Summary
Homeostatic generation of T cells, which occurs in the thymus, is controlled at least in part by endogenous cytokines and ligands. In addition, nutritional factors are other key regulators for the homeostasis of host immunity, but whether and how nutrition affects the homeostatic generation of thymocytes remains to be established. Here, we showed that vitamin B1 deficiency resulted in a bias toward the maturation of γδ thymocytes accompanied by decreased differentiation into double-positive thymocytes during thymic involution. These events were mediated through the increased production of TGF-β superfamily members due to the accumulation of branched-chain α-keto acids in thymic stromal cells. These findings revealed essential roles of vitamin B1 in the appropriate differentiation of T cells through the metabolism of thymic stromal cells.
Subject Areas: Cellular Physiology, Immunology, Cell Biology, Functional Aspects of Cell Biology
Graphical Abstract
Highlights
-
•
Vitamin B1 deficiency induces excessive BCKA production in the thymic stromal cells
-
•
BCKAs increase the production of TGF-β superfamily from thymic stromal cells
-
•
TGF-β superfamily induces unbalanced differentiation of thymocytes
-
•
Vitamin B1 deficiency induces thymic involution
Cellular Physiology; Immunology; Cell Biology; Functional Aspects of Cell Biology
Introduction
The thymus is the primary lymphoid organ for generating T cells, a process that is regulated through the interaction of endogenous molecules with thymocytes (e.g., Notch and T cell receptor [TCR]) and stromal cells (e.g., Delta ligand and self-peptides on major histocompatibility complex [MHC]) (Hogquist and Jameson, 2014; Takahama, 2006). Initially, thymocytes lack cell surface expression of both CD4 and CD8 (i.e., double-negative [DN] cells) and develop from stage DN1 to DN4 as TCR expression increases due to the interaction between Notch on thymocytes and Delta ligand on thymic stromal cells (Takahama, 2006). The strength of the TCR signal from the MHC on thymic stromal cells with Delta-Notch interaction drives the transition to CD4+CD8+ double-positive (DP) thymocytes (Hogquist and Jameson, 2014; Takahama, 2006). Moreover, transforming growth factor (TGF)-β superfamily members, including TGF-β1, and Activin A from thymic stromal cells arrest the development of thymocytes by preventing their progression from the DN to the DP stage (Licona-Limón et al., 2009; Takahama, 2006) and induce the apoptosis of DP thymocytes (Szondy et al., 2003). Furthermore, a strong TCR signal and high TGF-β superfamily activity during the DN stage accelerate the development or maturation of γδ thymocytes (Hogquist and Jameson, 2014; Woolf et al., 2007).
T cells have metabolic heterogeneity in regard to their various functions and development stages (Bantug et al., 2018; Buck et al., 2015). Indeed, naive T cells are relatively dependent on bioenergetic catabolism, using oxidative phosphorylation and fatty acid oxidation for homeostatic maintenance (Bantug et al., 2018; Buck et al., 2015). In contrast, to meet metabolic demands, activated and proliferating T cells generally depend on anabolism characterized by high mechanistic target of rapamycin (mTOR) activity and fatty acid synthesis and catabolism through glycolysis and amino acid metabolism (Bantug et al., 2018; Buck et al., 2015). Recently, it was reported that the homeostatic production of thymocytes was regulated through the metabolic states of mTOR in both thymocytes (Yang et al., 2018) and thymic stromal cells (Wang et al., 2016).
Accumulating evidence suggests that nutritional factors are other key regulators of T cell development and differentiation. Indeed, malnutrition induces thymic involution (Savino et al., 2007), and several nutrients, including zinc and vitamin A, are necessary for the maintenance of thymus (Cunningham-Rundles et al., 2005). Regarding vitamin A, retinoic acid signaling in thymic epithelial cells (TEC) is required to maintain an appropriate developmental balance from medullary (m) TEC to cortical (c) TEC and thus support thymocyte development (Wendland et al., 2018).
Vitamin B1 (thiamine) is an essential nutrient for the central metabolism rooted in pyruvate, branched amino acids, and ribose 5-phosphate as well as the citric acid cycle (Dhir et al., 2019). We previously reported that naive B cells in Peyer patches rely metabolically on the citric acid cycle, which has a high requirement for vitamin B1, to produce ATP for energy (Kunisawa et al., 2015). In contrast, IgA-producing plasma cells in the intestinal lamina propria utilize the glycolytic pathway for ATP production and thus have a decreased requirement for vitamin B1 (Kunisawa et al., 2015). Indeed, mice maintained on a vitamin B1-deficient diet showed significant reduction of naive B cells in Peyer patches without remarkable changes in IgA-producing plasma cells in the intestinal lamina propria (Kunisawa et al., 2015). Given that Peyer patches are the site in naive B cells for the class switching of IgM to IgA, especially to intestinal antigens, maintaining mice on a vitamin B1-deficient diet resulted in impaired intestinal IgA responses against orally immunized vaccine antigens (Kunisawa et al., 2015).
In the current study, we explored the immunologic roles of vitamin B1 in T cell development in thymus and found that vitamin B1 was highly required for appropriate production of TGF-β superfamily members from thymic stromal cells. This process was controlled through the metabolism of branched-chain amino acids.
Results
Vitamin B1 Supports the Homeostatic Development of T Cells in the Thymus
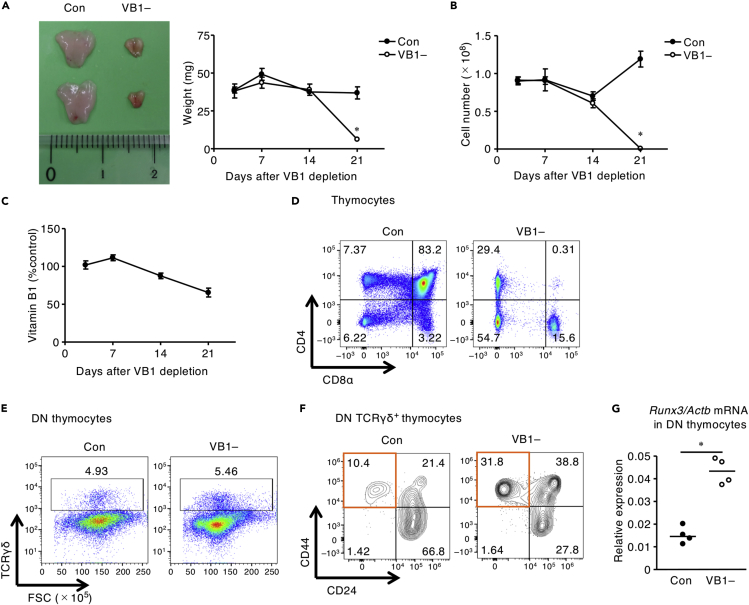
First, we evaluated whether dietary vitamin B1 affects T cell development in the thymus. Macroscopic analysis revealed that mice maintained on vitamin B1-deficient chow demonstrated remarkable decreases in the volume and weight of thymus and the total number of thymus cells, namely, thymic involution, compared with mice maintained on control chow (Figures 1A and 1B). Accordingly, the amount of vitamin B1 in thymus gradually decreased during the 3 weeks after dietary vitamin B1 was discontinued (Figure 1C). Flow cytometric analysis indicated that the proportion of DP thymocytes was decreased preferentially (Figure 1D). In contrast, vitamin B1 deficiency did not affect the overall proportion of TCRγδ+ cells among DN thymocytes (Figure 1E), but the proportion of cells that transitioned from immature, CD44–CD24+ cells to the mature, CD44+CD24– subset of TCRγδ+ DN thymocytes was increased (Figure 1F).
Figure 1.
Vitamin B1 Is Required to Inhibit Increases in Mature TCRγδ+ DN Thymocytes
(A–F) (A) Macroscopic analysis of thymus on day 21, thymus weight; (B) total number of thymocytes at days 3, 7, 14, and 21 of feeding mice a vitamin B1-deficient diet (VB1–) or control diet (Con); and (C) thymic vitamin B1 concentration at days 3, 7, 14, and 21 of feeding mice a vitamin B1-deficient diet (VB1–) relative to control diet. FACS plots of (D) CD4 and CD8α on live thymocytes gated on 7-AAD–, (E) of TCRγδ+ cells among DN thymocytes, and (F) of CD44 and CD24 among DN– TCRγδ+ thymocytes after 3 weeks of VB1– or Con diet. Scale, 1 cm. Horizontal lines indicate median values. p values were obtained by using the Mann-Whitney U-test (∗p < 0.05). The data shown are reproducible and are representative of two to five independent experiments.
(G) The levels of Runx3 mRNA in DN and DP thymocytes sorted from the VB1– and Con groups are shown. Horizontal lines indicate median values. p values were obtained by using the Mann-Whitney U-test (∗p < 0.05).
Given that increased expression of RUNX3 reportedly induces the maturation of DN TCRγδ+ thymocytes in thymus (Woolf et al., 2007), we assessed Runx3 mRNA expression in DN thymocytes by using qRT-PCR analysis. Runx3 mRNA expression levels in DN thymocytes were upregulated in vitamin B1-deficient mice compared with the control group (Figure 1G). These results suggest that vitamin B1 is required to control RUNX3 expression and thus maintain appropriate generation of DP and DN TCRγδ+ thymocytes.
High Requirement for Vitamin B1 in Thymic Stromal Cells
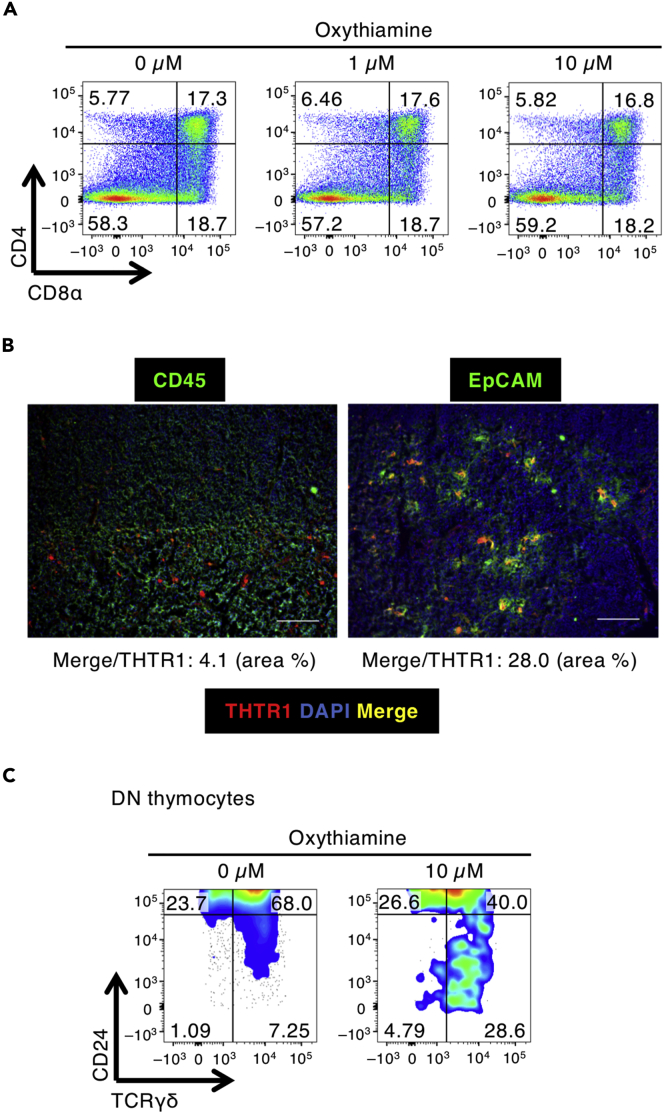
We investigated whether vitamin B1 affects thymocytes directly. When purified DN thymocytes were co-cultured with the bone marrow-derived OP9–DL1 cell line as stromal cells, they developed to single-positive and DP thymocytes (Lai et al., 2010). A similar pattern emerged when cultures were treated with oxythiamine, a vitamin B1 inhibitor (Figure 2A). Therefore, these results suggested that vitamin B1 inhibition did not directly affect thymocyte development in vitro, unlike in vivo vitamin B1 deficiency. Moreover, because we hypothesized that OP9–DL1 cells incubated with oxythiamine might not sufficiently recapitulate the in vivo thymic stromal environment in vitamin B1-deficient mice, we assessed the mRNA expression levels of thiamine transporter 1 (THTR1) (Dutta et al., 1999) in the OP9–DL1 cells and thymic stromal cells. Indeed, the levels of Thtr1 mRNA were significantly higher in thymic stromal cells than in OP9–DL1 cells (Figure S1). We then used immunohistochemistry to examine thiamine transporter 1 (THTR1) levels and showed that a population of EpCAM+ stromal cells expressed higher levels of THTR1 than did CD45+ thymocytes (Figure 2B). Given these findings, we then adopted a fetal thymic organ culture (FTOC) system to better mimic the thymic environment in vivo. Although treatment with oxythiamine did not significantly alter the proportion of DP thymocytes that developed from DN1–3 thymocytes (Figure S2), the proportion of CD24–, mature DN TCRγδ+ thymocytes increased (Figure 2C). This result indicates that the thymic environment created by stromal cells requires vitamin B1 for the appropriate control of thymocyte development.
Figure 2.
The Requirement for Vitamin B1 Is Higher in Thymic Stromal Cells Than in Thymocytes
(A) FACS plots of developing thymocytes sorted from DN1–3 to DP are shown after incubation for 7 days in the presence of oxythiamine and OP9–DL1 cells.
(B) Immunohistochemistry of murine thymus was performed by using monoclonal antibodies (mAbs) to either CD45 or EpCAM and THTR1. Scale bar, 100 μm. Merge area (yellow) was quantified as the color threshold of Merge (Y: 100, U: 0, and V: 120) and THTR1 (R: 100, G: 0, and B: 0) by using ImageJ.
(C) FACS plots of CD24– cells among DN TCRγδ+ thymocytes from sorted DN1–3 cells reconstituted in fetal thymic organ culture and incubated in the presence of oxythiamine. The data shown are reproducible and representative of five independent experiments.
Vitamin B1 Controls the Production of TGF-β Superfamily Members in Thymic Stromal Cells
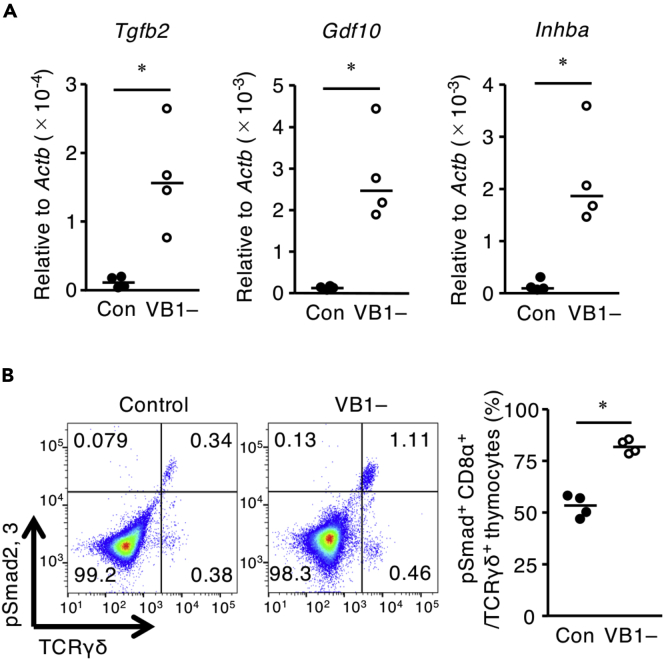
Both TGF-βs and Activin A (also known as Inhba), which belongs to the TGF-β superfamily, reportedly block the developmental transition of thymocytes from the DN to DP stage (Licona-Limón et al., 2009; Takahama et al., 1994) and induce the apoptosis of DP thymocytes (Szondy et al., 2003). Moreover, RUNX3 transcription is upregulated by TGF-β superfamily members through the phosphorylation of smad2 and smad3 (Klunker et al., 2009; Reis et al., 2013). Prompted by these reports and our current findings, we evaluated the effect of vitamin B1 deficiency on the production of TGF-β superfamily members in thymic stromal cells. Compared with the control group, thymic stromal cells from the vitamin B1-deficient condition had increased levels of Tgfb2, Gdf10, and Inhba mRNA (Figure 3A); mRNA transcript levels did not differ between the control and vitamin B1-deficient groups for any other TGF-β superfamily member (Figure S3). In addition, flow cytometric analysis consistently demonstrated that vitamin B1 deficiency increased the proportion of TCRγδ+ thymocytes expressing phosphorylated smad2 and smad3; these signaling molecules are induced by the TGF-β superfamily (Figure 3B). These results suggest that vitamin B1 modulates the expression of Tgfb2, Gdf10, and Inhba in thymic stromal cells, thus maintaining the homeostatic generation of DP and TCRγδ+ thymocytes through the phosphorylation of smad2 and smad3.
Figure 3.
Thymic Stromal Cells Require Vitamin B1 to Inhibit Excessive Production of the TGF-β Superfamily In Vivo
(A) The levels of Gdf10, Inhba, and Tgfb2 mRNAs in thymic stromal cells after 3 weeks of feeding mice a vitamin B1-deficient diet (VB1–) or control diet (Con). Horizontal lines indicate median values. p values were obtained by using the Mann-Whitney U-test (∗p < 0.05). The data shown are reproducible and representative of two independent experiments.
(B) FACS plots showing the ratios of cells positive for phosphorylated smad2, smad3+, and CD8α+ among TCRγδ+ thymocytes from VB1– and Con mice. Horizontal lines indicate median values. p values were obtained by using the Mann-Whitney U-test (∗p < 0.05).
Vitamin B1 Promotes the Metabolism of Branched-Chain α-Keto Acids in Thymic Stromal Cells
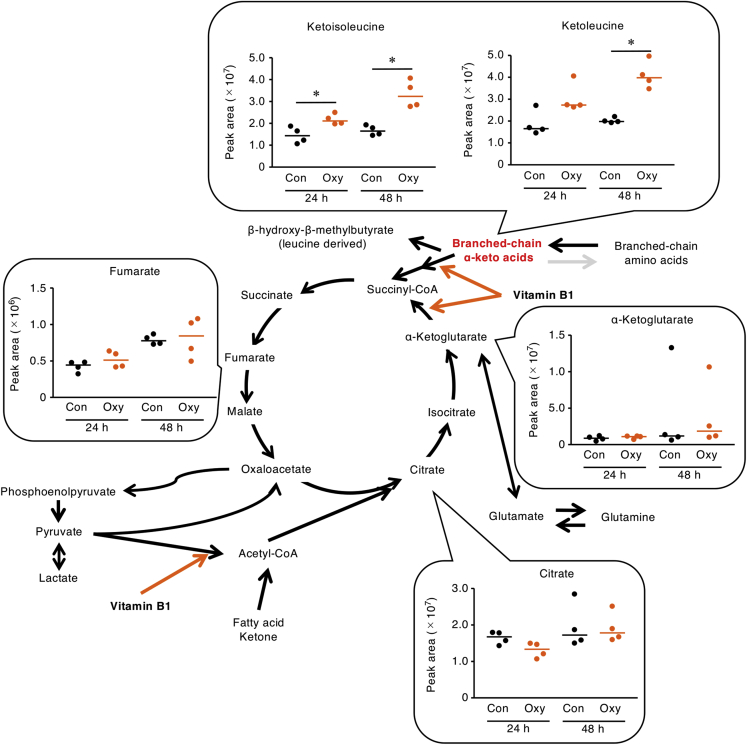
Vitamin B1 is well known as a necessary coenzyme for catalyzing the conversion of pyruvate to acetyl-CoA, of α-ketoglutarate to succinyl-CoA, of branched-chain α-keto acids (BCKAs) to branched-chain acyl-CoA, and of ribose 5-phosphate to glyceraldehyde 3-phosphate (Manoli and Venditti, 2016; Whitfield et al., 2018). Therefore, we wondered what metabolic changes might occur in thymic stromal cells. Metabolomic analysis using ion chromatography with Fourier transform mass spectrometry (IC-MS) showed that ketoleucine and ketoisoleucine, which are BCKAs that are generated as intermediate metabolites from branched-chain amino acids (BCAAs), were increased in the supernatant of thymic stromal cells that had been treated with oxythiamine; however, metabolites associated with the citric acid cycle and glycolysis remained unchanged (Figures 4 and S4).
Figure 4.
Vitamin B1 Is Required for Appropriate Metabolism of Branched-Chain α-Keto Acids in Thymic Stroma In Vitro
We used IC-MS to analyze citric-acid cycle metabolites in supernatants from murine thymic stromal cells that had been incubated with oxythiamine (Oxy) for 24 or 48 h. Horizontal lines indicate median values. Con, no-treatment control. ∗p < 0.05 (Mann-Whitney U-test).
BCKAs Induced Excessive Production of TGF-β Superfamily Members
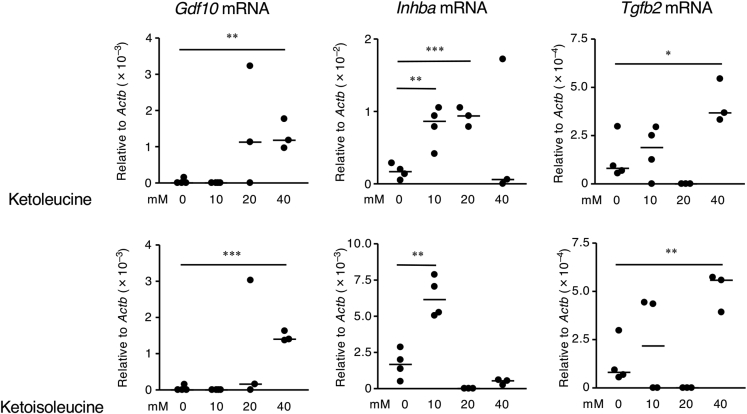
We investigated whether the BCKAs that accumulated in thymic stromal cells due to vitamin B1 insufficiency could increase the quantities of mRNAs encoding TGF-β superfamily members. Culturing thymic stromal cells in media containing ketoleucine and ketoisoleucine increased the mRNA expression of Inhba, Tgfb2, and Gdf10 (Figures 5 and S5). These results suggest that vitamin B1-mediated metabolism of BCAAs, especially BCKAs, controls the production of TGF-β superfamily members in thymic stromal cells.
Figure 5.
Branched-Chain α-Keto Acids Induce Excessive Production of the TGF-β Superfamily in Thymic Stromal Cells
Murine thymic stromal cells were incubated for 48 h with either ketoleucine or ketoisoleucine, which are branched-chain α-keto acids. The levels of Gdf10, Inhba, and Tgfb2 mRNAs in the thymic stromal cells were determined by using qRT-PCR. p values were obtained by using the two-tailed unpaired Student's t test (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). The data shown are reproducible and representative of two independent experiments.
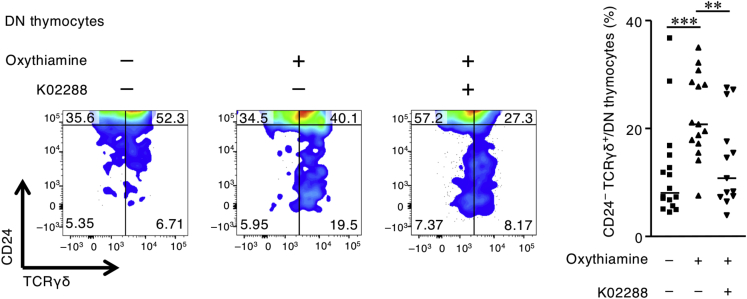
Activin A, TGF-β, and GDF10 act as ligands for the TGF-β superfamily receptors ALK4 and ActRIIA (to Activin A and GDF10), ALK5 (to TGF-β), and others (Heldin and Moustakas, 2016), whereas K02288 inhibits various TGF-β superfamily receptors (e.g., ALK2, ALK1, ALK6, ALK3, ActRIIA, ALK4, and ALK5) (Sanvitale et al., 2013). These activities allowed us to use FTOC to evaluate the effect of K02288 on the oxythiamine-induced maturation of DN γδ thymocytes. Treatment with K02288 canceled the oxythiamine-induced excessive maturation of DN γδ thymocytes in FTOCs (Figure 6). These results suggest that impaired thymocyte differentiation in the vitamin B1-deficient condition was mediated by TGF-β superfamily members.
Figure 6.
Vitamin B1 Inhibition Induces Excessive Maturation of γδ Thymocytes through TGF-β Superfamily Signaling in Fetal Thymic Organ Cultures
FACS plot of CD24– TCRγδ+ cells among DN developing thymocytes from sorted DN1–3 cells reconstituted in fetal thymic organ cultures incubated in the presence of oxythiamine, the TGF-β superfamily inhibitor K02288, or both agents. Horizontal lines indicate median values. p values were obtained by using the Mann-Whitney U-test (∗∗∗p < 0.001, ∗∗p < 0.01). The data shown are pooled from three independent experiments, which yielded reproducible data.
Changes in Peripheral T Lymphocytes
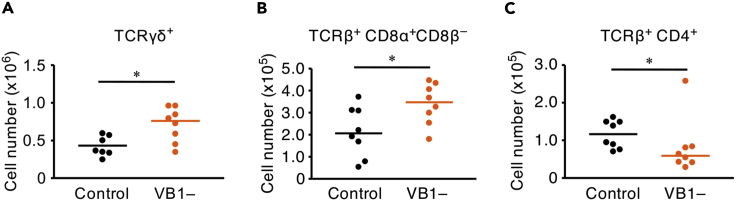
We next investigated the effect of vitamin B1 deficiency on the number of peripheral T lymphocytes in the gut, the site of numerous TCRγδ+ T lymphocytes, under immunologically naive conditions. Flow cytometric analysis revealed that vitamin B1 deficiency increased the proportion of CD8αα TCRγδ+ T lymphocytes in the small intestine (Figure 7A). A similar increase occurred in the CD8αα subpopulation of TCRβ+ T lymphocytes, an unconventional T lymphocyte subset derived from DN thymocytes (Konkel et al., 2011; Pobezinsky et al., 2012) (Figure 7B), whereas vitamin B1 deficiency decreased the number of CD4 TCRβ+ T lymphocytes in the small intestine (Figure 7C). In addition, we found that the numbers of CD4 and CD8α IFN-γ+ TCRβ+ T lymphocytes, which are the main functional subsets in spleen (Saxena et al., 2012), were reduced in the spleens of vitamin B1-deficient mice (Figure S7). These results suggest that vitamin B1 is required for the appropriate differentiation and function of T lymphocytes in peripheral tissues.
Figure 7.
Vitamin B1 Suppresses the Excessive Production of Unconventional T Lymphocytes and Maintains Conventional CD4 T Lymphocytes in Small Intestine
(A–C) The total number of (A) TCRγδ+, (B) CD8αα TCRβ+, and (C) CD4 TCRβ+ intestinal lymphocytes (i.e., lamina propria lymphocytes + intestinal epithelial lymphocytes) at 3 weeks after mice began a vitamin B1-deficient diet (VB1–) or control diet (Con). Horizontal lines indicate median values. p values were obtained by using the Mann-Whitney U-test (∗p < 0.05). The data shown are pooled from three independent experiments, which yielded reproducible data.
Discussion
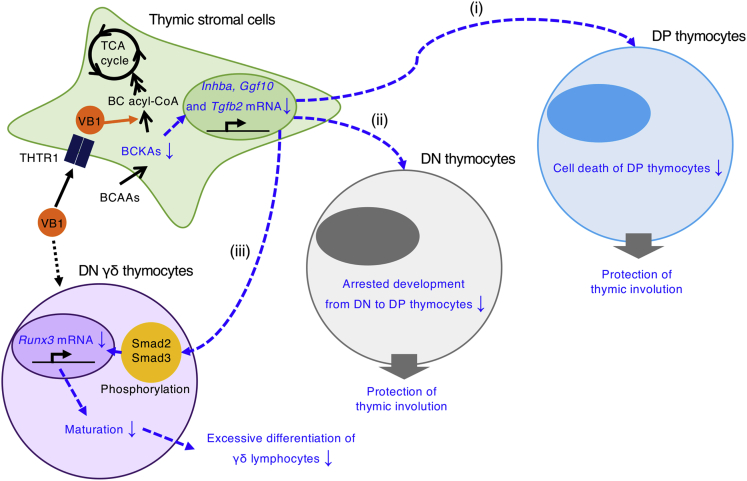
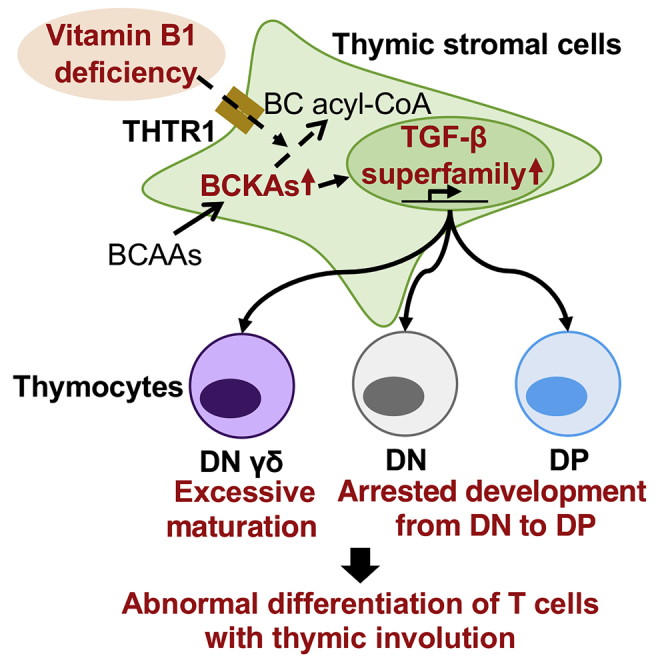
Here we showed that vitamin B1 modulates the metabolism of BCKAs and consequent production of TGF-β superfamily members in thymic stromal cells; these activities are required to maintain the appropriate differentiation of DP and γδ thymocytes (Figure 8). These findings extend current knowledge by revealing the novel role of vitamin B1 as an essential micronutrient for homeostatic development of immune cells.
Figure 8.
Hypothetical Scheme of the Mechanism Underlying Vitamin B1-Dependent Homeostatic Generation of Lymphocytes from Thymic Stromal Cells
Vitamin B1, which is transported through THTR1, is more highly required in thymic stromal cells than in thymocytes. Vitamin B1 suppresses overproduction of TGF-β superfamily members by promoting the metabolism of branched-chain α-keto acids in thymic stromal cells. Vitamin B1-dependent regulation of inappropriate TGF-β superfamily production (i) suppresses the apoptosis of DP thymocytes; (ii) protects against blockade of the DN to DP transition in thymocytes, which otherwise would cause spurious thymic involution; and (iii) prevents excess development of mature γδ thymocytes, leading to preferential differentiation of γδ lymphocytes.
Our current study showed that thymic stromal cells—but not CD45+ thymocytes—express THTR1 (Figure 2). Notably, THTR1 was not expressed in all thymic stromal cells (Figure 2B). In addition to cTEC and mTEC, recent studies using single-cell RNA sequencing have indicated that EpCAM+ thymic stromal cells comprise several functionally heterogeneous subsets, including tuft cells (Bornstein et al., 2018; Inglesfield et al., 2019; Miller et al., 2018). We found that some EpCAM+ thymic stromal cells preferentially express THTR1, to support appropriate development of thymocytes (Takahama, 2006).
Specificity protein 1 (Sp1) increases THTR1 in proximal tubular epithelial cells (Larkin et al., 2012), and p63 downregulates the activity of Sp1 in human nasal epithelial cells (Kaneko et al., 2017). The expression level of p63 in TECs is negatively correlated with that of FoxN1, one of the main differential transcription factors in TECs (Burnley et al., 2013). Therefore, the THTR1 content of TECs might be orchestrated through FoxN1-induced downregulation of p63 and thus upregulation of Sp1. Furthermore, compared with other cTECs and mTECs, the MHC class IIhigh cTECs among EpCAM+ thymic stromal cells show greater expression of FoxN1 mRNA (Nowell et al., 2011; O'Neill et al., 2016); this result implies that FoxN1-initiated control of THTR1 expression might be upregulated in MHC class IIhigh cTECs.
We found that vitamin B1 insufficiency increased BCKA levels and the production of some TGF-β superfamily members, including Inhba, Gdf10, and Tgfb2, in thymic stromal cells (Figures 3 and 5). Ketoleucine increased intracellular Ca2+ levels in rat cerebral cortex in vitro (Funchal et al., 2005). Moreover, increased levels of intracellular Ca2+, which induces TGF-β in the 3T3TβRII cell line (Xiao et al., 2008), activated p38 MAPK and ERK1/2 in bone marrow macrophages in vitro (Zhou et al., 2010). Why Inhba, Gdf10, and Tgfb2 mRNAs specifically are increased in thymic stromal cells remains unknown at this point. However, our current findings suggest that BCKAs, including ketoleucine and ketoisoleucine, may increase intracellular Ca2+ in thymic stromal cells, thus inducing MAPK- and ERK-mediated mRNA expression of TGF-β superfamily members, including Inhba, Gdf10, and Tgfb2.
We showed that dietary deficiency of vitamin B1 decreased the number of DP thymocytes and increased the numbers of mature γδ thymocytes in mice; these changes were associated with the increased expression of Runx3 mRNA and the phosphorylation of smad2 and smad3 in thymocytes (Figures 1 and 3). In support of our current findings, previous studies showed that various TGF-β superfamily members, including Activin A, GDF10, and TGF-β2, induce the phosphorylation of Smad2 and Smad3 (Heldin and Moustakas, 2016) and upregulate the expression of RUNX3 (Jin et al., 2004; Reis et al., 2013) and that TGF-β2 induces the RUNX3-mediated maturation of γδ thymocytes (Woolf et al., 2007). In addition, TGF-β and Activin A block thymocyte progression from the DN stage to the DP stage and induce the apoptosis of DP thymocytes (Licona-Limón et al., 2009; Szondy et al., 2003; Takahama et al., 1994).
Consistent with the in vivo results, treatment with oxythiamine increased the proportion of CD24– mature DN γδ thymocytes (Figure 2C) but did not significantly alter the proportion of DP thymocytes that developed from DN1–3 thymocytes (Figure S2). As an explanation of this apparent discrepancy, previous studies showed that fetal TECs had a greater rate of cell growth than those from adult thymus (Cowan et al., 2019) and that increased cell growth activity was associated with low dependency on BCAA catabolism in cardiomyocytes (Shao et al., 2018). Thus, under vitamin B1-deficient conditions, BCKA accumulation likely was lower in FTOCs than in adult thymus. In support of this notion, exogenous addition of BCKAs reduced the proportion of DP thymocytes in FTOCs (Figure S6). Furthermore, given that TGF-β receptors (i.e., Tgfβr1, Tgfβr2) were more highly expressed in TCRγδ+ thymocytes than in DP thymocytes (Do et al., 2010), DN γδ thymocytes might have greater sensitivity to the oxythiamine-induced production of BCKAs and TGF-β superfamily ligands.
In line with the immunologic phenotypes in the thymus, vitamin B1 deficiency decreased the population of DP thymocyte-derived CD4 TCRβ+ T lymphocytes in the gut (Figure 6). In contrast, we noted increases in the numbers of TCRγδ+ T lymphocytes and CD8αα TCRβ+ T lymphocytes (Figure 6), which developed from DN thymocytes through TGF-β-mediated RUNX3 expression (Konkel et al., 2011; Pobezinsky et al., 2012). Moreover, we showed that vitamin B1 deficiency led to a decrease in IFN-γ+ TCRβ+ T lymphocytes (Figure S7). Given that pyruvate dehydrogenase (PDH) induces the production of acetyl-CoA to induce the transcription of IFN-γ transcription (Peng et al., 2016) and that vitamin B1 is an essential co-enzyme for PDH (Kunisawa et al., 2015), vitamin B1 deficiency plausibly impaired PDH activity and consequently inhibited IFN-γ production.
Together, our current findings indicate that vitamin B1 is required for the appropriate differentiation of thymocytes, especially the development of DN cells into DP or γδ thymocytes. This regulation is mediated through control of the production of TGF-β superfamily members including Activin A, GDF10, and TGF-β2; this regulation is achieved by promoting the metabolism of BCKAs in thymic stromal cells. These findings provide new evidence of vitamin B1-mediated interaction between stromal and immune cells for the appropriate development of thymocytes.
Limitations of the Study
This study demonstrated that vitamin B1 was necessary for the appropriate metabolic functions in thymic stromal cells for the homeostatic differentiation of T cells. Although we proposed that a specific subset of thymic stromal cells expressing high levels of THTR1 were responsible for it, we could not specify them due to the experimental restriction of anti-THTR1 antibody.
Resource Availability
Lead Contact
Further information and requests should be directed by the Lead Contact, Jun Kunisawa (kunisawa@nibiohn.go.jp).
Materials Availability
New unique reagents were not generated in this study.
Data and Code Availability
The data in this study are available from the corresponding author on request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank RIKEN BRC CELL BANK for providing the OP9–DL1 cell line. We also thank our laboratory members for their helpful support. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) and the Japan Society for the Promotion of Science (JSPS) KAKENHI (nos. JP18H02150, JP18H02674, JP17K09604 [to J.K.], JP18K17997 [to K.H.], and JP19K07617 [to T.N.]); the Japan Agency for Medical Research and Development (nos. 17fk0108223h0002, 17fk0108207h0002, 17ek0210078h0002, 17ak0101068h0001, 17gm1010006s0101, 18ck0106243h0003, 19ek0410062h0001 [to J.K.], and 17ek0410032s0102 [to J.K.]); the Ministry of Health, Labour and Welfare of Japan (to J.K. and JP19KA3001 to K.H.); The Ministry of Health and Welfare of Japan and Public/Private R&D Investment Strategic Expansion PrograM: PRISM (to J.K.); Cross-ministerial Strategic Innovation Promotion Program: SIP (to J.K.); a grant for Joint Research Project of the Institute of Medical Science, the University of Tokyo (to J.K.); Astellas Foundation for Research on Metabolic Disorders (to J.K.); Nipponham Foundation for the Future of Food (to J.K.); the Canon Foundation (to J.K.); and Ono Medical Research Foundation (to J.K.).
Author Contributions
S.H. conceived and designed the study, performed experiments, analyzed data, and wrote the manuscript. K.S., J.A., J.I., Y.S., and T.T. helped to set up the IC-MS analyses. A.M. helped to perform immunologic analyses and contributed to discussions. T.N., K.H., and M.S. provided helpful suggestions and discussion. M.S. was the lead scientist (JST, ERATO, Suematsu Gas Biology) who established the infrastructure for metabolomics. J.K. conceived and supervised experiments, provided reagents, and contributed to manuscript preparation.
Declaration of Interests
The authors declare that they have no conflict of interest.
Published: September 25, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101426.
Supplemental Information
References
- Bantug G.R., Galluzzi L., Kroemer G., Hess C. The spectrum of T cell metabolism in health and disease. Nat. Rev. Immunol. 2018;18:19–34. doi: 10.1038/nri.2017.99. [DOI] [PubMed] [Google Scholar]
- Bornstein C., Nevo S., Giladi A., Kadouri N., Pouzolles M., Gerbe F., David E., Machado A., Chuprin A., Tóth B. Single-cell mapping of the thymic stroma identifies IL-25-producing tuft epithelial cells. Nature. 2018;559:622–626. doi: 10.1038/s41586-018-0346-1. [DOI] [PubMed] [Google Scholar]
- Buck M.D., O’Sullivan D., Pearce E.L. T cell metabolism drives immunity. J. Exp. Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnley P., Rahman M., Wang H., Zhang Z., Sun X., Zhuge Q., Su D.-M. Role of the p63-FoxN1 regulatory axis in thymic epithelial cell homeostasis during aging. Cell Death Dis. 2013;4:e932. doi: 10.1038/cddis.2013.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan J.E., Malin J., Zhao Y., Seedhom M.O., Harly C., Ohigashi I., Kelly M., Takahama Y., Yewdell J.W., Cam M. Myc controls a distinct transcriptional program in fetal thymic epithelial cells that determines thymus growth. Nat. Commun. 2019;10:5498. doi: 10.1038/s41467-019-13465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham-Rundles S., McNeeley D.F., Moon A. Mechanisms of nutrient modulation of the immune response. J. Allergy Clin. Immunol. 2005;115:1119–1128. doi: 10.1016/j.jaci.2005.04.036. quiz 1129. [DOI] [PubMed] [Google Scholar]
- Dhir S., Tarasenko M., Napoli E., Giulivi C. Neurological, psychiatric, and biochemical aspects of thiamine deficiency in children and adults. Front. Psychiatry. 2019;10:207. doi: 10.3389/fpsyt.2019.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do J., Fink P.J., Li L., Spolski R., Robinson J., Leonard W.J., Letterio J.J., Min B. Cutting edge: spontaneous development of IL-17-producing gamma delta T cells in the thymus occurs via a TGF-beta 1-dependent mechanism. J. Immunol. 2010;184:1675–1679. doi: 10.4049/jimmunol.0903539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta B., Huang W., Molero M., Kekuda R., Leibach F.H., Devoe L.D., Ganapathy V., Prasad P.D. Cloning of the human thiamine transporter, a member of the folate transporter family. J. Biol. Chem. 1999;274:31925–31929. doi: 10.1074/jbc.274.45.31925. [DOI] [PubMed] [Google Scholar]
- Funchal C., Zamoner A., dos Santos A.Q., Loureiro S.O., Wajner M., Pessoa-Pureur R. Alpha-ketoisocaproic acid increases phosphorylation of intermediate filament proteins from rat cerebral cortex by mechanisms involving Ca2+ and cAMP. Neurochem. Res. 2005;30:1139–1146. doi: 10.1007/s11064-005-7709-3. [DOI] [PubMed] [Google Scholar]
- Heldin C.-H., Moustakas A. Signaling receptors for TGF-β family members. Cold Spring Harb. Perspect. Biol. 2016;8:a022053. doi: 10.1101/cshperspect.a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist K.A., Jameson S.C. The self-obsession of T cells: how TCR signaling thresholds affect fate “decisions” and effector function. Nat. Immunol. 2014;15:815–823. doi: 10.1038/ni.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglesfield S., Cosway E.J., Jenkinson W.E., Anderson G. Rethinking thymic tolerance: lessons from mice. Trends Immunol. 2019;40:279–291. doi: 10.1016/j.it.2019.01.011. [DOI] [PubMed] [Google Scholar]
- Jin Y.-H., Jeon E.-J., Li Q.-L., Lee Y.H., Choi J.-K., Kim W.-J., Lee K.-Y., Bae S.-C. Transforming growth factor-beta stimulates p300-dependent RUNX3 acetylation, which inhibits ubiquitination-mediated degradation. J. Biol. Chem. 2004;279:29409–29417. doi: 10.1074/jbc.M313120200. [DOI] [PubMed] [Google Scholar]
- Kaneko Y., Kohno T., Kakuki T., Takano K.-I., Ogasawara N., Miyata R., Kikuchi S., Konno T., Ohkuni T., Yajima R. The role of transcriptional factor p63 in regulation of epithelial barrier and ciliogenesis of human nasal epithelial cells. Sci. Rep. 2017;7:10935. doi: 10.1038/s41598-017-11481-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunker S., Chong M.M.W., Mantel P.-Y., Palomares O., Bassin C., Ziegler M., Rückert B., Meiler F., Akdis M., Littman D.R. Transcription factors RUNX1 and RUNX3 in the induction and suppressive function of Foxp3+ inducible regulatory T cells. J. Exp. Med. 2009;206:2701–2715. doi: 10.1084/jem.20090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel J.E., Maruyama T., Carpenter A.C., Xiong Y., Zamarron B.F., Hall B.E., Kulkarni A.B., Zhang P., Bosselut R., Chen W. Control of the development of CD8αα+ intestinal intraepithelial lymphocytes by TGF-β. Nat. Immunol. 2011;12:312–319. doi: 10.1038/ni.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisawa J., Sugiura Y., Wake T., Nagatake T., Suzuki H., Nagasawa R., Shikata S., Honda K., Hashimoto E., Suzuki Y. Mode of bioenergetic metabolism during B cell differentiation in the intestine determines the distinct requirement for vitamin B1. Cell Rep. 2015;13:122–131. doi: 10.1016/j.celrep.2015.08.063. [DOI] [PubMed] [Google Scholar]
- Lai J.C.Y., Wlodarska M., Liu D.J., Abraham N., Johnson P. CD45 regulates migration, proliferation, and progression of double negative 1 thymocytes. J. Immunol. 2010;185:2059–2070. doi: 10.4049/jimmunol.0902693. [DOI] [PubMed] [Google Scholar]
- Larkin J.R., Zhang F., Godfrey L., Molostvov G., Zehnder D., Rabbani N., Thornalley P.J. Glucose-induced down regulation of thiamine transporters in the kidney proximal tubular epithelium produces thiamine insufficiency in diabetes. PLoS One. 2012;7:e53175. doi: 10.1371/journal.pone.0053175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licona-Limón P., Alemán-Muench G., Chimal-Monroy J., Macías-Silva M., García-Zepeda E.A., Matzuk M.M., Fortoul T.I., Soldevila G. Activins and inhibins: novel regulators of thymocyte development. Biochem. Biophys. Res. Commun. 2009;381:229–235. doi: 10.1016/j.bbrc.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoli I., Venditti C.P. Disorders of branched chain amino acid metabolism. Transl Sci. Rare Dis. 2016;1:91–110. doi: 10.3233/TRD-160009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C.N., Proekt I., von Moltke J., Wells K.L., Rajpurkar A.R., Wang H., Rattay K., Khan I.S., Metzger T.C., Pollack J.L. Thymic tuft cells promote an IL-4-enriched medulla and shape thymocyte development. Nature. 2018;559:627–631. doi: 10.1038/s41586-018-0345-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell C.S., Bredenkamp N., Tetélin S., Jin X., Tischner C., Vaidya H., Sheridan J.M., Stenhouse F.H., Heussen R., Smith A.J.H. Foxn1 regulates lineage progression in cortical and medullary thymic epithelial cells but is dispensable for medullary sublineage divergence. PLoS Genet. 2011;7:e1002348. doi: 10.1371/journal.pgen.1002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill K.E., Bredenkamp N., Tischner C., Vaidya H.J., Stenhouse F.H., Peddie C.D., Nowell C.S., Gaskell T., Blackburn C.C. Foxn1 is dynamically regulated in thymic epithelial cells during embryogenesis and at the onset of thymic involution. PLoS One. 2016;11:e0151666. doi: 10.1371/journal.pone.0151666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M., Yin N., Chhangawala S., Xu K., Leslie C.S., Li M.O. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science. 2016;354:481–484. doi: 10.1126/science.aaf6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobezinsky L.A., Angelov G.S., Tai X., Jeurling S., Van Laethem F., Feigenbaum L., Park J.-H., Singer A. Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nat. Immunol. 2012;13:569–578. doi: 10.1038/ni.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis B.S., Rogoz A., Costa-Pinto F.A., Taniuchi I., Mucida D. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4+ T cell immunity. Nat. Immunol. 2013;14:271–280. doi: 10.1038/ni.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanvitale C.E., Kerr G., Chaikuad A., Ramel M.-C., Mohedas A.H., Reichert S., Wang Y., Triffitt J.T., Cuny G.D., Yu P.B. A new class of small molecule inhibitor of BMP signaling. PLoS One. 2013;8:e62721. doi: 10.1371/journal.pone.0062721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino W., Dardenne M., Velloso L.A., Dayse Silva-Barbosa S. The thymus is a common target in malnutrition and infection. Br. J. Nutr. 2007;98(Suppl 1):S11–S16. doi: 10.1017/S0007114507832880. [DOI] [PubMed] [Google Scholar]
- Saxena A., Desbois S., Carrié N., Lawand M., Mars L.T., Liblau R.S. Tc17 CD8+ T cells potentiate Th1-mediated autoimmune diabetes in a mouse model. J. Immunol. 2012;189:3140–3149. doi: 10.4049/jimmunol.1103111. [DOI] [PubMed] [Google Scholar]
- Shao D., Villet O., Zhang Z., Choi S.W., Yan J., Ritterhoff J., Gu H., Djukovic D., Christodoulou D., Kolwicz S.C. Glucose promotes cell growth by suppressing branched-chain amino acid degradation. Nat. Commun. 2018;9:2935. doi: 10.1038/s41467-018-05362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szondy Z., Sarang Z., Molnar P., Nemeth T., Piacentini M., Mastroberardino P.G., Falasca L., Aeschlimann D., Kovacs J., Kiss I. Transglutaminase 2-/- mice reveal a phagocytosis-associated crosstalk between macrophages and apoptotic cells. Proc. Natl. Acad. Sci. U S A. 2003;100:7812–7817. doi: 10.1073/pnas.0832466100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat. Rev. Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- Takahama Y., Letterio J.J., Suzuki H., Farr A.G., Singer A. Early progression of thymocytes along the CD4/CD8 developmental pathway is regulated by a subset of thymic epithelial cells expressing transforming growth factor beta. J. Exp. Med. 1994;179:1495–1506. doi: 10.1084/jem.179.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.-X., Shin J., Wang S., Gorentla B., Lin X., Gao J., Qiu Y.-R., Zhong X.-P. mTORC1 in thymic epithelial cells is critical for thymopoiesis, T-cell generation, and temporal control of γδT17 development and TCRγ/δ recombination. PLoS Biol. 2016;14:e1002370. doi: 10.1371/journal.pbio.1002370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland K., Niss K., Kotarsky K., Wu N.Y.H., White A.J., Jendholm J., Rivollier A., Izarzugaza J.M.G., Brunak S., Holländer G.A. Retinoic acid signaling in thymic epithelial cells regulates thymopoiesis. J. Immunol. 2018;201:524–532. doi: 10.4049/jimmunol.1800418. [DOI] [PubMed] [Google Scholar]
- Whitfield K.C., Bourassa M.W., Adamolekun B., Bergeron G., Bettendorff L., Brown K.H., Cox L., Fattal-Valevski A., Fischer P.R., Frank E.L. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann. N. Y. Acad. Sci. 2018;1430:3–43. doi: 10.1111/nyas.13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf E., Brenner O., Goldenberg D., Levanon D., Groner Y. Runx3 regulates dendritic epidermal T cell development. Dev. Biol. 2007;303:703–714. doi: 10.1016/j.ydbio.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Xiao Y.Q., Freire-de-Lima C.G., Schiemann W.P., Bratton D.L., Vandivier R.W., Henson P.M. Transcriptional and translational regulation of TGF-β production in response to apoptotic cells. J. Immunol. 2008;181:3575–3585. doi: 10.4049/jimmunol.181.5.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Blanco D.B., Chen X., Dash P., Neale G., Rosencrance C., Easton J., Chen W., Cheng C., Dhungana Y. Metabolic signaling directs the reciprocal lineage decisions of αβ and γδ T cells. Sci. Immunol. 2018;3:eaas9818. doi: 10.1126/sciimmunol.aas9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Yuan X., Liu Q., Zhang X., Pan X., Zang L., Xu L. BAPTA-AM, an intracellular calcium chelator, inhibits RANKL-induced bone marrow macrophages differentiation through MEK/ERK, p38 MAPK and Akt, but not JNK pathways. Cytokine. 2010;52:210–214. doi: 10.1016/j.cyto.2010.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data in this study are available from the corresponding author on request.