Summary
Identification of safe and effective compounds to increase or activate UCP1 expression in brown or white adipocytes remains a potent therapeutic strategy to combat obesity. Here we reported that, glyburide, one of the FDA-approved drugs currently used to treat type 2 diabetes, can significantly enhance UCP1 expression in both brown and white adipocytes. Glyburide-fed mice exhibited a clear resistance to high-fat diet-induced obesity, reduced blood triglyceride level, and increased UCP1 expression in brown adipose tissue. Moreover, in situ injection of glyburide to inguinal white adipose tissue remarkably enhanced UCP1 expression and increased thermogenesis. Further mechanistic studies indicated that the glyburide effect in UCP1 expression in adipocytes was KATP channel independent but may involve the regulation of the Ca2+-Calcineurin-NFAT signal pathway. Overall, our findings revealed the significant effects of glyburide in regulating UCP1 expression and thermogenesis in adipocytes, which can be potentially repurposed to treat obesity.
Subject Areas: Human Metabolism, Molecular Biology
Graphical Abstract
Highlights
-
•
Glyburide significantly upregulated UCP1 expression in both brown and white adipocytes
-
•
Glyburide-fed mice exhibited a clear resistance to high-fat diet-induced obesity
-
•
The glyburide effect in UCP1 expression in adipocytes was KATP independent
-
•
Glyburide effect in UCP1 level was reversed by the Calcineurin-NFAT pathway inhibition
Human Metabolism; Molecular Biology
Introduction
Obesity is becoming a global pandemic as a result of sustained energy intake exceeding energy expenditure under a modern lifestyle. The discovery that the brown and beige adipose tissues can burn energy to heat instead of energy storing highlights pharmacological activation of brown adipose tissue or induction of beige adipose tissue as promising strategies to target obesity (Inagaki et al., 2016; Harms and Seale, 2013; Betz and Enerback, 2018). The energy burning properties of brown or beige adipocytes lies mostly in the expression of UCP1 (uncoupling protein 1), a protein that is highly expressed in brown and beige adipocytes and localized on the inner mitochondrial membrane. When activated, UCP1 uncouples oxidative respiration from ATP synthesis, resulting in heat generation (Fedorenko et al., 2012). Therefore, UCP1 serves as a promising molecular target to thermogenesis enhancement and obesity treatment. Although plenty of compounds have been reported to increase UCP1 expression in adipocytes (Andrade et al., 2014; Van Dam et al., 2015; Zhang et al., 2014; Lu et al., 2016; Feng et al., 2019), safe and effective compounds that can be used clinically are still lacking.
We have previously developed a cellular screening platform with the Ucp1-2A-GFP reporter cell lines, which allows high-throughput screenings using a fluorescence signal as readout (Qiu et al., 2018). Using this reporter line, we have identified a group of drugs that can upregulate UCP1 expression in brown adipocytes and reported sutent as an effective modulator of UCP1 expression in brown adipose tissue (Qiu et al., 2018). Sutent belongs to the tyrosine kinase (RTK) inhibitor family and has been used clinically to treat certain kidney, pancreatic, and gastrointestinal cancers. Although sutent treatment did not cause obvious adverse effect in our study in animals, side effects observed in patients renders it impossible for immediate repurposing to treat obesity.
It is very interesting that Glyburide, one of the drugs used in lowering blood glucose, has shown a strong effect in upregulating UCP1 in brown adipocytes in our screening. Glyburide is a sulfonylurea drug, also known as glibenclamide in Europe, which can directly bind to and block the SUR1 subunits of ATP-dependent potassium channels (KATP) and consequently increase insulin secretion from the pancreatic β cells. Glyburide has been approved to treat type 2 diabetes clinically (Malek and Davis, 2016), which has also been repurposed for the treatment of acute central nervous system injury recently (King et al., 2018).
Previous studies have indicated an interesting aspect of ions channels and the electrophysiological modulation in the regulation of thermogenesis activity in brown and beige adipocytes. For example, KCNK3, a two-pore-domain potassium channel, which promotes outward K+ current and limits Ca2+ influx, has been shown to affect thermogenesis in brown and beige adipocytes. KCNK3 is highly expressed in brown and beige adipose and is directly regulated by PRDM16. KCNK3 impairs thermogenesis via antagonizing NE-induced membrane depolarization and Ca2+ influx to decrease cAMP production (Chen et al., 2017; Pisani et al., 2016). TRPV2, a Ca2+-permeable non-selective cation channel, was also reported to play vital roles in brown adipose tissue (Sun et al., 2016). Lack of TRPV2 impaired thermogenesis in mouse brown adipose tissue. It is still unclear how TRPV2 activation modulates thermogenesis; however, the induction of thermogenic genes in response to β3-adrenergic receptor stimulation was decreased in TRPV2KO brown adipocytes and suppressed by reduced intracellular Ca2+ concentrations in wild-type brown adipocytes.
Combining the previous strong indication of glyburide in upregulating UCP1 in brown adipocytes and the interest whether the effect of glyburide is through KATP channel inhibiting, we decided to focus on the functional study of glyburide in adipocytes and to explore the underlying mechanism.
Results
Glyburide Enhanced UCP1 Expression in Adipocytes In Vitro
Our previous screening suggested strong upregulating effect of glyburide in UCP1 expression in brown adipocytes. We first validated the effect of glyburide in in vitro cultured brown adipocytes. Initially, mature brown adipocytes were treated with 10 μM glyburide at day 8 after the maintenance stage for 1 day (Figure 1A). The lipid content was not changed after glyburide treatment, indicating glyburide had no effect on adipocyte differentiation (Figure 1B). Consistent with the high-throughput screening results, glyburide treatment significantly increased UCP1 expression in brown adipocytes, at both the mRNA level and the protein level, compared with cells treated with DMSO (Figures 1C and 1D). Glyburide treatment also stimulated expression of other thermogenic or brown differentiation genes, including Cidea, Pgc1a, Ppara, and Prdm16 (Figure 1D). To know whether the glyburide effect is dependent on treatment time point, we then treated cells with glyburide for 3 days at the maintenance stage or 8 days along the whole differentiation process (Figures S1A and S1F). Under these two conditions, cells treated with glyburide both displayed increased UCP1 expression (Figures S1B–S1D and S1G–S1I) and enhanced expression of other thermogenic or brown differentiation genes (Figures S1C and S1H), although with no significant difference as compared with 1-day treatment at day 8 (Figures 1C and 1D) and with no significant change on adipocyte differentiation either (Figures S1E and S1J). Additionally, UCP1 expression was also significantly increased in day 8 brown adipocytes after glyburide treatment for as short as 4 h, indicating that glyburide stimulates UCP1 expression in a very short time in mature brown adipocytes (Figures S1K and S1L).
Figure 1.
Glyburide Promoted UCP1 Expression in Both Brown and White Adipocytes
(A) Schematic view of the brown adipocyte differentiation procedure and compound treatment protocol. Cells were treated with 10 μM glyburide or DMSO for 1 day after 7 days' differentiation and collected at day 8 for analysis.
(B) Assessment of lipid accumulation (evaluated by Oil Red O staining) in glyburide or DMSO-treated Ucp1-2A-GFP brown adipocytes. Scale bar, 200 μm.
(C) Western blot analysis of UCP1 protein expression in glyburide or DMSO-treated Ucp1-2A-GFP brown adipocytes.
(D) mRNA expression analyses of genes in glyburide or DMSO-treated Ucp1-2A-GFP brown adipocytes. n = 4.
(E) Gene Ontology analyses of regulated genes in brown adipocytes after glyburide treatment identified by RNA-seq.
(F) Schematic view of the white adipocyte differentiation procedure and compound treatment protocol. Cells were treated with 10 μM glyburide or DMSO for 4 days after 6 days' differentiation and collected at day 10 for analysis.
(G) Assessment of lipid accumulation (evaluated by Oil Red O staining) in glyburide or DMSO-treated Ucp1-2A-GFP white adipocytes. Scale bar, 200 μm.
(H) Western blot analysis of UCP1 protein expression in glyburide or DMSO-treated Ucp1-2A-GFP white adipocytes.
(I) mRNA expression analyses of genes in glyburide or DMSO-treated Ucp1-2A-GFP white adipocytes. n = 3.
(J) Gene Ontology analyses of regulated genes in white adipocytes after glyburide treatment identified by RNA-seq.
(K) Venn analysis of upregulated genes from brown and white adipocytes after glyburide treatment.
(L) Heatmap depicting upregulated genes related to lipid metabolism in brown and white adipocytes after glyburide treatment.
Bars show mean ± SEM. p Values are shown for indicated comparisons by the Student's t test. ∗p < 0.05, ∗∗p < 0.01. See also Figures S1 and S2.
We next tested the effect of glyburide in cultured white adipocytes. Mature white adipocytes were treated with glyburide for 4 days (Figure 1F), and the lipid accumulation was not changed after glyburide treatment (Figure 1G). We noticed significant increase in UCP1 expression upon glyburide treatment at both the mRNA and protein levels (Figures 1H and 1I). We also observed increased expression of other thermogenic genes, including Cidea and Pgc1a, indicating the induction of a browning process in white adipocytes (Figure 1I). Moreover, glyburide effect was also tested after treatment in the whole white adipocyte differentiation process. Cells were treated with glyburide for 8 days during the whole differentiation process for 8 days (Figure S2A). Results demonstrated that glyburide treatment significantly improved UCP1 expression (Figures S2B–S2D), and with a stronger UCP1 induction effect after longer time treatment (Figure S2D), with no significant change on adipocyte differentiation (Figure S2E).
To get a better understanding of glyburide effect, we next performed RNA sequencing (RNA-seq) for glyburide or DMSO-treated brown and white adipocytes. Gene ontology (GO) analysis revealed that glyburide treatment in brown adipocytes led to significant upregulation of in total 505 genes using a cutoff of p ≤ 0.05, which were enriched for lipid metabolic process, fat cell differentiation, and thermogenesis (Figure 1E). A total of 404 genes were upregulated in white adipocytes after glyburide treatment, which were also significantly enriched in lipid metabolic process, brown fat differentiation, oxidation reduction process, and others (Figure 1J). Among these upregulated genes, 100 genes were common genes regulated in both brown and white adipocytes after glyburide treatment (Figure 1K), including important regulators in lipid metabolism, such as Fgf21, Elovl3, Ppara, and Scd3 (Figure 1L). Altogether, these data demonstrated that glyburide treatment promoted UCP1 and thermogenesis gene expression in both brown and white adipocytes.
Glyburide Enhanced UCP1 Expression in Adipose Tissues In Vivo
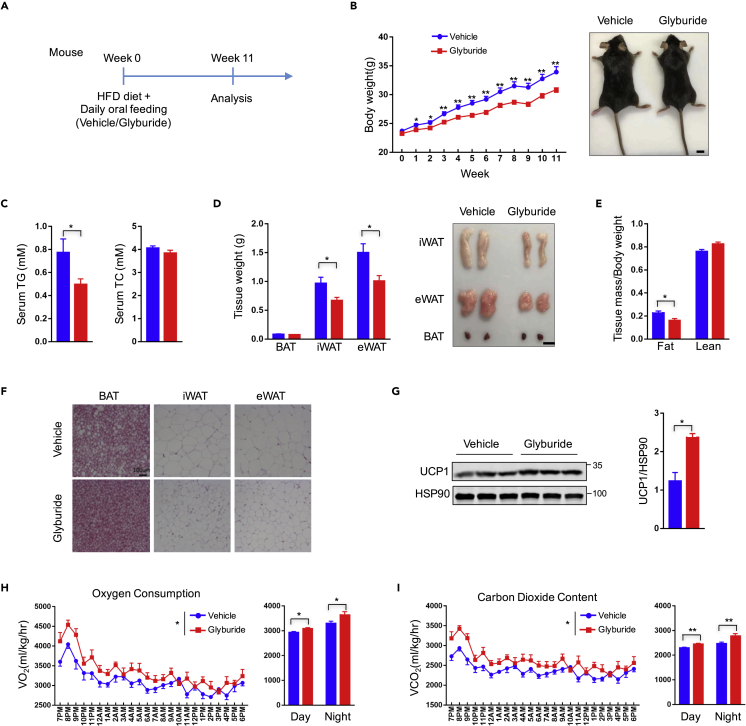
We next investigated the effect of glyburide treatment in vivo. Mice were fed with high-fat diet (HFD) and treated with glyburide at the dose of 2 mg/kg/day through oral administration for 11 week (Figure 2A). Glyburide treatment caused significant body weight loss (−9% after 11-week treatment) (Figure 2B), whereas no significant change was observed in food intake (Figure S3A). Serum analyses showed a clear reduction of total triglyceride (TG) (−35.92%), with no significant change of total cholesterol (TC) or free fatty acid contents in mice treated with glyburide (Figures 2C and S3B). Consistent with body weight loss, organ weights of BAT (brown adipose tissue, −9.76%), eWAT (epididymal white adipose tissue, −32.97%), and iWAT (inguinal white adipose tissue, −30.6%) were reduced significantly in glyburide-treated mice (Figure 2D). The reduction in body and organ weights were mostly caused by decrease in fat mass (−29.52%) as revealed by the NMR analysis (Figure 2E). Furthermore, histological analyses of adipose tissues displayed significantly reduced adipocyte size in BAT, eWAT, and iWAT (Figure 2F), although no significant change was observed in liver triglyceride or liver cholesterol content (Figure S3C). Consistent with the results in cultured cells, glyburide treatment resulted in significant increase of UCP1 expression in brown adipose tissue (Figure 2G). Metabolic analysis displayed a minor increase in O2 consumption after glyburide treatment, if calculated with normalization to body weight (Figures 2H and 2I); however, no significant change in net O2 consumption was observed (Figures S3G and S3H). In the meantime, there was minimal change in physical activity (Figure S3D). Besides, expression levels of other thermogenic genes, including Cidea, Dio2, Pgc1a, Ppara, and Prdm16, in brown adipose tissue were not changed after glyburide treatment (Figures S3E and S3F). Altogether, these results indicated that the phenotypic changes observed in mice after glyburide treatment are at least partly caused by improved UCP1 expression in brown adipose tissue.
Figure 2.
Glyburide Treatment Promoted Weight Loss of Mice under HFD
(A) Schematic view of mouse experiments. Mice were administrated 2 mg/kg glyburide or vehicle through daily oral feeding under HFD for 11 weeks.
(B) Body weights of mice treated with glyburide or vehicle under HFD (left) and representative image of mouse treated with glyburide or vehicle 11 weeks later (right). n = 12. Scale bar, 1 cm.
(C) Serum total triglyceride (TG) and total cholesterol (TC) content of mice measured after 11-week treatment with glyburide or vehicle. n = 12.
(D) Weights of different mouse adipose tissues (left) and representative fat images of different mouse adipose tissues (right) after 11-week treatment with glyburide or vehicle. n = 12. Scale bar, 1 cm.
(E) Body lean and fat composition of mice determined by NMR after 10-week treatment with glyburide or vehicle. n = 12.
(F) Representative HE staining images of BAT, iWAT, and eWAT. Scale bar, 100 μm.
(G) Western blot analysis of UCP1 protein expression level in mouse brown adipose tissues.
(H) Oxygen consumption measurement of mice in metabolic cages after 11-week treatment with glyburide or vehicle. n = 8.
(I) CO2 content measurement of mice in metabolic cages after 11-week treatment with glyburide or vehicle. n = 8.
Bars show mean ± SEM. p Values are shown for indicated comparisons by the Student's t test (B–E and G) or two-way ANOVA (H and I). ∗p < 0.05, ∗∗p < 0.01. See also Figures S3 and S4.
We also treated mice with a lower dose of 50 μg/kg/day via oral administration, which is more relevant to the therapeutic glyburide doses used clinically (Remedi and Nichols, 2008). Low-dose glyburide treatment did not cause significant change in either body weight or body composition in mice under HFD (Figures S4A and S4C). Besides, low-dose glyburide treatment led to no significant change in energy expenditure, as reflected by minimal change of O2 consumption and CO2 content, with the comparable physical activity (Figures S4E and S4G). Food intake monitoring did not show significant change after glyburide treatment (Figure S4B). Serum analysis indicated no significant change in total cholesterol or in total triglyceride level in mice treated with glyburide under HFD (Figure S4D). Mice were then dissected for further investigation. Overall, there was no significant change in total organ weight or adipocytes sizes in BAT, eWAT, or iWAT (Figures S4F and S4H). We then tested the insulin and blood glucose responses of animals to different doses of glyburide via oral administration. We found that 2 mg/kg glyburide caused a marked increase of insulin release and rapid drop of blood glucose level, whereas 50 μg/kg glyburide had no effect on the insulin and blood glucose contents of mice (Figures S4I and S4J), suggesting different drug sensitivity of glyburide between human beings and mice.
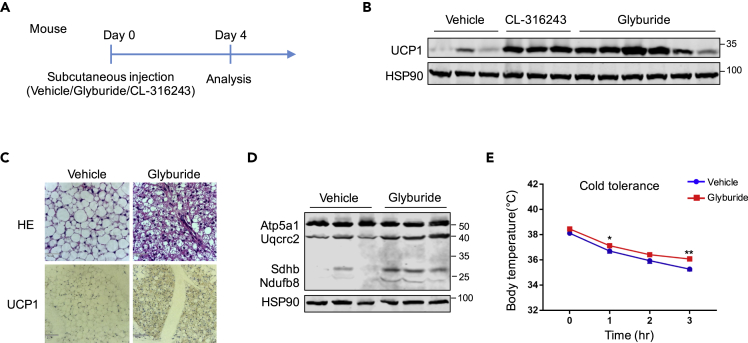
We suspected that the reason we did not observe a significant phenotypic change in animals after low-dose treatment of glyburide is because the drug cannot reach the adipose tissues, especially when delivered via oral administration. We thus next tested the effect of glyburide directly through in situ injection to inguinal white adipose depot. CL-316243 injection was used as a positive control. Four days after a single injection, mice were dissected and inguinal adipose tissues were obtained for UCP1 expression analysis (Figure 3A). Similar to CL-316243 treatment, we observed a remarkable increase in UCP1 expression in inguinal white adipose depot after glyburide treatment (Figure 3B). HE and UCP1 staining also displayed a clear browning phenomenon in inguinal adipose tissue, as indicated by smaller lipid droplets and enhanced UCP1 expression (Figure 3C). In consistency with increased UCP1 expression, levels of the OXPHOS proteins were also increased in glyburide-treated white adipose tissues (Figure 3D). Moreover, glyburide-treated mice displayed higher rectal temperature in the cold challenge experiment (Figure 3E). These results altogether demonstrated that glyburide treatment promoted white adipose browning in vivo.
Figure 3.
Glyburide Treatment Improved UCP1 Expression in Inguinal White Adipose Tissue
(A) Schematic view of mouse experiments. Mice were administrated 1 mg/kg glyburide, 1 mg/kg CL-316243, or vehicle through subcutaneous injection. Four days after a single injection, mice were dissected and inguinal white adipose tissues were obtained for further analysis.
(B) UCP1 protein expression analyses of inguinal white adipose tissue after compound treatment with as indicated.
(C) Representative images of H&E staining and UCP1 staining of inguinal white adipose tissue after glyburide or vehicle treatment. Scale bar, 210 μm.
(D) OXPHOS protein expression analyses in inguinal white adipose tissue of mice treated with glyburide or vehicle.
(E) Cold challenge experiment with vehicle or glyburide-treated mice. n = 8. Bars show mean ± SEM. p Values are shown for indicated comparisons by the Student's t test. ∗p < 0.05, ∗∗p < 0.01.
The Effect of Glyburide in UCP1 Upregulation Was KATP Independent
Glyburide is a second-generation sulfonylurea drug that has been used to treat patients with type 2 diabetes mellitus. It stimulates insulin secretion through the closure of ATP-sensitive potassium channels (KATP) on pancreatic β cells, raising intracellular calcium ion concentration (Gribble and Reimann, 2003). The KATP channel is a heteroctameric complex composed of four inwardly rectifying pore-forming K+ channel subunits (Kir6.x; Kir6.1 or Kir6.2) and four regulatory sulfonylurea receptor SUR ATP-binding cassettes subunits (SUR; SUR1 or SUR2) (Nichols, 2006). Diverse classes of KATP channels exist, which differ from each other in different tissues and cell types in terms of their nucleotide sensitivities, their biophysical properties, and their sensitivities to pharmacological agents (Foster and Coetzee, 2016). In pancreatic β cells Kir6.2/SUR1 are the major subunits expressed, in cardiac myocytes Kir6.2/SUR2A subunits, in smooth muscles SUR2B, whereas in adipose tissue Kir6.1/SUR2B are the major forms (Szeto et al., 2018). To directly assess whether KATP channel is involved in UCP1 upregulation by glyburide, we next knocked out either Kir subunits or SUR subunits of KATP channels using the CRISPR-Cas technology in adipocytes. We screened individual sgRNAs targeting Kir6.1, Kir6.2, Sur1, as well as Sur2 genes. All sgRNAs demonstrated high editing activity in brown adipocytes (Figure 4A). However, we did not observe significant change of UCP1 expression either at the mRNA level or the protein level after depleting two Kir genes (Kir-sgRNAs: Kir6.1-sgRNA and Kir6.2-sgRNA) or two Sur genes (Sur-sgRNAs: Sur1-sgRNA and Sur2-sgRNA) in brown adipocytes. In the meantime, glyburide treatment still upregulated UCP1 expression in Kir-sgRNAs and Sur-sgRNAs cells, showing no significant difference in comparison with the effect in control cells (Figures 4B–4D). These results demonstrated that, although glyburide was supposed to target KATP channel, it stimulated the expression of UCP1 in adipocytes in a KATP channel-independent manner. Moreover, Epac2, which is a guanine nucleotide exchange factor for the small guanosine triphosphatase Rap1, was reported to be another direct target of antidiabetic sulfonylurea drugs (Zhang et al., 2009). We therefore next tested whether glyburide increases UCP1 expression via targeting Epac2. We screened an sgRNA targeting the second exon of the mouse Epac2 gene, which showed high editing activity (Figure S5A). However, we did not observe significant increase of UCP1 expression in Epac2-KO brown adipocytes, and meanwhile, Epac2-KO did not abrogate the effect of glyburide in upregulating UCP1 expression (Figures S5B, S5C, and S5F).
Figure 4.
Glyburide Increased UCP1 Expression Independent of KATP Blockade
(A) Schematic view of CRISPR sgRNAs design for indicated genes and genome editing activity of selected sgRNAs as indicated.
(B) Western blot analyses of indicated proteins in CRISPR sgRNAs-treated brown adipocytes.
(C) Ucp1 mRNA expression analyses of brown adipocytes treated with CRISPRs and compounds as indicated. n = 3.
(D) UCP1 protein expression analyses of brown adipocytes treated with CRISPRs and compounds as indicated.
(E) The suggested target(s) and structure information of selected compounds.
(F) UCP1 protein expression analyses of brown adipocytes treated with compounds (10 μM) as indicated.
(G) Ucp1 mRNA expression analyses of brown adipocytes treated with compounds (10 μM) as indicated. n = 3.
(H) Ucp1 mRNA expression analyses of brown adipocytes treated with compounds as indicated. n = 3.
(I) UCP1 protein expression analyses of white adipocytes treated with compounds as indicated.
Bars show mean ± SEM. p Values are shown for indicated comparisons by the Student's t test. ∗p < 0.05, ∗∗p < 0.01. See also Figure S5.
To further confirm whether the glyburide effect in UCP1 upregulation is KATP independent or Epac2 independent, we chose another four compounds that can also target KATP or/and Epac2 (Figure 4E). However, only glyburide treatment was able to increase UCP1 expression in brown adipocytes, with little effect from other compounds (Figures 4F and 4G). Furthermore, treatment with a combination of four compounds had no effect in UCP1 expression or the UCP1 upregulation by glyburide (Figure 4H). Similarly, only glyburide, but none of the other four KATP inhibitors, was able to increase UCP1 expression in white adipocytes (Figure 4I). Taken together, these results suggest a unique feature of glyburide that upregulates UCP1 expression, which is KATP independent and Epac2 independent.
Caveolin was previously reported to be involved in the action of glimepiride, a sulfonylurea drug with very close structure with glyburide. Caveolin was tyrosine phosphorylated in isolated rat adipocytes in response to glimepiride treatment, playing important functions in transmembrane signal transduction (Muller, 2000; Muller et al., 2005). Moreover, caveolin-1 (Cav1) is expressed abundantly in adipose tissue and involved in many physiological processes. 3T3-L1 adipocytes overexpressing Cav1 were better differentiated (Chang et al., 2017). We thus next wanted to know whether the effect of glyburide is regulated through Cav1. We also targeted the mouse Cav1 gene using the CRISPR technology (Figure S5D). However, we did not observe significant change in UCP1 expression in Cav1-KO brown adipocytes with or without glyburide treatment (Figures S5E and S5F).
The Effect of Glyburide in UCP1 Upregulation Was Reversed by the Inhibition of the Ca2+-Calcineurin-NFAT Signal Pathway
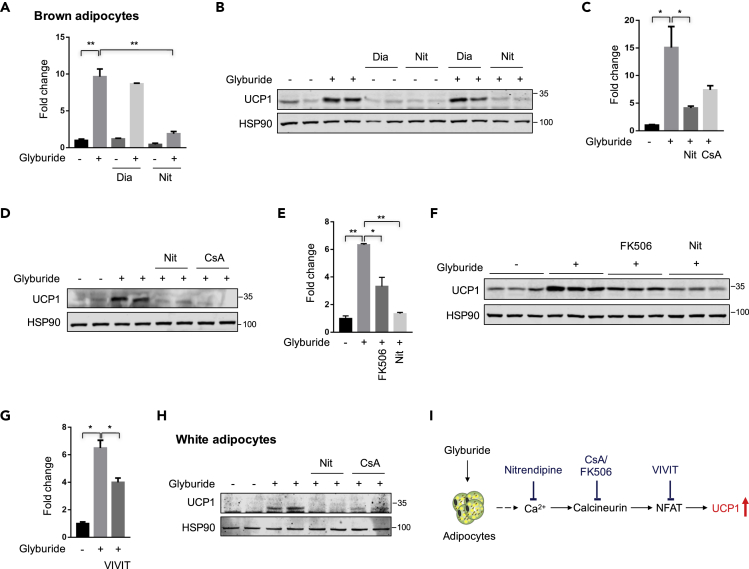
Glyburide can increase cytosolic Ca2+ levels in isolated rat adipocytes in a concentrated manner, which was blocked by nitrendipine, an L-type Ca2+ channel antagonist (Draznin et al., 1987, 1988). We thus treated the cells with nitrendipine to test whether the glyburide effect in UCP1 expression was mediated by the increase in intracellular Ca2+ levels. We also tested cells with another compound, diazoxide, a potassium channel activator, which was also reported to counteract the effect of glyburide in adipocytes (Shi et al., 1999). Results showed that nitrendipine (Nit) significantly blocked the UCP1 expression induced by glyburide treatment. However, diazoxide (Dia) did not cause significant change in UCP1 expression under basal level or upon glyburide treatment (Figures 5A and 5B), further confirming that the glyburide effect in UCP1 upregulating is not dependent on potassium channels but may involve regulation from intracellular Ca2+ levels. Transient receptor potential vanilloid 2 (TRPV2), a Ca2+-permeable non-selective cation channel, was reported to have critical roles in thermogenesis in mouse brown adipose tissue (Sun et al., 2016). We next tested whether the glyburide effect in UCP1 expression was regulated by the TRPV2 channel. We targeted the mouse Trpv2 gene using the CRISPR technology (Figure S5G). However, we did not observe significant change of UCP1 expression in Trpv2-KO brown adipocytes, with or without glyburide treatment (Figures S5H and S5I), suggesting that the glyburide upregulated UCP1 was not through TRPV2.
Figure 5.
Glyburide's Effect in UCP1 Upregulation Was Blocked by the Inhibition of Ca2+-Calcineurin-NFAT Signal Pathway
(A) Ucp1 mRNA expression analyses of brown adipocytes treated with compounds as indicated. n = 3.
(B) UCP1 protein expression analyses of brown adipocytes treated with compounds as indicated.
(C) Ucp1 mRNA expression analyses of brown adipocytes treated with compounds as indicated. n = 3.
(D) UCP1 protein expression analyses of brown adipocytes treated with compounds as indicated.
(E) Ucp1 mRNA expression analyses of brown adipocytes treated with compounds as indicated. n = 3.
(F) UCP1 protein expression analyses of brown adipocytes treated with compounds as indicated.
(G) Ucp1 mRNA expression analyses of brown adipocytes treated with compounds as indicated. n = 3.
(H) UCP1 protein expression analyses of white adipocytes treated with compounds as indicated.
(I) The schematic diagram of a working mechanism that glyburide regulates UCP1 expression in adipocytes. Compounds used for treatment were as following: 10 μM Glyburide, 10 μM Diazoxide (Dia), 30 μM Nitrendipine (Nit), 10 μM Cyclosporine A (CsA), 10 μM FK506 (Calcineurin inhibitor), 10 μM VIVIT (NFAT inhibitor). Bars show mean ± SEM. p Values are shown for indicated comparisons by the Student's t test. ∗p < 0.05, ∗∗p < 0.01.
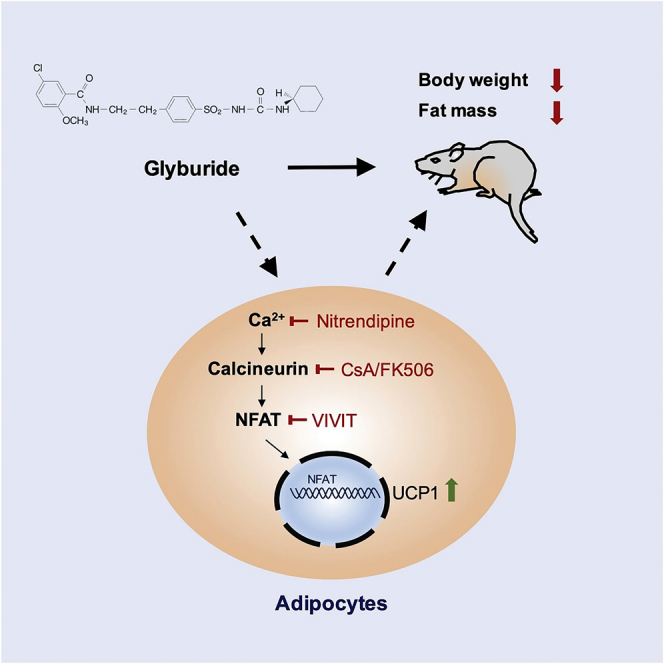
We next further tested whether glyburide upregulated UCP1 via the Ca2+-Calcineurin-NFAT signaling (Crabtree and Schreiber, 2009) by adding the calcineurin inhibitor, cyclosporine A (CsA) or FK506, to glyburide-treated brown adipocytes. Results showed that, similar to nitrendipine, both CsA and FK506 treatment effectively blocked the glyburide effect in UCP1 elevation (Figures 5C–5F). Moreover, the NFAT inhibitor, VIVIT, which is a cell-permeable peptide inhibitor of nuclear factor of activated T cells (NFAT) that selectively inhibits calcineurin-mediated dephosphorylation of NFAT (Ma et al., 2019), also significantly affected the increase of Ucp1 expression induced by glyburide treatment (Figure 5G). Similarly, in white adipocytes, the UCP1 upregulation upon glyburide treatment was also blocked by calcineurin inhibition (Figure 5H). Altogether, these data suggested that the glyburide effect in UCP1 upregulation could be reversed by the inhibition of Ca2+-Calcineurin-NFAT signal pathway (Figure 5I).
Discussion
Taken together, our study has identified that glyburide, a drug used in clinical treatment of type 2 diabetes, can effectively promote UCP1 expression in brown and white adipocytes, both in vitro and in vivo. The mechanism of UCP1 upregulation, however, is not mediated by inhibition of the KATP channel, a well-known target of glyburide. Although the direct target(s) of glyburide in upregulating UCP1 in adipocytes is still unknown, our results suggested the involvement of the Ca2+-Calcineurin-NFAT signal pathway in the underlying mechanism.
In our in vivo investigations, we found that, when treated with a high dose, glyburide can significantly decrease the gain of whole-body weight due to HFD feeding, and reduce blood triglyceride levels, at least partly due to improved UCP1 expression in brown adipose tissue. However, decreased body weight and blood lipids have not been observed in clinical patients taking glyburide. We suspect that the reason is mainly because the rather low efficiency of glyburide reaching adipose tissues by oral administration. This is similar to animal experiments treated with a low dose of glyburide, in which no obvious phenotypic change was observed. High dose of glyburide will certainly cause severe and life-threatening side effect as low blood glucose; it is therefore unacceptable to increase the dose in patients for the achievement of weight loss effect. As our results demonstrated that in situ injection of glyburide directly to the white adipose depots in animals had a strong effect in UCP1 upregulation and thermogenesis enhancement, different approaches for local adipose tissue delivering, for example, local delivery through a microneedle patch (Zhang et al., 2017), is essentially the way to go.
The effect of glyburide in adipocytes is not limited to the increase of UCP1 expression, but glyburide imposes a broad effect to the expression of many genes involved in lipid metabolism and thermogenesis, as revealed by the RNA-seq analyses. It is impressive that glyburide treatment led to a significant increase in Fgf21 expression in both brown and white adipocytes. FGF21 is a protein with multiple metabolic protective reactions, and indeed a bright star on the metabolic disease therapeutic horizon. In fact, several clinical trials are ongoing focusing on FGF21 therapy to metabolic diseases (Sanyal et al., 2019; Gaich et al., 2013; Kim et al., 2017). Our discovery that glyburide can boost the expression of Fgf21 in adipocytes revealed another beneficial aspect of glyburide treatment, besides the UCP1 upregulation and its long-term safety in vivo. However, in a different aspect, to what extent the upregulation of UCP1 explains in the whole phenotypic change upon glyburide treatment would need further investigation, ideally with the Ucp1 knockout animals.
The direct target(s) of glyburide in adipocyte is intriguing and remains undefined. Glyburide is known to stimulate insulin secretion through closure of the KATP channel in the pancreatic β cells; however, our results suggest that the KATP channel is not its target in adipocytes in regulating UCP1 expression. The direct comparison results of glyburide with other KATP targeting compounds also confirmed the conclusion and suggested different target(s) responsible for the effect of glyburide in UCP1 regulation. Our initial tests with several inhibitors suggest that the Ca2+-Calcineurin-NFAT signaling pathway may involve in the effect of glyburide. The Ca2+-Calcineurin-NFAT signaling pathway can transmit signals to the nucleus from a wide variety of receptors and is important to developmental events such as axon outgrowth, cardiac morphogenesis, and immune responses (Crabtree and Schreiber, 2009). Previous evidence indicated that the Regulator of Calcineurin 1 (RCAN1), a feedback inhibitor of the calcium-activated protein phosphatase calcineurin, was a negative regulator of UCP1 expression in white adipose tissue. Mice lacking Rcan1 were resistant to diet-induced obesity (Rotter et al., 2018). Besides, several reports also pointed out the possible important regulating function of NFAT in adipocyte biology. For example, mice deficient for Nfatc2 and Nfatc4 were protected from diet-induced obesity (Yang et al., 2006). And in human, polymorphisms for NFATc4 gene may confer certain protection or predisposition for the development of new-onset diabetes after kidney allograft transplantation (Chen et al., 2012). Also, genome-wide association studies (GWASs) indicated that the NFATc1, NFATc2, and NFATc4 loci are associated with a variety of metabolic traits (Ramos et al., 2014). However, to what extent the glyburide effects in adipocytes depend on the Ca2+-Calcineurin-NFAT signaling pathway is worth further investigation. Despite these unknowns, the findings that glyburide can increase UCP1 expression in adipocytes both in vitro and in vivo may shed light on new treatment of obesity and related metabolic diseases.
Limitations of the Study
In the present study, we showed that glyburide effect in UCP1 upregulation was KATP independent but may involve the regulation of Ca2+-Calcineurin-NFAT signaling pathway. However, further investigations are required to characterize the detailed mechanisms. Additionally, Ucp1 knockout mice will be helpful to establish the physiological relevance of UCP1 upregulation with the phenotypic change upon glyburide treatment in animals.
Resource Availability
Lead Contact
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Qiurong Ding (qrding@sibs.ac.cn).
Materials Availability
All unique materials generated from this study are available from the Lead Contact with a complete Materials Transfer Agreement.
Data and Code Availability
Raw data were deposited in NCBI Sequence Read Archive (SRA) with the accession number: PRJNA645978.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by grants from the National Key R&D Program of China (2017YFA0102800, 2017YFA0103700), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16030402), and the National Natural Science Foundation of China (31670829, 31971063).
Author Contributions
Y.Q. and Q.D. conceived and designed research studies, analyzed the data, prepared figures, and wrote the manuscript. Y.Q. performed all the experiments. Y.Y., Y.W., X.L., Z.F., X.Z., Y.C., and L.C. assisted with the mouse studies. Y.L., Y.Z., and L.L. provided advice. Q.D. supervised the project.
Declaration of Interests
The authors declare no competing interests.
Published: September 25, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101446.
Supplemental Information
References
- Andrade J.M., Frade A.C., Guimaraes J.B., Freitas K.M., Lopes M.T., Guimaraes A.L., De Paula A.M., Coimbra C.C., Santos S.H. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur. J. Nutr. 2014;53:1503–1510. doi: 10.1007/s00394-014-0655-6. [DOI] [PubMed] [Google Scholar]
- Betz M.J., Enerback S. Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease. Nat. Rev. Endocrinol. 2018;14:77–87. doi: 10.1038/nrendo.2017.132. [DOI] [PubMed] [Google Scholar]
- Chang C.C., Chen C.Y., Wen H.C., Huang C.Y., Hung M.S., Lu H.C., Chen W.L., Chang C.H. Caveolin-1 secreted from adipose tissues and adipocytes functions as an adipogenesis enhancer. Obesity (Silver Spring) 2017;25:1932–1940. doi: 10.1002/oby.21970. [DOI] [PubMed] [Google Scholar]
- Chen Y., Sampaio M.S., Yang J.W., Min D., Hutchinson I.V. Genetic polymorphisms of the transcription factor NFATc4 and development of new-onset diabetes after transplantation in Hispanic kidney transplant recipients. Transplantation. 2012;93:325–330. doi: 10.1097/TP.0b013e31823f7f26. [DOI] [PubMed] [Google Scholar]
- Chen Y., Zeng X., Huang X., Serag S., Woolf C.J., Spiegelman B.M. Crosstalk between KCNK3-mediated ion current and adrenergic signaling regulates adipose thermogenesis and obesity. Cell. 2017;171:836–848.e13. doi: 10.1016/j.cell.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree G.R., Schreiber S.L. SnapShot: Ca2+-calcineurin-NFAT signaling. Cell. 2009;138:210–210.e1. doi: 10.1016/j.cell.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draznin B., Kao M., Sussman K.E. Insulin and glyburide increase cytosolic free-Ca2+ concentration in isolated rat adipocytes. Diabetes. 1987;36:174–178. doi: 10.2337/diab.36.2.174. [DOI] [PubMed] [Google Scholar]
- Draznin B., Sussman K.E., Eckel R.H., Kao M., Yost T., Sherman N.A. Possible role of cytosolic free calcium concentrations in mediating insulin resistance of obesity and hyperinsulinemia. J. Clin. Invest. 1988;82:1848–1852. doi: 10.1172/JCI113801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko A., Lishko P.V., Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151:400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z., Wei Y., Zhang Y., Qiu Y., Liu X., Su L., Liang N., Yin H., Ding Q. Identification of a rhodanine derivative BML-260 as a potent stimulator of UCP1 expression. Theranostics. 2019;9:3501–3514. doi: 10.7150/thno.31951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster M.N., Coetzee W.A. KATP channels in the cardiovascular system. Physiol. Rev. 2016;96:177–252. doi: 10.1152/physrev.00003.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaich G., Chien J.Y., Fu H., Glass L.C., Deeg M.A., Holland W.L., Kharitonenkov A., Bumol T., Schilske H.K., Moller D.E. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013;18:333–340. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Gribble F.M., Reimann F. Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003;46:875–891. doi: 10.1007/s00125-003-1143-3. [DOI] [PubMed] [Google Scholar]
- Harms M., Seale P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- Inagaki T., Sakai J., Kajimura S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat. Rev. Mol. Cell Biol. 2016;17:480–495. doi: 10.1038/nrm.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A.M., Somayaji V.R., Dong J.Q., Rolph T.P., Weng Y., Chabot J.R., Gropp K.E., Talukdar S., Calle R.A. Once-weekly administration of a long-acting fibroblast growth factor 21 analogue modulates lipids, bone turnover markers, blood pressure and body weight differently in obese people with hypertriglyceridaemia and in non-human primates. Diabetes Obes. Metab. 2017;19:1762–1772. doi: 10.1111/dom.13023. [DOI] [PubMed] [Google Scholar]
- King Z.A., Sheth K.N., Kimberly W.T., Simard J.M. Profile of intravenous glyburide for the prevention of cerebral edema following large hemispheric infarction: evidence to date. Drug Des. Devel Ther. 2018;12:2539–2552. doi: 10.2147/DDDT.S150043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P., Zhang F.C., Qian S.W., Li X., Cui Z.M., Dang Y.J., Tang Q.Q. Artemisinin derivatives prevent obesity by inducing browning of WAT and enhancing BAT function. Cell Res. 2016;26:1169–1172. doi: 10.1038/cr.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.D., Jing J., Wang J.W., Mo Y.Q., Li Q.H., Lin J.Z., Chen L.F., Shao L., Miossec P., Dai L. Activation of the peroxisome proliferator-activated receptor gamma coactivator 1beta/NFATc1 pathway in circulating osteoclast precursors associated with bone destruction in rheumatoid arthritis. Arthritis Rheumatol. 2019;71:1252–1264. doi: 10.1002/art.40868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek R., Davis S.N. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin. Drug Metab. Toxicol. 2016;12:691–699. doi: 10.1080/17425255.2016.1187131. [DOI] [PubMed] [Google Scholar]
- Muller G. The molecular mechanism of the insulin-mimetic/sensitizing activity of the antidiabetic sulfonylurea drug Amaryl. Mol. Med. 2000;6:907–933. [PMC free article] [PubMed] [Google Scholar]
- Muller G., Schulz A., Wied S., Frick W. Regulation of lipid raft proteins by glimepiride- and insulin-induced glycosylphosphatidylinositol-specific phospholipase C in rat adipocytes. Biochem. Pharmacol. 2005;69:761–780. doi: 10.1016/j.bcp.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Nichols C.G. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- Pisani D.F., Beranger G.E., Corinus A., Giroud M., Ghandour R.A., Altirriba J., Chambard J.C., Mazure N.M., Bendahhou S., Duranton C. The K+ channel TASK1 modulates beta-adrenergic response in brown adipose tissue through the mineralocorticoid receptor pathway. FASEB J. 2016;30:909–922. doi: 10.1096/fj.15-277475. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Sun Y., Xu D., Yang Y., Liu X., Wei Y., Chen Y., Feng Z., Li S., Reyad-ul Ferdous M. Screening of FDA-approved drugs identifies sutent as a modulator of UCP1 expression in brown adipose tissue. EBioMedicine. 2018;37:344–355. doi: 10.1016/j.ebiom.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos E.M., Hoffman D., Junkins H.A., Maglott D., Phan L., Sherry S.T., Feolo M., Hindorff L.A. Phenotype-Genotype Integrator (PheGenI): synthesizing genome-wide association study (GWAS) data with existing genomic resources. Eur. J. Hum. Genet. 2014;22:144–147. doi: 10.1038/ejhg.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remedi M.S., Nichols C.G. Chronic antidiabetic sulfonylureas in vivo: reversible effects on mouse pancreatic beta-cells. PLoS Med. 2008;5:e206. doi: 10.1371/journal.pmed.0050206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter D., Peiris H., Grinsfelder D.B., Martin A.M., Burchfield J., Parra V., Hull C., Morales C.R., Jessup C.F., Matusica D. Regulator of Calcineurin 1 helps coordinate whole-body metabolism and thermogenesis. EMBO Rep. 2018;19:e44706. doi: 10.15252/embr.201744706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal A., Charles E.D., Neuschwander-Tetri B.A., Loomba R., Harrison S.A., Abdelmalek M.F., Lawitz E.J., Halegoua-Demarzio D., Kundu S., Noviello S. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet. 2019;392:2705–2717. doi: 10.1016/S0140-6736(18)31785-9. [DOI] [PubMed] [Google Scholar]
- Shi H., Moustaid-Moussa N., Wilkison W.O., Zemel M.B. Role of the sulfonylurea receptor in regulating human adipocyte metabolism. FASEB J. 1999;13:1833–1838. doi: 10.1096/fasebj.13.13.1833. [DOI] [PubMed] [Google Scholar]
- Sun W., Uchida K., Suzuki Y., Zhou Y., Kim M., Takayama Y., Takahashi N., Goto T., Wakabayashi S., Kawada T. Lack of TRPV2 impairs thermogenesis in mouse brown adipose tissue. EMBO Rep. 2016;17:383–399. doi: 10.15252/embr.201540819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto V., Chen N.H., Sun H.S., Feng Z.P. The role of KATP channels in cerebral ischemic stroke and diabetes. Acta Pharmacol. Sin. 2018;39:683–694. doi: 10.1038/aps.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam A.D., Nahon K.J., Kooijman S., Van Den Berg S.M., Kanhai A.A., Kikuchi T., Heemskerk M.M., Van Harmelen V., Lombes M., Van Den Hoek A.M. Salsalate activates brown adipose tissue in mice. Diabetes. 2015;64:1544–1554. doi: 10.2337/db14-1125. [DOI] [PubMed] [Google Scholar]
- Yang T.T., Suk H.Y., Yang X., Olabisi O., Yu R.Y., Durand J., Jelicks L.A., Kim J.Y., Scherer P.E., Wang Y. Role of transcription factor NFAT in glucose and insulin homeostasis. Mol. Cell Biol. 2006;26:7372–7387. doi: 10.1128/MCB.00580-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.L., Katoh M., Shibasaki T., Minami K., Sunaga Y., Takahashi H., Yokoi N., Iwasaki M., Miki T., Seino S. The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science. 2009;325:607–610. doi: 10.1126/science.1172256. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liu Q., Yu J., Yu S., Wang J., Qiang L., Gu Z. Locally induced adipose tissue browning by microneedle patch for obesity treatment. ACS Nano. 2017;11:9223–9230. doi: 10.1021/acsnano.7b04348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Zhang H., Li B., Meng X., Wang J., Zhang Y., Yao S., Ma Q., Jin L., Yang J. Berberine activates thermogenesis in white and brown adipose tissue. Nat. Commun. 2014;5:5493. doi: 10.1038/ncomms6493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data were deposited in NCBI Sequence Read Archive (SRA) with the accession number: PRJNA645978.