Abstract
Recently, the order Phaeomoniellales was established that includes fungi closely related to Phaeomoniella chlamydospora, a phytopathogen assumed to be the main causal agent of the two most destructive grapevine trunk diseases, Petri disease and esca. Other species of this order are reported as pathogens of other economically important crops, like olive, peach, apricot, cherry, plum, rambutan, lichee or langsat. However, they are rarely isolated and hence, little is known about their ecological traits and pathogenicity. During a 1-yr period of spore trapping in a German vineyard divided in minimally and intensively pruned grapevines, 23 fungal strains of the Phaeomoniellales were collected. Based on morphological and molecular (ITS, LSU and tub2) analyses the isolated strains were assigned to eight different species. Two species were identified as P. chlamydospora and Neophaeomoniella zymoides, respectively. The remaining six species displayed morphological and molecular differences to known species of the Phaeomoniellales and are newly described, namely Aequabiliella palatina, Minutiella simplex, Moristroma germanicum, Mo. palatinum, Neophaeomoniella constricta and N. ossiformis. A pathogenicity test conducted in the greenhouse revealed that except for P. chlamydospora, none of the species of the Phaeomoniellales isolated from spore traps is able to induce lesions in grapevine wood.
Keywords: esca, grapevine, new taxa, Phaeomoniella, phylogeny, spore trapping
INTRODUCTION
To separate Phaeomoniella chlamydospora (synonym Phaeoacremonium chlamydosporum; Crous et al. 1996) from the genus Phaeoacremonium based on significant morphological and molecular differences, Crous & Gams (2000) established the hyphomycete genus Phaeomoniella. Phaeomoniella chlamydospora is considered to be one of the main causal agents of Petri disease and esca, two grapevine trunk diseases (GTDs), which lead to high yield losses in grapevine industry all over the world (Bertsch et al. 2012, Fontaine et al. 2016, Gramaje et al. 2018). After the introduction of the genus Phaeomoniella, further species in this genus were described (Lee et al. 2006, Crous et al. 2008, Crous et al. 2011, Damm et al. 2010). Additionally, further unidentified Phaeomoniella species and related fungi appeared in many surveys focusing on fungal endophytes (Arnold et al. 2007, Botella & Diez 2010, Sánchez Márquez et al. 2011, Gueidan et al. 2014). Chen et al. (2015) established the order Phaeomoniellales to assemble all fungi with close affinity to the genus Phaeomoniella. Shortly thereafter, Crous et al. (2015) combined several Phaeomoniella species into new genera, and to date, the order Phaeomoniellales comprises 35 species in the following 12 genera: Aequabiliella, Celerioriella, Celothelium, Dolabra, Minutiella, Moristroma, Neophaeomoniella, Paraphaeoisaria, Paraphaeomoniella, Phaeomoniella, Pseudophaeomoniella and Xenocylindrosporium (Chen et al. 2015, Crous et al. 2015, Crous et al. 2016, Crous et al. 2018). Kirk (2015) described the family Phaeomoniellaceae with the type genus Phaeomoniella and an identical circumscription to the order. However, Celothelium, the type genus of the family Celotheliaceae (Aptroot et al. 2008) is also included in the circumscription of the family Phaeomoniellaceae. As the older name of the family has priority, Phaeomoniellaceae is an illegitimate name [ICN (Shenzhen) Art. 11.3 and Art. 52.1]. Moreover, in the phylogeny in Chen et al. (2015) there are no clades that would support the existence of two families.
Almost all known species of the Phaeomoniellales were isolated from plants. Several species, besides P. chlamydospora, are associated with wood diseases of economically important fruit crops. For example, Pseudophaeomoniella oleae and Ps. oleicola were isolated from discoloured xylem of wilting olive trees in Italy (Nigro et al. 2013, Crous et al. 2015). Aequabiliella effusa, Minutiella tardicola, M. pruni-avium, Celerioriella dura, C. prunicola, and Neophaeomoniella zymoides were isolated from necrotic wood of Prunus trees in South Africa and Germany (Damm et al. 2010, Bien & Damm 2020). Dolabra nepheliae is associated with stem canker disease of rambutan (Nephelium lappaceum; Booth & Ting 1964) and lychee (Litchi chinensis; Rossman et al. 2010), as well as corky bark disease of langsat (Lansium domesticum; Keith et al. 2013). Other species, such as Paraphaeomoniella capensis, Xenocylindrosporium kirstenboschense, N. niveniae and C. petrophiles were isolated from symptomatic leaves of various host plants (Crous et al. 2008,Crous et al. 2009,Crous et al. 2011,Crous et al. 2016). Other species were reported from wood or other substrates without association to a symptom or disease. For example, ascostromata of Moristroma quercinum and Mo. japonicum were discovered by Nordén et al. (2005) on canes and old stumps of oak trees. Moreover, N. zymoides and P. pinifoliorum as well as N. eucalypti were isolated from pine needles (Pinus densiflora) and stems of Eucalyptus globulus, respectively (Lee et al. 2006, Crous et al. 2015). Neophaeomoniella corymbiae and N. eucalyptigena were both isolated from eucalypt leaves, Corymbia citriodora and Eucalyptus pilularis, respectively (Crous et al. 2018). Paraphaeoisaria alabamensis was reported from acial galls of Cronartium quercuum (de Hoog & Morgan-Jones 1978). Furthermore, unknown species with affinity to the Phaeomoniellales were detected in a screening for lichen-associated fungi and multiple times in leaves of pine trees in Arizona (Peršoh & Rambold 2012, Bowman & Arnold 2018).
Despite efforts in revealing the ecological role and phylogeny of the Phaeomoniellales, the number of isolates and thus the information currently available is insufficient for concrete conclusions (Chen et al. 2015). Consequently, the isolation and examination of more fungi belonging to the Phaeomoniellales is indispensable.
During a 1-yr period of spore trapping in a German vineyard planted with minimally and intensively pruned grapevines cv. Riesling, several fungi were isolated that were presumed to belong to the Phaeomoniellales based on preliminary blastn searches with ITS sequences. Therefore, one objective of this study was to clarify the relationship of these fungi based on molecular data, to characterise the species morphologically and by means of DNA sequence data and monitor their occurrence depending on season and pruning method. In addition, since many species of the Phaeomoniellales can induce necrosis in woody tissue of their hosts and since the fungi were collected from spore traps located in vineyards, their potential threat to grapevine (Vitis vinifera) was investigated.
MATERIALS AND METHODS
Isolates
Fungal spores were trapped using glass slides coated with petroleum jelly (Balea Vaseline, DM, Karlsruhe, Germany) attached close (2 cm; Fig. 1) to branches of grapevine (Vitis vinifera) cv. Riesling. The vineyard was located close to the Julius Kühn-Institute in Siebeldingen, Germany (49°13′11.5″N 8°02′32.3″E). Half of the grapevines were trained in semi minimal pruned hedge (SMPH; Fig. 1A) and the other half in vertical shoot positioning (VSP; Fig. 1B). For each training system, four spore traps were placed randomly in the field.
Fig. 1.

Spore traps attached to grapevine plants cv. Riesling trained in SMPH (A) and VSP (B). Arrows indicate the spore traps (close-up views in the inserts).
Spore trapping was carried out from February 2016 to February 2017. Each week, glass slides were replaced by new ones. A sterile washing solution (NaCl 136.9 mM; KCl 2.7 mM; Na2HPO4 7.9 mM; KH2PO4 1.5 mM; Tween® 80, 0.01 %; in distilled water) was used to release the spores from the coated glass slides. Under sterile conditions, 25 mL washing solution was added to a 50 mL reaction tube containing one glass slide each. The tube was shaken vigorously by hand for about 30 s. Subsequently, the washing solution was passed through a filter system consisting of a 5.0 μm and a 0.45 μm filter (mixed cellulose ester membrane filter, ADVANTEC MFS Inc., Japan). Since fungi of the order Phaeomoniellales produce small conidia (< 5.0 μm), the 0.45 μm filter was used for further analysis. The filter was placed into a 2 mL reaction tube and washed with 500 μL washing solution. Subsequently, the washing solution was spread equally on two plates of malt yeast agar (MYA; 20 g/L malt extract, 1 g/L yeast extract, 20 g/L agar, 2.5 μg/mL chloramphenicol; Carl Roth, Karlsruhe, Germany) and incubated for 2 wk at 20 °C. Growing colonies were transferred to new MYA plates and identified by morphological and molecular analyses.
Cultures of newly described species are maintained in the culture collections of the Julius Kühn-Institute (JKI; www.julius-kuehn.de), the CBS culture collection of the Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands (WI; www.wi.knaw.nl) and the Senckenberg Museum of Natural History, Görlitz, Germany (GLMC; www.senckenberg.de). Type specimens of the species studied are deposited in the fungarium of the Senckenberg Museum of Natural History Görlitz, Germany (GLM). All descriptions are based on ex-holotype cultures, if not stated otherwise.
Local weather data (precipitation and temperature) were provided by the DLR Rhineland-Palatinate (www.dlr.rlp.de).
DNA extraction, PCR and sequencing
DNA extraction was performed according to the method of Tillett & Neilan (2000) from 2-wk-old cultures growing on MYA at 20 °C. Quality and quantity of the DNA were determined using a Spectrophotometer (Nanodrop 2000c, Thermo Fisher Scientific, Waltham, MA, USA). DNA was diluted to a final concentration of 100 μg/mL in distilled water.
For phylogenetic studies, three primer pairs were used to perform a polymerase chain reaction (PCR) amplifying the internal transcribed spacer regions 1 and 2 and intervening 5.8S rRNA (ITS: ITS5 and ITS4; White et al. 1990), the 28S rRNA (LSU: NL1 and NL4; O′Donnell 1993), and the beta-tubulin gene (tub2: BT2A and BT2B; Glass & Donaldson 1995). PCR reactions were set up as described in the KAPAHiFi™ hot start polymerase user manual (PEQLAB Biotechnologie GmbH, Erlangen, Germany). The reaction mixture consisted of 15.25 μL H2O, 5.0 μL 5× KAPAHiFi™ buffer, 0.75 μL KAPAHiFi™ dNTP mix (10 mM), 0.75 μL forward primer (10 μM), 0.75 μL reverse primer (10 μM), 0.5 μL KAPAHiFi™ HotStart DNA polymerase (1 U/μL) and 2 μL DNA template (5 ng/μL). For DNA amplification the following reaction steps were implemented on a SimpliAmp™ thermal cycler (Applied Biosystems, Darmstadt, Germany): 95 °C initial denaturation (5 min), 98 °C denaturation (20 s), 58 °C annealing (15 s), 72 °C extension (20 s), 72 °C final extension (1 min). The main amplification steps (denaturation - annealing - extension) were repeated 35 times. Afterwards electrophoresis was carried out for 45 min at 110 V using a 1.5 % agarose gel to check the quality and quantity of the amplicons. Amplicons were purified using the QIAquick PCR purification kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Sequencing reactions were set up using the ABI Prism Big Dye Terminator v. 3.1 cycle sequencing ready reaction kit (PE Biosystems, Foster City, CA, USA). Sequencing was performed on an ABI Prism 3130XL DNA sequencer. Sequence analyses and alignments were done with the program CLC Main Workbench v. 8.0 (Qiagen). ITS, tub2 and LSU sequences of the newly described fungi were deposited at NCBI GenBank (www.ncbi.nlm.nih.gov/genbank/).
Phylogenetic analyses
A concatenated ITS-LSU sequence alignment was constructed by separately aligning the sequences of the two loci and manually trimming the ends to achieve a uniform length of all sequences. Data of the tub2 gene were excluded from the phylogenetic analyses, since sequences of only a few reference strains were available.
MEGA 7 (Molecular Evolutionary Genetics Analysis v. 7.0; Kumar et al. 2015) was used for Maximum Likelihood (ML) and Maximum Parsimony (MP) analyses. A test was performed with MEGA 7 to find the most robust model for ML. Then a ML tree using tree bisection and reconnection (TBR) as branch-swapping algorithm was generated. Tree length, consistency index, retention index and composite index were calculated for the resulting tree. Finally, a ML tree containing bootstrap values (1 000 replications) calculated by both ML and MP analyses was chosen to visualise the phylogenetic relationship of the fungi investigated. Alignment and phylogenetic tree were lodged in TreeBASE (www.treebase.org, Study ID: 25809).
Morphological characterisation
To improve sporulation and pycnidial formation, fungal strains were cultivated on autoclaved grapevine wood pieces and autoclaved pine needles placed on SNA (synthetic nutrient-poor agar) medium (Nirenberg 1976) and incubated at 25 °C for up to 3 mo. Microscopic preparations were done in lactic acid with a Nikon Eclipse Ni-U light microscope with differential interference contrast (LM), or with a Nikon SMZ18 stereomicroscope (SM). Pictures were captured with Nikon Digital Sight DS-Fi2 cameras installed on the two microscopes mentioned above. In order to determine the size of fungal structures, 20 structures each were measured. Interpretation of colony characters was done after 2 wk of growth on potato dextrose agar (PDA; 40 g/L potato extract glucose agar, 5 μg/mL chloramphenicol; Carl Roth, Karlsruhe, Germany), oat meal agar (OA; 30 g/L oatmeal infusion, 20 g/L agar, 2.5 μg/mL chloramphenicol; Carl Roth, Karlsruhe, Germany) and MYA at 25 °C in the dark. The colour chart of Rayner (1970) was used to determine the colour of fungal colonies. To determine thermal tolerance and growth optima, the radial growth on MYA (three replicates) was measured at different temperatures, from 5 °C to 35 °C at 5 °C intervals, after 2 wk in the dark.
Pathogenicity
To investigate the potential threat of the trapped fungi to grapevine health, pathogenicity studies were performed on potted grapevines cv. Pinot noir and Müller-Thurgau in a greenhouse. Cuttings were hot-water treated at 50 °C for 30 min and then stored for 10 d at 10 °C for recovery. They were cut into smaller pieces with three buds each and planted into plastic boxes containing sterile soil. Incubation conditions in the greenhouse were 16 °C night time temperature, 24 °C day time temperature and 30 % relative humidity for 5 mo.
Young grapevine plants with shoot development were surface wounded with a sterilised scalpel by a diagonal cut of about 1 cm in length between the second and the third bud. After inoculating the wounds with a 20 μL spore suspension (~1 000 spores per 20 μL sterilised rainwater), they were sealed with plastic film to avoid evaporation and cross infection. One isolate of each species was tested, including Phaeomoniella chlamydospora as positive control. As negative control, plants were inoculated with sterile rainwater. For each species five plants were inoculated, and the experiment was repeated three times. Six months after inoculation, plants were cut longitudinally and lesions in the wood were measured up- and downwards from the inoculation point. Plants, which had dried out at the inoculation point, were excluded from the experiment. In a laminar flow cabinet, material from the lesion was removed with a sterile scalpel and placed on two MYA plates to verify the presence of the particular fungus. Fungi growing out from the lesion material were identified microscopically based on morphological characteristics.
For statistical evaluation of lesion length from the pathogenicity test, an analysis of variance (ANOVA) was conducted using the program RStudio v. 1.1.383 (RStudio Team 2016). Additionally, the re-isolation rate of the tested fungi, i.e. the percentage of the plants of which the fungus could be re-isolated, was determined.
RESULTS
Phylogenetic analyses
In total 23 fungal strains of the Phaeomoniellales were isolated in this study (Table 1). The phylogenetic analyses of the concatenated ITS-LSU sequence alignment comprised 45 strains, including reference strains and the outgroup Capronia epimyces (Table 2) and 919 characters. The ML tree with both ML and MP bootstrap values (1 000 replicates) is shown in Fig. 2 and consists of eleven main clades representing the ten genera of the Phaeomoniellales and Phaeomoniella pinifoliorum. Celothelium cinchonarum was excluded from the analysis due to missing sequence data. Strains JKI-Mz56, JKI-S09, JKI-Mz21, JKI-Mz20, JKI-Mz40, JKI-Mz34, JKI-Mz38, and JKI-Mz41 grouped with N. zymoides (ML/MP bootstrap support values: 98/97) in the Neophaeomoniella clade (100/100). Strains JKI-May02, JKI-May03 and JKI-May30 formed a sub-clade (85/97) within this clade, sister to the single strain sub-clade JKI-Mz35, and the sub-clade formed by N. corymbiae, N. eucalyptigena, N. eucalypti, N. niveniae and N. zymoides. The isolates JKI-Feb08, JKI-May05 and JKI-Ap04 grouped with P. chlamydospora in the Phaeomoniella clade (99/99). Strains JKI-Mz48, JKI-May29 and JKI-Ap36 grouped with Aequabiliella effusa in the Aequabiliella clade (100/100). However, the latter represented a single strain sub-clade. The same situation was found for strains JKI-Jn27 and JKI-Jn48 that formed a clade with Minutiella pruni-avium and M. tardicola, which formed single strain sub-clades within the Minutiella clade (99/100). Together with Moristroma japonicum and Mo. quercinum, strains JKI-Au02, JKI-Feb17 and JKI-Feb06 formed the Moristroma clade, in which isolate JKI-Feb06 grouped with Mo. quercinum and Mo. japonicum (81/86), while isolates JKI-Au02 and JKI-Feb17 formed a separate sub-clade within Moristroma (–/99). The Moristroma clade (100/100) was on a long branch, basal to all other genera in the Phaeomoniellales, except for Dolabra nepheliae strain CBS 122120.
Table 1.
Strains of the Phaeomoniellales studied with collection details and GenBank accession numbers.
| Species |
Accession no.a |
Collection date | Training systemb |
GenBank no.c |
||||
|---|---|---|---|---|---|---|---|---|
| JKI | CBS | GLMC | ITS | LSU | tub2 | |||
| Aequabiliella palatina | JKI-Mz48 | CBS 145007 | GLMC 1904 | 10 Mar. 2016 | SMPH | MH999505 | MH999528 | MK070468 |
| JKI-Ap36* | CBS 145018 | GLMC 1905 | 21 Apr. 2016 | SMPH | MH999506 | MH999529 | MK070469 | |
| JKI-May29 | – | GLMC 1906 | 5 May 2016 | SMPH | MH999507 | MH999530 | MK070470 | |
| Minutiella simplex | JKI-Jn27* | CBS 145008 | GLMC 1907 | 9 Jun. 2016 | SMPH | MH999508 | MH999531 | MK070471 |
| JKI-Jn38 | CBS 145009 | GLMC 1908 | 9 Jun. 2016 | SMPH | MH999509 | MH999532 | MK070472 | |
| Moristroma germanicum | JKI-Feb06* | CBS 145012 | GLMC 1911 | 3 Feb. 2017 | SMPH | MH999512 | MH999535 | MK070475 |
| Mo. palatinum | JKI-Feb17* | CBS 145010 | GLMC 1909 | 23 Feb. 2017 | SMPH | MH999510 | MH999533 | MK070473 |
| JKI-Au2 | CBS 145011 | GLMC 1910 | 9 Aug. 2016 | VSP | MH999511 | MH999534 | MK070474 | |
| Neophaeomoniella constricta | JKI-Mz35* | CBS 145015 | GLMC 1915 | 3 Mar. 2016 | SMPH | MH999516 | MH999539 | MK070479 |
| N. ossiformis | JKI-May02 | CBS 145014 | GLMC 1912 | 6 May 2016 | SMPH | MH999513 | MH999536 | MK070476 |
| JKI-May03* | CBS 145013 | GLMC 1913 | 6 May 2016 | VSP | MH999514 | MH999537 | MK070477 | |
| JKI-May30 | – | GLMC 1914 | 6 May 2016 | SMPH | MH999515 | MH999538 | MK070478 | |
| N. zymoides | JKI-Mz20 | – | GLMC 1916 | 3 Mar. 2016 | SMPH | MH999517 | MH999540 | MK070480 |
| JKI-Mz21 | CBS 145156 | GLMC 1917 | 3 Mar. 2016 | SMPH | MH999518 | MH999541 | MK070481 | |
| JKI-S09 | – | GLMC 1918 | 11 Feb. 2016 | VSP | MH999519 | MH999542 | MK070482 | |
| JKI-Mz56 | – | GLMC 1919 | 17 Mar. 2016 | SMPH | MH999520 | MH999543 | MK070483 | |
| JKI-Mz38 | – | GLMC 1920 | 3 Mar. 2016 | VSP | MH999521 | MH999544 | MK070484 | |
| JKI-Mz40 | – | GLMC 1921 | 3 Mar. 2016 | VSP | MH999522 | MH999545 | MK070485 | |
| JKI-Mz41 | CBS 145155 | GLMC 1922 | 3 Mar. 2016 | VSP | MH999523 | MH999546 | MK070486 | |
| JKI-Mz34 | – | GLMC 1923 | 3 Mar. 2016 | VSP | MH999524 | MH999547 | MK070487 | |
| Phaeomoniella chlamydospora | JKI-Ap04 | CBS 145016 | GLMC 1924 | 7 Apr. 2016 | SMPH | MH999525 | MH999548 | MK070488 |
| JKI-Feb08 | CBS 145017 | GLMC 1925 | 9 Feb. 2017 | SMPH | MH999526 | MH999549 | MK070489 | |
| JKI-May05 | – | GLMC 1926 | 6 May 2016 | VSP | MH999527 | MH999550 | MK070490 | |
aJKI: Culture collection of the Julius Kühn-Institute, Siebeldingen, Germany; CBS: Culture collection of the Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; GLMC: Culture collection of the Senckenberg Museum of Natural History Görlitz, Görlitz, Germany.
bTraining system of the grapevine at which the trap was attached to. SMPH: semi minimal pruned hedge; VSP: vertical shoot positioning.
cITS: internal transcribed spacers and intervening 5.8S DNA; LSU: 28S rDNA; tub2: partial beta-tubulin gene.
*Ex-type cultures.
Table 2.
Reference strains of the Phaeomoniellales with collection details and GenBank accession numbers.
| Species | Accession no.a | Host | Country |
GenBank no.b |
||
|---|---|---|---|---|---|---|
| ITS | LSU | tub2 | ||||
| Aequabiliella effusa | CBS 120883* | Prunus salicina | South Africa | GQ154598 | GQ154618 | KR260451 |
| Celerioriella dura | CBS 120882* | Pr. salicina | South Africa | GQ154597 | GQ154617 | – |
| C. petrophiles | CBS 142115* | Petrophile teretifolia | Australia | KY173394 | KY173487 | – |
| C. prunicola | CBS 120876* | Pr. salicina | South Africa | GQ154590 | GQ154615 | – |
| Dolabra nepheliae | CBS 122120* | Nephelium lappaceum | Malaysia | JQ004281 | GU332516 | – |
| Minutiella pruni-avium | CBS 145513* | Pr. avium | Germany | MN232957 | MN232925 | MN232985 |
| M. tardicola | CBS 121757* | Pr. armeniaca | South Africa | GQ154599 | GQ154619 | KR260454 |
| Moristroma japonicum | BN1674* | Quercus mongolica | Japan | AY254052 | AY254052 | – |
| Mo. quercinum | BN1678* | Q. robur | Sweden | AY254051 | AY254051 | – |
| Neophaeomoniella corymbiae | CBS 145092* | Corymbia citriodora | Australia | MK047457.1 | MK047507.1 | – |
| N. eucalypti | CBS 139919* | Eucalyptus globulus | USA | NR_138001 | KR476782 | – |
| N. eucalyptigena | CBS 145093* | E. pilularis | Australia | MK047458.1 | MK047508.1 | MK047584.1 |
| N. niveniae | CBS 131316* | Nivenia stokoei | South Africa | JQ044435 | JQ044454 | – |
| N. zymoides | CBS 114904* | Pinus densiflora | Korea | GQ154600 | GQ154620 | KR260455 |
| Paraphaeoisaria alabamensis | CBS 101.77B* | Cronartium quercuum | USA | MH861028 | MH872801 | – |
| Paraphaeomoniella capensis | CBS 123535* | Encephalartos altensteinii | South Africa | NR_137711 | FJ372408 | – |
| Phaeomoniella chlamydospora | CBS 229.95* | Vitis vinifera | Italy | FJ530942 | AF353609 | – |
| P. pinifoliorum | CBS 114903* | Pi. densiflora | Korea | DQ270240 | DQ270250 | – |
| Pseudophaeomoniella oleae | CBS 139191* | Olea europaea | Italy | NR_137966 | KP635971 | – |
| Ps. oleicola | CBS 139192* | O. europaea | Italy | KP411807 | KP635970 | – |
| Xenocylindrosporium kirstenboschense | CBS 125545* | En. friderici-guilielmi | South Africa | NR_132841 | GU229891 | – |
| Capronia epimycesc | CBS 601.96* | Picea sp. | Canada | AY156968 | AF050245 | – |
a BN: Botanical Institute, Göteborg University, Göteborg, Sweden; CBS: Culture collection of the Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands.
bITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: 28S rDNA; tub2: partial beta-tubulin gene.
cOutgroup.
*Types and ex-type cultures.
Fig. 2.
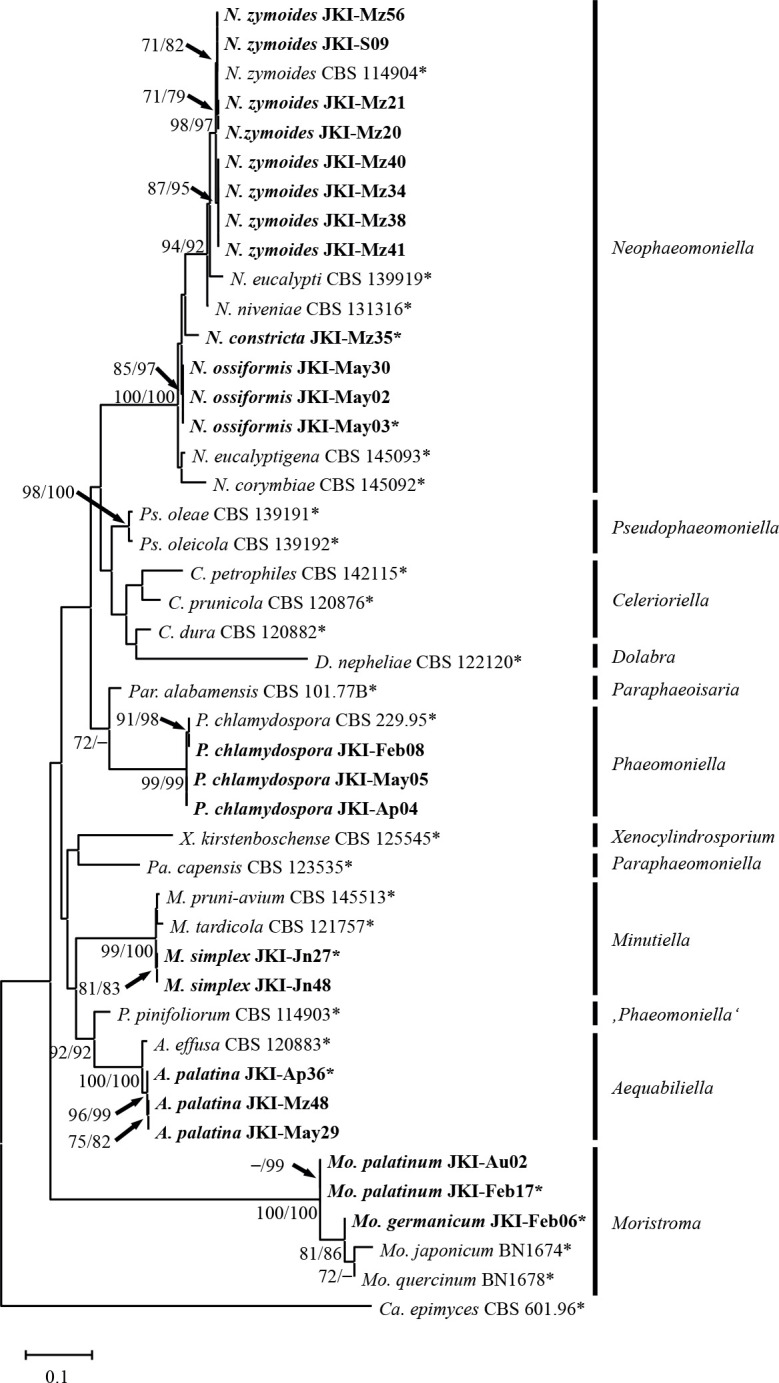
Maximum likelihood tree based on the concatenated ITS-LSU sequence alignment of the Phaeomoniellales. Bootstrap support values above 70 % of ML/MP analyses are shown at the nodes. Capronia epimyces strain CBS 601.96 was used as outgroup. Isolates analysed in this study are emphasised in bold. Numbers of types and ex-type cultures are marked with an asterisk.
Diversity and occurrence
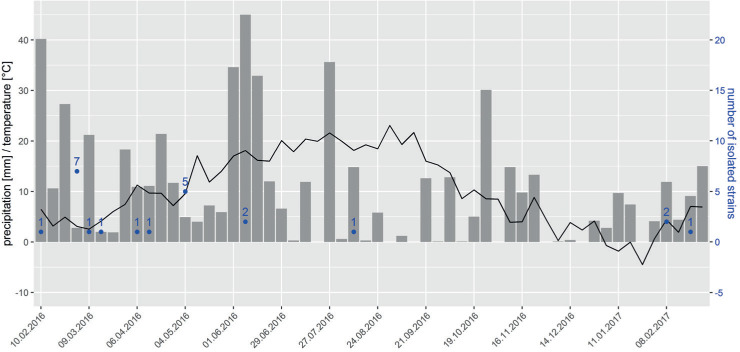
The strains were assigned to eight species in five genera: Aequabiliella, Minutiella, Moristroma, Neophaeomoniella and Phaeomoniella. Fifteen strains belonging to eight species originated from spore traps attached to SMPH trained grapevines and eight strains belonging to four species were isolated from traps attached to VSP trained grapevines. Spores were trapped between February and June, with one exception in August (JKI-Au2; Fig. 3).
Fig. 3.
Weekly precipitation [mm] (grey columns), average temperature [°C] (black line) and number of fungal strains collected (emphasised in blue) during the time of spore trapping.
Taxonomy
Six of the eight Phaeomoniellales species found in this study exhibited significant morphological and molecular differences to known species and are therefore described as new species (Figs 4–10).
Fig. 4.
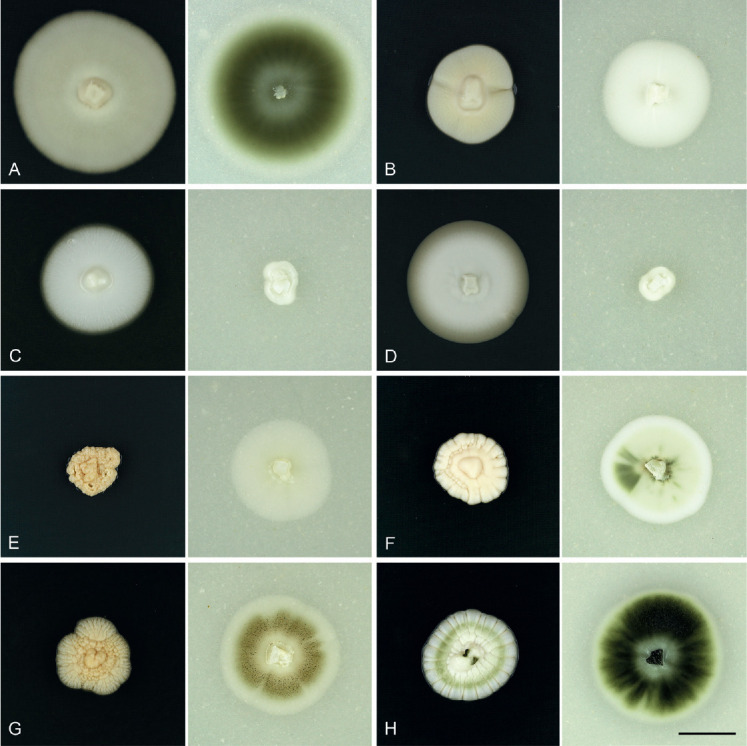
Cultures of Phaeomoniellales grown on PDA (left) and OA (right) for 4 wk at 20 °C in the dark. A. Aequabiliella palatina strain JKI-Ap36*. B. Minutiella simplex strain JKI-Jn27*. C. Moristroma germanicum strain JKI-Feb06*. D. Mo. palatinum strain JKI-Feb17*. E. Neophaeomoniella constricta strain JKI-Mz35*. F. N. ossiformis strain JKI-May03*. G. N. zymoides strain JKI-Mz41. H. Phaeomoniella chlamydospora strain JKI-Ap04. Scale bar: H = 15 mm; H applies to A–H. * Ex-type cultures.
Fig. 10.
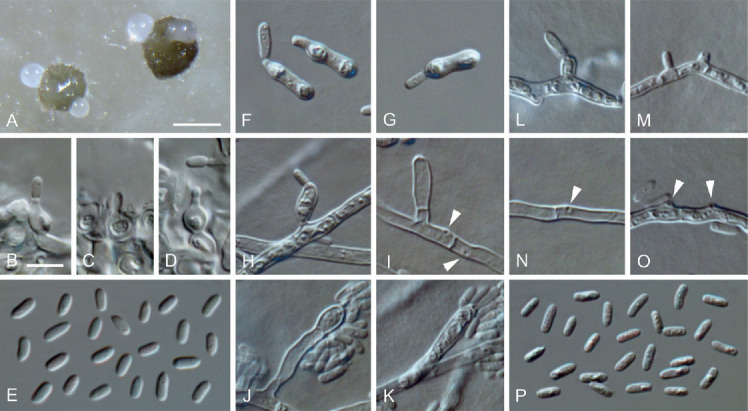
Neophaeomoniella ossiformis (ex-type culture JKI-May03). A. Pycnidia formed on OA. B–D. Conidiogenous cells from the inner cell wall of pycnidia. E. Conidia formed in pycnidia. F–G. Microcyclic conidiation. H–O. Conidiogenous cells on hyphal cells (arrows indicate conidiogenous openings). P. Conidia formed on hyphal cells. A: SM; B–P: LM. Scale bars: A = 100 μm; B = 5 μm; B applies to B–P.
Aequabiliella palatina C. Kraus, Damm, S. Bien, Vögele & M. Fisch., sp. nov. MycoBank MB828284. Figs 4A, 5.
Fig. 5.
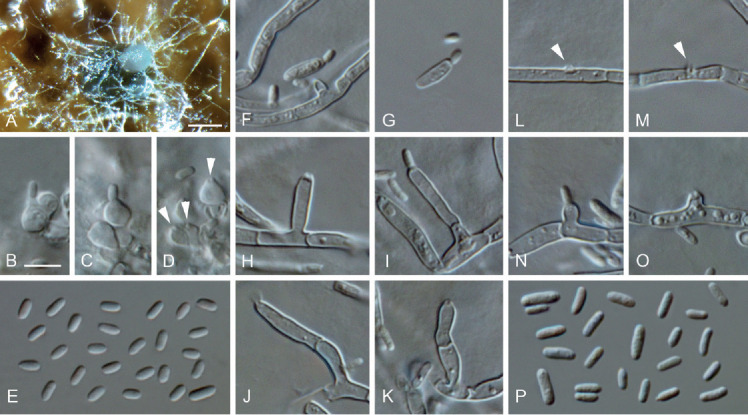
Aequabiliella palatina (ex-type culture JKI-Ap36). A. Conidia oozing from a pycnidium on grapevine wood. B–D. Conidiogenous cells lining the inner cell wall of pycnidia (arrows indicate conidiogenous openings). E. Conidia formed in pycnidia. F–G. Microcyclic conidiation. H–O. Conidiogenous cells on hyphal cells (arrows indicate conidiogenous openings on hyphal cells). P. Conidia generated on hyphal cells. A: SM; B–P: LM. Scale bars: A = 100 μm; B = 5 μm; B applies to B–P.
Etymology: Named after the federal state of Germany, Rhineland-Palatinate, in which the species was isolated (palatinus, adjective of Palatinatus = Pfalz).
Typus: Germany, Rhineland-Palatinate, Siebeldingen, °13′11.5″N 8°02′34.6″E, isolated from a spore trap attached to a grapevine shoot, 21 Apr. 2016, C. Kraus (GLM-F117490 holotype, culture ex-type JKI-Ap36 = CBS 145018 = GLMC 1905).
Vegetative hyphae hyaline, smooth-walled, 1.5–2.5 μm wide, septate, chlamydospores not observed. Sporulation abundant; conidia formed on hyphae, in pycnidia and by microcyclic conidiation. Conidiophores on hyphae hyaline, smooth-walled, mainly reduced to conidiogenous cells, rarely 2- or 3-celled, cylindrical to lanceolate, 13–41.5 × 2–2.5 μm. Conidiogenous cells enteroblastic, hyaline, smooth-walled, most frequently reduced to openings formed directly on hyphal cells, discrete phialides and adelophialides occasionally observed; collarettes inconspicuous; openings on hyphal cells 0.5–1.5 μm wide, periclinal thickening visible; discrete phialides cylindrical to cigar-shaped, 4.5–14 × 1.5–3 μm, adelophialides cylindrical, 1–8 × 1–3 μm. Conidia accumulated in heads around conidiogenous openings, hyaline, smooth-walled, aseptate, cylindrical to oblong-elliptical, sometimes slightly curved, smooth-walled, (3–)4(–6) × (1–)1.5(–2) μm, L/W ratio = 2.7.
Microcyclic conidiation observed on one side of swollen mother cells developed from primary conidia; conidiogenous cells hyaline, smooth-walled, aseptate, elongated obovate to oblong-elliptical, (5–)6(–6.5) × (1–)2(–2.5) μm, L/W ratio = 2.7.
Conidiomata pycnidial produced superficially on pine needles, grapevine wood and immersed in SNA medium after four wk, solitary or in groups, globose to subglobose, 110–330 μm diam, unilocular, opening by irregular rupture, pycnidial wall composed of textura angularis, 6–13 μm thick, 2–4 cell layers. Conidiophores reduced to conidiogenous cells lining the inner wall of pycnidia. Conidiogenous cells enteroblastic, hyaline, smooth-walled, discrete phialides, obpyriform 3.5–6.5 × 2.5–4 μm, collarettes inconspicuous; opening 0.5–2 μm. Conidia hyaline, smooth-walled, aseptate, cylindrical, elliptical to oblong-elliptical, (2.5–)3(–3.5) × (1–)1.5(–2) μm, L/W ratio = 1.9.
Culture characteristics: Colonies on PDA flat to raised, with entire to crenated margin, moist, buff to primrose, sparse, whitish, funiculose aerial mycelium in centre; reverse same colours. Colonies on OA flat, with entire to crenated margin, moist, olivaceous buff to olivaceous grey, olivaceous buff to olivaceous black in the centre, buff at the margin, aerial mycelium sparse; reverse same colours. Colonies on MYA flat, with crenated margin, moist, honey in centre, with a grey olivaceous to olivaceous black ring and a buff margin, aerial mycelium sparse, reverse same colours; 27–27.5 mm diam after 14 d on MYA (25 °C, in the dark), min 10 °C, max 30 °C, opt 25 °C.
Additional materials examined: Germany, Rhineland-Palatinate, Siebeldingen, °13′11.5″N 8°02′34.6″E, isolated from a spore trap attached to a grapevine shoot, 10 Mar. 2016, C. Kraus, JKI-Mz48 = CBS 145007 = GLMC 1904; Siebeldingen, °13′11.5″N 8°02′34.6″E, isolated from a spore trap attached to a grapevine shoot, 6 May 2016, C. Kraus, JKI-May29 = GLMC 1906.
Notes: Aequabiliella palatina is closely related to A. effusa, however, tub2, ITS and LSU sequences of the ex-type strain are only 94 % (21 nucleotides different), 98 % (9 nucleotides different) and 99 % (1 nucleotide different), respectively, identical to that of A. effusa (Damm et al. 2010, Úrbez-Torres et al. 2015). A blastn search of the ITS sequence resulted in 99 % (2–3 nucleotides different) accordance with unidentified fungal isolates from wood samples of Norway spruce and Scots pine taken in Finland (MG190556, Müller et al. 2018) and Western white pine taken in Montana, USA (JF705946, Larkin et al. 2012). Colonies of A. effusa are herbage-green, dark herbage-green to olivaceous on PDA, while those of A. palatina are buff to primrose. Microcyclic conidiation was only observed in A. palatina. Additionally, the temperature depending growth range differs between the two species: A. effusa can grow at temperatures between 5 °C and 35 °C, with an optimum at 30 °C, while the growth range of A. palatina is narrower, ranging from 10 °C to 30 °C, with an optimum at 25 °C.
Minutiella simplex C. Kraus, Damm, S. Bien, Vögele & M. Fisch., sp. nov. MycoBank MB828285. Figs 4B, 6.
Fig. 6.
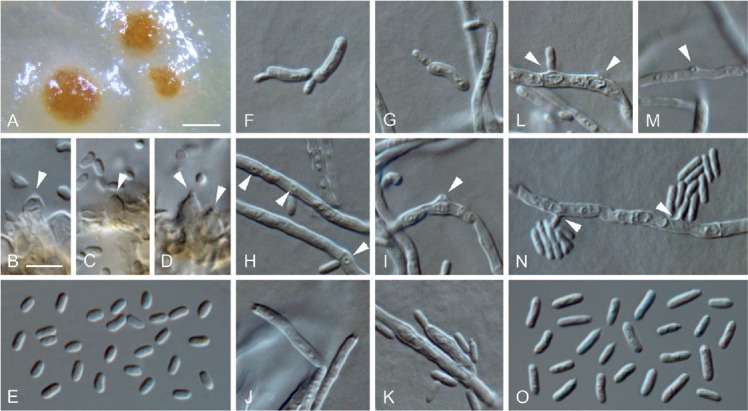
Minutiella simplex (ex-type culture JKI-Jn27). A. Pycnidia immersed in SNA. B–D. Conidiogenous cells lining the inner cell wall of pycnidia (arrows indicate conidiogenous openings). E. Conidia formed in pycnidia. F–G. Microcyclic conidiation. H–N. Conidiogenous cells on hyphal cells (arrows indicate conidiogenous openings). O. Conidia generated on hyphal cells. A: SM; B–O: LM. Scale bars: A = 200 μm; B = 5 μm; B applies to B–O.
Etymology: Named after the simple conidiogenous cells on hyphae.
Typus: Germany, Rhineland-Palatinate, Siebeldingen, °13′11.5″N 8°02′34.6″E, isolated from a spore trap attached to a grapevine shoot, 9 Jun. 2016, C. Kraus (GLM-F117492 holotype, culture ex-type JKI-Jn27 = CBS 145008 = GLMC 1907).
Vegetative hyphae hyaline, smooth-walled, 2–3.5 μm wide, septate, chlamydospores not observed. Sporulation abundant, conidia formed on hyphae, in pycnidia and by microcyclic conidiation. Conidiophores on hyphae hyaline, smooth-walled, reduced to conidiogenous cells. Conidiogenous cells enteroblastic, almost exclusively simple openings on hyphal cells, rarely discrete phialides, 10–18 × 2–3 μm; collarettes inconspicuous; openings on hyphal cells 0.5–1 μm wide. Conidia accumulated in heads around conidiogenous openings, hyaline, smooth-walled, aseptate, cylindrical, sometimes slightly curved, smooth-walled, (3–)4(–5) × (1–)1.5(–2) μm, L/W ratio = 2.4.
Microcyclic conidiation observed on one side of swollen mother cells developed from primary conidia; conidiogenous cells hyaline, smooth-walled, aseptate, cylindrical, obovate to oblong-elliptical, often curved, (5–)6(–7) × (1.5–)2(–2.5) μm, L/W ratio = 3.1.
Conidiomata pycnidial produced superficially on pine needles, grapevine wood and immersed in SNA medium after four to eight wk, mainly solitary, globose, subglobose to ellipsoidal, 120–300 μm diam, unilocular, opening by irregular rupture, pycnidial wall composed of textura angularis, 8–18 μm thick, 3–6 cell layers. Conidiophores reduced to conidiogenous cells. Conidiogenous cells enteroblastic, hyaline, discrete phialides; collarettes visible; discrete phialides obpyriform to ampulliform, 4–7 × 2–5 μm, openings 0.5–1.5 μm. Conidia hyaline, smooth-walled, aseptate, oblong-elliptical, sometimes slightly curved, (2.5–)3(–4) × (1–)1.5(–2) μm, L/W ratio = 2.2.
Culture characteristics: Colonies on PDA raised, with undulate to lobate margin, moist, buff to straw, lacking aerial mycelium, reverse same colours; on OA flat, with entire to crenated margin, moist, whitish to buff, funiculose to felty aerial mycelium in centre; reverse same colours; on MYA flat to raised, with crenated margin, moist, buff, hyaline, sparse aerial mycelium; reverse buff to luteous; 3–6.5 mm diam after 14 d on MYA (25 °C, in the dark), min 10 °C, max 25 °C, opt 20 °C.
Additional material examined: Germany, Rhineland-Palatinate, Siebeldingen, °13′11.5″N 8°02′34.6″E, isolated from a spore trap attached to a grapevine shoot, 9 Jun. 2016, C. Kraus, culture JKI-Jn38 = CBS 145009 = GLMC 1908.
Notes: Minutiella simplex is morphologically similar to M. tardicola and M. pruni-avium, however, differs in all three loci sequenced. Large subunit sequences of M. simplex differ in four and one nucleotides, ITS sequences in six and nine nucleotides and tub2 sequences in one and 33 nucleotides from those of M. tardicola and M. pruni-avium, respectively (Damm et al. 2010; Úrbez-Torres et al. 2015; Bien & Damm 2020). Furthermore, colonies of M. simplex have a higher growth rate than M. tardicola and form larger pycnidia. Additionally, M. simplex can grow between 10 °C and 25 °C with an optimum at 20 °C, while M. tardicola grows between 15 °C and 30 °C, preferring 25 °C (Damm et al. 2010).
Moristroma germanicum C. Kraus, Damm, S. Bien, Vögele & M. Fisch., sp. nov. MycoBank MB828287. Figs 4C, 7.
Fig. 7.
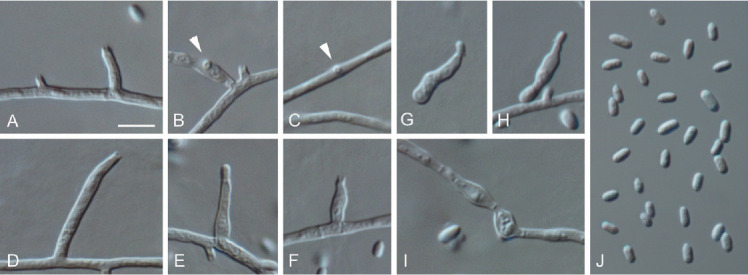
Moristroma germanicum (ex-type culture JKI-Feb06). A–F. Conidiogenous cells on hyphal cells (arrows indicate conidiogenous openings). G–H. Microcyclic conidiation. I. Inflated hyphal cells. J. Conidia formed on hyphal cells. A–J: LM. Scale bars: A = 5 μm; A applies to A–J.
Etymology: Named after the country the species was found in, Germany.
Typus: Germany, Rhineland-Palatinate, Siebeldingen, °13′11.5″N 8°02′34.6″E, isolated from a spore trap attached to a grapevine shoot, 3 Feb. 2017, C. Kraus (GLM-F117494 holotype, culture ex-type JKI-Feb06 = CBS 145012 = GLMC 1911).
Vegetative hyphae hyaline, smooth-walled, septate, 1–2.5 μm wide, partly inflated up to 5.5 μm, chlamydospores not observed. Sporulation abundant, conidia formed on hyphae and by microcyclic conidiation. Conidiophores on hyphae hyaline, smooth-walled, mainly reduced to conidiogenous cells, rarely 2–7-celled, cylindrical, 17–71 × 1–2 μm. Conidiogenous cells enteroblastic, hyaline, smooth-walled, often reduced to openings directly formed on hyphal cells, adelophialides and discrete phialides frequently observed; adelophialides cylindrical to lanceolate, sometimes conical, 2–21 × 1–2 μm; discrete phialides cylindrical to naviculate, 2.5–15.5 × 1.5–4 μm; collarettes conspicuous, cylindrical, 0.5–1.5 μm long, opening 0.5–1 μm, periclinal thickening observed. Conidia accumulated in heads around conidiogenous openings, hyaline, smooth-walled, aseptate, cylindrical, oblong-elliptical to obovate, (2.5–) 3(–5) × (1–)1.5(–2) μm, L/W ratio = 2.1.
Microcyclic conidiation observed on one side of swollen mother cells developed from primary conidia; conidiogenous cells hyaline, smooth-walled, aseptate, sometimes 2-celled, irregularly shaped or obovate (3–)4(–5) × (2–)2.5(–3) μm, L/W ratio = 1.6.
Conidiomata not observed.
Culture characteristics: Colonies on PDA flat to raised, with entire margin, moist, whitish to buff, aerial mycelium sparse; reverse same colours; on OA flat, with undulate margin, moist, whitish to buff, aerial mycelium sparse; reverse same colours; on MYA flat, with entire margin, moist, buff to primrose, aerial mycelium in the centre dense, whitish, funiculose; reverse same colours; 12.5–14 mm diam after 14 d on MYA (25 °C, in the dark), min 15 °C, max 30 °C, opt 25 °C.
Notes: Frequently occurring discrete phialides and the presence of inflated hyphal cells distinguishes Mo. germanicum from Mo. palatinum. The tub2 sequence of Mo. germanicum shows 96 % identity with Mo. palatinum (15 nucleotides different). The alignment displays 97 % identity (14 nucleotides different) of the ITS sequence of Mo. germanicum with that of Mo. quercinum, 93 % (31 nucleotides different) with that of Mo. palatinum and 93 % (33 nucleotides different) with that of Mo. japonicum. The LSU sequence of Mo. germanicum is two nucleotides different from Mo. quercinum (99 % identical), 9 nucleotides different (98 % identical) from Mo. japonicum and 11 nucleotides different (98 % identical) from Mo. palatinum. Due to missing sequence data and morphological data of the asexual morph, Mo. multisporum and Mo. polysporum, that were described from dead wood of Terminalia arjuna in India and from decorticated wood of Eucalyptus viminalis in Argentina, respectively, cannot be compared with the two Moristroma species described in this study (Sivanesan et al. 1988, Romero & Samuels 1991, Boonmee et al. 2011, Zhang et al. 2012).
Moristroma palatinum C. Kraus, Damm, S. Bien, Vögele & M. Fisch., sp. nov. MycoBank MB828286. Figs 4D, 8.
Fig. 8.
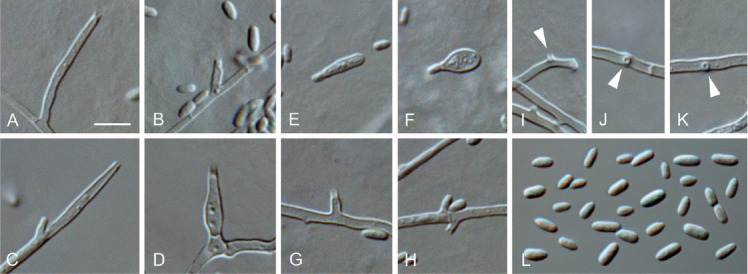
Moristroma palatinum (ex-type culture JKI-Feb17). A–D, G–K. Conidiogenous cells on hyphal cells (arrows indicate conidiogenous openings). E–F. Microcyclic conidiation. L. Conidia formed on hyphal cells. A–L: LM. Scale bars: A = 5 μm; A applies to A–L.
Etymology: Named after the federal state of Germany, Rhineland-Palatinate, in which the species was isolated.
Typus: Germany, Rhineland-Palatinate, Siebeldingen, °13′11.5″N 8°02′34.6″E, isolated from a spore trap attached to a grapevine shoot, 23 Feb. 2017, C. Kraus (GLM-F117495 holotype, culture ex-type JKI-Feb17 = CBS 145010 = GLMC 1909).
Vegetative hyphae hyaline, smooth-walled, 1–2.5 μm wide, septate, chlamydospores not observed. Sporulation abundant, conidia formed on hyphae, in pycnidia and by microcyclic conidiation. Conidiophores on hyphae hyaline, smooth-walled, reduced to conidiogenous cells. Conidiogenous cells enteroblastic, hyaline, smooth-walled, mostly reduced to openings directly formed on hyphal cells and adelophialides, discrete phialides rare; collarettes conspicuous, cylindrical, 0.5–1.5 μm long, opening 0.5–1.5 μm diam; adelophialides cylindrical to lanceolate, sometimes conical, discrete phialides cylindrical to lanceolate, 1.5–17 × 1–2.5 μm. Conidia accumulated in heads around conidiogenous opening, hyaline, smooth-walled, aseptate, cylindrical, elliptical, oblong-elliptical to obovate, (2–) 3(–3.5) × (1–)1.5(–2) μm, L/W ratio = 1.9.
Microcyclic conidiation observed on one side of swollen mother cells developed from primary conidia; conidiogenous cells hyaline, smooth-walled, aseptate, ellipsoidal to ovoidal, sometimes cylindrical, (5–)5.5(–6.5) × (2.5–)3(–4) μm, L/W ratio = 1.9.
Conidiomata pycnidial (not shown), rarely observed on pine needles after four wk, mainly solitary, superficial, ovoid, 40–90 μm diam, unilocular, with a central ostiole, pycnidial wall composed of textura angularis, 7–15 μm thick, 2–4 cell layers. Conidiophores reduced to conidiogenous cells lining the inner wall of the pycnidia. Conidiogenous cells enteroblastic, hyaline, smooth-walled, discrete phialides; collarettes conspicuous, cylindrical, 0.5–1 μm long; discrete phialides ampulliform, 3.5–6.5 × 1.5–3.5 μm, opening 0.5–1.5 μm. Conidia hyaline, smooth-walled, aseptate, oblong-elliptical to obovate, (2–)2.5(–3) × (1–) 1.5(–2.0) μm, L/W ratio = 1.7.
Culture characteristics: Colonies on PDA flat, with entire to undulate margin, moist to slimy, buff, aerial mycelium sparse; reverse same colours; on OA flat, with entire margin, moist, whitish to buff, dense, funiculose aerial mycelium in centre; reverse same colours; on MYA flat to raised, with undulate margin, moist, buff to primrose, sparse aerial mycelium; reverse same colours; 15–18 mm diam after 14 d on MYA (25 °C, in the dark), min 10 °C, max 30 °C, opt 25 °C.
Additional material examined: Germany, Rhineland-Palatinate, Siebeldingen, °13′11.5″N 8°02′34.6″E, isolated from a spore trap attached to a grapevine shoot, 9 Aug. 2016, C. Kraus, JKI-Au02 = CBS 145011 = GLMC 1910.
Notes: The ITS and LSU sequences of Moristroma palatinum are 93 % and 98 % identical with Mo. germanicum (31 and 11 nucleotides different), 91 % and 98 % with Mo. quercinum (42 and 13 nucleotides different) and 92 % and 97 % with Mo. japonicum (35 and 16 nucleotides different; Nordén et al., 2005), respectively. Additionally, the tub2 sequence of Mo. palatinum differs from Mo. germanicum by 15 nucleotides (95 % identical). Moreover, the absence of inflated hyphal cells and the lesser occurrence of discrete phialides distinguish Mo. palatinum from Mo. germanicum. The pycnidia of Mo. palatinum are smaller and conidiogenous cells in pycnidia are longer than those of Mo. quercinum and Mo. japonicum.
Neophaeomoniella constricta C. Kraus, Damm, S. Bien, Vögele & M. Fisch., sp. nov. MycoBank MB828288. Figs 4E, 9.
Fig. 9.
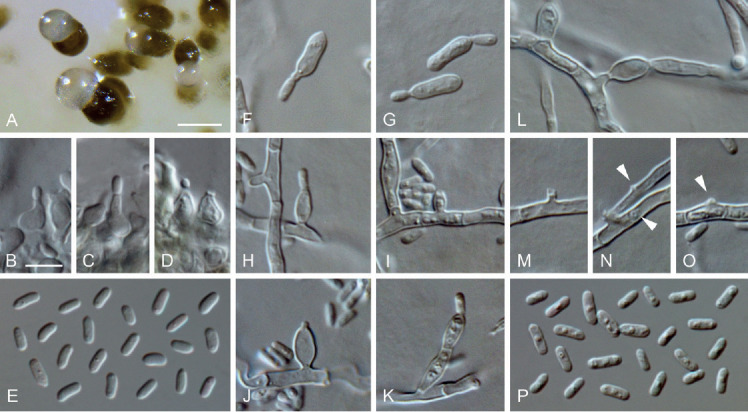
Neophaeomoniella constricta (ex-type culture JKI-Mz35). A. Pycnidia formed on OA. B–D. Conidiogenous cells from the inner cell wall of pycnidia. E. Conidia formed in pycnidia. F–G. Microcyclic conidiation. H–K, M–O. Conidiogenous cells on hyphal cells (arrows indicate conidiogenous openings). L. Inflated hyphal cell. P. Conidia formed on hyphal cells. A: SM; B–P: LM. Scale bars: A = 100 μm; B = 5 μm; B applies to B–P.
Etymology: Named after the conidiogenous cells that are often constricted at the septa.
Typus: Germany, Rhineland-Palatinate, Siebeldingen, °13′11.5″N 8°02′34.6″E, isolated from a spore trap attached to a grapevine shoot, 3 Mar. 2016, C. Kraus (GLM-F117500 holotype, culture ex-type JKI-Mz35 = CBS 145015 = GLMC 1915).
Vegetative hyphae hyaline, smooth-walled, 1–3 μm wide, septate, partly inflated up to 5.5 μm. Sporulation abundant; conidia formed on hyphae, in pycnidia and by microcyclic conidiation. Conidiophores on hyphae hyaline, smooth-walled, mainly reduced to conidiogenous cells, occasionally 2–6-celled conidiophores observed, often constricted at the septa, 4.5–32.5 × 2.5–3.5 μm. Conidiogenous cells enteroblastic, hyaline, smooth-walled, mostly openings formed directly on hyphal cells; sometimes discrete phialides, ellipsoidal, ovoidal to navicular, rarely cylindrical, 3.5–7.5 × 2.5–4 μm; collarettes sometimes visible, periclinal thickening inconspicuous. Conidia accumulated in groups around conidiogenous openings, hyaline, smooth-walled, aseptate, cylindrical to oblong-elliptical, sometimes slightly curved, (3–)4(–5) × (1–)1.5(–2) μm, L/W ratio = 2.7.
Microcyclic conidiation observed on one side of swollen mother cells developed from primary conidia; conidiogenous cells hyaline, smooth-walled, aseptate, rarely 2-celled, cylindrical to oblong-elliptical, (5–)6.5(–8) × (1.5–)2(–3) μm, L/W ratio = 3.0.
Conidiomata pycnidial, observed on pine needles, grapevine wood, on SNA and OA after four wk, solitary or in groups, superficial or immersed, globose, subglobose to ellipsoidal, 20–150 μm diam, unilocular, opening by irregular rupture, pycnidial wall composed of textura angularis, 8–19 μm thick, 2–6 cell layers. Conidiophores reduced to conidiogenous cells lining the inner cavity of the conidiomata. Conidiogenous cells enteroblastic, hyaline, smooth-walled, phialides discrete, ampulliform, 3.5–8 × 2.5–4 μm, collarettes inconspicuous, opening 0.5–1.5 μm wide. Conidia hyaline, smooth-walled, aseptate, cylindrical to oblong-elliptical, sometimes slightly curved, (3–)4(–5) × (1–)1.5(–2) μm, L/W ratio = 2.3.
Culture characteristics: Colonies on PDA raised, radially striate with lobate to crenated margin, moist, saffron to luteous, aerial mycelium sparse; reverse saffron; on OA flat, with entire margin, moist, in the centre greenish olivaceous to dark herbage green due to pycnidia formation, with a buff margin, aerial mycelium abundant, sometimes funiculose; reverse same colours; on MYA flat, with lobate margin, moist, saffron, aerial mycelium sparse; reverse same colours; 2–4.5 mm diam after 14 d on MYA (25 °C, in the dark), min 10 °C, max 25 °C, opt 20 °C.
Notes: The formation of up to 6-celled conidiophores, typically constricted at the septa, distinguishes N. constricta from its close relatives N. ossiformis, N. corymbiae, N. eucalyptigena, N. eucalypti, N. niveniae and N. zymoides. Within this group of slow-growing fungi studied here, N. constricta was the one with the slowest growth on PDA, which is an additional feature of this fungus (Fig. 4). Comparison of the tub2, ITS and LSU sequences showed that N. constricta is 93 % (24 nucleotides different), 98 % (10 nucleotides different) and 99 % (6 nucleotides different), respectively, identical with its closest relative N. ossiformis, while N. zymoides is 82 %, 95 % and 97 % identical. The ITS and LSU sequences of N. constricta are 93 % and 98 % identical with N. corymbiae (36 and 8 nucleotides different) and 97 % and 98 % with N. eucalyptigena (18 and 9 nucleotides different; Crous et al. 2018), respectively. A blastn search with the ITS sequence of N. constricta resulted in 97–99 % identity (8–16 nucleotides different) with sequences of eight fungal strains (identified as Eurotiomycetes, Phaeomoniella sp. or referred to as uncultured fungus), most of them described as endophytes of trees in Arizona (USA), New Zealand, New Mexico (USA) and Canada (KP202999, GQ999270, JN225892, KT264520, KT264593, KF742578, GQ153143, GQ153196; Hoffman & Arnold 2010, Johnston et al. 2012, Bérubé & Nicolas 2015, Chen et al. 2015). Additionally, 38 endophytic fungi isolated from leaves of pine trees in Arizona (USA) display a high accordance (98–99 %; 7–11 nucleotides different) with N. constricta (Bowman & Arnold 2018).
Neophaeomoniella ossiformis C. Kraus, Damm, S. Bien, Vögele & M. Fisch., sp. nov. MycoBank MB828289. Figs 4F, 10.
Etymology: Named after the shape of the conidia mother cells of the microcyclic conidiation that are sometimes ossiform.
Typus: Germany, Rhineland-Palatinate, Siebeldingen, °13′11.5″N 8°02′34.6″E, isolated from a spore trap attached to a grapevine shoot, 6 May 2016, C. Kraus (GLM-F117498 holotype, culture ex-type JKI-May03 = CBS 145013 = GLMC 1913).
Vegetative hyphae hyaline, smooth-walled, 1.5–3 μm wide, septate, partly inflated up to 8 μm wide. Sporulation abundant; conidia formed on hyphae, in pycnidia and by microcyclic conidiation. Conidiophores on hyphae hyaline, smooth-walled, mainly reduced to conidiogenous cells, occasionally 2–3-celled conidiophores observed, 6.5–22.5 × 2–3.5 μm. Conidiogenous cells enteroblastic, hyaline, smooth-walled, mostly reduced to openings directly formed on hyphal cells, discrete phialides sometimes observed, subglobose to elongate navicular, 4.5–11.5 × 2–3.5 μm, collarettes rarely observed, periclinal thickening inconspicuous. Conidia accumulated in heads around conidiogenous openings, hyaline, smooth-walled, aseptate, cylindrical, sometimes slightly curved, (2.5–)3.5(–4.5) × (1–)1.5(–2) μm, L/W ratio = 2.7.
Microcyclic conidiation observed on one side of swollen mother cells developed from primary conidia; conidiogenous cells hyaline, smooth-walled, aseptate, obovate to oblong-elliptical, sometimes ossiform, (6–)8(–10) × (1.5–)2.5(–3) μm, L/W ratio = 3.3.
Conidiomata pycnidial, produced superficially on pine needles, grapevine wood and immersed in SNA medium after four wk, mainly solitary, globose, subglobose to ellipsoidal, 40–360 μm diam, unilocular, opening by irregular rupture, pycnidial wall composed of textura angularis, 10–16 μm thick, 3–6 cell layers. Conidiophores reduced to conidiogenous cells lining the inner cavity of the pycnidium. Conidiogenous cells enteroblastic, hyaline, smooth-walled, phialides discrete, ampulliform to flask-shaped, 3.5–7 × 2.5–5 μm, opening 0.5–1.5 μm wide, collarettes inconspicuous, periclinal thickening not observed. Conidia hyaline, smooth-walled, aseptate, cylindrical to oblong-elliptical, sometimes slightly curved, (3–)3.5(–4) × (1–)1.5(–2) μm, L/W ratio = 2.3.
Culture characteristics: Colonies on PDA raised, radially striate with lobate to crenated margin, moist, buff, grey olivaceous in centre due to pycnidia formation, aerial mycelium in centre sparse, funiculose; reverse buff; on OA flat with entire edge, moist, buff to grey olivaceous with whitish margin, aerial mycelium sometimes formed in centre, sparse, funiculose; reverse same colours; on MYA raised, radially striate with lobate margin, moist, buff, grey olivaceous in centre due to pycnidia formation, aerial mycelium abundant, whitish, funiculose; reverse buff; 7–9 mm diam after 14 d on MYA (25 °C, in the dark), min 10 °C, max 25 °C, opt 20 °C.
Additional materials examined: Germany, Rhineland-Palatinate, Siebeldingen, °13′11.5″N 8°02′34.6″E, isolated from a spore trap attached to a grapevine shoot, 6 May 2016, C. Kraus, JKI-May02 = CBS 145014 = GLMC 1912; Siebeldingen, °13′11.5″N 8°02′34.6″E, isolated from a spore trap attached to a grapevine shoot, 6 May 2016, C. Kraus, JKI-May30 = GLMC 1914.
Notes: Neophaeomoniella ossiformis can be distinguished from the closely related N. constricta by its faster growth and shorter conidiophores that are usually less strongly constricted at the septa. The tub2, ITS and LSU sequences of N. ossiformis are 93 % (24 nucleotides different), 98 % (10 nucleotides different) and 99 % (6 nucleotides different) identical with the sequences of N. constricta. The ITS and LSU sequences of N. ossiformis are 94 % (32 nucleotides different) and 99 % (2 nucleotides different) identical with those of N. corymbiae, respectively, and 96 % (20 nucleotides different) and 99 % (4 nucleotides different) with those of N. eucalyptigena, respectively (Crous et al. 2018). A blastn search of the ITS sequence of N. ossiformis produced similar results as for N. constricta (see above).
Pathogenicity
Until the day of evaluation, i.e. after six months, infected plants showed no external symptoms of esca or other GTDs. However, longitudinal sections of the trunks unveiled discolorations at the inoculation site in all plants. The measured mean lesion length revealed significant differences between P. chlamydospora (22.7 ± 13.8 mm and 20.4 ± 11.2 mm, respectively) and the water control (6.1 ± 0.9 mm and 6.5 ± 1.7 mm, respectively) for both cultivars, Pinot noir and Müller-Thurgau (Table 3). However, the lesion length of the seven other examined species of the Phaeomoniellales showed no differences to the water control. For Pinot noir the produced mean lesions of the tested fungi ranged from 6.0 ± 1.2 mm (Mo. palatinum) to 7.5 ± 2.4 mm (N. zymoides). Similarly, for Müller-Thurgau the mean lesion varied between 6.2 ± 1.5 mm (Mo. palatinum) and 10.5 ± 4.1 mm (N. zymoides).
Table 3. Results of the pathogenicity test performed with eight species of the Phaeomoniellales. The test was conducted with grapevine varieties Pinot noir and Müller-Thurgau in a greenhouse. Shown is the mean total lesion length measured up- and downwards from the inoculation point in mm and the re-isolation rate. Significant differences (p < 0.001) between mean lesion lengths according to an ANOVA are indicated by different letters.
| Accession no.a | Fungal species |
Pinot noir |
Müller-Thurgau |
||
|---|---|---|---|---|---|
| Mean lesion length [mm] | Re-isolation rate [%] | Mean lesion length [mm] | Re-isolation rate [%] | ||
| JKI-Ap04 | Phaeomoniella chlamydospora | 22.7 ± 13.8A | 46.7 | 20.4 ± 11.2A | 66.7 |
| JKI-Ap36* | Aequabiliella palatina | 6.1 ± 2.4B | 14.3 | 7.2 ± 3.8B | 7.7 |
| JKI-Jn27* | Minutiella simplex | 6.6 ± 2.6B | 21.4 | 8.2 ± 2.9B | 61.5 |
| JKI-Feb06* | Moristroma germanicum | 6.0 ± 1.4B | 30 | 6.8 ± 1.1B | 38.5 |
| JKI-Feb17* | Mo. palatinum | 6.0 ± 1.2B | 0 | 6.2 ± 1.5B | 6.7 |
| JKI-Mz35* | Neophaeomoniella constricta | 7.2 ± 4.0B | 15.4 | 8.1 ± 2.2B | 14.3 |
| JKI-May03* | N. ossiformis | 7.1 ± 1.9B | 27.3 | 8.6 ± 3.1B | 50 |
| JKI-Mz41 | N. zymoides | 7.5 ± 2.4B | 15.4 | 10.5 ± 4.1B | 50 |
| – | Control (water) | 6.1 ± 0.9B | 0 | 6.5 ± 1.7B | 0 |
a JKI: Culture collection of the Julius Kühn-Institute, Siebeldingen, Germany.
*Ex-type cultures.
Wood pieces from the induced lesions were placed on MYA plates to verify the presence of the inoculated fungi in order to fulfil Koch’s postulates. Phaeomoniella chlamydospora was re-isolated most frequently (46.7 %) from infected Pinot noir plants. The seven other studied fungi reached a re-isolation rate between 14.3 % (A. palatina) and 30.0 % (Mo. germanicum). Moristroma palatinum could not be re-isolated from Pinot noir plants. Concerning the Müller-Thurgau vines, P. chlamydospora again showed the highest re-isolation rate with 66.7 %. The remaining fungi were recovered from 6.7 % (Mo. palatinum) to 61.5 % (M. simplex).
DISCUSSION
During this study, spore traps made of sticky glass slides were positive for fungi of the Phaeomoniellales in eleven out of 56 monitored wk (20 %). A similar trapping method was applied in Californian vineyards that were monitored from February to July; spores of Phaeomoniella chlamydospora were detected in nine out of 24 wk (38 %) (Eskalen & Gubler 2001), while Larignon & Dubos (2000) detected P. chlamydospora spores in 15 wk (15 %) during a 2-yr study with a sampling size of thirty collected glass slides each week. Both studies focused on P. chlamydospora spores and do not report the detection of other Phaeomoniellales. Since the detection rate was low in all three studies, it is assumed that in general the spore concentration of Phaeomoniellales in the air is relatively low or that the applied spore trapping method does not enable a higher trapping rate. Especially the trapping technique seems to be a critical point for successful monitoring of Phaeomoniellales. Although P. chlamydospora is common in South African vineyards, it was not detected by spore trapping, which could be due to the use of air sampling traps specifically made for the collection of wind distributed spores, e.g. Hirst or Burkard spore traps (Hirst 1952, Mostert et al. 2006, van Niekerk et al. 2010). However, slimy spores like those of P. chlamydospora and its relatives are more likely to be distributed by rain droplets, pruning scissors or even insects than by air (Aroca et al. 2010, Moyo et al. 2014). That may be the reason why glass slides with a sticky surface, closely attached to grapevines, are more effective in trapping spores of Phaeomoniellales than strict air sampling devices.
Only two of the eight identified species of the family Phaeomoniellales, namely P. chlamydospora and N. zymoides, were known species. Phaeomoniella chlamydospora is a known pathogen almost exclusively associated with grapevine; only once it was isolated from symptomatic wood of olive trees and from Convolvulus arvensis (Agustí-Brisach et al. 2010, Úrbez-Torres et al. 2013, Farr & Rossman 2018). Neophaeomoniella zymoides was first described from healthy pine needles and later isolated from necrotic wood of Prunus salicina (Lee et al. 2006, Damm et al. 2010). Two of the newly described species, N. constricta and N. ossiformis, are closely related to N. corymbiae, N. eucalypti, N. eucalyptigena, N. niveniae and N. zymoides, while Aequabiliella palatina and Minutiella simplex are closely related to A. effusa, M. pruni-avium and M. tardicola, respectively. The latter three were also associated with symptomatic wood of Prunus trees (Damm et al. 2010, Bien & Damm 2020). Moristroma germanicum and Mo. palatinum were assigned to the genus Moristroma; the other four species of this genus, Mo. japonicum, Mo. quercinum, Mo. multisporum and Mo. polysporum, had been described from branches of Quercus mongolica var. grossoserrata, Q. robur, dead wood of Terminalia arjuna and decorticated wood of Eucalyptus viminalis, respectively (Sivanesan et al. 1988, Romero & Samuels 1991, Nordén et al. 2005).
This is the first report of Phaeomoniellales other than P. chlamydospora in vineyards. Due to the harm, which this esca associated pathogen can cause, the question emerged, if the species detected are also pathogenic to grapevine. However, the pathogenicity test showed that only P. chlamydospora is able to induce necrosis in the wood of potted grapevine plants. This led to the conclusion that these species are not pathogenic to grapevine. Furthermore, it is likely that grapevine wood is not the natural habitat of these species, since they have to date never been found in or on any parts of grapevine plants (Casieri et al. 2009, González & Tello 2011, Hofstetter et al. 2012, Pancher et al. 2012, Bruez et al. 2014, Pinto et al. 2014, Bruez et al. 2016, Travadon et al. 2016, Farr & Rossman, 2018, Kraus et al. 2018). Based on the habitats of previously described Phaeomoniellales species, the new species might originate from woody plants. Five species belonging to four genera of the Phaeomoniellales have previously been collected from wood of Prunus trees in South Africa (Damm et al. 2010). One of these species, N. zymoides, was originally described from pine needles in Korea (Lee et al. 2006) and was the most abundant species in our study. Moreover, Bien & Damm (2020) recently isolated a fungus with high similarity to the ITS (100 % identical) and tub2 (99.8 % identical, 1 nucleotide different) sequence of M. simplex from necrotic wood of P. domestica in the region of Baden-Württemberg, Germany. Therefore, the Prunus trees planted close (in about 100 m distance) to the vineyard sampled could be one possible origin of the collected spores in this study. Two independent investigations on endophytic fungi in wood of pine trees in Montana, USA and Finland revealed unidentified fungal strains with high similarity (99 %, 2–3 nucleotides different) to the ITS sequence of A. palatina (Larkin et al. 2012, Müller et al. 2018). These findings may indicate that A. palatina also appears endophytically and that pine trees represent a possible host genus.
With the data generated in this study, the comparatively new order Phaeomoniellales will be extended by six new species. However, with the information at hand, the origin of the trapped fungal spores and their ecological traits remain basically unknown. The pathogenicity test conducted in this study does not support them to be pathogens of grapevine. However, this is no proof that these fungi do not live in or on grapevine wood. Further studies are necessary to reveal the origin and life style of the newly described species and their possible impact for example on economically important fruit crops.
ACKNOWLEDGEMENTS
We gratefully acknowledge the financial support of the Projektträger Jülich (PTJ) and the German Federal Ministery of Education and Research (BMBF). We want to thank the Karlsruhe Institute of Technology (KIT), Germany, especially Jan Maisch, for provision of equipment for microscopy. Furthermore, we want to thank Anita Kramm for her immense support with the greenhouse plants, as well as Sandra Biancu for her assistance with the microtome. Prof. Uwe Braun, Martin-Luther-Universität Halle-Wittenberg, Halle, Germany, is thanked for his support with Latin names and clearing the nomenclature problem regarding the family name.
REFERENCES
- Agustí-Brisach C, Gramaje D, León M, et al. , (2011). Evaluation of vineyard weeds as potential hosts of black-foot and Petri disease pathogens. Plant Disease 95: 803–810. [DOI] [PubMed] [Google Scholar]
- Aptroot A, Lücking R, Sipman HJM, et al. , (2008). Pyrenocarpous lichens with bitunicate asci. Bibliotheca Lichenologica 97: 1–162. [Google Scholar]
- Aroca A, Gramaje D, Armengol J, et al. , (2010). Evaluation of the grapevine nursery propagation process as a source of Phaeoacremonium spp. and Phaeomoniella chlamydospora and occurrence of trunk disease pathogens in rootstock mother vines in Spain. European Journal of Plant Pathology 126: 165–174. [Google Scholar]
- Arnold AE, Henk DA, Eells RL, et al. , (2007). Diversity and phylogenetic affinities of foliar fungal endophytes in loblolly pine inferred by culturing and environmental PCR. Mycologia 99: 185–206. [DOI] [PubMed] [Google Scholar]
- Bertsch C, Ramírez-Suero M, Magnin-Robert M, et al. , (2012). Grapevine trunk diseases: complex and still poorly understood. Plant Pathology 62: 243–265. [Google Scholar]
- Bérubé JA, Nicolas GG. (2015). Alien fungal species on asymptomatic live woody plant material imported into Canada. Canadian Journal of Plant Pathology 37: 67–81. [Google Scholar]
- Bien S, Damm U. (2020). Arboricolonus simplex gen. et sp. nov. and novelties in Cadophora, Minutiella and Proliferodiscus from Prunus wood in Germany. MycoKeys 63: 119–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonmee S, Zhang Y, Chomnunti P, et al. , (2011). Revision of lignicolous Tubeufiaceae based on morphological reexamination and phylogenetic analysis. Fungal Diversity 51: 63–102. [Google Scholar]
- Booth C, Ting TP. (1964). Dolabra nepheliae gen. nov., sp. nov., associated with canker of Nephelium lappaceum. Transactions of the British Mycological Society 47: 235–237. [Google Scholar]
- Botella L, Diez JJ. (2010). Phylogenic diversity of fungal endophytes in Spanish stands of Pinus halepensis. Fungal Diversity 47: 9–18. [Google Scholar]
- Bowman EA, Arnold AE. (2018). Distributions of ectomycorrhizal and foliar endophytic fungal communities associated with Pinus ponderosa along a spatially constrained elevation gradient. American Journal of Botany 105: 687–699. [DOI] [PubMed] [Google Scholar]
- Bruez E, Vallance J, Gerbore J, et al. , (2014). Analyses of the temporal dynamics of fungal communities colonizing the healthy wood tissues of esca leaf-symptomatic and asymptomatic vines. PLoS ONE 9: e95928 doi:101371/journalpone0095928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruez E, Baumgartner K, Bastien S, et al. , (2016). Various fungal communities colonise the functional wood tissues of old grapevines externally free from grapevine trunk disease symptoms. Australian Journal of Grape and Wine Research 22: 288–295. [Google Scholar]
- Casieri L, Hofstetter V, Viret O, et al. , (2009). Fungal communities living in the wood of different cultivars of young Vitis vinifera plants. Phytopathologia Mediterranea 48: 73–83. [Google Scholar]
- Chen K-H, Miadlikowska J, Molnár K, et al. , (2015). Phylogenetic analyses of eurotiomycetous endophytes reveal their close affinities to Chaetothyriales Eurotiales and a new order – Phaeomoniellales. Molecular Phylogenetics and Evolution 85: 117–130. [DOI] [PubMed] [Google Scholar]
- Crous PW, Gams W. (2000). Phaeomoniella chlamydospora gen. et comb. nov., a causal organism of Petri grapevine decline and esca. Phytopathologia Mediterranea 39: 112–118. [Google Scholar]
- Crous PW, Gams W, Wingfield MJ, et al. , (1996). Phaeoacremonium gen. nov. associated with wilt and decline diseases of woody hosts and human infections. Mycologia 88: 786–796. [Google Scholar]
- Crous PW, Luangsa-ard JJ, Wingfield MJ, et al (2018). Fungal Planet description sheets: 785–867. Persoonia 41: 238–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Shivas RG, et al. , (2011). Fungal Planet description sheets: 92–106. Persoonia 27: 130–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ. (2009). Xenocylindrosporium kirstenboschense. Persoonia 23: 200–201. [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. , (2016). Fungal Planet description sheets: 469–557. Persoonia 37: 218–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, et al. , (2015). Fungal Planet description sheets: 320–370. Persoonia 34: 167–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wood AR, Okada G, et al. , (2008). Foliicolous microfungi occurring on Encephalartos. Persoonia 21: 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Fourie PH, Crous PW. (2010). Coniochaeta (Lecythophora), Collophora gen. nov. and Phaeomoniella species associated with wood necroses of Prunus trees. Persoonia 24: 60–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskalen A, Gubler WD. (2001). Association of spores of Phaeomoniella chlamydospora, Phaeoacremonium inflatipes, and Pm. aleophilum with grapevine cordons in California. Phytopathologia Mediterranea 40: S429–S432. [Google Scholar]
- Farr DF, Rossman AY. (2018). Fungal Databases, U.S. National Fungus Collections ARS USDA https://ntars-gringov/fungaldatabases/ (accessed 22 April 2020).
- Fontaine F, Gramaje D, Armengol J, et al. , (2016). Grapevine trunk diseases. A review. 1st edn OIV Publications, Paris, France: ISBN: 979-10-91799-60-7. [Google Scholar]
- Glass LN, Donaldson GC. (1995). Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González V, Tello ML. (2011). The endophytic mycota associated with Vitis vinifera in central Spain. Fungal Diversity 47: 29–42. [Google Scholar]
- Gramaje D, Úrbez-Torres JR, Sosnowski MR. (2018). Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Disease 102: 12–39. [DOI] [PubMed] [Google Scholar]
- Gueidan C, Aptroot A, Cáceres MES, et al. , (2014). A reappraisal of orders and families within the subclass Chaetothyriomycetidae (Eurotiomycetes Ascomycota). Mycological Progress 13: 1027–1039. [Google Scholar]
- Hirst JM. (1952). An automatic volumetric spore trap. Annals of Applied Biology 39: 257–265. [Google Scholar]
- Hoffman MT, Arnold AE. (2010). Diverse bacteria inhabit living hyphae of phylogenetically diverse fungal endophytes. Applied and Environmental Microbiology 76: 4063–4075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter V, Buyck B, Croll D, et al. , (2012). What if esca disease of grapevine were not a fungal disease? Fungal Diversity 54: 51–67. [Google Scholar]
- Hoog GS de, Morgan-Jones G. (1978). Notes on Hyphomycetes. XXIII. Paraphaeoisaria alabamensis gen. et sp. nov. Mycotaxon 7: 133–138. [Google Scholar]
- Johnston PR, Johansen RB, Williams AF, et al. , (2012). Patterns of fungal diversity in New Zealand Nothofagus forests. Fungal Biology 116: 401–412. [DOI] [PubMed] [Google Scholar]
- Keith LM, Matsumoto TK, McQuate GT. (2013). First report of Dolabra nepheliae associated with corky bark disease of langsat in Hawaii. Plant Disease 97: 990. [DOI] [PubMed] [Google Scholar]
- Kirk PM. (2015). Nomenclatural novelties. Index Fungorum 265: 1–1. [Google Scholar]
- Kraus C, Voegele RT, Fischer M. (2018). Temporal development of the culturable endophytic fungal community in healthy grapevine branches and occurrence of GTD associated fungi. Microbial Ecology 77: 866–876. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2015). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larignon P, Dubos B. (2000). Preliminary studies on the biology of Phaeoacremonium. Phytopathologia Mediterranea 39: 184–189. [Google Scholar]
- Larkin BG, Hunt LS, Ramsey PW. (2012). Foliar nutrients shape fungal endophyte communities in Western white pine (Pinus monticola) with implications for white-tailed deer herbivory. Fungal Ecology 5: 252–260. [Google Scholar]
- Lee HB, Park JY, Jung HS, et al. , (2006). Phaeomoniella zymoides and Phaeomoniella pinifoliorum spp. nov., new acid-tolerant epiphytic fungi isolated from pine needles in Korea. Mycologia 98: 598–611. [DOI] [PubMed] [Google Scholar]
- Mostert L, Abeln ECA, Halleen F, et al. , (2006). Genetic diversity among isolates of Phaeomoniella chlamydospora on grapevines. Australasian Plant Pathology 32: 47–52. [Google Scholar]
- Moyo P, Allsopp E, Roets F, et al. , (2014). Arthropods vector grapevine trunk disease pathogens. Phytopathology 104: 1063–1069. [DOI] [PubMed] [Google Scholar]
- Müller MM, Henttonen HM, Penttilä R, et al. , (2018). Distribution of Heterobasidion butt rot in northern Finland. Forest Ecology and Management 425: 85–91. [Google Scholar]
- Nigro F, Boscia D, Antelmi I, et al. , (2013). Fungal species associated with a severe decline of olive in southern Italy. Journal of Plant Pathology 95: 659–668. [Google Scholar]
- Nirenberg H. (1976). Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem 169: 1–117. [Google Scholar]
- Nordén B, Sunhede S, Larsson E. (2005). New species of Moristroma (Ascomycetes) and phylogenetic position of the genus. Mycological Progress 4: 325–332. [Google Scholar]
- O’Donnell K. (1993). Fusarium and its near relatives In: The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. (Reynolds DR, Taylor JW, eds). CAB International; Wallingford, UK: 225–233. [Google Scholar]
- Pancher M, Ceol M, Corneo PE, et al. , (2012). Fungal endophytic communities in grapevines (Vitis vinifera L.) respond to crop management. Applied and Environmental Microbiology 78: 4308–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peršoh D, Rambold G. (2012). Lichen-associated fungi of the Letharietum vulpinae. Mycological Progress 11: 753–760. [Google Scholar]
- Pinto C, Pinho D, Sousa S, et al. , (2014). Unravelling the diversity of grapevine microbiome. PLoS ONE 9: e85622 doi:101371/journalpone0085622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner RW. (1970). A mycological colour chart. Commonwealth Mycological Institute; Kew UK. [Google Scholar]
- Romero AI, Samuels GJ. (1991). Studies on xylophilous fungi from Argentina: VI. Ascomycotina on Eucalyptus viminalis (Myrtaceae). Sydowia 43: 228–248. [Google Scholar]
- RStudio Team (2016). RStudio: Integrated development for R. RStudio, Inc., Boston, USA: http://wwwrstudiocom/ (accessed 16 July 2018). [Google Scholar]
- Rossman AY, Schoch CL, Farr DF, et al. , (2010). Dolabra nepheliae on rambutan and lychee represents a novel lineage of phytopathogenic Eurotiomycetes. Mycoscience 51: 300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez Márquez S, Bills GF, Zabalgogeazcoa I. (2011). Fungal species diversity in juvenile and adult leaves of Eucalyptus globulus from plantations affected by Mycosphaerella leaf disease. Annals of Applied Biology 158: 177–187. [Google Scholar]
- Sivanesan A, Rajak RC, Gupta RC. (1988). Thaxterina, a new tubeufiaceous genus with multispored asci from India. Transactions of the British Mycological Society 90: 662–665. [Google Scholar]
- Tillett D, Neilan BA. (2000). Xanthogenate nucleic acid isolation from cultured and environmental cyanobacteria. Journal of Phycology 36: 251–258. [Google Scholar]
- Travadon R, Lecomte P, Diarra B, et al. , (2016). Grapevine pruning system and cultivars influence the diversity of wood-colonizing fungi. Fungal Ecology 24: 82–93. [Google Scholar]
- Úrbez-Torres JR, Peduto F, Vossen PM, et al. , (2013). Olive twig and branch dieback: Etiology incidence and distribution in California. Plant Disease 97: 231–244. [DOI] [PubMed] [Google Scholar]
- Úrbez-Torres JR, Haag P, Bowen P, et al. , (2015). Development of a DNA-macroarray for the detection and identification of fungal pathogens causing decline of young grapevines. Phytopathology 105: 1373–1388. [DOI] [PubMed] [Google Scholar]
- Van Niekerk JM, Calitz FJ, Halleen F, et al. , (2010). Temporal spore dispersal patterns of grapevine trunk pathogens in South Africa. European Journal of Plant Pathology 127: 375–390. [Google Scholar]
- White TJ, Bruns T, Lee S, et al. , (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds). Academic Press; San Diego, USA: 315–322. [Google Scholar]
- Zhang Y, Crous PW, Schoch CL, Hyde KD. (2012). Pleosporales. Fungal Diversity 53: 1–221. [DOI] [PMC free article] [PubMed] [Google Scholar]