Summary
Stem cells are considered to be one of the greatest potential treatments to cure degenerative diseases. Stem cells injection for knee osteoarthritis (OA) is still a relatively new treatment and has not yet gained popularity. So, the effectiveness, safety and potential of mesenchymal stem cells (MSCs) for knee OA treatment is worthy to be explored. Explore the effectiveness and safety of mesenchymal stem cells (MSCs) in the treatment of knee osteoarthritis. We collected clinical trials using MSCs as treatment for knee OA (before April 2019), including randomized controlled trials (RCTs), retrospective studies and cohort studies. We searched PubMed, EMBASE, Cochrane Library, Web of Science and the ClinicalTrials.gov with keywords (Mesenchymal stem cells [MSCs], Knee osteoarthritis, Effectiveness and Safety), and then performed a systematic review and cumulative metaanalysis of all RCTs and retrospective comparative studies. To evaluate the effectiveness and safety of MSC in knee OA treatment, we applied visual analog scale score, Western Ontario and McMaster Universities Osteo-arthritis Index and adverse events. We included 15 RCTs, two retrospective studies and two cohort studies including a total of 584 knee OA patients in this study. We demonstrated that MSC treatment could significantly decrease visual analog scale in a 12-month follow-up study compared with controls (p < 0.001). MSC therapy also showed significant decreases in Western Ontario and McMaster Universities Osteoarthritis Index scores after the 6-month follow-up (p < 0.001). MSC therapy showed no difference compared with controls (p > 0.05) in adverse events. We suggest that MSC therapy could serve as an effective and safe therapy for clinical application in OA treatment.
The translational potential of this article
This study provided the best available evidence and a wider perspective to MSCs application in the management of knee OA. MSCs therapy will have great translational potential in the clinical treatment of various degenerative diseases once optimum formula and explicit target population are identified.
Keywords: Knee osteoarthritis, Mesenchymal stem cells, Meta-analysis
Introduction
Osteoarthritis (OA) is a degenerative disease, which can be classified into primary and secondary OA. Primary OA is OA without a clear cause. Secondary osteoarthritis has definite aetiology, including endocrine system disorder, anatomical structure abnormality, post-traumatic arthritis and inflammatory arthritis [1]. OA is characterized by reduction of articular chondrocytes and destruction of joint matrix [2]. Specifically, symptoms of OA include continuous chondrocyte cartilage damage [3], articular chondrocyte loss [4], subchondral microfracture [5], subchondral bone exposure [6], joint edge and subchondral bone hyperplasia [10]. Clinically OA patients suffer from slowly developing joint pain, joint stiffness, joint swelling, decreased joint range of movement and joint deformity [2,7]. Epidemiological statistics show that the overall prevalence of primary OA in people aged more than 40 years is 46.3%, 41.6% for male and 50.4% for female. Furthermore, the incidence rate in the middle-aged and elderly population can reach to 40% and 80%, respectively. A milder condition affects the quality of life, whereas a heavier condition can lead to disability. The disability rate can eventually reach 50% or higher [20,22]. Therefore, OA has become a serious health problem.
Currently, the treatment for knee OA is very limited. There are some conventional therapies for OA, including physiotherapy, nonsteroidal anti-inflammatory drugs, pain-relieving drugs, hyaluronic acid, platelet-rich plasma (PRP) or corticosteroid-based intra-articular injections, traditional Chinese medicine and knee arthroscopic surgery. All the above-mentioned treatments can only relieve symptoms, but cannot repair cartilage. As OA worsens, total knee arthroplasty is needed [24,25]. Stem cells therapy is a milestone in regenerative medicine for OA treatment.
Mesenchymal stem cells (MSCs) have self-renewal and multidirectional differentiation potential [26], and can exert therapeutic effects on various diseases through directed differentiation [27], regulation of immunity [28], anti-inflammatory, proangiogenesis [28], improvement of microenvironment [29] and promotion of regeneration [30]. MSCs have been used in the treatment of various diseases [31], such as premature ovarian failure, Parkinson's disease, nervous system damage and amyotrophic lateral sclerosis. MSCs therapy could be applied for OA treatment and have shown encouraging results [[26], [27], [28], [29], [30], [31]].
MSCs have not applied widely because of ethical issues in cell sources and expensive cell culture. Furthermore, its effectiveness and safety are worthy to be explored. The aim of this study is to collect high-quality clinical trials worldwide and to evaluate the efficacy and safety of stem cell therapy OA treatment, therefore to provide a convenient and effective treatment for patients with OA.
Evidence acquisition
A prospective protocol of objectives, literature-search strategy, inclusion and exclusion criteria, outcome measurements and statistical analysis methods were according to the Preferred Reporting Items for Systematic Reviews. Meta-analysis and Meta-analysis of Observational Studies in Epidemiology recommendations were applied for study reporting [32,33].
Literature-search strategy
A literature search was performed in April 2019 without restriction to regions, publication types or languages. The primary sources were the electronic databases of PubMed, EMBASE, Cochrane Library, Web of Science and the ClinicalTrials.gov. The following Medical Subject Headings (MeSH) terms and their combinations were searched in [Title/Abstract]: (Osteoarthritis OR Knee Osteoarthritis OR Osteoarthritis of Knee OR Knee, Osteoarthritis Of OR Knees, Osteoarthritis Of OR Osteoarthritis Of Knees) AND (Stem Cells OR Progenitor Cells OR Mother Cells OR Colony-Forming Unit Mesenchymal Stem Cell OR Wharton Jelly Cells OR Mesenchymal Stromal Cells OR Bone Marrow Mesenchymal Stem Cells OR IPS Cells OR Human Induced Pluripotent Stem Cells OR hiPSC OR Fibroblast-Derived Induced Pluripotent Stem Cells OR Fibroblast Derived Induced Pluripotent Stem Cells OR Fibroblast-Derived IPS Cells OR Teratocarcinoma Stem Cells OR Embryonal Carcinoma Cells) AND randomized controlled trial. The related articles' function was also used to broaden the search. Furthermore, we also applied manual searches of the reference lists of all retrieved studies, review articles and conference abstracts. When multiple reports describing the same population, the most recent or complete report was adopted.
Inclusion and exclusion criteria
Clinical trials, relevant review articles and postgraduate papers were examined to identify further relevant studies. Studies were eligible for inclusion if: (1) study must be published randomized controlled trials (RCTs), retrospective studies or cohort study in humans applying MSCs therapy for patients with knee OA; (2) the patient's detailed information was recorded both before and after therapy; (3) the study enrolled six or more patients; and (4) publication time of articles is limited to 2012–2019. Editorials, letters to the editor, review articles, case reports and animal experimental studies were excluded.
Data extraction and outcomes of interest
Data from the included studies were extracted and summarized independently by two authors (Junhui Zhang and Zhujian Lin). Any differences were resolved by the adjudicating senior authors (Hualiang Xu and Hong Chang).
The extracted study data features included the first author name, year, number of patients, mean age, body mass index, study design, outcome measure, follow-up time, stem cell origin, quality assessment, number of cells and method of experiment. The primary outcomes to assess effectiveness were performed by Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and visual analog scale (VAS). The incidence of adverse events (AEs) was used to evaluate the safety of MSCs. To make the results more credible, we use subgroup analysis to reduce heterogeneity.
Quality assessment and statistical analysis
Studies were rated for the level of evidence provided according to criteria by the Centre for Evidence-Based Medicine in Oxford, UK [34].
The methodological quality of RCTs was assessed by the Cochrane risk-of-bias tool [35]. The methodological quality of retrospective studies was assessed by the modified Newcastle–Ottawa scale [36,37], which consists of three factors: patient selection, comparability of the study groups and assessment of outcome. A score of 0–9 (allocated as stars) was allocated to each study except RCTs.
All the meta-analyses were performed using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK). The weighted mean difference (WMD) and odds ratio were used to compare continuous and dichotomous variables, respectively. All results were reported with 95% confidence intervals (CIs). For studies that presented continuous data as means and range values, the standard deviations were calculated using the technique described by Hozo et al. [38].
Statistical heterogeneity between studies was assessed using the Chi-squared test with significance set at p < 0.10, and heterogeneity was quantified using the I2 statistic. The random effects model was used if there was heterogeneity between studies; otherwise, the fixed effects model was used.
The subgroup analysis compared WOMAC and VAS score with different stem cell numbers and different stem cell sources. Sensitivity analyses were performed for high-quality studies. Funnel plots were used to screen for potential publication bias.
Evidence synthesis
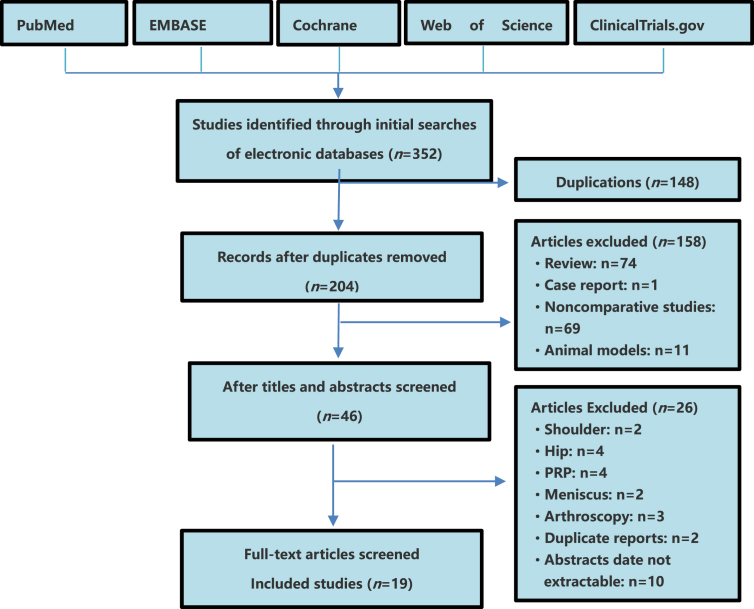
Nineteen studies including 584 knee OA patients (352 cases for MSC group and 232 cases for control group) fulfilled the predefined inclusion criteria and were included in the final analysis (Figure 1). Eighteen publications were full-text articles [[2], [3], [4], [5],[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19],21,23], and one publication was conference abstract [6].
Figure 1.
Flow diagram of studies identified, included and excluded. PRP = platelet-rich plasma.
Characteristics of eligible studies
The characteristics of included studies are shown in Table 1. Among the included studies, there were 15 RCTs [3,5,6,[8], [9], [10], [11], [12], [13], [14], [15],18,19,21,23], two retrospective studies [1,17] and two cohort studies [4,16]. All the 19 papers were fully published during the period from 2012 to 2019. The mean ages of patients enrolled were between 35 and 75 years. Sample size ranged from a minimum of nine to a maximum of 60 patients. In all the trials, MSC therapy was evaluated in knee OA patients with MSCs from fat in nine studies [1,4,8,13,[15], [16], [17], [18], [19]], MSCs from marrow in five studies [3,[9], [10], [11],14], peripheral blood stem cells in one study reported in a conference paper [21], and MSCs from foetus in four studies [5,6,12,23]. The patients received cell infusions from 1.89 × 106 to 100 × 106 cells. The injected route for MSC therapy was intra-articular injection.
Table 1.
Summary of studies using MSCs to treat KOA patients.
| Author | Year | Number of patients |
Mean age (year) |
BMI (kg/m2) | Study design | Outcome measure | Follow-up (mo) | Stem cell origin | Quality assessment | Number of cells (106) | Method of experiment | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSCs group (M/F) | Control group (M/F) | MSCs/Control | |||||||||||
| Koh et al. | 2012 | 25 (8/17) | 25 (8/17) | 54.2 ± 9.3/54.4 ± 11.3 | — | R | Lysholm, VAS | 16.4 | Fat | NOS | 1.89 | MSCs/PRP | [1] |
| Vega et al. | 2015 | 15 (6/9) | 15 (5/10) | 56.6/57.33 | — | RCT | VAS, WOMAC | 12 | Marrow | ROB | 40 | MSCs/HA | [3] |
| Pers et al. | 2016 | 18 (8/10) | — | 63.2 ± 4.1/63.2 ± 4.1 | 28.8 | CS | WOMAC, VAS, SF-36 | 6 | Fat | NOS | 2/10/50 | MSCs | [4] |
| Wang et al. | 2016 | 18 (10/8) | 18 (11/7) | 54.28/52.37 | 28.31 | RCT | Lysholm, WOMAC, SF-36 | 6 | Foetus | ROB | 20–30 | MSCs/HA | [5] |
| Matas et al. | 2017 | 18 | 9 | — | — | RCT | WOMAC, VAS, SF-36 | 12 | Foetus | ROB | 20 | MSCs/HA | [6] |
| Jo et al. | 2017 | 18 (3/15) | — | 61.8 ± 6.6 | 26 | RCT | WOMAC, VAS | 24 | Fat | ROB | 10/50/100 | MSCs | [8] |
| Shapiro et al. | 2017 | 25 (/18) | 25 (7/18) | 60 | 27.1 | RCT | VAS | 6 | Marrow | ROB | 52 mL | MSCs/NS | [9] |
| Bastos et al. | 2018 | 9 (4/5) | 9 (5/4) | 54.7 ± 7.2/60.4 ± 11.3 | — | RCT | KOOS | 12 | Marrow | ROB | 80–100 mL | MSCs/PRP | [10] |
| Liastani et al. | 2018 | 19 (12/7) | 24 (15/9) | 51.7 ± 9.2/54.7 ± 5.3 | 30.2 | RCT | WOMAC, VAS | 6 | Marrow | ROB | 40 | MSCs/NS | [11] |
| Forogh et al. | 2018 | 10 | 10 | 35–75 | <35 | RCT | VAS, KOOS | 6 | Foetus | ROB | 50–60 | MSCs/NS | [12] |
| Jones et al. | 2018 | 27 | 27 | 45–75 | <40 | RCT | WOMAC | 6 | Fat | ROB | 6 mL | MSCs | [13] |
| Gupta et al. | 2016 | 40 | 20 | 57.3 ± 9.4/54.9 ± 8.2 | 29.73 | RCT | WOMAC, VAS | 12 | Marrow | ROB | 25/50/75/100 | MSCs/HA | [14] |
| Kuah et al. | 2018 | 16 | 4 | 55 ± 5.15/55.0 ± 10.42 | 20–30 | RCT | WOMAC, VAS | 12 | Fat | ROB | — | MSCs/HA | [15] |
| Song et al. | 2018 | 18 | — | 55 | 24 | CS | WOMAC, SF-36 | 24 | Fat | NOS | 10/20/50 | MSCs | [16] |
| Zoran et al. | 2018 | 9 (3/6) | — | 63 ± 10.4 | 29.5 | RP | Lysholm, VAS | 18 | Fat | NOS | 5–10 | MSCs | [17] |
| Bait et al. | 2019 | 12 (3/9) | 12 (3/9) | 62.2 ± 6.5/63.2 ± 4.2 | 25.3 | RCT | WOMAC, VAS | 6 | Fat | ROB | 100 | MSCs/NS | [18] |
| Freitag et al. | 2019 | 20 (11/9) | 10 (5/5) | 54.6/51.5 | 31.6 | RCT | WOMAC | 12 | Fat | ROB | 95.1–103.9 | MSCs/NS | [19] |
| Khasru et al. | 2019 | 15 | 15 | 53 ± 11 | — | RCT | WOMAC, VAS | 6 | Blood | ROB | — | MSCs | [21] |
| Matas et al. | 2019 | 20 (9/11) | 9 (4/5) | 56.1 ± 6.8/54.8 ± 4.5 | 27.6 | RCT | WOMAC, VAS, SF-36 | 12 | Foetus | ROB | 20 | MSCs/NS/HA | [23] |
BMI = body mass index; CS = cohort study; HA = hyaluronic acid; KOA = knee osteoarthritis; KOOS = knee osteoarthritis outcome score; MSCs = mesenchymal stem cells; NOS = Newcastle–Ottawa Scale; NS = normal saline; PRP = platelet-rich plasma; R = retrospective; RCT = randomized controlled trial; ROB = The Cochrane collaboration's tool for assessing risk of bias; RP = retrospective design, prospective data collection; SF-36 = short form–36 health survey; VAS = visual analog scale; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
Methodological quality of included studies
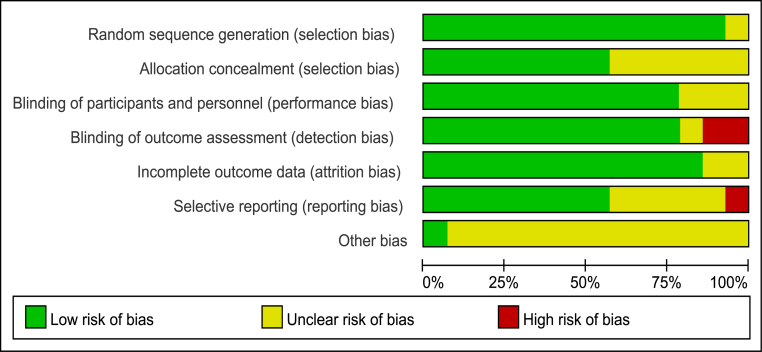
The methodological quality of RCTs was assessed by the Cochrane risk-of-bias tool, which consists of six factors: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data and selective reporting (Fig. 2). The methodological quality of retrospective studies was assessed by the modified Newcastle–Ottawa scale, which consists of three factors: patient selection, comparability of the study groups, and assessment of outcome. A score of 0–9 (allocated as stars) was allocated to each study (Table 2).
Figure 2.
Risk of bias graph—review authors' judgments about each risk of bias item presented.
Table 2.
Risk of bias in retrospective studies using modified Newcastle–Ottawa scale.
| Author (y) | Selection |
Comparability |
Outcome |
Quality score | ||||
|---|---|---|---|---|---|---|---|---|
| Assignment for treatmenta | Representative treatment group | Representative reference group | Comparable for 1,2,3,4b | Comparable for 5,6,7,8b | Assessment of outcome | Adequate follow-up | ||
| Koh (2012) | No | NA | NA | 1,2 | 6,7 | Yes | Yes | ★★★★ |
| Pers (2016) | No | NA | No | 1,2,3,4 | 6,7,8 | Yes | Yes | ★★★★★ |
| Jo (2017) | No | Yes | Yes | 1,2,3,4 | 6,7,8 | Yes | Yes | ★★★★★★★ |
| Zoran (2018) | No | Yes | Yes | 1,2,3,4 | 5,8 | Yes | Yes | ★★★★★★★ |
BMI = body mass index; NA = data not available; SF-36 = short form–36 health survey; VAS = visual analog scale; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
Comparability variables: 1 = age; 2 = gender; 3 = BMI; 4 = disease duration; 5 = Lysholm Knee Scoring Scale; 6 = WOMAC; 7 = SF-36; 8 = VAS.
Details of criteria for adequate random assignment of patients to treatments were provided.
If all characteristics were comparable, two stars; if two or three characteristics were comparable, one star; otherwise, no star.
Primary outcomes
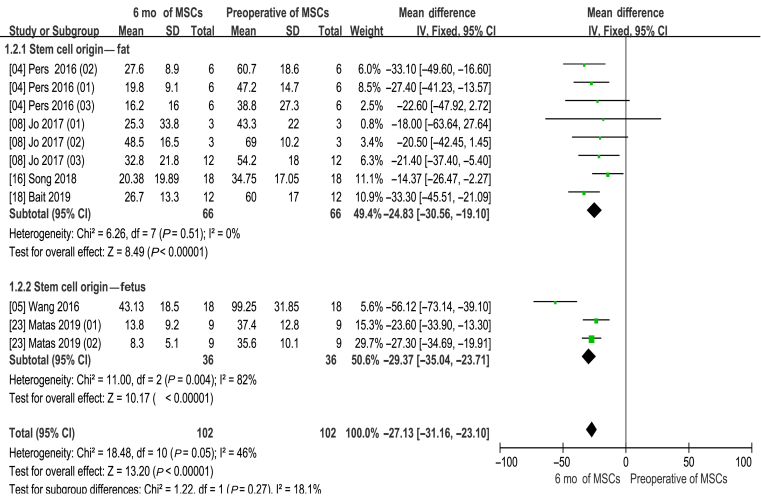
WOMAC 6 month
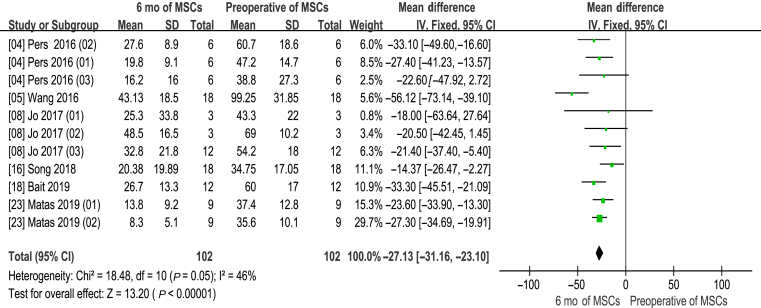
Information on the 6-month WOMAC improvement was available in six studies [4,5,8,16,18,23]. There are 11 experimental groups, including 102 patients. The mean difference of WOMAC changes was statistically significant, with value at −27.13 (95% CI −31.16 to −23.10, p < 0.00001). In addition, the corresponding I2 was 46%, indicating that the degree of variability among the trials was consistent with what would be expected by chance alone (Fig. 3).
Figure 3.
Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score (6 months of MSCs and preoperative of MSCs). CI = confidence interval; MSCs = mesenchymal stem cells; SD = standard deviation; IV = Inverse Variance methods.
VAS score
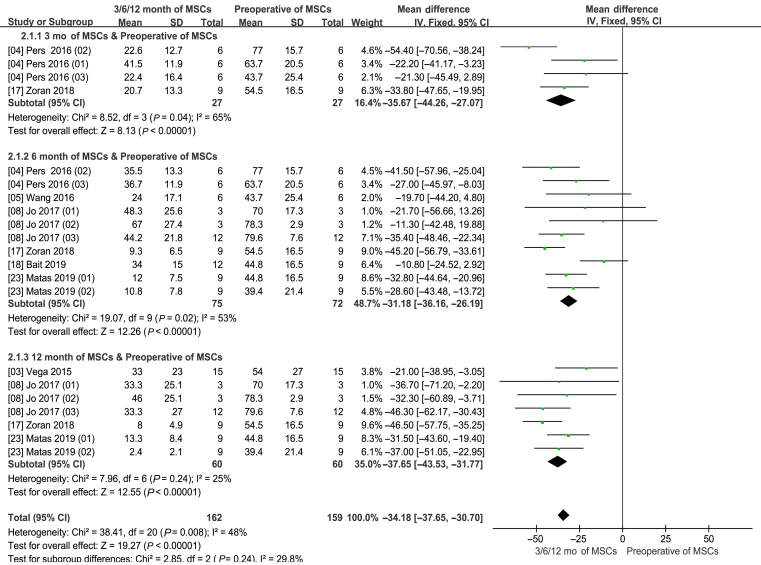
Information on the 3-month VAS improvement was available in two studies [4,17]. There are four experimental groups, including 27 patients. Information on the 6-month VAS improvement was available in six studies [4,5,8,17,18,23], with 10 experimental groups, including 75 patients. Information on the 12-month VAS improvement was available in four studies [3,8,17,23], with seven experimental groups, including a total of 60 patients.
The pooled data showed significant lower VAS scores in the MSCs group than the control group (WMD −34.18; 95% CI −37.65 to −31.77; p < 0.00001). In addition, the corresponding I2 was 48% (Fig. 4).
Figure 4.
Visual analog scale (VAS) score (3–6–12 months of MSCs and preoperative of MSCs). CI = confidence interval; MSCs = mesenchymal stem cells; SD = standard deviation; IV = Inverse Variance methods.
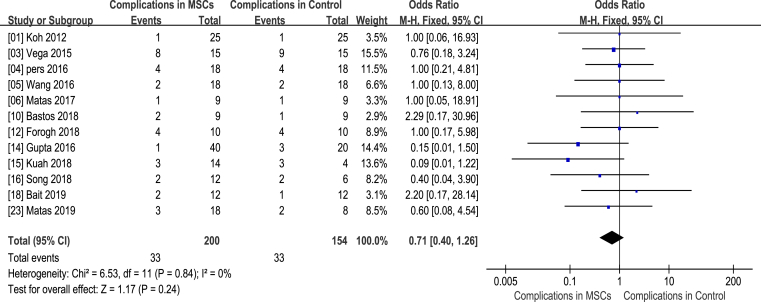
Complication
The clinical trials included in this meta-analysis reported several AEs, including pain at injection site [6], persistent bleeding [11,15], knee swelling [8], fracture [23], difficulty in moving knee [20], infection in knee [3,6,12], nervous system disorders [17], acute myocardial infarction [14,16], ileus [15] and small-intestine obstruction [26]. However, there was no statistical difference in what between the MSC treatment groups and controls (WMD 0.71; 95% CI 0.40–1.26; p = 0.24). In addition, the corresponding I2 was 0%, indicating that the degree of variability between the trials was consistent with what would be expected by chance alone. No serious adverse effects related to MSC implantation were developed in the 19 selected publications (Fig. 5).
Figure 5.
Safety assessment (MSCs and control groups). CI = confidence interval; MSCs = mesenchymal stem cells; M-H (Mantel-Haenszel) = Statistical Method for Calculating Odds Ratio of Binary Variables.
Subgroup analysis
Different cell origin of WOMAC score 6 months
There was no significant difference in this subgroup analysis compared with the original analysis (Fig. 6).
Figure 6.
WOMAC score of different cell origin (6 months of MSCs and preoperative of MSCs). CI = confidence interval; MSCs = mesenchymal stem cells; SD = standard deviation; IV = Inverse Variance methods.
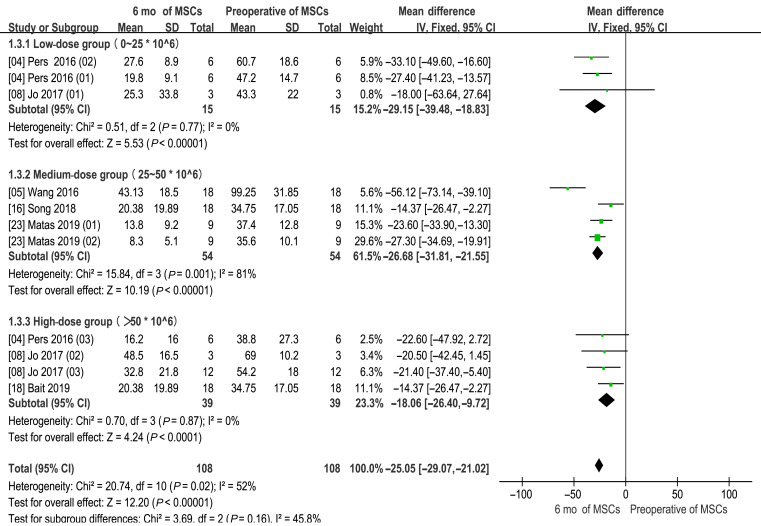
Different number of MSCs for WOMAC 6 months
There was no significant difference in this subgroup analysis compared with the original analysis (Fig. 7).
Figure 7.
WOMAC score of different number of cells (6 months of MSCs and preoperative of MSCs). CI = confidence interval; MSCs = mesenchymal stem cells; SD = standard deviation; IV = Inverse Variance methods.
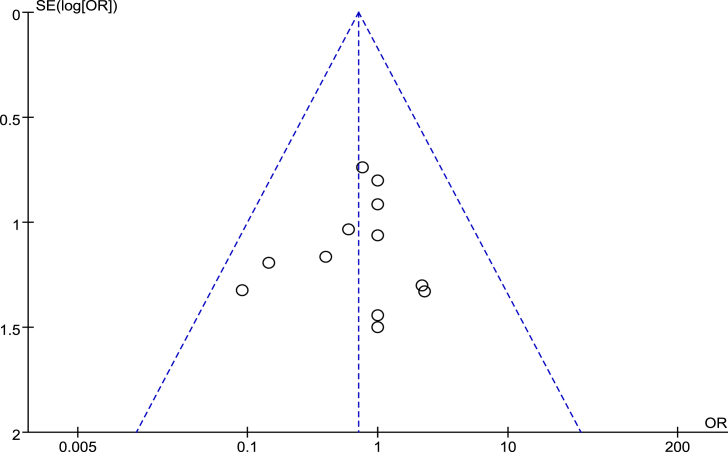
Sensitivity analysis and publication bias
Fifteen RCTs that assessed by the Cochrane risk-of-bias tool to complete bias analysis (Fig. 2) and four retrospective studies that scored six or more stars on the modified Newcastle–Ottawa scale were included in sensitivity analysis (Table 2). The degree of between-study heterogeneity is within reasonable limits [1,[3], [4], [5], [6],10,12,[14], [15], [16],18,23]. All the I2 were <50%. Fig. 8 shows a funnel plot of the studies included in this meta-analysis with AEs rates. All studies lied inside the 95% CIs, with an even distribution around the vertical, indicating no obvious publication bias.
Figure 8.
Funnel plots illustrating meta-analysis of complication rates. OR = odds ratio; SE = standard error.
Discussion
This meta-analysis covered 15 RCTs and four retrospective studies including 584 patients, and compared the efficacy of MSCs therapy and traditional treatment. The results showed that MSCs therapy was safe, effective and can significantly reduce postoperative pain.
OA is a degenerative disease characterized by reduction of articular chondrocytes and destruction of joint matrix [2]. Its clinical symptoms include slow progression of joint pain [7,9], tenderness [13], stiffness [25], joint swelling [20], limited movement [29], joint deformity, etc [5]. Knee OA is a progressive and degenerative condition, which will remain a serious clinical problem in orthopaedics unless significant advancements are made in regeneration technologies [2,7,20,22].
Current studies show that MSCs have the following functions: (1) interacting with the immune system and promote the immuno-regulation [26]; (2) migrating to the injury to enhance the tolerance of peripheral tissues, inhibit the release of inflammatory factors, promote the repair of injured tissues and increase the activity of injured cells [27,28]; (3) having great potential of multidirectional differentiation and reproductive activity [29]; and (4) secreting a variety of cytokines, such as transforming growth factor-β1, hepatocyte growth factor, fibroblast growth factor and vascular endothelial growth factor, which have an effect on anti-inflammatory, anti-apoptosis, anti-fibrosis, pro-angiogenesis, pro-mitosis, pro-wound healing, etc [30,31]. Therefore, the application of MSCs could be applied for OA treatment.
The results of this meta-analysis indicated that MSCs therapy could significantly reduce the VAS and WOMAC score, as well as improve the knee function and living quality for patients with OA. The research of Matas [39] showed that in a Phase I/II trial (NCT02580695), repeated Umbilical cord Mesenchymal Stem Cells treatment is safe, effective and superior to active comparator in knee OA after 1-year follow-up. Different injection doses [40] and different sources of MSCs [41] can alleviate pain and improve knee joint function in related studies [41]. In the study of Zhu et al. [22], the mechanism of action of MSCs may be attributed to the paracrine effect of stem cells. They evaluated the efficacy of MSC injections by macroscopic, histological and immunohistochemical analyses, and demonstrated that MSCs can achieve very good results in the treatment of knee OA [22].
Hyaluronic acid, PRP or corticosteroid are commonly used injection drugs for knee joint, which can also relieve pain and improve knee joint function [42]. The biggest advantage of MSCs is that they can improve and even repair cartilage [42], allowing damaged cartilage to regenerate, which is one of the biggest reasons for MSCs injection in knee could become a mainstream treatment in the future. Reports by Im [42] show that MSC-secreted factors target both synovium and articular chondrocytes to regulate anabolic and catabolic factors to induce/mediate tissue regeneration [42]. Other studies have shown that MSCs stimulate the proliferation and anabolic activity of articular chondrocytes, or promote the recruitment of stem cells/progenitor cells and cartilage differentiation [43]. In the objective evidence of cartilage repair, the related study mentioned that after cartilage healing, the degree of cartilage repair was confirmed by the degree of phase-contrast x-ray imaging. Phase-contrast x-ray imaging was found to be significantly improved after autologous MSC treatment and continued to improve during the second year of follow-up, indicating that MSCs contribute to cartilage repair or regeneration [8,44,45].
As a new treatment method, safety is the most important concern for patients. A number of researchers have evaluated the safety of MSCs. The safety of MSCs therapy has long been discussed and confirmed by numerous clinical trials. No significant adverse complication has been reported in any of the literatures in this review, and all included studies indicate that MSC injections in knee are safe. Most MSCs are low-immunogenic cells that rarely cause cellular immune responses in the body, causing side effects [[46], [47], [48]]. In a study of MSCs injection for up to 7 years of follow-up, patients had no adverse reaction [49].
In general, this meta-analysis was conducted at an appropriate time, because enough data have been accumulated for inspection by meta-analytical methods. We applied multiple strategies to identify studies, strict criteria to include and evaluate the methodological quality of the studies, and subgroup and sensitivity analyses to minimize the heterogeneity. Therefore, we are confident to provide the most up-to-date information in this field.
Limitation
There are some limitations in this meta-analysis: (1) WOMAC, VAS score and complications are subjective evaluation indexes. Although patients may answer the questionnaire truthfully, the risk of bias is unavoidable. (2) Included studies were worldwide, which performed by investigators with different levels and various methods to cultivate and preserve MSCs, with a risk of bias. (3) The sample capacity of all included studies is generally low. (4) Some clinical trials with negative results may not be published, which may also affect the results. It is expected that the evaluation of objective indicators would be adopted in the clinical trial design, such as examination of knee cavity effusion and magnetic resonance imaging examination of knee cartilage. We hope that our study would be of some help in the design of high-quality multicentre clinical trials in the future.
Future perspectives
With the gradual improvement of related technologies and processes, MSCs will be widely used in the clinical treatment of various degenerative diseases. However, it remains unclear which exact pathways and factors that participate in the mechanism of MSCs to repair the damaged knee joint cartilage. In recent years, related clinical trials are still limited. In addition, more clinical trials with the evaluation of the efficacy and safety of MSCs in the treatment of knee OA by subjective and objective indicators are needed. Furthermore, some factors related to therapeutic effect such as injection quantity, source and preservation of MSCs, and treatments combined MSCs with traditional knee injection therapy (sodium hyaluronate, glucocorticoid, PRP) are worthy to be explored to ensure the best therapeutic effect. Finally, with the continuous development of biomedical technology, individualized treatment is the trend of MSCs therapy in knee OA treatment.
Conclusions
A total of 19 selected publications regarding knee OA with 584 patients were included in the present meta-analysis. This analysis of MSC therapy in knee OA patients yielded encouraging results, with significant improvement in VAS, WOMAC and low rates of AEs. MSC therapy was shown to be effective, safe and has great potential as a clinical therapy for patients with knee OA. However, the safety and efficacy must be evaluated with a more rigorous, larger sample size validation before MSC therapy can be used in clinical practice.
Author contributions
Yancheng Song and Ling Kong had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Yancheng Song, Junhui Zhang: Conceptualization, Methodology. Junhui Zhang: Software. Yancheng Song: Validation. Ling Kong: Formal analysis. Hualiang Xu, Junhui Zhang, Wei Liu: Investigation, Resources. Junhui Zhang, Hualiang Xu, Zhujian Lin, Hong Chang: Data curation. Yancheng Song, Junhui Zhang: Writing - original draft. Ling Kong, Yancheng Song: Writing - review & editing. Ling Kong, Yancheng Song: Visualization and Supervision. Junhui Zhang: Project administration. Ling Kong, Yancheng Song: Funding acquisition.
Conflict of Interest
The authors have no conflicts of interest to disclose in relation to this article.
Acknowledgement
The authors have no acknowledgements to disclose and they received no funding for the work described in this article.
Contributor Information
Yancheng Song, Email: songyancheng@21cn.com.
Ling Kong, Email: kong_ling@grmh-gdl.cn.
References
- 1.Koh Y.G., Choi Y.J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. The Knee. 2012;19(6):902–907. doi: 10.1016/j.knee.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh P., Smith M. Osteoarthritis, genetic and molecular mechanisms. Biogerontology. 2002;3(1–2):85–88. doi: 10.1023/a:1015219716583. [DOI] [PubMed] [Google Scholar]
- 3.Vega Aurelio, Martín-Ferrero Miguel Angel, Canto Francisco Del, Alberca Mercedes, García Veronica, Munar Anna. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells. Transplantation. 2015;99(8):1681–1690. doi: 10.1097/TP.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 4.Pers Y.M., Rackwitz L., Ferreira R., Pullig O., Delfour C., Barry F. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl Med. 2016;5:847–856. doi: 10.5966/sctm.2015-0245. sctm.2015-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y.L., Jin W.X., Liu H.Y., Cui Y.H., Mao Q.C., Fei Z.Y. Curative effect of human umbilical cord mesenchymal stem cells by intra-articular injection for degenerative knee osteoarthritis. Zhongguo xiu fu chong jian wai ke za zhi = Zhongguo xiufu chongjian waike zazhi = Chinese journal of reparative and reconstructive surgery. 2016;30(12):1472. doi: 10.7507/1002-1892.20160305. [DOI] [PubMed] [Google Scholar]
- 6.Espinoza F., Matas J., Orrego M., Tapia R., Infante C., Khoury M. Allogeneic mesenchymal stromal cell (MSC) therapy for knee osteoarthritis (OA): a phase I/II randomized controlled trial. Cytotherapy. 2017;19(5):24. [Google Scholar]
- 7.Hootman Jennifer M., Helmick Charles G., Barbour Kamil E., Theis Kristina A., Boring Michael A. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015-2040. Arthritis Rheum. 2016;68(7):1582–1587. doi: 10.1002/art.39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jo C.H., Chai J.W., Jeong E.C., Oh S., Shin J.S., Shim H. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a 2-year follow-up study. Am J Sports Med. 2017;8(10) doi: 10.1177/0363546517716641. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro Shane A., Kazmerchak Shari E., Heckman Michael G., Zubair Abba C., O’Connor Mary I. A prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am J Sports Med. 2016;45(1):82. doi: 10.1177/0363546516662455. [DOI] [PubMed] [Google Scholar]
- 10.Bastos Ricardo, Mathias Marcelo, Andrade Renato, Bastos Raquel, Balduino Alex, Schott Vinicius. Knee Surgery, Sports Traumatology, Arthroscopy; 2018. Intra-articular injections of expanded mesenchymal stem cells with and without addition of platelet-rich plasma are safe and effective for knee osteoarthritis. [DOI] [PubMed] [Google Scholar]
- 11.Liastani M.G., Emadedin M., Labibzadeh N., Ghorbani Liastani M., Karimi A., Jaroughi N. Intra-articular implantation of autologous bone marrow-derived mesenchymal stromal cells to treat knee osteoarthritis: a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. BMC Muscoskel Disord. 2018;20(10):1238–1246. doi: 10.1016/j.jcyt.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Forogh B., Soltani S.K., Ahmadbeigi N., Kharazi H.H., Fallahzadeh K, Kashanid L. Safety and efficacy of allogenic placental mesenchymal stem cells for treating knee osteoarthritis: a pilot study. Bioimpacts. 2018;(8):89. doi: 10.1016/j.jcyt.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Jones I.A., Wilson M., Togashi R., Han B., Mircheff A.K., Thomas Vangsness C. A randomized, controlled study to evaluate the efficacy of intra-articular, autologous adipose tissue injections for the treatment of mild-to-moderate knee osteoarthritis compared to hyaluronic acid: a study protocol. BMC Muscoskel Disord. 2018;19(1):383. doi: 10.1186/s12891-018-2300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta P.K., Chullikana A., Rengasamy M., Shetty N., Pandey V., Agarwal V. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther. 2016;18(1):301. doi: 10.1186/s13075-016-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuah D., Sivell S., Longworth T., James K., Guermazi A., Cicuttini F. Safety, tolerability and efficacy of intra-articular Progenza in knee osteoarthritis: a randomized double-blind placebo-controlled single ascending dose study. J Transl Med. 2018;16(1):49. doi: 10.1186/s12967-018-1420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song Y., Du H., Dai C.X., Zhang L., Li S.K., Hunter D.J. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen Med. 2018;2:1–13. doi: 10.2217/rme-2017-0152. rme-2017-0152. [DOI] [PubMed] [Google Scholar]
- 17.Baščarević Zoran, Spasovski Duško, Spasovski Vesna, Stojiljković Maja, Vreća Miša, Andjelković Marina. Intra-articular injection of autologous adipose derived mesenchymal stem cells in treatment of knee osteoarthritis. J Gene Med. 2018;20(1):909. doi: 10.1002/jgm.3002. [DOI] [PubMed] [Google Scholar]
- 18.Woo-Suk Lee, Hwan Jin Kim, Kang-Il Kim, Gi Beom Kim, Wook Jin. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Translat Med. 2019;0:1–8. doi: 10.1002/sctm.18-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freitag Julien, Dan Bates, Wickham James, Kiran Kiran, Leesa Huguenin, Abi Tenen. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med. 2019;2:1–18. doi: 10.2217/rme-2018-0161. 1746-0751. [DOI] [PubMed] [Google Scholar]
- 20.Qingyun X., Kunzheng W., Fuxing P., Hanmin Z., Dadi J., Tianzun T. The survey of the prevalence of primary osteoarthritis in the population aged 40 years and over in China. Chin J Orthop. 2015;35(12):1206–1212. [Google Scholar]
- 21.Khasru M.R., Salek A.M., Marzen T., Siddip A., Islam M. Peripheral blood derived stem cells in OA knee at low resource setting: a phase II RCT. Osteoarthritis Cartilage. 2019;27:512. [Google Scholar]
- 22.Zhu Yu, Yuchen Wang, Bizeng Zhao, Xin Niu, Bin Hu, Qing Li. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther. 2017;8(1):64. doi: 10.1186/s13287-017-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matas J., Orrego M., Amenabar D., Infante C., Tapia-limonchi R., Cadiz M.I. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Translat Med. 2019;8:215–224. doi: 10.1002/sctm.18-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freitag J., Bates D., Boyd R., Shah K., Barnard A., Huguenin L. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy --a review. BMC Muscoskel Disord. 2016;17(1):230. doi: 10.1186/s12891-016-1085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones I.A., Togashi R., Wilson M.L., Heckmann N., Vangsness C.T. Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol. 2019;15(2):77–90. doi: 10.1038/s41584-018-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coulson-Thomas V.J., Coulson-Thomas Y.M., Gesteira T.F., Kao W.Y. Extrinsic and intrinsic mechanisms by which mesenchymal stem cells suppress the immune system. Ocul Surf. 2016;14(2):121–134. doi: 10.1016/j.jtos.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryu Jae-Sung, Jung Yeon-Hwa, Cho Mi-Young, Yeo Jee Eun, Choi Yun-Jin, Kim Yong Il. Co-culture with human synovium-derived mesenchymal stem cells inhibits inflammatory activity and increases cell proliferation of sodium nitroprusside-stimulated chondrocytes. Biochem Biophys Res Commun. 2014;447(4):715–720. doi: 10.1016/j.bbrc.2014.04.077. [DOI] [PubMed] [Google Scholar]
- 28.Barry F., Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol. 2013;9(10):584–594. doi: 10.1038/nrrheum.2013.109. [DOI] [PubMed] [Google Scholar]
- 29.Mamidi M.K., Das A.K., Zakaria Z., Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016;24(8):1307–1316. doi: 10.1016/j.joca.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Caplan A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2010;213(2):341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 31.Susanne G., Julia L. Tissue-engineering strategies to repair chondral and osteochondral tissue in osteoarthritis: use of mesenchymal stem cells. Curr Rheumatol Rep. 2014;16(10):452. doi: 10.1007/s11926-014-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., AIoannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stroup D.F., Berlin J.A., Morton S.C. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 34.Phillips B., Ball C., Sackett D., Badenoch D., Straus S., Haynes B. Oxford Centre for Evidence-Based Medicine Levels of Evidence (March 2009); 26 March 2009. Levels of Evidence and Grades of Recommendation.http://www.cebm.net/index.aspx?o=1025 Medicine Web site. [Google Scholar]
- 35.Higgins J., Green S. Cochrane Collaboration, John Wiley and Sons; New York, NY: 2008. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 36.Taggart D.P., D'Amico R., Altman D.G. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet. 2001;358:870–875. doi: 10.1016/S0140-6736(01)06069-X. [DOI] [PubMed] [Google Scholar]
- 37.Wells G.A., Shea B., O'Connell D., Peterson J., Welch V., Losos M. The Ottawa Hospital; 15 March 2012. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 38.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matas J., Orrego M., Amenabar D., Infante C., Tapia-Limonchi R., Cadiz M.I. Umbilical cord-derived mesenchymal stromal cells (MSCS) for knee osteoarthritis: repeated MSC dosing is superior to a single msc dose and to hyaluronic acid in a controlled randomized phase i/ii trial. Stem Cells Transl Med. 2018:1–10. doi: 10.1002/sctm.18-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui G., Wang Y., Li C., Wang W. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: a meta-analysis. Exp Therapeut Med. 2016;12:3390–3400. doi: 10.3892/etm.2016.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang Y.H., Liu H.W., Wu K.C., Ding D.C. Mesenchymal stem cells and their clinical applications in osteoarthritis. Cell Transplant. 2016;25(5):937–950. doi: 10.3727/096368915X690288. [DOI] [PubMed] [Google Scholar]
- 42.Im G.I. Tissue engineering in osteoarthritis: current status and prospect of mesenchymal stem cell therapy. BioDrugs. 2018;32(4):183–192. doi: 10.1007/s40259-018-0276-3. [DOI] [PubMed] [Google Scholar]
- 43.Saulnier N., Viguier E., Perrier-Groult E., Chenu C., Pillet E., Roger T. Intra-articular administration of xenogeneic neonatal mesenchymal stromal cells early after meniscal injury downregulates metalloproteinase gene expression in synovium and prevents cartilage degradation in a rabbit model of osteoarthritis. Osteoarthritis Cartilage. 2015;23(1):122–133. doi: 10.1016/j.joca.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Delgado-Enciso I., Paz-Garcia J., Valtierra-Alvarez J., Preciado‑Ramirez J., Almeida‑Trinidad R., Guzman‑Esquivel J. A phase I–II controlled randomized trial using a promising novel cell-free formulation for articular cartilage regeneration as treatment of severe osteoarthritis of the knee. Eur J Med Res. 2018;23(1) doi: 10.1186/s40001-018-0349-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao G., Zhang Z., Hu S., Zhang Z., Chang Z., Huang Z. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther. 2018;9(1) doi: 10.1186/s13287-018-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iijima H., Isho T., Kuroki H., Takahashi M., Aoyama T. Effectiveness of mesenchymal stem cells for treating patients with knee osteoarthritis: a meta-analysis toward the establishment of effective regenerative rehabilitation. NPJ Regen Med. 2018;3(1) doi: 10.1038/s41536-018-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamei N., Ochi M., Adachi N., Ishikawa M., Yanada S., Levin L.S. The safety and efficacy of magnetic targeting using autologous mesenchymal stem cells for cartilage repair. Knee Surg Sports Traumatol Arthrosc. 2018 doi: 10.1007/s00167-018-4898-2. [DOI] [PubMed] [Google Scholar]
- 48.Riester S.M., Denbeigh J.M., Lin Y., Jones D.L., Moou T.D., Lewallen E.A. Safety studies for use of adipose tissue-derived mesenchymal stromal/stem cells in a rabbit model for osteoarthritis to support a phase I clinical trial. Stem Cells Transl Med. 2017;6(3):1–13. doi: 10.5966/sctm.2016-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park Y.B., Ha C.W., Lee C.H., Yoon Y.C., Park Y.G. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med. 2017;6(2):613–621. doi: 10.5966/sctm.2016-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]