Summary
Integration of transforming growth factor β (TGF-β) signals with those of other pathways allows for precise temporal and spatial control of gene expression patterns that drive development and homeostasis. The Hippo pathway nuclear effectors, Taz/Yap, interact with the TGF-β transcriptional mediators, Smads, to control Smad activity. Key to TGF-β signaling is the nuclear localization of Smads. Thus, to investigate the role of Taz/Yap in Smad nuclear accumulation, we developed mathematical models of Hippo and TGF-β cross talk. The models were based on experimental measurements of TGF-β-induced changes in Taz/Yap and Smad subcellular localization obtained using high-throughput immunofluorescence (IF) imaging in the mouse mammary epithelial cell line, EpH4. Bayesian MCMC DREAM parameter estimation was used to quantify the uncertainty in estimates of the kinetic parameters. Variation of the model parameters and statistical analysis show that our modeling predicts that Taz/Yap can alter TGF-β receptor activity and directly or indirectly act as nuclear retention factors.
Subject Areas: Biological Sciences, Cell Biology, Computational Bioinformatics, Integrative Aspects of Cell Biology, Molecular Network
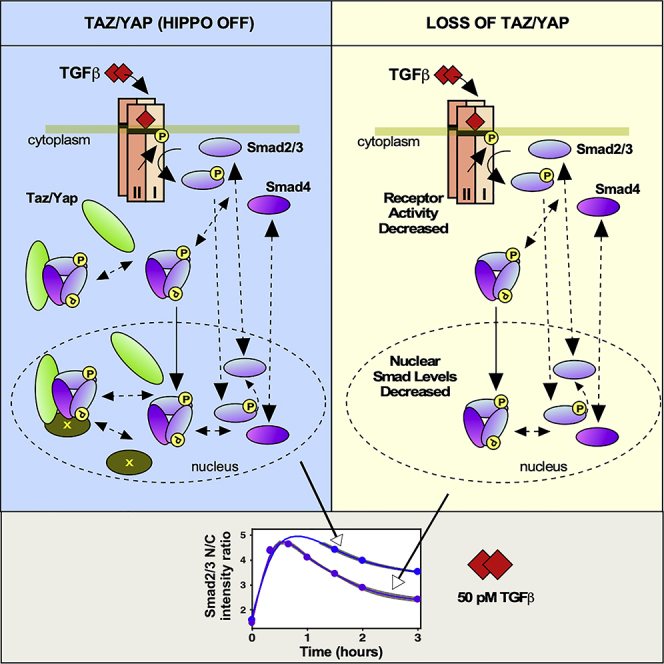
Graphical Abstract
Highlights
-
•
Taz/Yap modulate TGF-β-induced nuclear accumulation of Smad2/3 and Smad4
-
•
TGF-β does not affect Taz/Yap localization when Hippo activity is constant
-
•
Taz/Yap loss may alter activity of both Receptor and Smad nuclear retention factors
-
•
The mediator complex regulates Smad nuclear accumulation
Biological Sciences; Cell Biology; Computational Bioinformatics; Integrative Aspects of Cell Biology; Molecular Network
Introduction
Signal transduction pathways translate extracellular information to define cell outcomes. Signaling cross talk allows for plasticity and versatility in cellular responses and is essential for coordinating complex biological processes such as tissue patterning (McNeill and Woodgett, 2010; Attisano and Wrana, 2013; Beyer et al., 2013). Transforming growth factor β (TGF-β) superfamily members control a myriad of cellular activities including cell growth, cell differentiation, apoptosis, cellular homeostasis, and other cellular functions (Attisano and Wrana, 2002; Shi and Massague, 2003; Hata and Chen, 2016; Hill, 2016; Budi et al., 2017). TGF-β superfamily members are noted for their function as morphogens, in which gradients of ligand control the magnitude and timing of target gene activation that ultimately establishes cell fate. TGF-β ligand initiates canonical TGF-β signaling, which results in receptor activation. Smad2/3 interact with and are phosphorylated by receptor type I and then form a complex with Smad4, the pSmad2/3-Smad4 complex. The pSmad2/3-Smad4 complex translocates into the nucleus, binds to various proteins and DNA to regulate transcription (Attisano and Wrana, 2002; Shi and Massague, 2003; Feng and Derynck, 2005; Hata and Chen, 2016; Hill, 2016; Budi et al., 2017).
Proper development requires that cells also integrate signals from other pathways, such Hippo, a major regulator of tissue growth and organ size (Genevet and Tapon, 2011; Halder and Johnson, 2011; Barry and Camargo, 2013; Park and Guan, 2013; Yu et al., 2015; Misra and Irvine, 2018; Ma et al., 2019). Cues such as high cell density activate the Hippo pathway in which a core kinase cassette phosphorylates the transcriptional regulators, Taz/Yap, driving their cytoplasmic retention and inhibiting their transcriptional activities. Hippo and TGF-β pathways are intimately interconnected (Mauviel et al., 2012; Attisano and Wrana, 2013; Beyer et al., 2013). Taz/Yap interact with activated Smads, and when Hippo is active, cytoplasmically localized Taz/Yap binds and inhibits Smad nuclear accumulation and dampens TGF-β-induced transcription (Varelas et al., 2008; Varelas et al., 2010; Beyer et al., 2013; Narimatsu et al., 2015; Maiwald et al., 2016; Narimatsu et al., 2016). The key factor in TGF-β Smad signaling pathway is Smad nuclear accumulation. Indeed, precise nuclear concentration of Smads determines which genes are turned on and thus results in the specification of diverse cell fates (Gurdon and Bourillot, 2001; Schmierer and Hill, 2007; Strasen et al., 2018). However, the regulation of Smad nuclear accumulation is still poorly understood (Clarke et al., 2006; Clarke and Liu, 2008). Two hypotheses have been put forth to explain nuclear accumulation of Smads. In one, it is postulated that the different forms of Smads have different kinetics of nuclear import and export, such that the phosphorylated Smads accumulate in the nucleus (Schmierer and Hill, 2005; Hill, 2009). The second proposes that there are retention factors in the nucleus that have a higher affinity for phosphorylated Smads (Hoodless et al., 1999; Xu et al., 2002; Kang et al., 2003; Nicolás et al., 2004; ten Dijke and Hill, 2004; Xu, 2006; Hill, 2009). Thus, to understand signaling cross talk between Taz/Yap-mediated Hippo and TGF-β Smad signaling pathways, it is first necessary to investigate the role of Taz/Yap in Smad nuclear accumulation. However, given the many variables, gaining a broad understanding of how TGF-β/Hippo cross talk alters Smad nuclear accumulation is extremely challenging using only biological approaches.
The interplay of mathematical modeling with experiments is one of the central elements in systems biology, an approach that has been applied to the study of TGF-β/Smad signaling (Clarke et al., 2006; Zi and Klipp, 2007; Clarke and Liu, 2008; Schmierer et al., 2008; Zi et al., 2011; Strasen et al., 2018). Indeed, computational modeling of signaling cross talk can provide insights into the complex process and accept or reject proposed scenarios. A core part of mathematical modeling is estimating unknown parameters. The main problems for modeling of biological systems are uncertain parameters and noisy measurements, and so nonlinear optimization methods may not perform well to overcome the issues of trapping in local minima and convergence of the parameter estimation algorithm (Villaverde and Banga, 2014; Vrugt, 2016). Another option is Bayesian inference, which is based on the application of Bayes' theorem, which states that the posterior distribution of a parameter is proportional to the parameter's prior distribution multiplied by a likelihood function. Bayesian methods, and particularly Markov Chain Monte Carlo (MCMC) techniques, are extremely useful in uncertainty assessment and parameter estimation of biological models (Stuart, 2010; Kirk et al., 2016). Many MCMC approaches have been implemented to solve Bayesian inference problems (Craiu and Rosenthal, 2014). Among them, the scheme entitled Differential Evolution Adaptive Metropolis or DREAM has shown to be generally superior to other MCMC sampling approaches in various cases involving nonlinearity, high-dimensionality, and multimodality (Vrugt et al., 2008a; Vrugt et al., 2009). The DREAM strategy runs multiple different Markov chains simultaneously for global exploration and automatically tunes the scale and orientation of the proposal distribution in randomized subspaces during the search (Vrugt, 2016). This strategy deals very well with uncertainty and, if designed appropriately, avoids sensitivity to initial values and resolves convergence problem. In addition, it gives probability distributions instead of single values for unknown parameters, which makes comparing the parameters more meaningful (Vrugt et al., 2008a; Vrugt et al., 2009).
As experimental data are not perfect and models contain many unknown parameters, the structural identifiability of models must be investigated (Raue et al., 2009). To infer how well model parameters are estimated by the experimental data, here, we used the profile likelihood, a data-based method to detect structural and practical non-identifiability for reducing nonlinear models and designating likely candidates for reduction (Maiwald et al., 2016). By this approach, confidence intervals, which contain the true value of the parameter with a desired probability, and as a result appropriate prior distributions for unknown parameters in the MCMC algorithm can be derived. Experimental data and statistical analysis revealed that the level of Taz/Yap in the cytoplasm or nucleus is constant regardless of dose or time of TGF-β treatment, as long as Hippo pathway activity does not change. Mathematical modeling first showed that Taz/Yap is not involved in nuclear import of the phosphorylated Smad2/3 (pSmad2/3)-Smad4 complex. Next, we developed five mathematical models of Hippo and TGF-β cross talk to test diverse hypotheses including receptor activity alterations and/or the existence of nuclear retention factors to protect the Smad complex against nuclear phosphatases. Using the Bayesian model averaging (BMA) strategy (Vrugt et al., 2008b) to discriminate the five models, the best model was selected. This work showed that Taz/Yap was not involved in changing the rate of Smad import or export or in the molecular mechanism whereby Smad is phosphorylated. Rather, the modeling predicts that Taz/Yap alter Smad nuclear accumulation by acting either directly or indirectly as a retention factor and by altering TGF-β receptor activity through a post-translational mechanism.
Results
Taz/Yap Modulate the Nuclear Accumulation of Smad2/3 and Smad4
The precise nuclear concentration of Smads determines which genes are transcriptionally activated (Shi and Massague, 2003; Clarke et al., 2006; Zi et al., 2011; Hata and Chen, 2016). Two mechanisms have been proposed to explain nuclear Smad accumulation, namely, different nucleocytoplasmic shuttling kinetics for activated and inactive Smads (Schmierer and Hill, 2005; Hill, 2009) or the presence of nuclear retention factors (ten Dijke and Hill, 2004; Xu, 2006). Neither mechanism alone can completely account for existing data; thus, how Smad nuclear accumulation is regulated remains unclear. Hippo pathway activity impacts Smad activity; thus, to investigate the contribution of the Hippo pathway mediators, Taz/Yap to Smad function, we used EpH4, a TGF-β-responsive, normal mouse mammary epithelial cell line that has retained an intact Hippo pathway (Narimatsu et al., 2016) as a system to study TGF-β and Hippo cross talk. We first established experimental conditions to perturb the process in these cells by removing Taz/Yap from the nucleus by abrogating expression of both Taz and Yap using small interfering RNAs (siRNAs) (Figure 1A). The efficiency of knocking down Taz/Yap and concordant loss of expression of the Taz/Yap target genes, Ankrd1 and Cyr61, by qPCR was confirmed using pools or four individual siRNAs for each (Figures 1A and S1A–S1C). Knockdown efficiency was also verified by immunoblotting and immunofluorescence (IF) microscopy (Figures 1A and 1B), and the expected reduction of TGF-β-induced expression of Pai1 (Narimatsu et al., 2016) was also confirmed (Figure S1D). Density-induced polarization of epithelial cells can activate the Hippo pathway and leads to sequestration of Taz/Yap in the cytoplasm (Varelas et al., 2008; Narimatsu et al., 2015; Narimatsu et al., 2016); thus, as an alternative approach to remove Taz/Yap from the nucleus, varying concentrations of EpH4 cells were plated and Taz/Yap subcellular localization, protein levels, and phosphorylation were assessed. Immunofluorescence analysis confirmed that Taz/Yap localization to the cytoplasm increased with increasing cell density and a concordant, enhanced phosphorylation of Taz/Yap was observed at higher cell densities as monitored by immunoblotting (Figures S2A and S2B). We confirmed that Smad phosphorylation was equivalent at all selected cell densities (Figure S2C), whereas density-dependent Hippo pathway activation was achieved as determined by analyzing the expression of the Taz/Yap target genes, Ankrd1 and Cyr61 by qPCR (Figure S2D). Prolonged treatment of polarized epithelial cells with TGF-β results in re-localization of the receptor to the basolateral surface (Narimatsu et al., 2015; Narimatsu et al., 2016). The equivalent levels of Smad phosphorylation observed (Figure S2C) indicate TGF-β receptor re-localization, which has been observed to occur at longer time points (Narimatsu et al., 2015), has not yet been manifested. To investigate the contribution of Taz/Yap to Smad function, we next monitored endogenous Smad nuclear accumulation in EpH4 cells transfected with control or Taz/Yap siRNAs using quantitative, automated high-content IF microscopy. In brief, cells were treated with varying doses of TGF-β for 0–3 h and after fixation, the distribution of Taz/Yap and Smads, as a ratio of the nuclear to cytoplasmic localization, in individual cells was quantitated by automated imaging (Figures 1C, S1E, and S1F). This method has the advantage that endogenous, rather than transfected and tagged, Smad2 is tracked and because efficient knockdown of Taz/Yap at the single cell level can be simultaneously monitored. Interestingly, analysis of the nuclear to cytoplasmic ratio of Smad2/3 revealed that the peak levels and the rate of Smad2/3 nuclear accumulation to this peak for all TGF-β doses tested were not notably altered when Taz/Yap was removed (Figure 1D). However, beyond this peak, the nuclear levels of Smad2/3 declined more rapidly in the absence of Taz/Yap. Similar results were observed in the case of the Smad2/3 partner, Smad4 (Figure 1E). These results suggest that removing Taz/Yap facilitates nuclear export of Smad2/3 and Smad4. Analysis of Smad nuclear accumulation at low and high cell densities confirmed that activating Hippo in response to increased cell density also reduces Smad nuclear accumulation (Figures S2E and S2F). Altogether, these observations indicate that Taz/Yap are involved in promoting Smad nuclear accumulation.
Figure 1.
Analysis of the Nuclear Accumulation of Smad2/3 and Smad4 upon Loss of Taz/Yap Expression
(A) A schematic of the optimized protocol for knocking down Taz and Yap using siRNAs in EpH4 cells is shown (top). The efficiency of knocking down Taz/Yap and the reduction in expression level of the Taz/Yap target genes, Ankrd1 and Cyr61, was determined by qPCR with data plotted as the mean ± standard deviation of three independent experiments (left). Taz/Yap knockdown was confirmed by immunoblotting (right).
(B) Taz/Yap knockdown was verified by immunofluorescence microscopy.
(C) EpH4 cells were transfected with siCTL or siTaz/Yap and seeded in a 96-well plate. The localization of Taz/Yap in cells co-stained with DAPI was determined by immunofluorescence microscopy. The histogram from a representative experiment shows the intensity of Taz/Yap in transfected cells. Note that background intensity in the absence of cells is approximately 200–300 units, indicating a potent knockdown was achieved in the majority of cells.
(D and E) EpH4 cells transfected with siCTL or siTaz/Yap in 96-well plates were treated with different doses of TGF-β for varying times. Cells were fixed, nuclei visualized with DAPI, and Smad proteins stained with antibodies against Smad2/3 (D) or Smad4 (E) and visualized by immunofluorescence microscopy. Images were taken by confocal microscopy using In Cell Analyzer 6000. Smad protein localization of ~1,000 cells/well was quantified by automated image analysis. The mean (full circles) ± standard error of the mean (SEM, gray area) from at least nine independent biological experiments is shown.
The Loss of Smad Nuclear Accumulation Caused by Loss of Taz/Yap Is Unlikely to Be due to Protein Degradation
Taz/Yap may alter Smad nuclear accumulation by acting as retention factors, by affecting phosphorylation/dephosphorylation and/or import rate of Smads, or by promoting degradation of Smads or TGF-β receptors. To assess whether loss of Smad nuclear accumulation is due to degradation of receptors or other proteins involved in the pathway, we first examined the expression levels of Smads in EpH4 cells transfected with siCTL or siTaz/Yap. Immunoblotting analysis revealed that no change in the levels of these proteins was observed during the signaling time period (Figure 2A). To check for the contribution of protein degradation in general, transfected EpH4 cells were treated with MG132, a reversible proteasome inhibitor that inhibits the degradation of ubiquitin-conjugated proteins. The results show that treating cells with MG132 caused a general increase in the accumulation of nuclear Smads; however, the difference between siCTL and siTaz/Yap at all doses of TGF-β was still observed (Figures 2B and 2C) indicating that the loss of Smad nuclear accumulation in siTaz/Yap transfected cells is unlikely to be due to degradation of Smads, Taz/Yap or TGF-β receptors.
Figure 2.
The Loss of Smad Nuclear Accumulation in siTaz/Yap-Transfected Cells Is Unlikely to Be due to Degradation of Smads/Taz/Yap Proteins or TGF-β Receptors
(A) EpH4 cells were transfected with siControl (C) or siTaz/Yap (T/Y) and then treated with 5 or 10 pM of TGF-β for 1.5 h. Immunoblotting shows that knockdown of Taz/Yap does not affect the expression level of Smads.
(B and C) EpH4 cells transfected with siControl (siCTL) or siTaz/Yap were seeded in a 96-well plate and treated with 10 μM of the proteasome inhibitor, MG132, both during the 3-h serum starvation time and during TGF-β treatment. The localization of Smad2/3 (B) and Smad4 (C) in response to 5 and 50 pM of TGF-β at varying time points was quantified for all cells/well by automated image analysis in five biological replicates. The full and empty circles show the mean, and the gray area indicates the mean ± standard error of the mean (SEM). The results show that MG132 treatment increases Smads accumulation but that the difference between siControl and siTaz/Yap still remains.
Taz/Yap Knockdown Alters the Phosphorylation Status of Smads
We next investigated whether Taz/Yap knockdown affects phosphorylation or dephosphorylation of Smads. To do this, EpH4 cells were transfected with siCTL or siTaz/Yap and treated with two doses of TGF-β at varying time points. Immunoblotting experiments revealed that the initial rates of Smad phosphorylation are similar but that a more rapid decline in phosphorylation levels in cells transfected with siTaz/Yap, particularly at higher TGF-β doses, was observed (Figure 3). Dephosphorylation of Smads is thought to promote Smad nuclear export (ten Dijke and Hill, 2004; Xu, 2006); thus, these results are consistent with the IF experiments (Figure 1) that show constant Smad nuclear accumulation, up to the time of peak levels followed by a more rapid decrease in the levels of nuclear Smads upon the loss of Taz/Yap. These data also indicate that the initial levels of receptors are unaffected by loss of Taz/Yap.
Figure 3.
Taz/Yap Knockdown Alters Phosphorylation/Dephosphorylation of Smads
(A) EpH4 cells were transfected with siControl or siTaz/Yap and treated with two doses of TGF-β at varying times. A representative immunoblot is shown.
(B) Smad2/3 phosphorylation in seven different biological replicates was quantified by ImageJ software. The full circles show the mean, and the gray area indicates the mean ± the standard deviation of the different biological experiments.
Smad Nuclear Accumulation Is Not Altered in Cells Lacking Taz/Yap when TGF-β Receptors Are Inhibited by SB-431542
Our imaging results suggest that removing Taz/Yap facilitates Smad nuclear export. On the other hand, constitutive nucleocytoplasmic shuttling of Smads (Clarke et al., 2006) can allow for monitoring of receptor activity. To investigate if the increased loss of nuclear Smads induced by siTaz/Yap is due to changes in receptor activity, we inhibited TGF-β receptors by treating cells with SB-431542, an inhibitor of the TGF-β receptor type I (Inman et al., 2002). Analysis of the optimal timing and concentrations of SB-431542 to inhibit TGF-β receptors in EpH4 cells demonstrated that the compound was effective at 10 μM between 30 min and up to 3 h of treatment (Figures S3A and S3B). Thus, EpH4 cells transfected with siCTL or siTaz/Yap were treated with TGF-β (5 and 50 pM) continuously or after 30 min of TGF-β addition; cells were washed to remove ligand and then incubated without (wash) or with 10 μM of SB-431542. Automated quantitation of the nuclear to cytoplasmic ratio of Smad2/3 and Smad4 confirmed that loss of Taz/Yap increased the apparent export rate of Smads (Figure 4) as in the previous IF experiments (Figure 1). However, this difference was lost when TGF-β receptors were inhibited (Figure 4). Thus, continuous TGF-β receptor activity is required for Taz/Yap-mediated effects on Smad nuclear accumulation to be manifested.
Figure 4.
Altered Smad Nuclear Accumulation in the Absence of Taz/Yap Is Lost in the Presence of the TGF-β Receptor Inhibitor, SB-431542
EpH4 cells transfected with siCTL or siTaz/Yap (siT/Y) were treated with 5 or 50 pM TGF-β for the indicated times (full circles) or for 40 min after which TGF-β was washed out and fresh starvation media was added (A and B) or cells were incubated with 10 μM of SB-431542 for the indicated times (C and D; empty circles). All cells were simultaneously fixed, stained with DAPI, and Smads visualized using anti-Smad2/3 (A and C) or Smad4 (B and D) antibodies by immunofluorescence microscopy. The nuclear to cytoplasmic ratio of Smads was quantified by automated image analysis. The mean (full circles) ± standard error of the mean (SEM, gray area) from at least three independent biological experiments is shown.
The Nuclear to Cytoplasmic Ratio of Taz/Yap Is Constant Regardless of Dose or Timing of TGF-β Treatment when Hippo Pathway Activity Is Unaltered
To determine the localization pattern of Taz/Yap during the time course of TGF-β treatment, EpH4 cells transfected with siCTL or siTaz/Yap were treated with different doses of TGF-β from 1 to 50 pM at varying times from 0 to 3 h and the nuclear to cytoplasmic ratio of Taz/Yap was quantified. The results showed noisy, but constant, signals (Figure S3C). The probability density of the nuclear to cytoplasmic ratios of Taz/Yap for cells transfected with siCTL or siTaz/Yap at different doses and time points (for approximately 105 cells) show that, for cells in either of the two conditions, the ratio (roughly 2.5) is similar (Figure S3D). We next used linear mixed-effects modeling to test the effects of multiple factors on the mean of the vector of ratios (Pinero and Bates, 2000; Gelman and Hill, 2006). This modeling revealed that the ratio was independent of TGF-β time and dose (see Transparent Methods and Table S1). Moreover, stepwise regression modeling (Hox et al., 2017) to determine which variables to include in the model yielded a Ratio ∼1 + Id, where Id is the condition label, as the best model, thus also confirming that the ratio was independent of TGF-β (Table S2). We also experimentally confirmed that the nuclear to cytoplasmic ratio of Taz/Yap was independent of TGF-β treatment using subcellular fractionation followed by quantitation of immunoblots (Figure S3E). In contrast, the expected cell-density-induced cytoplasmic accumulation of Taz/Yap was readily detected using linear mixed-effects and stepwise regression modeling, whereas the time and dose of TGF-β did not have any influence on the Taz/Yap nuclear to cytoplasmic ratio (Figure S3F and Tables S3 and S4). Treating EpH4 cells with okadaic acid, a general phosphatase inhibitor that activates the Hippo pathway, (Hata et al., 2013) similarly reduced the ratio of nuclear to cytoplasmic of Taz/Yap (Figure S3G). Altogether, these results demonstrate that Taz/Yap localization is not altered by TGF-β and, thus, it is not necessary to consider changes in Taz/Yap localization in our modeling.
Mathematical Modeling of Smad Nuclear Accumulation Reveals Taz/Yap Is Not Involved in Nuclear Import of the Complex of pSmad2/3-Smad4
To compare nuclear import rates of the different forms of Smad complexes under control conditions, we turned to mathematical modeling. As Smads and Taz/Yap are sufficiently abundant, we selected a model using deterministic ordinary differential equations (ODE) based on the conservation of mass (Edelstein-Keshet, 2005). The proposed model contains binding of Taz/Yap to the complex of pSmad2/3-Smad4, the latter of which forms upon activation of the pathway by TGF-β addition (Figure 5 and Tables S5 and S6). To model the activated receptor, we used the impulse model (Chechik and Koller, 2009), a 7-parameter double-sigmoid function, one that captures activation and another that models destruction of functional TGF-β receptor complexes and post-translational mechanisms that provide negative feedback to the receptors that terminates signaling (Figure S4A). All other parameters are described in the Transparent Methods section and listed in Table S5.
Figure 5.
Models of TGF-β and Hippo Pathway Signaling Cross Talk
(A) The initial model contains binding of Taz/Yap to the complex of pSmad2/3-Smad4, which forms upon activation of the pathway by TGF-β addition. Since Taz/Yap localization is not altered by TGF-β and Taz/Yap is not involved in nucleocytoplasmic shuttling of the Smad complex, these steps (red arrows) are not considered in the simplified model.
(B) In the Receptor Activity Alteration (RAA) model, Taz/Yap still binds to the Smad complex; however, the binding has no effect on Smad nuclear accumulation, rather Taz/Yap alters receptor activity.
(C) In the Retention Factor (CRF or DRF) models there is a retention factor (X) in the nucleus, which binds to the complex of pSmad23-Smad4-Taz/Yap in a ligand-dependent manner. Factor X is either constant (Constant Retention Factor: CRF) or downregulated by the factor , in the range [0 1] upon knocking down Taz/Yap (Downregulated Retention Factor: DRF).
Next, we set out to define the inputs and outputs of the process, which in our model corresponds to the dose of TGF-β and the nuclear accumulation of Smads, respectively. Signaling output is critically dependent on TGF-β dose and timing; thus, we experimentally determined sub-saturation doses. Examination of Smad phosphorylation in EpH4 cells treated with varying doses of TGF-β for different times revealed that doses of TGF-β below 20 pM avoids saturation of Smad phosphorylation and that a duration of 3 h captures the dynamics of the process (Figures 6A–6D). Of note, the saturation dose was unaltered by loss of Taz/Yap indicating that endogenous ligand levels are similar in both conditions (Figure 6B). As our model requires the mass of Smad2/3, Smad4, and Taz/Yap, we converted the nuclear to cytoplasmic ratios obtained by immunofluorescence imaging into mass by taking into account the estimated volumes of the nucleus and cytoplasm (see Transparent Methods and Figures S4B–S4E).
Figure 6.
Determination of Below-Saturation TGF-β Doses for Model Input and Simulation of the Selected (RAADRF) Initial Model for Best Fitted Parameters
(A–D) Analysis of Smad phosphorylation in response to varying doses and times of TGF-β treatment. (A and B) EpH4 cells were transfected with siCTL or siTaz/Yap and treated with different doses of TGF-β for 30 min. (A) Smad phosphorylation was assessed by immunoblotting. (B) For quantitation, the intensity of each pSmad band was normalized to total Smad and then divided by the value of the sample with the highest pSmad levels (i.e., siCTL treated with 20 pM TGF-β) and was then multiplied by 100 to show percentage. Data, plotted as the mean ± standard deviation of four independent experiments, indicate that TGF-β doses below 20 pM should be used to avoid saturation of Smad phosphorylation. (C and D) EpH4 cells were plated at low density for 24 h and then were treated with TGF-β doses of 2.5, 5, 10, and 50 pM at varying time points. (C) Smad phosphorylation was assessed by immunoblotting. (D) Quantitation of Smad phosphorylation revealed the temporal patterns of signaling duration and allowed determination of the times of maximum phosphorylation for each dose of TGF-β, which occurred between 0.5 and 1 h. Simulation of the initial (RAADRF) selected model with the best fitted parameters.
(E) In cells transfected with siTaz/Yap, the concentration of free Smads in the cytoplasm or nucleus is higher than that of their counterparts in control cells.
(F) In siTaz/Yap transfected cells, cytoplasmic pSmad2/3 concentration is lower and there is no difference in pSmad2/3-Smad4 or nuclear pSmad2/3 as compared with controls. For nuclear pSmad2/3-Smad4, the mass is initially greater in siTaz/Yap than in control cells but then declines more rapidly, due to faster dephosphorylation.
(G) There is more pSmad2/3-Smad4-Taz/Yap in control cells than in cells transfected with siTaz/Yap in the cytoplasm, but no difference is observed in the nucleus for both conditions. Most of the complex in control cells is bound to the retention factor, X.
The signaling process, as in most biological contexts, is characterized by uncertain parameters and noisy measurements. In this regard, the Markov chain Monte Carlo (MCMC) Differential Evolution Adaptive Metropolis (DREAM) technique is extremely useful for modeling (Vrugt et al., 2008a; Vrugt et al., 2009) and has been applied to variety of non-linear, high-dimensionality cases, including both environmental systems and biological contexts (Mitchener et al., 2015; Vrugt, 2016; Perry et al., 2019). Thus, to estimate the unknown parameters in the model, we used the MCMC DREAM(ZS) algorithm (Vrugt, 2016), which includes special extensions to simplify inference of high-dimensional and CPU-intensive system models and to minimize the number of samples required for burn-in (see Transparent Methods).
In the absence of stimulation, phosphorylated Smads and receptors are at non-zero, basal levels. To find the initial concentrations of the corresponding model species, we calculated their steady-state levels in the absence of TGF-β where the derivatives of concentrations of different proteins were equal to zero as reported by others (Strasen et al., 2018) (see Transparent Methods). At each iteration of the MCMC DREAM(ZS) algorithm, the unknown initial values were estimated alongside with other unknown parameters of the model. To compare the import rates of the complexes pSmad2/3-Smad4 and pSmad2/3-Smad4-Taz/Yap, and , we estimated the ratio, alongside the other unknown model parameters for siCTL-transfected cells treated with three doses of TGF-β (2.5, 5, and 10 pM), selected for being below saturation, as well as in cells seeded at low or high cell densities and treated with 5 or 50 pM of TGF-β at different time points. The prior distribution for the ratio was selected in the range [0 5], which means that the import rate of the complex of pSmad2/3-Smad4-Taz/Yap can be up to five times more than the import rate of the pSmad2/3-Smad4 complex, which is a very conservative choice based on observed IF experiment results (Figures 1D and 1E). The estimated posterior distributions for the ratio, at the three aforementioned cases, shows that this rate is equal to zero and proposes that Taz/Yap has no role in the nuclear import of Smad complex (see Transparent Methods and Table S10). On the other hand, as the levels of Taz/Yap in the nucleus or cytoplasm, regardless of TGF-β treatment, are constant, then in the model,
| (Equation 1) |
and
| (Equation 2) |
where and are import and export rates of free (unbound) Taz/Yap, respectively. By assigning = 0, free Taz/Yap either does not shuttle or shuttles between the nucleus and cytoplasm by a constant ratio, which is specified by Hippo pathway activity. However, the import and export rates of Taz/Yap are not identifiable using the current data. Since Taz/Yap is not involved in nucleocytoplasmic shuttling of the Smad complex, its shuttling is irrelevant to the model, and without a loss of generality, we used the simplified model (Figure 5A and Table S7).
To conduct identifiability analysis, which is a major challenge in biological reaction networks modeled by differential equations, we used the profile likelihood, a data-based method to detect structural and practical non-identifiability for reducing nonlinear models and to designate likely candidates for reduction (Raue et al., 2009; Maiwald et al., 2016). Confidence intervals, which contain the true value of the parameter with a desired probability, result in derivation of appropriate prior distributions for unknown parameters in the MCMC DREAM(ZS) algorithm. Identifiability analysis of the simplified model revealed that all the model parameters are structurally and practically identifiable as they have well-defined confidence intervals (Raue et al., 2009) (Figure S5A). The statistical analysis of the estimated parameters, the initial values for different cytoplasmic and nuclear complexes, and the parameter correlation matrix for the simplified model are given in Tables S11–S13. The goodness of fit test and the residual analysis (Figure S5Bi-iii) validated the model and confirmed that the data fit the model appropriately. Receptor activity for the best fitted (Maximum A Posteriori: MAP) parameter values reveal that the maximum activity occurs around 1.3 h and thereafter, owing to negative regulation, the receptor activity declines (Figure S5Biv). The 95% uncertainty ranges for the model simulation data due to parameter and total uncertainty confirms the model captured the process dynamics well (Figure S5Bv-viii).
Taz/Yap Knockdown May Reduce Nuclear Smad Accumulation by Both Altering TGF-β Receptor Activity and through a Retention Factor
To investigate if Taz/Yap knockdown changes receptor activity, acts as a retention factor, or both, we proposed three different hypotheses and established appropriate mathematical models to test the scenarios. First, to test if Taz/Yap knockdown alters TGF-β receptor activity and thus affects Smad phosphorylation and nuclear accumulation, the Receptor Activity Alteration (RAA) hypothesis, we used a model (Figure 5B and Table S8) in which Taz/Yap still binds to the Smad complex; however, the binding has no effect on Smad nuclear accumulation, rather Taz/Yap alters receptor activity. The parameters and initial values of different Smad complexes of the model were estimated simultaneously for siCTL or siTaz/Yap transfected cells treated with 10 pM TGF-β, a sub-saturation dose that still shows differential accumulation at the two conditions. Except for the receptors, all the other parameters were the same. Of note, these parameters were similar to those reported in HaCaT cells in a study examining TGF-β signaling alone (Schmierer et al., 2008). The statistical analysis of the estimated parameters, the initial values, and the parameter correlation matrix are given in Tables S14–S17. The estimated parameter values showed that all the receptor parameters , , , , , and except (the offset time of the receptors) at the two conditions have the same probability distributions (Table S14). This observation suggests that a restricted model with all parameters the same except the parameter at two conditions may explain the data. To test this, we estimated the restricted model parameters assuming that the six aforementioned parameters in the receptor models are the same (Tables S14–S17). The goodness of fit test, residual analysis, and time series plot of 95% simulation uncertainty ranges suggested that both models explain the data appropriately. Comparison of the temporal patterns of receptor activity for the MAP values for both models reveals that receptors for both conditions initially have the same activity; however, at around the peak, receptor activity in cells transfected with siTaz/Yap declines faster (Figures S6A–S6D and Table S15) consistent with results from IF and Smad phosphorylation (Figures 1 and 3). To discriminate the two proposed models, we used the Bayesian Model Averaging (BMA) strategy, which weighs the different models such that the weighted estimate (model) is a better predictor of the observed system behavior (data) than any of the individual models of the ensemble (Vrugt et al., 2008b). With BMA, the full model was rejected in favor of the restricted model, which means that the full model does not fit the data significantly better than the restricted model. Thus, we accepted the restricted model for RAA hypothesis.
A second mechanism to explain differential Smad nuclear accumulation is the retention-factor hypothesis, in which there are binding factors in the nucleus that have a higher affinity for phosphorylated Smads and thereby stabilize the complex of pSmad2/3-Smad4-Taz/Yap. To determine whether the presence of a nuclear retention factor could explain the differential Smad nuclear accumulation in cells transfected with siCTL or siTaz/Yap, we used a model (Figure 5C and Table S9) in which receptor activity parameters do not change but there is an unknown factor (X) in the nucleus, which binds to the complex of pSmad2/3-Smad4-Taz/Yap in a TGF-β-dependent manner. Although the factor could have a temporal pattern, we considered the simplest case where the concentration of X is constant during signaling. Two different scenarios were considered, X is either constant (Constant Retention Factor or CRF hypothesis) or downregulated by factor , in the range [0 1] upon knocking down of Taz/Yap (Downregulated Retention Factor or DRF hypothesis). Statistical analysis of the estimated process parameters, initial values, and the parameter correlation matrices for the proposed models are summarized in Tables S18–S21. Validity of the models was confirmed by the residual analysis test and the chi-square goodness of fit (see Transparent Methods). The receptor activity in both models declines after 1 h. The 95% uncertainty ranges for the simulation data for the CRF and DRF models confirm that the measured data can be explained by both models (Figures S7A–S7D). Although both models fairly capture the process dynamics, applying the BMA strategy to discriminate the two proposed models (Vrugt et al., 2008b) rejected the DRF in favor of CRF. Simulation of the model for the best (MAP) parameters, using the initial values given in Table S18 at a dose of 10 pM TGF-β, so the role of the retention factor is manifested in a ligand-dependent manner shows that, until the peak, Smad nuclear accumulation is similar (Figure S7E) to the experimental results (see Figure 1). Altogether, our results show that existence a retention factor which protects the complex of pSmad in a ligand-dependent manner might be responsible for differential Smad nuclear accumulation.
Finally, to investigate whether both hypotheses may be valid, we used the same model but, in which both receptor parameters change and a nuclear retention factor exists, which is either constant, the Receptor Activity Alteration-Constant Retention Factor (RAACRF) hypothesis, or downregulated, the Receptor Activity Alteration-Downregulated Retention Factor (RAADRF) hypothesis, in cells lacking Taz/Yap. The parameters and initial values of both models were estimated (Tables S22 and S23) and there was no strong correlation between parameters (Tables S24 and S25). Validity of the models was confirmed by the residual analysis test, the chi-square goodness of fit test, and the 95% uncertainty ranges for the simulation data of both models (Figure S8). The receptor activity patterns at the two conditions show the same activity up to the peak, but this subsequently declines faster in cells transfected with siTaz/Yap (Figure S8). To discriminate the two proposed models, we used the BMA model selection strategy, which strongly rejected the RAACRF in favor of RAADRF.
So far, we have proposed and validated three different models to explain differential Smad nuclear accumulation in cells transfected with siCTL or siTaz/Yap. To compare to what extent the models fit the measured data, we used the BMA model selection strategy to find the best model among the three (Vrugt et al., 2008b). The estimated weights for the proposed models (Table S26) show that the RAA model is strongly rejected in favor of the other models, meaning that receptor activity alteration cannot be the sole factor responsible for differential nuclear accumulation. The RAADRF model was selected as the best model, whereas the Constant Retention Factor (CRF) model ranked between the two. The RAADRF model states that receptor activity will be reduced upon loss of Taz/Yap and that there is a Taz/Yap-dependent nuclear retention factor that is downregulated in the absence of Taz/Yap. Simulating the identified model for the best (MAP) parameters showed that, upon phosphorylation, Smads translocate to the nucleus immediately (Figure 6E). In the nucleus, owing to the excess amount of Taz/Yap in control cells, the mass of the pSmad2/3-Smad4-Taz/Yap-X complex is roughly 10 times more than the complex siTaz/Yap in transfected cells. This initially results in more, free, nuclear pSmad2/3-Smad4 in cells transfected with siTaz/Yap; however, after 1.5 h, this declines faster (Figures 6F and 6G).
The Mediator Complex Regulates Smad Nuclear Accumulation
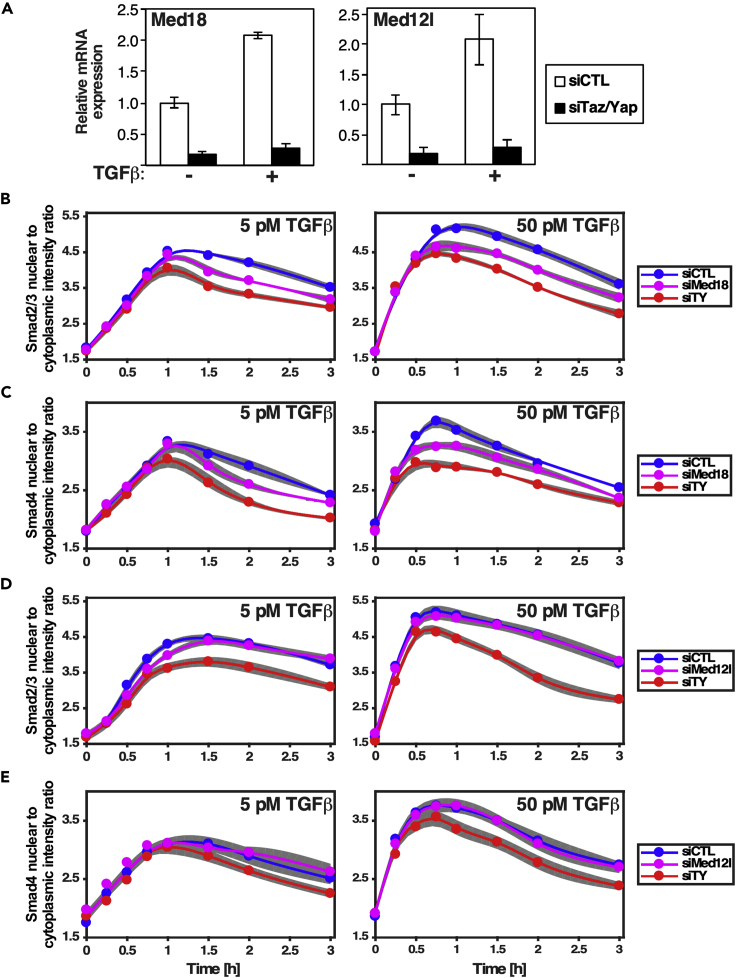
Our mathematical modeling indicates that an unknown nuclear retention factor may explain differential Smad nuclear accumulation in cells lacking Taz/Yap. Candidate retention factors include transcriptional regulatory components such as the mediator complex, which is responsible for recruiting RNA polymerase to promoters (Soutourina, 2018) and which has been reported to associate with Taz/Yap (Varelas et al., 2008; Galli et al., 2015). In the RAADRF model, the expression of this retention factor is Taz/Yap regulated. Thus, we analyzed the expression of mediator complex components in transcriptomic data obtained by RNA sequencing from control versus siTaz/Yap-transfected cells. This revealed that, of the 30 mediator (Med) complex subunits expressed in EpH4 cells, only Med18 and Med12l showed a decrease in expression of approximately 2-fold. In agreement, analysis by qPCR demonstrated that expression of Med18 and Med12l mediator complex subunits were markedly decreased upon knocking down Taz/Yap either in the presence or absence of TGF-β (Figure 7A). Moreover, examination of TGF-β-induced Smad nuclear accumulation by IF revealed that knocking down Med18 decreased TGF-β-induced nuclear accumulation of both Smad2/3 and Smad4 (Figures 7B and 7C), although loss of Med12l had no affect (Figures 7D and 7E). The pattern of Smad accumulation in cells lacking Med18 is consistent with the IF and Smad phosphorylation results (Figures 1 and 3), with peak levels and the rate of Smad nuclear accumulation to this peak for both TGF-β doses being unaltered; but beyond the peak, the nuclear levels of Smads declined more rapidly in the absence of Med18. Of note, the effect was not as dramatic as that observed for siTaz/Yap, indicating besides regulating Med18, Taz/Yap can also modulate Smads via alternative means, such as regulating receptor activity as predicted in the RAADRF model.
Figure 7.
Mediator Complex Components Differentially Regulate Smad Nuclear Accumulation
(A) Analysis by qPCR shows that the mediator complex subunits, Med12l and Med18, are regulated by Taz/Yap. Data are shown as the mean ± standard deviation of three independent experiments.
(B–E) EpH4 cells were transfected with siCTL, siTaz/Yap, siMed18, or siMed12l and treated with two doses of TGF-β (5 and 50 pM) at different time points. Localization of Smad2/3 (B and D) and Smad4 (C and E) was quantified using automated IF imaging. (B and C) Loss of Med18, mediator complex subunit 18, diminishes nuclear accumulation of Smads. (D and E) Loss of Med12l has no effect on Smad accumulation. The full circles and gray areas indicate the mean ± standard error of the mean (SEM) of five biological replicates, respectively.
Discussion
TGF-β and Hippo pathway cross talk controls transcriptional outcomes, and key to TGF-β signaling is the nuclear localization of Smads. Thus, to investigate the role of the Hippo pathway mediators, Taz/Yap in Smad nuclear accumulation, we used experimental data and computational models of Hippo and TGF-β cross talk to test diverse hypotheses. Quantitative automated high-content IF microscopy showed that Taz/Yap enhances the nuclear accumulation of Smad2/3 and Smad4. Treating siTaz/Yap transfected EpH4 cells with MG132, a reversible proteasome inhibitor, showed the reduction in nuclear accumulation was not due to degradation of Smads, Taz/Yap proteins or receptors. On the other hand, immunoblotting and IF experiments revealed that Taz/Yap knockdown does not alter the initial rate of TGF-β-induced Smad phosphorylation or Smad nuclear accumulation to the peak, but rather resulted in a more rapid decrease from peak levels of both phospho-Smad and nuclear-localized Smads. Inhibiting TGF-β receptor activity by treating cells with SB-431542, an inhibitor of the TGF-β receptor type I, showed that the differential rate of Smad exit from the nucleus in siCTL versus siTaz/Yap transfected cells was lost, indicating that changes in receptor activity at the later stages of the process contributed to the effect of loss of Taz/Yap. In terms of Taz/Yap, high-throughput IF microscopy, immunoblotting, and statistical analysis showed that Taz/Yap levels in the cytoplasm or nucleus were constant regardless of dose of TGF-β or time of treatment as long as Hippo pathway activity was constant. Thus, to better understand the mechanism of the cross talk, we next developed several mathematical models to test different hypotheses. We selected appropriate doses of TGF-β and timing of treatment to acquire time course data for different complexes in the cytoplasm and nucleus. To estimate the unknown parameters in the models, we used the Bayesian method, MCMC DREAM(ZS) technique (Vrugt, 2016), which has been implemented to analyze a variety of scenarios involving nonlinearity, high dimensionality, and multimodality (Vrugt et al., 2008a; Vrugt et al., 2009). Initial applications were primarily in the weather and environmental fields, but the algorithm has also been used to model biochemical reactions such as COX2 and most recently to understand the role of a scaffolding protein in modulating MAPK activity (Mitchener et al., 2015; Vrugt, 2016; Perry et al., 2019). Here, we applied the approach to study signaling pathway cross talk. Identifying an initial mathematical model showed that Taz/Yap is not involved in nuclear import of the complex of pSmad2/3-Smad4, and so, the initial model was reduced to a simplified model, which does not consider the nuclear import of the complex pSmad2/3-Smad4-Taz/Yap or Taz/Yap shuttling. The profile likelihoods of parameters of the simplified model show that all the parameters of the model are identifiable and the model is not reducible. Based on the experimental data, modeling, and statistical analysis, several different hypotheses were proposed to explain differential accumulation of Smads, three of which (RAA, CRF, and RAADRF) captured the process dynamics appropriately. The Receptor Activity Alteration (RAA) hypothesis suggests that the differential nuclear accumulation of Smads is due to a decline of receptor activity that occurs earlier in cells lacking Taz/Yap. The retention-factor hypothesis states that there are binding factors in the nucleus, which have a higher affinity for phosphorylated Smads and stabilize the complex of pSmad2/3-Smad4-Taz/Yap. Mathematical modeling confirmed that a retention factor, in which the levels are not regulated by Taz/Yap (i.e., the CRF hypothesis), was an appropriate model. Finally, a combination model, RAADRF, which proposes that the differential accumulation of Smads at two conditions might be due to both receptor activity alteration and existence of a Taz/Yap regulated retention factor also performed well. To compare to what extent these models fit the measured data, we used the BMA model selection strategy. While RAA was strongly rejected, CRF was ranked intermediately. The best model was determined to be RAADRF, that proposes that Taz/Yap is involved in receptor activity and that a nuclear Taz/Yap regulated retention factor, which stabilizes and protects the complex of Smad. Simulating the selected model shows there is more unphosphorylated Smads in both the cytoplasm and nucleus in Taz/Yap knocked down cells compared with the control. The concentration of pSmad2/3 in the cytoplasm is initially the same in siCTL and siTaz/Yap-transfected cells up to the peak but thereafter declines more rapidly in Taz/Yap-transfected cells. The concentration of nuclear pSmad2/3-Smad4 in siTaz/Tap cells is initially larger than controls owing to binding of this complex to Taz/Yap in control cells. However, it declines faster as the complex of pSmad2/3-Smad4 in control cells is protected by binding to Taz/Yap and subsequently to the factor X. Finally, roughly all of the nuclear pSmad2/3-Smad4-Taz/Yap in control cells is bound to the factor X. The simulation results confirm that receptor activity in Taz/Yap-transfected cells declines earlier and that the complex of pSmad2/3-Smad4 is protected by Taz/Yap and the factor, X. The RAADRF model was determined to be the best fit, but two proposed hypotheses (RAA and CRF) were not completely excluded. Applying the modeling approach we developed here in other cell lines may reveal that one of these alternate models would best fit the data in a different cell line. If so, this future work would have the potential to provide novel insights into context-dependent cross talk, a well-established characteristic of TGF-β signaling.
Our analysis indicated that differential TGF-β receptor activity upon loss of Taz/Yap may contribute to alterations in Smad nuclear accumulation. To date, phosphorylation, ubiquitination, SUMOylation, and N-linked glycosylation have been shown to modify the receptors post-translationally (Kim et al., 2012). Whether and how Taz/Yap may impact these events will require further investigation. Our results also predicted the existence of Taz/Yap-dependent retention factor(s). Candidate retention factors include transcription regulatory components or DNA-binding proteins. One such candidate is the mediator complex that is responsible for recruiting RNA polymerase to promoters and so affects maximum gene expression level (Soutourina, 2018). The mediator complex was reported to associate with Taz/Yap (Varelas et al., 2008; Galli et al., 2015). Our analysis revealed that the expression of Med18 and Med12l mediator complex subunits 18 and 12l were decreased upon knocking down Taz/Yap in EpH4 cells. Although Med12l had no effect on Smad nuclear accumulation, IF experiments confirmed that loss of Med18 reduced nuclear accumulation of both Smad2/3 and Smad4. However, as more dramatic effects were observed upon loss of Taz/Yap, the results indicate that other Taz/Yap-regulated factors such as other retention factors or receptor activity regulators are also likely be involved.
Limitations of the Study
Using the current data, the import and export rates of free Taz/Yap are not identifiable. However, as Taz/Yap is not involved in nucleocytoplasmic shuttling of the Smad complex, Taz/Yap shuttling is not relevant, and without a loss of generality, a simplified model was used. The nuclear retention factor could have a temporal pattern; however, based on available data, we only considered the simplest case in which the concentration of the retention factor is constant during signaling. Finally, an important factor that we were not able to directly investigate was the alteration of TGF-β receptor activity owing to the lack of availability of good receptor antibodies.
Resource Availability
Lead Contact
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Liliana Attisano (liliana.attisano@utoronto.ca).
Materials Availability
This study did not generate new unique materials.
Data and Code Availability
All data produced or analyzed for this study are included in the published article and its Supplemental Information files.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Dr. J.A. Vrugt for advice on the implementation of DREAM MCMC. This work was supported by Canada First Research Excellence Fund/Medicine by Design and Canadian Institute for Health Research (CIHR) Foundation grants FDN148455 and FDN143252 to L.A. and J.L.W. B.L. held a CIHR studentship and L.A. and J.L.W. were CRC Chairs.
Author Contributions
B.L. carried out experiments, developed the computational models, and wrote the manuscript. M.B. performed the imaging and developed the image analysis protocol. L.A. and J.L.W. supervised the study and wrote and edited the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: August 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101416.
Supplemental Information
References
- Attisano L., Wrana J.L. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- Attisano L., Wrana J.L. Signal integration in TGF-beta, WNT, and Hippo pathways. F1000Prime Rep. 2013;5:17. doi: 10.12703/P5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry E.R., Camargo F.D. The Hippo superhighway: signaling crossroads converging on the Hippo/Yap pathway in stem cells and development. Curr. Opin. Cell Biol. 2013;25:247–253. doi: 10.1016/j.ceb.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Beyer T.A., Narimatsu M., Weiss A., David L., Wrana J.L. The TGFbeta superfamily in stem cell biology and early mammalian embryonic development. Biochim. Biophys. Acta. 2013;1830:2268–2279. doi: 10.1016/j.bbagen.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Budi E.H., Duan D., Derynck R. Transforming growth factor-beta receptors and Smads: regulatory Complexity and functional versatility. Trends Cell Biol. 2017;27:658–672. doi: 10.1016/j.tcb.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Chechik G., Koller D. Timing of gene expression responses to environmental changes. J. Comput. Biol. 2009;16:279–290. doi: 10.1089/cmb.2008.13TT. [DOI] [PubMed] [Google Scholar]
- Clarke D.C., Betterton M.D., Liu X. Systems theory of Smad signalling. Syst. Biol. (Stevenage) 2006;153:412–424. doi: 10.1049/ip-syb:20050055. [DOI] [PubMed] [Google Scholar]
- Clarke D.C., Liu X. Decoding the quantitative nature of TGF-beta/Smad signaling. Trends Cell Biol. 2008;18:430–442. doi: 10.1016/j.tcb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craiu R.V., Rosenthal J.S. Bayesian computation via Markov chain Monte Carlo. Annu. Rev. Stat. Appl. 2014;1:179–201. [Google Scholar]
- Edelstein-Keshet L. SIAM; 2005. Mathematical Models in Biology. [DOI] [Google Scholar]
- Feng X.H., Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Galli G.G., Carrara M., Yuan W.C., Valdes-Quezada C., Gurung B., Pepe-Mooney B., Zhang T., Geeven G., Gray N.S., de Laat W. YAP drives growth by Controlling transcriptional pause release from dynamic enhancers. Mol. Cell. 2015;60:328–337. doi: 10.1016/j.molcel.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A., Hill J. Cambridge University Press; 2006. Data Analysis Using Regression and Multilevel/hierarchical Models. [Google Scholar]
- Genevet A., Tapon N. The Hippo pathway and apico-basal cell polarity. Biochem. J. 2011;436:213–224. doi: 10.1042/BJ20110217. [DOI] [PubMed] [Google Scholar]
- Gurdon J.B., Bourillot P.Y. Morphogen gradient interpretation. Nature. 2001;413:797–803. doi: 10.1038/35101500. [DOI] [PubMed] [Google Scholar]
- Halder G., Johnson R.L. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Chen Y.-G. TGF-β signaling from receptors to Smads. Cold Spring Harb. Perspect. Biol. 2016;8:a022061. doi: 10.1101/cshperspect.a022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata Y., Timalsina S., Maimaiti S. Okadaic Acid: a tool to study the hippo pathway. Mar. Drugs. 2013;11:896–902. doi: 10.3390/md11030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C.S. Nucleocytoplasmic shuttling of Smad proteins. Cell Res. 2009;19:36–46. doi: 10.1038/cr.2008.325. [DOI] [PubMed] [Google Scholar]
- Hill C.S. Transcriptional control by the SMADs. Cold Spring Harb. Perspect. Biol. 2016;8:a022079. doi: 10.1101/cshperspect.a022079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoodless P.A., Tsukazaki T., Nishimatsu S., Attisano L., Wrana J.L., Thomsen G.H. Dominant-negative Smad2 mutants inhibit activin/Vg1 signaling and disrupt axis formation in Xenopus. Dev. Biol. 1999;207:364–379. doi: 10.1006/dbio.1998.9168. [DOI] [PubMed] [Google Scholar]
- Hox J.J., Moerbeek M., Van de Schoot R. Routledge; 2017. Multilevel Analysis: Techniques and Applications. [Google Scholar]
- Inman G.J., Nicolas F.J., Callahan J.F., Harling J.D., Gaster L.M., Reith A.D., Laping N.J., Hill C.S. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- Kang Y., Chen C.-R., Massagué J. A self-enabling TGF-β response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Mol. Cell. 2003;11:915–926. doi: 10.1016/s1097-2765(03)00109-6. [DOI] [PubMed] [Google Scholar]
- Kim Y.-W., Park J., Lee H.-J., Lee S.-Y., Kim S.-J. TGF-β sensitivity is determined by N-linked glycosylation of the type II TGF-β receptor. Biochem. J. 2012;445:403–411. doi: 10.1042/BJ20111923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk P., Silk D., Stumpf M.P. Reverse engineering under uncertainty. In: Geris L., Gomez-Cabrero D., editors. Uncertainty in Biology. Springer; 2016. pp. 15–32. [Google Scholar]
- Ma S., Meng Z., Chen R., Guan K.-L. The Hippo pathway: biology and pathophysiology. Annu. Rev. Biochem. 2019;88:577–604. doi: 10.1146/annurev-biochem-013118-111829. [DOI] [PubMed] [Google Scholar]
- Maiwald T., Hass H., Steiert B., Vanlier J., Engesser R., Raue A., Kipkeew F., Bock H.H., Kaschek D., Kreutz C., Timmer J. Driving the model to its limit: profile likelihood based model reduction. PLoS One. 2016;11:e0162366. doi: 10.1371/journal.pone.0162366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauviel A., Nallet-Staub F., Varelas X. Integrating developmental signals: a Hippo in the (path) way. Oncogene. 2012;31:1743. doi: 10.1038/onc.2011.363. [DOI] [PubMed] [Google Scholar]
- McNeill H., Woodgett J.R. When pathways collide: collaboration and connivance among signalling proteins in development. Nat. Rev. Mol. Cell Biol. 2010;11:404–413. doi: 10.1038/nrm2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra J.R., Irvine K.D. The Hippo signaling network and its biological functions. Annu. Rev. Genet. 2018;52:65–87. doi: 10.1146/annurev-genet-120417-031621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchener M.M., Hermanson D.J., Shockley E.M., Brown H.A., Lindsley C.W., Reese J., Rouzer C.A., Lopez C.F., Marnett L.J. Competition and allostery govern substrate selectivity of cyclooxygenase-2. Proc. Natl. Acad. Sci. U S A. 2015;112:12366–12371. doi: 10.1073/pnas.1507307112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narimatsu M., Labibi B., Wrana J.L., Attisano L. Analysis of Hippo and TGFbeta signaling in polarizing epithelial cells and mouse embryos. Differentiation. 2016;91:109–118. doi: 10.1016/j.diff.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Narimatsu M., Samavarchi-Tehrani P., Varelas X., Wrana J.L. Distinct polarity cues direct Taz/Yap and TGFbeta receptor localization to differentially control TGFbeta-induced Smad signaling. Dev. Cell. 2015;32:652–656. doi: 10.1016/j.devcel.2015.02.019. [DOI] [PubMed] [Google Scholar]
- Nicolás F.J., De Bosscher K., Schmierer B., Hill C.S. Analysis of Smad nucleocytoplasmic shuttling in living cells. J. Cell Sci. 2004;117:4113–4125. doi: 10.1242/jcs.01289. [DOI] [PubMed] [Google Scholar]
- Park H.W., Guan K.L. Regulation of the Hippo pathway and implications for anticancer drug development. Trends Pharmacol. Sci. 2013;34:581–589. doi: 10.1016/j.tips.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry N.A., Kaoud T.S., Ortega O.O., Kaya A.I., Marcus D.J., Pleinis J.M., Berndt S., Chen Q., Zhan X., Dalby K.N. Arrestin-3 scaffolding of the JNK3 cascade suggests a mechanism for signal amplification. Proc. Natl. Acad. Sci. U S A. 2019;116:810–815. doi: 10.1073/pnas.1819230116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinero J., Bates D. Springer; 2000. Mixed-effects Models in S and S-PLUS (Statistics and Computing) [Google Scholar]
- Raue A., Kreutz C., Maiwald T., Bachmann J., Schilling M., Klingmuller U., Timmer J. Structural and practical identifiability analysis of partially observed dynamical models by exploiting the profile likelihood. Bioinformatics. 2009;25:1923–1929. doi: 10.1093/bioinformatics/btp358. [DOI] [PubMed] [Google Scholar]
- Schmierer B., Hill C.S. Kinetic analysis of Smad nucleocytoplasmic shuttling reveals a mechanism for transforming growth factor beta-dependent nuclear accumulation of Smads. Mol. Cell. Biol. 2005;25:9845–9858. doi: 10.1128/MCB.25.22.9845-9858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer B., Hill C.S. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat. Rev. Mol. Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- Schmierer B., Tournier A.L., Bates P.A., Hill C.S. Mathematical modeling identifies Smad nucleocytoplasmic shuttling as a dynamic signal-interpreting system. Proc. Natl. Acad. Sci. U S A. 2008;105:6608–6613. doi: 10.1073/pnas.0710134105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Soutourina J. Transcription regulation by the Mediator complex. Nat. Rev. Mol. Cell Biol. 2018;19:262. doi: 10.1038/nrm.2017.115. [DOI] [PubMed] [Google Scholar]
- Strasen J., Sarma U., Jentsch M., Bohn S., Sheng C., Horbelt D., Knaus P., Legewie S., Loewer A. Cell-specific responses to the cytokine TGFbeta are determined by variability in protein levels. Mol. Syst. Biol. 2018;14:e7733. doi: 10.15252/msb.20177733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart A.M. Inverse problems: a Bayesian perspective. Acta Numer. 2010;19:451–559. [Google Scholar]
- ten Dijke P., Hill C.S. New insights into TGF-beta-Smad signalling. Trends Biochem. Sci. 2004;29:265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Varelas X., Sakuma R., Samavarchi-Tehrani P., Peerani R., Rao B.M., Dembowy J., Yaffe M.B., Zandstra P.W., Wrana J.L. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat. Cell Biol. 2008;10:837–848. doi: 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]
- Varelas X., Samavarchi-Tehrani P., Narimatsu M., Weiss A., Cockburn K., Larsen B.G., Rossant J., Wrana J.L. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev. Cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Villaverde A.F., Banga J.R. Reverse engineering and identification in systems biology: strategies, perspectives and challenges. J. R. Soc. Interface. 2014;11:20130505. doi: 10.1098/rsif.2013.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrugt J.A. Markov chain Monte Carlo simulation using the DREAM software package: theory, concepts, and MATLAB implementation. Environ. Model. Softw. 2016;75:273–316. [Google Scholar]
- Vrugt J.A., Ter Braak C.J., Clark M.P., Hyman J.M., Robinson B.A. Treatment of input uncertainty in hydrologic modeling: doing hydrology backward with Markov chain Monte Carlo simulation. Water Resour. Res. 2008;44 doi: 10.1029/2007WR006720. [DOI] [Google Scholar]
- Vrugt J.A., Diks C.G., Clark M.P. Ensemble Bayesian model averaging using Markov chain Monte Carlo sampling. Environ. Fluid Mech. 2008;8:579–595. [Google Scholar]
- Vrugt J.A., Ter Braak C., Diks C., Robinson B.A., Hyman J.M., Higdon D. Accelerating Markov chain Monte Carlo simulation by differential evolution with self-adaptive randomized subspace sampling. Int. J. Nonlin. Sci. Num. Simulat. 2009;10:273–290. [Google Scholar]
- Xu L. Regulation of Smad activities. Biochim. Biophys. Acta. 2006;1759:503–513. doi: 10.1016/j.bbaexp.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Kang Y., Çöl S., Massagué J. Smad2 nucleocytoplasmic shuttling by nucleoporins CAN/Nup214 and Nup153 feeds TGF-β signaling complexes in the cytoplasm and nucleus. Mol. Cell. 2002;10:271–282. doi: 10.1016/s1097-2765(02)00586-5. [DOI] [PubMed] [Google Scholar]
- Yu F.X., Zhao B., Guan K.L. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zi Z., Feng Z., Chapnick D.A., Dahl M., Deng D., Klipp E., Moustakas A., Liu X. Quantitative analysis of transient and sustained transforming growth factor-β signaling dynamics. Mol. Syst. Biol. 2011;7:492. doi: 10.1038/msb.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zi Z., Klipp E. Constraint-based modeling and kinetic analysis of the Smad dependent TGF-β signaling pathway. PLoS One. 2007;2:e936. doi: 10.1371/journal.pone.0000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data produced or analyzed for this study are included in the published article and its Supplemental Information files.