Abstract
Background
Interleukin 22(IL-22), a recently identified, Th-22 associated cytokine has a key role in the production of human β defensin-2(hBD-2) and hence an indirect role in innate, nonspecific immunity. Hence this study was conducted as preliminary research to quantify and correlate the levels of IL-22 and hBD-2 in periodontal disease.
Materials and methods
Gingival crevicular fluid from subjects with chronic periodontitis (n = 27), gingivitis (n = 25) and healthy controls (n = 27) were obtained for the study. The periodontal status of each subject was assessed by criteria based on plaque index, gingival index, bleeding on probing, probing depth and clinical attachment loss. The levels of IL-22 and hBD-2 were measured in GCF samples with an enzyme-linked immunosorbent assay.
Result
Periodontal parameters were described and reported as mean values. The distribution and comparison of clinical parameters and gingival crevicular fluid IL-22, hBD-2 levels in healthy controls, gingivitis and chronic periodontitis groups were assessed using one way ANOVA test. Post Hoc Bonferroni test was used for intergroup comparisons. Association among the IL-22 , hBD-2 and clinical parameters in all three groups were examined using Pearson's correlation test.
The IL-22 level was significantly greater in chronic periodontitis group than healthy controls (P < 0.001) and gingivitis group (P < 0.001). The hBD-2 level was significantly higher in the chronic periodontitis group compared to gingivitis (P = 0.003) and healthy controls (P < 0.001). The results also showed a statistically significant correlation (P = 0.002) between IL-22 and hBD-2 concentration in chronic periodontitis group.
Conclusion
These results indicate the role of IL- 22 and hBD-2 in the innate immune response during periodontitis.
Keywords: Interleukin 22 protein, Human, Beta-defensin 2, Periodontal disease, Gingival crevicular fluid, Enzyme-linked immunosorbent assay
1. Introduction
Periodontitis is a chronic inflammatory disease which is multifactorial in origin as other than the microbial challenge a highly orchestrated host bacterial interaction leads to the disease initiation and progression. Numerous researchers have proved the role of host immune modulators like cytokines in the advancement or regression of the disease.1,2
The oral epithelium being the first line of defense is constantly stimulated by an array of microorganisms in the oral cavity and interestingly has been established to have immunomodulatory effects as well in addition to being just a physical barrier.3 Antimicrobial peptides are a group of peptides that are produced by these epithelia and are actively defensive against various microbes. The Human β defensin (hBD-2) proteins are low molecular weight protein belonging to the group of antimicrobial peptides and are locally produced by the keratinocytes of various tissues, especially skin and mucosa.4 These peptides have been shown to be produced in response to infection and inflammation in non-oral tissues.5 However studies have shown expression of these proteins in normal uninflamed tissues6 which could be due to constant exposure of oral epithelium to microbial pathogens.
Interleukin 22, a member of the IL-10 family of cytokines has been shown to be produced by activated T helper (Th) cells like the Th-17, Th-22 and activated Natural killer cells. This cytokine has been proven to have no direct action on immune cells and rather have targets amongst tissue cells like the keratinocytes and indirectly increase the innate, nonspecific immunity against pathogens.7 Wolk et al. observed that IL-22, in addition to increasing the proliferative capacity and other cellular activities of the keratinocytes, also elevated the production of hBD-2.8 He furthermore demonstrated an increased expression of IL-22 in diseased, inflamed skin and no expression of the cytokine in healthy skin partly reasoning the elevated expressions of hBD2,-3 in inflammatory skin diseases like Psoriasis.7
Interleukin- 22 has been studied in various systemic diseases like Pneumonia9 where it was noted that its expression was elevated on exposure to gram negative bacteria, Crohn's disease, wherein IL-22 promoted expression of pro-inflammatory cytokines.10 Radaeva et al. have shown in murine model that IL-22 cytokine serves as a protective factor against liver injury and also acts as a survival factor for hepatocytes.11 Wolk et al. have shown that IL-22 regulate few but important functions of keratinocytes like production of antimicrobial proteins, mobility and migration regulating proteins and differentiation – associated proteins.12 It also has been presented that IL-22 directly increased the transcription of hBD-2 production from keratinocytes isolated from dermal tissue.7,13 However there are not enough data on the role of IL-22 in periodontitis and in the production of hBD-2 form the gingival epithelium. Since IL-22 is involved in defensin production which in turn is an integral feature of the innate immune response of the periodontal tissues, we speculate that this cytokine might play a role in the etiopathogenesis of periodontitis. So this study was undertaken to better understand the expression levels of IL-22, hBD-2 in periodontal tissues.
2. Materials and methods
The study population consists of patients from the Department of Periodontology, Dr.D.Y.Patil Dental College and Hospital, Pimpri, Pune. The study protocol was designed in accordance with Helsinki declaration of 1975, as revised in 2013 and was approved by the university ethics committee (DYPV/EC/122/17).All participants willing to participate in our study signed an informed consent, were in good general heath, had no history of periodontal therapy, nor had they taken antibiotics/anti-inflammatory drugs within 6 months. Smokers, pregnant women, patients with underlying systemic disease were excluded from the study.
Sample size estimation was based on data provided by Chen et al., 2014 for expected means of GCF hBD-2 levels as measured by ELISA in chronic periodontitis, gingivitis and healthy control subjects (chronic periodontitis = 266.91 pg/mL ± 311.81 pg/mL, patients with chronic gingivitis:487.80 ± 420.66 pg/mL and healthy controls was 99.20 ± 99.98 pg/mL).14 Using a sample size of 25 subjects per group the overall estimated effect size was determined as f = 0.52 (F-test ANOVA) at p = 0.05 and 80% power, which was in the large range (>0.40) and thus deemed acceptable.15 Since there are no preliminary data for correlation of IL- 22 and hBD-2 in each these groups, we estimated the power of this sample to detect a significant large correlation effect (>0.5) and at n = 25, the sample had 80% power at p = 0.05 to determine a Pearson's correlation coefficient of 0.53.
2.1. Clinical measures
Clinical measurements made were plaque index (PI), gingival index (GI), bleeding on probing (BOP), probing depth (PD) and clinical attachment level (CAL). The measurements were taken at 6 sites per tooth and entered manually in the data sheet. Probing pocket depth and attachment level measurements were made to the nearest millimeter using an UNC 15 Probe.
A total of 79 subjects were recruited in the study and were grouped according to their periodontal status. Group 1 (Healthy controls) included 27 subjects with a plaque index (PI) of <1, gingival index (GI) of <1, probing depth (PD) of ≤ 3 mm, and no loss of attachment. Group 2 (Gingivitis) included 25 subjects with a PI of ≥1, GI of ≥1, PD ≤ 3 mm, clinical evidence of inflammation based on presence of BOP ≥ 50% of sites and no clinical attachment loss. Group 3 (Chronic periodontitis) had 27 subjects with a PI of ≥1, GI of ≥1, Probing depth (PD) ≥ 5 mm and clinical attachment level(CAL) ≥ 3 mm and presence of BOP ≥ 50% of sites with radiographic evidence of bone loss.16,17
2.2. GCF collection
GCF collection was done a day after the periodontal examination. In healthy individuals, sites with PD ≤ 3 mm and no signs of inflammation, in gingivitis patients, sites with bleeding on probing and in chronic periodontitis subjects, the sites with PD ≥ 5 mm were chosen for acquiring GCF. The area was isolated with gauze to avoid saliva contamination, after drying the area with air, supragingival plaque was removed with curette (Hu Friedy, USA), a calibrated volumetric microcapillary pipette (Sigma Aldrich) was placed at the entrance of the gingival sulcus, gently touching the gingival margin. From each test site, 2 μl of GCF was collected. The collected GCF was then transferred to a sterile Eppendorf tube and stored at −70oc until the assay. Micropipettes with contaminants like blood or saliva were discarded.18
The GCF level of IL-22, hBD-2 was assayed using commercially available ELISA kit (E Lab Biosciences) [Catalog no: E-EL-H0106 (IL-22), Catalog no: E-EL-H0996 (hBD-2]. The procedure was performed according to the instructions given in the kit.
2.3. Quantification of IL -22
Briefly, to a human IL-22 antibody precoated ELISA plate 100 μL of the standard or sample is added and incubated for 90 minutes at 37 °C and the free components are washed. 100 μL biotinylated detection antibody is added and incubated for 1 hour at 37 °C and washed. Then 100 μL of horse radish peroxide (HRP) conjugate is added and incubated for 30 minutes at 37 °C and washed. 90 μL of substrate reagent is added and incubated for 15 minutes at 37 °C followed by the addition of 50 μL stop solution. The optical density is measured with a spectrometry at a wave length of 450 nm immediately. The minimum detection limit for IL-22 is 9.38 pg/mL. The levels of IL-22 in each sample were determined using the concentration values of standards included in the kit contents. The results were expressed in pg/mL.
2.4. Quantification of hBD-2
Briefly, to a human hBD-2 antibody precoated ELISA plate 100 μL of the standard or sample is added and incubated for 90 minutes at 37 °C and the free components are washed. Add 100 μL biotinylated detection antibody is added and incubated for 1 hour at 37 °C and washed. Then 100 μL of horse radish peroxide (HRP) conjugate is added and incubated for 30 minutes at 37 °C and washed. 90 μL of substrate reagent is added and incubated for 15 minutes at 37 °C followed by the addition of 50 μL stop solution. The optical density is measured with a spectrometry at a wave length of 450 nm immediately. The minimum detection limit for hBD-2 is 37.50 pg/mL. The levels of hBD-2 in each sample were determined using the concentration values of standards included in the kit contents. The results were expressed in pg/mL.
3. Results
3.1. Statistical analysis
The statistical analysis was done using the Statistical package for the Social science (SPSS) for windows, version 19; SPSS Inc. Chicago, IL, USA. All the periodontal parameters were described and reported as mean values (±SD). The distribution and comparison of clinical parameters and gingival crevicular fluid IL-22, hBD-2 levels in healthy controls, gingivitis and chronic periodontitis groups were assessed using one way ANOVA test.
Post Hoc Bonferroni test was used for intergroup comparison of clinical parameters and gingival crevicular fluid (GCF) IL-22, hBD-2 levels in healthy controls, gingivitis and chronic periodontitis groups. Association among the IL-22 and hBD-2 and clinical parameters in all three groups were examined using Pearson's correlation test. Probability value of less than 0.05 was considered statistically significant for all analyses.
3.2. Clinical analysis
The healthy control group had 27 subjects with a mean age of 25.14 years and 25 gingivitis subjects with a mean age of 31.92 years and 27 patients with periodontitis with a mean age of 47.00 years. Periodontal parameters of the study groups are shown in Table 1. All clinical parameters were significantly higher in the chronic periodontitis group than the healthy and gingivitis groups (P < 0.05).
Table 1.
Distribution and comparison of study parameters in the three different study groups.(One-way analysis of variance [ANOVA];p < 0.05; significant)
| Study Parameters | Study groups | N | Mean | Std. Deviation | F | Sig |
|---|---|---|---|---|---|---|
| IL-22 Conc. (pg/ml) | Healthy control | 27 | 34.66667 | 19.479694 | 18.66 | <0.0001 |
| Gingivitis | 25 | 31.69920 | 5.123607 | |||
| Chronic periodontitis | 27 | 61.95556 | 27.433911 | |||
| Total | 79 | 43.05418 | 23.974608 | |||
| hBD-2 Conc. (pg/ml) | Healthy control | 27 | 268.14815 | 114.410766 | 9.58 | <0.0001 |
| Gingivitis | 25 | 294.96000 | 138.654390 | |||
| Chronic periodontitis | 27 | 487.40741 | 293.267436 | |||
| Total | 79 | 351.56962 | 220.840509 | |||
| Gingival Index | Healthy control | 27 | .64111 | .188931 | 522.7 | <0.0001 |
| Gingivitis | 25 | .46800 | .108704 | |||
| Chronic periodontitis | 27 | 2.32519 | .331156 | |||
| Total | 79 | 1.16190 | .876770 | |||
| Plaque Index | Healthy control | 27 | .62111 | .217792 | 729.7 | <0.0001 |
| Gingivitis | 25 | .48160 | .130916 | |||
| Chronic periodontitis | 27 | 2.46000 | .257965 | |||
| Total | 79 | 1.20544 | .934990 | |||
| Probing Depth (mm) | Healthy control | 27 | 1.59259 | .500712 | 299.4 | <0.0001 |
| Gingivitis | 25 | 1.94560 | .487631 | |||
| Chronic periodontitis | 27 | 5.91111 | 1.015178 | |||
| Total | 79 | 3.18025 | 2.107808 | |||
| Clinical Attachment Level (mm) | Healthy control | 27 | .00000 | .000000 | 735.2 | <0.0001 |
| Gingivitis | 25 | .00000 | .000000 | |||
| Chronic periodontitis | 27 | 6.19815 | 1.165019 | |||
| Total | 79 | 2.11835 | 3.034085 |
3.3. GCF IL-22 levels
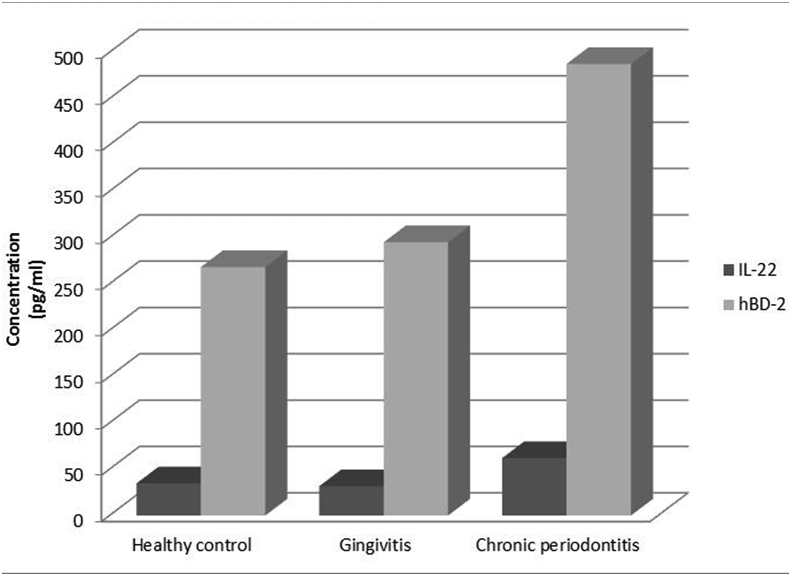
The mean GCF levels of IL-22 (Table 1, Graph 1) and the mean difference between groups are represented (Table 2). IL-22 levels were higher in the chronic periodontitis group than healthy control group (P < 0.05) and gingivitis group (P < 0.05).However, there were no statistical differences between healthy and gingivitis groups in terms of total amount of IL-22 in GCF.
Graph 1.
Gingival crevicular fluid levels of Interleukin-22 [in pg/mL] and Human β defensin-2 [in pg/mL] in healthy controls (Group 1), gingivitis (Group 2) and chronic periodontitis (Group 3).
Table 2.
Comparison of the study parameters in the three groups (Group 1- Healthy control; Group 2- Gingivitis; Group 3- Chronic periodontitis; Bonferroni post hoc test; *p < 0.05; significant).
| Study Parameters | (I) Groups | (J) Groups | Mean Difference (I-J) | Std. Error | Sig. |
|---|---|---|---|---|---|
| IL-22 Conc. (pg/ml) | Group1 | Group2 | 2.967467 | 5.520352 | 1.000 |
| Group3 | −27.288889* | 5.413151 | .000 | ||
| Group2 | Group1 | −2.967467 | 5.520352 | 1.000 | |
| Group3 | −30.256356* | 5.520352 | .000 | ||
| Group3 | Group1 | 27.288889* | 5.413151 | .000 | |
| Group2 | 30.256356* | 5.520352 | .000 | ||
| hBD-2 Conc. (pg/ml) | Group1 | Group2 | −26.811852 | 55.491797 | 1.000 |
| Group3 | −219.259259* | 54.414184 | .000 | ||
| Group2 | Group1 | 26.811852 | 55.491797 | 1.000 | |
| Group3 | −192.447407* | 55.491797 | .003 | ||
| Group3 | Group1 | 219.259259* | 54.414184 | .000 | |
| Group2 | 192.447407* | 55.491797 | .003 | ||
| Gingival Index | Group1 | Group2 | .173111* | .064175 | .026 |
| Group3 | −1.684074* | .062928 | .000 | ||
| Group2 | Group1 | -.173111* | .064175 | .026 | |
| Group3 | −1.857185* | .064175 | .000 | ||
| Group3 | Group1 | 1.684074* | .062928 | .000 | |
| Group2 | 1.857185* | .064175 | .000 | ||
| Plaque Index | Group1 | Group2 | .139511 | .058488 | .059 |
| Group3 | −1.838889* | .057352 | .000 | ||
| Group2 | Group1 | -.139511 | .058488 | .059 | |
| Group3 | −1.978400* | .058488 | .000 | ||
| Group3 | Group1 | 1.838889* | .057352 | .000 | |
| Group2 | 1.978400* | .058488 | .000 | ||
| Probing Depth(mm) | Group1 | Group2 | -.353007 | .198879 | .240 |
| Group3 | −4.318519* | .195017 | .000 | ||
| Group2 | Group1 | .353007 | .198879 | .240 | |
| Group3 | −3.965511* | .198879 | .000 | ||
| Group3 | Group1 | 4.318519* | .195017 | .000 | |
| Group2 | 3.965511* | .198879 | .000 | ||
| Clinical Attachment Level(mm) | Group1 | Group2 | .000000 | .189131 | 1.000 |
| Group3 | −6.198148* | .185458 | .000 | ||
| Group2 | Group1 | .000000 | .189131 | 1.000 | |
| Group3 | −6.198148* | .189131 | .000 | ||
| Group3 | Group1 | 6.198148* | .185458 | .000 | |
| Group2 | 6.198148* | .189131 | .000 |
3.4. GCF hBD-2 levels
The mean GCF levels of hBD-2(Table 1, Graph 1) and the mean difference between groups are represented (Table 2). The hBD−2 concentration was significantly higher in the chronic periodontitis group compared to gingivitis group (P = 0.003) and healthy control group (P < 0.05).However there were no statistical differences between healthy and gingivitis groups in terms of total amount of hBD-2 in GCF.
3.5. Correlations
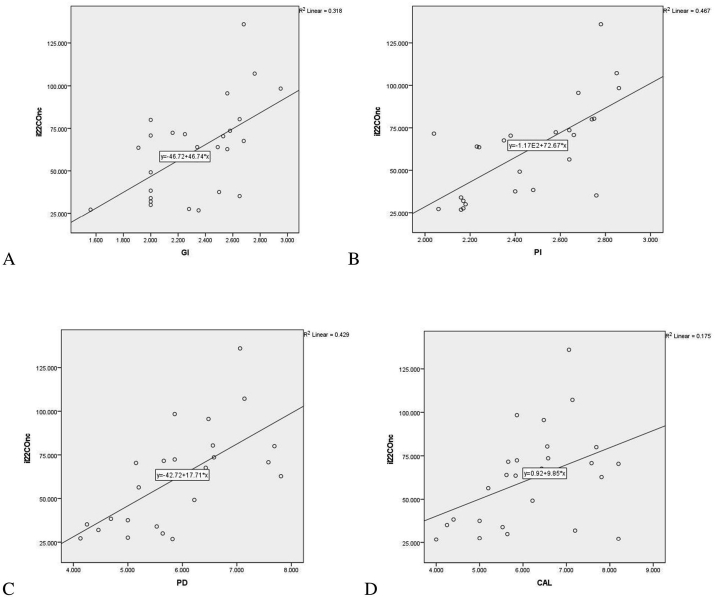
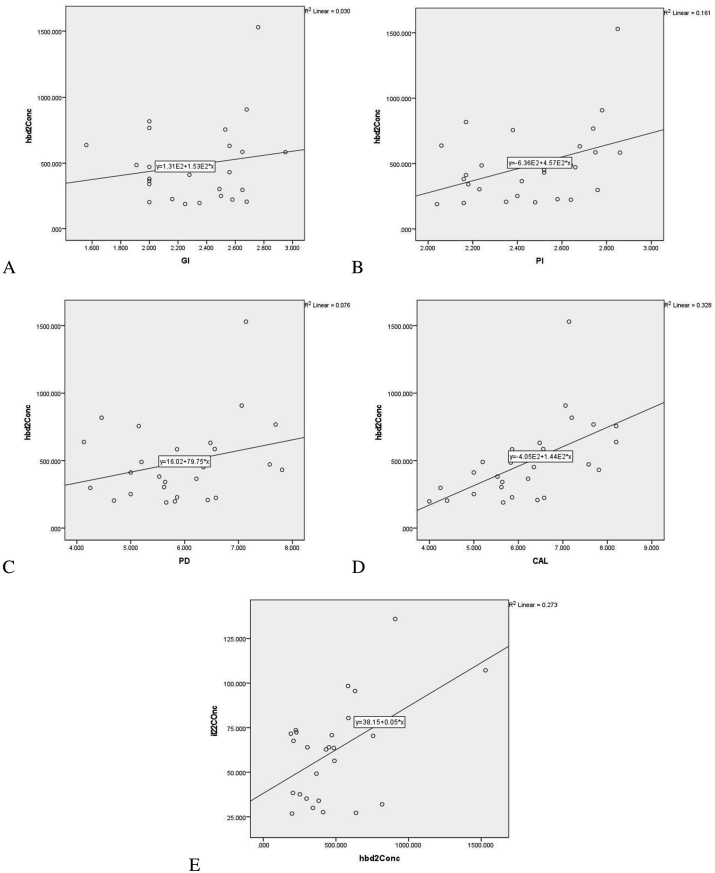
There was significant positive correlation between GCF total amount of IL-22 and GI (P < 0.01), PI (P < 0.01), PD (P < 0.00.01), CAL (P < 0.05) [Table 3, Graph 2(A, B, C, D)] in chronic periodontitis group (Group 3). Positive correlation between GCF total amount of hBD-2 and PI (P < 0.05) and CAL (P < 0.01) [Table 3, Graph 3(B, D)] was observed in chronic periodontitis group. Positive correlation was observed between IL-22 and hBD-2 levels in GCF of CP Group (P < 0.005) [Table 3, Graph 3(E)]. The correlation between clinical parameters and gingival crevicular fluid levels of hBD-2 and IL-22 in healthy controls and gingivitis group was statistically insignificant (data not shown).
Table 3.
Correlation between GCF IL-22, hBD-2 levels and clinical parameters in chronic periodontitis group. (Pearson's correlation coefficient - r; **.Correlation is significant at the 0.01 level (2-tailed);*. Correlation is significant at the 0.05 level (2-tailed)).
| GI (r) | PI (r) | PD (r) | CAL (r) | Correlation of IL-22 with hBD-2 (r) | |
|---|---|---|---|---|---|
| IL-22 | .564** | .683** | .655** | 0.418* | .522* |
| hBD-2 | .173 | .402* | 0.276 | .572** |
Graph 2.
Correlation between gingival crevicular fluid levels of Interleukin-22 [in pg/ml] and gingival index (A), plaque index(B), probing depth (C) [in mm] and clinical attachment level(D)[in mm] in chronic periodontitis group (Group 3).
Graph 3.
Correlation between gingival crevicular fluid levels of Human β defensin-2[in pg/ml] and gingival index(A), plaque index(B), probing depth (C)[in mm] and clinical attachment level(D)[in mm] in chronic periodontitis group (Group 3), (E) Correlation between gingival crevicular fluid levels of Interleukin-22[in pg/ml] and Human β defensin-2[in pg/ml] levels in chronic periodontitis group (Group 3).
4. Discussion
IL- 22, a recently identified cytokine has been proven to have a pivotal role in mucosal immunity.19,20 However, limited evidence exists for the role of IL-22 in periodontal tissues.21 hBD-2 on the other hand is a vastly studied antimicrobial peptide in the oral cavity. Various researchers have reported an increased expression of this cytokine in periodontally compromised patients.22, 23, 24
Our hypothesis in this study was that IL-22, a Th 22 cell associated cytokine having both pro-, as well an anti-inflammatory action contributing to the innate immunity in periodontal disease by aiding in the expression of hBD-2 from gingival keratinocytes. hBD-2 has been shown to be induced by various cytokines like TNF-α,IL-1 β, IL-17,IL-6 25, 26, 27 and also IL-22.28,12,13 The IL- 22 cytokine has been vastly studied in skin lesions like psoriasis and atopic dermatitis and shown to induce keratinocytes for hBD-2 secretion. In an in vitro study by Wolk et al. it has been shown that keratinocytes constitutively expressed hBD-2 and is markedly increased by induction with the IL-22 molecule.12 This positive correlation was also observed in Alveolar epithelial8 and hepatic cell line.29 All the above literature indicates a role of IL- 22 in the production of hBD-2 and in turn a role in the innate immune mechanism.
The present study demonstrated significantly elevated levels of IL -22 and a positive correlation with clinical parameters in chronic periodontitis subjects. hBD-2 GCF levels were also significantly elevated and positively correlated with most of the clinical parameters in chronic periodontitis subjects. To our best understanding this is the first study to have evaluated the levels of IL-22 and hBD-2 in periodontal disease.
The positive correlation between GCF IL-22 levels and clinical parameters in this study might be explained by the fact that microbial plaque stimulates inflammatory response. Due to constant microbial challenge, there is recruitment of a repertoire of Th cells. These T helper cells express a variety of cytokines amongst which, Th 22 and Th 17 subset of T helper cells have been shown to secrete IL-22 cytokine.
Our study results show a positive correlation between IL-22 and hBD-2 GCF levels in chronic periodontitis group. However there was no correlation between the molecules in healthy controls and gingivitis. Thus it is tempting to speculate that IL-22 might have a role in the expression of hBD-2 in periodontitis, further reconfirming the T cell mediated pathogenesis of this disease. Nevertheless, no statistical correlation between these two molecules in gingivitis could be explained by the fact that cytokines do not function in isolation but involve complex interactions with both pro- and anti-inflammatory effects.
This study however has a few limitations. Firstly, the cross sectional nature prevented us from assessing the exact nature of increase in levels of both the proteins. In addition, a potential bias could be that the study subjects were not age matched. Despite these drawbacks, a significant higher concentration of IL-22 and hBD-2 in chronic periodontitis leads us to speculate that IL-22 might have an effect on the elevated levels of hBD-2. However, these increased levels serve to be insufficient for control of the disease process due to multifactorial influences. This preliminary research warrants further studies investigating gene expression patterns of the selected molecules from gingival tissues to arrive at conclusive evidence for their role in the pathogenesis of periodontal disease. Furthermore, studies including other human β-defensin expression patterns are essential for a better understanding of the role of IL-22 and its interaction with hBD's mediated innate immune responses.
Support
This work was supported by a grant from Dr. D. Y. Patil Vidyapeeth, Pimpri, Pune [Grant Ref no: DPU/752(25)/2017].
Conflicting interest (if present, give more details)
Nil.
Acknowledgements
We thank Dr. Suneeta Panicker, Department of Microbiology, Dr. D. Y. Patil Arts, Commerce & Science College, Pune, for her technical assistance.
References
- 1.Graves D.T. The potential role of chemokines and inflammatory cytokines in periodontal disease progression. Clin Infect Dis. 1999 Mar;28(3):482–490. doi: 10.1086/515178. [DOI] [PubMed] [Google Scholar]
- 2.Garlet G.P. Destructive and protective roles of cytokines in periodontitis: a Re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010 Dec;89(12):1349–1363. doi: 10.1177/0022034510376402. [DOI] [PubMed] [Google Scholar]
- 3.Greer A., Zenobia C., Darveau R.P. Defensins and LL-37: a review of function in the gingival epithelium. Periodontol. 2013 Oct;63(1):67–79. doi: 10.1111/prd.12028. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schröder J.-M., Harder J. Human beta-defensin-2. Int J Biochem Cell Biol. 1999 Jun;31(6):645–651. doi: 10.1016/s1357-2725(99)00013-8. [DOI] [PubMed] [Google Scholar]
- 5.Liu A.Y., Destoumieux D., Wong A.V. Human β-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J Invest Dermatol. 2002 Feb;118(2):275–281. doi: 10.1046/j.0022-202x.2001.01651.x. [DOI] [PubMed] [Google Scholar]
- 6.Dale B.A., Krisanaprakornkit S. Defensin antimicrobial peptides in the oral cavity. J Oral Pathol Med. 2001 Jul;30(6):321–327. doi: 10.1034/j.1600-0714.2001.300601.x. [DOI] [PubMed] [Google Scholar]
- 7.Wolk K., Kunz S., Witte E., Friedrich M., Asadullah K., Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004 Aug;21(2):241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Li A., Gan Y., Wang R. IL-22 up-regulates β-defensin-2 expression in human alveolar epithelium via STAT3 but not NF-κB signaling pathway. Inflammation. 2015 Jun;38(3):1191–1200. doi: 10.1007/s10753-014-0083-z. [DOI] [PubMed] [Google Scholar]
- 9.Aujla S.J., Chan Y.R., Zheng M. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008 Mar;14(3):275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brand S., Beigel F., Olszak T. IL-22 is increased in active Crohn's disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006 Apr;290(4):G827–G838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- 11.Wolk K., Witte E., Wallace E. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006 May;36(5):1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 12.Radaeva S., Sun R., Pan H., Hong F., Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004 May;39(5):1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 13.Liang S.C., Tan X.-Y., Luxenberg D.P. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006 Oct 2;203(10):2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yong X., Chen Y., Tao R. Periodontopathogens and human β-defensin-2 expression in gingival crevicular fluid from patients with periodontal disease in Guangxi, China. J Periodontal Res. 2015 Jun;50(3):403–410. doi: 10.1111/jre.12220. [DOI] [PubMed] [Google Scholar]
- 15.Cohen J. In: Rev, editor. xv. Lawrence Erlbaum Associates, Inc; Hillsdale, NJ, US: 1977. p. 474. (Statistical Power Analysis for the Behavioral Sciences). [Google Scholar]
- 16.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967 Nov;38(6):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 17.Page R.C., Eke P.I. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007 Jul;78(7s):1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 18.Pradeep A.R., Kathariya R., Raghavendra N.M., Sharma A. Levels of pentraxin-3 in gingival crevicular fluid and plasma in periodontal health and disease. J Periodontol. 2011 May;82(5):734–741. doi: 10.1902/jop.2010.100526. [DOI] [PubMed] [Google Scholar]
- 19.Sugimoto K., Ogawa A., Mizoguchi E. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118(2):534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Y., Valdez P.A., Danilenko D.M. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008 Mar;14(3):282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 21.Kato-Kogoe N., Nishioka T., Kawabe M. The promotional effect of IL-22 on mineralization activity of periodontal ligament cells. Cytokine. 2012 Jul;59(1):41–48. doi: 10.1016/j.cyto.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Dommisch H., Acil Y., Dunsche A., Winter J., Jepsen S. Differential gene expression of human beta-defensins (hBD-1, -2, -3) in inflammatory gingival diseases. Oral Microbiol Immunol. 2005 Jun;20(3):186–190. doi: 10.1111/j.1399-302X.2005.00211.x. [DOI] [PubMed] [Google Scholar]
- 23.Vardar-Sengul S., Demirci T., Sen B.H., Erkizan V., Kurulgan E., Baylas H. Human β defensin-1 and -2 expression in the gingiva of patients with specific periodontal diseases. J Periodontal Res. 2007 Oct;42(5):429–437. doi: 10.1111/j.1600-0765.2006.00964.x. [DOI] [PubMed] [Google Scholar]
- 24.Lu Q., Jin L., Darveau R.P., Samaranayake L.P. Expression of human beta-defensins-1 and -2 peptides in unresolved chronic periodontitis. J Periodontal Res. 2004 Aug;39(4):221–227. doi: 10.1111/j.1600-0765.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 25.Harder J., Meyer-Hoffert U., Teran L.M. Mucoid Pseudomonas aeruginosa , TNF- α , and IL-1 β , but not IL-6, induce human β -Defensin-2 in respiratory epithelia. Am J Respir Cell Mol Biol. 2000 Jun;22(6):714–721. doi: 10.1165/ajrcmb.22.6.4023. [DOI] [PubMed] [Google Scholar]
- 26.Kao C.-Y., Chen Y., Thai P. IL-17 markedly up-regulates β-defensin-2 expression in human airway epithelium via JAK and NF-κB signaling pathways. J Immunol. 2004 Sep 1;173(5):3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 27.Zaga-Clavellina V., Martha R.V.-M., Flores-Espinosa P. In vitro secretion profile of pro-inflammatory cytokines IL-1β, TNF-α, IL-6, and of human beta-defensins (HBD)-1, HBD-2, and HBD-3 from human chorioamniotic membranes after selective stimulation with gardnerella vaginalis: cytokines and defensins IN human CHORIOAMNION. Am J Reprod Immunol. 2012 Jan;67(1):34–43. doi: 10.1111/j.1600-0897.2011.01054.x. [DOI] [PubMed] [Google Scholar]
- 28.Eyerich S., Eyerich K., Pennino D. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009 Dec;119(12):3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zenewicz L.A., Yancopoulos G.D., Valenzuela D.M., Murphy A.J., Karow M., Flavell R.A. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007 Oct;27(4):647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]