Abstract
Background
The aim of this study was to evaluate the clinical efficacy of Xuanfei Baidu Decoction (XBD) combined with conventional drug therapy compared with conventional medicine alone in patients with coronavirus disease 2019 (COVID-19).
Methods
Forty-two patients with COVID-19 were randomly assigned to XBD plus conventional medicine (n = 22) and conventional medicine alone (n = 20). Both groups were treated for 1 week. The primary endpoint was the disappearance rate of main symptoms (fever, cough, and fatigue).
Results
Compared with the conventional medicine, the disappearance rate of clinical symptoms such as fever, cough, fatigue and loss of appetite in the experimental group were significantly reduced (P < 0.05). The number of white blood cells and lymphocytes in the experimental group increased significantly (P < 0.05), which all returned to normal parameters. Meanwhile, the C-reactive protein and erythrocyte sedimentation rate in the experimental group were significantly reduced (P < 0.05).
Conclusion
XBD combined with conventional medicine may significantly improve patient's clinical symptoms, increase the number of white blood cells and lymphocytes to improve immunity, and also significantly reduce C-reactive protein and erythrocyte sedimentation rate to play an anti-inflammatory effect. However, it needs to be confirmed by a large sample study.
Clinical trial registration
China Clinical Trial Registry (ChiCTR2000034795).
Keywords: COVID-19, Herbal medicine, Randomized controlled trial, Integrative medicine, Chinese medicine
1. Introduction
At present, there is a lack of effective antiviral drugs, and vaccines are still being developed to treat coronavirus disease 2019 (COVID-19). China has been used integrated Chinese and Western medicine for treating patients with COVID-19. As the fourth edition of the guideline clearly pointed out that blind use of antibacterial drugs should be avoided.
It is generally believed by traditional Chinese medicine experts that COVID-19 is the pestilence in view of its epidemic and infectious nature. Combined with the occurrence time and geographical environment, this epidemic has the characteristics of external evils such as toxin evil, wet evil and cold evil. Therefore, in Chinese medicine, COVID-19 is referred to as “wet toxin pestilence”1, 2. Based on the above pathogenic characteristics, Academician Boli Zhang and Professor Qingquan Liu created Xuanfei Baidu Decoction. This decoction is derived from the famous traditional Chinese medicine book titled Treatise on Febrile and Miscellaneous Diseases, combined with clinical experience and pharmacological screening. The aim of this study was to evaluate the efficacy Xuanfei Baidu Decoction (XBD) combined with conventional medicine for treating patients with COVID-19.
2. Participants and methods
2.1. Type of study
This study was a pilot randomized controlled trial which has been registered with China Clinical Trial Registry (ChiCTR2000034795).
2.2. Inclusion criteria
The inclusion criteria for this trial were as follows:
-
-
Inpatients in Wuhan Pulmonary Hospital (from January 30 to February 10, 2020) who met the diagnostic criteria of COVID-19;
-
-
Age of 18-75 years old;
-
-
In mild and severe stages according to the fourth edition of guideline;
-
-
Agreed to participate in the trial;
-
-
No antibiotics were used during one cycle of this treatment.
2.3. Exclusion criteria
The exclusion criteria for this trial were as follows:
-
-
In the critical stage according to the fourth edition of guideline;
-
-
Acute respiratory diseases not caused by 2019-nCoV;
-
-
Asthma patients who needed daily treatment;
-
-
Severe lung interstitial lesions, bronchiectasis and other basic lung disease patients;
-
-
Accompanied by severe primary immunodeficiency disease, acquired immunodeficiency syndrome, congenital respiratory malformations, congenital heart disease, abnormal lung development and other basic diseases;
-
-
Pregnant women.
2.4. Randomization
According to the patient's hospitalization number, a simple random grouping was performed. The odd number was assigned to the experimental group, and the even number was assigned to the control group.
2.5. Intervention
For the experimental group, XBD (1 bag/time 200 ml, 2 times/day) was added on top of conventional medicine which were treated to the control group. The treatment time for both groups were 1 week. Antibiotics were disabled during the treatment of both groups, and other Chinese medicines were prohibited.
XBD was composed of the following herbs: “ma huang” 8 g,“xing ren” 15 g,“sheng shi gao” 30 g, “chang zhu” 10 g, “yi yi ren” 30 g, “huo xiang” 15 g, “hu zhang” 20 g, “ting li zi” 15 g, “ma bian cao” 30 g, “lu gen” 30 g, “qing hao” 25 g, “ju hong” 20 g, and “sheng gan cao” 10 g.
For the control group, conventional treatment was given, according to the treatment measures recommended by the “COVID-19 Prevention and Control Program (Trial)” issued by the National Health and Health Commission of China.
2.6. Outcome measures
2.6.1. The primary endpoint
The primary endpoint was the disappearance rate of main symptoms of COVID-19. The disappearance rate of the main symptoms in the experimental group and the control group were compared by evaluating 3 symptoms, fever, fatigue, and cough, after 1 week of treatment.
2.6.2. Secondary endpoints
The secondary endpoints were the disappearance rate of secondary symptoms, and the changes in white blood cells, lymphocytes, C-reactive protein, and erythrocyte sedimentation rate.
2.7. Safety monitoring
The occurrence of nausea, vomiting, diarrhea, rash and other adverse events after taking medicine were monitored and recorded.
2.8. Ethical concerns
This study followed and met the ethical requirements for conducting the clinical trial. The institute complies with the relevant regulations of the National Health Commission. The approval from the institutional review board was obtained from Wuhan Health Council (IRB number: 2020100).
2.9. Statistical analysis
For statistical analysis, SAS 9.4 software was used. All statistical tests used a two-sided test, with P < 0.05 indicating that the difference is statistically significant. The measurement data was described by . Quantitative data was compared using t test, and categorical data was compared using chi-square test or exact probability method.
3. Results
3.1. Patient characteristics
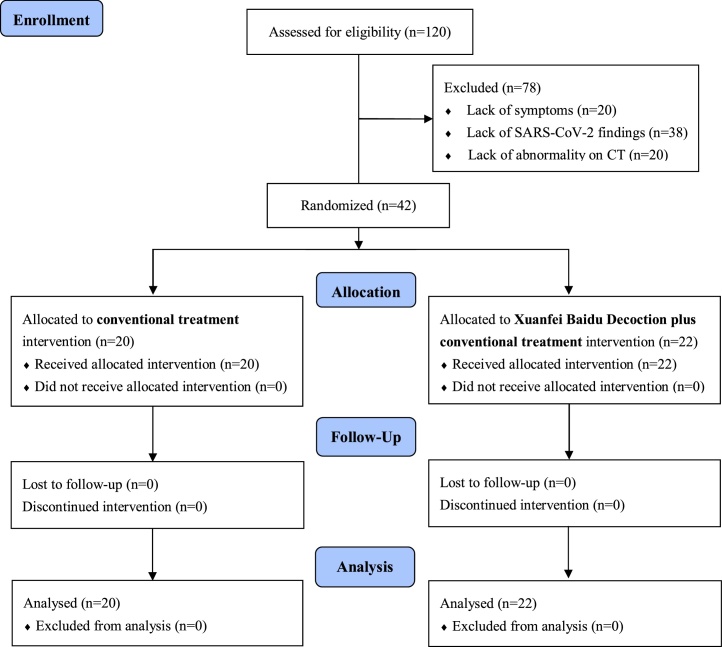
Of the 120 patients who were assessed for eligibility, 78 were excluded due to the lack of symptoms (not having fever, fatigue or coughing), SARS-CoV-2 assay findings, or radiologic abnormality on chest CT. Therefore, a total of 42 patients diagnosed with COVID-19 were included. The study flow chart is shown in Fig. 1.
Fig. 1.
Study flow chart.
There was no statistically significant difference between the two groups in terms of age, temperature, blood pressure, heart rate, blood oxygen saturation and other baseline data, and they were comparable (Table 1).
Table 1.
Demographic characteristics of participants
| XBD plus conventional medicine (n = 22) | Conventional medicine (n = 20) | Pvalue | |
|---|---|---|---|
| Age, years | 57.10 ± 14.00 | 62.40 ± 12.30 | 0.202 |
| Temperature, °C | 38.56 ± 0.68 | 38.38 ± 0.63 | 0.383 |
| Systolic pressure, mmHg | 123.90 ± 12.90 | 119.30 ± 14.4 | 0.290 |
| Diastolic pressure, mmHg | 75.30 ± 10.20 | 72.40 ± 9.80 | 0.361 |
| Heart rate, number/min | 88.59 ± 10.80 | 88.40 ± 11.60 | 0.978 |
| Oxyhemoglobin saturation, % | 84.28 ± 7.23 | 85.26 ± 6.98 | 0.925 |
3.2. Outcome measures
The disappearance rates of major symptoms including fever, cough and fatigue were significantly high in XBD plus conventional medicine group compared with conventional medicine alone (Table 2).
Table 2.
Disappearance rate of outcomes
| Outcomes | XBD plus conventional medicine | Conventional medicine | Pvalue |
|---|---|---|---|
| Major symptoms | |||
| Fever | 18/20 (90.0) | 11/18 (72.3) | 0.043 |
| Cough | 13/17 (76.5) | 7/18 (38.9) | 0.028 |
| Fatigue | 15/19 (78.9) | 6/14 (42.9) | 0.039 |
| Other symptoms | |||
| Loss of appetite | 9/12 (75.0) | 2/9 (22.2) | 0.024 |
| Shortness of breath | 8/10 (80.0) | 4/8 (50.0) | 0.201 |
| Chest tightness | 7/11 (63.6) | 2/9 (22.2) | 0.080 |
| Insomnia | 4/9 (44.4) | 2/7 (28.6) | 0.451 |
| Pharyngalgia | 3/5 (50.0) | 3/5 (50.0) | 1 |
| Chill | 5/9 (55.5) | 5/9 (55.5) | 1 |
| Headache | 3/5 (50.0) | 2/4 (50.0) | 1 |
| Nausea | 4/6 (60.7) | 3/5 (60.0) | 1 |
| Vomiting | 2/3 (66.7) | 2/3 (66.6) | 1 |
| Diarrhea | 5/7 (71.4) | 5/7 (71.4) | 1 |
| Biochemical parameters | |||
| White blood cell | 9/12 (75.0) | 3/11 (27.2) | 0.030 |
| Lymphocytes | 7/10 (70.0) | 2/10 (20.0) | 0.035 |
| C-reactive protein | 15/18 (83.3) | 5/17 (29.4) | 0.002 |
| Erythrocyte sedimentation rate. | 16/19 (84.2) | 6/16 (37.5) | 0.006 |
There were a significant improvement in the disappearance rate of loss of appetite in XBD plus conventional medicine group, while there was no significant difference in other symptoms including shortness of breath, chest tightness, insomnia, pharyngalgia, chill, headache, nausea, vomiting and diarrhea between the two groups (Table 2).
Regarding the main laboratory indicators, the increase rates of WBC and lymphocytes, the reduction rate of C-reactive protein, and erythrocyte sedimentation rate were favorable to XBD plus conventional medicine group (Table 2).
3.3. Safety monitoring
No obvious adverse reactions were seen in patients taking Chinese medicine.
4. Discussion
The results showed that the XBD combined with conventional medicine could significantly relieve clinical symptoms such as fever, cough, fatigue, loss of appetite, etc. From the laboratory data comparison results, this treatment can obviously increase the number of white blood cells and lymphocytes. Meanwhile, XBD may also significantly reduce C reactive protein and erythrocyte sedimentation rate, to play an obvious anti-inflammatory effect.
However, this study has several limitations. It had a small sample size, a short course of treatment, no strict random methods, and no blinding methods. Therefore, further exploration is needed in the future.
Chinese medicine participates in the treatment of this disease, paying more attention to the stage of the disease, and early treatment is a key period to reduce critical illness and mortality, and a large number of clinical literature is also proving that early use of Chinese medicine can improve the prognosis3, 4. In the absence of effective antiviral drugs, use traditional Chinese medicine to treat patients with COVID -19 may be considered.
Author contributions
Conceptualization: W-zX, GW; Methodology: W-zX, GW; Formal Analysis: JD, WA; Investigation: JD, WA; Data Curation: W-zX, GW; Writing–Original Draft: W-zX; Writing–Review & Editing: GW; Funding Acquisition: W-zX
Conflict of interest
The authors declare no conflict of interest.
Funding
None
Ethical statement
This research has been approved by Wuhan Health Council.
Data availability
The data will be made available upon request.
References
- 1.Liu J., Cui Y., Bai M., Zhang H., Jin Y., Lv P. Application of traditional Chinese medicine in prevention and treatment of COVID-19. Chinese Traditional and Herbal Drugs. 2020;51:860–865. doi: 10.7501/j.issn.0253-2670.2020.04.005. [DOI] [Google Scholar]
- 2.Miao Q., Cong X.D., Wang B., Wang Y.G., Zhang Z.D. Understanding and thinking of novel coronavirus pneumonia in traditional Chinese medicine. J Trad Chin Med. 2020;61:286–288. doi: 10.13288/j.11-2166/r.2020.04.003. [DOI] [Google Scholar]
- 3.Tong Tong, Ying-Qi Wu, Wei-Jian Ni, Ai-Zong Shen, Sheng Liu. The potential insights of Traditional Chinese Medicine on treatment of COVID-19. Chinese medicine. 2020:15. doi: 10.1186/s13020-020-00326-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J., Zhang Y. Traditional Chinese Medicine treatment of COVID-19. Complement Ther Clin Pract. 2020;39:101165. doi: 10.1016/j.ctcp.2020.101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be made available upon request.