Corresponding Author

Key Words: autonomic nervous system, cardiac contractility, optogenetics, vagal nerve stimulation
In Ancient Chinese philosophy, yin and yang express a concept of dualism, underlying how seemingly opposite or contrary forces may actually be complementary, interconnected, and interdependent in the natural world. This concept perfectly applies to cardiovascular autonomic control, at least in physiological conditions. Sympathetic and parasympathetic efferent neurons tightly collaborate in a closed-loop system to constantly adjust cardiac output to body demands based on sensory feedback and feedforward control loops and complex integrative mechanisms. In pathological conditions, when the homeostatic capability of the system is overcome, maladaptive responses occur, leading, in the great majority of cases, to excessive sympathoexcitation coupled with reduced parasympathetic output. As opposed to the classical theory of the centrally determined cardiac neuronal command, it is now well established that the first integration of cardiac sensory inputs occurs at the peripheral level, within interneurons of the intrinsic cardiac ganglia; these ganglia constitute the so-called intrinsic cardiac nervous system, whose structural and functional integrity is crucial to preserve cardiac function. Parasympathetic postganglionic neurons within each ganglion receive bilateral projection via the vagal nerves and in turn project to broad areas of preferential, albeit not exclusive, influence within the heart. Of note, despite the antiarrhythmic properties of vagal nerve stimulation (VNS) were first anecdotally reported in 1859, it was more than 100 years later that the existence of parasympathetic projections to the ventricles was first demonstrated. Left VNS acutely decreased left ventricular (LV) contractility and relaxation rate in vivo both in animal and in human paced hearts. The acute negative inotropic effect was blocked by muscarinic antagonism and attenuated by β-blockade, and the same was found for the increase in ventricular effective refractory period produced by VNS. The reduction in ventricular inotropism and the increase in effective refractory period duration in normal hearts were quantitatively related, underlying the importance of autonomic influences to regulate electromechanical coupling; ICaL inhibition by cholinergic muscarinic receptor signaling is thought to play a pivotal role in these effects. Nonetheless, the existence of a tonic inhibitory muscarinic influence on cardiac inotropy has long been questioned.
In 2016, Machhada et al. (1) demonstrated that atropine systemic administration under β-blockade and C1 level spinal cord transection increases LV contractility of anaesthetized rats. Preganglionic parasympathetic neurons of the nucleus ambiguous and the vagal dorsal motor nucleus (DVMN) were suggested to have a preferential control over pacemaker and ventricular tissue, respectively. From an anatomical point of view, canine studies showed that most efferent vagal fibers to the LV first cross the atrioventricular groove and then dive intramurally to the subendocardium, as opposed to efferent sympathetic fibers located throughout their course in the subepicardium along with coronary arteries. Both sympathetic and parasympathetic fibers run from the base to the apex of the heart; their different anatomical course originally led to speculate that parasympathetic dysfunction after myocardial infarction (MI) might be the result of subendocardial injury. This hypothesis is in overt disagreement with several preclinical studies showing beneficial effects of electrical right VNS applied after healed MI on overall survival, LV function, and ventricular arrhythmias susceptibility (2). Despite the large amount of data on the sympathetic component, information about chronic parasympathetic remodeling after MI has long been lacking. In 1988, Inoue and Zipes (3) demonstrated that a functional parasympathetic denervation occurs, in addition to the sympathetic one, distally to a transmural MI and within 180 min of coronary legation. Almost 30 years later, using a chronic post-MI porcine model, Vaseghi et al. (4) showed that in contrast to norepinephrine levels, cardiac acetylcholine levels remain preserved 6 to 8 weeks after MI in border zones and in viable myocardium of infarcted hearts. Yet, in vivo neuronal recordings from postganglionic parasympathetic neurons demonstrated abnormalities in resting firing frequency and in responses to stimuli. Overall, these data prove that parasympathetic cardiac neuronal network is anatomically intact but profoundly dysfunctional after a healed MI, therefore reinforcing the strong pathophysiological rationale for therapeutic interventions aimed to restore a proper cardiac vagal output. Of note, in the setting of electrical VNS a preferentially efferent, rather than afferent, fiber activation has been shown to depend on the shape and the orientation of the stimulating electrode as well as the frequencies, pulse widths, and currents used (5). Finding the most effective stimulation parameters is both challenging technically and extremely cumbersome to investigate clinically. Moreover, because an abnormal cardiovascular afferent signaling significantly contributes to the condition of autonomic imbalance, questions have been raised about the potential contribution of afferent mechanisms to the beneficial effects of electrical VNS observed in preclinical studies.
In this issue of JACC: Basic to Translational Science, Machhada et al. (6) have used the elegant technology of optogenetic stimulation to assess the effects of a 4-week program of intermittent (15 min every 48 h) DVMN neurons activation on LV contractility and exercise capacity in normal and in post-MI rats. Four groups were studied in order to guarantee that both experimental conditions (sham-MI and post-MI rats) had a control arm, represented by animals implanted with the optogenetic electrode but insensitive to blue light–induced DVMN activation. Echocardiography, direct hemodynamic assessment, and exercise stress test were performed in all groups at the end of the study period, therefore precluding the possibility to assess optogenetic VNS induced changes within the same group. In post-MI rats, despite an infarct size of at least 30% of LV mass, optogenetic VNS improved LV systolic function and exercise capacity as compared with post-MI rats with a similar infarct size not receiving VNS. In sham-MI animals, the group who received optogenetic VNS showed a better LV systolic function and exercise capacity, with no differences in resting mean arterial pressure. The favorable results obtained in post-MI rats agree with previous preclinical studies of electrical VNS in both ischemic and nonischemic heart failure models pursuing a preferentially efferent stimulation. Antiadrenergic (at both pre- and post-synaptic levels) as well as muscarinic and nicotinic cholinergic mechanisms of action for efferent VNS have been consistently demonstrated in models of cardiac pathology. Of note, as compared with the landmark study of Li et al. (7) (with electric stimulation of the right cervical vagus nerve), in this study optogenetic VNS was applied earlier after MI (e.g., 48 h vs. 14 days after coronary artery ligation) at a time when acute cardiac remodeling is expected to be still ongoing. The observed results, namely an almost normal LV systolic function 4 weeks after a large MI, are significant and reinforce the pathophysiological concept that the sooner the cardiac vagal output is restored, the better the outcome may be. The potential for an additional benefit of afferent mechanisms cannot be answered by this study but can be hypothesized based on the reduction of ischemia-reperfusion damage and related arrythmias demonstrated by transcutaneous auricular VNS.
The improvement in LV function caused by optogenetic VNS in sham MI is rather unexpected and worth discussing. In normal animals that have no reason to be characterized by autonomic imbalance, chronic optogenetic VNS of DVMN may potentially produce effects similar to those of physical training, with the important difference of a preferential effect on ventricular control as opposed to the combined effect of training on pacemaker and ventricular tissue. Because cardiac vagal activity is not completely abolished during physical effort at submaximal levels of exercise, an increased vagal output during a high-level background adrenergic activity might protect cardiomyocytes from cytoplasmatic calcium overload and improve calcium transient for contraction. Yet, this mechanism cannot explain the increased LV systolic function observed at rest. It must be acknowledged that same group had previously demonstrated that optogenetic stimulation of DVMN neurons in normal rats enhances myocardial contractility and responsiveness to β-adrenoceptor stimulation. These effects were associated with reduced myocardial expression of GRK2 (G protein–coupled receptor kinase 2) and β-arrestin 2, which are both involved in β-adrenergic receptor desensitization and internalization. Notably, β-arrestin 2 has also been identified as one of the key regulatory molecules involved in length-dependent enhancement of cardiac myofilament Ca2+ sensitivity (Frank-Starling mechanism) (8). Overall, parasympathetic overactivity mediated decrease in β-arrestin 2 may thus potentially lead to increased sympathetic responsiveness and less reliance on the Frank-Starling mechanism. Because parasympathetic DVMN neurons have been shown to preferentially control the ventricles, rather than the pacemaker area, this specific approach minimizes the heart rate lowering that would lead to increased cardiac filling and recruitment of the Frank-Starling mechanism (Figure 1). Intriguingly, back in 1986 (9), one of us demonstrated that older as compared with younger subjects had a higher percent increase in cardiac output during graded submaximal effort, despite a similar heart rate and mean arterial pressure response. A greater contribution of the Frank-Starling mechanism during exercise in older subjects was proposed to explain these findings. This hypothesis is in good agreement with the current previously mentioned data, considering that a preferential reduction of vagal output at the ventricular level is also suggested by the recently reported deterioration of parasympathetic DVMN neurons that occurs with aging. These findings are certainly worth further investigation.
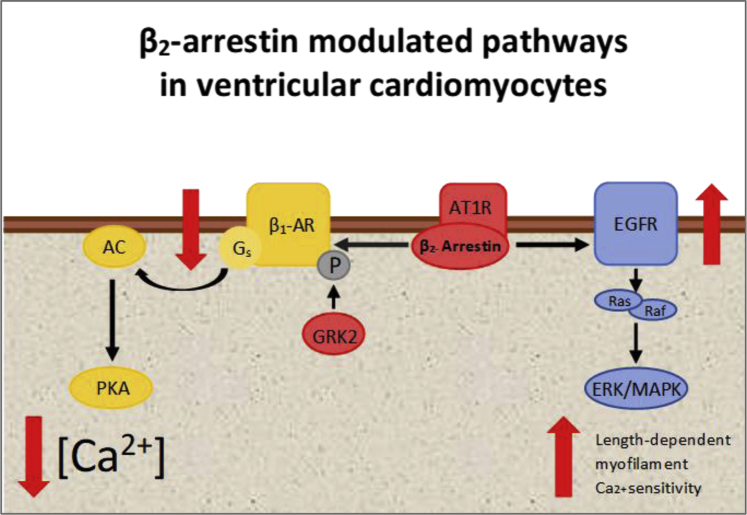
Figure 1.
Simplified Working Interpretation of β2- Arrestin Activation Impact on Ventricular Inotropism Based on Published Data
β2- arrestin is coupled to both G protein-coupled receptors (such as β-adrenoceptor) and not G protein-coupled receptors such as the epidermal growth factor receptor (EGFR). As detailed in the paper, Machhada et al. (6) recently proposed that β2-arrestin and G-protein-coupled receptor kinase 2 (GRK2) expression in ventricular cardiomyocytes is under parasympathetic control, with vagal withdrawal leading to upregulation, and enhanced vagal activity downregulation of expression. Once activated, GRK2 phosphorylate β-adrenoceptors and recruit β2-arrestin to block receptor coupling to G-proteins, leading to receptor desensitization and internalization. Additionally, other groups demonstrated that angiotensin II type 1 receptors (AT1Rs) can function as mechanosensors with a ligand-independent mechanism to activate β2-arrestin-dependent signaling and that both β2-arrestin and AT1Rs are key regulatory molecules in the Frank–Starling mechanism (8). AC = adenylyl cyclase; β1-AR = β-adrenoceptor; ERK = extracellular regulated kinase; GRK = G-protein coupled receptor kinase; MAPK = mitogen-activated protein kinase; P = phosphorylation site; PKA = cAMP-dependent protein kinase A.
Overall, the present study by Machhada et al. (6) reinforces the rationale for efferent VNS at the ventricular level in heart failure. It also draws attention to a novel additional pathway that might be involved in sympathetic-parasympathetic interaction at the cardiac level.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Machhada A., Marina N., Korsak A., Stuckey D.J., Lythgoe M.F., Gourine A.V. Origins of the vagal drive controlling left ventricular contractility. J physiol Action. 2016;594(14):4017–4030. doi: 10.1113/JP270984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Ferrari G.M. Vagal stimulation in heart failure. J Cardiovasc Transl Res. 2014;7:310–320. doi: 10.1007/s12265-014-9540-1. [DOI] [PubMed] [Google Scholar]
- 3.Inoue H., Zipes D.P. Time course of denervation of efferent sympathetic and vagal nerves after occlusion of the coronary artery in the canine heart. Circ Res. 1988;62:1111–1120. doi: 10.1161/01.res.62.6.1111. [DOI] [PubMed] [Google Scholar]
- 4.Vaseghi M., Salavatian S., Rajendran P.S. Parasympathetic dysfunction and antiarrhythmic effect of vagal nerve stimulation following myocardial infarction. JCI Insight. 2017;2 doi: 10.1172/jci.insight.86715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ardell J.L., Nier H., Hammer M. Defining the neural fulcrum for chronic vagus nerve stimulation: implications for integrated cardiac control. J Physiol. 2017;595:6887–6903. doi: 10.1113/JP274678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Machhada A., Hosford P.S., Dyson A., Ackland G.L., Mastitskaya S., Gourine A.V. Optogenetic stimulation of vagal efferent activity preserves left ventricular function in experimental heart failure. J Am Coll Cardiol Basic Trans Sci. 2020;5:799–810. doi: 10.1016/j.jacbts.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M., Zheng C., Sato T. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;104:120–124. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 8.Abraham D.M., Davis R.T., Warren C.M. β-arrestin mediates the Frank–Starling mechanism of cardiac contractility. Proc Natl Acad Sci U S A. 2016;113:14426–14431. doi: 10.1073/pnas.1609308113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mann D.L., Denenberg B.S., Gash A.K., Makler P.T., Bove A.A. Effects of age on ventricular performance during graded supine exercise. Am Heart J. 1986;111:108–115. doi: 10.1016/0002-8703(86)90561-2. [DOI] [PubMed] [Google Scholar]