Abstract
Objectives
In this research it was aimed to that evaluation of the effects of systemic metformin administration on the periimplant bone tissue response of TiAl6Va4 implants in experimental rat model.
Materials and method
Firstly TiAl6Va4 implants were inserted surgically in the metaphyseal part of the tibial bone and after, the rats were randomly separated into two groups: Controls (CNT) (n = 10) and Metformin group (M) (n = 10). No additional treatment was applied to the controls during the 4-week experimental period. Rats received 40 mg/kg metformin in every day during the four week experimental period in M group. At the end of the 28-day follow-up period, the TiAl6Va4 implants with surrounding bone were used for the histopathologic analysis. To analysis of the datas between M and CNT Student-T Test was used.
Result
Periimplant bone tissue filling ratios (%) were detected higher in M group compared with the CNT (P < 0.05).
Conclusion
Systemic administration of metformin may increases titanium implant osseointegration in non-diabetic rats.
Keywords: Osseointegration, Dental implant, Bone filling, Metformin
1. Introduction
Today, there are many studies on dental implantology that show high clinical success rates.1,2 Despite this, one of the most interesting topics in research related to implantology is improving the quality of osteointegration. The most important reasons for this are the long time period between insertion and loading and the presence of question marks regarding clinical success in cases where bone quality is insufficient.3,4
Previous studies were evaluated macro features,5 such as the shape and groove character of the implant, and micro features,6 such as the surface character of the implant.
The main purpose in the development of macro features is to provide ease of application and to increase the initial stability of the implant.5 The aim of studies about micro features is to increase the bone implant contact and, then, increasing the rapid progression of bone healing cells to the implant surface.7,8
Despite poor bone quality, predictable success of osseointegration, and for faster healing, includes biological products and biochemicals that are generally applied systemically or topically in previous research.9,10 For this purpose, the implant surface was covered in different materials, such as hydroxyapatite (HA), calcium phosphate, bioactive glass, and ceramics bioactive polymers.6 In addition, the following materials were used with the implants: platelet rich plasma (PRP),11 melatonin,10 recombinant human bone morphogenetic protein 2 (rhBMP-2)12, and bisphosphonates.9
Metformin is an anti-diabetic drug commonly used in the treatment of type 2 diabetes.13,14 It is effective on bone metabolism (osteoplast and osteoclast differentiation) by reducing the receptor activator of nuclear factor kappa B ligand (RANKL) expression and stimulating osteoprotegerin (OPG) expression.15
Previous studies suggest that Metformin has osteogenic effects on bone marrow progenitor cells,16 increases the osteoblastic activity and decreases osteoclastogenesis.17 Cortizo et al.18 detected that metformin has a potential osteogenic effect by stimulating the differentiation of preosteoblasts.
In a multicenter study conducted on diabetic patients, Metformin treatment was found to reduce bone resorption.19 Different studies have suggested that Metformin enhances osteogenic activity by promoting the differentiation of preosteoplasts to osteoblasts.18,20 Therefore, the purpose of this research was to detect the effects of systemic Metformin administration on the bone formation around titanium implants in rat tibial bones.
2. Material and method
2.1. Research design and animals
Approval for this experimental research was obtained from the Local Ethics Committee of the Animal Experiments of Firat University, Elazig, Turkey (Protocol No: 2016/154, Date:February 16, 2017). During the experimental period, the Helsinki Declaration on the protection of laboratory research animals was followed. This experimental research was conducted on 20 healthy, adult, female Sprague Dawley rats aged between 2.5 and 3 months and weighing from 220 to 230 g. All the rats selected for this research were in the same estrus period for standardization of the study.21 Vaginal smear method was used to determine the estrus periods of the rats.
The rats had constant access to food and water. Standard conditions were applied in the animal room (temperature of 22–24 °C, 12 h light or dark). During the study period, the animals were placed in plastic cages measuring 50 × 80 × 50 cm, with two rats in each cage.
For this research, TiAl6Va4 implants (Implance Dental Implant System, AGS Medical, Istanbul, Turkey), with a diameter of 2.5 mm and a length of 4 mm, were used.21 After two days of surgical procedures of the TiAl6Va4 implants, the rats were randomly separated into two groups, with similar mean weights in each group: the control (CNT) (n = 10) and the Metformin (n = 10) groups. No further treatment was given to the rats in the control group during the experimental phase of the research, while 40 mg/kg of metformin was applied with oral gavage every day to the metformin-experimental group for 28 days.
2.2. Surgical applications
All the surgical procedures were done under sterile conditions. Xylazine (5 mg/kg) (Rompun 2%, Bayer, Istanbul, Turkey) and Ketamine HCl (50 mg/kg) (Ketas, Eczacıbaşı-Warner Lambert, Istanbul, Turkey) were administered to all the experimental animals by intramuscular injection, and after the general anesthesia was given, surgical integration procedures were performed.
The surgical site was properly shaved and wiped with povidone iodine. Then, the skin on the tibial crest and periosteum in the right tibia were cut with a scalpel (no:15), and the tibial metaphyseal bone surface was exposed after the soft tissue dissection. The implant sockets were prepared with suitable drills with saline irrigation.
Preparing the implant socket involved ensuring that the socket was of sufficient size: 2.5 mm in diameter and 4 mm in length.21 Marking drill was used first, and then implant sockets were prepared with drills with diameters of 1.8 mm, 2.1 mm and 2.5 mm respectively. Machined surfaced TiAl6Va4 implants (Implance Dental Implant System, AGS Medical, Istanbul, Turkey) were inserted into corticocancellous bone in the metaphyseal part of the tibial bone, and primary stabilization was achieved. The incision site was closed with a vicryl 4-0 suture, and the periosteum and skin were primarily closed. Postoperatively, an antibiotic (50 mg/kg penicillin) and an analgesic (0.1 mg/kg tramadol hydrochloride) were given intramuscularly for three days to eliminate infection and pain.21 All surgery procedures were performed by the same person.
2.3. Histological analysis
No fatal complications (for example, wound formation and infection) were observed during the experimental period of our research. Within the 28-day experimental period, one rat died in the control groups (n = 9), Metformin groups (n = 10). At the end of the 28-day follow-up period, the rats were euthanized under deep anesthesia.22 The tibia samples containing the titanium implants were put into a 10% formalin solution for a week. All the non-decalcified histological procedures were completed at the Research Laboratory, Erciyes University Faculty of Dental Medicine in Kayseri, Turkey. The TiAl6Va4 implants with the surrounding bone tissue were embedded in 2-hydroxyethylmethacrylate for histological analysis, and an Exakt® microtome (Germany) was used for cutting. The samples were first cut in the middle, then each section was milled using an Exakt® grinder to obtain 50 μm thick sections for light microscopy analysis. Toluidine blue was used for analysis of histological bone filling (BF). A stereological software system (Nikon, Tokyo, Japan) was used by the author was calibrated and blinded to evaluate histomorphometry. Bone filling ratios for every TiAl6Va4 implant was calculated by the ratio of the amount of bone filled area to the total area of the region of interest. Region of interest area determined by 0,5 mm distance from the apical, distal and mesial part of every implant.23
2.4. Statistical analysis
SPSS 23.0 for Windows software (USA) was used for statistical analysis. Mean ± standard deviation were given for each group. The assumption of normal distribution of the data was analysed using the Kolmogorov Smirnov test, and Student-t test were used to detect the differences between the groups. The minimum number of animals required for the realization of the experimental design was determined by power analysis; when 8% deviation, type 1 error (α) 0.05 and type 2 error (β) (Power = 0.80) and animals were divided into groups, at least 9 rats were needed in each group.6,22 When P < 0.05, the data compared between the groups were considered statistically significant.
3. Result
According to the results of the histomorphometric analyses, the BF ratios of the rats in the Metformin group were 55.50 ± 14.034, and in the CNT group were 37.78 ± 13.017 (Table 1; Fig. 1, Fig. 2). In Metformin group BF ratio detected statistically higher when compared with the CNT (p < 0.05).
Table 1.
Bone Filling (BF) Ratio (%) of Control and Metformin group.
| Group | Bone Filling (Mean _Std Dev) (%) | p |
|---|---|---|
| Control group | 37.78 ± 13.017 | 0.011* |
| Metformin group |
55.50 ± 14.034 |
|
| Total | 20 |
Student's t-test p < 0.05.
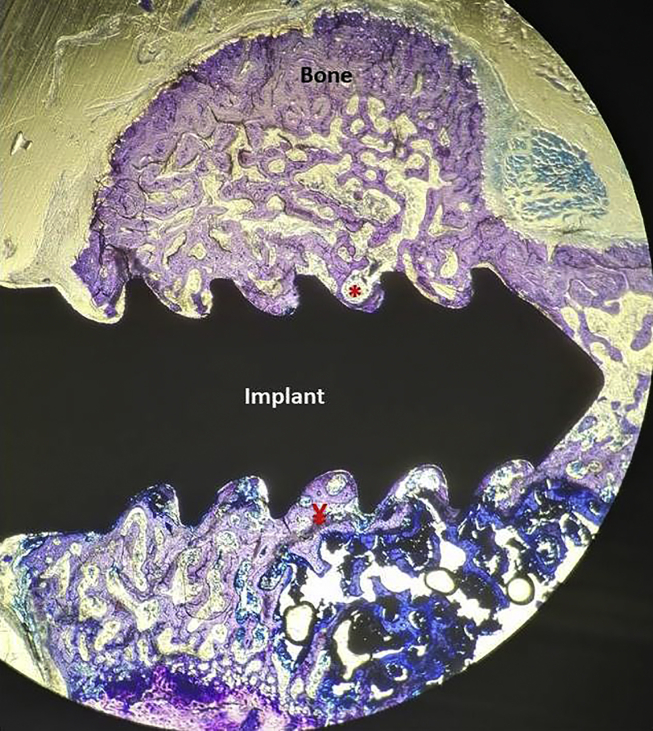
Fig. 1.
Section prepared without bone tissues decalcifying of the Control Group, (20 times magnification, Toluidin Blue). Bone filled areas were measured width 0.5 mm from the distal, mesial and apical part of implants. Total area of region of interest: Ă, non-bone areas: *, Bone filling areas: ¥ (Ă-*), Bone filling Ratios (%): ¥/Ăx100.
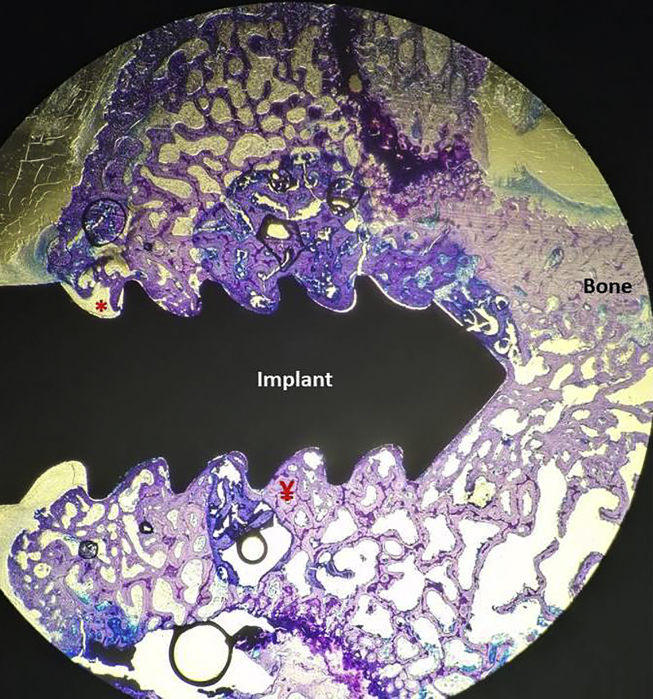
Fig. 2.
Section prepared without bone tissues decalcifying of the Metformin Group, (20 times magnification, Toluidin Blue). Bone filled areas were measured width 0.5 mm from the distal, mesial and apical part of implants. Total area of region of interest: Ă, non-bone areas: *, Bone filling areas: ¥ (Ă-*), Bone filling Ratios (%): ¥/Ăx100.
4. Discussion
Dental implants are generally used successfully in the rehabilitation of full or partial edentulous cases. But sometimes, early or late in the process, implant failure is observed. Therefore, studies to determine the factors that may hinder or help the bone healing process around titanium implants have increased recently. The effects of Metformin on bone metabolism have been investigated in many previous studies.13,15,24 Recent studies have shown that Metformin plays an important role in bone metabolism by affecting the differentiation of osteoblasts and osteoclasts.15,25 In addition, Metformin can suppress osteoclastogenesis in cell cultures in a dose-dependent manner by reducing RANKL expression and stimulating OPG expression.26 Other studies in animals, which have evaluated the effect of Metformin on bone, have shown that Metformin can increase bone mineral density and reduce bone loss in rats.17,27 In addition, Metformin enhanced bone healing in diabetic15 and non-diabetic rats.17,27
In the present research, we evaluated the effect of Metformin on the bone healing around TiAl6Va4 implants in non-diabetic rats. The finding of the research show that Metformin responds positively with the bone healing around titanium implants and enhances the osseointegration process. The BF ratio was detected high in the test animals-Metformin treated rats, when compared with the controls. The study results agree with other research on animals, which also shows that Metformin has a positive effect on osseointegration and bone repair in rats.15,25 A study in rats shows that Metformin prohibit glucocorticoid-induced bone resorption by preventing bone loss and increasing bone formation in the trabecular bone.28
As for the present study, previous research has demonstrated that other anti-diabetic drugs (for example, aminoguanidine and insulin) may control the negative effect of diabetes mellitus on the bone around implant in rats.29,30 The findings of this research were similar to those of Siqueira et al.,31 in that the level of bone implant connection (BIC) was similar in insulin-fed diabetic rats and non-diabetic control rats. Inouye al.25 examined the effects of hyperglycemia and Metformin on the healing of periimplant bone tissue. They divided thirty-six male rats into 3 equal groups: non-diabetic rats; no further treatment was performed during the study, diabetic rats and diabetic rats were given metformin (100 mg/kg/day water) for 4 weeks. The study findings suggest that in type 2 diabetes, bone healing is minimal, the remodeling of bone around the implant is not sufficient, but Metformin may improve bone healing.25 However, Boston et al.24 in non-diabetic rats, examined the effects of the systemic metformin application on the bone tissue response taking into account bone healing parameters. To experimental design twenty wistar albino rats were used and rats were divided into two groups: Controls and Metformin group (40 mg/kg/day by gavage). At thirty days after implant surgery, BIC were evaluated. The result of this study suggested that Metformin adversely affected osseointegration in non-diabetic rats by decreased the percentage of BIC and increasing level of RANKL around titanium implants. In addition, Serrao et al.15 assessed the possible effect of Metformin on the healing of bone surrounding implants rats. Rats (10 per group) were assigned to non diabetic, DM group (without metformin treatment) and DM + Metformin treatment group (40 mg/kg/day by gavage, starting on the 15th day after implant surgery). Rats were euthanized 30 days after implant surgery. The finding of this study revealed that Metformin had no effect on the decrease in bone healing due to hyperglycemia of diabetic rats in a histometric analysis but increased the level of OPG and reduced the RANKL/OPG ratio. The effects of Metformin on osseointegration in diabetic rats is different between studies. This difference may be due to the protocol of diabetes mellitus induction, the threshold of serum glucose level to determined diabetes mellitus, the glucose level when the implant was placed, and the method of evaluation of osseointegration.
The favorable effects of Metformin on bone could show differences in the mechanisms defined on diabetics.15,32 Adenosine monophosphate-activated protein kinase (AMPK) is important molecule on bone metabolism, which regulates osteoclastogenesis and cellular senescence.32,33 Aging bone cells produce a pro-inflammatory secretoma that reduces bone formation and increases bone resorption. Metformin eliminates aged cells or by reducing the production of pro-inflammatory secretomas; demonstrates protective bone loss in mice.32 Blümel et al.32 suggested that the use of Metformin was a protective factor for bone resorption even without type 2 diabetes. Metformin, due to its effect on AMPK, may have positive effects on bone metabolism by increasing osteoblastic activity and reducing osteoclastic activity.34 To the extent available, there is only one study examining Metformin's implant bone connection in non-diabetic rats. Since the results of the studies differ, more research is needed into the effect of Metformin on osteointegration in non-diabetic rats.
5. Conclusion
The findings of this research reveal that systemic Metformin application positively affects osseointegration by increasing the percentages of BF ratios. Statistically significant differences in BF were detected between the dental implants of the control and Metformin treatment groups during the four-week osseointegration period. However, further research is required to clear whether systemic Metformin application affects the BF in non-diabetic rats.
Funding
This work was funded by the Firat University Scientific Research Project Coordinatorship (DHF.18.06) Elazığ, Turkey.
References
- 1.Pariente L., Dada K., Daas M., Cok S., Dard M. Evaluation of the treatment of partially edentulous patients with bone level tapered implants: 24-month clinical and radiographic follow-up. J Oral Implantol. 2020;10:1563. doi: 10.1563/aaid-joi-D-19-00024. [DOI] [PubMed] [Google Scholar]
- 2.Kim M.J., Yun P.Y. The long-term evaluation of the prognosis of implants with acid-etched surfaces sandblasted with alumina: a retrospective clinical study. 2020;42:10. doi: 10.1186/s40902-020-00255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dursun C.K., Dursun E., Eratalay K. Effect of porous titanium granules on bone regeneration and primary stability in maxillary sinus: a human clinical, histomorphometric, and microcomputed tomography analyses. J Craniofac Surg. 2016;27:391–397. doi: 10.1097/SCS.0000000000002421. [DOI] [PubMed] [Google Scholar]
- 4.Dursun E., Keceli H.G., Uysal S., Güngör H., Muhtarogullari M., Tözüm T.F. Management of limited vertical bone height in the posterior mandible: short dental implants versus nerve lateralization with standard length implants. J Craniofac Surg. 2016;27:578–585. doi: 10.1097/SCS.0000000000002459. [DOI] [PubMed] [Google Scholar]
- 5.Vivan Cardoso M., Vandamme K., Chaudhari A. Dental implant macro-design features can impact the dynamics of osseointegration. Clin Implant Dent Relat Res. 2015;17:639–645. doi: 10.1111/cid.12178. [DOI] [PubMed] [Google Scholar]
- 6.Dundar S., Yaman F., Bozoglan A. Comparison of osseointegration of five different surfaced titanium implants. J Craniofac Surg. 2018;29:1991–1995. doi: 10.1097/SCS.0000000000004572. [DOI] [PubMed] [Google Scholar]
- 7.Park J.W. Osseointegration of two different phosphate ion-containing titanium oxide surfaces in rabbit cancellous bone. Clin Oral Implants Res. 2013;24(Suppl A100):145–151. doi: 10.1111/j.1600-0501.2011.02406.x. [DOI] [PubMed] [Google Scholar]
- 8.Du Z., Xiao Y., Hashimi S., Hamlet S.M., Ivanovski S. The effects of implant topography on osseointegration under estrogen deficiency induced osteoporotic conditions: histomorphometric, transcriptional and ultrastructural analysis. Acta Biomater. 2016;42:351–363. doi: 10.1016/j.actbio.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 9.Dundar S., Yaman F., Gecor O. Effects of local and systemic zoledronic acid application on titanium implant osseointegration: an experimental study conducted on two surface types. J Craniofac Surg. 2017;28:935–938. doi: 10.1097/SCS.0000000000003568. [DOI] [PubMed] [Google Scholar]
- 10.Dundar S., Yaman F., Saybak A. Evaluation of effects of topical melatonin application on osseointegration of dental implant: an experimental study. J Oral Implantol. 2016;42:386–389. doi: 10.1563/aaid-joi-D-16-00048. [DOI] [PubMed] [Google Scholar]
- 11.Fontana S., Olmedo D.G., Linares J.A., Guglielmotti M.B., Crosa M.E. Effect of platelet-rich plasma on the peri-implant bone response: an experimental study. Implant Dent. 2004;13:73–78. doi: 10.1097/01.id.0000116455.68968.29. [DOI] [PubMed] [Google Scholar]
- 12.Becker J., Kirsch A., Schwarz F. Bone apposition to titanium implants biocoated with recombinant human bone morphogenetic protein-2 (rhBMP-2). A pilot study in dogs. Clin Oral Invest. 2006;10:217–224. doi: 10.1007/s00784-006-0049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khajuria D.K., Patil O.N., Karasik D., Razdan R. Development and evaluation of novel biodegradable chitosan based metformin intrapocket dental film for the management of periodontitis and alveolar bone loss in a rat model. Arch Oral Biol. 2018;85:120–129. doi: 10.1016/j.archoralbio.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y.D., Park K.G., Lee Y.S. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes. 2008;57:306–314. doi: 10.2337/db07-0381. [DOI] [PubMed] [Google Scholar]
- 15.Serrao C.R., Bastos M.F., Cruz D.F., de Souza Malta F., Vallim P.C., Duarte P.M. Role of metformin in reversing the negative impact of hyperglycemia on bone healing around implants inserted in type 2 diabetic rats. Int J Oral Maxillofac Implants. 2017;32:547–554. doi: 10.11607/jomi.5754. [DOI] [PubMed] [Google Scholar]
- 16.Molinuevo M.S., Schurman L., McCarthy A.D. Effect of metformin on bone marrow progenitor cell differentiation: in vivo and in vitro studies. J Bone Miner Res. 2010;25:211–221. doi: 10.1359/jbmr.090732. [DOI] [PubMed] [Google Scholar]
- 17.Mai Q.G., Zhang Z.M., Xu S. Metformin stimulates osteoprotegerin and reduces RANKL expression in osteoblasts and ovariectomized rats. J Cell Biochem. 2011;112:2902–2909. doi: 10.1002/jcb.23206. [DOI] [PubMed] [Google Scholar]
- 18.Cortizo A.M., Sedlinsky C., McCarthy A.D., Blanco A., Schurman L. Osteogenic actions of the anti-diabetic drug metformin on osteoblasts in culture. Eur J Pharmacol. 2006;536:38–46. doi: 10.1016/j.ejphar.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 19.Stage T.B., Christensen M.H., Jorgensen N.R. Effects of metformin, rosiglitazone and insulin on bone metabolism in patients with type 2 diabetes. Bone. 2018;112:35–41. doi: 10.1016/j.bone.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Bahrambeigi S., Yousefi B., Rahimi M., Shafiei-Irannejad V. Metformin; an old antidiabetic drug with new potentials in bone disorders. Biomed Pharmacother. 2019;109:1593–1601. doi: 10.1016/j.biopha.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 21.Dundar S., Bozoglan A. Effects of restraint stress and high-fat diet on osseointegration of titanium implants: an experimental study. Braz Oral Res. 2020;34:e008. doi: 10.1590/1807-3107bor-2020.vol34.0008. [DOI] [PubMed] [Google Scholar]
- 22.Bozoglan A., Dundar S., Yildirim T.T. Effects of different levels of restraint stress on bone-implant contact. J Craniofac Surg. 2019;30:1294–1297. doi: 10.1097/SCS.0000000000005104. [DOI] [PubMed] [Google Scholar]
- 23.Nociti Junior F.H., Cesar Neto J.B., Carvalho M.D., Sallum E.A., Sallum A.W. Intermittent cigarette smoke inhalation may affect bone volume around titanium implants in rats. J Periodontol. 2002;73:982–987. doi: 10.1902/jop.2002.73.9.982. [DOI] [PubMed] [Google Scholar]
- 24.Bastos M.F., Serrao C.R., Miranda T.S., Cruz D.F., de Souza Malta F., Duarte P.M. Effects of metformin on bone healing around titanium implants inserted in non-diabetic rats. Clin Oral Implants Res. 2017;28:e146–e150. doi: 10.1111/clr.12960. [DOI] [PubMed] [Google Scholar]
- 25.Inouye K.A., Bisch F.C., Elsalanty M.E., Zakhary I., Khashaba R.M., Borke J.L. Effect of metformin on periimplant wound healing in a rat model of type 2 diabetes. Implant Dent. 2014;23:319–327. doi: 10.1097/ID.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 26.Lee Y.S., Kim Y.S., Lee S.Y. AMP kinase acts as a negative regulator of RANKL in the differentiation of osteoclasts. Bone. 2010;47:926–937. doi: 10.1016/j.bone.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Bak E.J., Park H.G., Kim M. The effect of metformin on alveolar bone in ligature-induced periodontitis in rats: a pilot study. J Periodontol. 2010;81:412–419. doi: 10.1902/jop.2009.090414. [DOI] [PubMed] [Google Scholar]
- 28.Zhao J., Li Y., Zhang H. Preventative effects of metformin on glucocorticoid-induced osteoporosis in rats. J Bone Miner Metabol. 2019;37(5):805–814. doi: 10.1007/s00774-019-00989-y. [DOI] [PubMed] [Google Scholar]
- 29.Guimaraes R.P., de Oliveira P.A., Oliveira A.M. Effects of induced diabetes and the administration of aminoguanidine in the biomechanical retention of implants: a study in rats. J Periodontal Res. 2011;46:691–696. doi: 10.1111/j.1600-0765.2011.01391.x. [DOI] [PubMed] [Google Scholar]
- 30.Margonar R., Sakakura C.E., Holzhausen M., Pepato M.T., Alba j R., Marcantonio j E. The influence of diabetes mellitus and insulin therapy on biomechanical retention around dental implants: a study in rabbits. Implant Dent. 2003;12:333–339. doi: 10.1097/01.id.0000086482.65273.b7. [DOI] [PubMed] [Google Scholar]
- 31.Siqueira J.T., Cavalher-Machado S.C., Arana-Chavez V.E., Sannomiya P. Bone formation around titanium implants in the rat tibia: role of insulin. Implant Dent. 2003;12:242–251. doi: 10.1097/01.id.0000074440.04609.4f. [DOI] [PubMed] [Google Scholar]
- 32.Blumel J.E., Arteaga E. Metformin use is associated with a lower risk of osteoporosis in adult women independent of type 2 diabetes mellitus and obesity. Redlınc Ix study. 2020:1–5. doi: 10.1080/09513590.2020.1718092. [DOI] [PubMed] [Google Scholar]
- 33.Khosla S., Farr J.N., Kirkland J.L. Inhibiting cellular senescence: a new therapeutic paradigm for age-related osteoporosis. J Clin Endocrinol Metab. 2018;103:1282–1290. doi: 10.1210/jc.2017-02694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiating L., Buyun J. vol. 2019. 2019. p. 9203934. (Role of Metformin on Osteoblast Differentiation in Type 2 Diabetes). [DOI] [PMC free article] [PubMed] [Google Scholar]