Corresponding Author

Key Words: aortic stenosis, bioprosthetic valves, end glycation valves, human serum albumin, structural valve deterioration
The only available option for the treatment of severe aortic stenosis is aortic valve replacement (AVR) by a prosthetic valve using open heart surgery or transcatheter valve replacement. Over 200,000 AVRs are performed each year in the United States. The bioprosthetic valves (BPVs) that are most frequently (>90%) used to replace the native aortic valve are themselves subject to structural valve deterioration (SVD), which limits their durability and may lead to heart failure, valve reintervention, or death. About one-third of patients undergoing AVR with a BPV have evidence of SVD at 10 years. The long-term durability of BPVs is becoming an even more crucial issue nowadays, as there is currently a strong trend for implanting BPVs in younger patients with longer life expectancy. There is thus an important and urgent need to unravel the factors and mechanisms responsible for SVD.
Extracellular matrix deterioration, collagen fiber disruption, and fibrocalcific remodeling are the main pathobiological processes leading to SVD. For a long time, these processes have been considered as purely passive and degenerative. However, recent studies suggest that active, and potentially modifiable, processes, such as lipid infiltration, inflammation, immune rejection, and active mineralization may be involved in the pathogenesis of SVD.
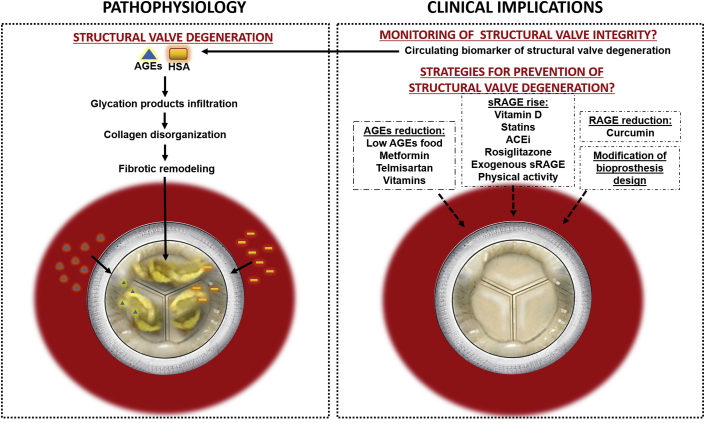
In this issue of JACC: Basic to Translational Science, Frasca et al. (1) present the results of an elegant translational research study investigating the possible detrimental effects of infiltrated glycation products and human serum albumin (HSA) leading to disorganization of BPV tissue. The authors demonstrated the presence of infiltrated advanced glycation end products (AGEs) and HSA in: 1) 46 explanted BPVs; 2) BPV bovine pericardium incubated in vitro in glyoxal and HSA; and 3) rat subdermal BPV implants. Second harmonic generation microscopy revealed structural disruption of collagen fibers and organization of BPVs subjected to glyoxal and HSA incubation. Subsequent analysis of hemodynamic performance on a commercial heart valve pulse duplicator of unimplanted surgical and transcatheter BPVs exposed to glyoxal and HSA demonstrated reduction in valve effective orifice area and increased pressure gradient and peak jet velocity (17.48%, 44.86%, and 7.62%, respectively in 30-day exposure to glyoxal medium). These results support the role of glycation and infiltration by HSA, the most abundant and glycation-susceptible circulating protein, in structural and functional degeneration of BPVs. These processes induce an adverse fibrotic remodeling of BPV tissues with disorganization of collagen fibers, leading to thickening and stiffening of valve leaflets (Figure 1).
Figure 1.
Role of Glycation Products and HSA in the Structural Valve Degeneration of Aortic Bioprostheses
Circulating AGEs and HSA infiltrate BPVs and cause collagen disorganization and fibrotic remodeling, ultimately leading to structural valve degeneration (SVD). Circulating levels of AGEs and HSA could help detecting and monitoring SVD. Strategies to reduce AGEs and RAGE and increase sRAGE as well as modification of BPV design could could contribute to prevent or slow SVD process. ACEi = angiotensin-converting enzyme inhibitor; AGE = advanced glycation end product; HAS = human serum albumin; RAGE = receptor for advanced glycation end products.
Several studies reported a strong association between type 2 diabetes mellitus and BPV SVD (2,3), and one of the potential causal mechanisms that has been proposed to explain this association was the high circulating levels of AGEs in patients with diabetes and their powerful pro-calcifying effect at the vascular and valvular levels. However, in the present study, the infiltration of AGEs and HSA within BPV leaflets resulted in fibrotic remodeling but with no significant calcification of BPV tissues. Moreover, in the small amount of patients with diabetes (17.8% of total explanted BPVs), no relationship was found between diabetes mellitus, BPV leaflet calcification, and the amount of infiltrated glycation products and HSA. These findings are counterintuitive because diabetes is an important factor contributing to production of AGEs and their receptor (receptor for advanced glycation end products [RAGE]), and hyperglycemia is a major determinant of albumin organ permeability in microcirculation and macrocirculation in patients with diabetes.
Younger age at the time of BPV implantation has been reported as one of the most important predictors of SVD and need for valve reintervention. Younger people generally have higher serum albumin concentration than do older individuals. Moreover, patients requiring AVR at a younger age most often have a bicuspid aortic valve. Some studies reported that circulating levels of AGEs are elevated in patients with bicuspid aortic valve, and particularly those with associated aortopathy (4). In the study by Frasca et al. (1), the proportion of patients with a native bicuspid valve at the time of BPV implantation was not reported, but this factor could have add an effect on the infiltration of glycation products in implanted BPVs. In light of these findings, it is plausible that accumulation of glycation products and HSA within BPV tissues is higher in younger versus older patients, which could therefore contribute to explain the faster SVD observed in younger patients.
Although BPVs are produced of chemically treated tissues to prevent cell infiltration, prostheses implanted in the circulation often elicit cellular responses. Cells from the healing process of the host, migrating cells from the sewing interface of the valve, and cells from the bloodstream that attach to the prosthesis are present on the inactivated tissues of BPVs. A great amount of immune cell infiltrate can be found in explanted BPVs developing SVD, supporting the chronic immune-mediated rejection of these tissues. In the present study, the authors used an elegant in vitro model to demonstrate the rapid uptake capacity of AGEs and HSA, as well as the inherent glycation capacity of intact BPVs. They found a comparable amount of AGE receptor ligand N-carboxymethyl-lysine infiltration in BPVs incubated with glyoxal and HSA despite less glyoxal uptake than in BPVs incubated with glyoxal alone. Glutaraldehyde fixation will normally sequester all available tissue lysine. However, infiltrated proteins and cells found in BPVs could be a substrate for increased glycation and SVD development. In the future, a biological in vitro model that would study glyoxal and HSA impact on explanted and degenerated BPVs could bring more evidence for an interaction and mechanisms between glycation products and infiltrated cellular component of the tissue and the ultimately applicable clinical responses to SVD.
During the past decade, transcatheter AVR has grown exponentially and is now rapidly expanding to low-risk populations. However, the long-term durability of transcatheter valves is still unknown, which is an important limitation in such population. The present study does not allow us to answer whether or not the durability as well as the mechanisms underlying SVD will be similar in transcatheter versus surgical BPVs, given that the majority of explanted BPVs in this study were surgical, with only 1 transcatheter valve. Further studies are needed to determine if there are any specifics in terms of factor or mechanism of SVD in transcatheter BPVs, which could translate into shorter or longer durability versus surgical BPVs.
One important clinical need is to develop blood biomarker of SVD that could be easily implemented in the clinical setting to identify patients at risk of SVD and to monitor the BPV structural and functional integrity during follow-up. In this context, the findings presented by Frasca et al. (1) raise the possibility that circulating levels of glycation products could serve as an early and sensitive marker of SVD, as well as of the risk of BPV failure and reintervention. This potential clinical implication of the results of Frasca et al. (1) needs to be validated in longitudinal cohorts of patients with aortic BPV.
There is, unfortunately, no medical treatment that can prevent or stop the progression of SVD, and our knowledge on the mechanisms leading to SVD is still largely incomplete. The study of Frasca et al. (1) cast some light on a novel mechanism that could be targeted by pharmacological therapies or by lifestyle modification. Indeed, the severity of AGE-RAGE–mediated cardiovascular disease can generally be attenuated with decreased AGEs consumption, suppression of RAGE, and increased levels of soluble receptor of RAGE (sRAGE). The reduction in the AGEs levels can be achieved by reduction in consumption of food containing AGEs, low temperature cooking, and shorter duration of cooking (Figure 1). AGE production can be reduced with drugs such as statins, metformin or telmisartan or by vitamins. Statins, angiotensin converting enzyme inhibitors, rosiglitazone, vitamin D, and physical activity can increase sRAGE levels (antagonist of RAGE-AGE effects), whereas curcumin is able to reduce RAGE (5). Exogenous administration of sRAGE can also have a beneficial impact on cardiovascular disease. In light of the results of Frasca et al. (1), these pharmacotherapeutic strategies should also be considered and tested in patients with BPV in order to prevent or slow SVD. One advantage of the context of BPV versus native aortic valve is that the treatment can be instituted immediately after BPV implantation, before any SVD process has yet started. Hence, randomized controlled trials using antiglycation therapies can be envisioned following AVR, with the goal of primary prevention of SVD.
Another future perspective for avoiding the deleterious effect of AGEs and HSA infiltration on BPV tissues would be to modify the design of the BPV per se, including, for example: 1) polymer coating at the surface of the BPV leaflet to prevent infiltration of glycation products and HSA; and 2) chemical and physical processes to stabilize the extracellular macromolecular components of the BPV tissues and therefore avoid deterioration related to infiltrated glycation products.
Footnotes
Dr. Pibarot has received funding from Edwards Lifesciences and Medtronic for echocardiography core laboratory analyses in the field of transcatheter aortic valve replacement with no direct personal compensation. Dr. Côté has reported that she has no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Frasca A., Xue Y., Kossar A.P. Glycation and serum albumin infiltration contribute to the structural degeneration of bioprosthetic heart valves. J Am Coll Cardiol Basic Trans Sci. 2020;5:755–766. doi: 10.1016/j.jacbts.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lorusso R., Gelsomino S., Luca F. Type 2 diabetes mellitus is associated with faster degeneration of bioprosthetic valve: results from a propensity score-matched Italian multicenter study. Circulation. 2012;125:604–614. doi: 10.1161/CIRCULATIONAHA.111.025064. [DOI] [PubMed] [Google Scholar]
- 3.Salaun E., Mahjoub H., Girerd N. Rate, timing, correlates, and outcomes of hemodynamic valve deterioration after bioprosthetic surgical aortic valve replacement. Circulation. 2018;138:971–985. doi: 10.1161/CIRCULATIONAHA.118.035150. [DOI] [PubMed] [Google Scholar]
- 4.Branchetti E., Bavaria J.E., Grau J.B. Circulating soluble receptor for advanced glycation end product identifies patients with bicuspid aortic valve and associated aortopathies. Arterioscler Thromb Vasc Biol. 2014;34:2349–2357. doi: 10.1161/ATVBAHA.114.303784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasad K., Tiwari S. Therapeutic interventions for advanced glycation-end products and its receptor-mediated cardiovascular disease. Curr Pharm Des. 2017;23:937–943. doi: 10.2174/1381612822666161006143032. [DOI] [PubMed] [Google Scholar]