Summary
Mitochondrial DNA (mtDNA) encodes thirteen core components of OXPHOS complexes, and its steady expression is crucial for cellular energy homeostasis. However, the regulation of mtDNA expression machinery, along with its sensing mechanism to energetic stresses, is not fully understood. Here, we identified SQSTM1/p62 as an important regulator of mtDNA expression machinery, which could effectively induce mtDNA expression and the effects were mediated by p38-dependent upregulation of mitochondrial ribosomal protein L12 (MRPL12) in renal tubular epithelial cells (TECs), a highly energy-demanding cell type related to OXPHOS. We further identified a direct binding site within the MRPL12 promoter to ATF2, the downstream effector of p38. Besides, SQSTM1/p62-induced mtDNA expression is involved in both serum deprivation and hypoxia-induced mitochondrial response, which was further highlighted by kidney injury phenotype of TECs-specific SQSTM1/p62 knockout mice. Collectively, these data suggest that SQSTM1/p62 is a key regulator and energetic sensor of mtDNA expression machinery.
Subject Areas: Biological Sciences, Cell Biology, Functional Aspects of Cell Biology, Molecular Biology
Graphical Abstract

Highlights
-
•
SQSTM1/p62 is an important regulator of mtDNA expression machinery
-
•
SQSTM1/p62 induces MRPL12 expression via activating p38/ATF2 signaling pathway
-
•
SQSTM1/p62 maintains TECs mitochondrial homeostasis and kidney function
Biological Sciences; Cell Biology; Functional Aspects of Cell Biology; Molecular Biology
Introduction
A major biological function of mitochondria is to provide energy in the form of ATP via oxidative phosphorylation (OXPHOS) (Cheng and Ristow, 2013). To maintain cellular energy homeostasis, the efficiency of mitochondrial OXPHOS must be under dedicated regulation to match different environmental or biological stresses, such as the changing nutrient availability and energy demands (Nunnari and Suomalainen, 2012; Gustafsson et al., 2016; Barshad et al., 2018a, 2018b). In this sense, the steady expression of the components constituting OXPHOS system, along with the fine-tuned adjustment in response to environmental challenges, is essential. The mammalian OXPHOS system is composed of about 90 proteins, among which 13 core constitutes of respiratory chain complexes are encoded by mitochondrial DNA (mtDNA) (Koc and Koc, 2012). During the past decades, the gene expression process of mtDNA has received extensive investigation, including the structure and inheritance of the mitochondrial genome, the modes of transcription and translation, and the coordinating pattern between nuclear-encoded and mitochondria-encoded genes (Nunnari and Suomalainen, 2012; Macao et al., 2015). However, modulators of mtDNA expression machinery, along with its adapting mechanisms to environmental challenges, have not been fully elucidated. To date, only a limited number of regulators have been identified. For example, PPARγ co-activator α (PGC1α) was shown to control mtDNA transcription by sensing cellular fluctuations of NAD+/NADH and AMP/ATP ratios (Bhargava and Schnellmann, 2017). NRF-1 and NRF-2 (GABP) could regulate expression of almost all complexes in the ETC (Scarpulla, 2008; Satoh et al., 2013). Besides, decreased TFAM protein levels in mitochondrial led to energetic defects and decreased mtDNA copy number, which is associated with a loss of SQSTM1/p62 (Seibenhener et al., 2013). In consideration of the complexity and plurality of energetic stresses that cells might encounter, it is reasonable to hypothesize that ample unknown regulators of mtDNA expression machinery are awaiting identification.
SQSTM1/p62, a 62-kDa protein widely expressed across different tissue types, is one of the first identified autophagy adaptors owing to its binding capability of ubiquitin and autophagy substrates, via its ubiquitin-associated domain and LC3-interacting region, respectively, which could control mitochondrial turnover via mediating mitophagy (Lamark et al., 2017). Current notions suggest that SQSTM1/p62 also harbors other functional motifs and participates in ample cellular processes, such as inflammation, tumorigenesis, and metabolism (Huang et al., 2018). The role of SQSTM1/p62 in metabolism was initially highlighted by the metabolic phenotype of systemic SQSTM1/p62 knockout animals, characterized as reduced metabolic rate, mature-onset obesity, and glucose intolerance (Rodriguez et al., 2006). Further study revealed that the mechanism responsible for such phenotypes might rely on the regulation of SQSTM1/p62 on brown adipose tissue (BAT) mitochondrial thermogenesis (Muller et al., 2013). In addition, a study performed in neuroblastoma cells demonstrated SQSTM1/p62 could affect mitochondrial complex I respiration (Zhang et al., 2019). Besides, via yet-unknown mechanisms, SQSTM1/p62 could regulate the availability of NADH for electron transfer chain (ETC) (Blacker and Duchen, 2016; Bartolome et al., 2017). Another newly published data showed SQSTM1/p62 could propel metabolic reprogramming from glycolysis to mitochondrial OXPHOS during neuroepithelial stem cell differentiation (Calvo-Garrido et al., 2019). These findings suggested that SQSTM1/p62 might have autophagy-independent impacts on mitochondrial function; however, the effects seem to be tissue specific and the concrete intermediate mechanisms require further clarification.
The mammalian mitochondrial ribosome consists of associated large (39S) and small (28S) ribosomal subunits (Sharma et al., 2003), with each subunit composed of tRNAs encoded by mtDNA and multiple mitochondrial ribosomal proteins (MRPs) encoded by nuclear DNA and imported into mitochondrial matrix. Recently, several MRPs have been implicated in multiple cellular processes such as cell proliferation, apoptosis, and cell cycle regulation outside of the ribosome (Andrea et al., 2016; Cavdar Koc et al., 2001; Kashuba et al., 2008). MRPL12 is one of the best studied MRPs and was recently identified to have distinct functions in mtDNA transcription by binding to mitochondrial RNA polymerase (POLRMT) in a free form (Surovtseva et al., 2011), whereas the precise regulating mechanism remains unclear.
In the present study, we evidenced that SQSTM1/p62 could effectively control OXPHOS and mtDNA expression, which was mediated by MRPL12. The effects of SQSTM1/p62 on MRPL12 expression was in a p38/ATF2 signaling-dependent manner, with a novel binding site within the promoter of MRPL12 to ATF2 being identified. Furthermore, the regulation of SQSTM1/p62 on mtDNA expression machinery participated in both serum deprivation and hypoxia-induced mitochondrial OXPHOS response. Finally, the role of SQSTM1/p62-mediated mtDNA expression was highlighted by tubular epithelial cells (TECs)-specific SQSTM1/p62 knockout mice, which exhibited obvious kidney injury including oliguria, increased serum creatinine, and blood urea nitrogen (BUN) levels. These results suggest that SQSTM1/p62 might act as a crucial modulator and energetic sensor of mtDNA expression machinery.
Results
SQSTM1/p62 Positively Controls OXPHOS and mtDNA Expression
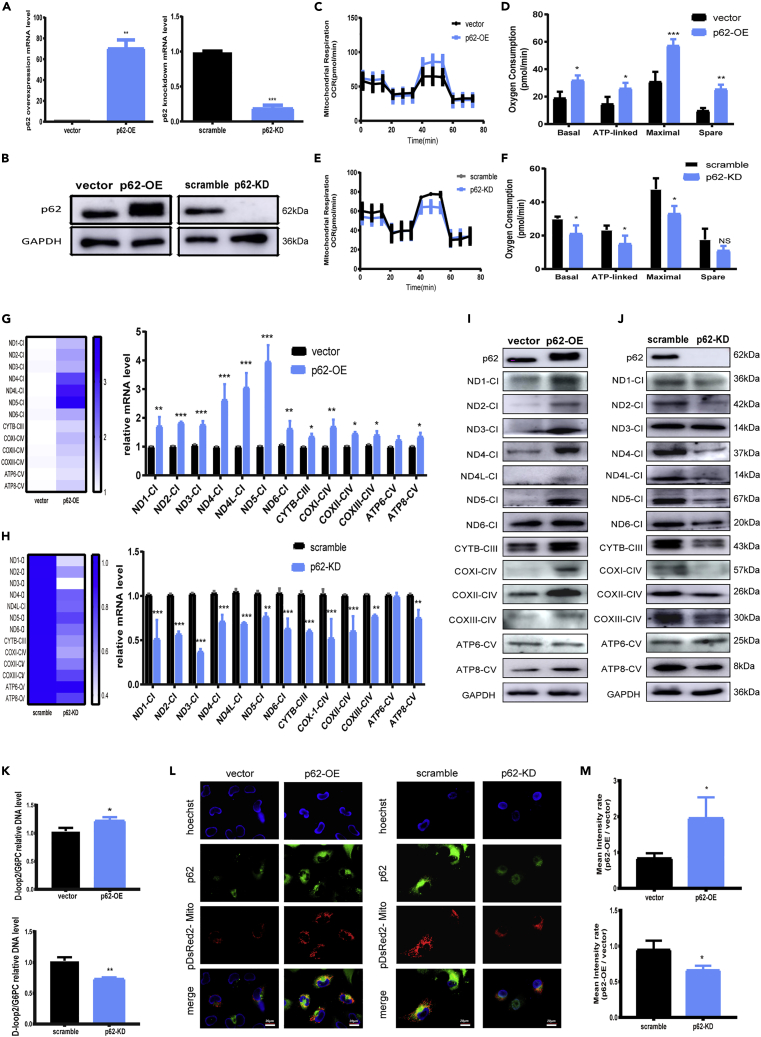
Cellular SQSTM1/p62 contents were either elevated or reduced by transfecting SQSTM1/p62-overexpressing plasmid or silencing siRNAs, which were verified by qRT-PCR and western blotting (Figures 1A and 1B). Then cell bioenergetic profiles were analyzed using Seahorse XF96 Flux Analyzer (Figures 1C–1F). SQSTM1/p62 overexpression induced a significant elevation of basal oxygen consumption rate (OCR), ATP-linked OCR, maximal respiration, and spare respiratory capacity, whereas SQSTM1/p62 silencing had opposite effects, suggesting SQSTM1/p62 could positively regulate mitochondrial OXPHOS.
Figure 1.
SQSTM1/p62 Positively Regulates OXPHOS and mtDNA Expression
(A and B) qRT-PCR and western blotting showed overexpression or knockdown efficiency of SQSTM1/p62 in HK-2 cells, β-actin was used as internal control in qRT-PCR and GAPDH was used as internal control in western blotting. Unpaired, two-tailed t tests were used, n = 3, ∗∗p < 0.01, ∗∗∗p < 0.001. SQSTM1/p62 was abbreviated into p62 in all figures.
(C and E) Bioenergetic profiles of HK-2 cells after SQSTM1/p62 transfection measured by Seahorse XF96.
(D and F) Mitochondrial OXPHOS were analyzed with basal respiration, ATP production, maximal respiration, and spare respiratory capacity, respectively in SQSTM1/p62 transfected HK2 cells. One-way ANOVA followed by Sidak's multiple comparisons test, n = 4 biological replicates, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
(G and H) mRNA levels of 13 mtDNA-coded OXPHOS components determined by qRT-PCR in SQSTM1/p62 transfected HK-2 cells, β-actin was used as internal control. Unpaired, two-tailed t tests were used, n = 3, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. The heatmaps indicated the results of the qRT-PCR.
(I and J) Protein levels of the 13 mtDNA-coded OXPHOS components (CI: ND1 to ND6; CIII: CYTB; CIV: COXI, COXII and COXIII; CV: ATP6 and ATP8) determined by western blotting in SQSTM1/p62 transfected HK-2 cells normalized to GAPDH.
(K) mtDNA copy numbers in SQSTM1/p62-overexpressed or knocked down HK-2 cells. G6PC was used as control. Unpaired, two-tailed t tests were used, n = 3, ∗p < 0.05, ∗∗p < 0.01.
(L) Mitochondria abundance detected by pDsRed2-Mito plasmid transfection in SQSTM1/p62-overexpressed or knocked down cells, and the nuclei were counterstained by Hoechst 33258. Scale bars, 20 μm.
(M) Mean intensity of L was calculated by Image Pro Plus, Unpaired t tests were used, n = 3, ∗p < 0.05.
Subsequently, we examined whether SQSTM1/p62 had impacts on mtDNA expression machinery. Transcriptional levels of thirteen mtDNA-encoded components (Figures 1G and 1H and Oligonucleotides were involved in Table S1) as well as some nDNA-encoded genes related to mitochondrial OXPHOS (Figure S1) were first determined by qRT-PCR. Overexpressing or knocking down SQSTM1/p62 induced elevations or reductions of steady-state mRNA levels of these components in various degrees, suggesting SQSTM1/p62 could positively control mtDNA expression. Western blotting analysis further confirmed the effects of SQSTM1/p62 on the protein level of these subunits (Figures 1I and 1J). Furthermore, as mtDNA transcription is known to be functionally linked to mtDNA replication (Barshad et al., 2018a, 2018b), the effects of SQSTM1/p62 on mitochondrial biogenesis were also determined. Quantification of mtDNA copy numbers (Figure 1K) and pDsRed2-Mito staining followed by intensity analysis (Figures 1L and 1M) confirmed the positive effects of SQSTM1/p62 on mitochondrial abundance. Collectively, these results clearly demonstrate that SQSTM1/p62 is a key regulator of OXPHOS and mtDNA expression coupled to mitochondrial abundance.
MRPL12 Mediates the Effects of SQSTM1/p62 on mtDNA Expression
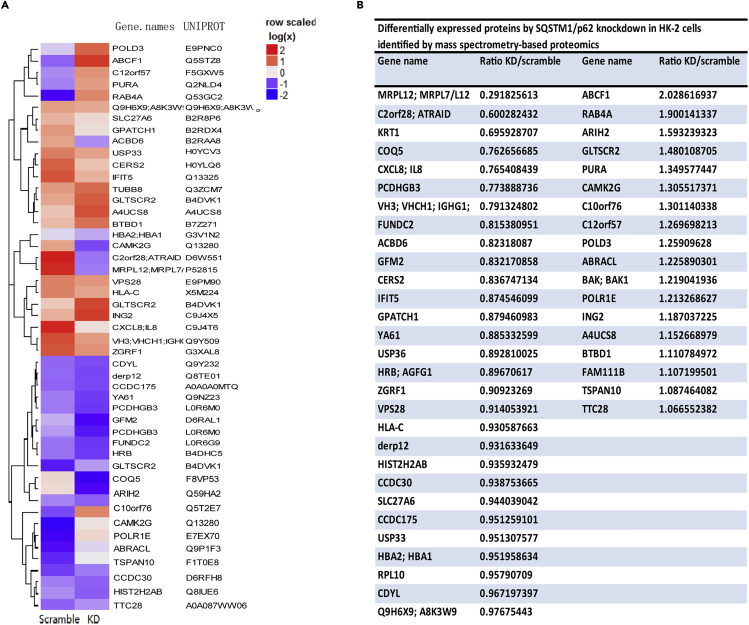
As the effects of SQSTM1/p62 on mtDNA expression were demonstrated, we continued to explore the possible underlying mechanisms. First, we performed a mass spectrometry-based proteomics approach to screen molecules that were differentially regulated by SQSTM1/p62 knockdown. A total of 47 proteins were identified with a 95% confidence interval in SQSTM1/p62 knocked down TECs and scramble siRNA transfected control cells (Figures 2A and 2B). Of particular interest is MRPL12, with its mutations reportedly leading to mitochondrial translation deficiency (Serre et al., 2013; Frei et al., 2005). These findings propelled us to hypothesize MRPL12 as the potential molecule mediating the effects of SQSTM1/p62 on mtDNA expression. Immunostaining and western blotting were performed to verify the proteomics results. A corresponding increase or decrease of MRPL12 protein levels after SQSTM1/p62 plus or minus was evidenced (Figures 3A–3D), and the mRNA level of MRPL12 was also altered (Figure 3E), with the expression of another mitochondrial ribosome protein MRPL11 unchanged (Figure 3F), indicating SQSTM1/p62 could indeed affect MRPL12 expression specifically.
Figure 2.
Mass Spectrometry-Based Proteomics
(A) The heatmap of M-S analysis. A total of 47 proteins were identified with a 95% confidence interval in SQSTM1/p62 knocked down TECs and scramble siRNA transfected control cells.
(B) The ratio of 47 protein expression in p62-KD and scramble.
Figure 3.
MRPL12 Mediates the Effects of SQSTM1/p62 on mtDNA Expression
(A–D) Expression of MRPL12 was detected on protein level by immunofluorescent staining (A and B) and western blotting (C and D) in SQSTM1/p62-overexpressed or knocked down HK-2 cells. Scale bars, 50 μm.
(E and F) mRNA levels of MRPL12 and MRPL11 detected by qRT-PCR in SQSTM1/p62-overexpressed or knocked down HK-2 cells. β-actin was used as internal control. Unpaired, two-tailed t tests were used, n = 3, ∗p < 0.05, ∗∗p < 0.01.
(G) Bioenergetic profiles measured by Seahorse XF96 in HK-2 cells co-transfected with SQSTM1/p62-overexpressing and MRPL12-silencing plasmids.
(H–K) Basal respiration (H), ATP production (I), maximal respiration (J), and spare respiratory capacity (K) in HK-2 cells co-transfected with SQSTM1/p62-overexpressing and MRPL12-silencing plasmids were identified, respectively. One-way ANOVA followed by Sidak's multiple comparisons test, n = 4 biological replicates, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
(L) Expression of subunits of OXPHOS complexes by western blotting in HK-2 cells co-transfected with SQSTM1/p62-overexpressing and MRPL12-silencing plasmids.
(M) mtDNA copy numbers in SQSTM1/p62-overexpressing and MRPL12-silencing duel-transfected HK-2 cells. Unpaired t tests were used, n = 3, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
(N and O) mRNA levels of ND1, CYTB, COXII, and ATP6 detected by qRT-PCR in MRPL12-overexpressed or knocked down HK-2 cells. β-actin was used as internal control. Unpaired, two-tailed t tests were used, n = 3, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
(P) Immunoblotting of POLRMT interacting with MRPL12 from SQSTM1/p62-overexpressed or knocked down HK-2 cells identified by coIP. Rabbit IgG was used as negative control.
The role of MRPL12 in the regulation of SQSTM1/p62 on mitochondrial OXPHOS was then determined in TECs by dual transfection of MRPL12-silencing and SQSTM1/p62 overexpressing plasmids. As shown in Figure 3G, SQSTM1/p62-induced mitochondrial respiration was effectively diminished by additional MRPL12 knockdown, which was evaluated by basal OCR (Figure 3H), ATP-linked OCR (Figure 3I), maximal respiration (Figure 3J), and spare respiratory capacity (Figure 3K). The SQSTM1/p62-induced expression of OXPHOS complex I, III, IV, and V was also compromised by the combination of MRPL12 knockdown (Figure 3L). In addition, SQSTM1/p62 was no longer able to boost the mtDNA copy number in MRPL12-depleted cells (Figure 3M). These results clearly evidenced that SQSTM1/p62-induced mtDNA expression was mediated by MRPL12. To further address the claim, MRPL12 overexpression or knockdown cells were prepared to sufficiently induce or suppress mtDNA expression (Figures 3N and 3O). Furthermore, as MRPL12 was known to interact with mitochondrial RNA polymerase POLRMT to modulate mtDNA encoded gene expression (Surovtseva et al., 2011), we also performed co-immunoprecipitation (coIP) study, which revealed that SQSTM1/p62 plus or minus led to a corresponding elevation or reduction of POLRMT binding to MRPL12 (Figure 3P).
SQSTM1/p62 Induces MRPL12 Expression via Activating p38/ATF2 Signaling Pathway
We continued to determine how SQSTM1/p62 affected MRPL12 expression in two aspects simultaneously. On one hand, SQSTM1/p62 is known to harbor several functional motifs and could interact with molecules of multiple signaling pathways (Long et al., 2017). Accordingly, in SQSTM1/p62-overexpressed cells, specific inhibitors or activators of these pathways were employed in the following experiments, including p38 inhibitor (SB203580), Nrf2 activator (NK252), mTOR inhibitor (Rapamycin), IRS-1/2 inhibitor (NT157), Erk 1/2 activator (Honokiol), and PKC inhibitor (Go6983) (Hisatsune et al., 2008; Saito et al., 2008; Lee et al., 2010; Yang et al., 2019; Kanayama et al., 2015; Reilly et al., 2015) (Figure 4A and detail information of the reagents was listed in Table S1), and the expression of MRPL12 was evaluated by western blotting (Figure 4B). Notably, p38 inhibitor and mTOR inhibitor effectively blocked SQSTM1/p62-induced MRPL12 expression, indicating these two pathways might be involved. On the other hand, considering that MRPL12 might be regulated by SQSTM1/p62 at the transcriptional level, website: http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3/ was used to identify putative transcription factor binding sites within the MRPL12 promoter region. Among the several potential transcription factors predicted, ATF2 is known as a downstream effector of p38, with the predicted promoter binding site of −2,344 bp to −2,336 bp (Figure 4C). Thus, a potential p38/ATF2-mediated transcriptional regulating mechanism was speculated.
Figure 4.
SQSTM1/p62 induces MRPL12 Expression via Activating p38/ATF2 Signaling Pathway
(A) Schematic diagram for SQSTM1/p62 domains and interacting partners. ① to ⑥ represent inhibitors or activators to the interacting partners as following: ① p38 inhibitor SB203580 (10 μM, Selleck); ② Nrf2 activator NK252 (20 μM, MedChemExpress); ③mTOR inhibitor rapamycin (10 μM, Selleck); ④ IRS-1/2 inhibitor NK157 (10 μM, Selleck); ⑤ ERK1/2 activator Honokiol (20 μM, Selleck); and ⑥ PKC inhibitor Go6983 (100 nM, Selleck).
(B) MRPL12 expression was detected by western blotting in SQSTM1/p62-overexpressed HK-2 cells treated with the above inhibitors or activators.
(C) A potential ATF2 binding site within the promoter region of human MRPL12 gene was identified by ALGGEN-PROMO (Version 3.0.2) between −2,344 bp and −2,336 bp upstream from the transcription start site.
(D–G) Western blotting for total p38 and ATF2 in SQSTM1/p62-overexpressed (D) or knocked down (F) HK-2 cells. GAPDH was used as internal control. (E) and (G) were grayscale analysis for (D) and (F) respectively. Unpaired t tests were used, n = 3, ∗∗p < 0.01.
(H–K) Western blotting for nuclear p-p38 and p-ATF2 in SQSTM1/p62-overexpressed (H) or knocked down (J) HK-2 cells. LaminB was used as internal control. (I) and (K) were grayscale analysis for (H) and (J) respectively. Unpaired t tests were used, n = 3, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
(L and M) Immunofluorescent staining for SQSTM1/p62 and p-ATF2 in SQSTM1/p62 transfected HK-2 cells. Nuclei were counterstained by Hoechst 33258. Scale bars, 20 μm. Mean intensity was calculated by Image Pro Plus and showed in histogram respectively. Unpaired t tests were used, n = 3, ∗p < 0.05, ∗∗p < 0.01.
Subsequently, we observed the effects of SQSTM1/p62 on p38/ATF2 signaling pathway. Although SQSTM1/p62 plus or minus had no effects on the total amounts of p38 and ATF2 in cytosol (Figures 4D–4G), nuclear abundance of phosphorylated p38 (p-p38) and phosphorylated ATF2 (p-ATF2) significantly increased or decreased (Figures 4H–4K). Such results were further confirmed by immunostaining study, as nuclear p-ATF2 staining was significantly enhanced by SQSTM1/p62 overexpression and diminished by SQSTM1/p62 suppression, respectively (Figures 4L and 4M), suggesting SQSTM1/p62 could activate p38 signaling and accordingly induce p-ATF2 to translocate into the nucleus. As SQSTM1/p62 was reported to enhance p38 activity by binding to p38 (Sudo et al., 2000; Saito et al., 2008), we further demonstrated the in situ interaction of SQSTM1/p62 with p38 by proximity ligation assay (PLA) (Figures 5A and 5B), which gives a positive green signal when the two proteins of interest are within 30–40 nm. As observed by in situ PLA, the colocalization of SQSTM1/p62 and p38 was significantly reduced in SQSTM1/p62 silenced cells, which was also confirmed by the coIP assay that the p38 binding to SQSTM1/p62 was diminished in SQSTM1/p62 knocked down TECs (Figure 5C). Thus, we demonstrated SQSTM1/p62-induced MRPL12 expression appeared to be mediated by the p38/ATF2 signaling pathway. This conclusion was then directly evaluated by analyzing MRPLl12 expression levels in the vector or p62-OE with and without p38 knockdown (Figure 5D) and p38 activation alone (Figure 5E).
Figure 5.
MRPL12 Is a Direct Target Gene of ATF2
(A and B) Interaction and quantification of SQSTM1/p62 and p38 in scramble transfected and SQSTM1/p62 knocked down HK-2 cells visualized by Duolink proximity ligation assay. The arrows indicated the interaction of SQSTM1/p. The green dots represents the interaction of SQSTM1/p62 and p38 which was also indicated by the arrows, the blue fluorescence represents the nuclei. Mean intensity was calculated by Image Pro Plus. Scale bars, 20 μm. Unpaired t tests were used, n = 3, ∗∗∗p < 0.001.
(C) Co-immunoprecipitation of SQSTM1/p62 and p38 in scramble transfected and SQSTM1/p62 knocked down HK-2 cells. Mouse-IgG was used as negative control.
(D) Western blotting for p62, p38, p-ATF2, MRPL12, and OXPHOS components in SQSTM1/p62 overexpressed with or without p38 knocked down HK-2 cells.
(E) Western blotting for OXPHOS components in p38-overexpressed or knocked down HK-2 cells.
(F) The binding of ATF2 to MRPL12 promoter DNA probe in HK-2 cells nuclear extract was identified by EMSA. A cold probe and a mutant probe were used as competitors.
(G) ChIP-PCR analysis for the binding of ATF2 to MRPL12 promoter performed on HK-2 cells chromatin using rabbit anti-ATF2 antibody and rabbit IgG was used as a negative control.
(H) The occupancy of MRPL12 promoter of ATF2 was enriched by SQSTM1/p62 overexpression and deprived by SQSTM1/p62 knockdown analyzed by quantitative PCR-based ChIP assay. Unpaired t tests were used, n = 3, ∗p < 0.05, ∗∗p < 0.01.
(I) Schematic structure of the MRPL12 luciferase reporter. The putative ATF2 binding site-directed mutagenesis from TGACGTCTT to GTCATAGG was shown.
(J) SQSTM1/p62 overexpression significantly increased the MRPL12 luciferase reporter activity. Two-tailed with unpaired t tests were used to compare these data points, n = 3, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
MRPL12 Is a Direct Target Gene of Transcription Factor ATF2
Then, the direct binding of ATF2 to the predicted sites within the MRPL12 promoter was evaluated as following. First, EMSA analysis demonstrated that ATF2 could indeed bind to the predicted sequence from −2,344 bp to −2,336 bp within the MRPL12 promoter (Figure 5F). Then, the binding of ATF2 was further proved by chromatin immunoprecipitation (ChIP) assay (Figure 5G), and SQSTM1/p62 plus or minus led to a significant elevation or reduction of ATF2 binding to MRPL12 (Figure 5H), suggesting SQSTM1/p62 did affect MRPL12 expression via ATF2-mediated transcription. Such conclusion was further confirmed by luciferase reporter assay. An MRPL12 promoter-driven luciferase reporter plasmid (MRPL12-Luci) and an ATF2-mutant MRPL12 promoter-driven luciferase reporter plasmid were generated, respectively (Figure 5I), and a significant elevation (2,835%) of MRPL12-Luci was evidenced (Figure 5J). Consistent with the findings of ChIP assay, SQSTM1/p62 overexpression also resulted in an additional enhancement of transcriptional activity in wild-type MRPL12-Luci transfected cells, not in mutant ones (Figure 5J). Collectively, these data evidenced that MRPL12 is a direct target gene of ATF2 and such mechanism is likely to be involved in the SQSTM1/p62-induced MRPL12 expression.
SQSTM1/p62-Mediated mtDNA Expression Participates in the Mitochondrial Response Induced by Energetic Stresses
The findings above demonstrated that SQSTM1/p62 could control mtDNA expression and OXPHOS and these effects appeared to be mediated via p38/ATF2 regulated MRPL12 transcription; then we wondered whether such mechanism participated in the mitochondrial adaptation to environmental energetic stresses, such as nutrients deprivation and hypoxia. As shown in Figures 6A and 6B, serum deprivation led to an impaired mitochondrial OXPHOS capacity, with significantly reduced basal OCR, ATP-linked OCR, maximal respiration, and spare respiratory capacity. Real-time qPCR revealed that mRNA levels of both SQSTM1/p62 and MRPL12 were also dramatically decreased under serum deprivation (Figures 6C and 6D). Concomitantly, protein levels of SQSTM1/p62, MRPL12, and mtDNA-encoded subunits of OXPHOS complexes also exhibited the same tendency (Figure 6E). Interestingly, overexpression of SQSTM1/p62 could effectively ameliorate serum deprivation-induced OXPHOS impairment (Figures 6F–6J), along with ameliorated cellular MRPL12 and OXPHOS complexes (Figure 6K) expressions.
Figure 6.
SQSTM1/p62-Mediated mtDNA Expression Participates in the Mitochondrial Response Induced by Energetic Stresses
(A) OCR in HK-2 cells was analyzed by Seahorse XF96 under serum deprivation (SD) for 48 h compared with normal condition (NC).
(B) Basal respiration, ATP production, maximal respiration, and spare respiratory capacity were identified, respectively. n = 4 biological replicates, one-way ANOVA followed by Sidak's multiple comparisons test, n = 4 biological replicates, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
(C and D) The mRNA levels of SQSTM1/p62 and MRPL12 were detected by qRT-PCR in HK-2 cells under 48 h serum deprivation. β-actin was used as internal control. Unpaired t tests were used, n = 3, ∗∗p < 0.01.
(E) The protein levels of SQSTM1/p62, MRPL12, and OXPHOS complexes detected by western blotting in HK-2 cells under serum deprivation.
(F–J) The amelioration of SQSTM1/p62 overexpression on serum deprivation induced OCR defects was evaluated by Seahorse XF96. The cells transfected with SQSTM1/p62 overexpression plasmid were treated by serum deprivation for 48 h. Basal respiration (G), ATP production (H), maximal respiration (I), and spare respiratory capacity (J) were identified, respectively. One-way ANOVA followed by Sidak's multiple comparisons test, n = 4 biological replicates, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
(K) Western blotting for SQSTM1/p62, MRPL12, and OXPHOS complexes in SQSTM1/p62-overexpressed cells under serum deprivation compared with controls.
(L and M) The mRNA levels of SQSTM1/p62 (L) and MRPL12 (M) were detected by qRT-PCR in HK-2 cells under hypoxia for 24 h. GAPDH was used as internal control. Unpaired t tests were used, n = 3, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
(N) The protein levels of SQSTM1/p62, MRPL12, and OXPHOS complexes in HK-2 cells were detected by western blotting under hypoxia for 24 h.
(O) Western blotting for SQSTM1/p62, MRPL12, and OXPHOS complexes in SQSTM1/p62-overexpressed cells under hypoxia compared with controls.
Then, we continued to determine whether SQSTM1/p62 also had a role in hypoxia-induced mitochondrial response. The mRNA levels of both SQSTM1/p62 and MRPL12 dramatically decreased upon hypoxia treatment (Figures 6L and 6M), accompanied by diminished protein contents of SQSTM1/p62, MRPL12, and OXPHOS complexes (Figure 6N). Furthermore, SQSTM1/p62 overexpression also effectively ameliorated hypoxia-induced expressions of MRPL12 and OXPHOS complexes (Figure 6O). In general, these data above suggested that SQSTM1/p62-induced MRPL12 transcription might be a key mechanism mediating both serum deprivation and hypoxia-induced mitochondrial response in TECs.
SQSTM1/p62 Is Essential in Maintaining TECs Mitochondrial Homeostasis and Kidney Function
Based on the in vitro findings above, we continued to investigate whether such mechanism might also play a role in the pathogenesis of energy stress-associated diseases, such as ischemic acute kidney injury (AKI). The expression of SQSTM1/p62 and MRPL12 was evaluated in renal samples from both patients with AKI complied with the KDIGO criteria (Table S2) and mice models by immunohistochemistry. As shown in Figure 6, ischemic attack led to an obvious tubulointerstitial injury (Figures 7A and 7B), accompanied by a significant decrease of SQSTM1/p62 and MRPL12, as well as protein subunits of OXPHOS complexes in renal biopsies of patients with AKI, especially in the tubulointerstitial area (Figure 7C). Similar findings were also observed in AKI mice renal samples compared with the control littermates (Figure 7D); the mean intensity of IHC images was statistically analyzed by ImageJ (Figure S2). All the results above suggested that SQSTM1/p62 and MRPL12 are involved in the pathogenesis of AKI which may be analogous to the in vitro study above.
Figure 7.
Tubulointerstitial Injuries in Both Patients with AKI and Mouse Model
(A and B) H&E staining for patients (A) and mice (B) with AKI compared with controls, with arrows indicating the tubulointerstitial injuries. Scale bars, 50 or 100 μm.
(C and D) Immunohistochemistry staining for SQSTM1/p62, MRPL12, and OXPHOS complexes (ND1, CYTB, COXII and ATP8) detected in patients (C) and mice (D) with AKI. Scale bars, 100 μm.
To evaluate the in vivo role of SQSTM1/p62 in ischemia-induced renal injuries, conditional TECs SQSTM1/p62 knockout mice (CKO) were then generated and the genotyping was first verified by PCR (Figure 8A). Immunofluorescent analysis also confirmed the specific deficiency of SQSTM1/p62 in TECs using CK18 as an epithelial marker (Figure 8B).
Figure 8.
TECs-Specific SQSTM1/p62 Knockout Mice Exhibited Kidney Injuries
(A) Genotyping of TECs-specific SQSTM1/p62 knockout mice. +/+ indicates homozygote.
(B) Immunofluorescent staining confirmed the deficiency of SQSTM1/p62 in renal epithelial tubules with CK18 as a marker. Scale bars, 100 μm.
(C) The morphology of SQSTM1/p62 TECs CKO mice and their kidneys at the age of 6 months compared with wild-type littermates.
(D) The body weights of SQSTM1/p62 CKO mice at the age of 2 and 6 months compared with wild-type littermates. n = 5, ∗p < 0.05.
(E–G) Urine volume (E), serum creatinine (F), and BUN (G) were measured in WT and CKO mice at the age of 2 and 6 months. Unpaired t tests were used, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
(H) H&E staining for WT and SQSTM1/p62 CKO mice. Scale bars, 100 μm.
(I) Electron microscopy analysis for TECs mitochondria in renal samples of WT and SQSTM1/p62 CKO mice. Scale bars, 2 μm.
(J) Mitochondria numbers per μm2 of (J) were counted using Image-Pro Plus 6.0. Unpaired t tests were used, n = 6, ∗p < 0.05.
(K) Oxygen consumption was recorded by Clark-type electrode in WT and SQSTM1/p62 CKO mice under normal or AKI treatments after the addition of substrates and inhibitors for Complex I (5 mM Glutamate plus 5 mM Malate and 2 mM Rotenone), Complex II + III (5 mM Succinate plus 5 mM Glyceraldehyde-3-P and 0.1 mM Antimycin), and Complex IV (1.2 mM TMPD and 6 mM KCN). n = 3, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
(L) Immunohistochemistry staining for p62, MRPL12, and subunits of OXPHOS complexes in renal samples of WT and SQSTM1/p62 CKO mice under normal or AKI treatments. Scale bars, 100 μm.
(M) Western blotting for p62, MRPL12, and OXPHOS complexes in renal samples of WT and SQSTM1/p62 CKO mice under normal or AKI treatments.
Unexpectedly, although no significant phenotypic alterations were observed at the age of 2 months, TECs SQSTM1/p62 CKO mice exhibited obvious kidney injuries at the age of 5 months, evidenced by oliguria, elevated serum creatinine, and BUN levels (Figures 8E–8G, and the information of the commercial assays was listed in Table S1). The body weights of CKO mice also decreased compared with their wild-type littermates at the age of 6 months (Figures 8C and 8D). Furthermore, although no obvious morphological alterations within tubulointerstitium were detected on the CKO mice by H&E staining (Figure 8H), electron microscope analysis demonstrated obvious mitochondrial injuries including irregularly arranging, mitochondrial swelling, and decreased mitochondrial abundance (Figures 8I and 8J).
Oxygen consumption determined by Clark-type oxygen electrode manifested that the mitochondrial respiration capacity was crippled in CKO mice, which was deteriorated after AKI modeling (Figure 8K). Concomitantly, immunohistochemistry study revealed that CKO mice exhibited a significant reduction of MRPL12, especially in tubulointerstitial area, as well as decreased expression of OXPHOS complexes, which were further decreased by additional loss of SQSTM1/p62 in TECs compared with AKI wild-type (WT) mice (Figure 8L), and the mean intensities were analyzed in Figure S3. These results were further confirmed by western blotting (Figure 8M).
Thereby, these in vivo findings provided further evidences supporting that SQSTM1/p62 is crucial for physiological regulation of mitochondrial OXPHOS, with p38/ATF2-mediated MRPL12 transcription and subsequent mtDNA expression as the key intermediate mechanism.
Discussion
Emerging data suggest SQSTM1/p62 might have autophagy-independent effects on mitochondrial function, such as regulating BAT mitochondrial uncoupling and controlling NADH availability for electron transfer chain (Muller et al., 2013; Bartolome et al., 2017). These make SQSTM1/p62 appearing as a multifaced modulator of mitochondria, possibly owing to its multiple functional motifs (Long et al., 2017). In the present study, we further developed that SQSTM1/p62 could also act as a crucial regulator and energetic sensor of mtDNA expression machinery, and such effects were mediated by MRPL12, which was newly identified to play a part in mtDNA expression. Via activating p38/ATF2 signaling, SQSTM1/p62 could induce MRPL12 transcription and accordingly promote mtDNA expression. Furthermore, SQSTM1/p62 and MRPL12-mediated mtDNA expression is also involved in mitochondrial response to energetic challenges including nutrients deprivation and hypoxia.
Although SQSTM1/p62 was found to participate in metabolic processes and energetic homeostasis, novel downstream ligands need to be found to establish the connection of mitochondrial dysfunction and the key domain of SQSTM1/p62. As a critical component of mitochondrial ribosome, MRPL12 could also exist in a ribosome-free form within mitochondria and directly bind to POLRMT, thereby stimulating mtDNA transcription (Surovtseva et al., 2011). And the overexpression of MRPL12 was also proved to be able to modulate mitochondrial gene expression in mammalian cells (Nouws et al., 2016). Similar to our findings that SQSTM1/p62 knockdown resulted in a reduced expression of complex subunits and impaired OXPHOS rate, MRPL12 mutation also exhibited damaged complex activities, with decreased synthesis of components comprising OXPHOS complexes (Serre et al., 2013). The similar phenotypes between SQSTM1/p62 knockdown and MRPL12 mutation provide further evidences supporting our proposal that MRPL12 may be the major downstream player in SQSTM1/p62-induced mtDNA expression. Furthermore, mitochondrial DNA transcription is known to be functionally coupled to mitochondrial biogenesis, and several components comprising mtDNA transcription apparatus concomitantly act as essential players in the process of mtDNA replication, such as mitochondrial transcription factor A (TFAM) and POLRMT (Koc and Koc, 2012; Kukat et al., 2015; Hao et al., 2016). Such notion was also evidenced by our results that SQSTM1/p62-induced mtDNA expression was accompanied by elevated mitochondrial abundance (Figures 1K and 1M).
MRPL12 is the first mitochondrial ribosomal protein to be characterized in mammals (Frei et al., 2005), and its mutations lead to growth retardation, neurological deterioration, and mitochondrial translation deficiency (Serre et al., 2013). In addition, an artificial intelligence analysis showed that MRPL12 plays central roles regarding the effects of calorie restriction on life extension (An et al., 2008). And MRPL12 was identified as one of the significant down-regulation mitochondrial proteins in prenatal stress-related mitochondrial biogenesis in the frontal cortex and hippocampus (Olszanecki and Basta-kaim, 2015). However, the physiological regulation of MRPL12 expression is little known so far. To our knowledge, our identification of ATF2 as its transcription factor is the first report concerning MRPL12 gene expression pattern. Via binding to the promoter of MRPL12 between −2,344 bp and −2,336 bp, ATF2 could effectively induce MRPL12 transcription (Figure 5). Furthermore, the effect of ATF2 is under the control of p38 signaling and accounts for SQSTM1/p62-induced mtDNA transcription. In addition, besides controlling inflammation, immunity, and proliferation, recent studies suggested p38 might be involved in multiple metabolic processes, such as gluconeogenesis, fatty acid β-oxidation, and insulin secretion (Bordicchia et al., 2012; Jager et al., 2007; Kawai et al., 2008). Through a few important transcription factors, p38 targets several proteins with prominent roles in mitochondrial function, such as uncoupling protein 1 (UCP1) (Cao et al., 2001; Nisoli et al., 2005), thereby enhancing mitochondrial OXPHOS and mtDNA expression. Our results established MRPL12 as a new downstream effector p38, thus mediating its effects on mitochondrial dysfunction in metabolic diseases.
It should be mentioned that, besides ATF2, several other transcription factors including NFE2L2/Nrf2 (nuclear factor, erythroid 2-like 2) and NF-κB (nuclear factor kappa B) were also predicted to be capable of binding to the MRPL12 promoter and might activate its transcription (data available on request). We did not explore these factors because among the main SQSTM1/p62-interacting signaling pathways, only p38 and mTOR signaling were evidenced to be involved in SQSTM1/p62's induction of MRPL12 expression (Figure 4B) (Kawai et al., 2008), whereas ATF2 is a known downstream effector of p38 signaling (Dodson et al., 2013). However, whether NFE2L2/Nrf2 and NF-κB might also act as potential transcription factors of MRPL12 is another interesting topic deserving future identification, as both molecules are known to be involved in ample cellular processes such as inflammation, stress response, and tumorigenesis (Bartolini et al., 2018; Mitchell et al., 2016).
When challenged by nutrient crises such as ischemia and hypoxia, cells are known to switch its energy production from mitochondrial OXPHOS to anaerobic glycolysis (Ham and Raju, 2017; Haran and Gross, 2014). Such effects could be achieved by concurrent upregulated synthesis of glycolysis enzymes and downregulated expression of OXPHOS components. This metabolic reprogramming is of significance in cell survival during the early phase of energy stresses, whereas persistent inhibition of OXPHOS might induce oxidative stress and cell apoptosis (Vempati et al., 2008; Mejlvang et al., 2018; Jiang et al., 2010). Several signaling pathways are known to be involved in this process, such as hypoxia-inducible factor 1α (HIF-1α) signaling and AMPK pathway (Palm and Thompson, 2017; Yamamoto et al., 2016). Our present study indicated that SQSTM1/p62-induced MRPL12 transcription might be another mechanism underlying. Both serum deprivation and hypoxia resulted in a significant decline of SQSTM1/p62 content along with concomitant impairments of p38 signaling, MRPL12 level, mtDNA expression, and OXPHOS activity, whereas replenishment of SQSTM1/p62 effectively ameliorated such abnormalities (Figure 5). Furthermore, energy stresses are known to induce the degradation of SQSTM1/p62 by inspiring autophagic flux (Kaushal and Shah, 2016; Zhou et al., 2018). Although the role of autophagic degradation could not be excluded, the decreased mRNA level of SQSTM1/p62 indicated that a transcriptional adapting mechanism of SQSTM1/p62 might exist as well (Figures 6C and 6L). It seems that SQSTM1/p62 might act as an active player participating in energy crises associated bioenergetic reprogramming, not only as a simple passive mediator secondary to the autophagic flux.
An unexpected finding of the present study is that TECs-specific SQSTM1/p62 knockout mice exhibited obvious kidney injury, characterized by oliguria and increased serum creatinine and BUN levels (Figures 8E–8G). Such results suggested that SQSTM1/p62 plays a key role in maintaining TECs homeostasis. As shown by Müller and colleagues, adipocyte-specific SQSTM1/p62 deficiency led to a significant impairment of BAT thermogenesis, whereas SQSTM1/p62 deficiency in liver or muscle had no obvious effects on the function of these organs (Muller et al., 2013). Taking all these findings into account, SQSTM1/p62 seems to be essential for those cell types in which cellular homeostasis heavily relies on mitochondria. Such proposal also coincides with the emerging role of SQSTM1/p62 in mitochondrial regulation. As to be mentioned, TECs-specific SQSTM1/p62 knockout mice exhibited mildly decreased body weights (Figure 8D) in contrast to the results observed in SQSTM1/p62 global-knockout mice (Muller et al., 2013). Such inconsistence might be due to the systemic metabolic disturbance induced by SQSTM1/p62 depletion as both obesity and diabetic states exhibited in SQSTM1/p62 global-knockout mice are known to be capable of inducing kidney hypertrophy. Finally, our identification that SQSTM1/p62 plays an essential role in TECs homeostasis suggested SQSTM1/p62 might be a potential target in intervening ischemic or hypoxic kidney injury. Although in vivo evidences employing SQSTM1/p62-overexpressing animals are lacking currently, our in vitro SQSTM1/p62-overexpressing studies in TECs (Figure 6), as well as several others' studies demonstrated that inhibiting autophagy, which could lead to cellular SQSTM1/p62 accumulation, all indicated that SQSTM1/p62 have protective effects on ischemic kidney injuries (Yamamoto et al., 2016; Kaushal and Shah, 2016).
In conclusion, our study suggested SQSTM1/p62 could act as a crucial modulator and energy sensor of mtDNA transcription and the effects are mediated by p38-ATF2 signaling-dependent MRPL12 expression.
Limitations of the Study
One limitation of the study is the lack of conditional TECs SQSTM1/p62 knockin mice. It remains to be seen if the increase in SQSTM1/p62 expression in the renal TECs elevates the OXPHOS in TECs and ameliorates the symptoms of AKI.
Another limitation of the study is that the effect of MRPL12 levels on mitochondrial gene expression via POLRMT has previously already been observed and we propose a direct signaling pathway back to p62.However, although we demonstrate that ATF2 can interact with a promoter region in the MRPL12 locus, it is not clear in what way this interaction affects MRPL12 gene expression and further experiments are needed to address the detailed mechanism.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Qiang Wan (wanqiang@sdu.edu.cn).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
Original samples, data, and transduced cells are available upon request.
Ethical Approval
All human samples obtained from human subjects were approved by the institutional review board of Cheeloo College of Medicine, Shandong University (ECSBMSSDU2018-1-045), and written informed consent was provided prior to enrollment into the study. All experiments were approved by institutional animal care and use committee of Shandong University.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81770729, 81970427, 91749111, 81570654, 31600699) and Shandong Province Taishan Scholar Project (tsqn20161073).
Author Contributions
Conceptualization, Q.W. and W.X.; Methodology, Y.M., S.Z., and J.Z.; Investigation, Y.M., S.Z., T.L., and X.G.; Data Curation, Y.M., S.Z., T.L., and H.F.; Writing – Original Draft, Y.M., Q.W., and W.X.; Writing – Review & Editing, Y.M., Q.W., W.X., T.L., and X.G.; Funding Acquisition, Q.W. and W.X.; Resources, Q.W., and J.Z.; Supervision, Q.W. and W.X.
Declaration of Interests
The authors declare no competing interests.
Published: August 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101428.
Contributor Information
Wei Xin, Email: vivienxin@126.com.
Qiang Wan, Email: wanqiang@sdu.edu.cn.
Supplemental Information
References
- An L., Approach A.I., Goertzel B., Pennachin C., Mudado M.D.A., Coelho L.D.S. Identifying the genes and genetic interrelationships underlying the impact of calorie restriction on maximum. Rejuvenation Res. 2008;11:735–748. doi: 10.1089/rej.2007.0627. [DOI] [PubMed] [Google Scholar]
- Andrea A.D., Gritti I., Nicoli P., Giorgio M., Doni M., Conti A., Bianchi V., Casoli L., Sabò A., Alexandre M. The mitochondrial translation machinery as a therapeutic target in Myc-driven lymphomas. Oncotarget. 2016;5:72415–72430. doi: 10.18632/oncotarget.11719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barshad G., Blumberg A., Cohen T., Mishmar D. Human primitive brain displays negative mitochondrial-nuclear expression correlation of respiratory genes. Genome Res. 2018;28:952–967. doi: 10.1101/gr.226324.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barshad G., Blumberg A., Cohen T., Mishmar D. Mitochondrial DNA transcription and its regulation: an evolutionary perspective. Trends Genet. 2018;34:682–692. doi: 10.1016/j.tig.2018.05.009. [DOI] [PubMed] [Google Scholar]
- Bartolini D., Dallaglio K., Torquato P., Piroddi M., Galli F. Nrf2-p62 autophagy pathway and its response to oxidative stress in hepatocellular carcinoma. Transl Res. 2018;193:54–71. doi: 10.1016/j.trsl.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Bartolome F., Esteras N., Martin-Requero A., Boutoleau-Bretonniere C., Vercelletto M., Gabelle A., Le Ber I., Honda T., Dinkova-Kostova A.T., Hardy J. Pathogenic p62/SQSTM1 mutations impair energy metabolism through limitation of mitochondrial substrates. Sci. Rep. 2017;7:1666. doi: 10.1038/s41598-017-01678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava P., Schnellmann R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017;13:629–646. doi: 10.1038/nrneph.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacker T.S., Duchen M.R. Investigating mitochondrial redox state using NADH and NADPH autofluorescence. Free Radic. Biol. Med. 2016;100:53–65. doi: 10.1016/j.freeradbiomed.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordicchia M., Liu D., Amri E.Z., Ailhaud G., Dessì-Fulgheri P., Zhang C., Takahashi N., Sarzani R., Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo-Garrido J., Maffezzini C., Schober F.A., Clemente P., Uhlin E., Kele M., Stranneheim H., Lesko N., Bruhn H., Svenningsson P. SQSTM1/p62-Directed metabolic reprogramming is essential for normal neurodifferentiation. Stem Cell Reports. 2019;12:696–711. doi: 10.1016/j.stemcr.2019.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Medvedev A.V., Daniel K.W., Collins S. beta-Adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein 1(UCP1) gene requires p38 MAP kinase. J. Biol. Chem. 2001;276:27077–27082. doi: 10.1074/jbc.M101049200. [DOI] [PubMed] [Google Scholar]
- Cavdar Koc E., Ranasinghe A., Burkhart W., Blackburn K., Koc H., Moseley A., Spremulli L.L. A new face on apoptosis: death-associated protein 3 and PDCD9 are mitochondrial ri- bosomal proteins. FEBS Lett. 2001;492:166–170. doi: 10.1016/s0014-5793(01)02250-5. [DOI] [PubMed] [Google Scholar]
- Cheng Z., Ristow M. Mitochondria and metabolic homeostasis. Antioxid. Redox Signal. 2013;19:240–242. doi: 10.1089/ars.2013.5255. [DOI] [PubMed] [Google Scholar]
- Dodson M., Darley-Usmar V., Zhang J. Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radic. Biol. Med. 2013;63:207–221. doi: 10.1016/j.freeradbiomed.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei C., Galloni M., Hafen E., Edgar B.A. The Drosophila mitochondrial ribosomal protein mRpL12 is required for Cyclin D/Cdk4-driven growth. EMBO J. 2005;24:623–634. doi: 10.1038/sj.emboj.7600523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson C.M., Falkenberg M., Larsson N.G. Maintenance and expression of mammalian mitochondrial DNA. Annu. Rev. Biochem. 2016;85:133–160. doi: 10.1146/annurev-biochem-060815-014402. [DOI] [PubMed] [Google Scholar]
- Ham P.B., Raju R. Mitochondrial function in hypoxic ischemic injury and influence of aging. Prog. Neurobiol. 2017;157:92–116. doi: 10.1016/j.pneurobio.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao X.D., Chen Z.L., Qu M.L., Zhao X.W., Li S.X., Chen P. Decreased integrity, content, and increased transcript level of mitochondrial DNA are associated with Keratoconus. PLoS One. 2016;11:e0165580. doi: 10.1371/journal.pone.0165580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haran M., Gross A. Balancing glycolysis and mitochondrial OXPHOS: lessons from the hematopoietic system and exercising muscles. Mitochondrion. 2014;19:3–7. doi: 10.1016/j.mito.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Hisatsune J., Nakayama M., Isomoto H., Kurazono H., Mukaida N., Asish K., Mukhopadhyay, Azuma T., Yamaoka Y., Jan S. Molecular characterization of Helicobacter pylori VacA induction of IL-8 in U937 cells reveals a prominent role for p38 MAPK in activating transcription factor-2, cAMP response element binding protein, and NF-kappaB activation. J. Immunol. 2008;180:5017–5027. doi: 10.4049/jimmunol.180.7.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Duran A., Reina-Campos M., Valencia T., Castilla E.A., Muller T.D., Tschop M.H., Moscat J., Diaz-Meco M.T. Adipocyte p62/SQSTM1 suppresses tumorigenesis through opposite regulations of metabolism in adipose tissue and tumor. Cancer Cell. 2018;33:770–784.e6. doi: 10.1016/j.ccell.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager S., Handschin C., St-Pierre J., Spiegelman B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., Liu K., Luo J., Dong Z. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am. J. Pathol. 2010;176:1181–1192. doi: 10.2353/ajpath.2010.090594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama M., Inoue M., Danzaki K., Hammer G., He Y.W., Shinohara M.L. Autophagy enhances NFkappaB activity in specific tissue macrophages by sequestering A20 to boost antifungal immunity. Nat. Commun. 2015;6:5779. doi: 10.1038/ncomms6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashuba E., Yurchenko M., Yenamandra S.P., Snopok B., Isaguliants M., Szekely L., Klein G. EBV-encoded EBNA-6 binds and targets MRS18-2 to the nucleus, resulting in the disruption of pRb-E2F1 complexes. Proc. Natl. Acad. Sci. U S A. 2008;105:5489–5494. doi: 10.1073/pnas.0801053105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal G.P., Shah S.V. Autophagy in acute kidney injury. Kidney Int. 2016;89:779–791. doi: 10.1016/j.kint.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai K., Saito A., Sudo T., Osada H. Specific regulation of cytokine-dependent p38 MAP kinase activation by p62/SQSTM1. J. Biochem. 2008;143:765–772. doi: 10.1093/jb/mvn027. [DOI] [PubMed] [Google Scholar]
- Koc E.C., Koc H. Regulation of mammalian mitochondrial translation by post-translational modifications. Biochim. Biophys. Acta. 2012;1819:1055–1066. doi: 10.1016/j.bbagrm.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Kukat C., Davies K.M., Wurmd C.A., Spåhra H., Bonekampa N.A., Kühla I., Joos F., Polosa P.L., Park C.B., Posse V., Falkenberg M. Cross-strand binding of TFAM to a single mtDNA molecule forms the mitochondrial nucleoid. Proc. Natl. Acad. Sci. U S A. 2015;112:11288–11293. doi: 10.1073/pnas.1512131112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamark T., Svenning S., Johansen T. Regulation of selective autophagy: the p62/SQSTM1 paradigm. Essays Biochem. 2017;61:609–624. doi: 10.1042/EBC20170035. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Pfluger P.T., Kim J.Y., Nogueiras R., Duran A., Pagès G., Pouysségur J., Tschöp M.H., Diaz-Meco M.T., Moscat J. A functional role for the p62-ERK1 axis in the control of energy homeostasis and adipogen- esis. EMBO Rep. 2010;11:226–232. doi: 10.1038/embor.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M., Li X., Li L., Dodson M., Zhang D., Zheng H. Multifunctional p62 effects underlie diverse metabolic diseases. Trends Endocrinol. Metab. 2017;28:818–830. doi: 10.1016/j.tem.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Macao B., Uhler J.P., Siibak T., Zhu X., Shi Y., Sheng W., Olsson M., Stewart J.B., Gustafsson C.M., Falkenberg M. The exonuclease activity of DNA polymerase g is required for ligation during mitochondrial DNA replication. Nat. Commun. 2015;6:7303. doi: 10.1038/ncomms8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejlvang J., Olsvik H., Svenning S., Bruun J.A., Abudu Y.P., Larsen K.B., Brech A., Hansen T.E., Brenne H., Hansen T. Starvation induces rapid degradation of selective autophagy receptors by endosomal microautophagy. J. Cell Biol. 2018;217:3640–3655. doi: 10.1083/jcb.201711002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S., Vargas J., Hoffmann A. Signaling via the NFkappaB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016;8:227–241. doi: 10.1002/wsbm.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T.D., Lee S.J., Jastroch M., Kabra D., Stemmer K., Aichler M., Abplanalp B., Ananthakrishnan G., Bhardwaj N., Collins S. p62 Links β-adrenergic input to mitochondrial function and thermogenesis. J. Clin. Invest. 2013;123:469–478. doi: 10.1172/JCI64209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli E., Tonello C., Cardile A., Cozzi V., Bracale R., Tedesco L., Falcone S., Valerio A., Cantoni O., Clementi E. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- Nouws J., Goswami A.V., Bestwick M., McCann B.J., Surovtseva Y.V., Shadel G.S. Mitochondrial ribosomal protein L12 is required for POLRMT stability and exists as two forms generated by alternative proteolysis during import. J. Biol. Chem. 2016;291:989–997. doi: 10.1074/jbc.M115.689299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnari J., Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszanecki R., Basta-kaim A. Maternal stress predicts altered biogenesis and the profile of mitochondrial proteins in the frontal cortex and hippocampus of adult offspring rats. Psychoneuroendocrinology. 2015;60:151–162. doi: 10.1016/j.psyneuen.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Palm W., Thompson C.B. Nutrient acquisition strategies of mammalian cells. Nature. 2017;546:234–242. doi: 10.1038/nature22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly S.M., Ahmadian M., Zamarron B.F., Chang L., Uhm M., Poirier B., Peng X., Krause D.M., Korytnaya E., Neidert A. A subcutaneous adipose tissue-liver signalling axis controls hepatic gluconeogenesis. Nat. Commun. 2015;6:6047. doi: 10.1038/ncomms7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A., Duran A., Selloum M., Champy M.F., Diez-Guerra F.J., Flores J.M., Serrano M., Auwerx J., Diaz-Meco M.T., Moscat J. Mature-onset obesity and insulin resistance in mice deficient in the signaling adapter p62. Cell Metab. 2006;3:211–222. doi: 10.1016/j.cmet.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Saito A., Kawai K., Takayama H., Sudo T., Osada H. Improvement of photoaffinity SPR imaging plat- form and determination of the binding site of p62/SQSTM1 to p38 MAP kinase. Chem. Asian J. 2008;3:1607–1612. doi: 10.1002/asia.200800099. [DOI] [PubMed] [Google Scholar]
- Satoh J., Kawana N., Yamamoto Y. Pathway analysis of ChIP-seq-based NRF1 target genes suggests a logical hypothesis of their involvement in the pathogenesis of neurodegenerative diseases. Gene Regul. Syst. Biol. 2013;7:139–152. doi: 10.4137/GRSB.S13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla R.C. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann. N. Y Acad. Sci. 2008;1147:321–334. doi: 10.1196/annals.1427.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibenhener M.L., Du Y., Diaz-Meco M.T., Moscat J., Wooten M.C., Wooten M.W. A role for sequestosome 1/p62 in mitochondrial dynamics, import and genome integrity. Biochim. Biophys. Acta. 2013;1833:452–459. doi: 10.1016/j.bbamcr.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre V., Rozanska A., Beinat M., Chretien D., Boddaert N., Munnich A., Rotig A., Chrzanowska-Lightowlers Z.M. Mutations in mitochondrial ribosomal protein MRPL12 leads to growth retardation, neurological deterioration and mitochondrial translation deficiency. Biochim. Biophys. Acta. 2013;1832:1304–1312. doi: 10.1016/j.bbadis.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M.R., Koc E.C., Datta P.P., Booth T.M., Spremulli L.L., Agrawal R.K. Structure of the mammalian mitochondrial ribosome reveals an expanded functional role for its component proteins. Cell. 2003;115:97–108. doi: 10.1016/s0092-8674(03)00762-1. [DOI] [PubMed] [Google Scholar]
- Sudo T., Maruyama M., Osad H. p62 functions as a p38 MAP kinase regulator. Biochembiophys Res. Commun. 2000;269:521–525. doi: 10.1006/bbrc.2000.2333. [DOI] [PubMed] [Google Scholar]
- Surovtseva Y.V., Shutt T.E., Cotney J., Cimen H., Chen S.Y., Koc E.C., Shadel G.S. Mitochondrial ribosomal protein L12 selectively associates with human mitochondrial RNA polymerase to activate transcription. Proc. Natl. Acad. Sci. U S A. 2011;108:17921–17926. doi: 10.1073/pnas.1108852108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vempati U.D., Torraco A., Moraes C.T. Mouse models of oxidative phosphorylation dysfunction and disease. Methods. 2008;46:241–247. doi: 10.1016/j.ymeth.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Takabatake Y., Kimura T., Takahashi A., Namba T., Matsuda J., Minami S., Kaimori J.Y., Matsui I., Kitamura H. Time-dependent dysregulation of autophagy: implications in aging and mitochondrial homeostasis in the kidney proximal tubule. Autophagy. 2016;12:801–813. doi: 10.1080/15548627.2016.1159376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Willis T.L., Button R.W., Strang C.J., Fu Y., Wen X., Grayson P.R.C., Evans T., Sipthorpe R.J., Roberts S.L. Cytoplasmic DAXX drives SQSTM1/p62 phase condensation to activate Nrf2-mediated stress response. Nat. Commun. 2019;10:3759. doi: 10.1038/s41467-019-11671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.L., Wang Z.Z., Shao Q.H., Zhang Z., Li L., Guo Z.Y., Sun H.M., Zhang Y., Chen N.H. RNAi-mediated knockdown of DJ-1 leads to mitochondrial dysfunction via Akt/GSK-3ss and JNK signaling pathways in dopaminergic neuron-like cells. Brain Res. Bull. 2019;146:228–236. doi: 10.1016/j.brainresbull.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Zhou J., Fan Y., Zhong J., Huang Z., Huang T., Lin S., Chen H. TAK1 mediates excessive autophagy via p38 and ERK in cisplatin-induced acute kidney injury. J. Cell Mol Med. 2018;22:2908–2921. doi: 10.1111/jcmm.13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original samples, data, and transduced cells are available upon request.