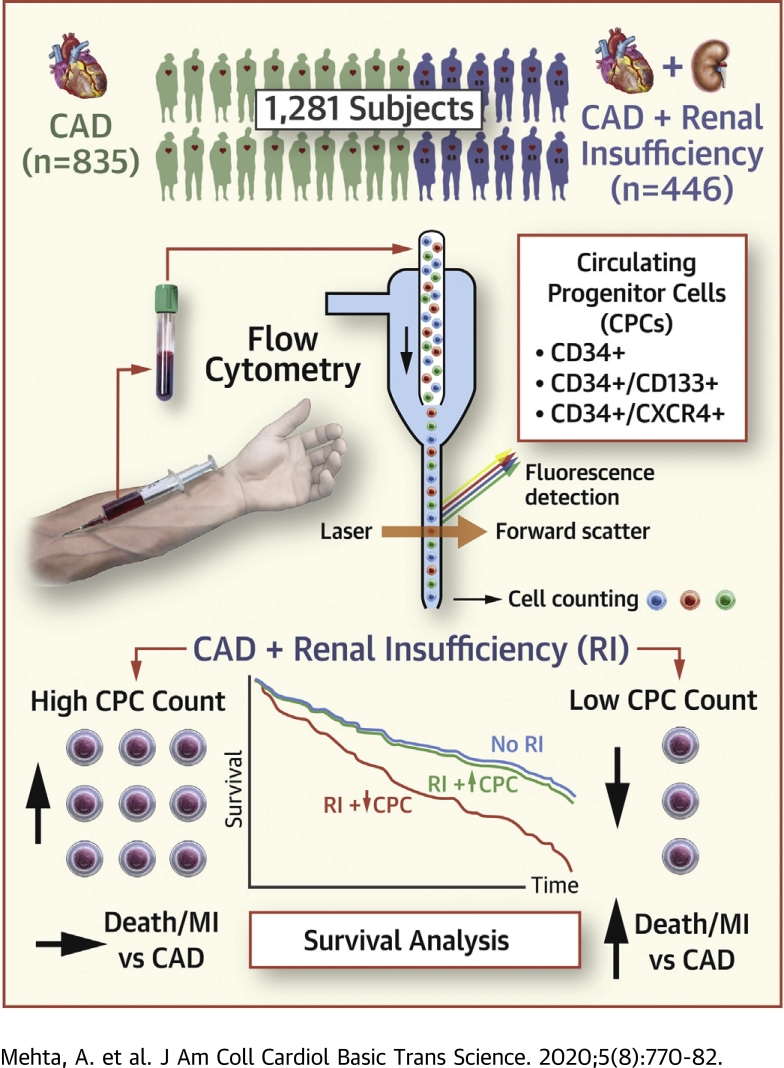
Visual Abstract
Key Words: coronary artery disease, outcomes, progenitor cells, regenerative capacity, renal insufficiency
Abbreviations and Acronyms: BNP, B-type natriuretic peptide; CAD, coronary artery disease; CD, cluster of differentiation; CI, confidence interval; CPC, circulating progenitor cell; CV, cardiovascular; CXCR4, chemokine (C-X-C motif) receptor 4; eGFR, estimated glomerular filtration rate; HR, hazard ratio; hsTnI, high-sensitivity troponin I; IDI, integrated discrimination index; MI, myocardial infarction; VEGF2R, vascular endothelial growth factor receptor 2
Highlights
-
•
Patients with CAD and renal insufficiency (eGFR <60 ml/min/1.73 m2) are at an increased risk of adverse outcomes.
-
•
CPC counts, an index of endogenous vascular regenerative capacity, may help stratify risk in patients with CAD and renal insufficiency.
-
•
Renal insufficiency is associated with lower CPC counts in old, but not young, patients with established CAD.
-
•
The increased risk of adverse outcomes with CAD and renal insufficiency is limited to patients with low, but not high, CPC counts.
Summary
Patients with coronary artery disease and renal insufficiency (RI) (estimated glomerular filtration rate <60 ml/min/1.73 m2) are at an increased risk of cardiovascular events. The contribution of regenerative capacity, measured as circulating progenitor cell (CPC) counts, to this increased risk is unclear. CPCs were enumerated as cluster of differentiation (CD) 45med+ mononuclear cells expressing CD34+, CD133+, CXCR4+ (chemokine [C-X-C motif] receptor 4), and VEGF2R+ (vascular endothelial growth factor receptor 2) epitopes in 1,281 subjects with coronary artery disease (35% with RI). Patients with RI and low (<median) hematopoietic CPCs (CD34+, CD34+/CD133+, and CD34+/CXCR4+) were at an increased risk of cardiovascular death or myocardial infarction events (hazard ratios: 1.75 to 1.80) during 3.5-year follow-up, while those with RI and high CPCs (>median) were at a similar risk as those without RI.
Patients with established coronary artery disease (CAD) and renal insufficiency are at an increased risk of adverse cardiovascular (CV) outcomes (1,2). Novel factors that contribute to this increased risk in patients with CAD and renal insufficiency are of considerable research interest. Circulating progenitor cells (CPCs) are mononuclear cells derived primarily from the bone marrow that contribute to vascular repair and regeneration largely through paracrine mechanisms (3, 4, 5). Circulating progenitors can be measured as mononuclear cells expressing the cluster of differentiation (CD) 34 epitope, and these cells have the potential to differentiate into endothelial, hematopoietic, and nonhematopoietic (mesenchymal, lacking CD45 expression) phenotypes (6,7). CD133 is a 5-transmembrane antigen seen on primitive stem cells that is lost during cellular maturation, and coexpression of CD34 with CD133 (CD34+/CD133+) identifies a hematopoietic CPC-enriched subpopulation (8,9). Coexpression of chemokine (C-X-C motif) receptor 4 (CXCR4) with CD34 (CD34+/CXCR4+) characterizes cells with capacity for tissue repair via homing of CPCs to SDF-1 (stromal-derived factor 1)–enriched hypoxic environments (10). Although CD34+/CD133+ and CD34+/CXCR4+ subtypes are enriched for hematopoietic progenitors, coexpression of vascular endothelial growth factor receptor 2 (VEGF2R) with CD34 (CD34+/VEGF2R+) identifies a rarer subpopulation of CPCs enriched for endothelial progenitors (11).
Previous work from our group has shown that presence of “traditional” CV risk factors including diabetes, hypertension, hypercholesterolemia, and smoking early in life is associated with higher CPC counts (12,13). This stimulation is likely a result of mobilization of progenitors from the bone marrow in response to the risk factor–mediated injury, and represents activation of the endogenous regenerative or reparative systems (5,13,14). It is worth noting that continuous exposure to risk factors with age leads to depletion in CPC counts (14). In this context, the association of renal insufficiency, a “nontraditional” CV risk factor, with CPC counts and the impact of age on this association has not been evaluated to date.
Furthermore, prior research from our group and others has shown that lower levels of CPCs are independently associated with a higher risk of adverse CV outcomes (15, 16, 17, 18), and small studies have reported similar findings in patients with end-stage renal disease (19, 20, 21, 22). Nonetheless, the predictive value of CPCs in patients with CAD and renal insufficiency, a high-risk group, has not been previously studied. Therefore, we sought to investigate: 1) the association between renal insufficiency, age, and CPC counts; and 2) the predictive value of CPCs among patients with CAD and renal insufficiency. We hypothesized that renal insufficiency would be inversely associated with CPC counts, and that lower CPC counts would contribute to the increased risk of adverse events in patients with renal insufficiency.
Methods
Study population
Participants enrolled in the Emory Cardiovascular Biobank, a prospective registry of patients undergoing cardiac catheterization for evaluation of CAD at 3 Emory Healthcare–affiliated hospitals were enrolled in this study (23). All participants provided written informed consent, and the study was approved by the Emory University Institutional Review Board. Subjects with acute myocardial infarction, severe valvular heart disease, organ transplantation, immunosuppressive medication use, leukocytosis (defined as white blood cell count >11,000 cells/μl), and active infection, inflammatory disorder, or cancer were excluded. A total of 1,281 subjects (446 with renal insufficiency) were analyzed in this study, and the data supporting our findings are available from the corresponding author upon reasonable request.
Participant characteristics
Participants were interviewed to collect information about demographic characteristics, smoking history, medical history, and CV medication (angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, aspirin, beta-blocker, clopidogrel, and statin) use as previously described (23). The prevalence of diabetes, hypertension, hypercholesterolemia, and established CV disease subtypes (CAD, heart failure [HF], and peripheral artery disease) was determined by physician diagnosis or treatment (23). Medical records were reviewed to extract ejection fraction information and confirm self-reported medical history. Weight and height were measured at enrollment, and body mass index was calculated by dividing weight by height (kg/m2). Complete blood count, including hemoglobin level measured as g/dl and white blood cell count measured as cells/μl, was measured at enrollment (23). Serum creatinine was measured at enrollment as well (23), and estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (24). Participants with eGFR <60 ml/min/1.73 m2 (GFR categories G3a, G3b, G4, and G5) were grouped together and classified as patients with renal insufficiency (25). Data regarding albuminuria were not collected. Circulating levels of high-sensitivity troponin I (hsTnI) and B-type natriuretic peptide (BNP) were measured using the Abbott ARCHITECT analyzer (Abbott Laboratories, North Chicago, Illinois) (26).
CPC assays
CPCs were measured in peripheral arterial blood samples collected in EDTA tubes before contrast administration for cardiac catheterization (17). Blood samples were prepared within 4 h of collection and incubated with fluorochrome-labeled monoclonal antihuman mouse antibodies to identify surface markers expressed on mononuclear cells before quantification using flow cytometry. A total of 300 μl of peripheral blood was incubated with 7 μl of FITC-CD34 (BD Biosciences, San Jose, California), PerCP-CD45 (BD Biosciences), PE-VEGFR2 (R and D Systems, Minneapolis, Minnesota), 5-μl APC-CD133 (Miltenyi, Bergish Gladbach, Germany), and 3-μl PE-Cy7-conjugated anti-CXCR4 (clone 12G5; EBioscience, San Diego, California) in the dark for 15 min (27). Then, 1.5-ml ammonium chloride lysing buffer was added to lyse red blood cells, following which 1.5-ml staining medium (phosphate-buffered saline with 3% heat-inactivated serum and 0.1% sodium azide) was added to stop the lysing reaction (27). Prior to flow cytometry, 100 μl of AccuCheck Counting Beads (Cat#: PCB100; Invitrogen, Carlsbad, California) were added to act as an internal standard for direct estimation of the concentration of target cell subsets (27). At least 2.5 million events were acquired from the cytometer. Flow cytometry data were analyzed using FlowJo software (Tree Star, Ashland, Oregon) with filter set at CD45med+ cells. This selection excludes CD45bright (lymphoblasts) and CD45– (nonhematopoietic progenitor) cells. CPC counts (CD34+, CD34+/CD133+, CD34+/CXCR4+, and CD34+/VEGF2R+) were measured using CD45med+ filter and are reported as cell counts per milliliter (Supplemental Figure 1) (27). A subset of 20 samples was analyzed on 2 occasions by 2 technicians, and percent repeatability coefficients (%) were calculated as the standard deviation of differences between pairs of measurements/mean of measurements × 100. The repeatability coefficients were 2.9%, 4.8%, 6.5%, and 21.6% for CD34+, CD34+/CD133+, CD34+/CXCR4+, CD34+/CD133+/CXCR4+, and CD34+/VEGF2R+, respectively (12).
Follow-up and outcomes
Study participants were prospectively followed for 2 outcomes of interest, a composite of CV death and nonfatal myocardial infarction (MI) events, and all-cause mortality. Follow-up data were available for 1,253 subjects (436 with renal insufficiency) and were obtained using annual phone contact, electronic medical record review, and the Social Security Death Index and state records (23). The cause of death was determined from medical record review or by direct contact with the participants’ family member(s). Cardiovascular death and nonfatal MI events were adjudicated by 2 independent cardiologists blinded to study data (23). Cardiovascular death was defined as death attributable to an ischemic CV cause such as fatal MI, stroke, or sudden death secondary to a presumed CV cause in this high-risk population (23). Nonfatal MI events were adjudicated using the third universal definition of MI (28).
Statistical analysis
Participant characteristics were reported as frequency and percentage for categorical variables and as mean ± SD or median (interquartile range) for continuous variables depending on distribution. Differences between subjects with and without renal insufficiency were evaluated using the chi-square test for categorical variables and using the independent-sample Student's t-test or Mann-Whitney U test, as appropriate, for continuous variables. Baseline characteristics were also described across CPC count categories.
CPC counts were non-normally distributed and were analyzed as continuous variables after log-transformation (log2[cell count+0.0001]). The association of renal insufficiency, eGFR, and participant characteristics with CPC counts was assessed using unadjusted linear regression models. The impact of aging on the association of renal insufficiency with CPC counts was evaluated in age- and multivariable-adjusted linear regression models by exploring the multiplicative interaction between renal insufficiency and age dichotomized at the median value (70 years) for participants with renal insufficiency. Beta coefficients in linear regression models were exponentiated to transform them to the linear scale. A similar approach was used to study the association of dialysis treatment with CPC counts.
The association of renal insufficiency with adverse outcomes in the overall cohort was studied using Cox proportional hazards regression models adjusted for age, sex, race, diabetes, current smoking, hypertension, hypercholesterolemia, body mass index, hemoglobin, white blood cell count, CAD history, heart failure history, peripheral artery disease history, angiotensin converting enzyme inhibitor or angiotensin-II receptor blocker use, aspirin use, beta-blocker use, clopidogrel use, and statin use. A stepwise regression approach using backward elimination with removal p threshold of 0.10 was utilized to analyze covariates in all Cox models, following which the independent variable of interest was added to the model. Association of lower CPC counts (log1/2-transformed, reflecting a 50% relative decrease in count) with CV death or MI and all-cause mortality in the overall cohort and the subgroup of participants with renal insufficiency was studied using multivariable-adjusted Cox models, and results were reported as hazard ratio (HR) and 95% confidence interval (CI). The improvement in risk discrimination afforded by CPC counts was studied using the integrated discrimination index (IDI). In a sensitivity analysis, Fine and Gray competing-risks regression models were used to analyze the CV death or MI outcome and all-cause mortality was treated as a competing event. In a second sensitivity analysis, multivariable-adjusted Cox models were further adjusted for circulating levels of hsTnI and BNP.
CPC subtypes associated with outcomes were dichotomized at their respective median counts and participants were categorized into those without renal insufficiency, and those with renal insufficiency and CPC counts above or below the median. The association of these categories with outcomes was analyzed using multivariable-adjusted Cox models and the survival function of participants in these categories was plotted. All analyses were performed using SPSS Statistics Version 25 (IBM Corporation, Armonk, New York), SAS Version 9.4 (SAS Institute, Cary, North Carolina), and R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). A 2-tailed p value < 0.05 was considered statistically significant.
Results
Baseline characteristics of study participants are described in Table 1. The mean age was 66 ± 13 years, 39% were women, 21% were black, and almost 35% (446) of the cohort had renal insufficiency, with 216 in category G3a (eGFR 45 to <60 ml/min/1.73 m2), 114 in G3b (eGFR 30 to <45 ml/min/1.73 m2), 37 in G4 (eGFR 15 to <30 ml/min/1.73 m2), and 79 in G5 (receiving dialysis and eGFR <15 ml/min/1.73 m2). Subjects with renal insufficiency were older (median age 70 years), more frequently black, and had a higher prevalence of diabetes, hypertension, heart failure, peripheral artery disease, beta-blocker use, and clopidogrel use, and higher white blood cell count and lower hemoglobin levels as compared with those without renal insufficiency (Table 1). Baseline characteristics of participants stratified my median CPC counts are described in Supplemental Tables 1A–D. Participants with higher counts for all CPC types were relatively younger and more frequently men.
Table 1.
Baseline Characteristics of Participants Stratified by Renal Insufficiency Status
| Characteristic | Overall (N = 1,281) | No Renal Insufficiency (n = 835) | Renal Insufficiency (n = 446) | p Value |
|---|---|---|---|---|
| eGFR, ml/min/1.73 m2 | 68.0 ± 26.7 | 83.4 ± 15.7 | 39.1 ± 17.6 | <0.001 |
| Age, yrs | 65.5 ± 13.1 | 63.4 ± 12.3 | 69.5 ± 13.4 | <0.001 |
| Male | 776 (60.6) | 517 (61.9) | 259 (58.1) | 0.187 |
| Black | 283 (22.1) | 162 (19.4) | 121 (27.1) | 0.002 |
| Diabetes | 513 (40.1) | 296 (35.5) | 217 (48.7) | <0.001 |
| Current smoking | 60 (4.7) | 47 (5.6) | 13 (2.9) | 0.036 |
| Hypertension | 1,156 (90.5) | 731 (87.9) | 425 (95.3) | <0.001 |
| Hypercholesterolemia | 951 (74.2) | 618 (74.0) | 333 (74.7) | 0.841 |
| Body mass index, kg/m2 | 29.4 ± 6.4 | 29.2 ± 6.4 | 29.6 ± 6.5 | 0.216 |
| Hemoglobin, g/dl | 13.0 ± 1.9 | 13.5 ± 1.8 | 12.3 ± 1.8 | <0.001 |
| White blood cells, cells/μl | 6,734 ± 1,793 | 6,640 ± 1,749 | 6,909 ± 1,861 | 0.015 |
| CAD history | 1,054 (82.3) | 685 (82.0) | 369 (82.7) | 0.818 |
| HF history | 451 (35.2) | 252 (30.2) | 199 (44.6) | <0.001 |
| PAD history | 246 (19.2) | 129 (15.4) | 117 (26.2) | <0.001 |
| Ejection fraction, % | 53.0 (12.8) | 54.0 (12.1) | 51.1 (13.8) | 0.001 |
| High-sensitivity troponin I, pg/ml | 6.5 (3.4–15.3) | 5.0 (2.9–10.2) | 12.1 (6.1–27.1) | <0.001 |
| B-type natriuretic peptide, pg/ml | 103.9 (43.9–270.0) | 74.9 (33.9–175.1) | 210.7 (91.2–662.7) | <0.001 |
| ACE inhibitor/ARB use | 662 (51.7) | 420 (50.3) | 242 (54.3) | 0.178 |
| Aspirin use | 1,006 (78.5) | 667 (79.9) | 339 (76.0) | 0.116 |
| Beta-blocker use | 937 (73.1) | 588 (70.4) | 349 (78.3) | 0.003 |
| Clopidogrel use | 488 (38.1) | 299 (35.8) | 189 (42.4) | 0.022 |
| Statin use | 895 (69.9) | 571 (68.4) | 324 (72.6) | 0.125 |
| CD34+, cells/ml | 1,627 (1,034–2,494) | 1,688 (1,080–2,501) | 1,507 (698–2,485) | 0.025 |
| CD34+/CD133+, cells/ml | 747 (451–1,019) | 756 (468–1,212) | 741 (418–1,184) | 0.116 |
| CD34+/CXCR4+, cells/ml | 794 (485–1,341) | 801 (519–1,382) | 762 (430–1,231) | 0.012 |
| CD34+/VEGF2R+, cells/ml | 41 (12–138) | 47 (13–146) | 36 (12–112) | 0.052 |
| Cardiovascular death/MI | 175 (14.0) | 86 (10.5) | 89 (20.4) | <0.001 |
| All-cause death | 234 (18.7) | 116 (14.2) | 118 (27.1) | <0.001 |
Values are mean ± SD, n (%), or median (interquartile range). Ejection fraction, high-sensitivity troponin I, and B-type natriuretic peptide were measured in 1,198, 1,251, and 1,130 participants, respectively.
ACE = angiotensin-converting enzyme; ARB = angiotensin II receptor blocker; CAD = coronary artery disease; CD = cluster of differentiation; CXCR4 = C-X-C chemokine (C-X-C motif) receptor type 4; eGFR = estimated glomerular filtration rate; HF = heart failure; MI = myocardial infarction; PAD = peripheral artery disease; VEGF2R = vascular endothelial growth factor receptor 2.
Association of renal iinsufficiency with CPC counts
The predictors of CPC counts in the overall cohort are listed in Supplemental Table 2. As reported previously, age and peripheral artery disease had strong inverse associations, while male sex and higher hemoglobin levels were positively correlated with all CPC counts (12,29). eGFR, as a continuous variable, correlated positively with CD34+, CD34+/CD133+, and CD34+/CXCR4+ counts, but not with CD34+/VEGF2R+ counts in unadjusted analyses (Supplemental Table 2). Thus, renal insufficiency was associated with a 10% to 11% lower CD34+, CD34+/CD133+, and CD34+/CXCR4+ counts in unadjusted linear regression models (Table 2), and no multiplicative interaction with CV medication use was seen (all p for interaction > 0.10).
Table 2.
Impact of Age on the Association of Renal Insufficiency With Circulating Progenitor Cell Counts
| CD34+ |
CD34+/CD133+ |
CD34+/CXCR4+ |
CD34+/VEGF2R+ |
|||||
|---|---|---|---|---|---|---|---|---|
| Beta (95% CI) (%) | p Value | Beta (95% CI) (%) | p Value | Beta (95% CI) (%) | p Value | Beta (95% CI) (%) | p Value | |
| Unadjusted | –10.1 (–16.9 to –2.8) | 0.008 | –11.4 (–19.4 to –2.6) | 0.013 | –11.3 (–19.1 to –2.6) | 0.012 | –18.1 (–38.2 to 8.6) | 0.165 |
| Model 1 | ||||||||
| Overall | 4.6 (–3.4 to 13.3) | 0.264 | 4.1 (–5.4 to 14.4) | 0.411 | 6.8 (–2.8 to 17.3) | 0.174 | 10.0 (–17.5 to 46.7) | 0.515 |
| Age <70 yrs | 3.1 (–7.3 to 14.5) | 0.575 | 6.1 (–5.8 to 19.5) | 0.330 | –0.3 (–12.2 to 13.3) | 0.967 | 9.7 (–24.4 to 59.3) | 0.626 |
| Age ≥70 yrs | –14.8 (–24.6 to –3.8) | 0.010 | –16.7 (–29.2 to –1.9) | 0.028 | –15.1 (–26.3 to –2.2) | 0.024 | –37.8 (–60.9 to –1.0) | 0.045 |
| Model 2 | ||||||||
| Overall | –1.3 (–8.9 to 7.1) | 0.759 | –2.8 (–12.0 to 7.2) | 0.567 | –1.3 (–10.5 to 9.0) | 0.801 | 5.2 (–22.4 to 42.6) | 0.745 |
| Age <70 yrs | 9.2 (–2.1 to 21.8) | 0.113 | 8.6 (–4.1 to 23.0) | 0.193 | 7.4 (–6.2 to 23.0) | 0.302 | 39.7 (–6.3 to 108.4) | 0.101 |
| Age ≥70 yrs | –14.5 (–24.4 to –3.4) | 0.012 | –15.7 (–28.7 to –0.4) | 0.045 | –12.8 (–24.4 to 0.6) | 0.061 | –35.2 (–59.6 to 4.0) | 0.072 |
Dependent variables are log-transformed CPC counts. Model 1 was adjusted for continuous age. Model 2 was adjusted for continuous age, sex, race, diabetes, current smoking, hypertension, hypercholesterolemia, body mass index, hemoglobin, white blood cell count, CAD history, HF history, PAD history, and cardiovascular medication use (ACE inhibitor/ARB, aspirin, beta-blocker, clopidogrel, and statin).
CI = confidence interval, other abbreviations as in Table 1.
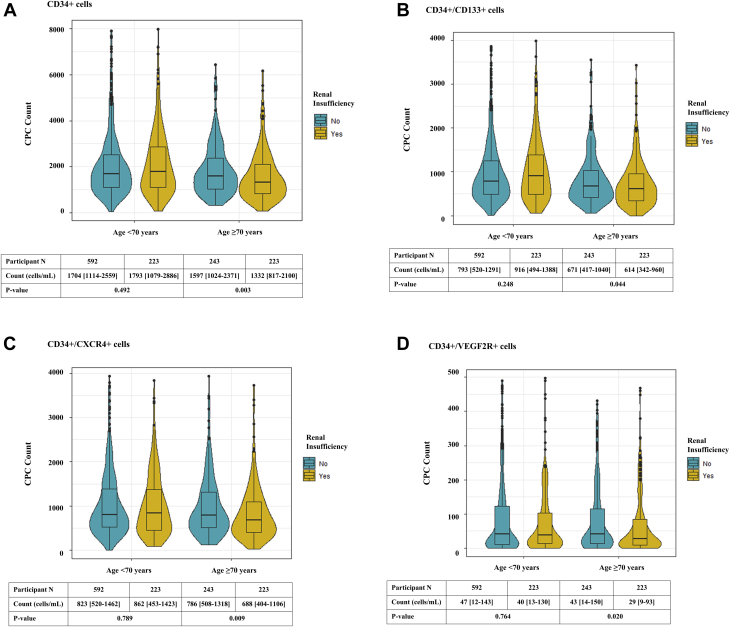
The relationship of renal insufficiency with CPC count was attenuated after adjustment for age (Table 2, model 1). Differences in CPC counts among participants with and without renal insufficiency across age percentiles are reported in Supplemental Figure 2. A consistent multiplicative interaction between renal insufficiency and median age (70 years) for CD34+ (p = 0.007), CD34+/CD133+ (p = 0.006), CD34+/CXCR4+ (p = 0.044), and CD34+/VEGF2R+ (p = 0.055) counts was observed. Thus, among older participants (≥70 years of age), presence of renal insufficiency was associated with a 14% to 35% lower CD34+, CD34+/CD133+, CD34+/CXCR4+ and CD34+/VEGF2R+ CPC counts, even after adjustment for age and other demographic and clinical characteristics, while no association was observed among those <70 years of age (Figure 1, Table 2). Similar results were observed for CD34+ and CD34+/CXCR4+ counts when participants were divided based on dialysis treatment (Supplemental Table 3).
Figure 1.
Impact of Age on the Association of Renal Insufficiency With Circulating Progenitor Cell Counts in Patients With Coronary Artery Disease
Circulating counts of (A) cluster of differentiation (CD) 34+ (CD34+), (B) CD34+/CD133+, (C) CD34+ and chemokine (C-X-C motif) receptor 4 (CD34+/CXCR4+), and (D) CD34+ and vascular endothelial growth factor receptor 2 (CD34+/VEGF2R+) cells were lower in older (≥70 years of age) but not in younger (<70 years of age) patients With renal insufficiency and coronary artery disease.
Outcomes with renal insufficiency
In the overall cohort, 175 CV death or MI and 234 all-cause death events were recorded during a median follow-up of 3.5 (interquartile range 1.5 to 5.2) years. In multivariable-adjusted Cox models, participants with renal insufficiency compared with those without were at a 48% higher risk of CV death or MI and 39% increased risk of all-cause mortality (Supplemental Table 4). The association of renal insufficiency with adverse outcomes was not attenuated after adding CPC counts to Cox models, and lower hematopoietic CPC counts (CD34+, CD34+/CD133+, and CD34+/CXCR4+) were associated with higher CV death or MI and all-cause mortality in the overall cohort (Supplemental Table 4).
CPC counts and outcomes in renal insufficiency
Among patients with CAD and renal insufficiency, a total of 89 CV death or MI events and 118 all-cause deaths were observed. CD34+, CD34+/CD133+, and CD34+/CXCR4+ counts were lower among participants that experienced a CV event as compared with those who did not (Supplemental Figure 3). Thus, lower CD34+, CD34+/CD133+, and CD34+/CXCR4+ counts were associated with higher CV death or MI and all-cause mortality rates in unadjusted Cox models (Table 3). In multivariable-adjusted models, the association between hematopoietic CPC counts and CV death or MI remained significant, while only CD34+/CD133+ counts were independently associated with all-cause death (Table 3). No associations between CD34+/VEGF2R+ counts and outcomes were observed (Table 3). There were no interactions of hematopoietic CPC counts with age or with dialysis use (all p for interaction > 0.05) for both outcomes.
Table 3.
Association Of Circulating Progenitor Cell Counts With Adverse Outcomes Among Patients With Renal Insufficiency
| CD34+ |
CD34+/CD133+ |
CD34+/CXCR4+ |
CD34+/VEGF2R+ |
|||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| CV death/MI | ||||||||
| Univariable | 1.44 (1.20–1.73) | <0.001 | 1.27 (1.16–1.38) | <0.001 | 1.38 (1.16–1.65) | <0.001 | 1.00 (0.94–1.06) | 0.972 |
| Multivariable | 1.30 (1.08–1.56) | 0.006 | 1.28 (1.16–1.43) | <0.001 | 1.28 (1.06–1.54) | 0.009 | 0.98 (0.91–1.04) | 0.478 |
| All-cause mortality | ||||||||
| Univariable | 1.35 (1.14–1.58) | <0.001 | 1.22 (1.12–1.33) | <0.001 | 1.26 (1.08–1.48) | 0.004 | 0.98 (0.93–1.04) | 0.507 |
| Multivariable | 1.18 (0.99–1.39) | 0.058 | 1.20 (1.08–1.33) | 0.001 | 1.09 (0.92–1.30) | 0.320 | 0.97 (0.92–1.03) | 0.335 |
Multivariable Cox models included log-transformed CPC count, eGFR, age, sex, race, diabetes, current smoking, hypertension, hypercholesterolemia, body mass index, hemoglobin, white blood cell count, CAD history, HF history, PAD history, ACE inhibitor/ARB use, aspirin use, beta-blocker use, clopidogrel use, and statin use as covariates. Stepwise Cox regression using backward elimination with model removal p threshold of 0.10 was used to analyze all covariates.
CV = cardiovascular; HR = hazard ratio; other abbreviations as in Table 1.
Age, eGFR, current smoking, heart failure history, and hemoglobin level were independent predictors of CV death or MI (Supplemental Table 5). Addition of CD34+, CD34+/CD133+, or CD34+/CXCR4+ counts to a baseline model comprising of these predictors results in significant improvements in IDI (0.181; 95% CI: 0.007 to 0.320; p = 0.040; 0.201; 95% CI: 0.042 to 0.309; p = 0.028; and 0.151; 95% CI: 0.004 to 0.254; p = 0.040; respectively) for CV death or MI. The independent predictors of all-cause death included age, eGFR, race, heart failure history, hemoglobin, and angiotensin converting enzyme inhibitor or angiotensin-II receptor blocker use (Supplemental Table 5). However, addition of CPC counts did not improve IDI for all-cause death.
First, in sensitivity analyses, the association of CPC counts with CV death or MI events was similar to Cox models when the Fine and Gray competing-risks regression approach was used (Supplemental Table 6). Second, hsTnI (HR: 1.10; 95% CI: 0.94 to 1.28; p = 0.223) and BNP (HR: 1.07; 95% CI: 0.89 to 1.28; p = 0.481) were not associated with CV death or MI in Cox regression models, while an independent association with all-cause death was seen with both hsTnI and BNP (HR: 1.15; 95% CI: 1.01 to 1.31; p = 0.039; and HR: 1.19; 95% CI: 1.02 to 1.39; p = 0.028, respectively). Importantly, further adjustment for hsTnI or BNP did not change the association of CPCs with outcomes (Supplemental Table 7).
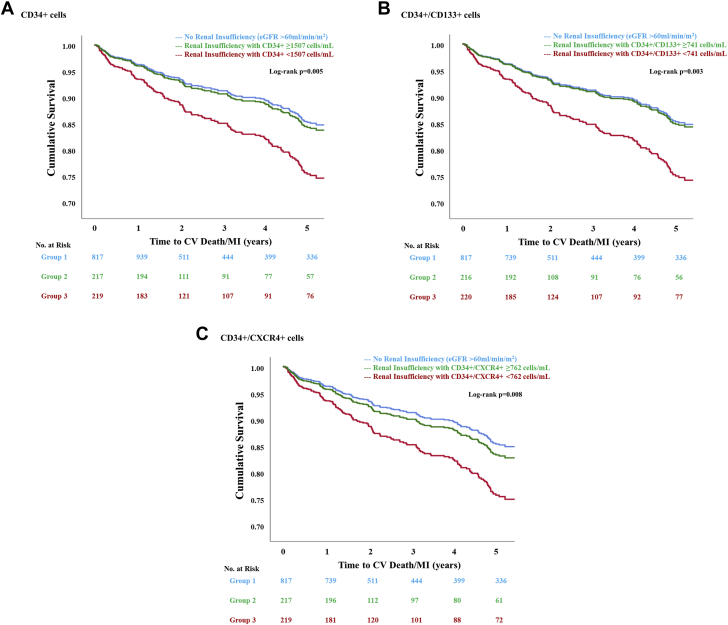
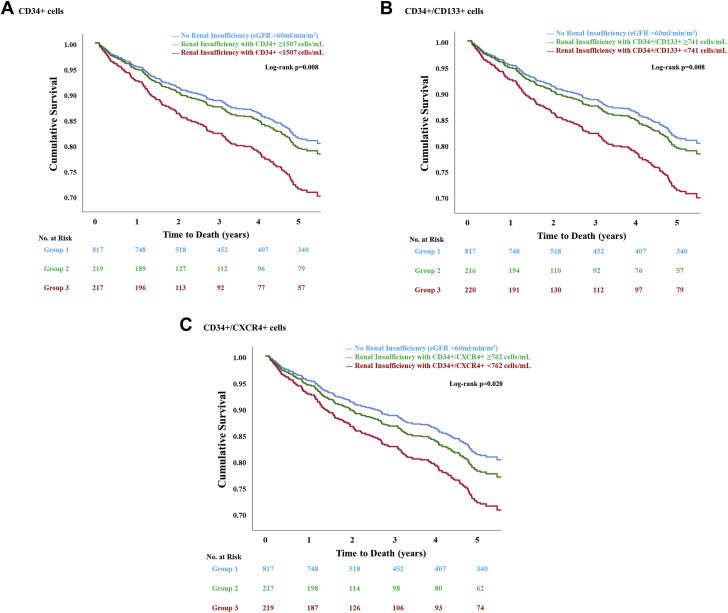
The risk of CV death or MI and all-cause mortality was similar among participants without renal insufficiency and those with renal insufficiency and high (≥median values) hematopoietic CPC counts (Table 4, Figures 2 and 3). In comparison, only participants with renal insufficiency and lower (<median values) hematopoietic CPC counts were at a 75% to 80% higher risk of CV death or MI and 57% to 63% higher risk of all-cause death as compared with those without renal insufficiency (Table 4). Similar associations were observed when Fine and Gray competing-risks regression models were used to analyze the CV death or MI outcome (Supplemental Table 8).
Table 4.
Adverse Outcomes Among Patients Categorized By Renal Insufficiency And Circulating Progenitor Cell Counts
| CV Death/MI |
All-Cause Mortality |
|||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| CD34+ | ||||
| No renal insufficiency | Reference | Reference | ||
| Renal insufficiency and count ≥1,507 cells/ml | 1.07 (0.70–1.64) | 0.752 | 1.12 (0.77–1.62) | 0.557 |
| Renal insufficiency and count <1,507 cells/ml | 1.76 (1.24–2.52) | 0.002 | 1.62 (1.19–2.22) | 0.002 |
| CD34+/CD133+ | ||||
| No renal insufficiency | Reference | Reference | ||
| Renal insufficiency and count ≥741 cells/ml | 1.03 (0.67–1.59) | 0.878 | 1.12 (0.78–1.61) | 0.553 |
| Renal insufficiency and count <741 cells/ml | 1.80 (1.26–2.56) | 0.001 | 1.63 (1.20–2.23) | 0.002 |
| CD34+/CXCR4+ | ||||
| No renal insufficiency | Reference | Reference | ||
| Renal insufficiency and count ≥762 cells/ml | 1.15 (0.76–1.75) | 0.504 | 1.19 (0.83–1.70) | 0.344 |
| Renal insufficiency and count <762 cells/ml | 1.75 (1.23–2.50) | 0.002 | 1.57 (1.15–2.15) | 0.005 |
Multivariable Cox models include renal insufficiency and circulating progenitor cell count category, eGFR, age, sex, race, diabetes, current smoking, hypertension, hypercholesterolemia, body mass index, hemoglobin, white blood cell count, CAD history, HF history, PAD history, ACE inhibitor/ARB use, aspirin use, beta-blocker use, clopidogrel use, and statin use as covariates. Stepwise Cox regression using backward elimination with model removal p threshold of 0.10 was used to analyze all covariates.
Figure 2.
Survival Function Curves for Cv Death or Mi Among Patients With Coronary Artery Disease Stratified by Renal Insufficiency and Circulating Progenitor Cell Counts
Patients with coronary artery disease, renal insufficiency, and (A) CD34+, (B) CD34+/CD133+, or (C) CD34+/CXCR4+ counts below the respective median cutoffs were at a higher risk of cardiovascular (CV) death or myocardial infarction (MI). Patients with coronary artery disease and renal insufficiency but circulating progenitor cell counts above the respective median cutoffs were at a similar risk of CV death or MI as those without renal insufficiency. eGFR = estimated glomerular filtration rate; other abbreviations as in Figure 1.
Figure 3.
Survival Function Curves For All-Cause Mortality Among Patients With Coronary Artery Disease Stratified By Renal Insufficiency And Circulating Progenitor Cell Counts
Patients with coronary artery disease, renal insufficiency, and (A) CD34+, (B) CD34+/CD133+, or (C) CD34+/CXCR4+ counts below the respective median cutoffs were at a higher risk of all-cause mortality. Patients with coronary artery disease and renal insufficiency but circulating progenitor cell counts above the respective median cutoffs were at a similar risk of all-cause mortality as those without renal insufficiency. Abbreviations as in Figure 1.
Discussion
In this large cohort study of patients with CAD, we found that the presence of renal insufficiency was associated with lower CPC counts among older participants (≥70 years of age) after adjusting for differences in demographic characteristics, risk factors, and medication use. We also demonstrated that low CPC count, indicative of impaired endogenous regenerative capacity, was an independent predictor of adverse CV outcomes among participants with renal insufficiency. In contrast, patients with renal insufficiency and higher CPC counts, indicative of preserved regenerative capacity, had similar outcomes as those without renal insufficiency. These observations suggest that the relatively poor outcomes in patients with CAD and renal insufficiency are related, at least in part, to impairment of endogenous regenerative capacity.
Renal insufficiency and CPC counts
Previous studies investigating the relationship between renal disease and CPC counts in patients without known CAD have reported 4% to 30% lower levels of CPCs in those with end-stage renal disease compared with healthy control subjects (30, 31, 32, 33, 34, 35, 36). Potential mechanisms underlying the lower CPC counts in renal disease include: 1) exposure to uremic toxins including beta(2)-microglobulin, indoxyl sulfate, and indole-3 acetic acid that inhibit CPC chemotactic mobility and promote their apoptosis (37,38); 2) induction of a permanent defect in bone marrow hematopoietic stem cell niche (39); 3) impaired PC mobilization and proliferation because of renal insufficiency–associated systemic inflammation and oxidative stress, demonstrated as an inverse association between CD34+/VEGF2R+ cells and circulating tumor necrosis factor-alpha and interleukin-6 levels among patients receiving hemodialysis (40); and 4) inhibitory effects of sera of patients with end-stage renal disease on CPC differentiation and migration (31) and effects of lower erythropoietin levels contributing to CPC deficiency in these patients (41).
The association of renal insufficiency with CPC counts in a large cohort of high-risk patients with CAD has not been well characterized. In a smaller study of 102 patients with stable angina and CAD, those with renal insufficiency were reported to have lower VEGF2R-expressing CPCs, but this study did not include women and the analyses were not adjusted for relevant confounders (42). In our study, we were adequately powered to investigate the independent association between renal insufficiency and CPC counts in patients with CAD. We found that this relationship is age-dependent, and the presence of renal function impairment is independently associated with lower CPC counts, but only in the older age group. This novel observation is concordant with previous studies that have shown that presence of CV risk factors at a relatively young age is associated with either normal or higher CPC counts, reflecting an endogenous reparative response to subclinical vascular injury related to risk factor exposure (12,13). This mobilization of CPCs into the peripheral circulation is exhausted at an older age after continued exposure to injurious stimuli (14). Overall, our results indicate that relatively young patients with CAD and renal insufficiency have similar CPC counts as those with CAD alone, whereas differences in CPC counts between the 2 groups become apparent at an older age.
CPC counts and outcomes in patients with CAD and renal insufficiency
Previous studies have shown that low CPC counts are associated with CV events in patients with CAD (15, 16, 17, 18) and in patients receiving dialysis treatment for end-stage renal disease (19, 20, 21, 22). For instance, in a seminal 2015 meta-analysis, Rigato et al. (18) demonstrated that low CPC counts are associated with ∼2-fold risk of CV events among patients with CAD. Maruyama et al. (19) showed that a low CD34+ count is associated with a 2.2-fold risk of CV events in patients receiving chronic hemodialysis. Our findings are consistent with these prior observations, as we observed that low hematopoietic CPC counts among patients with CAD and renal insufficiency are associated with ∼1.8-fold risk of CV events. Herein, we have demonstrated that CPC counts impact prognosis in patients with CAD and renal insufficiency, even before end-stage renal disease develops.
Our study participants underwent extensive phenotyping for 4 different CPC subtypes, and our results indicate that low counts of cell subtypes enriched for hematopoietic progenitors, CD34+, CD34+/CD133+, and CD34+/CXCR4+ cells, impact prognosis in this population. Previous research has shown that human CD133+ cells are protective in murine models of acute tubular and glomerular damage (43), and administration of CD133+ cells stimulates erythropoietic production and limits renal fibrosis after acute kidney injury (44). Additionally, SDF-1, the primary chemokine responsible for CXCR4+ cell mobilization from the bone marrow, is expressed in the normal kidney, and its expression is enhanced 24 h after renal ischemia (45,46). Taken together, these studies provide some pathophysiologic insight regarding the association of higher CD34+/CD133+ and CD34+/CXCR4+ counts with favorable prognosis in patients with CAD and renal insufficiency.
Last, we have also demonstrated that patients with renal insufficiency and high CPC counts have similar outcomes as those without renal insufficiency, indicating that an important mechanism underlying the increased CV risk in patients with CAD and renal insufficiency is impaired endogenous regenerative capacity that can be measured using CPC counts. Important implications of our results are that reduced regenerative capacity is a novel risk factor in patients with CAD and renal insufficiency and may be used as a biomarker to identify those at highest risk. Whether cell-based therapies would improve CV outcomes in this population remains to be investigated (45,47,48). Paradoxically, most clinical trials tend to exclude this high-risk population from CV cell therapy trials.
Study strengths
Strengths of our study include the large number of participants studied and the detailed exploration of the relationship between renal insufficiency and various CPC subtypes. Study participants were followed for more than 3 years for adjudicated CV events, and the independent association of CPC subtypes with outcomes was determined.
Study limitations
Potential limitations of the study are the use of estimated eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation, which, like all other creatinine-based estimating equations, remains imprecise. We did not investigate the association of CAD or renal insufficiency duration with CPC counts in this study. The functional activity of CPCs was not evaluated. However, previous studies have shown impaired PC function in renal disease (30,33). Finally, the observational nature of this analysis does not imply causation.
Conclusions
In conclusion, we found that renal insufficiency is independently associated with lower CPC counts among older patients with CAD. Lower CPC counts in patients with CAD and renal insufficiency are independently associated with an increased risk of adverse CV events, whereas those with preserved CPC counts have similar risk as those without renal insufficiency. Therapeutic interventions targeting endogenous regenerative capacity in this high-risk patient population are warranted.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Our study demonstrated that renal insufficiency is associated with impaired regenerative capacity, measured as decreased counts of CPCs, in older patients with coronary artery disease. Low CPC counts are independently associated with an increased risk of CV events and can stratify future CV risk in patients with CAD and renal insufficiency.
TRANSLATIONAL OUTLOOK: Future research studies should focus on deciphering the mechanistic basis of the association of renal insufficiency with impaired regenerative capacity in older patients with CAD. Randomized controlled trials to study the prognostic impact of therapeutic interventions that augment CPC counts in patients with CAD and renal insufficiency are warranted.
Acknowledgments
The authors would like to thank the Emory Cardiovascular Biobank participants and study coordinators.
Footnotes
Drs. Mehta, Tahhan, Dhindsa have been supported by the Abraham J. and Phyllis Katz Foundation (Atlanta, Georgia). Dr. Mehta is supported by American Heart Association postdoctoral fellowship award 19POST34400057. Dr. Quyyumi is supported by National Institutes of Health grants 1P20HL113451-01, 1R61HL138657-02, 1P30DK111024-03S1, 5R01HL095479-08, 3RF1AG051633-01S2, 5R01AG042127-06, 2P01HL086773-08, U54AG062334-01, 1R01HL141205-01, 5P01HL101398-02, 1P20HL113451-01, 5P01HL086773-09 1RF1AG051633-01, R01 NS064162-01, R01 HL89650-01, HL095479-01, 1DP3DK094346-01, and 2P01HL086773 and American Heart Association grant 15SFCRN23910003. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Grundy S.M., Stone N.J., Bailey A.L. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Gupta R., Birnbaum Y., Uretsky B.F. The renal patient with coronary artery disease: current concepts and dilemmas. J Am Coll Cardiol. 2004;44:1343–1353. doi: 10.1016/j.jacc.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T., Murohara T., Sullivan A. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 4.Asahara T., Masuda H., Takahashi T. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 5.Urbich C., Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 6.Lin Y., Weisdorf D.J., Solovey A., Hebbel R.P. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fadini G.P., Losordo D., Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gehling U.M., Ergun S., Schumacher U. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- 9.Yin A.H., Miraglia S., Zanjani E.D. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 10.Seeger F.H., Rasper T., Koyanagi M., Fox H., Zeiher A.M., Dimmeler S. CXCR4 expression determines functional activity of bone marrow-derived mononuclear cells for therapeutic neovascularization in acute ischemia. Arterioscler Thromb Vasc Biol. 2009;29:1802–1809. doi: 10.1161/ATVBAHA.109.194688. [DOI] [PubMed] [Google Scholar]
- 11.Alaiti M.A., Ishikawa M., Costa M.A. Bone marrow and circulating stem/progenitor cells for regenerative cardiovascular therapy. Transl Res. 2010;156:112–129. doi: 10.1016/j.trsl.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Al Mheid I., Hayek S.S., Ko Y.A. Age and human regenerative capacity impact of cardiovascular risk factors. Circ Res. 2016;119:801–809. doi: 10.1161/CIRCRESAHA.116.308461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fadini G.P., Mehta A., Dhindsa D.S. Circulating stem cells and cardiovascular outcomes: from basic science to the clinic. Eur Heart J. 2019 Dec 31 doi: 10.1093/eurheartj/ehz923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rauscher F.M., Goldschmidt-Clermont P.J., Davis B.H. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt-Lucke C., Rossig L., Fichtlscherer S. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 16.Werner N., Kosiol S., Schiegl T. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 17.Patel R.S., Li Q., Ghasemzadeh N. Circulating CD34+ progenitor cells and risk of mortality in a population with coronary artery disease. Circ Res. 2015;116:289–297. doi: 10.1161/CIRCRESAHA.116.304187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rigato M., Avogaro A., Fadini G.P. Levels of circulating progenitor cells, cardiovascular outcomes and death: a meta-analysis of prospective observational studies. Circ Res. 2016;118:1930–1939. doi: 10.1161/CIRCRESAHA.116.308366. [DOI] [PubMed] [Google Scholar]
- 19.Maruyama S., Taguchi A., Iwashima S. Low circulating CD34+ cell count is associated with poor prognosis in chronic hemodialysis patients. Kidney Int. 2008;74:1603–1609. doi: 10.1038/ki.2008.495. [DOI] [PubMed] [Google Scholar]
- 20.Lorenzen J., David S., Bahlmann F.H. Endothelial progenitor cells and cardiovascular events in patients with chronic kidney disease--a prospective follow-up study. PLoS One. 2010;5:e11477. doi: 10.1371/journal.pone.0011477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu C.L., Leu J.G., Liu W.C. Endothelial progenitor cells predict long-term mortality in hemodialysis patients. Int J Med Sci. 2016;13:240–247. doi: 10.7150/ijms.14209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee H.J., Kim W., Kim W.S. Circulating endothelial progenitor cell levels predict cardiovascular events in end-stage renal disease patients on maintenance hemodialysis. Nephron. 2015;130:151–158. doi: 10.1159/000430471. [DOI] [PubMed] [Google Scholar]
- 23.Ko Y.A., Hayek S., Sandesara P., Samman Tahhan A., Quyyumi A. Cohort profile: the Emory Cardiovascular Biobank (EmCAB) BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-018753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapter 1: definition and classification of CKD. Kidney Int Suppl (2011) 2013;3:19–62. doi: 10.1038/kisup.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samman Tahhan A., Sandesara P., Hayek S.S. High-sensitivity troponin I levels and coronary artery disease severity, progression, and long-term outcomes. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahar E.A., Mou L., Hayek S.S., Quyyumi A.A., Waller E.K. Flow cytometric data analysis of circulating progenitor cell stability. Data Brief. 2017;10:346–348. doi: 10.1016/j.dib.2016.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thygesen K., Alpert J.S., Jaffe A.S. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 29.Topel M.L., Hayek S.S., Ko Y.A. Sex differences in circulating progenitor cells. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi J.H., Kim K.L., Huh W. Decreased number and impaired angiogenic function of endothelial progenitor cells in patients with chronic renal failure. Arterioscler Thromb Vasc Biol. 2004;24:1246–1252. doi: 10.1161/01.ATV.0000133488.56221.4a. [DOI] [PubMed] [Google Scholar]
- 31.de Groot K., Bahlmann F.H., Sowa J. Uremia causes endothelial progenitor cell deficiency. Kidney Int. 2004;66:641–646. doi: 10.1111/j.1523-1755.2004.00784.x. [DOI] [PubMed] [Google Scholar]
- 32.Eizawa T., Murakami Y., Matsui K. Circulating endothelial progenitor cells are reduced in hemodialysis patients. Curr Med Res Opin. 2003;19:627–633. doi: 10.1185/030079903125002379. [DOI] [PubMed] [Google Scholar]
- 33.Krenning G., Dankers P.Y., Drouven J.W. Endothelial progenitor cell dysfunction in patients with progressive chronic kidney disease. Am J Physiol Renal Physiol. 2009;296:F1314–F1322. doi: 10.1152/ajprenal.90755.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueno H., Koyama H., Fukumoto S. Dialysis modality is independently associated with circulating endothelial progenitor cells in end-stage renal disease patients. Nephrol Dial Transplant. 2010;25:581–586. doi: 10.1093/ndt/gfp358. [DOI] [PubMed] [Google Scholar]
- 35.Krieter D.H., Fischer R., Merget K. Endothelial progenitor cells in patients on extracorporeal maintenance dialysis therapy. Nephrol Dial Transplant. 2010;25:4023–4031. doi: 10.1093/ndt/gfq552. [DOI] [PubMed] [Google Scholar]
- 36.Ozkok A., Yildiz A. Endothelial progenitor cells and kidney diseases. Kidney Blood Press Res. 2018;43:701–718. doi: 10.1159/000489745. [DOI] [PubMed] [Google Scholar]
- 37.Lin C.J., Wu C.J., Wu P.C. Indoxyl sulfate impairs endothelial progenitor cells and might contribute to vascular dysfunction in patients with chronic kidney disease. Kidney Blood Press Res. 2016;41:1025–1036. doi: 10.1159/000452604. [DOI] [PubMed] [Google Scholar]
- 38.Jourde-Chiche N., Dou L., Sabatier F. Levels of circulating endothelial progenitor cells are related to uremic toxins and vascular injury in hemodialysis patients. J Thromb Haemost. 2009;7:1576–1584. doi: 10.1111/j.1538-7836.2009.03540.x. [DOI] [PubMed] [Google Scholar]
- 39.Aleksinskaya M.A., Monge M., Siebelt M. Chronic kidney failure mineral bone disorder leads to a permanent loss of hematopoietic stem cells through dysfunction of the stem cell niche. Sci Rep. 2018;8:15385. doi: 10.1038/s41598-018-33979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozkok A., Aktas E., Yilmaz A. Decrease in endothelial progenitor cells associated with inflammation, but not with endothelial dysfunction in chronic hemodialysis patients. Clin Nephrol. 2013;79:21–30. doi: 10.5414/CN107318. [DOI] [PubMed] [Google Scholar]
- 41.Haller H., de Groot K., Bahlmann F., Elger M., Fliser D. Stem cells and progenitor cells in renal disease. Kidney Int. 2005;68:1932–1936. doi: 10.1111/j.1523-1755.2005.00622.x. [DOI] [PubMed] [Google Scholar]
- 42.Surdacki A., Marewicz E., Wieteska E. Association between endothelial progenitor cell depletion in blood and mild-to-moderate renal insufficiency in stable angina. Nephrol Dial Transplant. 2008;23:2265–2273. doi: 10.1093/ndt/gfm943. [DOI] [PubMed] [Google Scholar]
- 43.Bussolati B., Camussi G. Therapeutic use of human renal progenitor cells for kidney regeneration. Nat Rev Nephrol. 2015;11:695–706. doi: 10.1038/nrneph.2015.126. [DOI] [PubMed] [Google Scholar]
- 44.Aggarwal S., Grange C., Iampietro C., Camussi G., Bussolati B. Human CD133(+) renal progenitor cells induce erythropoietin production and limit fibrosis after acute tubular injury. Sci Rep. 2016;6:37270. doi: 10.1038/srep37270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goligorsky M.S., Yasuda K., Ratliff B. Dysfunctional endothelial progenitor cells in chronic kidney disease. J Am Soc Nephrol. 2010;21:911–919. doi: 10.1681/ASN.2009111119. [DOI] [PubMed] [Google Scholar]
- 46.Togel F., Isaac J., Hu Z., Weiss K., Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772–1784. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 47.Coppolino G., Cernaro V., Placida G., Leonardi G., Basile G., Bolignano D. Endothelial progenitor cells at the interface of chronic kidney disease: from biology to therapeutic advancement. Curr Med Chem. 2018;25:4545–4551. doi: 10.2174/0929867324666170920150134. [DOI] [PubMed] [Google Scholar]
- 48.Marcheque J., Bussolati B., Csete M., Perin L. Concise reviews: stem cells and kidney regeneration: an update. Stem Cells Transl Med. 2019;8:82–92. doi: 10.1002/sctm.18-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.