onabotulinumtoxinA helped manage spasticity, and clinicians (range: 94.6% [TBI]-98.8% [CP]) and patients (range: 88.4% [stroke]-91.2% [TBI]) would definitely/probably continue treatment. Treatment-related adverse events (TRAEs) and treatment-related serious adverse events (TRSAEs) were reported as follows: stroke: 10 TRAEs (2.2% patients), 3 TRSAEs (0.5%); MS: 5 TRAEs (4.2%), 0 TRSAEs; CP: 0 TRAEs, 0 TRSAEs; TBI: 1 TRAEs (2.2%), 0 TRSAEs; SCI: 0 TRAEs, 0 TRSAEs. No new safety signals were identified. High clinician- and patient-reported satisfaction were observed following individualized onabotulinumtoxinA treatment, as well as improved global function. Interim results from ASPIRE demonstrate etiology-specific similarities and differences in clinical approaches to manage spasticity.
Keywords: Botulinum toxins, Type A, Cerebral palsy, Cerebrovascular accident, Disability evaluation, Electrical stimulation, Electromyography, Multiple sclerosis, Muscle spasticity, Patient reported outcome measures, Spinal cord injuries, Stroke, Traumatic brain injury, Ultrasound
Graphical abstract
Highlights
-
•
ASPIRE found etiology-specific similarities and differences in real-world onabotulinumtoxinA utilization for spasticity.
-
•
Across all etiologies, there was high clinician- and patient-reported satisfaction with onabotulinumtoxinA treatment.
-
•
In DAS, all etiologies showed improved global function in lower limb mobility following onabotulinumtoxinA treatment.
-
•
Adverse event data varied by etiology of spasticity; however, no new safety signals were identified.
-
•
ASPIRE data may guide clinical strategies and educational programs to improve onabotulinumtoxinA spasticity management.
1. Introduction
Spasticity has been described as disordered sensorimotor control, resulting from an upper motor neuron lesion, presenting as intermittent or sustained involuntary activation of muscles (Pandyan et al., 2005, Tardieu et al., 1954). Spasticity affects coordinated movement of the shoulder, elbow, wrist, and finger flexors in the upper limb, and the ankle, knee, and hip in the lower limb, resulting in abnormal postures and limb pain (AANS, 2018, Mayer et al., 1997, Trompetto et al., 2014). Upper and/or lower limb spasticity can negatively impact a patient's ability to perform daily activities, leading to reduced health-related quality of life and significant socioeconomic impacts (Barin et al., 2018, Hughes and Howard, 2013, Lundstrom et al., 2010).
Spasticity is a disabling consequence of many central nervous system disorders, including stroke, multiple sclerosis (MS), cerebral palsy (CP), traumatic brain injury (TBI), spinal cord injury (SCI), and intracerebral hemorrhage (ICH). Approximately 17–43% of post-stroke patients experience spasticity (Wissel et al., 2013), while 34–84% of patients with moderate to severe TBI (Angulo-Parker and Adkinson, 2018, Wedekind and Lippert-Gruner, 2005), 31–78% of patients with SCI (Angulo-Parker and Adkinson, 2018, Finnerup, 2017, Holtz et al., 2017), 40–80% of patients with MS (Barnes et al., 2003, Collongues and Vermersch, 2013, Rizzo et al., 2004), and across their lifetime, 72–91% of patients with CP (Odding et al., 2006) are affected by spasticity. Many factors can influence the reported incidence of spasticity, including those that impact detection and/or influence appearance/severity. These include time from the event/diagnosis, the method(s) of diagnosis or the assessment scale(s) utilized, as well as various triggers that are extrinsic (eg, time of day, temperature, or fit of devices; Cheung et al., 2015, Hughes and Howard, 2013, Nair and Marsden, 2014) or intrinsic/patient-specific comorbid conditions (eg, bladder or bowel issues, menstrual cycle, skin, and fever; Cheung et al., 2015, Nair and Marsden, 2014).
Clinical treatment strategies to manage spasticity (see reviews: Kheder and Nair, 2012, Ward, 2008) often include several modalities (Mills et al., 2016, Wissel, 2018) to address the specific needs and goals of the individual patient. Individual treatment focuses on addressing symptoms, preventing further complications, restoring function, and improving quality of life (Esquenazi et al., 2013, Nair and Marsden, 2014). OnabotulinumtoxinA (BOTOX®, Allergan, an AbbVie company, North Chicago, Illinois, USA) is approved for use in the management of upper and lower limb spasticity in the U.S.A. and worldwide (Allergan, 2017) and is often used in combination with other supporting treatments, such as physical and occupational therapy. The use of onabotulinumtoxinA in controlled trials to treat post-stroke spasticity is well-established (eg, Brashear et al., 2002a, Kaji et al., 2010a, Kaji et al., 2010b, Patel, 2011). However, there are limited data describing the use of onabotulinumtoxinA in real-world clinical practice to treat spasticity across a range of etiologies (Esquenazi et al., 2017b). Real-world, observational data are necessary to improve educational programs on spasticity management and help guide clinical strategies to optimize patient care. The Adult SPasticity International REgistry (ASPIRE) study was developed to describe the clinical characteristics of adult patients being treated with onabotulinumtoxinA for spasticity in the real-world and its burden across several etiologies and geographical regions (Francisco et al., 2017). The goals of this 1-year interim analysis of ASPIRE were to describe the patterns of onabotulinumtoxinA utilization across several etiologies of spasticity and evaluate the effectiveness of onabotulinumtoxinA to treat spasticity across these etiologies via clinician- and patient-reported outcomes.
2. Methods
The ASPIRE study methods have been published previously (Francisco et al., 2017) and are described in brief below.
2.1. Study design and setting
ASPIRE is an international, multicenter, prospective, observational registry (NCT01930786). Data were collected by 74 treating clinicians across 54 international sites in North America, Europe, and Asia. OnabotulinumtoxinA treatments were administered at the clinician's discretion with no intervention from the sponsor in accordance with usual clinical practices and country-specific regulations. The design of ASPIRE included a 96-week study period, followed by a 12-week follow-up period for the collection of any additional safety data, for 108 weeks total. For this 1-year interim analysis, data from the first year of treatment, defined as 365 days after the first administration of onabotulinumtoxinA, were analyzed for each patient. For most patients, re-treatment is expected to occur approximately every 12 weeks according to the SmPC and package insert (Allergan, 2017, Allergan, 2018). However, the frequency of onabotulinumtoxinA treatments in real-world clinical practice varies according to many factors, including severity of spasticity, patient goals, the clinician's approach, administrative/logistical constraints, and others. Financial support was not provided by the sponsor for any treatment/treatment related costs. ASPIRE was conducted in accordance with all relevant regulatory requirements, including the Declaration of Helsinki and the Guidelines for Good Pharmacoepidemiology Practices (International Society for Pharmacoepidemiology [IPSE]).
2.2. Participants
Adult patients (≥18 years of age) that were either naïve or non-naïve to botulinum toxin(s) for spasticity were included in ASPIRE. Please refer to Francisco et al. (2017) for details on ASPIRE inclusion/exclusion criteria. Patients were treated with onabotulinumtoxinA for focal spasticity related to upper motor neuron syndrome during routine clinical practice. All patients were required to provide written informed consent. IRB/IEC approval was granted at each study site.
2.3. Outcomes and data sources
Patient demographics and clinical characteristic data, including etiology of spasticity, were collected at baseline. Etiology was not mutually exclusive, as more than one response was allowed per patient. Data from patients who reported more than one etiology were included for each identified etiology (eg, stroke and MS). The etiology(ies) identified at baseline were maintained across all treatment sessions for this analysis, regardless of changes in health status during the study. To investigate the impact of etiology of spasticity on onabotulinumtoxinA treatment patterns, utilization data were captured at each treatment session. To better understand the impact of onabotulinumtoxinA on patient-reported outcomes (PROs), changes in functional impairment were determined using the Disability Assessment Scale (DAS; Brashear et al., 2002b) and clinician- and patient-reported satisfaction data were gathered throughout the study. DAS was assessed by the clinician at treatment session 1 and at each subsequent treatment session. Clinician satisfaction was collected at each subsequent treatment session and patient satisfaction was collected 5±1 weeks post-treatment. Adverse event (AE) data were captured throughout the 1-year period and were summarized using the Medical Dictionary for Regulatory Activities (MedDRA) version 20.0 by system organ class and preferred term. Relationship to treatment and evaluation of potential distant spread of toxin were adjudicated by a panel of safety clinicians. For additional details on the assessment scales utilized in ASPIRE, as well as a complete list of data collected, refer to Francisco et al. (2017).
2.4. Statistical methods
No formal sample size/statistical power calculations were performed as analyses of the primary study objectives were descriptive and did not test specific hypotheses. Observed data are shown; no imputation of missing values was performed. Statistical significance was determined using ordinal logistic regression for DAS. Descriptive statistics were performed using SAS version 9.2 or higher (SAS Institute, Cary, NC, USA).
3. Results
3.1. Patient disposition, demographics, and clinical characteristics
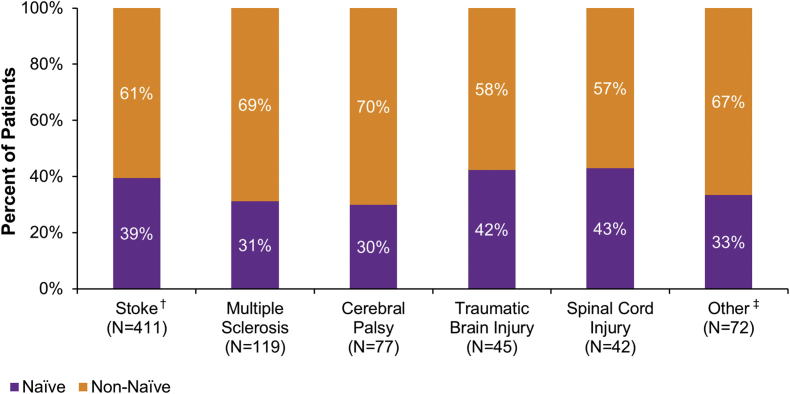
At the 1-year interim, 730 patients received at least one treatment of onabotulinumtoxinA to the upper and/or lower limb during at least one treatment session. The most common etiologies of spasticity at baseline were stroke (N=411/730 56.3% of patients), MS (N=119/730, 16.3%), CP (N=77/730, 10.5%), TBI (N=45/730, 6.2%), and SCI (N=42/730, 5.8%; Fig. 1). In total, 35 patients reported multiple etiologies at baseline: 34 patients reported 2 etiologies and 1 patient reported 3 etiologies. Etiologies in addition to those listed above were included in a category labeled “other”. Due to the diversity of underlying etiologies captured in other, meaningful conclusions about this category could not be drawn, and therefore, results are not presented in this manuscript. Approximately 1/3 of the total patient population was naïve to botulinum toxins for spasticity (Fig. 1). The proportion of patients naïve to botulinum toxin(s) was slightly higher in stroke, TBI, and SCI.
Fig. 1.
Etiology of spasticity at baseline. Within each etiology, the sum of naïve and non-naïve patients is 100%. Etiologies were not mutually exclusive, as more than one response was allowed per patient. †Stroke includes ischemic, hemorrhagic, and embolic stroke. ‡Other includes hereditary spastic paraparesis, stroke during aneurysm clipping, Chairi malformation, and hydrocephalus. N: number of patients.
Baseline patient demographics and clinical characteristics for each etiology are shown in Table 1. The mean age of patients ranged from 37.6 years old (CP) to 58.7 years old (stroke). Gender was evenly distributed in the stroke population, but was skewed towards more females in MS and CP, and more males in TBI and SCI, concordant with real-world prevalence and incidence. Race and BMI were similar across etiologies. The pattern of spasticity at baseline varied by etiology, with upper limb spasticity highest in stroke patients (N=377/411, 91.7%) and lowest in MS patients (N=40/119, 33.6%). Conversely, lower limb spasticity was highest in MS patients (N=114/119, 95.8%) and lowest in stroke patients (N=327/411, 79.6%). For each etiology, the percentage (number) of patients that withdrew consent and discontinued the study ≤365 days after their first treatment were as follows: 22.4% (N=92) of patients in stroke, 16.8% (N=20) in MS, 13% (N=10) in CP, 17.8% (N=8) in TBI, and 23.8% (N=10) in SCI.
Table 1.
Baseline patient demographics and clinical characteristics.
| Stroke (N=411) | MS (N=119) | CP (N=77) | TBI (N=45) | SCI (N=42) | |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean (SD) | 58.7 (14.1) | 53.1 (10.3) | 37.6 (13.4) | 42.8 (12.9) | 50.9 (16.0) |
| Median | 59.4 | 53.1 | 34.4 | 40.4 | 51.3 |
| Min, Max | 19.2, 93.2 | 24.7, 77.7 | 18.5, 68.1 | 20.2, 73.5 | 22.8, 93.2 |
| Gender, N (%) | |||||
| Female | 203 (49.4) | 83 (69.7) | 45 (58.4) | 16 (35.6) | 17 (40.5) |
| Male | 208 (50.6) | 36 (30.3) | 32 (41.6) | 29 (64.4) | 25 (59.5) |
| Race, N (%) | |||||
| Caucasian | 309 (75.2) | 99 (83.2) | 65 (84.4) | 32 (71.1) | 26 (61.9) |
| Black/African/Caribbean | 54 (13.1) | 9 ( 7.6) | 9 (11.7) | 5 (11.1) | 8 (19.0) |
| Asian | 32 ( 7.8) | 0 ( 0.0) | 1 ( 1.3) | 6 (13.3) | 5 (11.9) |
| Latino/Hispanic | 10 ( 2.4) | 1 ( 0.8) | 2 ( 2.6) | 1 ( 2.2) | 2 ( 4.8) |
| Other | 6 ( 1.4) | 10 ( 8.4) | 0 ( 0.0) | 1 ( 2.2) | 1 ( 2.4) |
| BMI (kg/m2), N | 351 | 96 | 61 | 38 | 36 |
| Mean (SD) | 27.2 (5.2) | 25.6 (5.4) | 27.4 (8.1) | 24.6 (4.8) | 26.0 (5.1) |
| Median | 26.2 | 24.7 | 25.6 | 23.1 | 25.3 |
| Min, Max | 17.3, 44.5 | 14.9, 42.2 | 15.5, 56.8 | 18.0, 37.1 | 17.2, 41.5 |
| Pattern of spasticity, N (%) | |||||
| Upper limb spasticity | 377 (91.7) | 40 (33.6) | 47 (61.0) | 35 (77.8) | 26 (61.9) |
| Lower limb spasticity | 327 (79.6) | 114 (95.8) | 69 (89.6) | 39 (86.7) | 35 (83.3) |
BMI: body mass index, CP: cerebral palsy, Max: maximum, Min: minimum, MS: multiple sclerosis, N: number of patients, SD: standard deviation, TBI: traumatic brain injury, SCI: spinal cord injury.
3.2. OnabotulinumtoxinA treatment utilization
The upper limb clinical presentations treated in ASPIRE included adducted/internally rotated shoulder, clenched fist, flexed elbow, flexed wrist, intrinsic plus hand, pronated forearm, and thumb-in-palm (refer to Mayer and Esquenazi, 2003, Simpson et al., 2017 for presentation descriptions). The lower limb clinical presentations treated in ASPIRE included adducted thigh, equinovarus foot, flexed hip, flexed knee, flexed toes, stiff extended knee, and striatal/hyperextended/hitchhiker toe (refer to Esquenazi et al., 2017a, Mayer and Esquenazi, 2003 for presentation descriptions). In the upper limb, clenched fist was treated most often in stroke, MS, and SCI, whereas flexed wrist and flexed elbow were most often treated in CP and TBI, respectively. (Fig. 2A). In the lower limb, equinovarus foot was treated most often in all etiologies (Fig. 2B).
Fig. 2.
Rank order of upper and lower limb clinical spasticity presentations. Within each etiology, clinical presentations (upper limb: A; lower limb: B) are listed in order of percentage of patients treated across all treatment sessions in the 1-year interim dataset: highest to lowest. Spasticity presentations are not mutually exclusive, and therefore, may add up to greater than100%. Data for “other” clinical presentations not predefined within the case report form, including for non-spasticity indications, are not shown. CP: cerebral palsy, MS: multiple sclerosis, n: number of treatment sessions per clinical presentation, N: number of patients, TBI: traumatic brain injury, SCI: spinal cord injury.
To compare onabotulinumtoxinA utilization across etiologies, the three most common upper limb presentations (ie, clenched fist, flexed elbow, and flexed wrist) and the three most common lower limb presentations (ie, equinovarus foot, flexed knee, adducted thigh) were identified based on percentage of patients treated for each presentation. Detailed utilization data, including number of patients treated, number of treatment sessions, dose, and injection localization methods are provided in Table 2 and Table 3, demonstrating the real-world diversity of onabotulinumtoxinA treatment, with findings of interest highlighted below. The additional presentations for the upper limb (Supplemental Table 1) and the lower limb (Supplemental Table 2) are provided in the supplementary materials.
Table 2.
OnabotulinumtoxinA treatment utilization for the most common upper limb clinical presentations.a
| Stroke (N=411) | MS (N=119) | CP (N=77) | TBI (N=45) | SCI (N=42) | |
|---|---|---|---|---|---|
| CLENCHED FIST | |||||
| Patients, N (%) | 290 (70.6) | 15 (12.6) | 23 (29.9) | 25 (55.6) | 17 (40.5) |
| Treatment sessions, n | 783 | 43 | 66 | 63 | 48 |
| Dose (U) | |||||
| Mean (SD) | 100 (60) | 105 (76) | 98 (96) | 127 (64) | 115 (61) |
| Mode | 100 | 50 | 100 | 100 | 100 |
| Min, Max | 10, 500 | 10, 350 | 10, 475 | 20, 300 | 25, 350 |
| Localization method, n (%)b | |||||
| Anatomical | 277 (35.4) | 32 (74.4) | 18 (27.3) | 11 (17.5) | 16 (33.3) |
| E-stim | 153 (19.5) | 14 (32.6) | 6 ( 9.1) | 17 (27.0) | 13 (27.1) |
| EMG | 430 (54.9) | 21 (48.8) | 41 (62.1) | 29 (46.0) | 23 (47.9) |
| Ultrasound | 208 (26.6) | 8 (18.6) | 9 (13.6) | 25 (39.7) | 19 (39.6) |
| FLEXED ELBOW | |||||
| Patients, N (%) | 277 (67.4) | 15 (12.6) | 29 (37.7) | 26 (57.8) | 12 (28.6) |
| Treatment sessions, n | 716 | 42 | 82 | 63 | 19 |
| Dose (U) | |||||
| Mean (SD) | 116 (75) | 163 (118) | 101 (56) | 126 (46) | 151 (96) |
| Mode | 100 | 100 | 100 | 100 | 100 |
| Min, Max | 15, 600 | 25, 500 | 20, 300 | 20, 225 | 30, 350 |
| Localization method, n (%)b | |||||
| Anatomical | 221 (30.9) | 26 (61.9) | 20 (24.4) | 10 (15.9) | 2 (10.5) |
| E-stim | 102 (14.2) | 1 ( 2.4) | 11 (13.4) | 17 (27.0) | 1 ( 5.3) |
| EMG | 403 (56.3) | 9 (21.4) | 52 (63.4) | 33 (52.4) | 10 (52.6) |
| Ultrasound | 168 (23.5) | 11 (26.2) | 7 ( 8.5) | 18 (28.6) | 8 (42.1) |
| FLEXED WRIST | |||||
| Patients, N (%) | 221 (53.8) | 8 (6.7) | 29 (37.7) | 13 (28.9) | 8 (19.0) |
| Treatment sessions, n | 551 | 18 | 92 | 33 | 16 |
| Dose (U) | |||||
| Mean (SD) | 79 (58) | 54 (22) | 80 (51) | 95 (41) | 97 (65) |
| Mode | 100 | 60 | 50 | 100 | 100 |
| Min, Max | 10, 500 | 20, 100 | 12, 300 | 25, 200 | 25, 250 |
| Localization method, n (%)b | |||||
| Anatomical | 156 (28.3) | 16 (88.9) | 23 (25.0) | 3 ( 9.1) | 9 (56.3) |
| E-stim | 87 (15.8) | 1 ( 5.6) | 16 (17.4) | 12 (36.4) | 2 (12.5) |
| EMG | 328 (59.5) | 3 (16.7) | 46 (50.0) | 21 (63.6) | 9 (56.3) |
| Ultrasound | 152 (27.6) | 2 (11.1) | 16 (17.4) | 8 (24.2) | 3 (18.8) |
CP: cerebral palsy, E-stim: electrical stimulation, EMG: electromyography, Max: maximum, mL: milliliter, Min: minimum, MS: multiple sclerosis, N: number of patients, n: number of treatment sessions, SCI: spinal cord injury, SD: standard deviation, TBI: traumatic brain injury, U: units of onabotulinumtoxinA.
For each etiology, the upper limb spasticity presentations were first ranked by number of patients treated: highest to lowest. Next, the number of times each spasticity presentation was included in the top 3 ranking was counted and the 3 presentations with the highest number of top 3 rankings are shown here. Upper limb spasticity presentations are not mutually exclusive, and therefore, may add up to greater than 100%.
Localization methods were not mutually exclusive and may have been influenced by availability of equipment at the site. “Anatomical” localization refers to palpation. Data represents the sum across all treatment sessions in the 1-year interim analysis.
Table 3.
OnabotulinumtoxinA treatment utilization for the most common lower limb clinical presentations.a
| Stroke (N=411) | MS (N=119) | CP (N=77) | TBI (N=45) | SCI (N=42) | |
|---|---|---|---|---|---|
| EQUINOVARUS FOOT | |||||
| Patients, N (%) | 217 (52.8) | 72 (60.5) | 38 (49.4) | 28 (62.2) | 20 (47.6) |
| Treatment sessions, n | 577 | 209 | 100 | 65 | 51 |
| Dose (U) | |||||
| Mean (SD) | 223 (131) | 206 (124) | 162 (116) | 223 (109) | 277 (168) |
| Mode | 300 | 300 | 100 | 150 | 200 |
| Min, Max | 15, 630 | 20, 875 | 20, 480 | 50, 450 | 60, 900 |
| Localization method, n (%)b | |||||
| Anatomical | 175 (30.3) | 113 (54.1) | 22 (22.0) | 11 (16.9) | 22 (43.1) |
| E-stim | 180 (31.2) | 60 (28.7) | 22 (22.0) | 42 (64.6) | 13 (25.5) |
| EMG | 288 (49.9) | 113 (54.1) | 52 (52.0) | 21 (32.3) | 16 (31.4) |
| Ultrasound | 155 (26.9) | 37 (17.7) | 17 (17.0) | 10 (15.4) | 13 (25.5) |
| FLEXED KNEE | |||||
| Patients, N (%) | 39 (9.5) | 32 (26.9) | 26 (33.8) | 12 (26.7) | 9 (21.4) |
| Treatment sessions, n | 80 | 99 | 66 | 17 | 24 |
| Dose (U) | |||||
| Mean (SD) | 143 (86) | 181 (122) | 150 (89) | 154 (60) | 165 (84) |
| Mode | 100 | 150 | 100 | 150 | 200 |
| Min, Max | 15, 500 | 20, 550 | 30, 325 | 75, 300 | 50, 300 |
| Localization method, n (%)b | |||||
| Anatomical | 46 (57.5) | 67 (67.7) | 19 (28.8) | 1 ( 5.9) | 10 (41.7) |
| E-stim | 10 (12.5) | 2 ( 2.0) | 0 ( 0.0) | 5 (29.4) | 1 ( 4.2) |
| EMG | 49 (61.3) | 45 (45.5) | 49 (74.2) | 9 (52.9) | 15 (62.5) |
| Ultrasound | 4 ( 5.0) | 6 ( 6.1) | 5 ( 7.6) | 3 (17.6) | 4 (16.7) |
| ADDUCTED THIGH | |||||
| Patients, N (%) | 20 (4.9) | 38 (9.2) | 17 (22.1) | 7 (15.6) | 9 (21.4) |
| Treatment sessions, n | 43 | 107 | 43 | 15 | 22 |
| Dose (U) | |||||
| Mean (SD) | 112 (54) | 173 (112) | 163 (94) | 166 (114) | 140 (66) |
| Mode | 100 | 200 | 100 | 150 | 100 |
| Min, Max | 20, 240 | 25, 500 | 40, 550 | 35, 400 | 50, 300 |
| Localization method, n (%)b | |||||
| Anatomical | 17 (39.5) | 77 (72.0) | 19 (44.2) | 2 (13.3) | 13 (59.1) |
| E-stim | 11 (25.6) | 8 ( 7.5) | 1 ( 2.3) | 4 (26.7) | 4 (18.2) |
| EMG | 18 (41.9) | 38 (35.5) | 23 (53.5) | 9 (60.0) | 11 (50.0) |
| Ultrasound | 3 ( 7.0) | 8 ( 7.5) | 0 ( 0.0) | 2 (13.3) | 1 ( 4.5) |
CP: cerebral palsy, E-stim: electrical stimulation, EMG: electromyography, Max: maximum, mL: milliliter, Min: minimum, MS: multiple sclerosis, N: number of patients, n: number of treatment sessions, SCI: spinal cord injury, SD: standard deviation, TBI: traumatic brain injury, U: units of onabotulinumtoxinA.
For each etiology, the lower limb spasticity presentations were first ranked by number of patients treated: highest to lowest. Next, the number of times each spasticity presentation was included in the top 3 ranking was counted and the 3 presentations with the highest number of top 3 rankings are shown here. Lower limb spasticity presentations are not mutually exclusive, and therefore, may add up to greater than 100%.
Localization methods were not mutually exclusive and may have been influenced by availability of equipment at the site. “Anatomical” localization refers to palpation. Data represents the sum across all treatment sessions in the 1-year interim analysis.
Clenched fist was most often treated in stroke (70.6% of patients), followed by TBI (55.6%), SCI (40.5%), CP (29.9%), and MS (12.6%; Table 2). A mean dose of 98 U (CP) to 127 U (TBI) of onabotulinumtoxinA per treatment session was observed, with the most common dose (mode) being 100 U in all etiologies except MS (50 U). Of the injection guidance techniques available, electromyography (EMG) was most often utilized by clinicians to locate the site(s) for injection in CP (62.1% of treatment sessions), stroke (54.9%), SCI (47.9%), and TBI (46.0%). However, anatomical localization was utilized most often in MS (74.4%).
Flexed elbow was most often treated in stroke (67.4% of patients), followed by TBI (57.8%), CP (37.7%), SCI (28.6%), and MS (12.6%; Table 2). A mean dose of 101 U (CP) to 163 U (MS) of onabotulinumtoxinA per treatment session was observed, with the most common dose being 100 U in all etiologies. EMG was most often utilized by clinicians to locate the site(s) for injection in CP (63.4% of treatment sessions), stroke (56.3%), SCI (52.6%), and TBI (52.4%), while anatomical localization was utilized most often in MS (61.9%).
Flexed wrist was most often treated in stroke (53.8% of patients), followed by CP (37.7%), TBI (28.9%), SCI (19.0%), and MS (6.7%; Table 2). A mean dose of 54 U (MS) to 97 U (SCI) of onabotulinumtoxinA per treatment session was observed, with the most common dose being 100 U (stroke, TBI, and SCI). EMG was most often utilized by clinicians to locate the site(s) for injection in TBI (63.6% of treatment sessions), stroke (59.5%), and CP (50.0%). However, anatomical localization was most often utilized in MS (88.9%) and anatomical localization and EMG were equally utilized in SCI (56.3% for both methods).
Equinovarus foot was most often treated in TBI (62.2% of patients), followed by MS (60.5%), stroke (52.8%), CP (49.4%), and SCI (47.6%; Table 3). A mean dose of 162 U (CP) to 277 U (SCI) of onabotulinumtoxinA per treatment session was observed, with the most common dose being 300 U (stroke and MS). EMG was most often utilized by clinicians to locate the site(s) for injection in CP (52.0% of treatment sessions) and stroke (49.9%), while both EMG and anatomical were equally utilized in MS (54.1% for both methods). Anatomical localization was utilized most often in SCI (43.1%) and electrical-stimulation (E-stim) in TBI (64.6%).
Flexed knee was most often treated in CP (33.8% of patients), followed by MS (26.9%), TBI (26.7%), SCI (21.4%), and stroke (9.5%; Table 3). A mean dose of 143 U (stroke) to 181 U (MS) of onabotulinumtoxinA per treatment session was observed, with the most common dose being 100 U (stroke and CP) or 150 U (MS and TBI). EMG was most often utilized by clinicians to locate the site(s) for injection in CP (74.2% of treatment sessions), SCI (62.5%), stroke (61.3%), and TBI (52.9%). However, anatomical localization was utilized most often in MS (67.7%).
Adducted thigh was most often treated in CP (22.1% of patients), followed by SCI (21.4%), TBI (15.6%), MS (9.2%), and stroke (4.9%; Table 3). A mean dose of 112 U (stroke) to 173 U (MS) of onabotulinumtoxinA per treatment session was observed, with the most common dose being 100 U (stroke, CP, and SCI). EMG was most often utilized by clinicians to locate the site(s) for injection in TBI (60.0% of treatment sessions), CP (53.5%), and stroke (41.9%), while anatomical localization was utilized most often in MS (72.0%) and SCI (59.1%).
The total body (ie, upper limb and/or lower limb) mean cumulative dose of onabotulinumtoxinA per treatment session ranged from 53 to 1038 U (Table 4). The mean cumulative dose in each etiology was as follows: 406 U in TBI, 371 U in stroke, 344 U in MS, 343 in SCI, and 296 U in CP. The most common (mode) cumulative dose of onabotulinumtoxinA utilized by clinicians was 300 U (MS, TBI, and SCI).
Table 4.
Mean cumulative dose of onabotulinumtoxinA per treatment session.
| Stroke (N=411) | MS (N=119) | CP (N=77) | TBI (N=45) | SCI (N=42) | |
|---|---|---|---|---|---|
| Dose (U) | |||||
| Mean (SD) | 371 (186) | 344 (177) | 296 (145) | 406 (152) | 343 (186) |
| Mode | 200 | 300 | 400 | 300 | 300 |
| Min, Max | 53, 1038 | 59, 850 | 60, 620 | 175, 927 | 100, 900 |
CP: cerebral palsy, Max: maximum, Min: minimum, MS: multiple sclerosis, N: number of patients, SCI: spinal cord injury, SD: standard deviation, TBI: traumatic brain injury, U: units of onabotulinumtoxinA.
3.3. Effectiveness
3.3.1. Disability Assessment Scale (DAS)
To evaluate functional impairment resulting from spasticity, DAS scores were collected by the treating clinician. Compared to treatment session 1, DAS scores improved significantly in stroke patients at subsequent treatments for nearly all subscales following treatment with onabotulinumtoxinA, indicative of decreased functional impairment/improved global functional over time, in both the upper limb (all comparisons, P < 0.0001; Table 5) and the lower limb (all comparisons, P < 0.05 except for hygiene; Table 6). In CP patients, DAS scores improved over time for the subscale of pain in the upper limb (P < 0.05; Table 5) and for the subscales of dressing, limb posture, and mobility in the lower limb following onabotulinumtoxinA treatment (P < 0.05; Table 6). In MS, TBI, and SCI patients, DAS scores improved over time for the subscale of mobility in the lower limb following treatment with onabotulinumtoxinA (P < 0.05; Table 6); however, no other significant changes were observed. Complete DAS data (treatment sessions 1–4) for each etiology are provided in Supplemental Tables 3–7.
Table 5.
Disability Assessment Scale (DAS) for the upper limb.a
| Stroke (N=411) |
MS (N=119) |
CP (N=77) |
TBI (N=45) |
SCI (N=42) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tx1 (N=410)c | Tx4 (N=152) | Tx1 (N=116) | Tx4 (N=47) | Tx1 (N=77) | Tx4 (N=28) | Tx1 (N=45) | Tx4 (N=15) | Tx1 (N=42) | Tx4 (N=15) | |
| Dressing, N (%) | ||||||||||
| 0 - No disability | 62 (15.1) | 27 (17.8) | 76 (65.5) | 27 (57.4) | 34 (44.2) | 9 (32.1) | 15 (33.3) | 6 (40.0) | 21 (50) | 5 (33.3) |
| 1 - Mild disability | 100 (24.4) | 63 (41.4) | 16 (13.8) | 4 ( 8.5) | 16 (20.8) | 6 (21.4) | 8 (17.8) | 4 (26.7) | 9 (21.4) | 5 (33.3) |
| 2 - Moderate disability | 169 (41.2) | 50 (32.9) | 10 ( 8.6) | 8 (17.0) | 19 (24.7) | 10 (35.7) | 16 (35.6) | 4 (26.7) | 7 (16.7) | 3 (20.0) |
| 3 - Severe disability | 79 (19.3) | 12 ( 7.9) | 14 (12.1) | 8 (17.0) | 8 (10.4) | 3 (10.7) | 6 (13.3) | 1 ( 6.7) | 5 (11.9) | 2 (13.3) |
| Odds ratio | 3.8 | 1.2 | 2.2 | 2.5 | 0.5 | |||||
| 95% CI | 2.4, 6.0 | 0.4, 3.9 | 0.7, 7.3 | 0.6, 11.4 | 0.1, 2.4 | |||||
| F-valueb | 15.9 | 0.6 | 1.5 | 0.8 | 1.1 | |||||
| P-value | < 0.0001 | 0.606 | 0.227 | 0.493 | 0.910 | |||||
| Hygiene, N (%) | ||||||||||
| 0 - No disability | 105 (25.6) | 53 (34.9) | 81 (69.8) | 29 (61.7) | 38 (49.4) | 11 (39.3) | 16 (35.6) | 6 (40.0) | 21 (50.0) | 5 (33.3) |
| 1 - Mild disability | 106 (25.9) | 42 (27.6) | 10 ( 8.6) | 6 (12.8) | 17 (22.1) | 6 (21.4) | 14 (31.1) | 6 (40.0) | 10 (23.8) | 6 (40.0) |
| 2 - Moderate disability | 120 (29.3) | 41 (27.0) | 12 (10.3) | 5 (10.6) | 13 (16.9) | 7 (25.0) | 8 (17.8) | 2 (13.3) | 5 (11.9) | 2 (13.3) |
| 3 - Severe disability | 79 (19.3) | 16 (10.5) | 13 (11.2) | 7 (14.9) | 9 (11.7) | 4 (14.3) | 7 (15.6) | 1 ( 6.7) | 6 (14.3) | 2 (13.3) |
| Odds ratio | 2.3 | 3.0 | 1.4 | 2.9 | 0.6 | |||||
| 95% CI | 1.5, 3.6 | 0.8, 10.7 | 0.4, 4.3 | 0.7, 12.9 | 0.1, 2.7 | |||||
| F-value | 9.6 | 1.6 | 1.8 | 1.0 | 2.7 | |||||
| P-value | < 0.0001 | 0.198 | 0.158 | 0.396 | 0.051 | |||||
| Limb Posture, N (%) | ||||||||||
| 0 - No disability | 60 (14.6) | 21 (13.8) | 80 (69.0) | 27 (57.4) | 35 (45.5) | 7 (25.0) | 11 (24.4) | 4 (26.7) | 19 (45.2) | 4 (26.7) |
| 1 - Mild disability | 62 (15.1) | 49 (32.2) | 11 ( 9.5) | 5 (10.6) | 8 (10.4) | 8 (28.6) | 6 (13.3) | 2 (13.3) | 6 (14.3) | 3 (20.0) |
| 2 - Moderate disability | 173 (42.2) | 59 (38.8) | 13 (11.2) | 7 (14.9) | 24 (31.2) | 9 (32.1) | 18 (40.0) | 8 (53.3) | 10 (23.8) | 5 (33.3) |
| 3 - Severe disability | 115 (28.0) | 23 (15.1) | 12 (10.3) | 8 (17.0) | 10 (13.0) | 4 (14.3) | 10 (22.2) | 1 ( 6.7) | 7 (16.7) | 3 (20.0) |
| Odds ratio | 3.2 | 2.1 | 1.3 | 2.3 | 0.7 | |||||
| 95% CI | 2.1, 5.0 | 0.6, 7.0 | 0.4, 4.0 | 0.6, 9.3 | 0.1, 3.2 | |||||
| F-value | 19.2 | 1.9 | 1.4 | 1.0 | 0.4 | |||||
| P-value | < 0.0001 | 0.137 | 0.240 | 0.403 | 0.729 | |||||
| Pain, N (%) | ||||||||||
| 0 - No disability | 146 (35.6) | 76 (50.0) | 89 (76.7) | 33 (70.2) | 48 (62.3) | 14 (50.0) | 23 (51.1) | 8 (53.3) | 21 (50.0) | 8 (53.3) |
| 1 - Mild disability | 108 (26.3) | 46 (30.3) | 14 (12.1) | 5 (10.6) | 19 (24.7) | 8 (28.6) | 12 (26.7) | 6 (40.0) | 9 (21.4) | 4 (26.7) |
| 2 - Moderate disability | 97 (23.7) | 23 (15.1) | 7 ( 6.0) | 4 ( 8.5) | 9 (11.7) | 5 (17.9) | 7 (15.6) | 1 ( 6.7) | 10 (23.8) | 0 ( 0.0) |
| 3 - Severe disability | 59 (14.4) | 7 ( 4.6) | 6 ( 5.2) | 5 (10.6) | 1 ( 1.3) | 1 ( 3.6) | 3 ( 6.7) | 0 ( 0.0) | 2 ( 4.8) | 3 (20.0) |
| Odds ratio | 3.6 | 1.5 | 1.0 | 1.7 | 1.1 | |||||
| 95% CI | 2.3, 5.8 | 0.4, 5.4 | 0.3, 3.0 | 0.4, 6.9 | 0.3, 4.4 | |||||
| F-value | 19.5 | 0.2 | 2.7 | 1.3 | 2.5 | |||||
| P-value | < 0.0001 | 0.912 | 0.048 | 0.271 | 0.064 | |||||
CI: confidence interval, CP: cerebral palsy, MS: multiple sclerosis, N: number of patients, SCI: spinal cord injury, TBI: traumatic brain injury, Tx: treatment session.
DAS objectively evaluates functional impairment resulting from spasticity across several subscales, including dressing, hygiene, limb posture, and pain (Brashear et al., 2002b). Patients were scored on a 4-point scale (range: 0–3) for each subscale, where a “0” represents no disability and a “3” represents severe disability (normal activities limited). DAS was assessed by the clinician at treatment session 1 and at each subsequent treatment session. However, to allow for comparison across etiologies, only treatment sessions 1 and 4 are shown here. Complete DAS data tables are provided in the supplemental materials for each etiology.
Data were analyzed using ordinal logistic regression. The F-value and level of significance (P-value) are shown for each subscale for the comparison across treatment sessions 1 to 4.
Data from treatment session 1 for each etiology were used as the reference for statistical analysis.
Table 6.
Disability Assessment Scale (DAS) for the lower limb.a
| Stroke (N=411) |
MS (N=119) |
CP (N=77) |
TBI (N=45) |
SCI (N=42) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tx1 (N=409)c | Tx4 (N=150) | Tx1 (N=117) | Tx4 (N=54) | Tx1 (N=77) | Tx4 (N=31) | Tx1 (N=45) | Tx4 (N=14) | Tx1 (N=42) | Tx4 (N=15) | |
| Dressing, N (%) | ||||||||||
| 0 - No disability | 131 (32.0) | 40 (26.7) | 14 (12.0) | 11 (20.4) | 18 (23.4) | 10 (32.3) | 15 (33.3) | 7 (50.0) | 11 (26.2) | 5 (33.3) |
| 1 - Mild disability | 126 (30.8) | 63 (42.0) | 36 (30.8) | 12 (22.2) | 21 (27.3) | 13 (41.9) | 12 (26.7) | 6 (42.9) | 11 (26.2) | 5 (33.3) |
| 2 - Moderate disability | 116 (28.4) | 39 (26.0) | 32 (27.4) | 19 (35.2) | 25 (32.5) | 6 (19.4) | 12 (26.7) | 1 ( 7.1) | 15 (35.7) | 3 (20.0) |
| 3 - Severe disability | 36 ( 8.8) | 8 ( 5.3) | 35 (29.9) | 12 (22.2) | 13 (16.9) | 2 ( 6.5) | 6 (13.3) | 0 ( 0.0) | 5 (11.9) | 2 (13.3) |
| Odds ratio | 1.6 | 1.9 | 3.5 | 2.5 | 2.2 | |||||
| 95% CI | 1.0, 2.5 | 0.8, 4.5 | 1.3, 9.8 | 0.6, 11.0 | 0.5, 9.5 | |||||
| F-valueb | 2.8 | 1.4 | 3.1 | 0.6 | 0.6 | |||||
| P-value | 0.041 | 0.252 | 0.029 | 0.649 | 0.594 | |||||
| Hygiene, N (%) | ||||||||||
| 0 - No disability | 196 (47.9) | 70 (46.7) | 28 (23.9) | 17 (31.5) | 27 (35.1) | 18 (58.1) | 22 (48.9) | 8 (57.1) | 18 (42.9) | 6 (40.0) |
| 1 - Mild disability | 98 (24.0) | 40 (26.7) | 25 (21.4) | 8 (14.8) | 24 (31.2) | 7 (22.6) | 9 (20.0) | 3 (21.4) | 11 (26.2) | 5 (33.3) |
| 2 - Moderate disability | 82 (20.0) | 33 (22.0) | 35 (29.9) | 17 (31.5) | 18 (23.4) | 4 (12.9) | 9 (20.0) | 3 (21.4) | 7 (16.7) | 3 (20.0) |
| 3 - Severe disability | 33 ( 8.1) | 7 ( 4.7) | 29 (24.8) | 12 (22.2) | 8 (10.4) | 2 ( 6.5) | 5 (11.1) | 0 ( 0.0) | 6 (14.3) | 1 ( 6.7) |
| Odds ratio | 1.2 | 1.5 | 4.4 | 1.2 | 1.1 | |||||
| 95% CI | 0.8, 2.0 | 0.7, 3.6 | 1.5, 13.2 | 0.2, 6.6 | 0.3, 5.2 | |||||
| F-value | 2.0 | 0.4 | 2.6 | 1.5 | 0.5 | |||||
| P-value | 0.108 | 0.736 | 0.056 | 0.218 | 0.709 | |||||
| Limb Posture, N (%) | ||||||||||
| 0 - No disability | 109 (26.7) | 41 (27.3) | 15 (12.8) | 9 (16.7) | 14 (18.2) | 7 (22.6) | 9 (20.0) | 5 (35.7) | 10 (23.8) | 4 (26.7) |
| 1 - Mild disability | 104 (25.4) | 56 (37.3) | 22 (18.8) | 13 (24.1) | 15 (19.5) | 13 (41.9) | 11 (24.4) | 3 (21.4) | 10 (23.8) | 4 (26.7) |
| 2 - Moderate disability | 148 (36.2) | 42 (28.0) | 43 (36.8) | 17 (31.5) | 33 (42.9) | 8 (25.8) | 16 (35.6) | 5 (35.7) | 13 (31.0) | 5 (33.3) |
| 3 - Severe disability | 48 (11.7) | 11 ( 7.3) | 37 (31.6) | 15 (27.8) | 15 (19.5) | 3 ( 9.7) | 9 (20.0) | 1 ( 7.1) | 9 (21.4) | 2 (13.3) |
| Odds ratio | 2.6 | 2.2 | 3.0 | 1.5 | 2.3 | |||||
| 95% CI | 1.6, 4.0 | 1.0, 5.0 | 1.2, 7.9 | 0.4, 6.2 | 0.5, 9.5 | |||||
| F-value | 7.6 | 2.1 | 4.0 | 0.6 | 2.0 | |||||
| P-value | < 0.0001 | 0.099 | 0.009 | 0.614 | 0.129 | |||||
| Pain, N (%) | ||||||||||
| 0 - No disability | 201 (49.1) | 83 (55.3) | 37 (31.6) | 14 (25.9) | 32 (41.6) | 14 (45.2) | 22 (48.9) | 8 (57.1) | 17 (40.5) | 6 (40.0) |
| 1 - Mild disability | 103 (25.2) | 40 (26.7) | 23 (19.7) | 18 (33.3) | 21 (27.3) | 7 (22.6) | 10 (22.2) | 6 (42.9) | 7 (16.7) | 5 (33.3) |
| 2 - Moderate disability | 74 (18.1) | 23 (15.3) | 31 (26.5) | 13 (24.1) | 13 (16.9) | 8 (25.8) | 9 (20.0) | 0 ( 0.0) | 10 (23.8) | 1 ( 6.7) |
| 3 - Severe disability | 31 ( 7.6) | 4 ( 2.7) | 26 (22.2) | 9 (16.7) | 11 (14.3) | 2 ( 6.5) | 4 ( 8.9) | 0 ( 0.0) | 8 (19.0) | 3 (20.0) |
| Odds ratio | 1.9 | 2.0 | 1.9 | 2.5 | 1.6 | |||||
| 95% CI | 1.2, 3.1 | 0.9, 4.2 | 0.7, 5.2 | 0.5, 12.4 | 0.3, 7.6 | |||||
| F-value | 10.7 | 1.5 | 1.9 | 0.5 | 0.4 | |||||
| P-value | < 0.0001 | 0.205 | 0.127 | 0.663 | 0.755 | |||||
| Mobility, N (%) | ||||||||||
| 0 - No disability | 90 (22.0) | 25 (16.7) | 4 ( 3.4) | 4 ( 7.4) | 10 (13.0) | 4 (12.9) | 6 (13.3) | 4 (28.6) | 8 (19.0) | 4 (26.7) |
| 1 - Mild disability | 73 (17.8) | 43 (28.7) | 10 ( 8.5) | 9 (16.7) | 11 (14.3) | 12 (38.7) | 6 (13.3) | 3 (21.4) | 3 ( 7.1) | 4 (26.7) |
| 2 - Moderate disability | 165 (40.3) | 63 (42.0) | 42 (35.9) | 20 (37.0) | 28 (36.4) | 10 (32.3) | 21 (46.7) | 7 (50.0) | 14 (33.3) | 5 (33.3) |
| 3 - Severe disability | 81 (19.8) | 19 (12.7) | 61 (52.1) | 21 (38.9) | 28 (36.4) | 5 (16.1) | 12 (26.7) | 0 ( 0.0) | 17 (40.5) | 2 (13.3) |
| Odds ratio | 2.1 | 4.3 | 5.2 | 7.2 | 8.0 | |||||
| 95% CI | 1.3, 3.3 | 1.7, 11.3 | 1.9, 14.8 | 1.5, 34.3 | 1.8, 34.6 | |||||
| F-value | 4.4 | 4.1 | 4.9 | 2.8 | 3.8 | |||||
| P-value | 0.004 | 0.007 | 0.003 | 0.049 | 0.013 | |||||
CI: confidence interval, CP: cerebral palsy, MS: multiple sclerosis, N: number of patients, SCI: spinal cord injury, TBI: traumatic brain injury, Tx: treatment session.
DAS objectively evaluates functional impairment resulting from spasticity across several subscales, including dressing, hygiene, limb posture, and pain (Brashear et al., 2002b). Patients were scored on a 4-point scale (range: 0–3) for each subscale, where a “0” represents no disability and a “3” represents severe disability (normal activities limited). DAS was assessed by the clinician at treatment session 1 and at each subsequent treatment session. However, to allow for comparison across etiologies, only treatment sessions 1 and 4 are shown here. Complete DAS data tables are provided in the supplemental materials for each etiology.
Data were analyzed using ordinal logistic regression. The F-value and level of significance (P-value) are shown for each subscale for the comparison across treatment sessions 1 to 4.
Data from treatment session 1 for each etiology were used as the reference for statistical analysis.
3.3.2. Clinician and patient satisfaction
In ASPIRE, clinicians and patients were asked a series of questions to determine their satisfaction with onabotulinumtoxinA treatment for spasticity (Fig. 3). Clinicians reported extreme satisfaction/satisfaction that onabotulinumtoxinA helped their patient's spasticity (range: 87.4% [SCI] to 94.2% [CP] of treatment sessions; Fig. 3A). Likewise, patients reported extreme satisfaction/satisfaction that onabotulinumtoxinA helped their spasticity (range: 67.6% [TBI] to 89.7% [SCI]; Fig. 3B). In addition, clinicians reported extreme satisfaction/satisfaction that onabotulinumtoxinA helped manage their patient's spasticity-related pain, if present (range: 82.7% [TBI] to 93.5% [CP]; Fig. 3C), with patients showing high agreement with this statement (range: 82.6% [stroke and TBI] to 87.0% [SCI]; Fig. 3D). Furthermore, clinicians reported extreme satisfaction/satisfaction that onabotulinumtoxinA helped their patients participate in therapy/exercise (range: 85.2% [MS] to 95.5% [CP]; Fig. 3E), with patients showing high agreement with this statement as well (range: 74.2% [TBI] to 81.5% [SCI]; Fig. 3F). Lastly, the majority of clinicians (range: 94.6% [TBI] to 98.8% [CP]; Fig. 3G) and patients (range: 88.4% [stroke] to 91.2% [TBI]; Fig. 3H) responded that they would definitely/probably continue to use onabotulinumtoxinA to manage spasticity.
Fig. 3.
Clinician- and patient-reported satisfaction with onabotulinumtoxinA treatment for spasticity. Clinicians (at each subsequent treatment session; A, C, E, G) and patients (5±1 weeks post-treatment; B, D, F, H) were asked a series of questions to determine their satisfaction with the previous onabotulinumtoxinA (referred to as BOTOX in the original questionnaire) treatment for spasticity. For figures C–F, the percentage of clinicians and patients were recalculated to exclude those that indicated that the question was “not applicable”. CP: cerebral palsy, MS: multiple sclerosis, n: number of treatment sessions, TBI: traumatic brain injury, Tx: treatment session, SCI: spinal cord injury.
3.4. Safety
At the 1-year interim, AE and serious AE data varied by etiology of spasticity and are provided in Supplemental Table 8. In addition, the total number of treatment-related AEs varied across etiologies (stroke: 10 events [2.2% of patients]; MS: 5 events [4.2%], CP: 0 events [0.0%]; TBI: 1 event [2.2%]; SCI: 0 events [0.0%]), with muscular weakness being the most common treatment-related AE reported (Table 7). Few treatment-related serious AEs were reported (Table 7) and included one case each of dysphagia, muscular weakness, and slow speech in 2 male patients from the stroke population. Neither case was considered related to the distant spread of toxin as adjudicated by a panel of safety clinicians.
Table 7.
Treatment-related adverse events and treatment-related serious adverse eventsa
| Stroke (N=411) |
MS (N=119) |
CP (N=77) |
TBI (N=45) |
SCI (N=42) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients, N (%) |
Events, n | Patients, N (%) |
Events, n | Patients, N (%) |
Events, n | Patients, N (%) |
Events, n | Patients, N (%) |
Events, n | |
| All TRAEsb | ||||||||||
| Muscular weakness | 4 (1.0) | 4 | 3 (2.5) | 3 | – | – | – | – | – | – |
| Dysphagia | 2 (0.5) | 2 | – | – | – | – | – | – | – | – |
| Asthenia | 1 (0.2) | 1 | – | – | – | – | – | – | – | – |
| Drug tolerance | 1 (0.2) | 1 | – | – | – | – | – | – | – | – |
| Dry mouth | – | – | 1 (0.8) | 1 | – | – | – | – | – | – |
| Grip strength decreased | – | – | – | – | – | – | 1 (2.2) | 1 | – | – |
| Influenza-like illness | – | – | 1 (0.8) | 1 | – | – | – | – | – | – |
| Peripheral edema | 1 (0.2) | 1 | – | – | – | – | – | – | – | – |
| Slow speech | 1 (0.2) | 1 | – | – | – | – | – | – | – | – |
| All TRSAEsb | ||||||||||
| Dysphagia | 1 (0.2) | 1 | – | – | – | – | – | – | – | – |
| Muscular weakness | 1 (0.2) | 1 | – | – | – | – | – | – | – | – |
| Slow speech | 1 (0.2) | 1 | – | – | – | – | – | – | – | – |
CP: cerebral palsy, MS: multiple sclerosis, n: number of adverse events, N: number of patients, SCI: spinal cord injury, TBI: traumatic brain injury, TRAE: treatment-related adverse events, TRSAE: treatment-related serious adverse events.
All TRAE and TRSAE data are shown.
For each TRAE and TRSAE, the number of patients that reported the event were totaled across all etiologies and arranged in descending order.
4. Discussion
The majority of published data describing the use of onabotulinumtoxinA to treat spasticity are from controlled trials in post-stroke patients (eg, Brashear et al., 2002a, Kaji et al., 2010a, Kaji et al., 2010b, Patel, 2011, Wein et al., 2018). Real-world, observational data examining onabotulinumtoxinA utilization across other etiologies of spasticity, in addition to stroke, are needed to improve clinical approaches and optimize patient care. The aims for this 1-year interim analysis of the ASPIRE study were to describe the real-world patterns of onabotulinumtoxinA utilization across several etiologies of spasticity and evaluate the effectiveness of onabotulinumtoxinA to treat spasticity across these etiologies via clinician- and patient-reported outcomes.
OnabotulinumtoxinA utilization data from ASPIRE revealed similarities and differences in clinical approaches to treat spasticity across five common etiologies – stroke, MS, CP, TBI, and SCI. OnabotulinumtoxinA utilization data from ASPIRE are highly generalizable and reflect the complexity of treating patients in the real-world. In clinical practice, dosing decisions can be impacted by country-specific dosing regulations, treatment history, severity of spasticity, number and location of muscles targeted, and experience of the treating clinician, amongst others (Brin, 1997, Esquenazi et al., 2017b, Simpson et al., 2017, Sunnerhagen et al., 2013).
In this 1-year analysis of ASPIRE, clenched fist was the most commonly treated upper limb presentation in stroke, MS, and SCI, while flexed wrist and flexed elbow were most commonly treated in CP and TBI, respectively. Within the lower limb, equinovarus foot was the most commonly treated presentation in all etiologies. The dose ranges of onabotulinumtoxinA utilized by clinicians showed consistency within the upper limb versus the lower limb. Specifically, across the three most common upper limb presentations (clenched fist, flexed elbow, and flexed wrist), dose ranges were highest when treating post-stroke patients. In contrast, across the three most common lower limb presentations (equinovarus foot, flexed knee, and adducted thigh), dose ranges were highest when treating patients with MS. There are several possible explanations for the observed variability in dosing between etiologies. For example, differences in spasticity presentation (eg, higher weakness associated with a specific etiology), location and size of muscles targeted (eg, MS patients were often treated for lower limb spasticity in ASPIRE, with larger muscles generally found in the lower limb), or severity of spasticity (eg, a specific etiology may typically present with greater severity) could all affect the dose of onabotulinumtoxinA utilized by clinicians.
Furthermore, onabotulinumtoxinA dosing for the most common clinical presentations in the upper limb were generally lower and less variable between etiologies compared to the lower limb. For example, the most common dose (mode) of onabotulinumtoxinA utilized, which was similar to the mean dose, was 100 U to treat clenched fist (4 out of 5 etiologies), flexed elbow (all etiologies), and flexed wrist (3 out of 5 etiologies). In contrast, common dosing patterns were less apparent for the lower limb presentations of equinovarus foot, flexed knee, and adducted thigh. For example, the most common dose of onabotulinumtoxinA utilized for equinovarus foot varied by etiology, with 300 U utilized in stroke and MS, 200 U in SCI, 150 U in TBI, and 100 in CP. Despite the variability in doses of onabotulinumtoxinA utilized by clinicians, the total body (ie, upper and/or lower limb) mean cumulative dose of onabotulinumtoxinA per treatment session (range: 296 U [CP] to 406 U [TBI]) was near or below that recommended on the product label (400 U; Allergan, 2017). Combined, these dosing data from ASPIRE demonstrate the individualized approach used by clinicians when treating patients with onabotulinumtoxinA from various etiologies of spasticity. Indeed, an individualized and flexible approach to botulinum toxin therapy has been discussed in the literature, as a single approach is unlikely to suit all patients or disease states (Wissel, 2018).
Injection localization methods, such as EMG, E-stim, and ultrasound, have been recommended to improve the localization of target muscles for injection (Picelli et al., 2012, Picelli et al., 2014,bib_Picelli_et_al_2014,Picelli et al., 2012, Simpson et al., 2017, Walker et al., 2015). A review by Chan et al. (2017) found level I evidence that using injection localization methods improved patient outcomes (eg, MMAS, range of motion, limb placement, and Tardieu Scale) compared to manual needle placement alone. In ASPIRE, EMG was utilized by clinicians in approximately half of all treatment sessions for the majority of presentations in both the upper and lower limb in stroke, CP, TBI, and SCI. In contrast, clinicians treating MS showed a preference for anatomical localization compared to instrumented guidance techniques. As these are real-world data, they reflect the injection techniques used by treating clinicians in actual practice, which may be indicative of their preferences, but also may have been influenced by the equipment that was available at the study sites. Finally, these data suggest that there may be an educational opportunity to inform clinicians of the benefits of guided localization methods to improve patient outcomes with botulinum toxin therapy.
To assess the effectiveness of onabotulinumtoxinA to treat upper and/or lower limb spasticity, PRO data were collected throughout the course of the study from all etiologies. Functional impairment was assessed by the clinician at each treatment session using DAS (Brashear et al., 2002b) and clinician- and patient-reported satisfaction data were gathered following each onabotulinumtoxinA treatment. At the 1-year interim analysis, among stroke patients, DAS scores were significantly reduced in the upper and lower limbs across the subscales of dressing, hygiene (excluding lower limb), limb posture, pain, and mobility, demonstrating improved global function and quality of life following repeated onabotulinumtoxinA treatment. Additionally, among MS, CP, TBI, and SCI patients, DAS scores significantly improved in the lower limb for the subscale of mobility following onabotulinumtoxinA treatment. These changes in DAS are a meaningful finding for patients, as increased disability on the DAS has been associated with reductions in health-related quality of life in post-stroke spasticity patients (Doan et al., 2012). Improvements in DAS are likely a reflection of goal selection (ie, active vs. passive) and influenced by clinical presentations that are common to specific etiologies, time to treatment after onset of spasticity, and many other factors.
Across all etiologies, the majority of patients and clinicians reported that they were satisfied with onabotulinumtoxinA treatment and would continue to use onabotulinumtoxinA to treat spasticity. Patients and clinicians in ASPIRE reported that onabotulinumtoxinA treatment helped reduce their/their patient's spasticity-related pain and helped them/their patients participate in therapy/exercise. Spasticity-related pain has been associated with reduced quality of life (Andresen et al., 2016, Harrison and Field, 2015), as well as economic losses due to decreased work productivity (Lang, 2003). Importantly, adjunct therapies have been shown to improve patient outcomes, including a reduction in hypertonicity and improvements on the Modified Ashworth Scale over time, following treatment with botulinum toxins (Giovannelli et al., 2007, Mills et al., 2016, Rosales et al., 2016). Interestingly, patients with TBI consistently reported the lowest satisfaction with onabotulinumtoxinA treatment and its ability to help them participate in therapy/exercise or help their spasticity-related pain, yet these patients reported that they were the most willing to continue onabotulinumtoxinA treatment compared to all other etiologies. The findings of decreased satisfaction in patients with TBI may be due, in part, to the higher complexity of spasticity patterns often observed in this patient population. Treating TBI patients may require different clinical approaches (eg, more frequent dosing) and/or higher mean doses of onabotulinumtoxinA, which may be restricted by country-specific dosing regulations and/or insurance coverage.
Data from this 1-year interim analysis of ASPIRE support the safety of onabotulinumtoxinA to treat adult spasticity across several etiologies, including stroke, MS, CP, TBI, and SCI. OnabotulinumtoxinA demonstrated an acceptable safety profile, with no new safety signals identified. Adverse event data captured in this analysis of ASPIRE are consistent with safety data within the literature and global labelling (Allergan, 2017, Brashear et al., 2002a, Childers et al., 2004, Kaji et al., 2010b).
Limitations of the ASPIRE study have been discussed previously (Francisco et al., 2017). Of relevance to this interim analysis, the number of patients within each etiology was not preordained and varied according to patient recruitment at the study sites, resulting in uneven sample sizes amongst etiologies. Due to this variability, results in post-stroke patients are likely more robust due to the larger sample size (N=411) compared to the other etiologies (eg, TBI [N=45] or SCI [N=42]). In addition, the number of treatments received in a 1-year period varies among patients due to several factors, such as a patient's severity of spasticity, experience of clinician, previous response to treatment (ie, duration of effect), and other factors. Therefore, data from later treatment sessions (within the 1-year interim) should be interpreted with caution due to lower sample sizes. Future analyses from ASPIRE will need to determine the impact of etiology of spasticity on other factors, such as treatment adherence, as well as describe the patterns of onabotulinumtoxinA utilization and clinician- and patient-reported outcomes for the completed 2-year study.
5. Conclusions
High clinician- and patient-reported satisfaction were observed across all etiologies of spasticity following individualized onabotulinumtoxinA treatment in ASPIRE, as well as improved global function based on DAS. These real-world data highlight the similarities and differences in treating patients across several common etiologies, providing an important educational tool to help guide clinical strategies on the use of onabotulinumtoxinA to treat spasticity.
Data Sharing Statement
Data reported in this manuscript are available within the article and its supplementary materials. Allergan, an AbbVie company, will share de-identified patient-level data and/or study-level data, including protocols and clinical study reports, for phase 2–4 trials completed after 2008 that are registered on ClinicalTrials.gov or EudraCT. The indication studied in the trial must have regulatory approval in the United States and/or the European Union and the primary manuscript from the trial must be published prior to data sharing. To request access to the data, the researcher must sign a data use agreement. All shared data are to be used for non-commercial purposes only. More information can be found at http://www.allerganclinicaltrials.com/.
CRediT authorship contribution statement
Gerard E. Francisco: Conceptualization, Methodology, Investigation, Writing - review & editing, Visualization. Daniel S. Bandari: Investigation, Writing - review & editing, Visualization. Ganesh Bavikatte: Investigation, Writing - review & editing, Visualization. Wolfgang H. Jost: Investigation, Writing - review & editing, Visualization. Emily McCusker: Writing - original draft, Visualization. Joan Largent: Methodology, Formal analysis, Writing - review & editing, Visualization. Aleksej Zuzek: Writing - review & editing, Visualization, Project administration, Funding acquisition. Alberto Esquenazi: Conceptualization, Methodology, Investigation, Writing - review & editing, Visualization.
Declaration of competing interest
Financial arrangements of the authors with companies whose products may be related to the present manuscript are listed below, as declared by the authors. GEF: consulted/received research grants from Allergan, an AbbVie company, Merz, and Ipsen; DSB: consulted/speaker for Accorda, Biogen, Genentech, Genzyme, EMD-Serono, Mallinckrodt and Teva, and received research support from Biogen, Genentech, Allergan, an AbbVie company, Genzyme and Med-day; GB: served on a steering committee/consulted for Allergan, an AbbVie company; WHJ: consulted/speaker for Allergan, an AbbVie company, Ipsen, and Merz; EM and AZ: full-time AbbVie employees; JL: full-time IQVIA (formerly QuintilesIMS) employee, the contract research organization responsible for the management and statistical analysis of ASPIRE, and a former full-time Allergan employee; AE: consulted for Allergan, an AbbVie company, Ipsen, and Merz, and received research grants from Allergan, an AbbVie company, and Ipsen.
Acknowledgements
The authors sincerely thank the patients who participated in this study and acknowledge the investigators and staff who contributed to the study conduct. Thank you to Vedanta Research (Kristina Fanning, PhD and Michael Reed, PhD) for their assistance with additional statistical analyses. This study was sponsored by Allergan (prior to its acquisition by AbbVie). Writing and editorial assistance was provided to the authors by Monica R.P. Elmore of AbbVie. All authors met the ICMJE authorship criteria. Neither honoraria nor any other form of compensation was provided for authorship.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.toxcx.2020.100040.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- AANS . American Association of Neurological Surgeons; 2018. Spasticity. [Google Scholar]
- Allergan . 2017. BOTOX® [Package Insert] [Google Scholar]
- Allergan . 2018. BOTOX® 100 Units Summary of Product Characteristics (SmPC) [Google Scholar]
- Andresen S.R. Pain, spasticity and quality of life in individuals with traumatic spinal cord injury in Denmark. Spinal Cord. 2016;54:973–979. doi: 10.1038/sc.2016.46. [DOI] [PubMed] [Google Scholar]
- Angulo-Parker F.J., Adkinson J.M. Common etiologies of upper extremity spasticity. Hand Clin. 2018;34:437–443. doi: 10.1016/j.hcl.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Barin L. The disease burden of Multiple Sclerosis from the individual and population perspective: which symptoms matter most? Mult Scler Relat Disord. 2018;25:112–121. doi: 10.1016/j.msard.2018.07.013. [DOI] [PubMed] [Google Scholar]
- Barnes M.P. Spasticity in multiple sclerosis. Neurorehabilitation Neural Repair. 2003;17:66–70. doi: 10.1177/0888439002250449. [DOI] [PubMed] [Google Scholar]
- Brashear A. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke. N. Engl. J. Med. 2002;347:395–400. doi: 10.1056/NEJMoa011892. [DOI] [PubMed] [Google Scholar]
- Brashear A. Inter- and intrarater reliability of the Ashworth Scale and the Disability Assessment Scale in patients with upper-limb poststroke spasticity. Arch. Phys. Med. Rehabil. 2002;83:1349–1354. doi: 10.1053/apmr.2002.35474. [DOI] [PubMed] [Google Scholar]
- Brin M.F. Dosing, administration, and a treatment algorithm for use of botulinum toxin A for adult-onset spasticity. Spasticity Study Group. Muscle Nerve Suppl. 1997;6:S208–220. doi: 10.1002/(sici)1097-4598(1997)6+<208::aid-mus15>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Chan A.K. Does the method of botulinum neurotoxin injection for limb spasticity affect outcomes? A systematic review. Clin. Rehabil. 2017;31:713–721. doi: 10.1177/0269215516655589. [DOI] [PubMed] [Google Scholar]
- Cheung J. Patient-identified factors that influence spasticity in people with stroke and multiple sclerosis receiving botulinum toxin injection treatments. Physiother. Can. 2015;67:157–166. doi: 10.3138/ptc.2014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers M.K. Dose-dependent response to intramuscular botulinum toxin type A for upper-limb spasticity in patients after a stroke. Arch. Phys. Med. Rehabil. 2004;85:1063–1069. doi: 10.1016/j.apmr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Collongues N., Vermersch P. Multiple sclerosis spasticity: 'state-of-the-art' questionnaire survey of specialized healthcare professionals. Expert Rev. Neurother. 2013;13:21–25. doi: 10.1586/ern.13.10. [DOI] [PubMed] [Google Scholar]
- Doan Q.V. Relationship between disability and health-related quality of life and caregiver burden in patients with upper limb poststroke spasticity. Pharm. Manag. PM R. 2012;4:4–10. doi: 10.1016/j.pmrj.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Esquenazi A. Evidence-based review and assessment of botulinum neurotoxin for the treatment of adult spasticity in the upper motor neuron syndrome. Toxicon. 2013;67:115–128. doi: 10.1016/j.toxicon.2012.11.025. [DOI] [PubMed] [Google Scholar]
- Esquenazi A. OnabotulinumtoxinA for lower limb spasticity: guidance from a Delphi panel approach. Pharm. Manag. PM R. 2017;9:960–968. doi: 10.1016/j.pmrj.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Esquenazi A. Patient registry of spasticity care world: data analysis based on physician experience. Am. J. Phys. Med. Rehabil. 2017;96:881–888. doi: 10.1097/PHM.0000000000000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerup N.B. Neuropathic pain and spasticity: intricate consequences of spinal cord injury. Spinal Cord. 2017;55:1046–1050. doi: 10.1038/sc.2017.70. [DOI] [PubMed] [Google Scholar]
- Francisco G.E. Adult Spasticity International Registry Study: methodology and baseline patient, healthcare provider, and caregiver characteristics. J. Rehabil. Med. 2017;49:659–666. doi: 10.2340/16501977-2245. [DOI] [PubMed] [Google Scholar]
- Giovannelli M. Early physiotherapy after injection of botulinum toxin increases the beneficial effects on spasticity in patients with multiple sclerosis. Clin. Rehabil. 2007;21:331–337. doi: 10.1177/0269215507072772. [DOI] [PubMed] [Google Scholar]
- Harrison R.A., Field T.S. Post stroke pain: identification, assessment, and therapy. Cerebrovasc. Dis. 2015;39:190–201. doi: 10.1159/000375397. [DOI] [PubMed] [Google Scholar]
- Holtz K.A. Prevalence and effect of problematic spasticity after traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 2017;98:1132–1138. doi: 10.1016/j.apmr.2016.09.124. [DOI] [PubMed] [Google Scholar]
- Hughes C., Howard I.M. Spasticity management in multiple sclerosis. Phys. Med. Rehabil. Clin. 2013;24:593–604. doi: 10.1016/j.pmr.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Kaji R. Botulinum toxin type A in post-stroke lower limb spasticity: a multicenter, double-blind, placebo-controlled trial. J. Neurol. 2010;257:1330–1337. doi: 10.1007/s00415-010-5526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji R. Botulinum toxin type A in post-stroke upper limb spasticity. Curr. Med. Res. Opin. 2010;26:1983–1992. doi: 10.1185/03007995.2010.497103. [DOI] [PubMed] [Google Scholar]
- Kheder A., Nair K.P. Spasticity: pathophysiology, evaluation and management. Practical Neurol. 2012;12:289–298. doi: 10.1136/practneurol-2011-000155. [DOI] [PubMed] [Google Scholar]
- Lang A.M. Botulinum toxin type A therapy in chronic pain disorders. Arch. Phys. Med. Rehabil. 2003;84:S69–73. doi: 10.1053/apmr.2003.50121. [DOI] [PubMed] [Google Scholar]; quiz S74-65. DOI:10.1053/apmr.2003.50121.
- Lundstrom E. Four-fold increase in direct costs of stroke survivors with spasticity compared with stroke survivors without spasticity: the first year after the event. Stroke. 2010;41:319–324. doi: 10.1161/STROKEAHA.109.558619. [DOI] [PubMed] [Google Scholar]
- Mayer N.H., Esquenazi A. Muscle overactivity and movement dysfunction in the upper motoneuron syndrome. Phys. Med. Rehabil. Clin. 2003;14:855–883. doi: 10.1016/S1047-9651(03)00093-7. vii-viii. [DOI] [PubMed] [Google Scholar]
- Mayer N.H. Common patterns of clinical motor dysfunction. Muscle Nerve Suppl. 1997;6:S21–35. [PubMed] [Google Scholar]
- Mills P.B. Systematic review of adjunct therapies to improve outcomes following botulinum toxin injection for treatment of limb spasticity. Clin. Rehabil. 2016;30:537–548. doi: 10.1177/0269215515593783. [DOI] [PubMed] [Google Scholar]
- Nair K.P., Marsden J. The management of spasticity in adults. BMJ. 2014;349:g4737. doi: 10.1136/bmj.g4737. [DOI] [PubMed] [Google Scholar]
- Odding E. The epidemiology of cerebral palsy: incidence, impairments and risk factors. Disabil. Rehabil. 2006;28:183–191. doi: 10.1080/09638280500158422. [DOI] [PubMed] [Google Scholar]
- Pandyan A.D. Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil. Rehabil. 2005;27:2–6. doi: 10.1080/09638280400014576. [DOI] [PubMed] [Google Scholar]
- Patel A.T. Successful treatment of long-term, poststroke, upper-limb spasticity with onabotulinumtoxinA. Phys. Ther. 2011;91:1636–1641. doi: 10.2522/ptj.20100370. [DOI] [PubMed] [Google Scholar]
- Picelli A. Botulinum toxin injection into the forearm muscles for wrist and fingers spastic overactivity in adults with chronic stroke: a randomized controlled trial comparing three injection techniques. Clin. Rehabil. 2014;28:232–242. doi: 10.1177/0269215513497735. [DOI] [PubMed] [Google Scholar]
- Picelli A. Botulinum toxin type A injection into the gastrocnemius muscle for spastic equinus in adults with stroke: a randomized controlled trial comparing manual needle placement, electrical stimulation and ultrasonography-guided injection techniques. Am. J. Phys. Med. Rehabil. 2012;91:957–964. doi: 10.1097/PHM.0b013e318269d7f3. [DOI] [PubMed] [Google Scholar]
- Rizzo M.A. Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult. Scler. 2004;10:589–595. doi: 10.1191/1352458504ms1085oa. [DOI] [PubMed] [Google Scholar]
- Rosales R.L. Botulinum toxin as early intervention for spasticity after stroke or non-progressive brain lesion: a meta-analysis. J. Neurol. Sci. 2016;371:6–14. doi: 10.1016/j.jns.2016.10.005. [DOI] [PubMed] [Google Scholar]
- Simpson D.M. OnabotulinumtoxinA injection for poststroke upper-limb spasticity: guidance for early injectors from a Delphi panel process. Pharm. Manag. PM R. 2017;9:136–148. doi: 10.1016/j.pmrj.2016.06.016. [DOI] [PubMed] [Google Scholar]
- Sunnerhagen K.S. Assessing and treating functional impairment in poststroke spasticity. Neurology. 2013;80:S35–44. doi: 10.1212/WNL.0b013e3182764aa2. [DOI] [PubMed] [Google Scholar]
- Tardieu G. [Research on a technic for measurement of spasticity] Rev. Neurol. (Paris) 1954;91:143–144. [PubMed] [Google Scholar]
- Trompetto C. Pathophysiology of spasticity: implications for neurorehabilitation. BioMed Res. Int. 2014;2014:354906. doi: 10.1155/2014/354906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker H.W. Botulinum toxin injection techniques for the management of adult spasticity. Pharm. Manag. PM R. 2015;7:417–427. doi: 10.1016/j.pmrj.2014.09.021. [DOI] [PubMed] [Google Scholar]
- Ward A.B. Spasticity treatment with botulinum toxins. J. Neural. Transm. 2008;115:607–616. doi: 10.1007/s00702-007-0833-2. [DOI] [PubMed] [Google Scholar]
- Wedekind C., Lippert-Gruner M. Long-term outcome in severe traumatic brain injury is significantly influenced by brainstem involvement. Brain Inj. 2005;19:681–684. doi: 10.1080/02699050400025182. [DOI] [PubMed] [Google Scholar]
- Wein T. OnabotulinumtoxinA for the treatment of poststroke distal lower limb spasticity: a randomized trial. Pharm. Manag. PM R. 2018;10:693–703. doi: 10.1016/j.pmrj.2017.12.006. [DOI] [PubMed] [Google Scholar]
- Wissel J. Towards flexible and tailored botulinum neurotoxin dosing regimens for focal dystonia and spasticity - insights from recent studies. Toxicon. 2018;147:100–106. doi: 10.1016/j.toxicon.2018.01.018. [DOI] [PubMed] [Google Scholar]
- Wissel J. Toward an epidemiology of poststroke spasticity. Neurology. 2013;80:S13–19. doi: 10.1212/WNL.0b013e3182762448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.