Introduction
Topical mechlorethamine remains one of the most effective therapies for the treatment of early- stage mycosis fungoides (MF) with overall response rates between 59% and 94.7%.1, 2, 3 Its use can be limited by the development of cutaneous reactions at sites of application.4 It is not uncommon for dermatitis to occur and lead to noncompliance to the treatment. During our clinical experience with mechlorethamine gel (Valchlor 0.016% gel [USA], Ledaga [chlormethine gel] [EU]), we have identified 3 presentations of contact dermatitis (CD) in patients undergoing therapy. In each case presented, topical steroids were not used, and the current US Food and Drug Administration guidelines for mechlorethamine gel followed. Patients were enrolled in the Mechlorethamine Induced Contact Dermatitis Avoidance Study (MIDAS) (NCT03380026). Understanding these 3 presentations may help dermatologists address the issue and manage CD associated with mechlorethamine gel.
Case reports
Case 1: Mild-to-moderate CD not requiring cessation of mechlorethamine gel
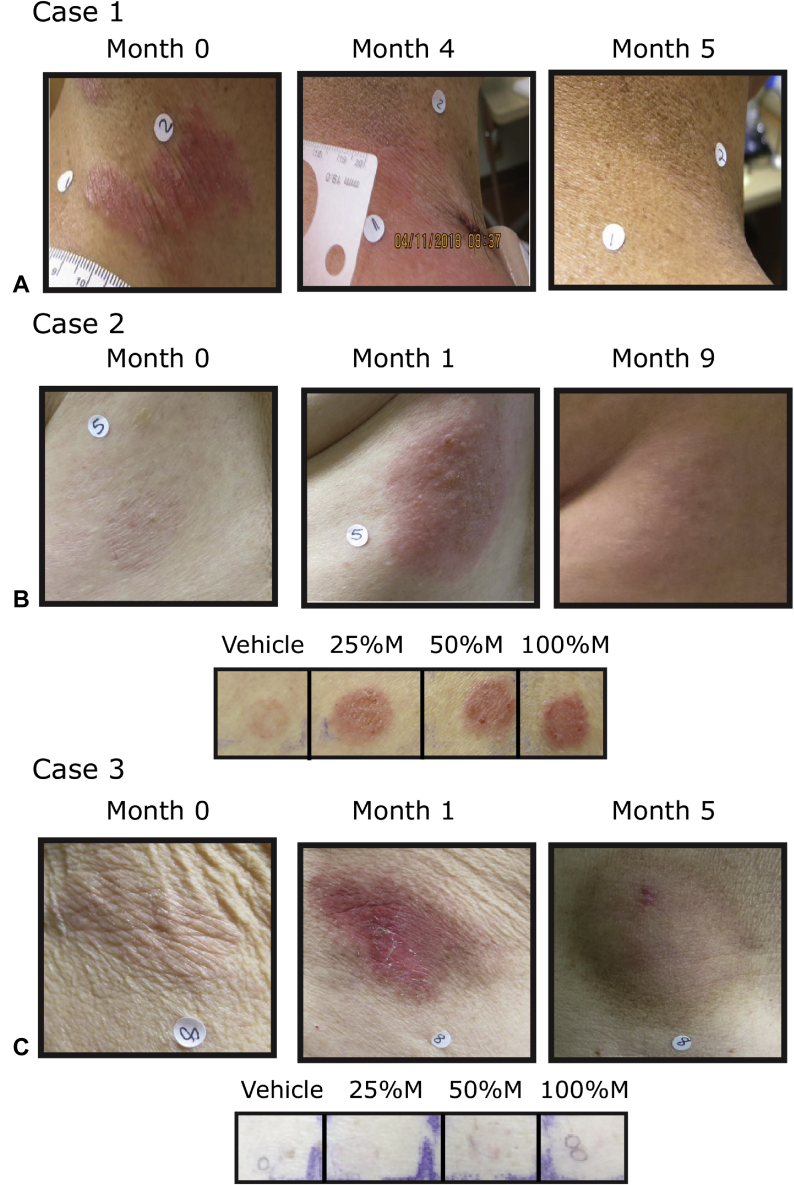
A 49-year-old man with stage IB folliculotropic MF, who previously did not respond to ultrapotent topical steroids, psoralen ultraviolet A, total skin electron beam therapy, bexarotene capsules, interferon-α and interferon-γ, topical carmustine, and topical imiquimod, was treated with mechlorethamine gel once daily for 4 months (Fig 1, A). Despite the development of an erythematous CD that was burning and pruritic after 1 month of therapy and peaking in month 3, the patient did not require a change in dosing or discontinuation of therapy. After a 1-month treatment hold period, at the 5-month timepoint, the patient had a complete response by composite assessment of index lesions (CAILS) (Fig 1, A). Patch testing found an allergic CD (data not shown). Mechlorethamine gel was restarted at month 5, and the patient continued to tolerate daily application over multiple areas with complete clearance of treated lesions through 18 months of follow-up (data not shown).
Fig 1.
A-C, Three cases of CD caused by mechlorethamine gel treatment of MF. Patients were treated for the indicated number of months. Patch testing was performed with varying concentrations (v/v) of mechlorethamine (M). After 96 hours, final results were obtained and photographs taken. Baseline (no treatment) is month 0. Cases 1 (not shown) and 2 show allergic CD, and case 3 irritant by CD patch testing.
Case 2: CD with delayed type hypersensitivity reaction
A 72-year-old woman with stage IA MF, who did not respond to ultrapotent topical steroids, topical bexarotene, and topical tazarotene, was treated once daily with mechlorethamine gel (Fig 1, B). During her second month of treatment, blisters developed with pain that led to a treatment interruption. After her CD resolved (14 days after the initial interruption), she restarted mechlorethamine gel at a reduced frequency of once every 3 days. She again had blisters, interrupted therapy, and restarted after the CD cleared. The patient then restarted once-weekly therapy with mechlorethamine gel but could not tolerate treatment at this reduced frequency either. Patch testing found allergic contact dermatitis (ACD) to mechlorethamine and not the vehicle control (Fig 1, B). Biopsy of the CD showed acute spongiosis with eosinophils consistent with ACD (data not shown). Over the 4-month period, she was treated for approximately 2.5 months with mechlorethamine gel. She was off all therapy from months 4 through 9. Despite having poor tolerance to the drug, she had a complete response by CAILS on a follow-up visit in month 9 (Fig 1, B). She remains clear through 18 months of follow-up.
Case 3: CD without delayed type hypersensitivity reaction
An 81-year-old man with stage IB MF, who previously did not respond to ultrapotent topical steroids, was treated with once-daily mechlorethamine gel. After 1 month of treatment, erosions and redness developed at several treated lesions on the legs and trunk that led to treatment interruption (Fig 1, C). After his CD recovered (14 days after the initial interruption), he restarted mechlorethamine gel every third day. Over the next several months, he was able to increase his frequency to every other day and then to daily use, which ultimately led to a recurrence of CD. Patch testing supported an irritant CD (ICD) (Fig 1, C). Biopsy also supported ICD (data not shown). The patient was treated with mechlorethamine gel for 3.5 months out of the planned 4 months of therapy. At month 5, after stopping mechlorethamine for 1 month, most of his lesions were resolved and the patient had a partial response by CAILS.
Discussion
We identified 3 distinctive patterns of dermatitis in patients receiving topical mechlorethamine gel. The first we term mild-to-moderate dermatitis, as the eruption does not have blisters, erosions, or sufficient pain or itch requiring discontinuation of therapy. The MF in these patients may appear worse because of the dermatitis, but, ultimately, the patient is able to continue daily therapy. The second pattern is an ACD with blisters and pain that leads to discontinuation of daily therapy. We rate this reaction as a severe dermatitis, as the mechlorethamine must be stopped. Although some of these patients can restart therapy at less-frequent dosing, others will not tolerate mechlorethamine gel. Even with complete intolerance (eg, blisters after 1 application), some of the patients will have response to the drug. Generally, when ACD is identified through patch testing, dermatologists recommend complete avoidance of the allergen. However, when that allergen is a chemotherapy that is treating a cancer, this may not be the best approach if the patient can tolerate the ACD. Our current approach is to reduce dosing and perform use testing in patients to determine if they will tolerate restarting mechlorethamine gel. The third pattern also involves a severe dermatitis but is caused by an ICD. In these patients, there is a better likelihood of restarting the mechlorethamine gel with decreased dosing frequency than that for the ACD. Patch testing may be helpful to identify these patients. Studies are underway that may help define molecular signatures to identify the reaction patterns in these patients.
It is important to maximize tolerance of mechlorethamine gel for multiple reasons: (1) We currently have no way to determine whose disease will progress from early stage to later stage. (2) Therapies are expensive and therefore should be used to maximal effect. (3) Mechlorethamine gel is a highly effective therapy. From our current experience treating MF with topical mechlorethamine gel, we make the following recommendations:
| Mild-to-moderate dermatitis may not require suspension of treatment but may require emollients or topical steroids or decreased dosing frequency (eg, three times per week dosing). Indeed, some dermatologists routinely include topical steroids with mechlorethamine gel therapy. |
| For severe dermatitis, discontinuation will be necessary. Severe dermatitis may be caused by ICD or ACD. Restarting patients at a decreased frequency is helpful in testing tolerability, and those who do not have immediate, severe reactions upon retrial generally will tolerate continuation of therapy. In some cases, patients can resume standard dosing after a trial of reduced dosing. |
| Lastly, a high level of expertise in evaluating the dermatitis associated with mechlorethamine gel is necessary to ensure compliance (potentially at a reduced schedule) and optimize treatment benefit. Dermatologists are best suited for this task and should recognize that not all red reactions on the skin are the same. Although some patients may be unable to tolerate the therapy as typically prescribed, partial responses and complete responses are possible in patients with dermatitis reactions. |
Footnotes
Authors Gilmore and Alexander-Savino contributed equally to this article.
Funding sources: None.
Conflicts of interest: Drs Gilmore and Poligone receive research support from Helsinn Healthcare.
References
- 1.Kim Y.H., Martinez G., Varghese A., Hoppe R.T. Topical nitrogen mustard in the management of mycosis fungoides: update of the Stanford experience. Arch Dermatol. 2003;139(2):165–173. doi: 10.1001/archderm.139.2.165. [DOI] [PubMed] [Google Scholar]
- 2.Lessin S.R., Duvic M., Guitart J. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149(1):25–32. doi: 10.1001/2013.jamadermatol.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsay D.L., Parnes R.E., Dubin N. Response of Mycosis Fungoides to topical chemotherapy with mechlorethamine. JAMA Dermatol. 1984;120(12):1585–1590. [PubMed] [Google Scholar]
- 4.Esteve E., Bagot M., Joly P. A prospective study of cutaneous intolerance to topical mechlorethamine therapy in patients with cutaneous T-cell lymphomas. French Study Group of Cutaneous Lymphomas. Arch Dermatol. 1999;135(11):1349–1353. doi: 10.1001/archderm.135.11.1349. [DOI] [PubMed] [Google Scholar]