Abstract
Objective
The transfer of peripheral nerves originating above the level of injured spinal cord into the nerves/roots below the injury is a promising approach. It facilitates the functional recovery in lower extremity, bladder/bowel and sexual function in paraplegics. We assessed anatomical feasibility of transfer of lower intercostal nerves to S2 ventral root in human cadaver for management of neurogenic bladder dysfunction in patients with spinal cord injury.
Methods
Study was performed in five formalin fixed cadavers. Cadavers were placed in prone position. A transverse incision was made along 11th ribs on both sides and 10th, 11th Intercostal nerves (ICN) and subcostal nerve were harvested up to maximum possible length. In four cadavers the ventral root of S2 was exposed by endoscope and in one by the standard open laminectomy. Intercostal nerves were brought down to lumbo-sacral region, S2 ventral root was cut cranially and feasibility of intercostal to S2 anastomosis was assessed.
Results
The mean length of intercostal nerves was 18.4 cm for the 10th 19.5 cm for the 11th and 22.15 cm for the subcostal nerve. The length of harvested nerve and the nerve length necessary to perform sacral roots neurotization were possible in all cases by only by subcostal nerve while T11 and T10 ICN fall short of the required length.
Conclusion
For Spinal cord lesions located at the conus, subcostal nerve could be connected to ventral root of S2 in an attempt to restore bladder function while 10th and 11th ICN had enough length to neurotize lumbar plexus.
Keywords: Cauda equina, Intercostal nerve, Nerve transfer, Neurotization, Spinal cord injury, Neurogenic bladder
1. Introduction
The transfer of peripheral nerves originating above the level of the injured spinal cord into the nerves/roots below the injury is a promising approach. It facilitates the motor/sensory recovery in lower-extremity, bladder/bowel and sexual function in complete paraplegics. Even the partial recovery after this procedure will have long term impact in terms of rehabilitation, reduction in cost, reduced morbidity/mortality and over all improved quality of life (QOL) of spinal cord injury (SCI) patients.
Apart from the loss of ambulation, neurogenic bladder dysfunction is one of the most devastating consequences of SCI. Incidence and pattern of neurogenic bladder dysfunctions depends upon the level of SCI injury and is roughly 70–84%.1 Commonly encountered complications of the neurogenic bladder are recurrent urinary tract infections, vesico-ureteric reflux, renal stones and renal impairment which may be caused by the pathology itself or secondary to use of indwelling catheters.2 It adversely affects the social and physical quality of life of the patients and renal impairment is still among the most important causes of death in SCI patients. Regaining bladder function in patients with SCI improves quality of life and helps prevent clinical complications.3 Therefore, neurogenic bladder dysfunction has to be managed aggressively. The purpose of our study was to assess anatomical feasibility of transfer of lower intercostal nerves to S2 ventral root extradurally in human cadaver for management of neurogenic bladder dysfunction in patients with SCI at or below conus medullaris.
2. Material and methods
A cadaveric study was performed in the Department of Anatomy and Department of Surgery, in a tertiary referral center. Institutional ethical committee approval was taken before the study. Five formalin fixed cadavers (3 males and 2 females with age ranging from 45 to 60 years) were used in the study. All the cadavers with normal anatomy in the lower dorsal, lumbar and sacral region were included. Cadavers with previous surgery or trauma in this region were excluded. Cadavers were placed in the prone position. A transverse incision was made along 11th ribs on both sides. 10th, 11th and subcostal nerves were harvested up to maximum possible length or till its division by using the technique described by Vialle et al.4
In four cadavers the ventral root of S2 was exposed by Neuro-endoscope and in one the standard open laminectomy was used. In the prone position of the cadaver, L5S1 space was identified by making a 2.5 cm skin incision 1 cm lateral to the midline. Deep fascia was cut in line of skin incision and paraspinal muscle was dissected up to lamina. 23 mm diameter tubular endoscopic sheath was inserted and fixed with holder. After docking, ipsilateral L5S1 lamina was identified. High-speed drill was used for flavectomy of L5 and sacral lamina up to S3. Then S1 and S2 roots were identified and traced up to exiting foramina. After sufficient mobilisation, S2 root was cut as proximal as possible and the feasibility of intercostal to S2 anastomosis was assessed. Same procedure was repeated on the opposite side. In open dissection midline skin incision of 5 cm length was made from L5 to S3. The Bilateral paraspinal muscle were dissected, L5 to S3 spinous process and lamina were exposed. Laminectomy was done with the help of bone nibbler and Kerrison punch in standard fashion. After removal of ligament flavum, bilateral S1 to S3 root were exposed and mobilised proximally to gain maximum length. Intercostal nerves were brought down to the exposed lumbo-sacral region. S2 ventral root was cut cranially and the feasibility of intercostal to S2 anastomosis was assessed (Fig. 1).
Fig. 1.
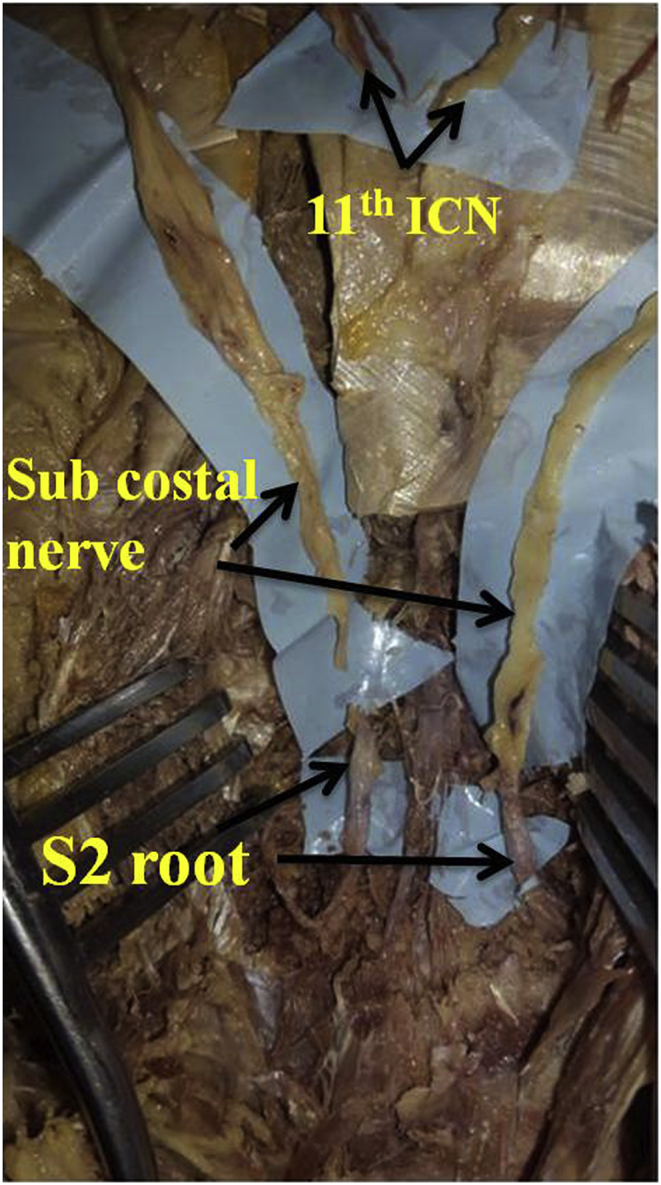
Feasibility of anastomosis of Intercostal nerves to S2 ventral root.
3. Results
The total and mean length of intercostal nerves harvested was 18.2 (range 16.5–19.5 cm) cm for the 10th ICN, 19.5 cm (range 18–20.5 cm) for the 11th ICN and 21.8 cm (range 21–22.5 cm) for the subcostal nerve on the left side. The mean total length of intercostal nerve harvested was 18.6 (17.5–19.5 cm) cm for the 10th ICN, 19.5 cm (18–21.5 cm) for the 11th intercostal nerve ICN and 22.5 cm (19.5–24 cm) for the subcostal nerve on the right side (Table-1).
Table 1.
Total and mean length of lower ICNs on both sides.
| Cadavers | Length of Intercostal nerves (cm) |
|||||
|---|---|---|---|---|---|---|
| 10th ICN | 11th ICN | Subcostal nerve | ||||
| Right | Left | Right | Left | Right | Left | |
| 1 | 17.5 | 16.5 | 18.5 | 18.5 | 19.5 | 21 |
| 2 | 19 | 18 | 21.5 | 20.5 | 23.5 | 21.5 |
| 3 | 18.5 | 18.5 | 20.5 | 20 | 22.5 | 21.5 |
| 4 | 18.5 | 19.5 | 18 | 20.5 | 23 | 22.5 |
| 5 | 19.5 | 18.5 | 19 | 18 | 24 | 22.5 |
| Mean length | 18.6 | 18.2 | 19.5 | 19.5 | 22.5 | 21.8 |
The length of the harvested nerve and the nerve length necessary to perform sacral roots neurotization were possible in all cases by subcostal nerve while T11 and T10 ICN fell short of the required length (Fig. 2). The data about harvested intercostal nerve lengths and required length are summarized in Table −2.
Fig. 2.
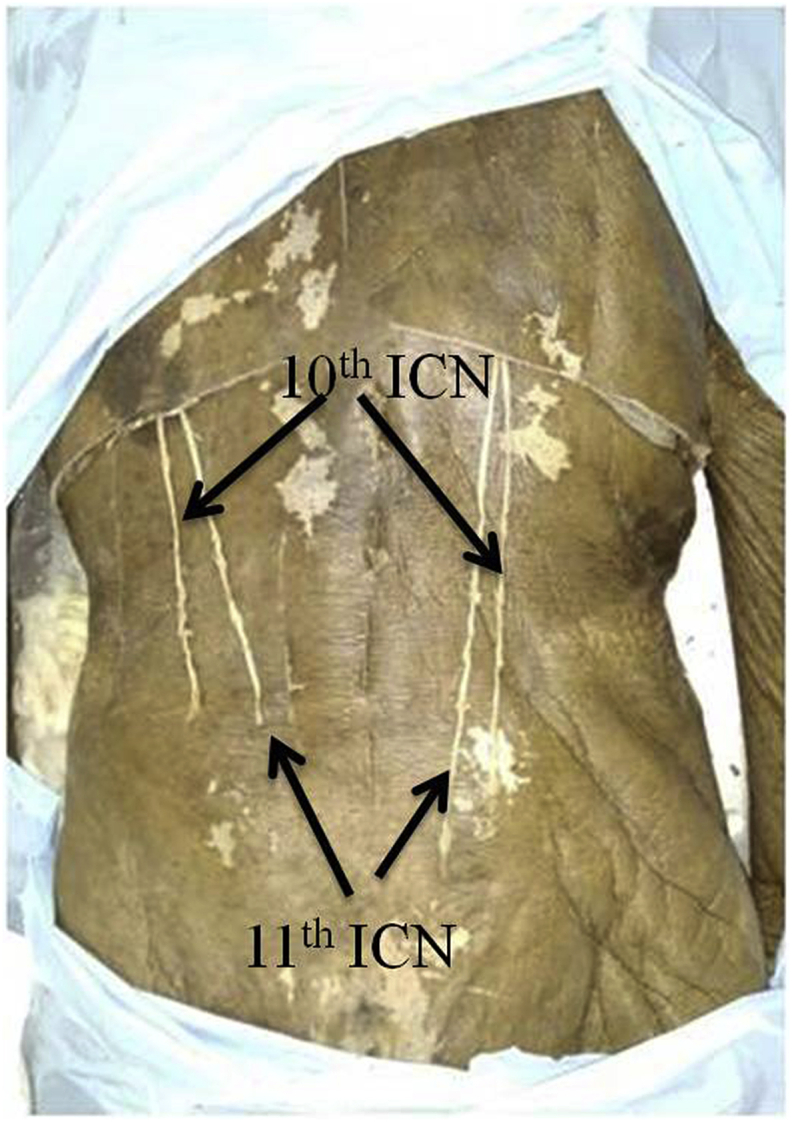
Sacral roots neurotization possible by subcostal nerve while T11 and T10 ICN fall short of the required length.
Table 2.
Details of cadaveric dissection showing mean length of lower ICN.
| S no | Intercostal nerve | N | Mean length (cm) of right ICN |
Mean Length (cm) of left ICN |
Feasibility of transfer | ||
|---|---|---|---|---|---|---|---|
| Actual length | Necessary length to reach S2 root | Actual length | Necessary length to reach S2 root | ||||
| 1 | 10th | 5 | 18.6 | 22.3 | 18.2 | 21.6 | Not feasible |
| 2 | 11th | 5 | 19.5 | 22.3 | 19.5 | 21.6 | Not feasible |
| 3 | Subcostal nerve | 5 | 22.5 | 22.3 | 21.8 | 21.6 | Feasible |
4. Discussion
Damage to the spinal cord (suprasacral/sacral lesion including cauda equina lesions) or pudendal nerve results in bladder and bowel dysfunction. The conservative options for management of bladder dysfunction include timed voiding, the Valsalva and Credé maneuvers, Anticholinergic medications, clean intermittent catheterization (CIC) and/or an indwelling catheter (IC). The surgical modalities to treat neurogenic bladder for restoration of bladder functionality includes sacral root repair/transfer, peripheral nerve transfer to sacral roots/pelvic/pudendal nerves, Direct detrusor muscle reinnervation, and Artificial somatic-to-autonomic reflex pathway. These techniques re-establish and/or create new pathways between the bladder and the spinal cord. These approaches have had some success but also limitations. These techniques have reduced the incidence of UTIs, renal failure and mortality after SCI in humans, however restoration of bladder function by these techniques though promising but yet to be a standard care of treatment.
Neurogenic bladder presents significant health problems in patients with SCI and urinary tract problems with continuous or clean intermittent catheterization are well known. Additionally, many QOL studies have shown that regaining bladder/bowel functions is the second priority after motor recovery for SCI patients.3 There are several techniques like sacral rhizotomy, sacral root repair/transfer, detrusor myoplasty, pudendal nerve neurotization, creation of artificial skin–CNS–bladder pathway and functional nerve stimulation (Brindley technique) described in the literature for the management of neurogenic bladder.5, 6, 7, 8, 9, 10, 11, 12, 13, 14 However, most of them have significant limitations in terms of being studies with small number of patients, limited gain of function, sacrifice of function of donor nerve, technical difficulties and poor reproducibility of technique.
Recently nerve transfers have been used to bypass spinal cord injury.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 The intercostal nerve originating above the level of injury is transferred to nerve roots or peripheral nerves below the level of injured segment. The evolution of nerve transfer to restore function in neurogenic bladder occurred in stages. After animal experimentation,14, 15, 16, 17, 18 their feasibility was assessed in cadavers19, 20, 21, 22, 23 and positive preclinical results prompted researchers to apply these strategies in human.24, 25, 26 Many of these studies showed encouraging results but still no nerve transfer technique had been established as standard surgical procedure for management of neurogenic bladder dysfunction. The peripheral nerves which can be used for neurotization are hypogastric, obturator, genitofemoral and branches from femoral nerves. The limitations of these procedures include limited axonal regeneration, difficult approach for exposure, loss of function of nerve, neuroma formation and most of them were not tested in humans.27
The Majority of the spinal trauma patients have an injury in lumbar region sparing the intercostal nerves hence they can be used as donor nerves to bypass the injured levels of the cord. Thus, 10th, 11th and subcostal nerve can be used to perform a lumbar-sacral neurotization. Historically, two strategies have been used; first strategy was to neurotize lumbar roots to recover functional motor power of lower limb.15,28,29 Second strategy was to neurotize sacral roots for restitution of bladder and sexual functions.13,14,18,25,26
Intercostal nerves had been used as donor in various sheep, cat, rat, rabbits and dog models. Post-neurotization axonal regeneration was demonstrated by histological analysis and retrograde labelling of tracers as well as functional improvement in terms of raised intravesical pressures which showed respiratory variation, stimulation of the transferred nerves led to bladder contraction, and sectioning of the transferred nerves abolished this function. All these findings suggest that there was good re-innervation of target muscles.14, 15, 16, 17, 18
The cadaveric studies assessing the anatomic feasibility of ICN transfer to lumbosacral roots showed that ICN 10–12 can reach comfortably up to lumbar roots and can be used to neurotize lumbar roots in a tension free manner but for sacral root they will either require a nerve graft or shorter intra-dural route.19, 20, 21, 22, 23 (Table-3).
Table 3.
Results from different cadaver studies of nerve transfer for the treatment of SCI.
| Studies | Number | Nerve | Mean length cm | Spinal level |
|---|---|---|---|---|
| Malik & Buhr21 | unknown | T11 | Unknown | L5- sufficient length to reach |
| Vorstman et al.23 | 4 | T10 | 22 | S3- reached by intradural route |
| T11 | 21 | S3- reached by intradural route | ||
| T12 | 20 | S3- did not reach | ||
| Court et al.22 | 30 | T9 | 17.86 | Sufficient length to reach the lumber roots |
| T10 | 16.95 | |||
| T11 | 15.75 | |||
| Vialle et al.20 | 100 | 9th | 17.96 | Sufficient length to reach the Lumber roots |
| 10th | 17.14 | |||
| 11th | 15.94 | |||
| Haque et al.19 | 4 | T6 | 16.4 ± 1.9 18 ± 1.8 18.7 ± 2.0 18.8 ± 3.4 19.6 ± 3.4 18.8 ± 2.1 15.8 ± 1.7 |
Reached T12 |
| 4 | T7 | Reached L1 | ||
| 6 | T8 | Reached L2 | ||
| 6 | T9 | Reached L2,3 | ||
| 6 | T10 | at least L-3 | ||
| 5 | T11 | at least L-3 | ||
| 4 | T12 | at least L-3 | ||
| Agarwal et al. (current study) | 10 | T10 | 18.4 | sufficient length to reach Lumber roots |
| T11 | 19.5 | sufficient length to reach Lumber roots | ||
| T12 | 22.15 | sufficient length to reach the S2 |
Multiple lumbar-sacral root neurotization with lower intercostal nerves has been done to treat neurologic deficits following spinal cord lesions in human. Patients who underwent transfer of the 2–4 ICNs to the L1–4 nerve roots showed recovery of lower-extremity motor function to permit ambulation with supportive device.27 One year after transferring the bilateral T11and subcostal nerve to the bilateral S2–3 nerve roots, patients had significant improvements in bladder function, sacral sensations, and sacral reflexes.25,26
In our study the subcostal nerve reached easily to sacral roots while 11th and 10th ICN fell short in length to reach up to sacral roots. 11th and 10th ICN can neurotize lumbar plexus but if these two ICN have to be used for sacral roots neurotization they will either require a nerve graft or shorter but more technically demanding intradural route. After harvesting subcostal nerve has enough length to reach S2 root by subcutaneous tunnel. If the ventral root of S2 was harvested by open laminectomy and cut cranially, both the nerve ends can be brought out easily on the skin surface and extradural anastomosis can be performed in a tension free manner. But if the ventral root of S2 was harvested by endoscope than fibrin glue can be used to anastomose nerve ends extradurally hence avoiding the more hazardous subdural route with its attendant likely complications. The disadvantages of intradural nerve root anastomosis include thin roots making them more vulnerable to injury during their separation and micro suturing, risk of CSF leak, pseudomeningocele, meningitis, and arachnoiditis.30, 31, 32, 33 The advantages with extradural transfer and anastomosis is that extradural nerve roots are thick, hold the sutures better which helps in better axonal regeneration and less distance to the target organ leading to faster functional recovery.33, 34, 35 However, in extradural procedures, identification of ventral and dorsal roots is a challenging task but ventral roots are always located ventral to dorsal roots outside the dural sac.36
The rationale of using ICN is the intercostal nerves are fairly long nerves; contain large numbers (2000) of motor fibres, remain preserved in lumbosacral injury and taking of intercostal nerves above the injury site does not cause any neurologic deficit.37,38 At least 40% axons need to be innervated for a meaningful recovery. S2 ventral root contains 4766 ± 1035 fibres, thus ICN containing 2000 motor fibres has the potential to provide innervations of required number of axons.39 The ICN nerve transfer procedures give better results for restitution of bladder function as compared to regaining motor power to lower limbs. The reason may be the discrepancy in size of the nerves and the distance of the muscle endplate for lower limbs from the proximal spinal cord lesion.
In cases of incomplete spinal cord injury direct cooptation of nerves may not be feasible due to fear of losing the intact functions. In these cases end to side neurorrhaphy was tried by many authors with good outcomes for sensory recovery and poor to the modest outcome for motor recovery.40,41 The probable reasons for the unsatisfactory outcome may be the slow axon growth after end-to-side neurorrhaphy and the difficulty of regenerating axons to match properly sensory and motor fibres in a mixed nerve.42 Therefore end-to-side technique can reasonably be considered as a valid treatment of nerve repair for sensory recovery.
Despite the encouraging results, there are certain limitations of neurotization of lumbar/sacral roots by the ICN. The length of the ICNs is inconsistent therefore it may not reach to target nerve and the amount of successfully regenerating axons appeared to be low in clinical studies.43 Many cord injuries are incomplete and spontaneous functional recovery may occur over time therefore patients with incomplete paraplegia are not suitable for this treatment. Performing such bypasses/neurotization i.e. dissection and micro-suturing between peripheral nerves and rootlets, is technically demanding. Despite those limitations, neurotization forms the foundation for the repair strategies and future research following spinal cord injury. Even if patients do not get total recovery, any improvement in function can improve QOL. It must be emphasized that these surgeries are well tolerated and have shown good results with regard to safety and feasibility.
5. Conclusion
For Spinal cord lesions located at the conus, functional ICN could be connected to sacral roots to bypass the injury in an attempt to restore bladder function. This study proves the feasibility of transfer of subcostal nerve to neurotize ventral root of S2 extradurally in tension free manner for management of neurogenic bladder while 10th and 11th ICN did not reach to ventral root of S2 and can be used to neurotize lumber plexus. Time has come to change the traditional concept that nothing can be done for complete SCI patients and more aggressive neurorestorative strategies, from bench to bed side, are the need of the hour.
Funding sources
None.
Declaration of competing interest
Nil.
References
- 1.Hamid R., Averbeck M.A., Chiang H. Epidemiology and pathophysiology of neurogenic bladder after spinal cord injury. World J Urol. 2018;36:1517–1527. doi: 10.1007/s00345-018-2301-z. [DOI] [PubMed] [Google Scholar]
- 2.Taweel W.A., Seyam R. Neurogenic bladder in spinal cord injury patients. Res Rep Urol. 2015;10:85–99. doi: 10.2147/RRU.S29644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal P, Mishra A N, Wankhede S, Mukati P, Sharma D. Priorities of functions following spinal cord injury (SCI) JCOT in press. 10.1016/j.jcot.2019.08.001. [DOI]
- 4.Vialle R., Court C., Harding I., Lepeintre Jf, Khouri N., Tadie M. Multiple plexus neurotizations of the ninth, tenth, and eleventh intercostal nerves. Clin Anat. 2006;19:51–58. doi: 10.1002/ca.20148. [DOI] [PubMed] [Google Scholar]
- 5.Brindley G.S. The first 500 patients with sacral anterior root stimulator implants: general description. Paraplegia. 1994;32:795–805. doi: 10.1038/sc.1994.126. [DOI] [PubMed] [Google Scholar]
- 6.Xiao C.G. Reinnervation for neurogenic bladder: historic review and introduction of a somatic-autonomic reflex pathway procedure for patients with spinal cord injury or spina bifida. Eur Urol. 2006;49:22–28. doi: 10.1016/j.eururo.2005.10.004. discussion 28–29. [DOI] [PubMed] [Google Scholar]
- 7.Barbe M.F., Ruggieri M.R. Innervation of parasympathetic postganglionic neurons and bladder detrusor muscle directly after sacral root transection and repair using nerve transfer. Neurourol Urodyn. 2011;30:599–605. doi: 10.1002/nau.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuite G.F., Storrs B.B., Homsy Y.L. Attempted bladder reinnervation and creation of a scratch reflex for bladder emptying through a somatic-to-autonomic intradural anastomosis. J Neurosurg Pediatr. 2013;12:80–86. doi: 10.3171/2013.4.PEDS12302. [DOI] [PubMed] [Google Scholar]
- 9.Gasparini M.E., Schmidt R.A., Tanagho E.A. Selective sacral rhizotomy in the management of the reflex neuropathic bladder: a report on 17 patients with long-term follow up. J Urol. 1992;148:1207–1210. doi: 10.1016/s0022-5347(17)36862-3. [DOI] [PubMed] [Google Scholar]
- 10.Burns A.S., Rivas D.A., Ditunno J.F. The management of neurogenic bladder and sexual dysfunction after spinal cord injury. Spine. 2001;26(24 Suppl):S129–S136. doi: 10.1097/00007632-200112151-00022. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Amaya S.M., Barbe M.F., de Groat W.C. Neural reconstruction methods of restoring bladder function. Nat Rev Urol. 2015;12:100–118. doi: 10.1038/nrurol.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin H., Hou C., Zhen X., Xu Z. Clinical study of reconstructed bladder innervation below the level of spinal cord injury to produce urination by Achilles tendon-to-bladder reflex contractions. J Neurosurg Spine. 2009;10:452–457. doi: 10.3171/2009.1.SPINE08540. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal P., Sharma D., Wankhede S., Jain P.C., Agrawal N.L. Sciatic Nerve to Pudendal Nerve Transfer: Anatomical Feasibility for a New Proposed Technique. Indian J Plast Surg. 2019 May;52(2):222–225. doi: 10.1055/s-0039-1688513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal P., Husain S., Wankhede S., Sharma D. Rectus abdominis detrusor myoplasty (RADM) for acontractile/hypocontractile bladder in spinal cord injury patients: preliminary report. J Plast Reconstr Aesthet Surg. 2018;71:736–742. doi: 10.1016/j.bjps.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Zhao S., Beuerman R.W., Kline D.G. Neurotization of motor Nerves innervating the lower extremity by utilizing the lower Intercostal nerves. J Reconstr Microsurg. 1997;13:39–45. doi: 10.1055/s-2008-1063939. [DOI] [PubMed] [Google Scholar]
- 16.Vialle R., Lacroix C., Harding I., Loureiro M.C., Tadié M. Motor and sensitive axonal regrowth after multiple intercosto- neurotizations in a sheep model. Spinal Cord. 2010;48:367–374. doi: 10.1038/sc.2009.144. [DOI] [PubMed] [Google Scholar]
- 17.TokS SchmidUD., FerbertA DavenportT. Intercosto spinal nerve anastomosis. An experimental study in dogs. Spine. 1991;16:463–466. doi: 10.1097/00007632-199104000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Sundin T., Carlsson C.A. Reconstruction of severed ventral roots innervating the urinary bladder. An experimental study in cats II. Regeneration studies. Scand J Urol Nephrol. 1972;6:185–196. doi: 10.3109/00365597209133635. [DOI] [PubMed] [Google Scholar]
- 19.Haque R.M., Malone H.R., Bauknight M.W. Spinal cord bypass surgery with intercostal and spinal accessory nerves: an anatomical feasibility study in human cadavers. Laboratory investigation. J Neurosurg Spine. 2012;16:178–186. doi: 10.3171/2011.9.SPINE10378. [DOI] [PubMed] [Google Scholar]
- 20.Vialle R., Lepeintre J.F., Court C., Loureiro M.C., Lacroix C., Tadié M. Anatomical feasibility of using the ninth, 10th, and 11th intercostal nerves for the treatment of neurological deficits after damage to the spinal cord. J Neurosurg Spine. 2006;4:225–232. doi: 10.3171/spi.2006.4.3.225. [DOI] [PubMed] [Google Scholar]
- 21.Malik H.G., Buhr A.J. Intercostal nerve transfer to nerve roots. Part I: development of an animal model and cadaver studies. Spine. 1978;4:410–415. doi: 10.1097/00007632-197909000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Court C., Vialle R., Lepeintre J.F., Tadié M. The thoracoabdominal intercostal nerves: an anatomical study for their use in neurotization. Surg Radiol Anat. 2005;27:8–14. doi: 10.1007/s00276-004-0281-8. [DOI] [PubMed] [Google Scholar]
- 23.Vorstman B., Schlossberg S., Landy H., Kass L. Nerve crossover techniques for urinary bladder reinnervation: animal and human cadaver studies. J Urol. 1987;137:1043–1047. doi: 10.1016/s0022-5347(17)44356-4. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S., Johnston L., Zhang Z. Restoration of stepping-forward and ambulatory function in patients with paraplegia: rerouting of vascularized intercostal nerves to nerve roots using selected interfascicular anastomosis. Surg Technol Int. 2003;11:244–248. [PubMed] [Google Scholar]
- 25.Livshits A., Catz A., Folman Y. Reinnervation of the neurogenic bladder in the late period of the spinal cord trauma. Spinal Cord. 2004;42:211–217. doi: 10.1038/sj.sc.3101574. [DOI] [PubMed] [Google Scholar]
- 26.Sievert K.D., Xiao C.G., Hennenlotter J. Voluntary micturition after intradural nerve anastomosis. Urologe. 2005;44:756–761. doi: 10.1007/s00120-005-0849-x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S., Wang Y., Johnston L. Restoration of function in complete spinal cord injury using peripheral nerve rerouting: a summary of procedures. Surg Technol Int. 2008;17:287–291. [PubMed] [Google Scholar]
- 28.Hauge E.N. The anatomical basis of a new method for re-innervation of the gluteal region in paraplegics. Acta Physiol Scand Suppl. 1991;603:19–21. [PubMed] [Google Scholar]
- 29.Lang E.M., Borges J., Carlstedt T. Surgical treatment of lumbosacral plexus injuries. J Neurosurg Spine. 2004;1:64–71. doi: 10.3171/spi.2004.1.1.0064. [DOI] [PubMed] [Google Scholar]
- 30.Hasue M. Pain and the nerve root. An interdisciplinary approach. Spine. 1993;18:2053–2058. doi: 10.1097/00007632-199310001-00022. [DOI] [PubMed] [Google Scholar]
- 31.Narotam P.K., Qiao F., Nathoo N. Collagen matrix duraplasty for posterior fossa surgery: evaluation of surgical technique in 52 adult patients. Clinical article. J Neurosurg. 2009;111:380–386. doi: 10.3171/2008.10.JNS08993. [DOI] [PubMed] [Google Scholar]
- 32.Shaffrey C.I., Spotnitz W.D., Shaffrey M.E., Jane J.A. Neurosurgical applications of fibrin glue: augmentation of dural closure in 134 patients. Neurosurgery. 1990;26:207–210. [PubMed] [Google Scholar]
- 33.Zhou X., Liu Y., Ma J., Sui T., Ge Y., Cao X. Extradural nerve anastomosis technique for bladder reinnervation in spinal cord injury: anatomical feasibility study in human cadavers. Spine. 2014;39:635–641. doi: 10.1097/BRS.0000000000000208. [DOI] [PubMed] [Google Scholar]
- 34.Hernández-García J.M., Reina M.A., Prats-Galino A., De Andrés J.A. Morphologic study of nerve root and types of needle used in transforaminal injections. Reg Anesth Pain Med. 2011;36:278–281. doi: 10.1097/AAP.0b013e31821770d4. [DOI] [PubMed] [Google Scholar]
- 35.Reina M.A., Villanueva M.C., Machés F., Carrera A., López A., De J.A. The ultrastructure of the human spinal nerve root cuff in the spine. Anesth Analg. 2008;106:339–344. doi: 10.1213/01.ane.0000295803.31074.dc. [DOI] [PubMed] [Google Scholar]
- 36.Yang K., Chen H., Tang J. Anatomical feasibility of extradural transferring S2 and S3 ventral roots to S1 ventral root for restoring neurogenic bladder in spinal cord injury. Spine. 2018;43:E1046–E1052. doi: 10.1097/BRS.0000000000002613. [DOI] [PubMed] [Google Scholar]
- 37.Asfazadourian H., Tramond B., Dauge M.C., Oberlin C. Morphometric study of the upper intercostal nerves: practical application for neurotisations in traumatic brachial plexus palsies. Ann Hand Up Limb Surg. 1999;18:243–253. doi: 10.1016/s0753-9053(99)80037-4. [DOI] [PubMed] [Google Scholar]
- 38.Carlsson C.A., Sundin T. Reconstruction of efferent pathways to the urinary bladder in a paraplegic child. Rev Surg. 1967;24:73–76. [PubMed] [Google Scholar]
- 39.Liu Y., Zhou X., Ma J., Ge Y., Cao X. The diameters and number of nerve fibers in spinal nerve roots. J Spinal Cord Med. 2015;38:532–537. doi: 10.1179/1079026814Z.000000000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertelli J.A., Ghizoni M.F. Nerve repair by end to side coaptation or fascicular transfer: a clinical study. J Reconstr Microsurg. 2003;19:313–318. doi: 10.1055/s-2003-42499. [DOI] [PubMed] [Google Scholar]
- 41.Viterbo F., Ripari W.T. Nerve grafts prevent paraplegic pressure ulcers. J Reconstr Microsurg. 2008;24:251–253. doi: 10.1055/s-2008-1078692. [DOI] [PubMed] [Google Scholar]
- 42.De Sá J.M., Mazzer N., Barbieri C.H., Barreira A.A. The end-to-side peripheral nerve repair: functional and morphometric study using the peroneal nerve of rats. J Neurosci Methods. 2004;15:45–53. doi: 10.1016/j.jneumeth.2003.12.018. 136. [DOI] [PubMed] [Google Scholar]
- 43.Dam-Hieu P., Liu S., Tadié M. Experimental bypass surgery between the spinal cord and caudal nerve roots for spinal cord injuries. Neurochirurgie. 2004;50:500–514. doi: 10.1016/s0028-3770(04)98331-2. [DOI] [PubMed] [Google Scholar]


