Abstract
The alarming global increase in drug-resistant strains plagues the global fight to end tuberculosis (TB), especially in developing countries. The often reported poor treatment outcomes, sequelae, and lack of best practice guidelines in drug-resistant spinal TB poses a significant challenge in its efficient management. While multi-drug chemotherapy is still the primary modality of treatment, surgical intervention is essential in specific scenarios. With limited data on management and outcomes in drug-resistant spinal TB, there is no consensus on the appropriate therapy regarding the number and duration of drugs and therapeutic endpoints of this conundrum. In this light of limited evidence, we have performed a systematic computerized search using the Cochrane Database of Systematic Reviews, Scopus, Embase, Web of Science, and PubMed databases and studies published over the past 30 years on drug-resistance in spinal TB have been analyzed. This systematic review aims to review the current epidemiology, clinical features, updates in clinical diagnostics and chemotherapy, surgical management, and outcomes in drug-resistant spinal TB. We also consolidate potential areas of action and emphasize the need for research and large scale trials in the management of drug-resistant spinal TB.
Keywords: Spinal tuberculosis, Multi drug-resistance, Epistasis, Anti-tubercular therapy, Treatment outcome
1. Introduction
Tuberculosis is a significant cause of ill-health and continues to be the top infectious killer worldwide, with an estimated 1.2 million deaths (2018) in non-HIV individuals.1 Extra-pulmonary TB has low prevalence (3%), with skeletal TB contributing to 10% of those cases. Spinal TB is the most frequent site of skeletal involvement, contributing to more than 50% of all skeletal TB.2 The emergence of drug-resistance is a serious health concern and is the greatest barrier to the global community in achieving the targets of the “End TB strategy.“3 WHO has formulated the guidelines of management of drug-resistant TB only very recently, which has not been widely adopted into clinical practice, especially in the spine community.4 The poor bioavailability of oral drugs in skeletal tissues necessitates extended treatment, posing compliance issues, complicating further the problem of drug-resistance, resulting in poor outcomes of spinal TB.5 In a retrospective analysis of 145 patients with spinal TB managed conservatively, nine patients had poor outcomes with seven of them having drug-resistance. Similarly, 19/23 patients with poor results amongst the 638 patients managed surgically had drug-resistance.6 The presence of drug-resistance strain is one of the most import factors in determining recurrence of spinal tuberculosis following treatment.7
2. Defining drug-resistance
Mycobacterium tuberculosis is monoresistant if the strains are resistant to any of the two most effective anti-tuberculous drugs (Isoniazid/Rifampicin). It is multiresistant (MDR) if both Isoniazid and Rifampicin are not effective, and polyresistant (if in addition to these drugs, the bacterium is not sensitive to any other first-line drugs). The term extensively drug-resistance (XDR) is used when, in addition to Isoniazid and Rifampicin, the bacterium is resistant to any one of the fluoroquinolones and one of the injectables used in the MDR-TB regime. Pre-XDR is used when in addition to Rifampicin and Isoniazid, the strain is additionaly resistant either to a fluoroquinolone (Pre-XDR FQ) or aminogycosidse (Pre-AM).The term total drug-resistance or pan resistance is not used routinely.
3. Epidemiology
In 2018, the global burden of TB was estimated at 10 million individuals with an alarming incidence of Rifampicin resistance in half a million new cases, of which 78% had MDR-TB.1 The global estimate of MDR-TB is around 3.4% of all new cases and 18% of all previously treated cases. Unfortunately, the three countries which share the highest global burden of MDR-TB include India (27%), China (14%), and The Russian Federation (9%). These figures are more worrisome considering the fact that India contributes to most (25%) of the gap existing between the number of cases being reported and the estimated global burden of cases because of underdiagnosis and underreporting of cases. To complicate this scenario, globally, only 51% of the bacteriologically confirmed cases of TB underwent testing for resistance. China and India together have accounted for 43% of the global gap between incidence and treatment enrolment. Among the positive TB cultures reported in India, only 48% of newly detected and 65% of previously treated cases was tested for Rifampicin resistance in 2018.8 This simply means that India faces yet another challenge because of the undetected drug-resistant cases.
While pulmonary TB cases are being reported more regularly, underreporting of extra-pulmonary forms of TB is highly prevalent. Empirical TB is still being prescribed in certain localities around the world, and even in patients diagnosed with spinal TB, there is a considerable variation amongst practicing surgeons on diagnosing drug-resistance. This necessarily points out that the real incidence of MDR/XDR TB in Spine remains unknown in India and many parts of the world.
With the global migration, increasing incidences of MDR Spine TB in other continents is not a surprise. WHO European region with 53 member states reported a considerable variation in the notification of TB from 2.8 in Italy to 123 in Kazakhstan per 100,000 population in 2010. They witnessed a deep rise of MDR-TB up to 13.7% in new cases and 48.7% in previously treated cases.9 XDR-TB occurred in 12.2% of the cases, and overall success rate of MDR-TB was only 56.3% in 2010. In a retrospective analysis of 967 patients treated for spinal TB, drug susceptibility testing using the proportional method revealed drug-resistance in 49 patients, amongst which 48.98% had multidrug-resistance strains, and 2 had extensively drug-resistant strains.10 To achieve the WHO’s ‘End TB Strategy’ vision of “A world free of tuberculosis – zero deaths, disease, and suffering due to tuberculosis,” efforts should be taken to first detect and treat all cases of drug-resistant TB.
Mechanisms and genetic understanding of drug-resistance in TB: The four main causes of drug-resistance are 1) inadequate or incomplete treatment, 2) non-adherence to treatment, 3) genetic predisposition and 4) coinfection with HIV.
Primary: Drug-resistance is said to be primary or existing naturally if it occurs when the patient has not been exposed to that particular drug. Such an innate resistance is believed to be a rare cause of drug-resistance in spinal TB. The average frequency of denovo Isoniazid resistance is 1 in 106, and for Rifampicin resistance is 1 in 108, and this is the basis of why multiple drugs are administered during antitubercular chemotherapy.11
Secondary: Secondary or acquired resistance has been implicated to have occurred following the exposure of a strain to chemotherapy. In an extensive series of 111 proven cases of drug-resistant strains, Mohan et al. reported a prevalence of 78.37% MDR and 2.7% XDR strains. Among the 111 patients, 58 (52.2%) had taken some form of anti-TB treatment in the past, and 48 were identified to have MDR strains.12 In addition, all eight patients who were defaulters had MDR-TB. Among the first-line drugs, Isoniazid had the highest and Pyrazinamide, the least incidence of drug-resistance. More importantly, they reported a high prevalence of drug-resistance in many second-line drugs in spinal TB, which was not reported before and therefore stressed the need for drug susceptibility testing in all cases of spinal TB.
In a tertiary unit with specialized spine services in South India, a total of 478 patients with culture positive spinal TB were analyzed for prevalence of drug-resistance.13 24/478 (5.02%) had mono resistance, 7/478 (1.46%) had poly resistance, 21/478 (4.4%) had multi-drug-resistance (MDR), and 4 patients (0.83%) has extensively drug-resistant (XDR) strains. 80% of all resistant strains were secondary and innate resistance was rare.
4. Mechanisms of evolution of drug-resistance in mycobacterium tuberculosis
Various extrinsic and intrinsic factors are important in the evolution of drug-resistance in TB.14,15 Economic and social situation of individuals and quality of TB control programs are important extrinsic factors which influence the spread of drug-resistance. Intrinsic factors particularly mutation rate, drug-resistance associated mutation, fitness cost of resistance mutations, compensatory mutations and epistasis influence evolution. Biological fitness, compensatory mutation and epistasis are important mechanisms involved in development of drug-resistance.16
The panel of mutations in M.tuberculosis is highly diverse and it depends on the affected gene and the genetic background.16 Few specific genetic mutations involving specific genes are predominant and their mutation frequency also varies in clinical isolates (Table 1). Exposure of bacterial cells to sub-lethal levels of bactericidal antibiotics promote cellular mutagenesis leading to increased mutation in other genes.17 This phenomenon mediated through increased production of reactive oxygen species might play a key role in the emergence of multidrug-resistance phenotypes in pathogenic bacteria like M. tuberculosis. Both isoniazid and ethionamide require activation by redox enzymes to become inhibitory.18 This process produces reactive oxygen and radicals that exert mycobactericidal activity. But once a mutant survives the killing action of reactive oxygen, these same chemical matters would enhance its mutability leading to additional drug mutations.19
Table 1.
Genes associated with drug resistance and mutation frequency for each gene in clinical isolates of Mycobacterium tuberculosis
| Group | Drug | Gene associated with drug resistance | Mutation frequency in isolates (%) |
|---|---|---|---|
| First line anti-TB drugs | Rifampicin (R) | rpoB encoding for β-subunit of RNA polymerase | 90–100 |
| Isoniazid (H) | katG encoding for catalase-peroxidase | 40–97 | |
| inhA encoding for fatty acid enoyl acyl carrier protein reductase A (InhA) | 8–64 | ||
| Ethambutol (E) | embB encoding for arabinosyl transferase | 47–89 | |
| Pyrazinamide (Z) | pncA encoding for pyrazinamidase | 44–97 | |
| Streptomycin (S) | rrs encoding for 16S rRNA subunit | 12–26 | |
| rpsL encoding for S12 ribosomal protein | 40–68 | ||
| gidB encoding for 7-methylguanosine methyltransferase | 5–13 | ||
| Second line anti-TB drugs | Amikacin, Kanamycin, Capreomycin | rrs encoding for 16S rRNA | 40–90 |
| Kanamycin | eis encoding for aminoglycoside acetyltransferase | 28–80 | |
| Capreomycin | tlyA encoding for 2′-O- methyltransferase | 4–13 | |
| Ofloxacin, levofloxacin, moxifloxacin, gatifloxacin | gyrA encoding for DNA gyrase subunit A | 70–90 | |
| gyrB encoding for DNA gyrase subunit B | 0–11 | ||
| Ethionamide |
inhA encoding for fatty acid enoyl acyl carrier protein reductase A (InhA) |
33–62 | |
| ethA encoding for EthA | 46–72 | ||
|
ethR encoding for transcriptional repressor EthR, NADH-ACP |
0–4 |
Different mutations cause different levels of drug-resistance and affect fitness cost even when they are located on the same codon (Table 2). Epistasis between drug-resistant mutations and between drug-resistance-associated mutations and compensatory mutations play an important role in emergence and spread of highly resistant strains. In a review by Nguyen et al.,20 the authors concluded that strains carrying multiple mutations reveal an increased ability to acquire other resistances or compensatory mutations.
Table 2.
Fitness cost and compensatory mechanisms in specific genetic mutations involved in evolution of resistance to Mycobacterium tuberculosis
| Gene mutation(s) | Fitness cost | Compensatory mechanism |
|---|---|---|
| inhA | Reduced biosynthesis of fatty acids | Secondary mutation in inhA promoter |
| inhA promoter | No | No |
| katG | Reduced protection against oxidative damage | Overexpression of ahpC by mutation in its promoter |
| rpoB | Decreased efficiency of DNS transcription | Secondary mutation in rpoA, rpoC or rpoB |
| rpsL & rrs | Impaired ribosome performance | Unknown |
| pncA | Unknown | Unknown |
| embB | Reduced cell wall biosynthesis efficiency | Secondary mutation in embABC operon |
| gyrA & gyrB | Reduced DNA supercoiling, reduced DNS replication | Secondary mutation in gyrA or gyrB |
| Eis | No | No |
5. Diagnostics in drug-resistance
Spinal tuberculosis being paucibacillary lesions, the yield of bacilli using gold standard culure methods (Lowenstein Jensen (LJ) medium) is only around 50% and the time taken is around 6 weeks. Middlebrook agar has a better turn around time of 10–12 days. BACTEC-46, BACTEC MGIT-96 and Septi-chek AFB are alternative culture methods with a much lesser turn around time. Drug susceptibility testing (DST) is then performed in culture positive specimens over 3 week and is done in three methods. Absolute concentration method and resistance-ratio method determine minimal inhibhitory concentration (MIC) and the proportion method determines the critical proportion for sensitive or resistance strains.21 In this background of poor culture positivity and time consumptive culture based detection of drug-resistance, and huge possibility of missing out on drug-resistant strains resulting in treatment failure, potential alternatives to DST are being explored.
5.1. DNA line probe assays
Gene based detection have made considerable progress and allow simultaneous detection of different mutations using multiple probes. The targets of these Line probe assays (LPA) are usually mutations in rpoB (INNO LiPA Rif.TB; Innogenetics, Ghent, Belgium) or rpoB and katG (GenoType MTBDR; Hain Lifescience GmbH, Nehren, Germany). GenoType MTBDRplus (Hain Lifescience GmbH, Nehren, Germany) endorsed by WHO in 2008 was developed with additional ability to detect wild type rpoB gene and mutations in promotor region of inhA gene. Later in 2010 GeneXpert MTB/RIF assay was recommended by WHO and multiple studies have found it superior in terms of earliest results (less than 48 h), concordance with DST in detecting Rifampicin resistance and ability to diagnose TB. The only drawback is its ability to diagnose only Rifampicn resistance. To overcome this Nipro NTM + MDRTB detection kit 2 (Nipro, Osaka, Japan) was developed and endorsed by WHO in 2016 which detects resistance to Rifampicin (rpoB), Isoniazid (inhA promotor, katG, fabG1 and furA), pyrazinamide (pncA), and fluoroquinolones (gyrA). The MeltPro TB assay, was developed by Zeesan Biotecheh (Xiamen, China), which detects resistance to main first-line and second-line anti-TB drugs. Its efficacy in diagnosing MDR-TB is around 86.7% and in XDR-TB is around 71.4%.22
5.2. Xpert MTB/RIF and xpert MTB/RIF ultra
Xpert MTB/RIF (Cepheid, United States) is a semi-quantitative PCR technique which amplifies the 81 bp hot spot region of rpoB gene to detect Rifampicin resistance. It is the most common and widely used molecular diagnostic method in TB since WHO’s endorsement in 2010 for use in adult pulmonary TB and later in 2013 for children and some forms of extra-pulmonary TB. The Xpert MTB/RIF Ultra is an advanced version which has better amplification and additional targets for identification (IS1081 and IS6110) of TB Bacilli.
5.3. Sequencing
Next-generation sequencing and targeted-gene sequencing are advanced molecular diagnostics which can study the genotype of the bacteria in a single run and are being developed. Some of them are under trial and WHO has published guidelines for its use in detecting drug-resistance in TB. However cost, expertise and standardization are the current limitations which will be overcome in the coming decades as they have immense potential.23
While bacterial cultures are referred to as the gold standard to diagnose drug-resistance, sequencing-based PCR and DNA detection techniques are reported to be rapid with better sensitivity and specificity.
6. Conventional versus molecular diagnostics in diagnosing spinal MDR-TB
The use of Xpert MTB/RIF in extra-pulmonary forms of TB has been recommended by WHO since 2013. In a study comparing bacterial cultures and PCR based detection of common drug-resistant genes (rpoB, rpsL, and katG), Si et al. identified mutations in 17 patients and drug-resistance in 11 samples out of the 50 cases of spinal TB in a mean duration of 6 days compared to only 7 samples in 34 days using modified Lowenstein-Jensen medium and absolute concentration method.24 Xu et al. in a retrospective analysis of 152 patients of Spinal TB, identified a culture positivity of only 50%, among which 30.3% of patients were determined to have drug-resistance using BACTEC MGIT 960 system (Becton-Dickinson, Sparks, Maryland) and proportion method on Lowenstein-Jensen medium.25
In 2018, first line probe assays in India revealed a 6% prevalence of MDR-TB; however, the incidence of Isoniazid resistance was (7.3%) much higher than Rifampicin resistance (1.03%) questioning the reliability of relying only on GeneXpert which detects only Rifampicin resistance potentially resulting in missing out a huge number of Isoniazid monoresistant cases.26 Further, on performing second line probe assay, around 29.6% of cases were Fluoroquinolone resistant, and 6.3% had XDR TB. In a study by Held et al. GeneXpert had a sensitivity of 95.6% and specificity of 96.2% in diagnosing spinal TB.27 GeneXpert results were obtained within 48 h, compared to 35 days using standard GenoType MTBDR-plus. In a series of 40 cases of spinal TB, Patil et al. reported Rifampicin resistance in 3 cases, while only one had culture positivity. Presuming others as MDR-TB, treatment was initiated with second-line drugs, and for the one case with DST results, individualized chemotherapy was initiated, and all three had achieved healing.
Wang et al. compared the efficacy of Xpert, MeltPro TB, smear positivity and histopathology in culture-positive cases of spinal TB and found Xpert to have the highest sensitivity of 98.79%, followed by histopathology (84.24%), MeltPro TB (69.70%) and lastly by smear positivity (36.3%).28 However, recent reports of Xpert analysis in drug-resistant spinal TB have shown false positive results of Rifampicin resistance in comparison to traditional DST and also have reported maximum amount of resistance to Isoniazid which can be missed in Xpert analysis highlighting the fact, that one should never rely on Xpert analysis alone and DST should be performed in all cases.29,30
Controversy exists regarding the sampling of appropriate tissue in spinal TB as whether to sample pus, granulation tissue, or caseous necrotic tissue. Li et al. in his study of 223 patients with spinal TB, found GeneXpert to be superior to standard culture techniques in achieving a diagnosis.31 They also found that pus and granulation tissue had the highest yield both in traditional culture and molecular diagnostics.
Current molecular diagnostics such as INNO-LiPA, Genotype-MDR-TBplus, and Xpert MTB/RIF are useful in the early detection of Isoniazid and Rifampicin resistance. They cannot detect resistance to other tuberculosis drugs, and therefore surgeons have to still rely on the time-consuming DST. MeltPro TB, and DNA Microarray based gene chips are being designed to detect drug-resistance to other groups of drugs; however, their efficiency is not high in identifying resistance to second-line drugs.32
7. Lack of consensus in spinal MDR-TB management
Naturally occurring drug-resistant strains (primary drug-resistance) were detected as early as the 1950s and monotherapy, non-compliance to therapy, and global migration of patients infected with drug-resistant stains mainly from Asia pacific region were the main risk factors considered for secondary drug-resistance.33 The burden of MDR-TB in the spine has increased rapidly, starting from very few case reports of 1990s.34 Rafailidis et al. reported a case of tuberculous spondylodiscitis, where CT guided fine needle aspiration was unsuccessful in achieving diagnosis and managed it by anterior corpectomy and later reported drug-resistant strains in the tissue obtained.35 He had discussed the importance of aggressive management in such cases in order to procure adequate tissue, which now seems an overkill, given the efficacy of transpedicular biopsy in sampling diseased tissues. Surgical intervention, Fluoroquinolone use, and no previous treatment were identified as factors favoring better outcomes in the management of pulmonary MDR-TB in a meta-analysis.36 Whether aggressive treatment in the form of surgical clearance of the infected area is essential in managing spinal TB is a matter of controversy. Further, the optimal treatment strategy and duration of chemotherapy remains unknown owing to the lack of high-quality randomized control trials in Spinal MDR-TB.37
8. Treatment outcomes in spinal MDR-TB
The general belief is that outcomes of spinal MDR-TB are poor when compared to drug-sensitive cases. In 2009, Pawar et al. were the first to report on outcomes of 25 cases of culture-proven spinal MDR-TB.38 They highlighted the need for a long duration of treatment to achieve healing for at least 18–24 months and reported drug-related complications in more than 50% of the cases. Of note was a higher incidence in children (7/25), and 75% of spinal MDR-TB cases had resistance to more than three drugs. Only four patients required surgery owing to mechanical instability and neurological deterioration and not due to failure to the response. The study, for the first time, indicated the high prevalence of multi-drug-resistant strains. It recommended drug susceptibility testing to be done in all cases of suspected spinal TB to diagnose drug-resistant spinal TB at the earliest to avoid delay in initiation of appropriate line of ATT.
Li et al. in their retrospective analysis of 35 spinal MDR-TB, showed the feasibility of performing surgery safely in these patients.39 More importantly, they showed that 61.5% of the retreated cases received previous irregular chemotherapy. This could be due to financial restrictions, non-availability of drugs, compliance-related issues, or/and inadequate follow-up of those initiated on chemotherapy. 33/35 patients achieved complete cure in this surgical group and the results are comparable to the good outcomes in 19/25 patients primarily managed conservatively in the previously mentioned study, indicating that both conservative and surgical options yield good outcomes.
On the other hand, in a case series of 15 spinal TB patients refractory to 5 months of chemotherapy, Jain et al. could obtain positive cultures only in three cases, among which two had multi-drug-resistance.40 Presuming drug-resistance in the other negative cultures, second-line ATT was initiated in all cases, and 14 of the 15 patients showed excellent results after 48 months of chemotherapy. This study brings out two important factors, one being the very low sensitivity of routine cultures and the other being presumptive consideration of drug-resistance in refractory cases and initiation of second-line drugs if there is no growth in culture, provided histopathology, clinical and radiological picture is suggestive of spinal TB.
Wu et al. compared the clinical outcomes of two groups of spinal MDR-T, one identified by standard DST following culture and the other by molecular diagnostics (Genotype MDR-TBplus and Xpert MTB/RIF). They found significantly better outcomes with lesser complications in patients who were started on MDR ATT therapy following molecular diagnostics, because the time to initiation of treatment was only 5 days compared to 73 days following culture and DST.41
9. Elderly and the young
While pediatric TB (0–14 years) contributes up to around 15–20% of the total burden,42 with an estimated 3.2% of them being affected with MDR-TB, the exact incidence of spinal MDR-TB is unknown.43 With estimates of 14.8% being resistant to Isoniazid and mortality as high as 13.6%; it is alarming that the reports of children with MDR-TB represent only 3.5% of the annual estimated incidence.43 It has been well established that pediatric population is at a higher risk for deformity progression and neurological complications both during the active and healed phase of TB.44 Further, owing to the increased metabolic pathways and consequences of ineffective treatment in children which include the development of drug-resistance and treatment failure, the WHO recommends an increase of dosage in the pediatric population.45 In this background, the long duration of treatment and their adverse effects on children can be compounding factors for poor outcomes in spinal MDR-TB.
Seddon et al. in his retrospective analysis of children being treated for TB in a high burden setting located in South Africa identified 11 children between 2004 and 2010 to have been treated with spinal MDR-TB and analyzed the outcomes of 7 children who were treated with injectibles and multi-drug chemotherapy for an average of 18 months.46 Except for one patient who developed severe spinal deformity, five of them completed treatment, and one patient was nearing completion without added complications. However, the authors brought out the issue of significant delay in the initiation of appropriate therapy, which happened only after the failure of the first-line drug regime. They recommended DST to be done for patients and also brought out the lack of knowledge on the pharmacokinetics of second-line drugs and their safety in children.
Recently Arockiaraj et al. in his series of 6 Spinal MDR-TB affected children, achieved good outcomes with only chemotherapy in three and a combination of surgery and chemotherapy in three patients.47 All of these six patients had prior treatment with ATT chemotherapy and underwent both Xpert MTB/RIF assay and DST. Early detection of drug-resistance was made based on Xpert MTB/RIF results within an average of 10.5 days from the presentation, and combination chemotherapy initiated and modified later based on DST. The duration of chemotherapy was 18–24 months with one injectable (initiation phase of 6 months), and a combination of one first-line drug, fluoroquinolone, second-line drug, and Class V- drugs was used.
A constant rise in life expectancy has resulted in an aging population living with co-morbidities, and the presence of underlying acute/chronic diseases, immunosuppression and malnutrition has increased the incidence of spinal TB in geriatric population.48 Increasing incidence of MDR-TB in transplant recipients demands a high index of suspicion in those on immunosuppressive drugs.49
10. Chemotherapy and updates
Multidrug chemotherapy has drastically altered the course of TB disease and brought down the mortality from 30 to 50% before the advent of chemotherapy to less than 1% and also has improved healing in surgically treated patients from 30% to 96%. It is vital for medical professionals to understand the basis of multi-drug chemotherapy in TB to ensure appropriate prescription of medications, as the problem of multi-drug-resistance is believed to be a man-made tragedy primarily arising out of inappropriate therapy rather than primary resistance. Mycobacterium Tuberculosis can exist in four types; extracellular rapidly dividing bacilli, extracellular intermittent or slowly dividing bacilli, intracellular intermittently dividing bacilli, and dormant bacilli. While Isoniazid, Streptomycin and Ethambutol are active against extracellular rapidly acting bacteria, Rifampicin acts against extracellular slow-growing bacilli, and Pyrazinamide penetrates the cell wall and acts against intracellular organisms.
The drug efficacy of second-line drugs and toxicity profile is inferior, and their usage warrants appropriate follow-ups for monitoring compliance, dosage, and adverse effects, and outcome of TB.It is essential that an expert physician is involved in the management of MDR-TB, especially in the elderly and pediatric population. The emergency of HIV as a pandemic has resulted in the resurgence of spinal TB and drug-resistance. It is pertinent to understand that antiretroviral drugs and antitubercular chemotherapy have several drug interactions and can reduce the efficacy of either of the drugs.
Xu et al. in his retrospective analysis of 19 cases of drug-resistant spinal TB, identified 16 patients with MDR-TB and reported successful outcomes with a combination of surgery and DST guided individualized chemotherapy, given over 18–24 months.25 In another series of 15 cases (10 adults and 5 children) with drug-resistant spinal TB, Desai et al. successfully treated 14/15 of the patients with chemotherapy alone for 24 months.50 The principles behind the tailored selection of drugs included a selection of one sensitive first-line drug, an injectable for six months, a quinolone, 1-s line drug such as ethionamide and cycloserine, and lastly, other drugs such as amoxicillin clavulanate or linezolid and clofazimine. It is recommended that no single drug be added to a previous failing regime, and a minimum of four drugs previously not used should be administered in MDR-TB. Further, two drugs in the same pharmacological group need to be avoided. Recently Li et al. evaluated the penetration of oral 600 mg linezolid in Spinal MDR-TB and found it to be good evn after 24 h of administration.51 WHO also recommends its usage in long term MDR-TB regime and future trials are required in MDR-TB between the WHO recommended long term and short term regimes.
The category of drugs available for chemotherapy is enlisted in Table 3. Most recently, the WHO has made reccomendations and has provided guidelines in mamagement of drug-resistant TB Table 4.
Table 3.
Chemotherapeutic drugs in tuberculosis.
| WHO 2016 TB drug classification | ||
|---|---|---|
| First Line oral anti TB drugs | Isoniazid, Pyrazinamide, Ethambutal,Rifampicin, | |
| Second line drugs | ||
|
Moxifloxacin, Levofloxacin, Gatifloxacin | |
|
Amikacin, Kanamycin, Capreomycin, Streptomycin | |
|
Ethionamide/Prothionamide, Cycloserine/Terizidone, Linezolid, Clofazimine | |
|
D1 | Pyrazinamide, Ethambutol, High dose Isoniazid |
| D2 | Bedaquiline, Delamanid | |
| D3 | P-Aminosalicylic Acid,Imipenem–Cilastatin, Meropenem Amoxycillin-Clavulonate, Thioacetazone |
|
| WHO Classification And Recommendation Of Drugs Recommended For Use In Longer MDR-Tb Regimes- 2019 | ||
| Group A: Include all three medicines | Levofloxacin Or Moxifloxacin | |
| Bedaquiline | ||
| Linezolid | ||
| Group B: Add one or both medicines | Clofazimine | |
| Cycloserine Or Terizidone | ||
| Group C: Add to complete the regimen and when medicines from Groups A and B cannot be used | Ethambutol | |
| Delamanid | ||
| Pyrazinamide | ||
| Imipenem–Cilastatin Or Meropenem | ||
| Amikacin Or Streptomycin | ||
| Ethionamide Or Prothionamide | ||
| P-Aminosalicylic Acid | ||
Group A: fluoroquinolones (levofloxacin and moxifloxacin), bedaquiline and linezolid were considered highly effective and strongly recommended for inclusion in all regimens unless contraindicated.
Group B: clofazimine and cycloserine or terizidone were conditionally recommended as agents of second choice.
Group C: included all other medicines that can be used when a regimen cannot be composed with Group A and B agents. The medicines in Group C are ranked by the relative balance of benefit to harm usually expected of each.
Table 4.
WHO recommendations in pulmonary MDR-TB treatment.
| Drug resistance group | Recommendations |
|---|---|
| Rifampicin susceptible but Isoniazid resistance |
|
| MDR-RR TBa |
|
| Duration of long term regime | 18–20 months to which modifications can be done based on response to therapy |
| Shorter regime (2018) | 9–12 months can be used (seven agents in a 4-month intensive phase and four agents in a 5-month continuation phase) provided
|
|
Initiation Phase – Kanamycin + Moxifloxacin + Clofazimine + Ethionamide + Pyrazinamide + Ethambutol + High dose Isoniazid (Km-Mfx–Cfz–Eto–Z–E–Hh) Continuation phase - Moxifloxacin + Clofazimine + Pyrazinamide + Ethambutol (Mfx–Cfz–Z–E) |
|
| Shorter regime modification (2019) | All oral regime would suffice if bedaquiline is used and therefore Kanamycin or any injectable is not required |
| Anti retroviral therapy | In case of coinfection with HIV,ATT to be started at the earliest preferably within 8 weeks of initiation of TB chemotherapy even in lower CD4 counts |
| Novel treatment regime in XDR Tbb (2019) | BPal regime- 6–9 months of treatment with three drugs Bedaquiline + Pretomanid + Linezolid |
Multi drug resistance with Rifampicin Resistance.
Extensively drug resistant Tb.
11. Newer drugs
Recently Bedaquiline fumarate has been approved by US-FDA and WHO to be used in MDR pulmonary TB. Bedaquiline is known to prolong Qtc interval, and therefore baseline ECG, electrolyte values (calcium, magnesium, and potassium) need to be recorded and carefully monitored periodically. The original WHO recommendation is for 24 weeks, and recently, the safety profile and efficacy of prolonged treatment (>190 days) were assessed, which yielded favorable outcomes and was overall well tolerated.52 However, the emergence of bedaquiline resistance raises a considerable concern for its usage in Spinal MDR-TB to avoid the development and spread of bedaquiline resistant strains.53
Similarly, the efficacy of Delamanid in pulmonary MDR-TB has been analyzed and seems promising, and its use in spinal MDR-TB has no contraindications but needs larger trials.54 While the addition of injectibles in the treatment of MDR-TB is most recommended, a recent trial using non-injectable regimen containing either of bedaquiline/delamanid or both in Rifampicin resistant pulmonary TB showed good results in the adolescent population.55 More recently, WHO has recommended a short course regime in which Bedaquiline replaced injectables. However there have been no such trials at this point of time in Spinal MDR-TB.
11.1. Adjunctive therapy in spinal MDR-TB
Host-directed therapy (HDT) has been described in managing both drug-sensitive and resistant pulmonary TB.56 Their efficacy as beneficial adjuncts in Spinal MDR-TB is unknown. Immunomodulation as a part of the treatment protocol in spinal TB has been reported by Jain et al. were they report usage of Levamisole (2 mg/kg/day) for three days in a weekly interval for seven cycles. Besides, two doses of 0.1 ml of BCG vaccine were administered intradermally for two months in addition to five oral drugs (Rifampicin, Isoniazid, Ofloxacin, Ethionamide, Cycloserine) and one injectable (Kanamycin/Amikacin).40
11.2. Surgical indications
Given the lack of guidelines in managing Spinal MDR-TB and well-documented reports of both surgery and conservative modalities, currently, indications of surgery can be considered the same as that of non-resistant spinal TB which generally include; lack of response to chemotherapy, instability, deformity, gross neurological deficits, spine at risk signs and incapacitating pain not allowing ambulation.2 In the absence of culture positivity, drug susceptibility cannot be performed and in such cases where there is no improvement after initiating chemotherapy, surgery can be considered for mainly two reasons, one to reduce the microbial load by giving a good clearance of diseased focus, which also improves penetration of drugs at the affected site and the second being adequate procurement of tissue for subjecting to both traditional DST and molecular methods (Fig. 1).
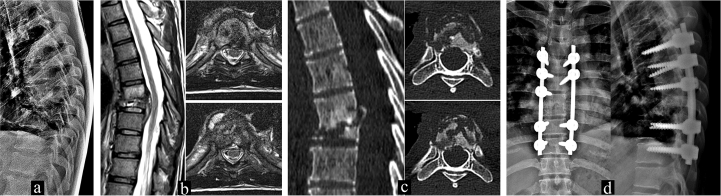
Fig. 1.
A 32 year old male recently diagnosed as HIV positive individual with a CD count of 223 cells/mm3 presented with complaints of backpain referred to anterior chest wall. A) Plain radiography shows complete collapse of T8 vertebra with a local kyphotic deformity of 34°. B) MRI shows involvement of T7 and T8 vertebra with paravertebral abscess and C) Sagittal CT demonstrating vertebra plana of T8 with fragmentation of vertebrae in the axial cuts. D) Postoperative imaging of this patient who underwent debridement and posterior stabilization due to unrelenting pain not allowing ambulation. Histopathology was confirmative of Tuberculosis and Gene Xpert analysis revealed Rifampicin resistance. The case was reported and registered under the government RNTCP program andinitiated on second-line drugs. DST results are awaited and the regime will be altered based on it.
12. Lack of consensus and research
There is no consensus on whether pulmonary MDR-TB and spinal MDR-TB can be treated on the same line, and this raises concern as it is well established that penetration of drugs into bones is more inadequate than soft tissues.57 While surgical debridement can overcome this issue, especially in children who are at a higher risk for neurological complications, deformity progression, and drug toxicity, there is no high-quality data at this point of time to support this view especially when there are enough reports of good outcomes by chemotherapy alone.
13. Conclusion
The fight against spinal MDR-TB necessitates increased awareness and the importance of timely recognition of this entity. Appropriately designed chemotherapeutic regimes are successful in achieving good outcomes and no additional surgery is required for clearance of disease foci. The efficacy of shorter regimes with novel drugs such as Bedaquiline and Delamanid in spinal MDR-TB needs to be analyzed. The problem of secondary drug-resistance due to irregular therapy should be kept in mind and it is vital to notify TB cases and strictly monitor for adverse affects. Molecular diagnostics for early recognition and additional drug suceptibilty should be universally performed in all cases of spinal TB.
Funding statement
No funding granted or received.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.World Health Organization . World Health Organization; 2019. Global Tuberculosis Report 2019. [Google Scholar]
- 2.Rajasekaran S., Soundararajan D.C.R., Shetty A.P., Kanna R.M. Spinal tuberculosis: current concepts. Global Spine J. 2018;8(4_suppl):96S–108S. doi: 10.1177/2192568218769053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO The End TB Strategy. http://www.who.int/TB/strategy/en/ WHO, April 24, 2020.
- 4.World Health Organization WHO consolidated guidelines on drug-resistant tuberculosis treatment. 2019. http://www.ncbi.nlm.nih.gov/books/NBK539517/ [PubMed]
- 5.Pandita A., Madhuripan N., Pandita S., Hurtado R.M. Challenges and controversies in the treatment of spinal tuberculosis. J Clin Tuberc Mycobact Dis. 2020;19:100151. doi: 10.1016/j.jctube.2020.100151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao G., Rao J., Cai Y. Analysis of treatment and prognosis of 863 patients with spinal tuberculosis in guizhou province. BioMed Res Int. 2018;2018:1–8. doi: 10.1155/2018/3265735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang B., Kong L., Zhu Z. Recurrent complex spinal tuberculosis accompanied by sinus tract formation: causes of recurrence and clinical treatments. Sci Rep. 2018;8(1):6933. doi: 10.1038/s41598-018-25142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO Tuberculosis Country Profiles. http://www.who.int/TB/country/data/profiles/en/ WHO, June 28, 2020.
- 9.Dara M., Dadu A., Kremer K., Zaleskis R., Kluge H.H.P. Epidemiology of tuberculosis in WHO European Region and public health response. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2013;22(Suppl 4):549–555. doi: 10.1007/s00586-012-2339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi T., Zhang Z., Dai F. Retrospective study of 967 patients with spinal tuberculosis. Orthopedics. 2016;39(5):e838–e843. doi: 10.3928/01477447-20160509-03. [DOI] [PubMed] [Google Scholar]
- 11.Rajasekaran S., Khandelwal G. Drug therapy in spinal tuberculosis. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2013;22(Suppl 4):587–593. doi: 10.1007/s00586-012-2337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohan K., Rawall S., Pawar U.M. Drug-resistance patterns in 111 cases of drug-resistant tuberculosis spine. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2013;22(Suppl 4):647–652. doi: 10.1007/s00586-012-2154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arockiaraj J., Balaji G.S., Cherian V.M. Drug-resistant skeletal tuberculosis in a tertiary care centre in South India. J Clin Orthop Trauma. 2018;9(Suppl 1):S44–S48. doi: 10.1016/j.jcot.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehner B. Molecular mechanisms of epistasis within and between genes. Trends Genet TIG. 2011;27(8):323–331. doi: 10.1016/j.tig.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Eldholm V., Pettersson J.H.-O., Brynildsrud O.B. Armed conflict and population displacement as drivers of the evolution and dispersal of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2016;113(48):13881–13886. doi: 10.1073/pnas.1611283113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molecular detection of mutations associated with first- and second-line drug-resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis, Antimicrob Agents Chemother. https://aac.asm.org/content/55/5/2032 [DOI] [PMC free article] [PubMed]
- 17.Kohanski M.A., DePristo M.A., Collins J.J. Sublethal antibiotic treatment leads to multidrug-resistance via radical-induced mutagenesis. Mol Cell. 2010;37(3):311–320. doi: 10.1016/j.molcel.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito K., Yamamoto K., Kawanishi S. Manganese-mediated oxidative damage of cellular and isolated DNA by isoniazid and related hydrazines: non-Fenton-type hydroxyl radical formation. Biochemistry. 1992;31(46):11606–11613. doi: 10.1021/bi00161a046. [DOI] [PubMed] [Google Scholar]
- 19.Smith T., Wolff K.A., Nguyen L. Molecular biology of drug-resistance in Mycobacterium tuberculosis. Curr Top Microbiol Immunol. 2013;374:53–80. doi: 10.1007/82_2012_279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen Q.H., Contamin L., Nguyen T.V.A., Bañuls A.-L. Insights into the processes that drive the evolution of drug-resistance in Mycobacterium tuberculosis. Evol Appl. 2018;11(9):1498–1511. doi: 10.1111/eva.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain A.K., Jaggi K.R., Bhayana H., Saha R. Drug-resistant spinal tuberculosis. Indian J Orthop. 2018;52(2):100–107. doi: 10.4103/ortho.IJOrtho_306_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pang Y., Dong H., Tan Y. Rapid diagnosis of MDR and XDR tuberculosis with the MeltPro TB assay in China. Sci Rep. 2016;6(1):25330. doi: 10.1038/srep25330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen T.N.A., Anton-Le Berre V., Bañuls A.-L., Nguyen T.V.A. Molecular diagnosis of drug-resistant tuberculosis; A literature review. Front Microbiol. 2019;10 doi: 10.3389/fmicb.2019.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Si J., Wang Z., Wang Z., Li H. Sequencing-based detection of drug-resistant Mycobacterium tuberculosis in patients with spinal tuberculosis. Arch Orthop Trauma Surg. 2012;132(7):941–945. doi: 10.1007/s00402-012-1506-7. [DOI] [PubMed] [Google Scholar]
- 25.Xu L., Jian-Zhong X., Xue-Mei L., Bao-Feng G. Drug susceptibility testing guided treatment for drug-resistant spinal tuberculosis: a retrospective analysis of 19 patients. Int Surg. 2013;98(2):175–180. doi: 10.9738/INTSURG-D-12-00004.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.India TB Report 2019.pdf. https://TBcindia.gov.in/WriteReadData/India%20TB%20Report%202019.pdf
- 27.Held M., Laubscher M., Zar H.J., Dunn R.N. GeneXpert polymerase chain reaction for spinal tuberculosis. Bone Jt J. 2014;96-B(10):1366–1369. doi: 10.1302/0301-620X.96B10.34048. [DOI] [PubMed] [Google Scholar]
- 28.Wang G., Dong W., Lan T. Diagnostic accuracy evaluation of the conventional and molecular tests for Spinal Tuberculosis in a cohort, head-to-head study. Emerg Microb Infect. 2018;7(1):109. doi: 10.1038/s41426-018-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Upadhyay M., Patel J., Kundnani V., Ruparel S., Patel A. Drug sensitivity patterns in Xpert-positive spinal tuberculosis: an observational study of 252 patients. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. Published online February. 2020;13 doi: 10.1007/s00586-020-06305-x. [DOI] [PubMed] [Google Scholar]
- 30.Patel J., Upadhyay M., Kundnani V., Merchant Z., Jain S., Kire N. Diagnostic efficacy, sensitivity, and specificity of Xpert MTB/RIF assay for spinal tuberculosis and rifampicin resistance. Spine. 2020;45(3):163–169. doi: 10.1097/BRS.0000000000003225. [DOI] [PubMed] [Google Scholar]
- 31.Tang L., Feng S., Gao R. A comparative study on the role of Xpert MTB/RIF in testing different types of spinal tuberculosis tissue specimens. Genet Test Mol Biomarkers. 2017;21(12):722–726. doi: 10.1089/gtmb.2017.0149. [DOI] [PubMed] [Google Scholar]
- 32.Lyu J., Wu W., Cheng P. A chip for detecting tuberculosis drug-resistance based on polymerase chain reaction (PCR)-Magnetic bead molecule platform. Front Microbiol. 2018;9:2106. doi: 10.3389/fmicb.2018.02106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vartanian A.J., Alvi A. Multidrug-resistant cervical tuberculosis. Am J Otolaryngol. 1999;20(4):252–256. doi: 10.1016/s0196-0709(99)90010-8. [DOI] [PubMed] [Google Scholar]
- 34.Cherifi S., Guillaume M.P., Peretz A. Multidrug-resistant tuberculosis spondylitis. Acta Clin Belg. 2000;55(1):34–36. doi: 10.1080/17843286.2000.11754270. [DOI] [PubMed] [Google Scholar]
- 35.Rafailidis P.I., Avramopoulos I., Sapkas G., Falagas M.E. Multidrug-resistant tuberculous spondylodiscitis: need for aggressive management and drug susceptibility testing of Mycobacterium tuberculosis isolates. J Infect. 2006;52(2):e35–e37. doi: 10.1016/j.jinf.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 36.Johnston J.C., Shahidi N.C., Sadatsafavi M., Fitzgerald J.M. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PloS One. 2009;4(9) doi: 10.1371/journal.pone.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suárez-García I., Noguerado A. Drug treatment of multidrug-resistant osteoarticular tuberculosis: a systematic literature review. Int J Infect Dis. 2012;16(11):e774–e778. doi: 10.1016/j.ijid.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Pawar U.M., Kundnani V., Agashe V., Nene A., Nene A. Multidrug-resistant tuberculosis of the spine--is it the beginning of the end? A study of twenty-five culture proven multidrug-resistant tuberculosis spine patients. Spine. 2009;34(22):E806–E810. doi: 10.1097/BRS.0b013e3181af7797. [DOI] [PubMed] [Google Scholar]
- 39.Li L., Zhang Z., Luo F. Management of drug-resistant spinal tuberculosis with a combination of surgery and individualised chemotherapy: a retrospective analysis of thirty-five patients. Int Orthop. 2012;36(2):277–283. doi: 10.1007/s00264-011-1398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jain A.K., Dhammi I.K., Modi P., Kumar J., Sreenivasan R., Saini N.S. Tuberculosis spine: therapeutically refractory disease. Indian J Orthop. 2012;46(2):171–178. doi: 10.4103/0019-5413.93685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu W., Lyu J., Cheng P. Improvement in clinical outcome and infection control using molecular diagnostic techniques for early detection of MDR tuberculous spondylitis: a multicenter retrospective study. Emerg Microb Infect. 2017;6(11):e97. doi: 10.1038/emi.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marais B.J., Obihara C.C., Warren R.M., Schaaf H.S., Gie R.P., Donald P.R. The burden of childhood tuberculosis: a public health perspective. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2005;9(12):1305–1313. [PubMed] [Google Scholar]
- 43.Jenkins H.E. Global burden of childhood tuberculosis. Pneumonia. 2016;8 doi: 10.1186/s41479-016-0018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajasekaran S. Kyphotic deformity in spinal tuberculosis and its management. Int Orthop. 2012;36(2):359–365. doi: 10.1007/s00264-011-1469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ridge A., Grzemska M., Hill S., Gie R., World Health Organization . 2010. Rapid Advice: Treatment of Tuberculosis in Children.http://whqlibdoc.who.int/publications/2010/9789241500449_eng.pdf [PubMed] [Google Scholar]
- 46.Seddon J.A., Donald P.R., Vlok G.J., Schaaf H.S. Multidrug-resistant tuberculosis of the spine in children—characteristics from a high burden setting. J Trop Pediatr. 2012;58(5):341–347. doi: 10.1093/tropej/fmr104. [DOI] [PubMed] [Google Scholar]
- 47.Arockiaraj J., Robert M., Rose W., Amritanand R., David K.S., Krishnan V. Early detection and analysis of children with multidrug-resistant tuberculosis of the spine. Asian Spine J. 2019;13(1):77–85. doi: 10.31616/asj.2017.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maeda Y., Izawa K., Nabeshima T., Yonenobu K. Tuberculous spondylitis in elderly Japanese patients. J Orthop Sci Off J Jpn Orthop Assoc. 2008;13(1):16–20. doi: 10.1007/s00776-007-1197-z. [DOI] [PubMed] [Google Scholar]
- 49.Huaman M.A., Brawley R., Ashkin D. Multidrug-resistant tuberculosis in transplant recipients: case report and review of the literature. Transpl Infect Dis. 2017;19(2) doi: 10.1111/tid.12672. [DOI] [PubMed] [Google Scholar]
- 50.Desai U., Joshi J.M. Extrapulmonary drug-resistant tuberculosis at a drug-resistant tuberculosis center, Mumbai: our experience - hope in the midst of despair! Lung India off Organ Indian Chest. For Soc. 2019;36(1):3–7. doi: 10.4103/lungindia.lungindia_192_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y., Huang H., Dong W. Penetration of linezolid into bone tissue 24 h after administration in patients with multidrug-resistant spinal tuberculosis. PloS One. 2019;14(10) doi: 10.1371/journal.pone.0223391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guglielmetti L., Jaspard M., Dû D.L. Long-term outcome and safety of prolonged bedaquiline treatment for multidrug-resistant tuberculosis. Eur Respir J. 2017;49(3) doi: 10.1183/13993003.01799-2016. [DOI] [PubMed] [Google Scholar]
- 53.Veziris N., Bernard C., Guglielmetti L. Rapid emergence of Mycobacterium tuberculosis bedaquiline resistance: lessons to avoid repeating past errors. Eur Respir J. 2017;49(3) doi: 10.1183/13993003.01719-2016. [DOI] [PubMed] [Google Scholar]
- 54.8131480597Guidelines for use of Delamanid for treatment of DR-TB in India.pdf. https://TBcindia.gov.in/WriteReadData/l892s/8131480597Guidelines%20for%20use%20of%20Delamanid%20for%20treatment%20of%20DR-TB%20in%20India.pdf
- 55.Mohr-Holland E., Reuter A., Furin J. Injectable-free regimens containing bedaquiline, delamanid, or both for adolescents with rifampicin-resistant tuberculosis in Khayelitsha, South Africa. EClinicalMedicine. 2020;20 doi: 10.1016/j.eclinm.2020.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zumla A., Rao M., Dodoo E., Maeurer M. Potential of immunomodulatory agents as adjunct host-directed therapies for multidrug-resistant tuberculosis. BMC Med. 2016;14 doi: 10.1186/s12916-016-0635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu P., Zhu Q., Jiang J. Distribution of three antituberculous drugs and their metabolites in different parts of pathological vertebrae with spinal tuberculosis. Spine. 2011;36(20):E1290. doi: 10.1097/BRS.0b013e31820beae3. [DOI] [PubMed] [Google Scholar]