Introduction
Alopecia areata (AA) is considered an autoimmune condition of unknown etiology. One inciting event may be a breakdown of the immune privilege of the hair follicle that allows for autoreactive T cells to cause inflammatory hair loss. We present an unusual case of alopecia totalis after the use of permanent hair dye.
Case report
A 60-year-old man presented with sudden onset of patchy hair loss after coloring his hair at home using a permanent hair dye. He had used the same dye one time prior without problems. After the second application, there was mild burning on the scalp but no other problems. However, after 2 weeks, large patches of hair loss appeared suddenly (Fig 1). A dermatologist diagnosed AA, and the patient was started on intralesional kenalog, topical clobetasol, and minoxidil. The patient denied personal or family history of AA or other autoimmune disorders. Three weeks after kenalog injections, there was worsening hair loss, and after online research, the patient decided to perform a “use test” on his arm with the dye that caused the hair loss. He subsequently had severe contact dermatitis of his hands, arm, and face but no additional hair loss on the arm. The extensive dermatitis along with worsening hair loss prompted his dermatologist to start oral prednisone (60 mg × 7 days and 40 mg × 7 days), which resolved the contact dermatitis but failed to improve the AA.
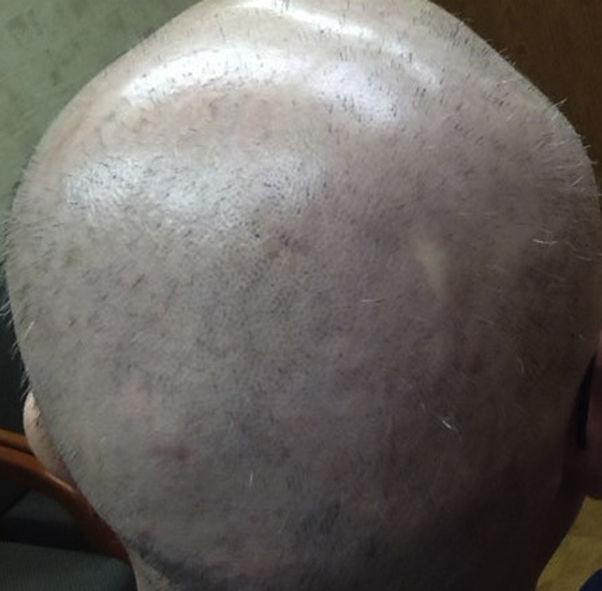
Fig 1.
Patchy nonscarring AA on initial presentation.
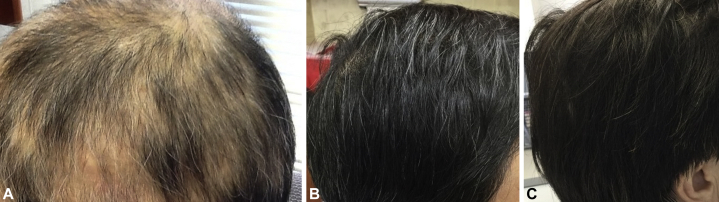
The patient was referred to our office where we found alopecia totalis with exclamation point hairs but normal eyebrow, eyelash, and beard hair (Fig 2). Given the lack of hair growth after 1 course of intralesional kenalog and oral prednisone, we initiated oral cyclosporine 300 mg daily. Although new hair regrowth occurred within 1 week, the patient became concerned about the potential risk of cyclosporine and stopped the medication. He requested an alternative treatment, and we offered contact sensitization with diphenylcyclopropenone (DPCP). Initial sensitization with 2% DPCP solution was started. The patient did not follow up for DPCP treatment but returned 3 weeks later after reinitiating cyclosporine on his own. Continued hair regrowth was noted with cyclosporine, but follow-up laboratory values showed elevations in creatinine after 2 months. The patient was started on sulfasalazine, 500 mg twice a day, while tapered off cyclosporine. He continued to have new hair growth, and the sulfasalazine dose was increased to 1500 mg (Fig 3, A). Complete regrowth of hair occurred within 7 months (Fig 3, B). Lastly, although the patient was advised never to color his hair again, he elected to try a “safe and natural” hair dye. He has successfully colored his hair with “pure” henna (red henna [Lawsonia inermis] and black henna [Indigofera tinctoria]) without paraphenylenediamine (PPD) (Fig 3, C).
Fig 2.
Alopecia totalis with exclamation point hairs 1 month after initial hair loss.
Fig 3.
A, Partial regrowth of hair at 3 months after starting treatment with cyclosporine. B, Complete regrowth of hair following treatment with cyclosporine and sulfasalazine. C, Hair colored with henna.
Discussion
This is a case of a persistent patient who did not always follow the treatment plan and shows an unusual case of alopecia totalis following the use of permanent black hair dye. The current molecular etiology of AA is based on activation of natural killer gene 2D expressing CD8+ T helper cell (Th) type 1 and Th17 populations in response to an unknown hair follicle antigen following disruption of hair follicle immune privilege. In association, there are elevated levels of inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interferon gamma (INF-γ), and interleukin (IL)-17, IL-2, and IL-15. In comparison, contact or type IV hypersensitivity reaction, while also a predominantly Th1-type reaction, appears to be mainly mediated by activated CD4+ T cells with upregulation of IL-2, IL-8, IL-10, TNF-α and IFN-γ. Thus, there are both overlapping and distinct cell populations and cytokines mediating the 2 inflammatory conditions.
Type IV hypersensitivity has not been shown to cause AA. In fact, AA is often treated with contact sensitization using squaric acid dibutylester or DPCP. The underlying efficacy of contact sensitization may involve antigenic competition or cytokine inhibition, whereby an exaggerated hypersensitivity reaction paradoxically elicits suppressor T cells or cytokines that inhibit the primary autoimmune reaction.1 Although it is still unclear what effect any particular cytokine plays in the suppression of AA during contact sensitization, increases in IL-10, a Th2 cytokine, can inhibit the Th1 responses in AA.2 In addition, elevations in TNF-α and TGF-β can diminish the Th1 response in AA.3
It is not clear why contact sensitivity to hair dye was associated with onset of AA in our patient. The most common sensitizer in hair dye is PPD. Both CD4+ and CD8+ T cells producing IFN, IL-17, and IL-22 are believed to be involved in contact dermatitis related to PPD.4 The “use test” performed by our patient showed classic type IV hypersensitivity reaction to the hair dye. However, he initially had only patchy hair loss without skin inflammation consistent with AA, which was confirmed by biopsy. The current theory of AA theorizes that disruption of the immune privilege of the hair follicle normally present in the anagen follicle but disrupted during normal catagen may inadvertently expose the immune system to hair follicle antigens that in genetically susceptible persons can trigger AA.5 Our patient had irritation with the hair dye, and this may have been sufficient to disrupt the normal hair follicle immune privilege, which may have increased the risk of AA. A previous study found skin barrier disruption with down-regulation of tight junction and stratum corneum proteins after PPD exposure.6
Although most patients with AA can have spontaneous regrowth of hair, our patient had rapid regrowth of hair with cyclosporine followed by sulfasalazine. Cyclosporine is found to decrease T-cell activation and has been used to treat both AA contact dermatitis.7,8 The patient was successfully transitioned to sulfasalazine, which showed 25.6% response in a study of 39 AA patients.9 Sulfasalazine has been found to induce apoptosis in a human T-lymphocyte cell line and in human peripheral blood T lymphocytes.10
We present an unusual case of alopecia totalis associated with concurrent hypersensitivity to permanent hair dye and discuss a brief overview of the complex etiology of AA. The hair loss was successfully treated with cyclosporine and sulfasalazine.
Footnotes
Funding sources: none.
Conflicts of interest: None disclosed.
References
- 1.Freyschmidt-Paul P., Happle R., McElwee K.J., Hoffman R. Alopecia areata: treatment of today and tomorrow. J Invest Dermatol Symp Proc. 2003;8:12–17. doi: 10.1046/j.1523-1747.2003.12165.x. [DOI] [PubMed] [Google Scholar]
- 2.Fiorentino D.F., Bond M.W., Mosmann T.R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckley D.A., Du Vivier A.W. Therapeutic use of topical contact sensitizers in benign dermatoses. Br J Dermatol. 2001;145:385–405. doi: 10.1046/j.1365-2133.2001.04399.x. [DOI] [PubMed] [Google Scholar]
- 4.Gibson A., Kim S.H., Faulkner L. In vitro priming of naıve T-cells with p-phenylenediamine and Bandrowski's base. Chem Res Toxicol. 2015;28:2069–2077. doi: 10.1021/acs.chemrestox.5b00294. [DOI] [PubMed] [Google Scholar]
- 5.McElwee K.J., Gilhar A., Tobin D.J. What causes alopecia areata? Exp Dermatol. 2013;2:609–626. doi: 10.1111/exd.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meisser S.S., Altunbulakli C., Bandier J. Skin barrier damage after exposure to para-phenylenediamine. J Allergy Clin Immunol. 2020;145:619–631. doi: 10.1016/j.jaci.2019.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Sung C.T., McGowan M.A., Machler B.C., Jacob S.E. Systemic treatments for allergic contact dermatitis: the final frontier. Dermatitis. 2019;30:46–53. doi: 10.1097/DER.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 8.Lai V.W.Y., Chen G., Gin D., Sinclair R. Cyclosporin for moderate-to-severe alopecia areata: a double-blind, randomized, placebo-controlled clinical trial of efficacy and safety. J Am Acad Dermatol. 2019;81:694–701. doi: 10.1016/j.jaad.2019.04.053. [DOI] [PubMed] [Google Scholar]
- 9.Rashidi T., Mahd A.A. Treatment of persistent alopecia areata with sulfasalazine. Int J Dermatol. 2008;47:850–852. doi: 10.1111/j.1365-4632.2008.03700.x. [DOI] [PubMed] [Google Scholar]
- 10.Liptay S., Fulda S., Schanbacher M. Molecular mechanisms of sulfasalazine induced T-cell apoptosis. Br J Pharmacol. 2002;137:608–620. doi: 10.1038/sj.bjp.0704870. [DOI] [PMC free article] [PubMed] [Google Scholar]