Abstract
Objective
Circulating glucose may relate to affective and physical feeling states reflective of emotional disorder symptoms. No prior studies have investigated within-day associations between glucose and subsequent affective and physical feeling states (positive affect, negative affect, and fatigue) as they occur naturally among healthy adolescents; this pilot study assessed these associations by combining data collected from ecological momentary assessment (EMA) and continuous glucose monitors (CGM).
Methods
Participants (N=15, mean age=13.1[±1.0] years, 66.7% female, 40.0% Hispanic, 66.7% healthy weight) wore a CGM for 7–14 days. Simultaneously, participants reported on their current positive affect, negative affect, and fatigue randomly during specified windows up to 7 times daily via EMA. CGM-measured mean interstitial glucose was calculated during the time windows (mean minutes=122.5[±47.3]) leading up to each EMA prompt. Multilevel models assessed within-subject (WS) associations between mean interstitial glucose since the previous EMA prompt and EMA-reported affective and physical feeling states at the current prompt.
Results
Participants provided 532 interstitial glucose-matched EMA reports of affective and physical feeling states. During intervals when interstitial glucose was higher than one’s usual, higher positive affect (WS β=0.01, p<0.0001, f2=0.02) and lower fatigue (WS β=−0.01, p<0.0001, f2=0.09) were subsequently reported. Interstitial glucose was unrelated to negative affect (WS β=−0.002, p=0.10, f2=0.01). Associations were weakened, but remained significant following further adjustment for time of day.
Conclusions
Though effect sizes were small, within-person variations in interstitial glucose may relate to subsequent affective and physical feeling states among healthy youth. Investigations using similar methodologies in larger, more diverse samples are warranted.
Keywords: blood glucose, mood, real-time, within-subject, youth
Introduction
Adolescence is characterized by rapid physical maturation and increased emotional variability [1, 2]. Consequently, adolescents are at increased risk for emotional health problems, including the development of depressive and anxiety disorders [3, 4]. It is estimated the prevalence of depression and anxiety is highest among adolescents compared to all other age groups [5, 6]. Even at subclinical levels, emotional disorder symptoms during adolescence can continue into adulthood and influence long-term outcomes such as lower educational attainment and substance misuse [7–9]. In an attempt to address this public health concern, investigators have focused on gaining a better understanding of the modifiable risk factors associated with emotional disorder symptoms in youth.
Common symptoms of emotional disorders include changes in affective and physical feeling states, such as lower levels of positive affect, higher levels of negative affect, and increased fatigue [10, 11]. Results from previous studies of behavioral correlates of affective and physical feeling states indicate that more physical activity is related to momentary increases in positive affect and decreases in negative affect and fatigue [12, 13]. Additional energy balance behaviors such as sedentary behavior and dietary intake may also influence affective and physical feeling states. For example, more sedentary time is acutely related to lower positive affect (e.g., in the subsequent 30 minutes), and youth with higher negative affect consume more pastries, sweets, and fast food [14, 15].
Although the behavioral factors relating to emotional disorder symptoms in youth are commonly investigated, the biological correlates are relatively understudied. It is plausible that the associations between health behaviors and affective and physical feeling states can be partially explained by biological underpinnings. Experimental and observational studies among healthy samples of children and young adults have demonstrated that blood glucose levels are acutely related to subsequent affective and physical feeling states [16–18]. One in-lab study among children demonstrated that participants reported feeling happier following a low glycemic index meal (vs. high glycemic index meal) and less sluggish following the high glycemic index meal in the 90 to 140 minutes following meal administration [16]. Another in-lab study reported that both acutely and over 2 hours, ingestion of a glucose drink and subsequent higher blood glucose levels were associated with feeling less tense/anxious [17]. Conversely, observational studies among individuals diagnosed with Type 1 and Type 2 diabetes in free-living settings have shown that higher average blood glucose and greater blood glucose variability are related to poorer affective states [19, 20]. Given that glucose is the brain’s main energy source that has been implicated in regulatory processes (including emotion regulation) [21, 22] and the mixed findings among prior studies, it is important to understand the associations between glucose levels and acute affective states in the naturalistic setting among healthy adolescents.
Recent advancements in technology have made studying the free-living, within-day associations between affective and physical feeling states and glucose levels possible. Ecological momentary assessment (EMA) is a real-time data capture strategy which prompts participants to report on factors such as affective states and physical feeling states in defined time windows throughout the day using a smartphone [23]. EMA can reduce recall errors and biases, is ecologically valid, and allows investigators to assess acute temporal associations [23]. Similarly, some continuous glucose monitors (CGM), small devices worn on the arm that capture real-time interstitial glucose levels, are less burdensome for participants (e.g., do not require multiple finger sticks per day), and provide more granular information compared to traditional glucose data collection strategies such as the use of a glucometer [24]. To date, no studies have combined EMA and CGM to investigate interstitial glucose-affective and physical feeling state associations. Therefore, the aim of this pilot study was to conduct exploratory analyses assessing the within-day associations between interstitial glucose, measured with CGM, and subsequently-reported affective and physical feeling states, measured via EMA, among healthy adolescents in a free-living environment. This study may elucidate potential biological factors related to markers of emotional health and can inform recommendations for future psychosomatic research that incorporates EMA and CGM.
Methods
Participants
The participants in this report were a subsample (N=15) of youth, ages 11–15 years, from the Mothers’ and Their Children’s Health (MATCH) study. Briefly, the MATCH study was a three-year longitudinal cohort study of maternal stress on child obesity risk. Mother-child dyads were recruited via flyers and in-person staff visits at public elementary schools and various community events. The inclusion criteria for mother-child dyads of the MATCH study were (1) the child is in 3rd to 6th grade (ages 8–12 years old) at baseline, (2) more than half of the child’s custody belongs to the mother, and (3) both the mother and child are able to read English or Spanish. Dyads were excluded from the MATCH cohort if the mother or the child (1) was taking medications for thyroid function or psychological conditions, (2) had a health condition that limited physical activity, (3) was enrolled in a special education program, (4) was currently using oral or inhalant corticosteroids for asthma, (5) was pregnant, (6) the child was classified as underweight by a body mass index percentile of <5% adjusted for sex and age, or (7) the mother worked more than two weekday evenings (between 5–9pm) per week or more than eight hours on any weekend day. The detailed MATCH study procedures are described in detail elsewhere [25].
Participants enrolled in the MATCH study were recruited for the Sedentary Behavior and Health Outcomes (SBHO) study, a randomized-crossover trial investigating the metabolic effects of interrupting sitting (ClinicalTrials.gov registration no. NCT03153930). Each participant was required to be enrolled in the MATCH cohort across all six waves (three years) and be in good general health. Participants diagnosed with cardiac or pulmonary disease, allergies to metals, evidence of impaired fasting blood glucose (blood glucose >100 mg/dL), endocrinologic disorders, or taking medications for Attention-Deficit/Hyperactivity Disorder were excluded. All study procedures were approved by the University of Southern California Institutional Review Board.
Procedures
Baseline screening visit
A baseline screening was conducted by trained staff to determine study enrollment eligibility. After parental consent and child assent were provided, demographic information was collected, fasting blood glucose was measured, and anthropometric measures including height, weight, and waist circumference were taken in duplicate. After verifying eligibility for the SBHO study, participants returned to the University of Southern California Diabetes and Obesity Research Institute lab on two separate occasions to complete in-lab metabolic measures. A sub-sample of participants enrolled in the SBHO study were given the opportunity to participate in a free-living data collection for two, week-long observational periods (totaling 14 days) on a rolling basis until the target sample size (N=15) was achieved. For the purposes of this report, data collected during the free-living observational period will be included in the analyses.
EMA
Participants in the free-living sub-study of the in-lab SBHO study were provided a Moto G mobile phone (Motorola Mobility, Chicago, IL) with the Movisens EMA app pre-downloaded for use for the duration of the study. Participants were randomly prompted within one-hour time windows to report on their affective and physical feeling states via EMA. EMA prompts occurred four times per day on weekdays except during school hours, and seven times per day on weekend days, between the hours of 7am and 8pm. The mobile device would prompt the participant via sound and/or vibration to stop his/her current activity and answer the EMA survey, which took approximately two minutes to complete. Participants were given the option to ignore the EMA prompts if they were engaging in an incompatible activity at the time of the prompt, such as sleeping or after-school activities.
CGM
A CGM (Abbott FreeStyle Libre Pro) was placed into the subcutaneous tissue on the back of the upper arm, capturing interstitial glucose levels every 15 minutes for up to 7 days for two separate weeks. Study staff monitored CGM compliance via text message with participants. If the CGM was damaged or fell off, a study team member went to the home to apply a new device. After seven days, the CGM was retrieved by study staff and data were downloaded onto a computer.
During either the first or the second observational week (based on participant randomization for the SBHO study mentioned above), participants were provided a wrist-worn device (LYCOS Life) which prompted them to interrupt their sitting every 30 minutes. This was done to assess feasibility of prompting interruptions in sedentary behavior in the free-living environment. Seven participants received the LYCOS Life during the first observational week, and eight participants received the LYCOS Life during the second observational week; participants were instructed to proceed with their normal daily routines during weeks when they were not instructed to wear the LYCOS Life. Randomization order did not differ by participant characteristics (data not shown). Compensation in the form of a pre-loaded Greenphire ClinCard (https://greenphire.com/clincard/) was provided to the participants’ parents at the end of each observational week when the devices were returned to a study team member.
Measures
Affective and physical feeling states
EMA items were adapted from the Positive and Negative Affect Schedule for Children (PANAS-C) [26] to capture momentary affective states, consistent with previous EMA studies [12, 14]. To assess positive affect, participants were prompted with two items, “Right before the phone went off, how HAPPY and JOYFUL were you feeling?” Negative affect was measured by three items, “Right before the phone went off, how STRESSED, MAD, and SAD were you feeling?” Participants were asked to respond to each affective state as outlined above. Response options included “0=Not at all,” “1=A little bit,” “2=Quite a bit,” and “3=Extremely.” Fatigue was measured with four EMA items taken from the Profile of Mood States for Adolescents [27], “Right before the phone went off, how TIRED, SLEEPY, EXHAUSTED, and WORN OUT were you feeling?”. Response options for each item ranged from not at all (0) to extremely (3). Responses for the happy and joyful items were averaged to create a positive affect composite score (within-subject internal consistency reliability, ω=0.90). Similarly, responses for the stressed, mad, and sad items were averaged to create a negative affect composite score (ω=0.81), and the tired, sleepy, exhausted, and worn out responses were averaged to create a fatigue score (ω=0.88). Therefore, the potential scores for all three variables could range from 0 to 3 during each EMA prompt.
Interstitial glucose
The CGM (Abbott FreeStyle Libre Pro) used in this study measures interstitial glucose every 15 minutes for up to 14 days. The accuracy between CGM measures and capillary blood glucose measures has been established [28]. After each observational period, the CGM data were downloaded to a study computer using the FreeStyle Libre software (version 1.0). To match the EMA prompting schedule and minimize the amount of unused CGM data, average interstitial glucose levels were calculated for the time period in between each EMA prompt (e.g., from the start time of prompt one to the start time of prompt two) and treated as a predictor of EMA-reported affective and physical feeling states immediately thereafter (e.g., those reported at prompt two). EMA prompts were approximately two hours apart (122.5 [±47.3] minutes); paralleling this time frame, past two-hour average interstitial glucose was calculated for the first EMA prompt of each day. Similarly, since EMA prompting did not occur during usual school hours (e.g., between the hours of 9am and 3pm during weekdays), past two-hour interstitial glucose during weekdays was instead calculated for the first afternoon prompt during weekdays. Data values occurring on days with less than 24-hours of CGM measures (including the first and last observational days of the study) were removed prior to analysis [29]. Therefore, these partial-day CGM data were missing at the day-level (rather than the momentary level) in the current study.
Covariates
A priori covariates included self-reported demographic characteristics including age (years; continuous), sex (female; yes vs. no), ethnicity (Hispanic; yes vs. no), and highest maternal education (college graduate or higher; yes vs. no), which served as a proxy for socioeconomic status. Additionally, body mass index (BMI) percentile calculated from the anthropometric measures taken at the baseline screening visit using the EpiInfo (version 7.2.2.6), day of week (weekend day; yes vs. no), and experimental condition (SIT; yes vs. no) were included as covariates in all models. Lastly, linear time of day (chronological EMA prompt number within the day, which ranges from 1–7; continuous) was further adjusted for in an additional set of models.
In addition to the a priori covariates, EMA-reported sleep quality, physical activity, sedentary behavior, dietary intake, and social context were tested as covariates in all models, given their potential to confound interstitial glucose-affective and physical feeling state associations [14, 30–37]. Sleep quality was measured with the EMA item “Compared to a typical night over the past month, how well did you sleep last night?” Response options included “much worse than usual,” “a little worse than usual,” “about the same as usual,” “a little better than usual,” and “much better than usual.” Physical activity was measured by the EMA item “Please choose the ONE main physical activity you were doing just before the phone went off.” Response options included “exercise,” “sports,” “walking/biking for transport,” “active house chores,” and “none of these things.” Similarly, sedentary behavior was captured by the EMA item “Please choose the ONE main sedentary activity you were doing just before the phone went off,” with response options including “TV/movies/videos,” “texting,” “social media (Facebook, Snapchat, Instagram, Tumblr, etc.),” “videogames,” “computer/tablet use,” “homework/reading,” “hanging out/chatting,” “art/painting/coloring,” “riding in the car/bus,” and “none of these things.” Participants reported on dietary intake of typical high glycemic index foods consumed by youth via the EMA item “Over the last 30 minutes, which of these things have you eaten? (choose all that apply).” Response options included “salty snacks/fried side dishes (e.g., chips, fries),” “sweets (e.g., pastries, candy, cookies, frozen dessert),” “soda or energy drinks (not including diet),” “fruit juice,” “bread/grains,” and “none of these things.” Lastly, social context was captured with the EMA item “Who were you with just before the phone went off?” with response options including family members, friends, classmates, or being alone. Each of the above variables that were tested as covariates were treated as dichotomous (much/a little worse sleep quality than usual; yes vs. no, engagement in any physical activity; yes vs. none of these things, engagement in any sedentary behavior; yes vs. none of these things, dietary intake of any high glycemic index food; yes vs. none of these things, and being alone; yes vs. no).
Statistical Analysis
Descriptive statistics of the study sample are found in Table 1. These included means or frequencies of demographic characteristics, baseline fasting blood glucose, week-level interstitial glucose levels measured by the CGM, affective states, and fatigue. Mean (standard error) affective state/fatigue score by interstitial glucose levels were also calculated. EMA prompt compliance was calculated as the proportion of prompts completed out of the total number of prompts. Separate multilevel logistic regression models investigated whether age, sex (female; yes vs. no), ethnicity (Hispanic; yes vs. no), weight status (healthy weight vs. overweight/obese), maternal education (college graduate or higher; yes vs. no), day of week (weekend; yes vs. no), person-mean interstitial glucose, person-mean positive affect, person-mean negative affect, or person-mean fatigue predicted momentary EMA prompt compliance (yes vs. no) and CGM data, which were missing at the day level (yes vs. no). Because EMA data were missing at the momentary level, multilevel logistic regression models also tested whether time of day was related to compliance at any given EMA prompt.
Table 1.
Participant characteristics (N=15)
| Characteristic | Mean (SD) or N (%) |
|---|---|
| Baseline | |
| Age (Years) | 13.1 (1.0) |
| Female | 10 (66.7%) |
| Hispanic Ethnicity | 6 (40.0%) |
| BMI Percentile | 55.4 (32.1) |
| Maternal Education (College Graduate) | 11 (73.3%) |
| Fasting Blood Glucose (mg/dL) | 87.6 (5.7) |
| Week-Level | |
| Interstitial Glucose (mg/dL) | 101.8(16.2) |
| Positive Affect | 1.6 (1.0) |
| Negative Affect | 0.3 (0.6) |
| Fatigue | 0.6 (0.7) |
The associations between CGM-measured interstitial glucose and subsequent EMA-reported affective states and fatigue were assessed using random-intercept linear mixed models with autocorrelated residual structures using PROC MIXED in SAS v9.4; random slopes (which allow for between-person differences in associations between the predictor and outcome) were also specified in each model and retained only if model fit improved as determined by changes in AIC and BIC. Linear mixed models adjust for the clustering of observations within each participant [38, 39]; and the first-order autocorrelated residual structures are a more parsimonious method for adjusting for the outcome variable at t-1 [40]. Mixed models allow for the partitioning of variances such that between-subject (level 2) and within-subject (level 1) versions of the predictor variable (interstitial glucose) can be created. This is done by grand-mean and person-mean centering, respectively [41]. The present analyses tested within-subject (momentary [past ~120 minute] variation from one’s own week-level mean) interstitial glucose as a predictor of EMA-reported positive affect, negative affect, and fatigue in three separate models, controlling for between-subject interstitial glucose and all a priori covariates mentioned above except for time of day (model A). Time of day was further adjusted for in an additional set of three models (model B). See the supplemental text for reproducible SAS code for the multilevel models specified in the present study. EMA-reported physical activity, sedentary behavior, dietary intake, and social context were tested separately as covariates in each model and retained in the model if significant confounding was present. Lastly, Cohen’s f2, a measure of local effect size appropriate for multilevel models, was calculated [42]. Due to the possibility of the model results being sensitive to the interstitial glucose time frame selected, sensitivity analyses using within-subject past 60-minute and past 30-minute interstitial glucose as separate predictors of EMA-reported affective and physical feeling states were conducted.
Results
Participant and data flow
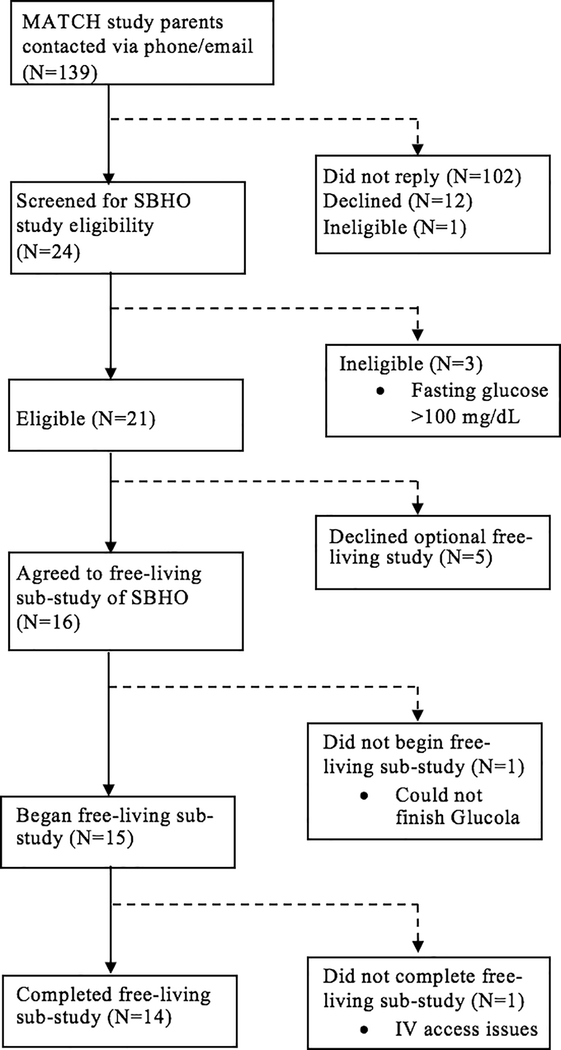
A total of 15 adolescents participated in the free-living study. Fourteen participants completed both week-long observational periods, while one participant only completed one week of free-living observation (Figure 1). Participants received a total of 1030 EMA prompts, of which 637 were completed (61.8% compliance; range: 32.5%−88.0%). One hundred and five of the completed EMA prompts corresponded with excluded CGM data during non-24 hour CGM days and were not included in the analyses; thus, the analytic sample in this report consists of 532 interstitial glucose-matched EMA reports of affective and physical feeling states (level 1) across 15 participants (level 2).
Figure 1.
Study participant flow
Sample characteristics and device compliance
The mean age of the sample was ~13 years (range: 11 to 15 years old). More than half of the sample was female (66.7%). Ten (66.7%) participants were of healthy weight and just below half of the sample was of Hispanic ethnicity (40.0%). Of the remaining non-Hispanic participants, 20% were white, 20% were black, 13.3% were more than one race, and 6.7% had an unknown race. Mean fasting blood glucose obtained during the baseline screening visit was 87.6 mg/dL (range: 77.9 to 94.6 mg/dL), while the mean (SD) interstitial glucose in the approximate two-hour time period prior to the EMA prompt was 101.8 (16.2) mg/dL (range: 59.8–162.0 mg/dL). Supplemental Figure 1 presents the average interstitial glucose levels in the two-hour time period prior to the EMA prompt across the day (7am-8pm). On average, the mean amplitude of glycemic excursions (MAGE; a day-level glucose variability metric, calculated as the arithmetic average of the “amplitudes” above and below 1 standard deviation of one’s mean glucose) [43] was 41.7 mg/dL in our sample; consistent with what has been previously reported among another sample of healthy youth [44]. Refer to Table 1 for mean week-level affective and physical feeling states reported by participants.
Results from multilevel logistic regression analyses for EMA prompt compliance (yes. vs. no) indicated that EMA prompt compliance did not differ by demographic characteristics (age: below mean age=59.2%, above mean age=65.8%; sex: male=65.4%, female=60.0%; ethnicity: non-Hispanic=63.7%, Hispanic=59.2%; maternal education: less than college education=67.6%, college education or greater=59.7%), weight status (healthy weight=58.6%, overweight/obese=68.8%), or day of week (weekday=63.0%, weekend day=60.4%). EMA prompt compliance was not statistically different between those below the grand-mean versus above the grand-mean for interstitial glucose (56.3% vs. 69.3%), positive affect (63.4% vs. 59.3%), negative affect (64.5% vs. 58.1%), and fatigue (65.3% vs. 57.2%). Momentary EMA prompt compliance was related to the time of day such that participants were more likely to complete EMA prompts later in the day (OR=1.1 95%CI 1.0–1.1, p=0.02).
Participant age (OR=0.7, 95%CI 0.5–1.1, p=0.10), sex (OR=0.8, 95% CI 0.3–2.1, p=0.59), ethnicity (OR=0.8, 95% CI 0.3–2.1, p=0.65), maternal education (OR=0.6, 95% CI 0.2–1.7, p=0.30), and weight status (OR=0.9, 95% CI 0.3–2.6, p=0.88) were not associated with missing CGM data. CGM data were more likely to be missing on weekend days compared to weekdays (OR=4.2, 95% CI 2.9–6.1, p<0.0001); this is because participants usually began the observational period of the study on a weekend day when there was no school. Therefore, the CGM data values occurring on days with less than 24 hours of wear (e.g., the first observational day of the study) that were removed prior to analyses were more likely to occur on weekend days. Average participant interstitial glucose (OR=1.0, 95%CI 0.95–1.0, p=0.16), positive affect (OR= 0.7, 95% CI 0.4–1.2, p=0.19), negative affect (OR 0.7, 95% CI 0.2–2.1, p=0.58), and fatigue (OR 0.6, 95% CI 0.2–1.7, p=0.33) were not associated with missing CGM data.
Interstitial glucose and subsequent affective and physical feeling states
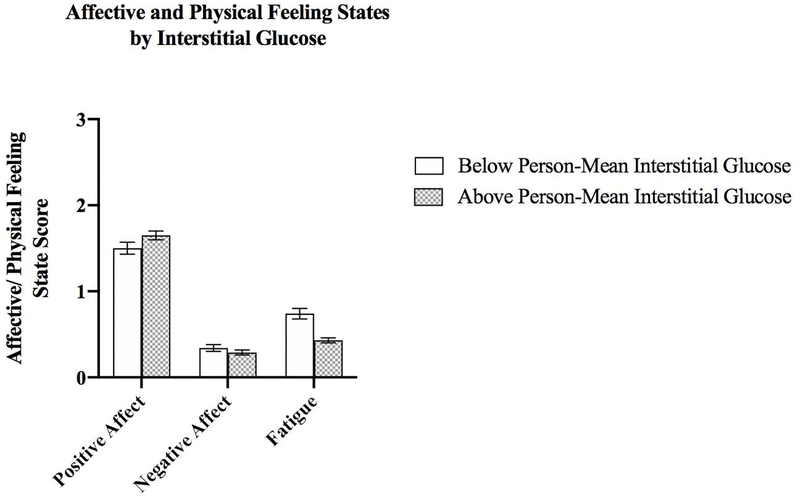
Figure 2 presents mean affective and fatigue scores stratified by occasions when participants were above and below their own mean interstitial glucose level. The mean (SE) positive affect score on occasions when participants were below their own mean interstitial glucose was 1.50 (0.07), compared to 1.65 (0.05) on occasions when participants had higher interstitial glucose than their own mean; an unadjusted multilevel model indicated this difference was significant (WS β=0.01, p<0.0001). The positive association between interstitial glucose and subsequent positive affect remained statistically significant after adjusting for a priori covariates (WS β=0.01, p<0.0001, f2=0.02; Table 2, Model A). Once models were further adjusted for time of day, the association was weakened, but remained significant (WS β=0.005, p=0.03, f2=0.00; Table 2, Model B).
Figure 2.
Mean (SE) affective and physical feeling state scores by within-subject glucose (L1 N=532, L2 N=15)
Table 2.
Multilevel model estimates (β [SE]) for CGM-measured glucose predicting EMA-reported affective and physical feeling states (L1 N=532, L2 N=15)
| Positive Affect | Negative Affect | Fatigue | |||||
|---|---|---|---|---|---|---|---|
| Model A | Model B | Model A | Model B | Model A | Model B | ||
| Fixed Effects | Glucose (WS) | 0.01 (0.002)*** | 0.005 (0.002)* | −0.002 (0.001) | −0.003 (0.002) | −0.01 (0.002)*** | −0.01 (0.002)** |
| Glucose (BS) | −0.02 (0.01)** | −0.02 (0.01)** | 0.01 (0.01) | 0.01 (0.01) | 0.01 (0.01)* | 0.01 (0.01)** | |
| Age | 0.43 (0.21) | 0.43 (0.21) | −0.12(0.12) | −0.12 (0.12) | −0.01 (0.12) | −0.01 (0.12) | |
| Sexa | 0.61 (0.74) | 0.60 (0.74) | 1.09 (0.43)* | 1.09 (0.43)* | 1.35 (0.41)** | 1.36 (0.40)** | |
| Ethnicityb | 0.38 (0.67) | 0.38 (0.67) | −1.01 (0.38)* | −1.01 (0.38)* | −1.12 (0.36)* | −1.12 (0.36)* | |
| BMI Percentile | −0.002 (0.01) | −0.002 (0.01) | −0.02 (0.01)* | −0.02 (0.01)* | −0.03 (0.01)** | −0.03 (0.01)** | |
| Maternal Educationc | 0.31 (0.72) | 0.30 (0.72) | −0.80 (0.41) | −0.81 (0.41) | −1.13 (0.39)* | −1.13(0.39)* | |
| Weekendd | −0.09 (0.06) | −0.02 (0.06) | 0.07 (0.05) | 0.08 (0.05) | 0.004 (0.05) | −0.05 (0.05) | |
| In-Lab Conditione | −0.11 (0.07) | −0.12 (0.07) | −0.02 (0.06) | −0.02 (0.06) | 0.08 (0.06) | 0.09 (0.06) | |
| Time of day | N/A | 0.08 (0.01)*** | N/A | 0.004 (0.01) | N/A | −0.07 (0.01)*** | |
| Random Effects | Intercept (τ00) | 0.53 (0.26) | 0.53 (0.26) | 0.17(0.09) | 0.17(0.09) | 0.15(0.08) | 0.15(0.08) |
| Autocorrelation | 0.17(0.05) | 0.17(0.05) | 0.30 (0.04) | 0.30 (0.04) | 0.13(0.05) | 0.15(0.05) | |
| Residual (σ2e) | 0.38 (0.03) | 0.36 (0.02) | 0.21 (0.01) | 0.21 (0.01) | 0.26 (0.02) | 0.24 (0.02) |
Female=1
Hispanic=1
College graduate or higher=1
Weekend day=1
SIT=1, participants were randomized to uninterrupted sitting or sitting+ walking condition (ClinicalTrials.gov registration no. NCT03153930)
Note. Model A adjusts for between-subject interstitial glucose, age, sex, Ethnicity, BMI percentile, maternal education, weekend, and in-lab condition. Model B includes the same covariates as Model A and further adjusts for time of day.
p<0.05
p<0.01
p<0.0001
The mean (SE) negative affect score when participants were below their own mean interstitial glucose was 0.34 (0.04) and 0.29 (0.03) when participants were above their own mean glucose level (Figure 2). The association between interstitial glucose and negative affect was not significant in the unadjusted (WS β=−0.002, p=0.09) or adjusted (WS β=−0.002, p=0.10, f2=0.01) models (Table 2, Model A), including the model additionally controlling for time of day (WS β=−0.003, p=0.11, f2=0.01; Table 2, Model B).
Mean (SE) fatigue ratings were higher on occasions when participants had lower (vs. higher) than usual interstitial glucose (0.74 [0.06] vs. 0.43 [0.03], respectively; Figure 2). Unadjusted (WS β=−0.01, p<0.0001) and adjusted (WS β=−0.01, p<0.0001, f2=0.09) model estimates were significant between interstitial glucose and subsequent fatigue score (Table 2, Model A). This association was also weakened, but remained significant following further adjustment for time of day (WS β=−0.01, p=0.002, f2=0.02; Table 2, Model B).
EMA-reported sleep quality, physical activity, sedentary behavior, dietary intake, and social context were not significant covariates and were therefore excluded from the models. Sensitivity analyses following the removal of the participant with the lowest EMA prompt compliance (32.5%) were conducted and results were unchanged. Model results from the sensitivity analyses using within-subject past 60-minute and past 30-minute interstitial glucose as predictors of current affective/physical feeling states are presented in Supplemental Tables 1 and 2; associations remained comparable to those presented in Table 2. Lastly, random slopes did not improve model fit for each affective/feeling state outcome, and therefore were not retained in the models.
Discussion
To our knowledge, this is the first study to examine the within-day associations between CGM-measured interstitial glucose and subsequent EMA-reported affective and physical feeling states in the free-living environment among adolescents. The exploratory findings of this pilot study indicate that deviations from one’s own mean interstitial glucose can acutely impact subsequent affective and physical feeling states. After occasions when interstitial glucose was higher than usual, participants reported greater positive affect and less fatigue via EMA; it is important to note, however, that effect sizes were small. Also noteworthy is that the strength of the associations between interstitial glucose and subsequent positive affect and fatigue were weakened after time of day was accounted for. Therefore, time of a day may be an important cofounder to consider when assessing free-living associations between interstitial glucose and affective/physical feeling states. We did not find associations between interstitial glucose and subsequently-reported negative affect in our sample. Our findings parallel experimental evidence where healthy participants reported feeling less tense and more energetic in the two-hour time period following the administration of a glucose drink [17]. However, our findings among our healthy population are in contrast to an observational study of adolescents with Type 1 diabetes, where higher average daily blood glucose was related to lower positive affect and higher negative affect [20].
Differential associations between glucose levels and affective and physical feeling states among individuals with diabetes compared to healthy individuals are expected, considering potential differences in the mechanisms linking these variables. The blood glucose-affective state relationship among those with diabetes could be mediated by perceived diabetes task competence and self-efficacy [20, 45]. Previous studies among individuals with diabetes (with higher average daily blood glucose compared to our healthy sample) suggest that elevated blood glucose levels may result in feelings of incompetence or reduced self-efficacy at diabetes self-management, leading to subsequently-induced negative affective states [20, 45]. Similarly, noncompliance with diabetes management behaviors related to negative outcome expectancies (e.g., poorer blood glucose control) [46, 47] may worsen negative affective states [48]. Therefore, we may not have observed interstitial glucose-negative affect associations because our sample of healthy adolescents may not associate higher-than-usual glucose or glucose-raising behaviors with negative outcomes, thus, affective states did not appear to worsen as glucose levels rose. However, an important consideration is our small sample size of healthy youth; future confirmatory studies should continue to test the relation between glucose and negative affect, particularly among at-risk populations such as those with pre-diabetes and diabetes. Within a normal range, higher glucose levels may not relate to negative affect, whereas when glucose levels are at a higher range (e.g., within patients with pre-diabetes or diabetes), hyperglycemia may be associated with negative affect.
Our findings suggest that affective and physical feeling states improve following time periods when interstitial glucose is higher than usual among healthy adolescents, which may be explained by neurological mechanisms. Glucose plays an integral role in brain functioning, and is the brain’s main energy source [49]. It is believed that glucose is responsible for the production of neurotransmitters and is a precursor for neurotransmitter synthesis in the long-term [50]. Acutely, blood glucose can also influence levels of dopamine in the nucleus accumbens [51], resulting in subjective feelings of well-being [52]. Similarly, when blood glucose is limited (e.g., experimentally induced hypoglycemia), reduced positive affect and increased fatigue are subsequently reported, even up to 30 minutes after the restoration of euglycemia [53]. The affective and physical feeling state changes following occasions where extremely low glucose is experienced may be attributed to neuroglycopenia, a shortage of glucose in the brain resulting in altered neuronal function [54]. Altogether, our findings of reduced fatigue following higher than usual interstitial glucose levels align with the expected physiologic responses in the brain. Future research should aim to experimentally test potential mechanisms further linking interstitial glucose and affective/physical feeling states.
The findings of the present study do not suggest that EMA-reported energy balance behaviors (e.g., physical activity, sedentary behavior, dietary intake) confound the associations between interstitial glucose levels and acute affective/physical feeling states. Other studies have shown that physical activity lowers glucose [55] and increases subjective experiences of positive affective states [12, 13]; whereas sedentary behaviors and the consumption of carbohydrate-rich foods raise glucose levels [56, 57] and often result in subsequently poorer affective states [14, 15]. Taken together with our findings that higher-than-usual interstitial glucose may relate to more positive affect and less fatigue, there is still a need for a greater understanding of how energy balance behaviors and biological factors may independently and interactively influence acute affective and physical feeling states. The interplay between behavioral and biological factors in influencing acute affective states and physical feeling states is likely to be complex and dynamic, which our study design may not have captured entirely. Future intensive longitudinal studies among larger level 1 and level 2 samples (to be powered to test mediation and moderation) should consider combining EMA, CGM, and objective activity monitors (accelerometers) to gain a better understanding of these complex relationships across different time periods ranging from hours to days. Furthermore, future EMA and CGM studies should consider using a sensor-informed, event-contingent EMA prompting schedule where participants are prompted to complete an EMA survey following occasions when glucose rises or falls below pre-specified thresholds; this approach may better at capturing the timing of associations compared the random sampling method employed in the current study.
Additionally, this study raises several other important methodological suggestions for future confirmatory studies of interstitial glucose-affective state associations. Observational studies combining EMA and CGM should attempt to gain a better understanding of the potential confounding/moderating role that time of day may play in interstitial glucose-affective state associations. After controlling for time of day, our significant associations were weakened. The present study treated time of day as linear, however it is plausible that time of day may influence the strength of glucose-affective state associations differently across the day; interstitial glucose and affective/physical feelings states may only be associated with one another during specific times throughout the day due to naturally-occurring, simultaneous, yet independent diurnal patterning. The sample size of the present study limited our ability to use more dynamic statistical models, such as time-varying effect models (TVEMs), which model associations across time non-parametrically [58]; future studies among larger samples should consider using TVEMs to increase our understanding of how time of day influences interstitial glucose-affective state associations. In addition, future studies should continue to test how the interstitial glucose time frame selection may influence the strength of associations observed. Although we tested time frames in interstitial glucose ranging from the past 30 to ~120 minutes, which each yielded comparable associations with affective/physical feeling states, it is still plausible that larger (or smaller timeframes) could impact the strength of associations observed. Lastly, future studies (particularly among more diverse samples, including clinical and nonclinical participants) should continue to test random slopes and account for the possibility that the strength and direction of associations could differ between participants.
There are several study strengths. The novel method of our pilot study demonstrates the scalability and feasibility of combining EMA and CGM in order to gain a better understanding of interstitial glucose and affective/physical feeling state associations as they occur naturally in the free-living environment. Additional research using these methodologies in larger and more diverse samples of adolescents, including those with Type 1 and Type 2 diabetes is warranted. Adolescence is a developmental period where glycemic control deteriorates [59], affective states become more variable and negative [60], and the prevalence of emotional disorders rises [4]; providing a unique developmental opportunity for investigators study the associations between interstitial glucose, affective and physical feeling states, and long-term emotional health risk.
This study is not without its limitations. Our sample of 15 adolescents limits the generalizability of our findings to other populations and our ability to draw between-person inferences (due to limited level 2 statistical power). Our findings cannot be extrapolated beyond the hours of 7am and 8pm (given restrictions to EMA prompting to only during non-school hours), participants were more likely to comply with EMA prompts later in the day, and our overall EMA compliance rate was slightly below the reported compliance rates among similar samples [61]. As with other EMA studies, we had large amounts of missing data that were not missing at random (due to noncompliance to EMA prompts), and therefore imputation strategies were not used [61]. To increase EMA prompt compliance in future studies, investigators should consider prompting participants fewer times per day over a longer study duration. Previous studies indicate that sampling frequency is inversely related to EMA prompt compliance among nonclinical samples of youth [61]. Other methods such as shortening the length of the EMA surveys themselves (asking participants fewer question during a given EMA prompt), monitoring EMA compliance during the observational period and sending out reminder texts if compliance drops below a certain threshold, and embedding engaging/entertaining content (news, jokes, etc.) into the EMA surveys may also be avenues for increasing EMA prompt compliance. Additionally, the composite affective and physical feeling state scores were calculated from only two to four items, however this is consistent with previous EMA studies to limit participant burden [12]. Due to our study sample/design, interstitial glucose data were more likely to be missing on weekend days compared to weekdays, limiting the generalizability of our findings to weekends. Future studies using CGM should begin the study observational periods on weekdays, rather than weekend days, considering that the first observational day of CGM data are typically removed. Lastly, we did not assess the potential bi-directionality of interstitial glucose-affective state associations. Future studies should consider assessing how interstitial glucose and affective states may influence each other over time using the methodological recommendations for combining EMA and CGM outlined above.
Conclusions
This pilot study suggests that variations from one’s own mean level of interstitial glucose were related to subsequently reported positive affect and fatigue, but were not related to negative affect among healthy, non-diabetic adolescents. After accounting for time of day, these associations persisted, but were weakened in strength. This study provides ecologically-valid exploratory evidence for biological factors potentially contributing acute affective experiences and physical feeling states, while simultaneously demonstrating the scalability of combining novel intensive longitudinal data capture strategies for future confirmatory studies among larger samples.
Supplementary Material
Highlights.
Ecological momentary assessment and continuous glucose monitors can be integrated
Interstitial glucose may relate to subsequent affective and physical feeling states
Adjusting for time of day can attenuate some of the above associations
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K01DK11306), the National Cancer Institute (T32CA009492), the National Heart, Lung, and Blood Institute (R01HL119255), and the University of Southern California Office of the Provost.
Footnotes
Competing Interests
The authors have no competing interests to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spear LP, The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews, 2000. 24(4): p. 417–463. [DOI] [PubMed] [Google Scholar]
- 2.Christie D and Viner R, Adolescent development. BMJ (Clinical research ed.), 2005. 330(7486): p. 301–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim-Cohen J, et al. , Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Archives of general psychiatry, 2003. 60(7): p. 709–717. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, et al. , Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry, 2005. 62(6): p. 593–602. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, et al. , Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry, 2005. 62(6): p. 617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessler RC, et al. , Prevalence, persistence, and sociodemographic correlates of DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry, 2012. 69(4): p. 372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson S, et al. , Age of onset and course of major depressive disorder: associations with psychosocial functioning outcomes in adulthood. Psychol Med, 2015. 45(3): p. 505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beesdo-Baum K and Knappe S, Developmental epidemiology of anxiety disorders. Child and Adolescent Psychiatric Clinics, 2012. 21(3): p. 457–478. [DOI] [PubMed] [Google Scholar]
- 9.Copeland WE, et al. , Adult functional outcomes of common childhood psychiatric problems: a prospective, longitudinal study. JAMA psychiatry, 2015. 72(9): p. 892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joiner TE Jr, Catanzaro SJ, and Laurent J, Tripartite structure of positive and negative affect, depression, and anxiety in child and adolescent psychiatric inpatients. Journal of Abnormal Psychology, 1996. 105(3): p. 401. [DOI] [PubMed] [Google Scholar]
- 11.Brown TA, Chorpita BF, and Barlow DH, Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. Journal of abnormal psychology, 1998. 107(2): p. 179. [DOI] [PubMed] [Google Scholar]
- 12.Dunton GF, et al. , Momentary Assessment of Affect, Physical Feeling States, and Physical Activity in Children. Health psychology : official journal of the Division of Health Psychology, American Psychological Association, 2014. 33(3): p. 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao Y, Shonkoff ET, and Dunton GF, The acute relationships between affect, physical feeling states, and physical activity in daily life: a review of current evidence. Frontiers in Psychology, 2015. 6: p. 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen CKF, et al. , Relationships among affective states, physical activity, and sedentary behavior in children: Moderation by perceived stress. Health Psychology, 2018. 37(10): p. 904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mason TB, et al. , Momentary affect, stress coping, and food intake in mother–child dyads. Health Psychology, 2019. 38(3): p. 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Micha R, Rogers PJ, and Nelson M, Glycaemic index and glycaemic load of breakfast predict cognitive function and mood in school children: a randomised controlled trial. British journal of nutrition, 2011. 106(10): p. 1552–1561. [DOI] [PubMed] [Google Scholar]
- 17.Benton D and Owens D, Is raised blood glucose associated with the relief of tension? Journal of psychosomatic research, 1993. 37(7): p. 723–735. [DOI] [PubMed] [Google Scholar]
- 18.Kohn N, et al. , In a sweet mood? Effects of experimental modulation of blood glucose levels on mood-induction during fMRI. Neuroimage, 2015. 113: p. 246–256. [DOI] [PubMed] [Google Scholar]
- 19.Penckofer S, et al. , Does glycemic variability impact mood and quality of life? Diabetes technology & therapeutics, 2012. 14(4): p. 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortenberry KT, et al. , Perceived diabetes task competence mediates the relationship of both negative and positive affect with blood glucose in adolescents with type 1 diabetes. Annals of Behavioral Medicine, 2009. 37(1): p. 1–9. [DOI] [PubMed] [Google Scholar]
- 21.Beedie CJ and Lane AM, The role of glucose in self-control: Another look at the evidence and an alternative conceptualization. Personality and Social Psychology Review, 2012. 16(2): p. 143–153. [DOI] [PubMed] [Google Scholar]
- 22.Gailliot MT and Baumeister RF, The physiology of willpower: Linking blood glucose to self-control. Personality and social psychology review, 2007. 11(4): p. 303–327. [DOI] [PubMed] [Google Scholar]
- 23.Shiffman S, Stone AA, and Hufford MR, Ecological momentary assessment. Annu Rev Clin Psychol, 2008. 4: p. 1–32. [DOI] [PubMed] [Google Scholar]
- 24.Olczuk D and Priefer R, A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 2018. 12(2): p. 181–187. [DOI] [PubMed] [Google Scholar]
- 25.Dunton GF, et al. , Investigating within-day and longitudinal effects of maternal stress on children’s physical activity, dietary intake, and body composition: Protocol for the MATCH study. Contemporary Clinical Trials, 2015. 43: p. 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laurent J, et al. , A measure of positive and negative affect for children: scale development and preliminary validation. Psychological assessment, 1999. 11(3): p. 326. [Google Scholar]
- 27.Crichton A, et al. , Fatigue in child chronic health conditions: a systematic review of assessment instruments. Pediatrics, 2015. 135(4): p. e1015–e1031. [DOI] [PubMed] [Google Scholar]
- 28.Bailey T, et al. , The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes technology & therapeutics, 2015. 17(11): p. 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paing AC, et al. , The associations of sedentary time and breaks in sedentary time with 24-hour glycaemic control in type 2 diabetes. Preventive medicine reports, 2018. 12: p. 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mikolajczyk RT, El Ansari W, and Maxwell AE, Food consumption frequency and perceived stress and depressive symptoms among students in three European countries. Nutrition Journal, 2009. 8(1): p. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gellar L and Nansel TR, High and low glycemic index mixed meals and blood glucose in youth with type 2 diabetes or impaired glucose tolerance. The Journal of pediatrics, 2009. 154(3): p. 455–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkins GP, et al. , Cardiometabolic Correlates of Physical Activity and Sedentary Patterns in U.S. Youth. Med Sci Sports Exerc, 2017. 49(9): p. 1826–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang S, et al. , Intra-and inter-individual differences in adolescent depressive mood: The role of relationships with parents and friends. Journal of abnormal child psychology, 2018. 46(4): p. 811–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arigo D, et al. , The social context of the relationship between glycemic control and depressive symptoms in type 2 diabetes. Chronic illness, 2015. 11(1): p. 33–43. [DOI] [PubMed] [Google Scholar]
- 35.Garcia C, et al. , L atina Adolescent Sleep and Mood: An Ecological Momentary Assessment Pilot Study. Journal of Child and Adolescent Psychiatric Nursing, 2014. 27(3): p. 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Botega NJ, et al. , Psychiatric morbidity among medical in-patients: a standardized assessment (GHQ-12 and CIS-R) using ‘lay’ interviewers in a Brazilian hospital. Soc Psychiatry Psychiatr Epidemiol, 1995. 30(3): p. 127–31. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Y, et al. , Association between sleep architecture and glucose tolerance in children and adolescents. Journal of diabetes, 2015. 7(1): p. 10. [DOI] [PubMed] [Google Scholar]
- 38.Raudenbush S and Bryk AS, A hierarchical model for studying school effects. Sociology of education, 1986: p. 1–17. [Google Scholar]
- 39.Raudenbush SW and Bryk AS, Hierarchical linear models: Applications and data analysis methods. Vol. 1 2002: Sage. [Google Scholar]
- 40.Kenny DA and Zautra A, The trait-state-error model for multiwave data. Journal of consulting and clinical psychology, 1995. 63(1): p. 52. [DOI] [PubMed] [Google Scholar]
- 41.Curran PJ and Bauer DJ, The disaggregation of within-person and between-person effects in longitudinal models of change. Annu Rev Psychol, 2011. 62: p. 583–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selya AS, et al. , A practical guide to calculating Cohen’s f2, a measure of local effect size, from PROC MIXED. Frontiers in psychology, 2012. 3: p. 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baghurst PA, Calculating the mean amplitude of glycemic excursion from continuous glucose monitoring data: an automated algorithm. Diabetes technology & therapeutics, 2011. 13(3): p. 296–302. [DOI] [PubMed] [Google Scholar]
- 44.Dasari PS, et al. , Glycemic variability is associated with markers of vascular stress in adolescents. The Journal of pediatrics, 2016. 172: p. 47–55. e2. [DOI] [PubMed] [Google Scholar]
- 45.Holmes CS, et al. , Predictors of youth diabetes care behaviors and metabolic control: a structural equation modeling approach. J Pediatr Psychol, 2006. 31(8): p. 770–84. [DOI] [PubMed] [Google Scholar]
- 46.Nouwen A, et al. , Longitudinal motivational predictors of dietary self-care and diabetes control in adults with newly diagnosed type 2 diabetes mellitus. Health Psychology, 2011. 30(6): p. 771. [DOI] [PubMed] [Google Scholar]
- 47.Carels RA, et al. , The relationship between self-monitoring, outcome expectancies, difficulties with eating and exercise, and physical activity and weight loss treatment outcomes. Annals of Behavioral Medicine, 2005. 30(3): p. 182–190. [DOI] [PubMed] [Google Scholar]
- 48.Carver CS and Scheier MF, Origins and functions of positive and negative affect: a control-process view. Psychological review, 1990. 97(1): p. 19. [Google Scholar]
- 49.van de Rest O, van der Zwaluw NL, and de Groot LC, Effects of glucose and sucrose on mood: a systematic review of interventional studies. Nutrition reviews, 2017. 76(2): p. 108–116. [DOI] [PubMed] [Google Scholar]
- 50.Mergenthaler P, et al. , Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends in neurosciences, 2013. 36(10): p. 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bello NT and Hajnal A, Alterations in blood glucose levels under hyperinsulinemia affect accumbens dopamine. Physiology & behavior, 2006. 88(1–2): p. 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blum K, Thanos PK, and Gold MS, Dopamine and glucose, obesity, and reward deficiency syndrome. Frontiers in psychology, 2014. 5: p. 919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gold AE, et al. , Changes in mood during acute hypoglycemia in healthy participants. Journal of personality and social psychology, 1995. 68(3): p. 498. [DOI] [PubMed] [Google Scholar]
- 54.Owens DS, Parker PY, and Benton D, Blood glucose and subjective energy following cognitive demand. Physiology & behavior, 1997. 62(3): p. 471–478. [DOI] [PubMed] [Google Scholar]
- 55.Cockcroft EJ, et al. , High intensity interval exercise is an effective alternative to moderate intensity exercise for improving glucose tolerance and insulin sensitivity in adolescent boys. J Sci Med Sport, 2015. 18(6): p. 720–4. [DOI] [PubMed] [Google Scholar]
- 56.Fletcher EA, et al. , Effects of breaking up sitting on adolescents’ postprandial glucose after consuming meals varying in energy: a cross-over randomised trial. Journal of science and medicine in sport, 2018. 21(3): p. 280–285. [DOI] [PubMed] [Google Scholar]
- 57.Ambrosini GL, et al. , Dietary patterns and markers for the metabolic syndrome in Australian adolescents. Nutrition, metabolism and cardiovascular diseases, 2010. 20(4): p. 274–283. [DOI] [PubMed] [Google Scholar]
- 58.Li R, et al. , TVEM (Time-Varying Effect Modeling) SAS Macro Users’ Guide. 2015.
- 59.Helgeson VS, et al. , Brief report: trajectories of glycemic control over early to middle adolescence. Journal of pediatric psychology, 2010. 35(10): p. 1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silvers JA, et al. , Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion (Washington, D.C.), 2012. 12(6): p. 1235–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wen CKF, et al. , Compliance With Mobile Ecological Momentary Assessment Protocols in Children and Adolescents: A Systematic Review and Meta-Analysis. Journal of Medical Internet Research, 2017. 19(4): p. e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.