Abstract
Objective
Lumbar degenerative spinal ailments are the most important causes for chronic low back pain. Modic changes (MC) are vertebral bone marrow signal intensity changes seen on MRI, commonly in association with degenerative disc disease (DDD). Despite being widely studied, majority of issues concerning MC are still controversial. The current narrative, evidence-based review comprehensively discusses the various aspects related to MC.
Literature search
An elaborate search was made using keywords “Modic changes”, “lumbar Modic changes”, “Modic changes in lumbar spine”, and “vertebral Endplate Spinal Changes”, on pubmed and google (scholar.google.com) databases on the 3rd of March 2020. We identified crucial questions regarding Modic changes and included relevant articles pertaining to these topics for this narrative review.
Results
The initial search using the keywords “Modic changes”, “lumbar Modic changes”, “Modic changes in lumbar spine”, and “vertebral Endplate Spinal Changes” on pubmed yielded a total of 568, 412, 394 and 216 articles on “pubmed” database, respectively. A similar search using the aforementioned keywords yielded a total of 3650, 3548, 3726 and 21570 articles on “google scholar” database. The initial screening involved exclusion of duplicate articles, articles unrelated to MC, animal or other non-clinical studies, and articles in non-English literature based on abstracts or the titles of articles. This initial screening resulted in the identification of 405 articles. Full manuscripts were obtained for all these selected articles and thoroughly scrutinised at the second stage of article selection. All articles not concerning Modic changes, not pertaining to concerned questions, articles concerning other degenerative phenomena, articles discussing cervical or thoracic MC, case reports or animal studies, articles in non-English language and duplicate articles were excluded. Review articles, randomised controlled trials and level 1 studies were given preference. Overall, 69 articles were included in this review.
Conclusion
Modic change (MC) is a dynamic phenomenon and its true etiology is still not definitely known. Disc/end plate injury, occult discitis and autoimmune reactions seem to trigger an inflammatory cascade, which leads to their development. Male sex, older age, diabetes mellitus, genetic factors, smoking, obesity, spinal deformities, higher occupational loads and DDD are known risk factors. There is no conclusive evidence on the causative role of MC in chronic low back pain (LBP) or any influence on the long term outcome in patients with LBP or lumbar disc herniations (LDH). Patients with MC have been reported to have less satisfactory outcome following conservative treatment or discectomy, although the evidence is still unclear.
Keywords: Modic changes, Low back pain, End plate injury, Bone marrow lesion, Degenerativedisc disease
1. Introduction
In 1988, Modic described three types of vertebral marrow signal changes adjacent to endplates on magnetic resonance imaging (MRI).1,2 The clinical relevance of this endplate-marrow phenomenon has been debated over years. Even after two decades since the initial description, there is no lucid consensus regarding the etiology, patho-physiology, clinical significance and surgical relevance of Modic changes (MC) and most issues concerning this phenomenon in degenerative spinal disorders are still controversial.3,4
The current article is a comprehensive review discussing diverse aspects of MC including its historical background, etiology, pathobiology, epidemiology and treatment implications, as well as evidence in the current literature on these issues.
2. Literature search
An elaborate search was made using keywords “Modic changes”, “lumbar Modic changes”, “Modic changes in lumbar spine”, and “vertebral Endplate Spinal Changes”, on pubmed and google (scholar.google.com) databases on the 3rd of March 2020. We identified crucial questions regarding MC and included relevant articles pertaining to these topics.
3. Results
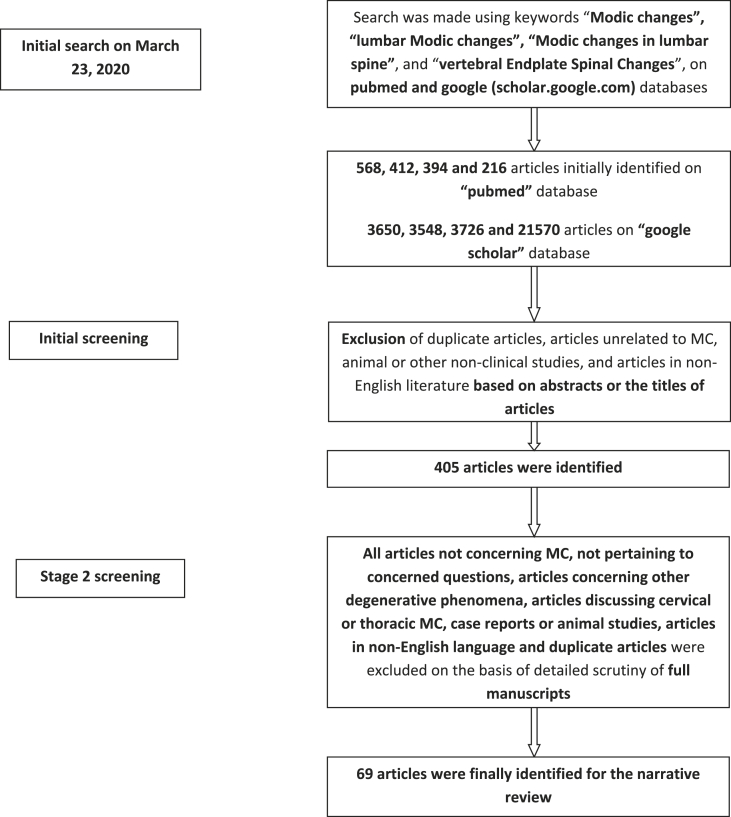
The initial search using the keywords “Modic changes”, “lumbar Modic changes”, “Modic changes in lumbar spine”, and “vertebral Endplate Spinal Changes” on pubmed yielded a total of 568, 412, 394 and 216 articles on “pubmed” database, respectively. A similar search using the aforementioned keywords yielded a total of 3650, 3548, 3726 and 21570 articles on “google scholar” database. The initial screening involved exclusion of duplicate articles, articles unrelated to MC, animal or other non-clinical studies, and articles in non-English literature based on abstracts or the titles of articles. This initial screening resulted in the identification of 405 articles. Full manuscripts were obtained for all these selected articles and thoroughly scrutinised at the second stage of article selection. All articles not concerning Modic changes, not pertaining to concerned questions, articles concerning other degenerative phenomena, articles discussing cervical or thoracic MC, case reports or animal studies, articles in non-English language and duplicate articles were excluded. Review articles, randomised controlled trials and level 1 studies were given preference (Fig. 1). Finally, 69 articles were included in this review. We did not perform any screening [Methodological Index for non-randomized studies (MINORS) or Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria] for including articles.
Fig. 1.
Flowchart depicting the methodology of article selection.
3.1. Low back pain and historic background of MC
Low back pain (LBP) is a common disabling condition, which afflicts 80% of adults during their lifetime.5 Lumbar degenerative disc disease (DDD) is an important cause for LBP.6 With the advent of MRI, the complex relationship between disc, vertebral body, end plates and facet joints; as well as their individual roles in the pathogenesis of LBP have received tremendous attention.7
The disc is avascular with sparse nerve distribution and lacks a strong physiological basis as a primary pain generator.8 Over years, the attention has therefore shifted to other anatomical structures like endplates to explain the etiological basis of LBP.9 Vertebral endplate is a thin interface between bone marrow (BM) and disc; and any loss of its integrity can potentially trigger a cascade of degenerative events.10,11 The endplate is rich in neural elements, and therefore can be a pain generator.12 The two main arms of endplate research are Modic changes (MC) and endplate lesions (Schmorl’s nodes/fractures/erosions/calcifications).13,14
The concept of MC is well-known over the past 30 years and is one of the highly controversial topics till date.15, 16, 17, 18, 19, 20 The current literature review comprehensively discusses various issues regarding MC including historical details, etiology, patho-biology, clinical features, causative role in back pain and other treatment considerations.
In 1987, Roos et al.21 first described BM signal intensity changes immediately adjacent to degenerated discs on MRI. They attributed these changes to DDD itself, rather than infection or tumors. A year later, Modic et al.1,2 classified these signal changes into three categories, still popularly called Modic’s changes (MC) types I, II and III. The basis for this classification was the appearance of lesion on T1-and T2-weighted spin-echo (T1WI and T2WI) sequences on MRI. T1WI is the sequence characterized by short repetition time (TR)/short echo time (TE), while T2WI is characterized by long TR/long TE ratio.22
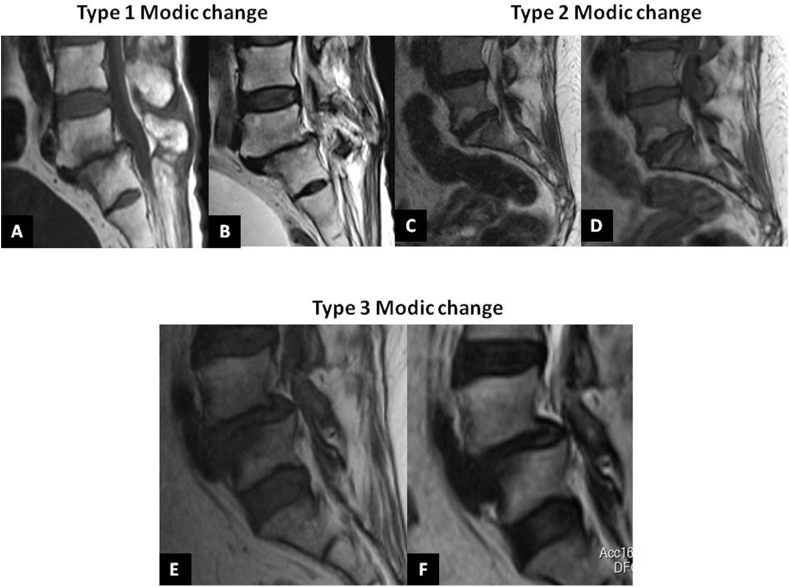
MC type I is characterized by hypo- and hyper-intense signal intensities on T1WI and T2WI, respectively. Histologically, in MC I lesions, endplate is disrupted, fibrous tissue replaces BM amidst thickened trabeculae and disc-bone interface is filled with vascularised granulation tissue.1,2 These changes represent BM edema and inflammation. MC II is defined by hyper-intense signal on T1WI and T2WI. Apart from the aforementioned findings of MC I, MC type II specimens additionally demonstrate fatty marrow replacement. These findings represent conversion of normal hematopoietic marrow into fatty, yellow BM. Type III MC is characterized by hypo-intense signals on both T1WI and T2WI MRI, related to sub-chondral bone sclerosis (Fig. 2).23 Histo-morphometric analysis of specimens from Modic lesions have demonstrated that MC I is characterised by high bone turnover, type II shows decreased bone turnover and type III lesions are stable. These histological features highlight the dynamic interactions between bone and marrow compartments in MC. These findings have also been confirmed by bone scintigraphy.22,24, 25, 26
Fig. 2.
Modic type I change: A - Mid sagittal MRI section (L5-S1) showing hypo-intense signal intensity on T1 weighted image (T1WI) and B – Para sagittal MRI (L5-S1) showing hyper-intense bone marrow lesion on T2WI. C and D - MC type II lesion (L5-S1) showing hyper-intense signal intensity on T1WI and T2WI parasagittal sections, respectively. E and F - Type III MC (at L4-L5) showing hypo-intense signal intensity on both T1WI and T2WI parasagittal sections, respectively. G and H - Type III MC (at L4-L5) showing hypo-intense signal intensity on both T1WI and T2WI parasagittal sections, respectively.
3.1.1. Results of original Modic’s study
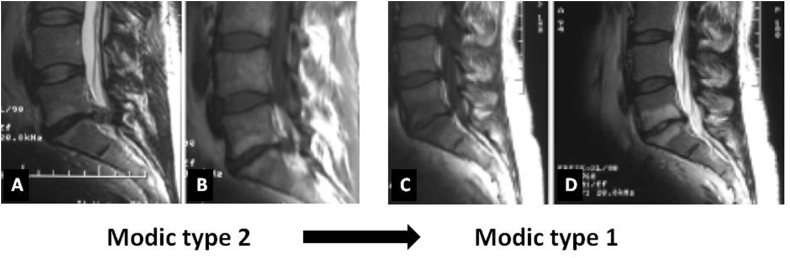
In his original study, Modic1,2 had studied 474 lumbar MR images. Four and 16% of images showed MC types I and II, respectively. Only less than 1% of MRI showed Modic type III changes. Modic followed his patients with type I and II changes longitudinally. Five out of six patients with MC I progressed onto type II over 2–3 years, while 10 patients with MC II remained stable. Modic postulated these signal intensity changes to be a spectrum of vertebral BM changes associated with DDD. He concluded that these 3 types represent different stages of the same pathological process (Fig. 3).
Fig. 3.
MRI showing interval change of Modic lesion at L5-S1 over a period of 9 months. A and B – T2-and T1WI showing parasagittal sections of a typical Modic type 2 lesion. C and D – Mid sagittal sections of T1-and T2WI showing evolution of the same lesions to a larger sized Modic type 1 lesion.
3.2. Etiology and patho-biology of MC
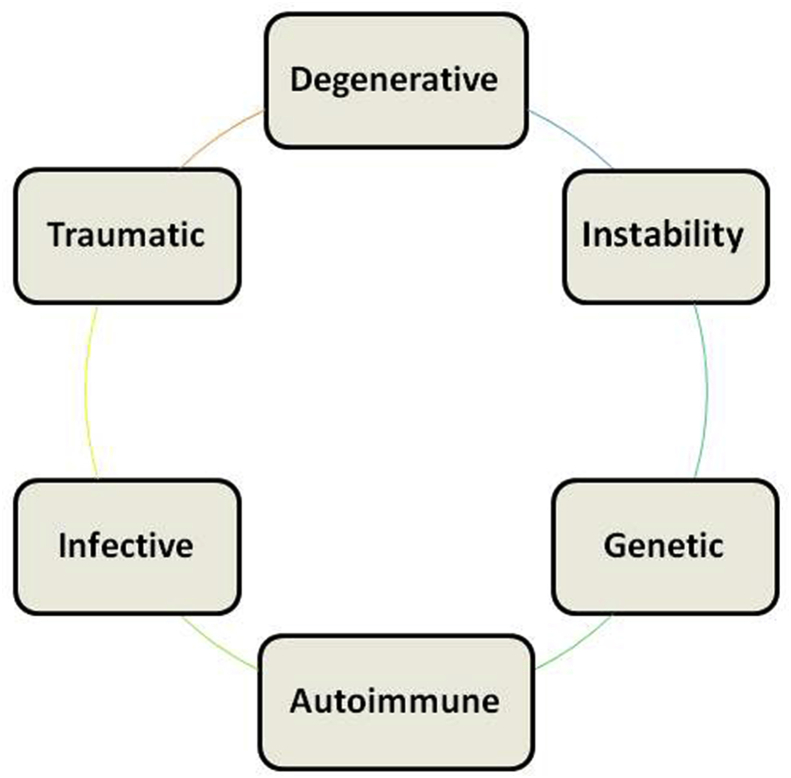
The elucidation of etiology of MC is complicated by multi-factorial patho-physiology and dynamic presentation (Fig. 4). It remains unclear why some patients with DDD develop MC, while others do not. Different factors may pave way for the development of different types and also for the conversion of one MC to another.27 The common risk factors include male sex, older age, duration and severity of diabetes mellitus, genetic factors, smoking, obesity, spinal deformities and higher occupational loads. The most common risk factor for MC is the presence of DDD. There is a high probability of identifying MC in patients with other degenerative findings like disc degeneration (DD), disc herniation (DH) and endplate defects (ED).28, 29, 30
Fig. 4.
Diagram showing multi-factorial etiology of Modic changes.
a) Degenerative disc disease (DDD): Although MC is found adjacent to degenerated discs, DDD alone is not a sufficient trigger for its development.31, 32, 33 The literature demonstrates a significant association of endplate damages (ED) and large DH with MC.23,31,32,34 ED leads to a cascade of degenerative interactions between disc and vertebra. They lead to increased intra-osseous pressure, affect metabolite transport, cause hydraulic vertebral body-disc coupling, and flow of inflammatory mediators from disc to BM. Such events lead to BM lesions adjacent to endplate.35,36 Similar changes in acute fractures heal with time, as the inflammatory stimulus is short-lived. However in degenerative pathologies and in situations of persistent stimuli, there is an accumulative damage to BM, leading to a “frustrated healing response” within bone.37,38 These phenomena may lead to MC type I lesions. Lv et al.9 showed that the size of ED might significantly increase the size of MC lesions, as well as accelerate facet joint (FJ) tropism and degeneration. High incidence of MC I has also been demonstrated at the same levels as large DH.
b) Low-virulent infective discitis: Biological plausibility of infectious etiology of MC emanates from the knowledge of nature of disc including poor vascularity, anerobic environment and low healing capacity.3 It has been demonstrated that damage to peripheral disc can make it vulnerable to infections from organisms of low virulence like Cutibacterium acnes.39, 40, 41 Bacteremia may occur during innocuous daily activities like brushing of teeth.39 While good immune response and aerobic environment within blood and BM curtail bacterial infection, disc may provide a conducive environment for local infection.42 Discitis may result in production of bacterial metabolites and cytokines, which leads to inflammation within adjacent marrow.43 Elevated C-reactive protein (CRP) has been reported in MC I lesions.42
Recently, Manniche et al.44 demonstrated the presence of Cutibacterium acnes biofilm and inflammation in disc tissues of patients with disc herniation and no other signs of infection using fluorescent in situ hybridization (FISH) method. They concluded that a certain subgroup of patients with MC may have an underlying infection of low virulence and may benefit from antibiotic therapy. In another recent systematic review, Jha et al.45 also reported a similar conclusion and emphasized upon the need for further clinical studies to determine the role of infectious etiology in a subset of MC. Nevertheless, certain other prospective studies in the recent literature have questioned the purported association between infection and degeneration changes, and have offered only a moderate support to the disc infection hypothesis in the pathology of MC.46, 47, 48, 49
c) Auto-immunity: After the embryonic development of disc, nucleus pulposus (NP) never comes in contact with systemic circulation. NPs therefore maintain their immunological privilege and carry Fas ligand, capable of inducing apoptosis. When end plate damage occurs, NP co-locates with BM immune cells.50 Following exposure to immune system, NP cells get recognised as “foreign” and an autoimmune reaction gets triggered. In such tissues, high expression of cytokines, macrophages and activated T- and B-cells have been detected.51 Disc cartilage proteoglycans can also trigger autoimmune response through enhanced lymphocyte transformation and monocyte infiltration.52
d) Genetic association: Genetic basis for lumbar DDD has been evaluated in various studies. Rajasekaran et al.53,54 critically reviewed the genetic associations of DDD-related morphological phenotypes like DD, DH, MC, ED and Schmorl’s nodes (SN). They reported genetic association of MC with vitamin D receptor (VDR) and matrix-metalloproteinase-20 (MMP20) genes.55 Biczo15 reported significant associations between specific lumbar DDD endo-phenotypes and VDR variants genes. The search for “magic molecular bullet” which may target DDD-related changes has been a topic for advanced research globally.
3.2.1. Final common pathway – Inflammatory cascade
Although disc/endplate injury, discitis and autoimmune reaction may trigger the inflammatory cascade, development of MC is not uniform. This discrepancy is due to variability in the inflammatory potential of disc material and the ability of BM to respond. There are many similarities between the pathogenesis of BM lesions in osteoarthritis (OA) and MCs.
Some final common pathways for this inflammatory cascade in MC include:
a. Toll-like receptors (TLR): Higher TNFα/IL-1β levels in degenerated discs enhance the TLR2-mediated NF-Kß-responsive gene transcription, as well as IL-6 and IL-8 production.56,57 These cytokines have been significantly associated with MC.
b. TLRs are receptors for bacterial cell wall and damage-associated molecular proteins (DAMP). DAMP molecules are extra-cellular matrix (ECM) molecules, which are released from necrotic cells and play a crucial role in DDD.57
c. These cytokines released from degenerated discs enhance the osteoclastic factors like RANKL, M-CSF etc. and result in altered bone turnover within BM.58
d. The response of BM to inflammatory mediators depends on the composition of BM itself. High marrow adipose tissue (MAT) content leads to increased saturated fatty acids (FA) and oxidised low-density lipoproteins (LDLs), which can cause adipogenesis and fatty marrow conversion, as seen in MC II lesions.59 High MAT may also sometimes cause continued PPARγ activation leading to chronic osteogenesis and a situation similar to MC III.60
e. Hyperloading-related mechanical injury can complement the aforementioned biological factors and contribute to MC pathobiology.61
In short, structural damage triggers an inflammatory cascade in disc, which allows microbial infiltration and/or immune-mediated reactions. These inflammatory reactions cause nociceptive stimuli and activate intra-cellular signalling pathways leading to adipogenesis and osteoclastogenesis. The development of different types of MC depends on the severity and duration of inflammatory stimuli, as well as the degree to which BM can respond.57, 58, 59, 60, 61
3.3. Epidemiology
3.3.1. MC in asymptomatic population
Literature reports a huge variation in the prevalence of MC. In the asymptomatic population, the reported prevalence is around 0.5%–47.1%.62, 63, 64 Majority of the studies indicate that MCs are more common in male patients. Recently, Chen et al.12 observed that MC I are more prevalent in males, while the female counterparts tend to develop MC II more frequently. There is no known ethnic predilection for MC across the globe. Kanna et al.62 recently reported a prevalence of 13% in a cohort of 809 Indian patients, while Vredeveld et al.63 reported 28.6% prevalence of MC among European military personnel. In general, most studies report MC II lesions as commonest sub-type followed by MC I. However, some studies have shown relatively higher prevalence of MC I lesion. This discrepancy may be secondary to a relatively younger study population or greater prevalence of LBP.
Kanna et al.62 reported that 60%, 30%, 5% and 5% of patients presented with single-, two-, three- and four- or multi-level MC, respectively. MC were commonly observed at lower lumbar (L4-S1 levels).1 In the study by Kanna et al.,62 L4-5 was the most commonly involved level (30.7% patients), followed by L5-S1 (26.3%), L3-4 (23.9%), L2-3 (12.4%) and L1-2 (6.8%). The prevalence of MCs, especially MC II has been shown to increase with age and a significant proportion of these lesions develop in patients ≥50 years.65,66 In general, the prevalence varies between 0.5 and 1.9% in adolescents and young adults, as against 5.8–47.1% in middle-aged or older adults.16,65,67, 68, 69 MCs in lumbar MRI are more prevalent in anterior third of vertebral body,31 especially in association with severe DDD or DH. Morphologically, MCs are symmetrical cephalad and caudad to the disc.32,33 Recent studies have demonstrated strong associations between sagittal spino-pelvic alignment and MC. While Zehra et al.14 demonstrated significantly greater prevalence of MC in patients with low pelvic incidence (≤42°), Chen et al.12 showed greater association of high lumbar lordosis (LL) and higher L4-5 lordotic angle with MC.
3.3.2. MC and low back pain (LBP)
Previous systematic reviews by Jensen70 (2008), Zhang71 (2008) and Brijikji72 (2015) demonstrated a significant association between MC and non-specific LBP. While Brinjikji et al.72 found an association between LBP and MC I only, the other two studies did not find any difference between the types of MC.70,71 The overall prevalence of MC in patients with LBP has been reported to vary between 8 and 80.1%.65,70, 71, 72 Among the three types, MC I has been most commonly associated with LBP.65
In 2018, Feng et al.73 reported that ED, DD and MC were all risk factors for severe LBP. Studies have also reported that LBP patients with MC demonstrate a clinically different symptomatology as compared with those without MC.31 In general, those with positive MC report greater frequency and duration of LBP.70 In the meta-analysis by Brijinkji,72 MRI findings including MC I, disc bulge, spondylolysis, DH and DD were more prevalent in individuals ≤50 years with LBP, while “any MC”, lumbar stenosis, high intensity zones (HIZ), annular fissures and spondylolisthesis showed no association with axial pain. They cautioned against interpreting this as causative evidence and purported that these imaging findings may be only considered as candidate biomarkers for LBP.
However, more recent literature indicates a much less lucid association between LBP and MC. A recent meta-analysis by Herlin et al.65 involving 31 major studies revealed no statistically significant association between all types of MC or their sizes and prevalence or severity of low back pain or activity limitation. In a population-based study involving 478 participants,74 there was no statistically significant correlation between presence of MC and LBP over the past 12 months, or during lifetime. Among all MRI parameters, ED demonstrated significant association with lifetime LBP and significantly contributed to the worst pain intensity during previous 12 months. They concluded that ED, rather than MC or DD was the commonly overlooked, independent factor for LBP and a major confounder for other MRI findings.
In short, there is no consensus on whether MC lesions have a causal association with chronic LBP. One of the major issues highlighted in recent literature is the heterogeneity in the nomenclature of MC and other endplate lesions (fractures, erosions, calcifications and Schmorl’s nodes). In a survey involving 55 participants, 84% and 80% of clinicians and researchers respectively, acknowledged significant variations in the existing nomenclature and understanding of endplate pathologies and emphasized upon the need for standardized nomenclature.14
3.3.3. Modic changes and LDH
In a retrospective study involving patients with LDH, Shan et al.75 reported 41.2% prevalence of MC in patients with symptomatic LDH. Among them, type II changes (seen in 30.6% of all patients) were significantly more common than type I (25.7% patients). Chen et al.12 reported greater association of disc bulge with MC I, although no significant association was noticed between MC and other patterns of LDH (extrusions, protrusions or sequestration).
3.3.4. Pitfalls in diagnosing MC lesion
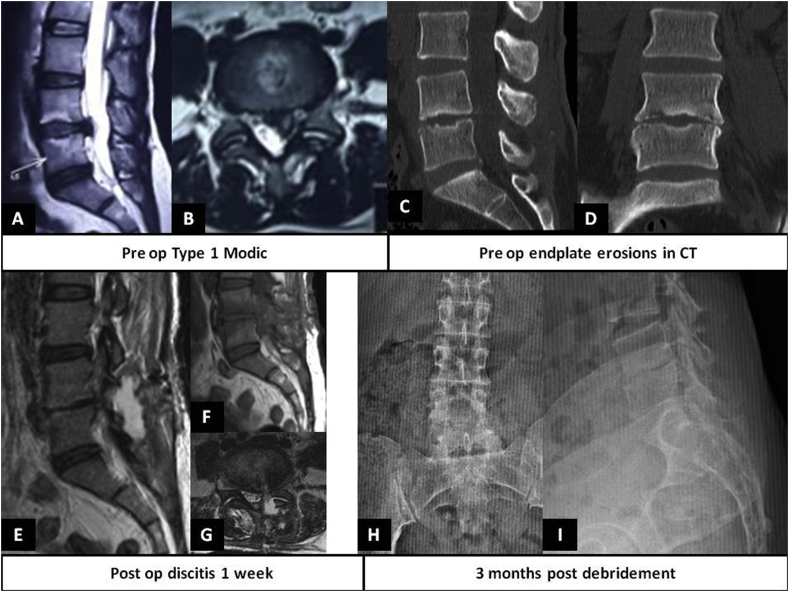
One of the major pitfalls in diagnosing MC is to differentiate it from spondylodiscitis (Fig. 5). Studies have evaluated the possible imaging findings on non-enhanced MRI which may distinguish these two entities. Schwarz-Nemec et al.76 reported that endplate contour, extent of edema, and T1-signal ratios of MC I (extent – 31.96%, T1 signal ratio – 0.83), early spondylodiscitis (56.42%; 0.60) and severe spondylodiscitis (91.84%; 0.61) are significantly different. In patients with vertebral marrow edema, presence of irregular, yet intact T1-W endplate contour has a high accuracy for diagnosing MC I. The role of short tau inversion recovery (STIR) MRI in Modic’s lesion has been discussed. Recently, Kristoffersen et al.77 reported good inter-observer reliability for the interpretation of STIR sequence signal hyper-intensity in Modic’s lesion. Such STIR hyper-intense MRI lesions may indicate ongoing inflammation or edema which has been correlated with painful MC or early infections. Whenever infection is suspected, computerised tomography (CT – to identify bony defects better) and gadolinium contrast-enhanced MRI should be obtained.
Fig. 5.
Pre-operative MRI showing A. T2WI mid-sagittal section revealing large L4-5 disc extrusion and Modic type 1 changes, B. T2WI axial section revealing large L4-5 left sided disc extrusion, C and D. Mid sagittal and coronal CT images revealing significant end plate erosions of adjacent endplates of L4 and L5 vertebrae, E, F, G – Post-operative MRI (at 3 weeks) T2WI, T1WI (parasagittal sections) and axial section at L4-5 disc space showing signs of early infection and possible left sided fluid/abscess collection, H,I – AP and lateral plain radiographs at 3 months post-debridement period showing significant collapse of L4-5 disc space.
Another important differential diagnosis to be considered in young patients with MC I is inflammatory spondyloarthropathy (SpA).78,79 Nguyen et al.78 concluded that despite certain morphological similarities on MRI between MC I and early SpA, these two lesions are completely distinct clinical entities; and patients with MC I do not fulfil all the clinical, biological and imaging criteria for SpA. Canella et al.79 reported that MC I and Andersson’s lesions can sometimes be incorrectly differentiated on the basis of MRI alone in patients with SpA; and a combined diagnostic approach involving clinical, laboratory and radiological evaluations may be necessary to a precise distinction.
3.4. Treatment implications in the presence of MC
Based on the available evidence, MCs are generally considered as “incidental” lesions on MRI and it is unclear if the presence of MC itself is an indication for treatment in patients with chronic LBP. Recent systematic reviews have not demonstrated any influence of MC on long-term outcomes of LBP.80 In the 13-year follow-up study by Udby et al.,80 it was observed that MCs were not negatively associated with long-term pain, sick-leave or disability. In fact, they concluded that LBP patients with MC had significantly less chronic disability. However, these lesions co-exist with other degenerative changes in the lumbar spine (LDH, spondylolisthesis and scoliosis), which may warrant non-surgical/surgical treatment. In a systematic review by Jensen et al.,81 it was concluded that the evidence regarding the clinical importance of MC in determining the ideal treatment option for a patient with low back pain is still largely unclear.
3.4.1. MC and conservative treatment
Conservative treatment (including physical therapy) should obviously be the first line of management in all patients with MC and chronic LBP, although some studies have demonstrated that patients with MC (MC II > I) respond poorly to conservative treatment.32,82 Studies have evaluated roles of bisphosphonate, hyperbaric oxygen and antibiotics, however there is no conclusive evidence on any of these modalities.83,84 Shan et al.75 demonstrated poor response to conservative measures or poor resolution of LDH in patients with concomitant MC. They postulated that the presence of hyaline cartilage makes herniated disc fragment less amenable to vascular infiltration and spontaneous resorption.
3.4.2. MC and antibiotics
In 2013, Albert43 purported the role of infection (Cutibacterium acnes and Corynebacterium propinquum) in the pathophysiology of MC and endorsed the administration of antibiotic therapy (3-month course of amoxicillin-clavulanate) in a select group of patients with MC. Over the past years, concerns have been raised by clinicians in view of potential risk of study bias, conflicting results in subsequent studies and the prospect of treating large number of LBP patients with long-term, high-dose antibiotics.65,85 A recent multi-centered, randomised, Norwegian study has refuted this claim and strongly discouraged the use of antibiotics in MC.46
3.4.3. MC and surgical treatment
DH and MC often co-exist. It is of utmost importance that prior to planning discectomy or fusion in patients with DH and MC, possibility of infection should be thoroughly investigated. Patients with MC I seem to demonstrate poor outcome following micro-discectomy. This has led to the belief that pain in these patients is not only discogenic, but also vertebrogenic or endplate-origin. Increased number of PGP-9.5 nerve fibers and TNF-α positive cells inside MC type I and II endplates may contribute to endplate-origin pain.86, 87, 88 Djuric et al.8 demonstrated the importance of signs of disc inflammation in MC. They showed that patients with pre-operative disc inflammation had less satisfactory outcome after discectomy. Xu et al.89 also observed that patients with MC (especially type I) showed a deteriorating trend in post-operative back pain after trans-foraminal per-cutaneous endoscopic discectomy (TF-PELD). In a study by Lv9 involving patients who underwent TF-PELD, it was observed that progression in ED, rather than worsening DD or MC resulted in progressive deterioration in pain scores and functional disability until 6 weeks post-operatively.
A 2-year prospective study by Bostelman90 evaluated the natural course of MC following micro-discectomies. Interestingly, they concluded that evolution of MC showed a complex pattern, instead of the progressively worsening trend as commonly believed. Majority of MC retained the same sub-type as pre-operative status. There was significant conversion from one type to another (including both upward and downward trends), especially at 12 months in MC II and between 12 and 24 months in MC I. They re-iterated the fact that MCs are dynamic lesions and an assumption of its universal worsening following micro-discectomy should be reconsidered.
Some studies have evaluated whether all patients with MC and DH require lumbar fusion, however there is no strong evidence in favour of such an approach.76,77,90, 91, 92 In a prospective study, Esposito et al.93 demonstrated that the type of MC might influence the outcome following lumbar fusion surgeries. In their cohort, patients with MC II demonstrated poor outcome following fusion, while patients with MC I and III types showed significant improvement in pain and disability. In a retrospective study by Wang94 involving patients undergoing trans-foraminal lumbar fusion, it was concluded that although presence of MC did not negatively influence fusion rates, cage subsidence rates were greater in those with both Modic’s I and II lesions. Liu et al.91 demonstrated that patients with MC and endplate sclerosis had significantly reduced cage subsidence following oblique lumbar inter-body fusion. In a prospective, multicenter, trial involving patients undergoing “lumbar decompression-alone” or “lumbar decompression with fusion”, Ulrich92 reported no significant difference in post-operative clinical outcome (until 36 months post-operatively) between patients with MC and those without MC.
Thus, although current literature indicates that patients with MC tend to show relatively poorer outcome following conservative treatment or discectomies, there is no significant evidence favouring definitive fusion procedures or recommending alternative strategies for managing these lesions.76,77,90, 91, 92
4. Conclusion
Despite such extensive research, our understanding of MCs is still incomplete. Disc/end plate injury, occult discitis and autoimmune reactions seem to trigger an inflammatory cascade, which leads to their development. Male sex, older age, diabetes mellitus, genetic factors, smoking, obesity, spinal deformities, higher occupational loads and DDD are known risk factors. There is no conclusive evidence on the causative role of MC in chronic LBP or any influence on the long-term outcome in patients with LBP or LDH. Patients with MC generally have less satisfactory outcome following conservative treatment or discectomy, although the evidence is still unclear. Overall, there is huge scope for advanced research on this phenomenon in the years ahead. Large scale, multi-center trials in future can help us understand MC better, bring to light its clinical and surgical relevance and pave way for better management of LBP.
Contributor Information
Vibhu Krishnan Viswanathan, Email: drvibu007@gmail.com.
Ajoy Prasad Shetty, Email: ajoyshetty@gmail.com.
S. Rajasekaran, Email: rajasekaran.orth@gmail.com.
References
- 1.Modic M.T., Steinberg P.M., Ross J.S., Masaryk T.J., Carter J.R. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1 Pt 1):193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 2.Modic M.T., Masaryk T.J., Ross J.S., Carter J.R. Imaging of degenerative disk disease. Radiology. 1988;168(1):177–186. doi: 10.1148/radiology.168.1.3289089. [DOI] [PubMed] [Google Scholar]
- 3.Georgy M., Stern M., Murphy K. What is the role of the bacterium propionibacterium acnes in type 1 modic changes? A review of the literature. J Assoc Can Radiol. 2017;68(4):419–424. doi: 10.1016/j.carj.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Martínez-Quiñones J.V., Aso-Escario J., González-García L., Consolini F., Arregui-Calvo R. Are modic changes able to help us in our clinical practice? A study of the modic changes in young adults during working age. Clin Spine Surg. 2017;30(6):259–264. doi: 10.1097/BSD.0000000000000195. [DOI] [PubMed] [Google Scholar]
- 5.Balagué F., Mannion A.F., Pellisé F., Cedraschi C. Non-specific low back pain. Lancet Lond Engl. 2012;379(9814):482–491. doi: 10.1016/S0140-6736(11)60610-7. [DOI] [PubMed] [Google Scholar]
- 6.Andersson G.B. Epidemiological features of chronic low-back pain. Lancet Lond Engl. 1999;354(9178):581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 7.Rahyussalim A.J., Zufar M.L.L., Kurniawati T. Significance of the association between disc degeneration changes on imaging and low back pain: a review article. Asian Spine J. 2020;14(2):245–257. doi: 10.31616/asj.2019.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Djuric N., Yang X., Ostelo R.W.J.G. Disc inflammation and Modic changes show an interaction effect on recovery after surgery for lumbar disc herniation. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2019;28(11):2579–2587. doi: 10.1007/s00586-019-06108-9. [DOI] [PubMed] [Google Scholar]
- 9.Lv B., Yuan J., Ding H. Relationship between endplate defects, modic change, disc degeneration, and facet joint degeneration in patients with low back pain. BioMed Res Int. 2019;2019 doi: 10.1155/2019/9369853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.el Barzouhi A., Vleggeert-Lankamp C.L.A.M., van der Kallen B.F. Back pain’s association with vertebral end-plate signal changes in sciatica. Off J North Am Spine Soc. 2014;14(2):225–233. doi: 10.1016/j.spinee.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 11.Urban J.P.G., Smith S., Fairbank J.C.T. Nutrition of the intervertebral disc. Spine. 2004;29(23):2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y., Bao J., Yan Q., Wu C., Yang H., Zou J. Distribution of Modic changes in patients with low back pain and its related factors. Eur J Med Res. 2019;24(1):34. doi: 10.1186/s40001-019-0393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y., Videman T., Battié M.C. Morphometrics and lesions of vertebral end plates are associated with lumbar disc degeneration: evidence from cadaveric spines. J Bone Joint Surg Am. 2013;95(5):e26. doi: 10.2106/JBJS.L.00124. [DOI] [PubMed] [Google Scholar]
- 14.Zehra U., Cheung J.P.Y., Bow C. Spinopelvic alignment predicts disc calcification, displacement, and Modic changes: evidence of an evolutionary etiology for clinically-relevant spinal phenotypes. JOR Spine. 2020;3(1) doi: 10.1002/jsp2.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biczo A., Szita J., McCall I., Varga P.P., Genodisc Consortium. Lazary A. Association of vitamin D receptor gene polymorphisms with disc degeneration. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2020;29(3):596–604. doi: 10.1007/s00586-019-06215-7. [DOI] [PubMed] [Google Scholar]
- 16.Mok F.P.S., Samartzis D., Karppinen J., Fong D.Y.T., Luk K.D.K., Cheung K.M.C. Modic changes of the lumbar spine: prevalence, risk factors, and association with disc degeneration and low back pain in a large-scale population-based cohort. Off J North Am Spine Soc. 2016;16(1):32–41. doi: 10.1016/j.spinee.2015.09.060. [DOI] [PubMed] [Google Scholar]
- 17.Pang H., Bow C., Cheung J.P.Y. The UTE disc sign on MRI: a novel imaging biomarker associated with degenerative spine changes, low back pain, and disability. Spine. 2018;43(7):503–511. doi: 10.1097/BRS.0000000000002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lotz J.C., Fields A.J., Liebenberg E.C. The role of the vertebral end plate in low back pain. Global Spine J. 2013;3(3):153–164. doi: 10.1055/s-0033-1347298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown M.F., Hukkanen M.V., McCarthy I.D. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg Br. 1997;79(1):147–153. doi: 10.1302/0301-620x.79b1.6814. [DOI] [PubMed] [Google Scholar]
- 20.Kuslich S.D., Ulstrom C.L., Michael C.J. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin N Am. 1991;22(2):181–187. [PubMed] [Google Scholar]
- 21.de Roos A., Kressel H., Spritzer C., Dalinka M. MR imaging of marrow changes adjacent to end plates in degenerative lumbar disk disease. AJR Am J Roentgenol. 1987;149(3):531–534. doi: 10.2214/ajr.149.3.531. [DOI] [PubMed] [Google Scholar]
- 22.Perilli E., Parkinson I.H., Truong L.-H., Chong K.C., Fazzalari N.L., Osti O.L. Modic (endplate) changes in the lumbar spine: bone micro-architecture and remodelling. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2015;24(9):1926–1934. doi: 10.1007/s00586-014-3455-z. [DOI] [PubMed] [Google Scholar]
- 23.Albert H.B., Briggs A.M., Kent P., Byrhagen A., Hansen C., Kjaergaard K. The prevalence of MRI-defined spinal pathoanatomies and their association with modic changes in individuals seeking care for low back pain. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2011;20(8):1355–1362. doi: 10.1007/s00586-011-1794-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuisma M., Karppinen J., Haapea M., Lammentausta E., Niinimäki J., Tervonen O. Modic changes in vertebral endplates: a comparison of MR imaging and multislice CT. Skeletal Radiol. 2009;38(2):141–147. doi: 10.1007/s00256-008-0590-9. [DOI] [PubMed] [Google Scholar]
- 25.Schmid G., Witteler A., Willburger R., Kuhnen C., Jergas M., Koester O. Lumbar disk herniation: correlation of histologic findings with marrow signal intensity changes in vertebral endplates at MR imaging. Radiology. 2004;231(2):352–358. doi: 10.1148/radiol.2312021708. [DOI] [PubMed] [Google Scholar]
- 26.Järvinen J., Niinimäki J., Karppinen J., Takalo R., Haapea M., Tervonen O. Does bone scintigraphy show Modic changes associated with increased bone turnover? Eur J Radiol Open. 2020;7 doi: 10.1016/j.ejro.2020.100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson K.J., Dagher A.P., Eckel T.S., Clark M., Reinig J.W. Modic changes on MR images as studied with provocative diskography: clinical relevance--a retrospective study of 2457 disks. Radiology. 2009;250(3):849–855. doi: 10.1148/radiol.2503080474. [DOI] [PubMed] [Google Scholar]
- 28.Karppinen J., Solovieva S., Luoma K., Raininko R., Leino-Arjas P., Riihimäki H. Modic changes and interleukin 1 gene locus polymorphisms in occupational cohort of middle-aged men. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2009;18(12):1963–1970. doi: 10.1007/s00586-009-1139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farshad-Amacker N.A., Hughes A.P., Aichmair A., Herzog R.J., Farshad M. Determinants of evolution of endplate and disc degeneration in the lumbar spine: a multifactorial perspective. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2014;23(9):1863–1868. doi: 10.1007/s00586-014-3382-z. [DOI] [PubMed] [Google Scholar]
- 30.Karchevsky M., Schweitzer M.E., Carrino J.A., Zoga A., Montgomery D., Parker L. Reactive endplate marrow changes: a systematic morphologic and epidemiologic evaluation. Skeletal Radiol. 2005;34(3):125–129. doi: 10.1007/s00256-004-0886-3. [DOI] [PubMed] [Google Scholar]
- 31.Kjaer P., Korsholm L., Bendix T., Sorensen J.S., Leboeuf-Yde C. Modic changes and their associations with clinical findings. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2006;15(9):1312–1319. doi: 10.1007/s00586-006-0185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen T.S., Bendix T., Sorensen J.S., Manniche C., Korsholm L., Kjaer P. Characteristics and natural course of vertebral endplate signal (Modic) changes in the Danish general population. BMC Muscoskel Disord. 2009;10:81. doi: 10.1186/1471-2474-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudli S., Fields A.J., Samartzis D., Karppinen J., Lotz J.C. Pathobiology of modic changes. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2016;25(11):3723–3734. doi: 10.1007/s00586-016-4459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen T.S., Kjaer P., Korsholm L. Predictors of new vertebral endplate signal (Modic) changes in the general population. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2010;19(1):129–135. doi: 10.1007/s00586-009-1184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoganandan N., Larson S.J., Gallagher M., Pintar F.A., Reinartz J., Droese K. Correlation of microtrauma in the lumbar spine with intraosseous pressures. Spine. 1994;19(4):435–440. doi: 10.1097/00007632-199402001-00009. [DOI] [PubMed] [Google Scholar]
- 36.Dudli S., Haschtmann D., Ferguson S.J. Persistent degenerative changes in the intervertebral disc after burst fracture in an in vitro model mimicking physiological post-traumatic conditions. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2015;24(9):1901–1908. doi: 10.1007/s00586-014-3301-3. [DOI] [PubMed] [Google Scholar]
- 37.Rajasekaran S., Babu J.N., Arun R., Armstrong B.R.W., Shetty A.P., Murugan S. ISSLS prize winner: a study of diffusion in human lumbar discs: a serial magnetic resonance imaging study documenting the influence of the endplate on diffusion in normal and degenerate discs. Spine. 2004;29(23):2654–2667. doi: 10.1097/01.brs.0000148014.15210.64. [DOI] [PubMed] [Google Scholar]
- 38.Adams M.A., Freeman B.J., Morrison H.P., Nelson I.W., Dolan P. Mechanical initiation of intervertebral disc degeneration. Spine. 2000;25(13):1625–1636. doi: 10.1097/00007632-200007010-00005. [DOI] [PubMed] [Google Scholar]
- 39.Albert H.B., Kjaer P., Jensen T.S., Sorensen J.S., Bendix T., Manniche C. Modic changes, possible causes and relation to low back pain. Med Hypotheses. 2008;70(2):361–368. doi: 10.1016/j.mehy.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 40.Stirling A., Worthington T., Rafiq M., Lambert P.A., Elliott T.S. Association between sciatica and Propionibacterium acnes. Lancet Lond Engl. 2001;357(9273):2024–2025. doi: 10.1016/S0140-6736(00)05109-6. [DOI] [PubMed] [Google Scholar]
- 41.Wedderkopp N., Thomsen K., Manniche C., Kolmos H.J., Secher Jensen T., Leboeuf Yde C. No evidence for presence of bacteria in modic type I changes. Acta Radiol Stockh Swed. 1987;50(1):65–70. doi: 10.1080/02841850802524485. 2009. [DOI] [PubMed] [Google Scholar]
- 42.Rannou F., Ouanes W., Boutron I. High-sensitivity C-reactive protein in chronic low back pain with vertebral end-plate Modic signal changes. Arthritis Rheum. 2007;57(7):1311–1315. doi: 10.1002/art.22985. [DOI] [PubMed] [Google Scholar]
- 43.Albert H.B., Lambert P., Rollason J. Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2013;22(4):690–696. doi: 10.1007/s00586-013-2674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manniche C., O’Neill S. New insights link low-virulent disc infections to the etiology of severe disc degeneration and Modic changes. Future Sci OA. 2019;5(5) doi: 10.2144/fsoa-2019-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jha S.C., Sairyo K. The role of Propionibacterium acnes in and Modic type 1 changes : A literature review. J Med Investig JMI. 2020;67(12):21–26. doi: 10.2152/jmi.67.21. [DOI] [PubMed] [Google Scholar]
- 46.Bråten L.C.H., Rolfsen M.P., Espeland A. Efficacy of antibiotic treatment in patients with chronic low back pain and Modic changes (the AIM study): double blind, randomised, placebo controlled, multicentre trial. BMJ. 2019;367:l5654. doi: 10.1136/bmj.l5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmed-Yahia S., Decousser J.-W., Flouzat-Lachaniette C.H. Is the discopathy associated with Modic changes an infectious process? Results from a prospective monocenter study. PloS One. 2019;14(8) doi: 10.1371/journal.pone.0221030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rigal J., Thelen T., Byrne F. Prospective study using anterior approach did not show association between Modic 1 changes and low grade infection in lumbar spine. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2016;25(4):1000–1005. doi: 10.1007/s00586-016-4396-5. [DOI] [PubMed] [Google Scholar]
- 49.Fritzell P., Welinder-Olsson C., Jönsson B. Bacteria: back pain, leg pain and Modic sign-a surgical multicentre comparative study. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2019;28(12):2981–2989. doi: 10.1007/s00586-019-06164-1. [DOI] [PubMed] [Google Scholar]
- 50.Kaneyama S., Nishida K., Takada T. Fas ligand expression on human nucleus pulposus cells decreases with disc degeneration processes. Off J Jpn Orthop Sci Assoc. 2008;13(2):130–135. doi: 10.1007/s00776-007-1204-4. [DOI] [PubMed] [Google Scholar]
- 51.Wang F., Jiang J.-M., Deng C.-H., Wang F.-L., Fu Z.-Z., Zhang Z.-F. Expression of Fas receptor and apoptosis in vertebral endplates with degenerative disc diseases categorized as Modic type I or II. Injury. 2011;42(8):790–795. doi: 10.1016/j.injury.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 52.Geiss A., Larsson K., Junevik K., Rydevik B., Olmarker K. Autologous nucleus pulposus primes T cells to develop into interleukin-4-producing effector cells: an experimental study on the autoimmune properties of nucleus pulposus. Off J Publ Orthop Res Soc. 2009;27(1):97–103. doi: 10.1002/jor.20691. [DOI] [PubMed] [Google Scholar]
- 53.Kanna R.M., Shetty A.P., Rajasekaran S. Patterns of lumbar disc degeneration are different in degenerative disc disease and disc prolapse magnetic resonance imaging analysis of 224 patients. Off J North Am Spine Soc. 2014;14(2):300–307. doi: 10.1016/j.spinee.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 54.Rajasekaran S., Kanna R.M., Reddy R.R. How reliable are the reported genetic associations in disc degeneration?: the influence of phenotypes, age, population size, and inclusion sequence in 809 patients. Spine. 2016;41(21):1649–1660. doi: 10.1097/BRS.0000000000001847. [DOI] [PubMed] [Google Scholar]
- 55.Zehra U., Bow C., Lotz J.C. Structural vertebral endplate nomenclature and etiology: a study by the ISSLS Spinal Phenotype Focus Group. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2018;27(1):2–12. doi: 10.1007/s00586-017-5292-3. [DOI] [PubMed] [Google Scholar]
- 56.Klawitter M., Hakozaki M., Kobayashi H. Expression and regulation of toll-like receptors (TLRs) in human intervertebral disc cells. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2014;23(9):1878–1891. doi: 10.1007/s00586-014-3442-4. [DOI] [PubMed] [Google Scholar]
- 57.Piccinini A.M., Midwood K.S. DAMPening inflammation by modulating TLR signalling. Mediat Inflamm. 2010 doi: 10.1155/2010/672395. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Torkki M., Majuri M.-L., Wolff H. Osteoclast activators are elevated in intervertebral disks with Modic changes among patients operated for herniated nucleus pulposus. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2016;25(1):207–216. doi: 10.1007/s00586-015-3897-y. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz A.V., Sigurdsson S., Hue T.F. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J Clin Endocrinol Metab. 2013;98(6):2294–2300. doi: 10.1210/jc.2012-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ott C., Jacobs K., Haucke E., Navarrete Santos A., Grune T., Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–429. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nuttall M.E., Shah F., Singh V., Thomas-Porch C., Frazier T., Gimble J.M. Adipocytes and the regulation of bone remodeling: a balancing act. Calcif Tissue Int. 2014;94(1):78–87. doi: 10.1007/s00223-013-9807-6. [DOI] [PubMed] [Google Scholar]
- 62.Kanna R.M., Shanmuganathan R., Rajagopalan V.R. Prevalence, patterns, and genetic association analysis of modic vertebral endplate changes. Asian Spine J. 2017;11(4):594–600. doi: 10.4184/asj.2017.11.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vredeveld T., Teitsma X.M., Mert A., Van der Wurff P. Prevalence of modic changes in active duty military men with lumbar disk herniation who were scheduled for surgery. J Manip Physiol Ther. 2012;35(8):622–628. doi: 10.1016/j.jmpt.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 64.Albert H.B., Sorensen J.S., Christensen B.S., Manniche C. Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): a double-blind randomized clinical controlled trial of efficacy. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2013;22(4):697–707. doi: 10.1007/s00586-013-2675-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herlin C., Kjaer P., Espeland A. Modic changes-Their associations with low back pain and activity limitation: a systematic literature review and meta-analysis. PloS One. 2018;13(8) doi: 10.1371/journal.pone.0200677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y., Videman T., Battié M.C. Modic changes: prevalence, distribution patterns, and association with age in white men. Off J North Am Spine Soc. 2012;12(5):411–416. doi: 10.1016/j.spinee.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kjaer P., Leboeuf-Yde C., Sorensen J.S., Bendix T. An epidemiologic study of MRI and low back pain in 13-year-old children. Spine. 2005;30(7):798–806. doi: 10.1097/01.brs.0000157424.72598.ec. [DOI] [PubMed] [Google Scholar]
- 68.Koyama K., Nakazato K., Min S. Radiological abnormalities and low back pain in gymnasts. Int J Sports Med. 2013;34(3):218–222. doi: 10.1055/s-0032-1316366. [DOI] [PubMed] [Google Scholar]
- 69.Kuisma M., Karppinen J., Niinimäki J. Modic changes in endplates of lumbar vertebral bodies: prevalence and association with low back and sciatic pain among middle-aged male workers. Spine. 2007;32(10):1116–1122. doi: 10.1097/01.brs.0000261561.12944.ff. [DOI] [PubMed] [Google Scholar]
- 70.Jensen T.S., Karppinen J., Sorensen J.S., Niinimäki J., Leboeuf-Yde C. Vertebral endplate signal changes (Modic change): a systematic literature review of prevalence and association with non-specific low back pain. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2008;17(11):1407–1422. doi: 10.1007/s00586-008-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y.-H., Zhao C.-Q., Jiang L.-S., Chen X.-D., Dai L.-Y. Modic changes: a systematic review of the literature. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2008;17(10):1289–1299. doi: 10.1007/s00586-008-0758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brinjikji W., Diehn F.E., Jarvik J.G. MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2015;36(12):2394–2399. doi: 10.3174/ajnr.A4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feng Z., Liu Y., Yang G., Battié M.C., Wang Y. Lumbar vertebral endplate defects on magnetic resonance images: classification, distribution patterns, and associations with modic changes and disc degeneration. Spine. 2018;43(13):919–927. doi: 10.1097/BRS.0000000000002450. [DOI] [PubMed] [Google Scholar]
- 74.Chen L., Battié M.C., Yuan Y., Yang G., Chen Z., Wang Y. Lumbar vertebral endplate defects on magnetic resonance images: prevalence, distribution patterns, and associations with back pain. Off J North Am Spine Soc. 2020;20(3):352–360. doi: 10.1016/j.spinee.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 75.Shan Z., Fan S., Xie Q. Spontaneous resorption of lumbar disc herniation is less likely when modic changes are present. Spine. 2014;39(9):736–744. doi: 10.1097/BRS.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 76.Schwarz-Nemec U., Friedrich K.M., Stihsen C. Vertebral bone marrow and endplate Assessment on MR imaging for the differentiation of modic type 1 endplate changes and infectious spondylodiscitis. J Clin Med. 2020;9(3) doi: 10.3390/jcm9030826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kristoffersen P.M., Vetti N., Storheim K. Short tau inversion recovery MRI of Modic changes: a reliability study. Acta Radiol Open. 2020;9(1) doi: 10.1177/2058460120902402. 2058460120902402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nguyen C., Bendeddouche I., Sanchez K. Assessment of ankylosing spondylitis criteria in patients with chronic low back pain and vertebral endplate Modic I signal changes. J Rheumatol. 2010;37(11):2334–2339. doi: 10.3899/jrheum.100165. [DOI] [PubMed] [Google Scholar]
- 79.Canella C., Schau B., Ribeiro E., Sbaffi B., Marchiori E. MRI in seronegative spondyloarthritis: imaging features and differential diagnosis in the spine and sacroiliac joints. AJR Am J Roentgenol. 2013;200(1):149–157. doi: 10.2214/AJR.12.8858. [DOI] [PubMed] [Google Scholar]
- 80.Udby P.M., Bendix T., Ohrt-Nissen S. Modic changes are not associated with long-term pain and disability: a cohort study with 13-year follow-up. Spine. 2019;44(17):1186–1192. doi: 10.1097/BRS.0000000000003051. [DOI] [PubMed] [Google Scholar]
- 81.Jensen R.K., Leboeuf-Yde C. Is the presence of modic changes associated with the outcomes of different treatments? A systematic critical review. BMC Muscoskel Disord. 2011;12:183. doi: 10.1186/1471-2474-12-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jensen O.K., Nielsen C.V., Sørensen J.S., Stengaard-Pedersen K. Type 1 Modic changes was a significant risk factor for 1-year outcome in sick-listed low back pain patients: a nested cohort study using magnetic resonance imaging of the lumbar spine. Off J North Am Spine Soc. 2014;14(11):2568–2581. doi: 10.1016/j.spinee.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 83.Koivisto K., Karppinen J., Haapea M. The effect of zoledronic acid on serum biomarkers among patients with chronic low back pain and modic changes in lumbar magnetic resonance imaging. Diagn Basel Switz. 2019;9(4) doi: 10.3390/diagnostics9040212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen Y., Yang H., Zhang L., Wang Y., Zou J. Analyzing the influence of modic changes on patients with lower back pain undergoing conservative treatment. Pain Res Manag. 2019;2019 doi: 10.1155/2019/8185316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lings S. Antibiotics for low back pain? Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2014;23(2):469–472. doi: 10.1007/s00586-013-2977-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lurie J.D., Moses R.A., Tosteson A.N.A. Magnetic resonance imaging predictors of surgical outcome in patients with lumbar intervertebral disc herniation. Spine. 2013;38(14):1216–1225. doi: 10.1097/BRS.0b013e31828ce66d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ohtori S., Inoue G., Ito T. Tumor necrosis factor-immunoreactive cells and PGP 9.5-immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back Pain and Modic Type 1 or Type 2 changes on MRI. Spine. 2006;31(9):1026–1031. doi: 10.1097/01.brs.0000215027.87102.7c. [DOI] [PubMed] [Google Scholar]
- 88.Fields A.J., Liebenberg E.C., Lotz J.C. Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. Off J North Am Spine Soc. 2014;14(3):513–521. doi: 10.1016/j.spinee.2013.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu J., Li Y., Wang B. Percutaneous endoscopic lumbar discectomy for lumbar disc herniation with modic changes via a transforaminal approach: a retrospective study. Pain Physician. 2019;22(6):E601–E608. [PubMed] [Google Scholar]
- 90.Bostelmann R., Petridis A., Fischer K., Vajkoczy P., Bostelmann T., Barth M. New insights into the natural course and clinical relevance of Modic changes over 2 years following lumbar limited discectomy: analysis of prospective collected data. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2019;28(11):2551–2561. doi: 10.1007/s00586-019-05988-1. [DOI] [PubMed] [Google Scholar]
- 91.Liu J., Ding W., Yang D. Modic changes (MCs) associated with endplate sclerosis can prevent cage subsidence in oblique lumbar interbody fusion (OLIF) stand-alone. World Neurosurg. 2020 doi: 10.1016/j.wneu.2020.02.047. Published online February 17. [DOI] [PubMed] [Google Scholar]
- 92.Ulrich N.H., Burgstaller J.M., Gravestock I. The influence of endplate (Modic) changes on clinical outcomes in lumbar spinal stenosis surgery: a Swiss prospective multicenter cohort study. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2020;10 doi: 10.1007/s00586-020-06364-0. Published online March. [DOI] [PubMed] [Google Scholar]
- 93.Esposito P., Pinheiro-Franco J.L., Froelich S., Maitrot D. Predictive value of MRI vertebral end-plate signal changes (Modic) on outcome of surgically treated degenerative disc disease. Results of a cohort study including 60 patients. Neurochirurgie. 2006;52(4):315–322. doi: 10.1016/s0028-3770(06)71225-5. [DOI] [PubMed] [Google Scholar]
- 94.Wang M.Y., Xu L., Qiu Y. Effect of Modic changes on fusion rate and cage subsidence after transforaminal lumbar interbody fusion. Zhonghua Yixue Zazhi. 2019;99(47):3703–3709. doi: 10.3760/cma.j.issn.0376-2491.2019.47.006. [DOI] [PubMed] [Google Scholar]