Summary
Epithelial-mesenchymal transition (EMT) is a critical process that occurs during the embryonic development, wound healing, organ fibrosis and the onset of malignancy. Emerging evidence suggests that the EMT is involved in the invasion and metastasis of cancers. The inflammatory reaction antecedent to fibrosis in the onset of oral submucous fibrosis (OSF) and the role of EMT in its malignant transformation indicates a hitherto unexplored involvement of EMT. This review focuses on the role of EMT markers which are regulators of the EMT mediated complex network of molecular mechanisms involved in the pathogenesis of OSF and OSCC. Further the gene enrichment analysis and pathway analysis supports the association of the upregulated and downregulated genes in various EMT regulating pathways.
Keywords: Oral submucous fibrosis (OSF), Epithelial-mesenchymal transition (EMT), Oral squamous cell carcinoma (OSCC), Type II EMT, Signaling pathways in EMT, Transcription factors in EMT, Functional enrichment
1. Introduction
Oral submucous fibrosis (OSF) is a chronic mucosal condition occurring predominantly among the Indians, possibly due to prolonged use of areca nut, leading to marked rigidity and inability to open the mouth [1]. The pathogenesis of OSF is not clearly understood, but there is compelling evidence to suggest that OSF is a result of collagen deregulation [2]. Therefore, an increase in collagen formation concomitant with reduced collagen degradation is one of the plausible explanations for the onset of this condition [2]. An alarming complication associated with OSF is the higher risk of transforming to oral squamous cell carcinoma (OSCC). It has been described that the pathological changes in the connective tissue of OSF are likely to affect the overlying epithelium and induce EMT [1].
OSCC is the most common type of oral cancer, which accounts for 3.8% of all the cancers and 3.6% of cancer deaths [3]. Despite its ease of access for diagnosis and treatment, the mortality rate remains high because of the increased risk of developing second primary malignancy, which is the leading cause of death in patients with head and neck cancer [4]. It is well-established that EMT contributes to the acquisition of invasive behavior, essential for metastasis and invasion [5].
Although the experimental evidence points to the association of EMT in the mechanism of metastasis, its specific role in human cancer needs to be further explored [6]. Identification of signature genes influencing EMT may unravel novel pathways, which are critical to the progression of oral cancer. These markers of EMT may prove to be efficient targets to control the further spread and improve the prognosis of OSCC. With this background, this review focuses on the mechanisms and signaling pathways that direct the change in the gene expression signatures inducing EMT in OSF and OSCC.
2. Epithelial to mesenchymal transition (EMT)
Epithelial to mesenchymal transition (EMT) is a biological process involving the transition of a polarized epithelial cell into a cell that has the characteristics of a mesenchymal phenotype [7]. EMT is crucial for developmental milestones such as gastrulation of the metazoans, neural crest formation, and heart morphogenesis [8]. EMT is shown to be elicited following chronic inflammation and during wound healing [9]. The role of EMT has increasingly gained significance as an essential process in fibrosis and carcinogenesis. Considering the involvement of EMT in various physiological and pathological mechanisms, three types of EMTs have been described [7].
Type 1 EMT is associated with implantation, embryo formation and organ development. This type of EMT generates mesenchymal cells that have the ability to undergo a further transition to form secondary epithelia [7]. Type II EMT is associated with inflammation related to wound healing, organ fibrosis and tissue regeneration [7]. Type III EMT is associated with tumor formation, progression and metastasis [7]. Although the molecular basis for all the three types of EMT remains the same, the type I EMT is a physiological process during organogenesis, which further undergoes MET (Mesenchymal to epithelial transition) to form the secondary epithelia, hence reversal in the expression of EMT inducing genes may be seen. Type II EMT presents with upregulation of ECM proteins and transcription factors bring about the phenotypic switch to induce fibrosis. Type III EMT exhibits change in the phenotype with the upregulation of EMT associated genes to promote tissue invasion and metastasis of cancer cells [10].
EMT is a process in which there is a reduced expression of epithelial genes (E-cadherin) and an increase in the expression of mesenchymal genes (N-cadherin) and EMT transcription factors. Together with an altered localization of the β-catenin, the epithelial cells lose their phenotype and intercellular adhesions [11]. Besides, there is an increased expression of Vimentin [12] signifying a mesenchymal change in the cytoskeleton. An increase in Tenascin [13] implies that the matrix deposition enables the migration of cells. Significantly, the matrix metalloproteinase 9 (MMP9) [11] overexpression demonstrates the disruption of the basement membrane and the proneness of cells to infiltrate the underlying stroma [14,15].
The inflammatory reaction antecedent to fibrosis and the role of EMT in fibrogenesis and malignant transformation in other organs [[16], [17], [18]], points to the involvement of EMT in the pathogenesis of OSF and its malignant transformation [1,9]. The inflammatory cytokines produced in response to the inflammation may mediate the progression of OSF via various EMT pathways [19]. The membranous loss of E-cadherin [20], β- catenin [11], Cytokeratin 5(CK 5), and Cytokeratin 14(CK14) [21] with an overwhelming expression of vimentin [22], N-cadherin [11], and α- Smooth muscle actin (α- SMA) [23,24] seen in OSF further confirms the role of EMT in OSF (Table 1).
Table 1.
Molecular events regulating EMT in the pathogenesis of OSF and OSCC.
| OSF | OSCC | |
|---|---|---|
| Epithelial markers | • ↓ E –cadherin (membranous loss) (CDH1) [20] | • ↓ E –cadherin (membranous loss) (CDH1) [43,139] |
| • ↓ Cytokeratin 5, 14 (CK5, CK14) (KRT 5, 14) [21] | • ↑ Cytokeratin 5, 14 (CK5, CK14) (KRT 5, 14) [140] | |
| Mesenchymal markers | • ↑Vimentin (VIM) [22] | • ↑ Vimentin (VIM) [69] |
| • ↑N- cadherin (CDH2) [11] | • ↑ N- Cadherin (CDH2) [139] | |
| • ↑Alpha Smooth muscle Actin (α-SMA)(ACTA2) [42] | • ↑Alpha Smooth muscle Actin (α-SMA)(ACTA2) [141] | |
| Transcription factors | • ↑ SNAI-1 [142] | • ↑ SNAI-1 [143], SNAI-2 [144] |
| • ↑ Twist [11,37] | • ↑ Twist [41] | |
| • ↑ ZEB1 [23] | • ↑ ZEB1 [43] | |
| • ↑ LEF-1 [48] |
3. Transcription factors inducing EMT
Several transcription factors have been shown to induce EMT by repressing the transcription of cell adhesion molecules and driving epithelial cell reprogramming. These transcription factors bind to the promoter region of the CDH1 gene encoding E- cadherin and thus initiate EMT [5]. An important attribute of EMT is the loss of expression of cell-cell adhesion molecule, E-cadherin. Among the transcription factors directly contributing to this process includes snail super family of zinc-finger transcription factors, Snail1 and Snail2 (also known as Slug) [25], zinc finger E-box–binding homeobox (ZEB) family with the ZF (zinc finger) class of homeodomain transcription factors ZEB1 [26], ZEB2 [27] and TWIST1 gene, which encodes a basic helix-loop-helix (bHLH) transcription factor [28] and lymphoid enhancer binding factor-1 (LEF-1) [29] (Table 1).
The Snail1 and Snail2 (Slug) belong to the snail superfamily consisting of highly conserved C terminal domain with zinc fingers that bind to the E-box motif in the target gene promoters [25]. The transcription factors of the Snail family plays an important role in EMT through the functional inhibition and suppression of E-cadherin expression by binding to the promoter region of the E-cadherin gene [30]. However, in the fibrotic buccal mucosa fibroblasts, snail binds to the E-box in the α-SMA promoter and brings about upregulation of myofibroblasts expression, thus perpetuating fibrosis [31]. Stromal Snail positivity has been reported in OSCC due to the presence of fibro-/myofibroblasts generated from the dedifferentiated carcinoma cells [32]. An inverse correlation between the E-cadherin and snail expression has been observed in OSCC cells in vitro [33]. The cells lines that exhibit upregulated Snail expression along with the downregulation of E-cadherin and desmoglein 2 show higher invasive potential implicating the role of Snail transcription factor in driving the epithelial cell reprogramming [33,34]. TGF-β was shown to upregulate Snail (SNAI1) and Slug (SNAI2) expression in OSCCs and thereby promote chemo- resistance to anti-cancer drugs [35]. The upregulation of both Snail and Slug in OSCC cells showed decreased sensitivity to anti-cancer drugs. Hence knock down of Slug and Snail would suppress its chemo-resistance to anti-cancer drugs [35].
Twist, a basic helix-loop-helix domain-containing transcription factor functions as a transcription repressor to activate EMT [36,37]. Ectopic expression of Twist has resulted in the loss of E-cadherin mediated cell-cell adhesion, activation of mesenchymal markers, and gain of cell motility [36]. Twist was shown to be upregulated in fibroblasts of lung tissue in idiopathic pulmonary fibrosis patients [38]. Further, the arecoline treated cells show enhanced expression of Twist and myofibroblast transdifferentiation, with the silencing of Twist being able to reverse this phenomenon [37]. The role of Twist playing a critical role in the progression and metastasis of head and neck carcinomas [39], including OSCC, has been demonstrated [40,41]. Twist overexpression has been associated with clinical outcomes such as advanced clinical stage, presence of lymph node metastasis, distant metastasis and local recurrence [39].
Zinc finger E-box binding homeobox 1 (ZEB1) is a well-known activator of the EMT programme [23]. ZEB1 functions as a transcription repressor that negatively regulates the expression of polarity markers, such as E-cadherin, MucI and Pkp3 [32]. ZEB1 plays a pathogenic role in the induction of the myofibroblast activity of buccal mucosal fibroblasts (BMFs) by binding to the promoter region of α-SMA and hence inducing myofibroblasts transdifferentiation and promoting fibrosis [42]. In OSCC, a negative correlation exists between ZEB1 and E- cadherin expression [43]. Overexpression of ZEB1 and loss of E-cadherin expression is shown to be associated with local recurrence, lymph node metastasis and advanced pathological grading [43]. ZEB-1 promotes EMT by interacting with acetyltransferases p300/pCAF and SMADs to activate the target genes that contribute to mesenchymal differentiation [44]. Upregulated of ZEB1 has been noted in recurrent OSCC cases compared to primary lesions, indicating its role as marker of tumor recurrence [45].
Lymphoid enhancer- binding factor 1 (LEF1), a member of the T-cell Factor (TCF)/LEF1 family of transcription factors, is a downstream mediator of the Wnt/β-catenin signaling pathway that promotes the transcription of the Wnt target genes [46]. It has an essential role in EMT by activating the transcription of N-cadherin, Vimentin and Snail [46]. During embryogenesis, EMT is executed by the binding of SMAD2-P-SMAD4-LEF1 complex to three binding regions in the E- cadherin promoter leading to its transcriptional silencing [47]. Activation of the Wnt-β catenin pathway promotes the transcription of downstream target genes such as c-myc, LBH, Oct4, Nanog and LEF1 in various carcinomas [46]. In OSCC, LEF1 is over expressed in the moderate and poorly differentiated carcinomas [48]. Overexpressed LEF1 maintains the cancer cells in undifferentiated embryonic stem cell morphology [29]. This may be the mechanism by which LEF1 promotes tumor invasion and its overexpression is associated with poor prognosis [48].
4. Signaling pathways in EMT
4.1. TGF-β activated Smad signaling in EMT
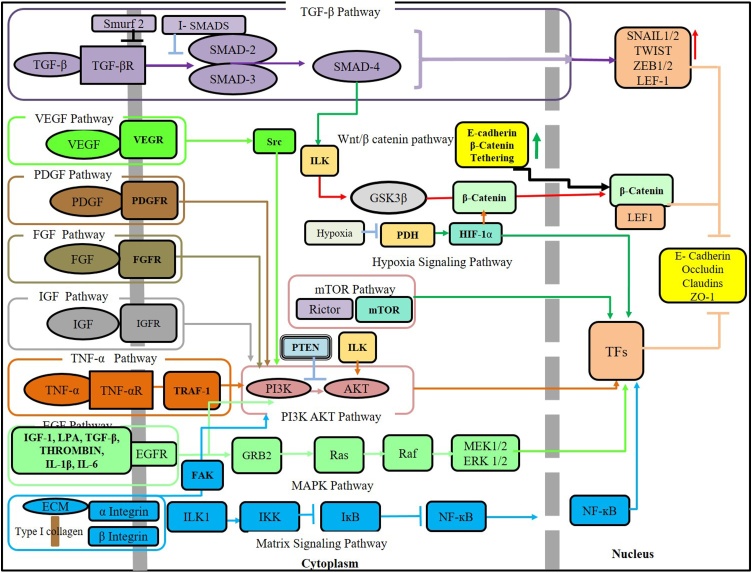
The canonical signaling pathway for TGF-β involves the Smad transcription activators. TGF-β pathway is the most common pathway that induces EMT. The signaling pathway is activated by TGF-β superfamily of ligands, which includes the 3 isoforms for TGF β and 6 isoforms of Bone morphogenetic protein (BMP2−7) [5]. Binding of TGF-β to the cell membrane receptor TGF βR, activates type II TGF-βR to Trans phosphorylate and activate the type I TGF-βR. This recruits the receptor-activated Smads, the R- Smads (Smad2/3), which are phosphorylated to form a complex with Smad4. In the BMP signaling, Smad 1/Smad 5 form complex with Smad4. This trimeric Smad complex translocates into the nucleus and bind to the promoter region of the target genes and thus activates or represses the transcription of regulatory genes [49].
The inhibitory Smads (I-Smads), Smad6 and Smad7 are negative regulators. They negatively regulate the Smad activation by competing with Smad2 and Smad3 or Smad1 and Smad5 for binding to the type I TGF-βR [50]. I- Smads also recruits the E3 ubiquitin-protein ligases Smurf1 and Smurf2 for proteasomal degradation of Smad proteins. It acts by forming complexes with smurfs in the nucleus, translocates to the plasma membrane and induces ubiquitination and proteasomal degradation of the TGFβ receptors hence terminating Smad-mediated signaling [51] (Fig. 1).
Figure 1.
Signaling pathways promoting epithelial mesenchymal transition (EMT) in oral submucous fibrosis.
TGF-β induces EMT through transcription factors like Snail1 and ZEB1. The downstream mediator Smad3-Smad4 complex translocates into the nucleus and interacts with the transcriptional repressor Snail1 to form a complex which then targets the promoters of genes encoding E-cadherin and Occludin [52]. Several feedback loops between transcription factors and microRNAs also regulate the TGF-β induced EMT. A Double-negative feedback loop exists between Snail1/miR-34 and ZEB1/miR-200 and an autocrine feedback loop between TGF- β /miR-200, which brings about EMT changes [53].
TGF–β is upregulated in OSF tissues [[54], [55], [56]] and its activation is shown by the nuclear localization of p-SMAD2 [57]. The extracts of areca nut induce TGF-β signaling in epithelial cells with increased levels of p-SMAD2, indicating the induction of TGF-β ligand (TGF-β2) and its activator Thrombospondin1 (THBS-1) leading to activation of TGF-β pathway [58]. Thus there is a pro fibrotic cascade involving TGF- β pathway triggered in epithelium that influences the underlying submucosa for a fibrotic response [59]. Also there is down regulation of BMP7 in OSF as induced by TGF-β, suppressing the antifibrotic effect of BMP7 [57]. In OSCCs, BMP 7, 2 [60] expression is associated with the tumor differentiation and lymph node metastasis and hence indicative of poor prognosis [60].
Defective TGF-β Smad signaling pathway may lead to loss of proliferation inhibitory effect of TGF-β. Loss or decreased expression of the TGF-β receptors effect the regulatory function of TGF-β, hence is associated with carcinogenesis and tumor progression [61]. TGF-β treated OSCC cell lines showed EMT changes characterized by transformation to fibroblasts like cells with downregulation of E -cadherin and upregulation of Vimentin [62]. TGF-β also induces THBS-1 in OSCC, which promotes the migration of cancer cells and upregulates the MMPs thereby favoring the OSCC invasion [60] (Table 2).
Table 2.
Signaling pathways regulating epithelial mesenchymal transition (EMT) in Oral submucous fibrosis (OSF) and oral squamous cell carcinoma (OSCC).
| Pathways | OSF | OSCC |
|---|---|---|
| TGF-β signaling | ↑ Transforming growth factor β1 & β2 [59,145] | ↑ Transforming growth factor β1 & β2 (TGFB1 & TGFB2) [150,151] |
| ↑ Collagen type 1(COL1A1) [113,146] | ↑ Collagen type 1, 4 (COL1A1, COL4A1) [127] | |
| ↑ Type I plasminogen activator inhibitor (PAI-1) [147] | ↑ Type I plasminogen activator inhibitor (PAI-1) [152] | |
| ↑ Thrombospondin1 (THBS1) [148] | ↑ Thrombospondin1 (THBS1) [151] | |
| ↓ Bone Morphogenetic protein 7 (BMP7) [57] | ↑ Bone Morphogenetic protein 7, 2 (BMP7, 2) [153] | |
| ↑ SMAD2 [149] | ↓ SMAD7 [154] | |
| RTK signaling | ↑ Insulin-like growth factor-1 (IGF1) [94] | ↑ Insulin-like growth factor-1 (IGF1) [97] |
| ↑ Vascular endothelial growth factor (VEGF) [155] | ↑ Vascular endothelial growth factor (VEGF) [102] | |
| ↑ Basic fibroblast growth factor (bFGF) [86] | ↑ Basic fibroblast growth factor (bFGF) [89] | |
| ↑ Fibroblast growth factors 2 (FGF2) [156] | ↑ Fibroblast growth factors 2(FGF2) [156] | |
| ↑ Epidermal growth factor (EGF) [90] | ↑ Epidermal growth factor (EGF) [93,158] | |
| ↑ Tumor necrosis factor α (TNF-α)(TNFa) [157] | ↑ Tumor necrosis factor α (TNF-α) | |
| (TNFa) [159] | ||
| Hypoxia signaling pathway | ↑ Hypoxia-inducible factor-1α (HIF-1α) [133] | ↑ Hypoxia-inducible factor-1α (HIF-1α) [135] |
| Wnt signaling | ↓ Secreted frizzled related proteins 1, 5 (SFRP1,5) [160] | ↓Secreted frizzled related proteins 1,4, 5 (SFRP1, SFRP4, SFRP5) [110] |
| ↓ -β - catenin (membranous loss) (CTNNB1) [11] | ↓ -β - catenin (membranous loss) (CTNNB1) [161] | |
| ↓ Wnt Inhibitory factor (WIF1) [107] | ↓ Wnt Inhibitory factor (WIF1) [108] | |
| ↓Dickkopf WNT signaling pathway inhibitor 3 (DKK3) [105,106] | ↓ Dickkopf WNT signaling pathway inhibitor 3 (DKK3) [108] | |
| MAPk/ERK pathway | ↑c-myc (MYC) [80] | ↑ c-myc (MYC) [110] |
| Akt/mTOR pathway | ↓ Phosphatase and tensin homolog (PTEN) [72] | ↓ Phosphatase and tensin homolog (PTEN) [162,163] |
| ↑ PHLPP2 [[68]] | ||
| Matrix signaling pathway | ↑ αvβ6 Integrin (ITGB6) [125] | ↑ αvβ6 Integrin (ITGB6) [127] |
| ↑ β1 integrin (ITGB1) [126] | ↑ β1 integrin (ITGB1) [126] | |
| ↓ Matrix Metallopeptidase 1 (MMP1) [114,164] | ↑ Matrix Metallopeptidase 1, 2, 7, 9 (MMP1, MMP2, MMP7, MMP9) [116] | |
| ↑ Matrix Metallopeptidase 2, 9 (MMP2, MMP9) [113] | ↑ Nuclear factor-kappa B (NF-κB) (NFKB1) [165] | |
| ↑ Nuclear factor-kappa B (NF-κB) (NFKB1) [128] |
4.2. Non-Smad signaling in TGF-β-induced EMT
TGF-β induces EMT alternatively by initiating Non-Smad signaling, which leads to the activation of pathways that are more commonly considered as the effectors pathways of receptor tyrosine kinase (RTK) signaling, such as PI3K/Akt, Erk, and p38 (Mitogen-activated protein kinase) MAPK, and Rho-GTPases pathways [63]. Activation of non -Smad pathways can occur as an indirect response to Smad -mediated gene expression induced by TGF β. Direct activation of non- Smad signaling can occur through the interaction of signaling mediators directly with the TGF-β receptors or through other adopter proteins [50].
4.3. PI3 kinase/Akt /mTOR signaling in EMT
Activation of PI3 kinase/Akt signaling by TGF-β plays a significant role in inducing EMT. TGF-β activates phosphoinositide 3-kinase (PI3K) through its receptors or trans-activation through epidermal growth factor (EGF) and platelet –derived growth factor (PDGF) receptors. PI3K on activation phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5- trisphosphate (PIP3), a phospholipid membrane protein that binds Akt. Upon binding, Akt is phosphorylated and activated by phosphoinositide- dependent kinase 1 (PDK1). Phosphatase and tensin homolog (PTEN) facilitates the dephosphorylation of PIP3 [5]. Loss of function and mutation of PTEN is observed in various carcinomas [64]. Integrin-linked kinase (ILK) on activation may alternatively mediate the phosphorylation of Akt through integrins. Akt induces EMT in SCCs by promoting the transcription of snail through nuclear factor-kB (NF-kB) [65]. TGF-β brings about the change in cell size and protein content during EMT by activation of mammalian target of rapamycin complex 1 (mTORC1) and mTORC2 through Akt to bring about cell migration and invasion [66,67] (Fig. 1).
Arecoline, the major active ingredient in the betel nut is involved in the pathogenesis of OSF. Downregulation of Akt/mTOR pathway in HacaT cells is seen on treatment with arecoline, executed through suppression in phosphorylation of AKT, mTOR and eukaryotic initiation factor 4E–binding protein 1(4E-BP1) [68]. The arecoline induced downregulation of the Akt/mTOR pathway is mediated through the upregulation of PH domain Leucine-rich repeat Protein Phosphatase 2 (PHLPP2), an upstream target of Akt. siRNA-mediated knockdown of PHLPP2 recovered the phosphorylation state of Akt, as well as attenuated the effect of arecoline on cell viability [68]. Inverse correlation between p-Akt and E-cadherin expression is observed in OSCC. Akt activation represses E-cadherin gene transcription by upregulation of the transcription repressors SNAIL, TWIST [41] and Smad interacting protein 1 (SIP1) [69]. Akt is associated with invasiveness, enhancement of proliferation, growth and anti-apoptosis, hence upregulation of Akt was associated with poor prognosis in patient with OSCC [70]. Upregulation of Akt and PI3K with inactivation of PTEN is reported to be induced by tobacco components such as nicotine [71]. Progressive decrease in expression of PTEN is observed in OSF, suggesting TGF-β mediated loss of PTEN that results in decreased apoptosis, increased survival of fibroblast leading to fibrosis [72]. The genes associated with the PI3K/AKT pathway, including PI3K, Akt, RAS and PTEN, are infrequently found to be mutated in Head and neck squamous cell carcinoma (HNSCC) and are rarely reported in OSCC cases [73] (Table 2).
4.4. MAPK/ERK pathway in EMT
MAP kinases represent the cytoplasmic components of the signaling pathway that are activated by tyrosine kinases and the G protein-coupled receptors. The activation of the MAPK pathway by the family of TGF-β proteins are weaker than those induced by the receptor tyrosine kinase (RTK) ligands. Erk1/2 MAPK signaling is activated by TGF-β through the association of ShcA (Src homology and collagen A adaptor protein A) with TGF-βRI and subsequent phosphorylation at tyrosine and serine, which provides a docking site for the growth factor receptor-bound protein 2 (Grb2) and the son of sevenless (SOS) proteins hence initiating the MAPK pathway [49,74]. The ShcA/ Grb2/SOS complex converts G protein, such as Ras into its active GTP-bound form, which binds Raf kinase. The MAP kinase pathway is composed of three consecutive kinases (MAPKKK, MAPKK, and MAPK) leading to its phosphorylation to MEK (MAPKK), which on further phosphorylation forms MAPK (ERK). MAPK now functions as an enzyme and translocates into the nucleus to bring about phosphorylation and activation of various transcription factors to induce EMT [75,76]. The TGFβ induced pathway stabilizes SNAIL1 by inhibiting GSK3β, thus increasing SNAIL1 activity, and repression of E- cadherin [49] (Fig. 1).
Areca nut and arecoline induce the activation of the ERK/JNK/p38 MAPK pathways, through phosphorylation of p-Akt, p-ERK and p-p38 and activation of these pathways play an important role in OSF and OSCC [77,78]. These pathways regulate the expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) to promote wound healing and fibrosis [77]. Upregulation of the downstream targets like c-myc has also been reported in OSF, where the expression of c-myc may be correlated with the progressive cellular transformation in these precancerous conditions [79,80]. Induction of Ras/ERK pathways by EGF reduces the interferon-α mediated apoptosis of epidermoid carcinoma cells, indicating the survival of DNA damaged cells via this pathway [81].
Mutation in Ras or Raf oncogenes leads to the activation of ERK1/ERK2 pathways in many cancers [82]. But there have been discrepancies pertaining to ERK1/ERK2 expression in oral cancer and HNSCC [83], however, this may be due to the differentiation stage of the tumor, where poorly differentiated tumors present with decreased phosphorylation of ERK leading to increase in cell proliferation and cancer progression [84].
4.5. Receptor tyrosine kinase (RTK) signaling in EMT
RTK signaling pathway can be activated by various growth factors such as epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF) and insulin-like growth factor (IGF). These growth factors bind to the external domain of RTK, inducing the dimerization and subsequent auto phosphorylation of the tyrosine residue in the receptor. Hence activating the downstream signaling pathways such as PI3K/Akt/mTOR and ERK/MAPK pathway [5,49] (Fig. 1).
Basic Fibroblast growth factor-2 (bFGF-2) induces EMT by decreasing the expression of cytokeratin and E- cadherin and inducing the expression of vimentin, FSP-1 and αSMA [85]. bFGF is upregulated in the early stages of OSF [86], with increased expression in fibroblasts and endothelial cells. The expression in fibroblasts may be due to the heparan sulfate, which shows enrichment of bFGF-binding domains in fibrotic lesions, and these regions may play an important role in the fibrogenesis through their interaction with endogenous bFGF [87]. The increased bFGF expressivity in endothelial cells along with fibroblasts may potentiate the leukocyte recruitment to inflammation by enhancing endothelial adhesion molecule expression [88]. OSCCs showed the increased intensity of bFGF staining in the invasive fonts indicating the role of cancer cells in producing the bFGF. However, the expression was regardless of its clinical characteristics. bFGF promotes the production of proteinases and enhances the invasive capabilities of the cancer cells [89].
While increased expression of EGFR was noted in the stratum spinosum of the epithelium, TGF-α was restricted to stratum germinativum, indicating an upregulation of TGF-α initially and then exerting a paracrine effect of the non-proliferative cells to increase the expression of cell surface receptor [80]. There was an upregulation of both the TGF- α and EGF in the precancerous lesions like OSF and oral leukoplakia, seen with the increase in the degree of dysplasia, implying the activation of RTK pathways and activation of oncogenes such as c-fos and c-myc subsequently [90]. Areca nut extract (ANE) induces activation of RTK signaling by activating the upstream epidermal growth factor receptor (EGFR), Src and Ras signaling pathways [91]. Increased expression of EGFR has been reported in OSCC [92] and is usually associated with poor prognosis and outcome [93].
IGF-1 is a profibrogenic growth factor which is overexpressed in fibroblasts derived from OSF [94] similar to that seen in lung fibrosis [95] and systemic sclerosis [96]. This induces excessive production of collagen and ECM in OSF [94]. The OSCC cell lines express high levels of IGF-2 and IGF-1R, while the normal mucosa expresses IGF-1. Thus IGF-2 has a significant role in controlling the proliferation of oral carcinoma cells [97]. Studies on head and neck cancer have shown no alteration in the level of expression of IGF2, E2F3 and IGF1 but there is an evidence of the upregulation of pro-apoptotic IGF1 binding protein 3 (IGFBP3) [98]. IGFBP3 regulates the IGF1 by blocking its anti-apoptotic function and increasing its half –life. IGFBP3 may also effect activation of IGF1 signaling in these carcinomas [98] (Table 2).
Angiogenesis is an important phenomenon in precancerous conditions as it favors the nutrition and growth of the dysplastic cells, usually initiated through angiogenic stimulants such as Vascular endothelial growth factor (VEGF) [99]. Neovascularization induced by VEGF is important for tumor growth and metastasis [99]. OSF demonstrated increased expression of VEGF, indicative of hypoxia-induced angiogenesis in fibrous connective tissue stroma [100]. VEGF expression by epithelial cells in OSF may promote growth via an autocrine proliferative effect on the atrophic epithelium supporting its survival and potential to undergo malignant change [100]. In OSCCs, higher co-localisation of VEGF was seen in tumoral blood microvessels in the invasive fronts, well few studies showed an association with tumor differentiation [101,102].
4.6. Wnt signaling in EMT
Wnt signals are transduced through the binding of Wnt proteins to the extracellular domain of Fizzled (Fz) protein, in the presence of co-factors such as low-density-lipoprotein-related protein5/6 (LRP5/6) which is required to mediate the canonical Wnt signal [103]. In the absence of signaling, β- catenin is degraded by the β-catenin destruction complex, which includes Axin, tumor suppressor adenomatosis polyposis coli (APC), glycogen synthase kinase 3β (GSK- 3β) and casein kinase 1α (CK1α) [5,103]. Phosphorylation of β-catenin by this complex drives it for ubiquitination and subsequent proteolytic degradation [103]. In case of Wnt signaling, the binding of Wnt protein to the receptor complex will result in the phosphorylation of LRP5/6 by glycogen synthase kinase 3β and recruitment of cytoplsmic phosphoprotein Dishevelled (Dsh/Dvl) and Axin, which prevents the formation destruction complex unable to phosphorylate β-catenin thereby leading to its accumulation in the cytoplasm and translocation into the nucleus. The nuclear β-catenin interacts with transcription factors T-cell factor/lymphocyte enhancer factor (TCF/LEF) and inhibits the transcription of E -cadherin to bring about EMT [5] (Fig. 1).
A group of secreted Wnt antagonists has been implicated in the regulation of the Wnt/β-catenin-signaling pathway, including Wnt inhibitory factor 1, secreted frizzled-related protein (SFRP), and the Dickkopf families [104]. The expression of SERP1 and SERP5 was seen to reduce in OSF undergoing malignant change and was associated with the loss of membranous β- catenin expression. The loss of SERP1 and SERP 5 expression was due to promoter methylation [104]. Dickkopf WNT signaling pathway inhibitor 3 (DKK3) showed upregulation in OSF progressing to OSCC and a rare mutation of DKK3 was observed in OSCC, along with increased copy numbers [105]. However in OSF there was decrease in DKK3 expression seen with further decline with progression in disease [106]. The expression of Wnt Inhibitory factor (WIF1), an antagonist of the Wnt signaling is downregulated in OSF and OSCC due to methylation [107]. WIF1 methylation is associated with a poorer prognosis in OSCC patients [108]. WIF1 is considered a potential epigenetic biomarker indicative of early changes in OSCC [107]. 4-Nitroquinoline carcinogen used to generate premalignant lesions and OSCC in Axin2-CreER; YFP mice. Tamoxifen was applied to induce Cre activity, which leads to the labeling of cancer-initiating cells (CICs). Immunohistochemical studies revealed co-expression of βcatenin and LEF1 in OSCC, suggesting activation of Wnt/2-catenin signaling. Increased Axin2 fluorescence was visualized in basal cells in OSCC, thus being able to confirm that Wnt-responsive CICs in OSCC contribute its malignant progression [109].
The downstream signaling molecules such as Wnt3a, β‑catenin, secreted frizzled‑related proteins sFRP‑1, sFRP‑2, sFRP‑4, sFRP‑5, Wnt inhibitory factor 1, dickkopf‑1, c‑myc, and cyclin‑D1 studied in OSCC did not show significant expression except for c-myc [110]. Aberrant expression of β-catenin in OSCC was considered not to be due to mutation or epigenetic changes but due to focal or transient expression of β-catenin in OSCC due to various underlying mechanisms [111] (Table 2).
4.7. Matrix signaling in EMT
The signaling pathways initiated in ECM is the result of its interaction with epithelium and it may facilitate the motility of the cells exhibiting migratory phenotype in the connective tissue through the remodeling of matrix [5]. EMT signaling pathways induces various MMPs such as MMP-2 and MMP-9 which cleaves the type IV collagen in the basal lamina and also has a role in weakening the adherens junctions of the epithelial cells and thereby promoting the phenotypic change and tumor invasion [112]. In OSF, the upregulation of MMP-2 and MMP-9 has resulted in the decrease in type IV collagen on the progression of the disease, however the subsequent accumulation of type I collagen was evident [113]. Further, the overexpression of MMP9 may result in complete destruction of the basement membrane (BM) due to degradation of collagen type-IV, which may stimulate the OSF towards malignancy [113]. However, the expression of MMP1 was decreased in OSF, favoring the condition of fibrosis [114]. In OSCC, upregulation of MMP1, MMP2, MMP7 and MMP9 is seen, owing to their role in the degradation of BM and ECM and association with the clinical outcome such as regional lymph node and/or distant metastasis [115,116] (Table 2).
Integrin binding to ECM proteins will activate intercellular cascades that induce EMT. The importance of type I collagen in matrix signaling inducing metastasis has been reported in carcinomas of lungs, breast and esophagus [117,118]. Binding of type I collagen to integrin activates intercellular cascades causing phosphorylation of IkB (inhibitor of kB) in an ILK- dependent manner. This, in turn increases the nuclear translocation of active NF-κB, which promotes the expression of SNAI1 and LEF1 to induce EMT [119]. Similarly, the binding of type I collagen to Discoidin domain receptor 1/2 (DDR1/2) increases NF-κB and activation of transcription factor LEF-1 to initiate EMT [120]. Abundance of collagen activates JNK pathways [121] and also canonical and noncanonical TGF-β signaling pathways [122] (Fig. 1).
ECM remodeling is noted in OSF and significant alteration in the expression of ECM molecules is demonstrable as the disease progresses. With the progression of OSF from early to advanced stages, Type III collagen and type IV [123] are replaced with type I collagen leading to the accumulation of type I collagen in the connective tissue [124]. Upregulation of vβ6 in oral keratinocytes induced by arecoline promotes the invasive and migratory characteristics. 80% of the OSF transformed to OSCC shows a higher αvβ6 expression suggestive of an underlying EMT change promoting malignant transformation in OSF [125]. Arecoline induces the upregulation of αvβ6, promoting the trans-differentiation of oral fibroblasts into myofibroblasts [125]. β1 integrin is has also shown to be upregulated in OSF [126]. Activation of integrin by type I collagen to induce EMT changes in OSF needs to be studied further. The binding of integrin αvβ8 with type Ⅰ collagen activates the downstream MEK/ERK signaling pathway, thereby facilitating the proliferation and invasion of OSCC cells in vivo [127]. The expression of αvβ6 essentially has a role in cell proliferation, adhesion and migration in the progression of OSCC [127]. The downstream NF-κB is upregulated in epithelial cells, fibroblasts and inflammatory cells in OSF. NF-κB overexpression is associated with persistent chronic inflammation indicating the role of inflammation in inducing fibrosis [128]. The invasive and metastatic potential of OSCC cells is enhanced by Tumor necrosis factor α (TNFα) via the NF-κB signaling pathway. NF-κB regulates the expression of MMPs especially MMP9 which degrades the ECM to enhance the tumor invasion [129].
4.8. Hypoxia signaling in EMT
Fibrosis and cancerous tissues are triggered by hypoxia resulting in a phenotypic change promoting EMT [5]. Hypoxia-inducible factor-1α (HIF-1α) is one such transcription factor that regulates oxygen homeostasis. Normally, it undergoes ubiquitination and subsequent proteasomal degradation with a short half-life of 5 min [130]. Under hypoxic condition it stabilizes and interacts with coactivators such as p300/CBP to modulate its transcriptional activity [130]. HIF-1α induces EMT by binding to the promoter region of ZEB1 [131], SNAIL1 [132] and hence increasing its trans activity and expression (Fig. 1).
OSF exhibits the upregulation of HIF-1α, which further progresses with dysplastic changes. Functional HIF-1α helps in cell survival and proliferation during the early stages of carcinogenesis under hypoxic conditions [133,134]. There is an increase in HIF-1α in OSCC which is attributed to the genetic changes in the tumor cells as well the tumor hypoxia which results in the stabilization of HIF-1α [134]. HIF-1α regulates various angiogenic-stimulating cytokines, growth factors and genes regulating angiogenesis [135]. (Table 2).
5. Gene enrichment analysis
Gene enrichment analysis to analyze the molecular functions, biological processes and cellular components of the upregulated and downregulated genes was performed with the g:Profiler [136]. Association of gene with disease and protein- protein interaction was identified by Metascape tool [137]. Pathway enrichment on wikiPathway cancer was performed by WebGestalt [137].
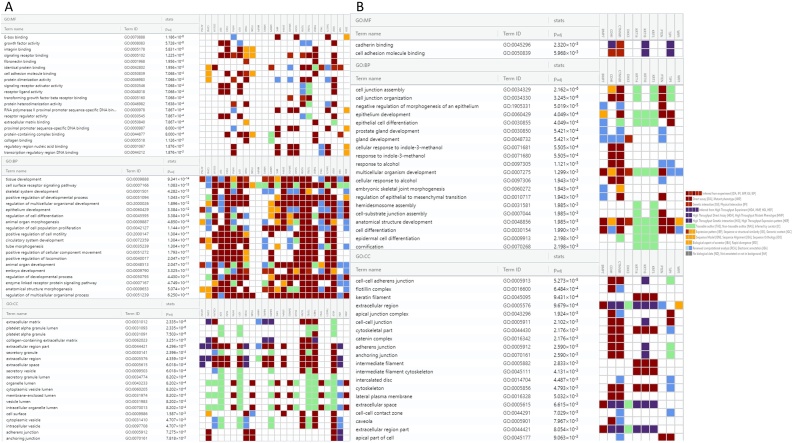
Gene enrichment and pathway analysis identified the upregulated genes to be involved in 47 molecular functions, 885 biological process, 24 cellular components significantly (FDR < 0.05 (Fig. 2A). The downregulated genes were involved in 2 Molecular functions, 56 Biological process, 24 cellular components significantly (FDR < 0.05) (Fig. 2B). Top 20 gene ontology features based on FDR is represented in Fig. 3. Gene ontology exposed that the genes were commonly involved in growth factor activity, signaling receptor binding, regulation of cell differentiation, cadherin binding and epithelial cell differentiation.
Figure 2.
Gene Ontology covering three domains showing top 20 biological processes, molecular functions and cellular components among (A) Upregulated gene sets (B) Downregulated gene sets.
Figure 3.
Gene Ontology and Protein–Protein Interactions showing (A) Bar graph of enriched Gene ontology for overexpressed list of genes (B) Bar graph of enriched Gene ontology for downregulated list of genes. (C) Protein–Protein Interaction of overexpressed genes, (D) Protein- Protein Interaction of downregulated genes.
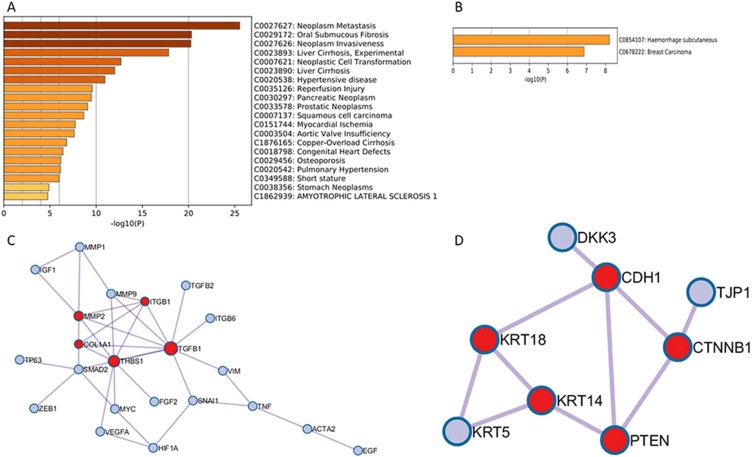
Evaluation of the association of differentially expressed genes with disease showed the involvement of upregulated genes with oral submucous fibrosis (OSF) and squamous cell carcinoma (SCC) (Fig. 3A). The genes are also seen to be associated with neoplasm metastasis, neoplasm invasiveness etc. Protein‑protein interaction (PPI) network was constructed with Molecular Complex Detection (MCODE) algorithm. For upregulated genes, the interaction of ITGB1, MMP2, COL1A1, THBS1, and TGFB1 was significant. (Indicated in red, Fig. 3C). In downregulated genes, CDH1, CTNNB1, KRT14, KRT18, PTEN were seen to be interacting significantly (Indicated in red, Fig. 3D). The pathway analysis revealed the participation of overexpressed genes in TGF-β signaling pathway and Epithelial-Mesenchymal Transition pathways. Downregulated genes were mainly involved in Wnt Signaling Pathway, DNA Damage Response and CDK-β catenin activity (Table 3).
Table 3.
The pathways involved in upregulated genes (A) downregulated genes (B).
| Description | P Value | FDR |
|---|---|---|
| (A) Pathways involved in upregulated genes | ||
| TGF-β Signaling in Thyroid Cells for Epithelial-Mesenchymal Transition | 0.0000010501 | 0.000078755 |
| Pathways in Bladder Cancer | 0.0000042588 | 0.00012569 |
| Epithelial to mesenchymal transition in colorectal cancer | 0.0000050276 | 0.00012569 |
| TGF-β Receptor Signaling | 0.00041368 | 0.0077565 |
| TGF- β Signaling Pathway | 0.00052635 | 0.0078953 |
| Angiogenesis | 0.0024025 | 0.030031 |
| Photodynamic therapy-induced NF-kB survival signaling | 0.0071459 | 0.072530 |
| Pathways in Type 2 papillary renal cell carcinoma | 0.0077366 | 0.072530 |
| Chromosomal and microsatellite instability in colorectal cancer | 0.0094417 | 0.078681 |
| Pathways in clear cell renal cell carcinoma | 0.016513 | 0.11709 |
| (B) Pathways involved in downregulated gene | ||
| Wnt Signaling Pathway | 0.049612 | 0.41343 |
| DNA Damage Response (only ATM dependent) | 0.047317 | 0.41343 |
| Epithelial to mesenchymal transition in colorectal cancer | 0.010704 | 0.16056 |
| LncRNA involvement in canonical Wnt signaling and colorectal cancer | 0.039286 | 0.41343 |
| ncRNAs involved in Wnt signaling in hepatocellular carcinoma | 0.028737 | 0.35921 |
| Pathways in Endometrial cancer | 0.00066794 | 0.031752 |
| TGF-β Signaling in Thyroid Cells for Epithelial-Mesenchymal Transition | 0.056162 | 0.42122 |
| Wnt/ β-catenin Signaling Pathway in Leukemia | 0.0026673 | 0.050012 |
| Regulation of Wnt/B-catenin Signaling by Small Molecule Compounds | 0.0012701 | 0.031752 |
| H19 action Rb-E2F1 signaling and CDK- β-catenin activity | 0.00099900 | 0.031752 |
Pathway enrichment performed with WebGestalt.
6. Conclusions
Transcription factors act synergistically to bring about the epithelial cell reprogramming. Regulation of these factors control the expression of critical genes and identification of the downstream targets. [5] Evidence suggests a cross talk between various signaling pathways and some studies suggest the inhibition of single transcription factor is enough to block EMT [138]. EMT has detrimental role in the progression of fibrosis and cancer metastasis. Poor prognosis clinical outcomes of oral cancer combined with the development of drug resistance makes it critical to identify suitable targets to prevent the induction of EMT. A thorough understanding of signaling pathways involved in EMT and the tumor microenvironment in OSF and OSCC paves for newer therapeutic strategies. Whilst the systematic analysis of the association of genes with disease showed its involvement in OSF and SCC, pathway analysis showed the participation of upregulated and downregulated genes with various EMT regulating pathways.
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Funding
Science and Engineering Research Board (SERB) - EMR/2017/002792.
References
- 1.Ekanayaka R.P., Tilakaratne W.M. Oral submucous fibrosis: review on mechanisms of pathogenesis and malignant transformation oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;122:192–199. doi: 10.1016/j.oooo.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Rajalalitha P., Vali S. Molecular pathogenesis of oral submucous fibrosis—a collagen metabolic disorder. J Oral Pathol Med. 2005;34:321–328. doi: 10.1111/j.1600-0714.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 3.Shield K.D., Ferlay J., Jemal A., Sankaranarayanan R., Chaturvedi A.K., Bray F. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin. 2017;67:51–64. doi: 10.3322/caac.21384. [DOI] [PubMed] [Google Scholar]
- 4.Krisanaprakornkit S., Iamaroon A. Epithelial-mesenchymal transition in oral squamous cell carcinoma. ISRN Oncol. 2012;2012:10. doi: 10.5402/2012/681469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez D.M., Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7 doi: 10.1126/scisignal.2005189.Signaling. re8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zidar N., Boštjančič E., Malgaj M., Gale N., Dovšak T., Didanovič V. The role of epithelial-mesenchymal transition in squamous cell carcinoma of the oral cavity. Virchows Arch. 2018;472:237–245. doi: 10.1007/s00428-017-2192-1. [DOI] [PubMed] [Google Scholar]
- 7.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larue L., Bellacosa A. Epithelial – mesenchymal transition in development and cancer : role of phosphatidylinositol 3’ kinase / AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 9.Yanjia H., Xinchun J. The role of epithelial – mesenchymal transition in oral squamous cell carcinoma and oral submucous fibrosis. Clin Chim Acta. 2007;383:51–56. doi: 10.1016/j.cca.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Zeisberg M., Neilson E.G. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183.protected. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das R.K., Anura A., Pal M., Bag S., Majumdar S., Barui A. Epithelio-mesenchymal transitional attributes in oral sub-mucous fibrosis. Exp Mol Pathol. 2013;95:259–269. doi: 10.1016/j.yexmp.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Mendez M.G., Kojima S., Goldman R.D. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2016;24:1838–1851. doi: 10.1096/fj.09-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tak J., Rao N.N., Chandra A., Gupta N. Immunohistochemical analysis of tenascin expression in different grades of oral submucous fibrosis. J Oral Maxillofac Pathol. 2015;19:291–296. doi: 10.4103/0973-029X.174645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee K., Nelson C.M. 1st ed. vol. 294. Elsevier Inc.; 2012. (New insights into the regulation of epithelial – mesenchymal transition and tissue fibrosis). [DOI] [PubMed] [Google Scholar]
- 15.Scanlon C.S., Van Tubergen E.A., Inglehart R.C., D’Silva N.J. Biomarkers of epithelial- mesenchymal transition in squamous cell carcinoma. J Dent Res. 2013;92:114–121. doi: 10.1177/0022034512467352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kriz W., Kaissling B., Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest. 2011;121:468–474. doi: 10.1172/JCI44595.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyun K., Koo G., Han H., Sohn J., Choi W. Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget. 2016;7:24677–24687. doi: 10.18632/oncotarget.8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horowitz J.C., Thannickal V.J. Epithelial-mesenchymal interactions in pulmonary fibrosis. Semin Respir Crit Care Med. 2006;27:600–612. doi: 10.1055/s-2006-957332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma M., Shetty S.S., Radhakrishnan R. Oral submucous fibrosis as an overhealing wound: implications in malignant transformation. Recent Pat Anticancer Drug Discov. 2018;13 doi: 10.2174/1574892813666180227103147. [DOI] [PubMed] [Google Scholar]
- 20.Pal M., Ray A.K., Sengupta S., Paul R.R., Barui A., Chatterjee J. Assessment of malignant potential of oral submucous fibrosis through evaluation of p63, E-cadherin and CD105 expression. J Clin Pathol. 2010;63:894–899. doi: 10.1136/jcp.2010.078964. [DOI] [PubMed] [Google Scholar]
- 21.Ranganathan K., Kavitha R., Sawant S.S., Vaidya M.M. Cytokeratin expression in oral submucous fibrosis--an immunohistochemical study. J Oral Pathol Med. 2006;35:25–32. doi: 10.1111/j.1600-0714.2005.00366.x. [DOI] [PubMed] [Google Scholar]
- 22.Nayak M.T., Singh A., Desai R.S., Vanaki S.S. Immunohistochemical analysis of vimentin in oral submucous fibrosis. J Cancer Epidemiol. 2013;2013 doi: 10.1155/2013/549041. 6 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang Y.-C., Tsai C.-H., Lai Y.-L., Yu C.-C., Chi W.-Y., Li J.J. Arecoline-induced myofibroblast transdifferentiation from human buccal mucosal fibroblasts is mediated by ZEB1. J Cell Mol Med. 2014;18:698–708. doi: 10.1111/jcmm.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angadi P.V., Kale A.D., Hallikerimath S. Evaluation of myofibroblasts in oral submucous fibrosis: Correlation with disease severity. J Oral Pathol Med. 2011;40:208–213. doi: 10.1111/j.1600-0714.2010.00995.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., Shi J., Chai K., Ying X., Zhou B. The role of snail in EMT and tumorigenesis. Curr Cancer Drug Targets. 2013;13:963–972. doi: 10.2174/15680096113136660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y., El-Naggar S., Darling D.S., Higashi Y., Dean D.C. ZEB1 links epithelial-mesenchymal transition and cellular senescence. Development. 2008;7135:579–588. doi: 10.1002/ana.22528.Toll-like. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanrahan K., O’Neill A., Prencipe M., Bugler J., Murphy L., Fabre A. The role of epithelial-mesenchymal transition drivers ZEB1 and ZEB2 in mediating docetaxel-resistant prostate cancer. Mol Oncol. 2017;11:251–265. doi: 10.1002/1878-0261.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Liu J., Ying X., Lin P.C., Zhou B.P. Twist-mediated epithelial-mesenchymal transition promotes breast tumor cell invasion via inhibition of Hippo Pathway. Sci Rep. 2016;6:24606. doi: 10.1038/srep24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun L., Liu T., Zhang S., Guo K., Liu Y. Oct4 induces EMT through LEF1/β-catenin dependent WNT signaling pathway in hepatocellular carcinoma. Oncol Lett. 2017;13:2599–2606. doi: 10.3892/ol.2017.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batlle E., Sancho E., Francí C., Domínguez D., Monfar M., Baulida J. The transcription factor Snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 31.Yang H.-W., Lu M.-Y., Chiu Y.-W., Liao Y.-W., Huang Y.-F., Ju Chueh P. Hinokitiol ablates myofibroblast activation in precancerous oral submucous fibrosis by targeting Snail. Environ Toxicol. 2018;33:454–462. doi: 10.1002/tox.22531. [DOI] [PubMed] [Google Scholar]
- 32.Franz M., Spiegel K., Umbreit C., Richter P., Berndt A., Sven A.A. Expression of Snail is associated with myo W broblast phenotype development in oral squamous cell carcinoma. Histochem Cell Biol. 2009;131:651–660. doi: 10.1007/s00418-009-0559-3. [DOI] [PubMed] [Google Scholar]
- 33.Yokoyama K., Kamata N., Hayashi E., Hoteiya T., Ueda N., Fujimoto R. Reverse correlation of E-cadherin and snail expression in oral squamous cell carcinoma cells in vitro. Oral Oncol. 2001;37:65–71. doi: 10.1016/S1368-8375(00)00059-2. [DOI] [PubMed] [Google Scholar]
- 34.Kume K., Haraguchi M., Hijioka H., Ishida T., Miyawaki A., Nakamura N. The transcription factor Snail enhanced the degradation of E-cadherin and desmoglein 2 in oral squamous cell carcinoma cells. Biochem Biophys Res Commun. 2013;430:889–894. doi: 10.1016/j.bbrc.2012.12.060. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura R., Ishii H., Endo K., Hotta A., Fujii E., Miyazawa K. Reciprocal expression of slug and snail in human oral cancer cells. PLoS One. 2018;13:1–14. doi: 10.1371/journal.pone.0199442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J., Mani S.A., Weinberg R.A. Exploring a new twist on tumor metastasis. Cancer Res. 2006;66:4549–4552. doi: 10.1158/0008-5472.CAN-05-3850. [DOI] [PubMed] [Google Scholar]
- 37.Lee Y., Yang L.-C., Hu F.-W., Peng C.-Y., Yu C.-H., Yu C.-C. Elevation of Twist expression by arecoline contributes to the pathogenesis of oral submucous fibrosis. J Formos Med Assoc. 2016;115:311–317. doi: 10.1016/j.jfma.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Tan J., Tedrow J.R., Nouraie M., Dutta J.A., Miller D.T., Li X. Loss of Twist1 in the mesenchymal compartment promotes increased fibrosis in experimental lung injury by enhanced expression of CXCL12. J Immunol. 2017;198:2269–2285. doi: 10.4049/jimmunol.1600610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuo X., Luo H., Chang A., Li D., Zhao H., Zhou Q. Is overexpression of TWIST, a transcriptional factor, a prognostic biomarker of head and neck carcinoma? Evidence from fifteen studies. Sci Rep. 2015;5:18073. doi: 10.1038/srep18073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wushou A., Pan H.Y., Liu W., Tian Z., Wang L.Z., Shali S. Correlation of increased twist with lymph node metastasis in patients with oral squamous cell carcinoma. J Oral Maxillofac Surg. 2012;70:1473–1479. doi: 10.1016/j.joms.2011.06.212. [DOI] [PubMed] [Google Scholar]
- 41.de Freitas Silva B.S., Yamamoto F.P., Pontes F.S.C., Cury S.E.V., Fonseca F.P., Pontes H.A.R. TWIST and p-Akt immunoexpression in normal oral epithelium, oral dysplasia and in oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal. 2012;17:29–34. doi: 10.4317/medoral.17344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang Y.-C., Lin C.-W., Yu C.-C., Wang B.-Y., Huang Y., Hsieh Y. Resveratrol suppresses myofibroblast activity of human buccal mucosal fibroblasts through the epigenetic inhibition of ZEB1 expression. Oncotarget. 2016;7:12137–12149. doi: 10.18632/oncotarget.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao X., Sun S. Clinicopathological significance of ZEB-1 and E-cadherin proteins in patients with oral cavity squamous cell carcinoma. Onco Targets Ther. 2017;10:781–790. doi: 10.2147/OTT.S111920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmalhofer O., Brabletz S., Brabletz T. E-cadherin, β-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28:151–166. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 45.Ho C.M., Hu F.W., Lee S.S., Shieh T.M., Yu C.H., Lin S.S. ZEB1 as an indicator of tumor recurrence for areca quid chewing-associated oral squamous cell carcinomas. J Oral Pathol Med. 2015;44:693–698. doi: 10.1111/jop.12286. [DOI] [PubMed] [Google Scholar]
- 46.Santiago L., Daniels G., Wang D., Deng F.-M., Lee P. Wnt signaling pathway protein LEF1 in cancer, as a biomarker for prognosis and a target for treatment. Am J Cancer Res. 2017;7:1389–1406. [PMC free article] [PubMed] [Google Scholar]
- 47.Nawshad A., Medici D., Liu C.-C., Hay E.D. TGFbeta3 inhibits E-cadherin gene expression in palate medial-edge epithelial cells through a Smad2-Smad4-LEF1 transcription complex. J Cell Sci. 2007;120:1646–1653. doi: 10.1242/jcs.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su M.C., Chen C.T., Huang F.I., Chen Y.L., Jeng Y.M., Lin C.Y. Expression of LEF1 is an independent prognostic factor for patients with oral squamous cell carcinoma. J Formos Med Assoc. 2014;113:934–939. doi: 10.1016/j.jfma.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 49.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758.Molecular. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Derynck R., Zhang Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 51.Biernacka A., Dobaczewski M., Frangogiannis N.G. TGF-β signaling in fibrosis. Growth Factors. 2011;29:196–202. doi: 10.3109/08977194.2011.595714.TGF-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albanell J., Pietras K., Virtanen I., Philipson L., Philip L., Crystal R.G. A SNAIL1– SMAD3/4 transcriptional repressor complex promotes TGF-β mediated epithelial–mesenchymal transition. Nat Cell Biol. 2009;11:943–950. doi: 10.1038/ncb1905.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan Z., Kui Z., Ping Z. Reviews and prospectives of signaling pathway analysis in idiopathic pulmonary fibrosis. Autoimmun Rev. 2014;13:1020–1025. doi: 10.1016/j.autrev.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 54.Rai A., Ahmad T., Parveen S., Parveen S., Faizan M.I., Ali S. Expression of transforming growth factor beta in oral submucous fibrosis. J Oral Biol Craniofacial Res. 2020;10:166–170. doi: 10.1016/j.jobcr.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pant I., Rao S.G., Kondaiah P. Role of areca nut induced JNK / ATF2 / Jun axis in the activation of TGF- β pathway in precancerous Oral Submucous Fibrosis. Sci Rep. 2016;6:34314. doi: 10.1038/srep34314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kamath V.V., Krishnamurthy S., Satelur K.P., Rajkumar K. Transforming growth factor-β1 and TGF-β2 act synergistically in the fibrotic pathway in oral submucous fibrosis : an immunohistochemical observation. Indian J Med Paediatr Oncol. 2015;36:111–116. doi: 10.4103/0971-5851.158842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khan I., Agarwal P., Thangjam G.S., Radhesh R., Rao S.G., Kondaiah P. Role of TGF-β and BMP7 in the pathogenesis of oral submucous fibrosis. Growth Factors. 2011;29:119–127. doi: 10.3109/08977194.2011.582839. [DOI] [PubMed] [Google Scholar]
- 58.Khan I., Kumar N., Pant I., Narra S., Kondaiah P. Activation of TGF pathway by Areca nut constituents: a possible cause of oral submucous fibrosis. PLoS One. 2012;7:1–12. doi: 10.1371/journal.pone.0051806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pant I., Kumar N., Khan I., Rao S.G., Kondaiah P. Role of areca nut induced TGF-β and epithelial-mesenchymal interaction in the pathogenesis of oral submucous fibrosis. PLoS One. 2015;10:1–19. doi: 10.1371/journal.pone.0129252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Titidej A., Eshghyar N., Jolehar M., Jolehar M. Prognostic new marker (bone morphogenetic protein 7) in squamous cell carcinoma. J Contemp Dent Pract. 2018;19:675–679. [PubMed] [Google Scholar]
- 61.Pathway F.-B.S.S., Peng H., Shintani S., Kim Y., Wong D.T. Loss of p12 CDK2-AP1 expression in human oral squamous cell carcinoma with disrupted transforming growth. Neoplasia. 2006;8:1028–1036. doi: 10.1593/neo.06580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meng W., Xia Q., Wu L., Chen S., He X., Zhang L. Downregulation of TGF-beta receptor types II and III in oral squamous cell carcinoma and oral carcinoma-associated fibroblasts. BMC Cancer. 2011;11:88. doi: 10.1186/1471-2407-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y.E. Non-Smad signaling pathways of the TGF-β family. Cold Spring Harb Perspect Biol. 2017;9:1–18. doi: 10.1101/cshperspect.a022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carracedo A., Pandolfi P.P. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–5541. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 65.Julien S., Puig I., Caretti E., Bonaventure J., Nelles L., Van Roy F. Activation of NF-κB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene. 2007;26:7445–7456. doi: 10.1038/sj.onc.1210546. [DOI] [PubMed] [Google Scholar]
- 66.Lamouille S., Derynck R. Cell size and invasion in TGF-β-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol. 2007;178:437–451. doi: 10.1083/jcb.200611146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lamouille S., Connolly E., Smyth J.W., Akhurst R.J., Derynck R. TGF—induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. J Cell Sci. 2012;125:1259–1273. doi: 10.1242/jcs.095299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gu L., Xie C., Peng Q., Zhang J., Li J., Tang Z. Arecoline suppresses epithelial cell viability through the Akt/mTOR signaling pathway via upreguL.ation of PHLPP2. Toxicology. 2019;419:32–39. doi: 10.1016/j.tox.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 69.Grille S.J., Bellacosa A., Upson J., Klein-szanto A.J., Van Roy F., Lee-kwon W. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63:2172–2178. [PubMed] [Google Scholar]
- 70.Lim J., Kim J.H., Paeng J.Y., Kim M.J., Hong S.D., Lee J.I. Prognostic value of activated Akt expression in oral squamous cell carcinoma. J Clin Pathol. 2005;58:1199–1205. doi: 10.1136/jcp.2004.024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.West K.A., Brognard J., Clark A.S., Linnoila I.R., Yang X., Swain S.M. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI200316147.Introduction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Angadi P.V., Krishnapillai R. Evaluation of PTEN immunoexpression in oral submucous fibrosis: role in pathogenesis and malignant transformation. Head Neck Pathol. 2012;6:314–321. doi: 10.1007/s12105-012-0341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cohen Y., Goldenberg-Cohen N., Shalmon B., Shani T., Oren S., Amariglio N. Mutational analysis of PTEN/PIK3CA/AKT pathway in oral squamous cell carcinoma. Oral Oncol. 2011;47:946–950. doi: 10.1016/j.oraloncology.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 74.Lee M.K., Pardoux C., Hall M.C., Lee P.S., Warburton D., Qing J. TGF-β activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 2007;26:3957–3967. doi: 10.1038/sj.emboj.7601818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McCain J. The MAPK (ERK) pathway. Pharm Ther. 2012;38:346–353. doi: 10.1159/000154811. [DOI] [Google Scholar]
- 76.Zhang W., Liu H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2006;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 77.Dai J.P., Chen X.X., Zhu D.X., Wan Q.Y., Chen C., Wang G.F. Panax notoginseng saponins inhibit areca nut extract-induced oral submucous fibrosis in vitro. J Oral Pathol Med. 2014;43:464–470. doi: 10.1111/jop.12158. [DOI] [PubMed] [Google Scholar]
- 78.Chang M.C., Wu H.L., Lee J.J., Lee P.H., Chang H.H., Hahn L.J. The induction of prostaglandin E 2 production, interleukin-6 production, cell cycle arrest, and cytotoxicity in primary oral keratinocytes and KB cancer cells by areca nut ingredients is differentially regulated by MEK/ERK activation. J Biol Chem. 2004;279:50676–50683. doi: 10.1074/jbc.M404465200. [DOI] [PubMed] [Google Scholar]
- 79.Eversole L.R., Philip Sapp J. c-myc Oncoprotein expression in oral precancerous and early cancerous lesions. Eur J Cancer Part B Oral Oncol. 1993;29:131–135. doi: 10.1016/0964-1955(93)90035-D. [DOI] [PubMed] [Google Scholar]
- 80.Srinivasan M., Jewell S.D. Quantitative estimation of PCNA, c-myc, EGFR and TGF-α in oral submucous fibrosis - an immunohistochemical study. Oral Oncol. 2001;37:461–467. doi: 10.1016/S1368-8375(00)00115-9. [DOI] [PubMed] [Google Scholar]
- 81.Caraglia M., Tagliaferri P., Marra M., Giuberti G., Budillon A., Di Gennaro E. EGF activates an inducible survival response via the RAS-& Erk-1/2 pathway to counteract interferon-α-mediated apoptosis in epidermoid cancer cells. Cell Death Differ. 2003;10:218–229. doi: 10.1038/sj.cdd.4401131. [DOI] [PubMed] [Google Scholar]
- 82.Schreck R., Rapp U.R. Raf kinases: oncogenesis and drug discovery. Int J Cancer. 2006;119:2261–2271. doi: 10.1002/ijc.22144. [DOI] [PubMed] [Google Scholar]
- 83.Aguzzi A., Maggioni D., Nicolini G., Tredici G., Gaini R.M., Garavello W. MAP kinase modulation in squamous cell carcinoma of the oral cavity. Anticancer Res. 2009;29:303–308. [PubMed] [Google Scholar]
- 84.Uzgare A.R., Kaplan P.J., Greenberg N.M. Differential expression and/or activation of p38MAPK, erk1/2, and jnk during the initiation and progression of prostate cancer. Prostate. 2003;55:128–139. doi: 10.1002/pros.10212. [DOI] [PubMed] [Google Scholar]
- 85.Strutz F., Zeisberg M., Ziyadeh F.N., Yang C.-Q., Kalluri R., Muller G.A. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002;61:1714–1728. doi: 10.1046/j.1523-1755.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- 86.Bishen K.A., Radhakrishnan R., Satyamoorthy K. The role of basic fibroblast growth factor in oral submucous fibrosis pathogenesis. J Oral Pathol Med. 2008;37:402–411. doi: 10.1111/j.1600-0714.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 87.Baird A., Schubert D., Ling N., Guillemin R. Receptor- and heparin-binding domains of basic fibroblast growth factor. Proct Natl Acad Sci USA. 1988;85:2324–2328. doi: 10.1073/pnas.85.7.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zittermann S.I., Issekutz A.C. Basic fibroblast growth factor (bFGF, FGF-2) potentiates leukocyte recruitment to inflammation by enhancing endothelial adhesion molecule expression. Am J Pathol. 2006;168:835–846. doi: 10.2353/ajpath.2006.050479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hase T., Kawashiri S., Tanaka A., Nozaki S., Noguchi N., Kato K. Correlation of basic fibroblast growth factor expression with the invasion and the prognosis of oral squamous cell carcinoma. J Oral Pathol Med. 2006;35:136–139. doi: 10.1111/j.1600-0714.2006.00397.x. [DOI] [PubMed] [Google Scholar]
- 90.Srinivasan M., Jewell S.D. Evaluation of TGF-α and EGFR expression in oral leukoplakia and oral submucous fibrosis by quantitative immunohistochemistry. Oncology. 2001;61:284–292. doi: 10.1159/000055335. [DOI] [PubMed] [Google Scholar]
- 91.Chang M.C., Chen Y.J., Chang H.H., Chan C.P., Yeh C.Y., Wang Y.L. Areca nut components affect COX-2, cyclin B1/cdc25C and keratin expression, PGE2 production in keratinocyte is related to reactive oxygen species, CYP1A1, Src, EGFR and Ras signaling. PLoS One. 2014;9:1–12. doi: 10.1371/journal.pone.0101959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bernardes V.F., Gleber-Netto F.O., Sousa S.F., Silva T.A., Aguiar M.C.F. Clinical significance of EGFR, Her-2 and EGF in oral squamous cell carcinoma: a case control study. J Exp Clin Cancer Res. 2010;29:1–7. doi: 10.1186/1756-9966-29-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Temam S., Kawaguchi H., El-Naggar A.K., Jelinek J., Tang H., Liu D.D. Epidermal growth factor receptor copy number alterations correlate with poor clinical outcome in patients with head and neck squamous cancer. J Clin Oncol. 2007;25:2164–2170. doi: 10.1200/JCO.2006.06.6605. [DOI] [PubMed] [Google Scholar]
- 94.Tsai C., Yang S., Chen Y.-J., Chou M.-Y., Chang Y.-C. The upregulation of insulin-like growth factor-1 in oral submucous fibrosis. Oral Oncol. 2005;41:940–946. doi: 10.1016/j.oraloncology.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 95.Uh S., Inoue Y., King T.E., Chan E.D., Newman L.S., Riches D.W.H. Morphometric analysis of insulin-like growth Factor-I localization in lung tissues of patients with idiopathic pulmonary fibrosis. Am J Respiir Crit Care Med. 1998;158:1626–1635. doi: 10.1164/ajrccm.158.5.9804025. [DOI] [PubMed] [Google Scholar]
- 96.Harrison N.K., Cambrey A.D., Myers A.R., Southcolts A.M., Black C.M., Du Bois R.M. Insulin-like growth factor-I is partially responsible for fibroblast proliferation induced by bronchoalveolar lavage fluid from patients with systemic sclerosis. Clin Sci. 1994;86:141–148. doi: 10.1042/cs0860141. [DOI] [PubMed] [Google Scholar]
- 97.Brady G., Crean S., Naik P., Kapas S. Upregulation of IGF-2 and IGF-1 receptor expression in oral cancer cell lines. Int J Oncol. 2007;31:875–881. [PubMed] [Google Scholar]
- 98.Zhi X., Lamperska K., Golusinski P., Schork N.J., Luczewski L., Golusinski W. Expression levels of insulin-like growth factor 1 and 2 in head and neck squamous cell carcinoma. Growth Horm IGF Res. 2014;24:137–141. doi: 10.1016/j.ghir.2014.04.003.Expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hunasgi S., Koneru A., Vanishree M., Manvikar V. Coalition of E‑cadherin and vascular endothelial growth factor expression in predicting malignant transformation in common oral potentially malignant disorders. J Oral Maxillofac Pathol. 2018;22:40–47. doi: 10.4103/jomfp.JOMFP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Desai R.S., Mamatha G.S., Khatri M.J., Shetty S.J. Immunohistochemical expression of vascular endothelial growth factor (VEGF) and its possible role in tumour progression during malignant transformation of atrophic epithelium in oral submucous fibrosis. Curr Angiogenes. 2012;1:347–353. [Google Scholar]
- 101.Pirici D., Simionescu C., Raica M., Stepan A., Ribatti D., Mogoanta L. VEGF expression and angiogenesis in oral squamous cell carcinoma: an immunohistochemical and morphometric study. Clin Exp Med. 2010;10:209–214. doi: 10.1007/s10238-010-0095-4. [DOI] [PubMed] [Google Scholar]
- 102.Margaritescu C., Pirici D., Simionescu C., Mogoanta L., Raica M., Stinga A. VEGF and VEGFRs expression in oral squamous cell carcinoma. Rom J Morphol Embryol. 2009;50:527–548. [PubMed] [Google Scholar]
- 103.Komiya Y., Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou S., Mashrah M., Zhu Y., Liu J., He Z., Xiang T. Deregulation of secreted frizzled-related proteins is associated with aberrant β -catenin activation in the carcinogenesis of oral submucous fibrosis. Onco Targets Ther. 2015;8:2923–2931. doi: 10.2147/OTT.S91460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mashrah M., Yao Z., Zhang X., Zhang C., Zhou S., Guo F. Expression pattern of DKK3, dickkopf WNT signaling pathway inhibitor 3, in the malignant progression of oral submucous fibrosis. Oncol Rep. 2016;37:979–985. doi: 10.3892/or.2016.5307. [DOI] [PubMed] [Google Scholar]
- 106.Mashrah M., Yao Z. Aberrant DKK3 expression in the oral leukoplakia and oral submucous fibrosis : a comparative immunohisto- chemical study. Eur J Histochem. 2016;60:2629. doi: 10.4081/ejh.2016.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou S., Chen L., Mashrah M., Zhu Y., He Z., Hu Y. Expression and promoter methylation of Wnt inhibitory factor-1 in the development of oral submucous fibrosis. Oncol Rep. 2015;34:2636–2642. doi: 10.3892/or.2015.4264. [DOI] [PubMed] [Google Scholar]
- 108.Sarbak J., Kostrzewska-poczekaj M., Mielcarek-kuchta D., Baer-dubowska W. The negative regulators of Wnt pathway — DACH1, DKK1, and WIF1 are methylated in oral and oropharyngeal cancer and WIF1 methylation predicts shorter survival. Tumor Biol. 2015;36:2855–2861. doi: 10.1007/s13277-014-2913-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Menon R., Li C.C., Li M.Z. Wnt signaling in the oral cancer initialing cells. Oral Maxillofac Pathol. 2017;124:e202. doi: 10.1016/j.oooo.2017.06.015. [DOI] [Google Scholar]
- 110.Marimuthu M., Andiappan M., Wahab A., MR M., Balakrishnan A., Shanmugam S. Canonical wnt pathway gene expression and their clinical correlation in oral squamous cell carcinoma. Indian J Dent Res. 2018;29:291–297. doi: 10.4103/ijdr.IJDR. [DOI] [PubMed] [Google Scholar]
- 111.Lo Muzio L., Goteri G., Vinella A., Mastrangelo F., Carcinoma O. Beta-catenin gene analysis in oral squamous cell carcinoma. Int J Immunopathol Pharmacol. 2005;18:33–38. [PubMed] [Google Scholar]
- 112.Radisky E.S., Radisky D.C. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:201–212. doi: 10.1007/s10911-010-9177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Katarkar A., Prodhan C., Mukherjee S., Ray J.G., Chaudhuri K. Role of matrix metalloproteinase-9 polymorphisms in basement membrane degradation and pathogenesis of oral submucous fibrosis. Meta Gene. 2018;16:255–263. doi: 10.1016/j.mgene.2018.04.001. [DOI] [Google Scholar]
- 114.Mishra G., Ranganathan K. Matrix metalloproteinase-1 expression in oral submucous fibrosis: an immunohistochemical study. Indian J Dent Res. 2010;21:320–325. doi: 10.4103/0970-9290.70785. [DOI] [PubMed] [Google Scholar]
- 115.de Vicente J.C., Lequerica-Fernández P., Santamaría J.F.M. Expression of MMP-7 and MT1-MMP in oral squamous cell carcinoma as predictive indicator for tumor invasion and prognosis. J Oral Pathol Med. 2007;36:4115–4124. doi: 10.1111/j.1600-0714.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 116.Katayama A., Bandoh N., Kishibe K., Takahara M., Ogino T., Nonaka S. Expressions of matrix metalloproteinases in early-stage oral squamous cell carcinoma as predictive indicators for tumor metastases and prognosis. Clin Cancer Res. 2004;10:634–640. doi: 10.1158/1078-0432.ccr-0864-02. [DOI] [PubMed] [Google Scholar]
- 117.Heino J. Cellular signaling by collagen-binding integrins. Adv Exp Med Biol. 2014;819:143–155. doi: 10.1007/978-94-017-9153-3. [DOI] [PubMed] [Google Scholar]
- 118.Fang S., Dai Y., Mei Y., Yang M., Hu L., Yang H. Clinical significance and biological role of cancer-derived Type I collagen in lung and esophageal cancers. Thorac Cancer. 2019;10:277–288. doi: 10.1111/1759-7714.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Medicia D., Nawshad A. Type I collagen promotes epithelial-mesenchymal transition through ILK-dependent activation of NF-κB and LEF-1. Matrix Biol. 2011;29:161–165. doi: 10.1016/j.matbio.2009.12.003.Type. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Walsha L.A., Nawshadb A., Medicia D. Discoidin domain receptor 2 is a critical regulator of epithelial- mesenchymal transition. Matrix Biol. 2011;30:243–247. doi: 10.1016/j.matbio.2011.03.007.Discoidin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shintani Y., Hollingsworth M.A., Johnson K.R., Wheelock M.J. Collagen I promotes metastasis in pancreatic Cancer by activating c-Jun NH 2 -Terminal kinase 1 and up-regulating N-Cadherin expression. Cancer Res. 2006;66:11745–11754. doi: 10.1158/0008-5472.CAN-06-2322. [DOI] [PubMed] [Google Scholar]
- 122.Garamszegi N., Garamszegi S.P., Walford E., Schneiderbauer M.M. Extracellular matrix-induced transforming growth factor- b receptor signaling dynamics. Oncogene. 2010;29:2368–2380. doi: 10.1038/onc.2009.514. [DOI] [PubMed] [Google Scholar]
- 123.PA R, CW VW, J B, D S Distribution of procollagen type III, collagen type VI and tenascin in oral submucous fibrosis (OSF) J Oral Pathol Med. 1994;23:394–398. doi: 10.1111/j.1600-0714.1994.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 124.Utsunomiya H., Tilakaratne W.M., Oshiro K., Maruyama S., Suzuki M., Ida-Yonemochi H. Extracellular matrix remodeling in oral submucous fibrosis: its stage-specific modes revealed by immunohistochemistry and in situ hybridization. J Oral Pathol Med. 2005;34:498–507. doi: 10.1111/j.1600-0714.2005.00339.x. [DOI] [PubMed] [Google Scholar]
- 125.Moutasim K.A., Jenei V., Sapienza K., Marsh D., Weinreb P.H., Violette S.M. Betel-derived alkaloid up-regulates keratinocyte alphavbeta6 integrin expression and promotes oral submucous fibrosis. J Pathol. 2011;223:366–377. doi: 10.1002/path.2786. [DOI] [PubMed] [Google Scholar]
- 126.Veeravarmal V., Austin R.D., Nagini S., Nassar M.H.M. Expression of β1integrin in normal epithelium, oral submucous fibrosis and oral squamous cell carcinoma. Pathol Res Pract. 2018;214:273–280. doi: 10.1016/j.prp.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 127.Hayashido Y., Kitano H., Sakaue T., Fujii T. Overexpression of integrin αv facilitates proliferation and invasion of oral squamous cell carcinoma cells via MEK/ERK signaling pathway that is activated by interaction of integrin αvβ8 with type Ⅰ collagen. Int J Oncol. 2014;45:1875–1882. doi: 10.3892/ijo.2014.2642. [DOI] [PubMed] [Google Scholar]
- 128.Ni W.-F., Tsai C.-H., Yang S.-F., Chang Y.-C. Elevated expression of NF- kB in oral submucous fibrosis – evidence for NF- kB induction by safrole in human buccal mucosal fibroblasts. Oral Oncol. 2007;43:557–562. doi: 10.1016/j.oraloncology.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 129.Tang D., Tao D., Fang Y., Deng C., Xu Q., Zhou J. TNF-alpha promotes invasion and metastasis via NF-Kappa B pathway in oral squamous cell carcinoma. Med Sci Monit Basic Res. 2017;23:141–149. doi: 10.12659/MSMBR.903910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Masoud G.N., Li W. HIF-1 α pathway : role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang W., Shi X., Peng Y., Wu M., Zhang P., Xie R. HIF-1 α promotes epithelial-mesenchymal transition and metastasis through direct regulation of ZEB1 in colorectal Cancer. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu Y., Tan J., Xie H., Wang J., Meng X., Wang R. HIF-1α regulates EMT via the Snail and b -catenin pathways in paraquat poisoning-induced early pulmonary fibrosis. J Cell Mol Med. 2016;20:688–697. doi: 10.1111/jcmm.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tilakaratne W.M., Iqbal Z., Teh M.T., Ariyawardana A., Pitiyage G., Cruchley A. Upregulation of HIF-1α in malignant transformation of oral submucous fibrosis. J Oral Pathol Med. 2008;37:372–377. doi: 10.1111/j.1600-0714.2007.00625.x. [DOI] [PubMed] [Google Scholar]
- 134.Chaudhary M., Bajaj S., Bohra S., Swastika N., Hande A. The domino effect : role of hypoxia in malignant transformation of oral submucous fibrosis. J Oral Maxillofac Pathol. 2015;19:122–127. doi: 10.4103/0973-029X.164519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liang J., Zhang Z., Liang L., Shen Y., Ouyang K. HIF-1α regulated tongue squamous cell carcinoma cell growth via regulating VEGF expression in a xenograft model. Ann Transl Med. 2014;2:1–7. doi: 10.3978/j.issn.2305-5839.2014.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kull M., Peterson H., Hansen J., Vilo J.G. Profiler — a web-based toolset for functional profiling of gene lists from large-scale experiments. Nucleic Acids Res. 2007;35:193–200. doi: 10.1093/nar/gkm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhou Y., Zhou B., Pache L., Chang M., Khodabakhshi A.H., Tanaseichuk O. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10:1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xie L., Law B.K., Chytil A.M., Brown K.A., Aakre M.E., Moses H.L. Activation of the erk pathway is required for TGF-β1-Induced EMT in vitro. Neoplasia. 2004;6:603–610. doi: 10.1593/neo.04241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Angadi P.V., Patil P.V., Angadi V., Mane D., Shekar S., Hallikerimath S. Immunoexpression of epithelial mesenchymal transition proteins in oral squamous cell carcinoma. Int J Surg Pathol. 2016;24 doi: 10.1177/1066896916654763. [DOI] [PubMed] [Google Scholar]
- 140.Frohwitter G., Buerger H., Diest P.J.V.A.N., Korsching E., Kleinheinz J., Fillies T. Cytokeratin and protein expression patterns in squamous cell carcinoma of the oral cavity provide evidence for two distinct pathogenetic pathways. Oncol Lett. 2016;12:107–113. doi: 10.3892/ol.2016.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Smitha A., Rao K., Umadevi H.S., Smitha T., Sheethal H.S., Vidya M.A. Immunohistochemical study of α ‑ smooth muscle actin expression in oral leukoplakia and oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2019;23:59–64. doi: 10.4103/jomfp.JOMFP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Su T.R., Liao Y.W., Hsieh P.L., Tsai L.L., Fang C.Y., Lin T. Butylidenephthalide abrogates the myofibroblasts activation and mesenchymal transdifferentiation in oral submucous fibrosis. Environ Toxicol. 2018;33:686–694. doi: 10.1002/tox.22557. [DOI] [PubMed] [Google Scholar]
- 143.Lee S.S., Tsai C.H., Yu C.C., Chang Y.C. Elevated snail expression mediates tumor progression in Areca quid chewing-associated oral squamous cell carcinoma via reactive oxygen species. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zheng M., Jiang Y., Chen W., Li K., Liu X., Gao S. Snail and Slug collaborate on EMT and tumor metastasis through miR-101-mediated EZH2 axis in oral tongue squamous cell carcinoma. Oncotarget. 2015;6:6797–6810. doi: 10.18632/oncotarget.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kamath V.V., Satelur K.P., Rajkumar K., Krishnamurthy S. Transforming growth factor beta 1 in oral submucous fibrosis: An immunohistochemical study – understanding the pathogenesis. J Dent Res Rev. 2014;1:75–80. doi: 10.4103/2348-2915.133942. [DOI] [Google Scholar]
- 146.Chen C., Méndez E., Houck J., Fan W., Doody D., Yueh B. Gene expression profiling identifies genes predictive of oral squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:2152–2162. doi: 10.1158/1055-9965.EPI-07-2893.Gene. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang S., Hsieh Y., Tsai C. The upregulation of type I plasminogen activator inhibitor in oral submucous fibrosis. Oral Oncol. 2003;39:367–372. doi: 10.1016/s1368-8375(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 148.Hu Y., Jian X., Peng J., Jiang X., Li N., Zhou S. Gene expression profiling of oral submucous fibrosis using oligonucleotide microarray. Oncol Rep. 2008;20:287–294. [PubMed] [Google Scholar]
- 149.Zagabathina S., Ramadoss R., Ah H.P., Krishnan R. Comparative evaluation of SMAD-2 expression in oral submucous fibrosis and reactive oral lesions. Asian Pac J Cancer Prev. 2020;21:399–403. doi: 10.31557/APJCP.2020.21.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Iamaroon A., Pattamapun K., Piboonniyom S. Aberrant expression of Smad4, a TGF- β signaling molecule, in oral squamous cell carcinoma. J Oral Sci. 2006;48:105–109. doi: 10.2334/josnusd.48.105. [DOI] [PubMed] [Google Scholar]
- 151.Pal S.K., Thi C., Nguyen K., Morita K., Miki Y., Kayamori K. THBS1 is induced by TGFB1 in the cancer stroma and promotes invasion of oral squamous cell carcinoma. J Oral Pathol Med. 2016;45:730–739. doi: 10.1111/jop.12430. [DOI] [PubMed] [Google Scholar]
- 152.Baker E.A., Leaper D.J., Hayter J.P., Dickenson A.J. Plasminogen activator system in oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 2007;45:623–627. doi: 10.1016/j.bjoms.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 153.Gao Q., Tong W., Luria J.S., Wang Z., Nussenbaum B., Effects P.H.K. Effects of bone morphogenetic protein-2 on proliferation and angiogenesis in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2010;39:266–271. doi: 10.1016/j.ijom.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 154.Ge W.L., Xu J.F., Hu J. Regulation of oral squamous cell carcinoma proliferation through crosstalk between SMAD7 and CYLD. Cell Physiol Biochem. 2016;38:1209–1217. doi: 10.1159/000443069. [DOI] [PubMed] [Google Scholar]
- 155.Sharada P., Swaminathan U., Nagamalini B.R., Kumar K.V., Ashwini B.K., Lavanya V. Coalition of E-cadherin and vascular endothelial growth factor expression in predicting malignant transformation in common oral potentially malignant disorders. J Oral Maxillofac Pathol. 2018;22:40–47. doi: 10.4103/jomfp.JOMFP_13_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nayak S., Goel M.M., Makker A., Bhatia V., Chandra S., Kumar S. Fibroblast growth factor (FGF-2) and its receptors FGFR-2 and FGFR-3 may Be putative biomarkers of malignant transformation of potentially malignant oral lesions into oral squamous cell carcinoma. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chiu C.-J., Chiang C.-P., Chang M.-L., Chen H.-M., Hahn L.-J., Hsieh L.-L. Associaion beiween geneic polymorphism of tumor necrosis Factor-α and risk of oral submucous fibrosis, a pre-cancerous condilion of oral Cancer. J Dent Res. 2001;80:2055–2059. doi: 10.1177/00220345010800120601. [DOI] [PubMed] [Google Scholar]
- 158.Andressa F., Ribeiro P., Noguti J., Tizuko C., Oshima F., Ribeiro D.A. Effective targeting of the epidermal growth factor receptor (EGFR) for treating oral Cancer: a promising approach. Anticancer Res. 2014;34:1547–1552. [PubMed] [Google Scholar]
- 159.Nakano Y., Kobayashi W., Sugai S., Kimura H. Expression of tumor necrosis factor- α and Interleukin-6 in oral squamous cell carcinoma. Jpn J Cancer Res. 1999;90:858–866. doi: 10.1111/j.1349-7006.1999.tb00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhou S., Chen L., Mashrah M., Zhu Y., Liu J., Yang X. Deregulation of secreted frizzled-related proteins is associated with aberrant beta-catenin activation in the carcinogenesis of oral submucous fibrosis. Onco Targets Ther. 2015;8:2923–2931. doi: 10.2147/OTT.S91460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Eckert A.W., Schütze A., Lautner M.H.W., Taubert H., Schubert J. HIF-1 α is a prognostic marker in oral squamous cell carcinomas. Int J Biol Markers. 2010;25:87–92. doi: 10.1177/172460081002500205. [DOI] [PubMed] [Google Scholar]
- 162.Kurasawa Y., Shiiba M., Nakamura M., Fushimi K., Ishigami T., Bukawa H. PTEN expression and methylation status in oral squamous cell carcinoma. Oncol Rep. 2008;19:1429–1434. [PubMed] [Google Scholar]
- 163.Rahmani A., Alzohairy M., Babiker A.Y., Rizvi M.A., Elkarimah- H.G. Clinicopathological significance of PTEN and bcl2 expressions in oral squamous cell carcinoma. Int J Clin Exp Pathol. 2012;5:965–971. [PMC free article] [PubMed] [Google Scholar]
- 164.Chaudhary A.K., Pandya S., Mehrotra R., Bharti A.C., Jain S., Singh M. Functional polymorphism of the MMP-1 promoter (-1607 1G/2G) in potentially malignant and malignant head and neck lesions in an Indian population. Biomarkers. 2010;15:684–692. doi: 10.3109/1354750X.2010.511267. [DOI] [PubMed] [Google Scholar]
- 165.Bano N., Yadav M., Mohania D., Das Id B.C. The role of NF- κ B and miRNA in oral cancer and cancer stem cells with or without HPV16 infection. PLoS One. 2018;13 doi: 10.1371/journal.pone.0205518. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]