Abstract
Introduction
Back pain is a common ailment affecting individuals around the globe. Animal models to understand the back pain mechanism, treatment modalities, and spinal cord injury are widely researched topics worldwide. Despite the presence of several animal models on disc degeneration and Spinal Cord Injury, there is a lack of a comprehensive review.
Material and method
A methodological narrative literature review was carried out for the study. A total of 1273 publications were found, out of which 763 were related to spine surgery in animals. The literature with full-text availability was selected for the review. Scale for the Assessment of Narrative Review Articles (SANRA) guidelines was used to assess the studies. Only English language publications were included which were listed on PubMed. A total of 113 studies were shortlisted (1976–2019) after internal validation scoring.
Result
The animal models for spine surgery ranged from rodents to primates. These are used to study the mechanisms of back pain as well as spinal cord injuries. The models could either be created surgically or through various means like use of electric cautery, chemicals or trauma. Genetic spine models have also been documented in which the injuries are created by genetic alterations and knock outs. Though the dorsal approach is the most common, the literature also mentions the anterior and lateral approach for spine surgery animal experiments.
Conclusion
There are no single perfect animal models to represent and study human models. The selection is based on the application and the methodology. Careful selection is needed to give optimum and appropriate results.
Keywords: Animal spine models, Spinal cord injury, IVD degeneration, Animal ethics
1. Introduction
Back pain is a common ailment affecting individuals around the globe.1 A few of the recent studies have stated that a majority of disability-adjusted life years (DALYs) lost by an individual are due to low back pain. As the postural problems in the individuals continue to rise, the DALYs lost due to low back pain are expected to increase in due course of time.2, 3, 4, 5, 6
Studies using animal models have been widely researched to understand the mechanism of spinal diseases and their biomechanics. Though the literature in the animal spinal model is limited, a few of the developed models exist in rabbits, sheep, goats, pigs, and dogs.7, 8, 9, 10 History has witnessed animal models with little empathy in the earlier studies to revolutions in the present time. A few of the studies have mentioned about the tissue engineering for disc degeneration models, local hormonal injections, autologous cell implantation, and gene transfer.11
From the days of little rehabilitation for spinal injury models to the establishment of animal physiotherapy laboratories, the animal spine model research has shown tremendous growth.12 This narrative review discusses the history of animal spine models, the existing experimental models, and the future it holds.
2. Material and Methods
A methodological narrative literature review was carried out for the study. The authors independently assessed the methodological quality of each study. The blinding of studies was performed to the best of authors knowledge to avoid biases due to institutions, authors, and journals.
The data were independently extracted from each study utilizing a predesignated data extraction form. Core data entailed study characteristics, the presence of funding, ethical approval, operative characteristics, type of anesthesia, animal model, approaches and outcomes. The corresponding author was contacted for additional or missing data. In the event, the lead study author could not be contacted or was nonresponsive, only the published data parameters were noted.
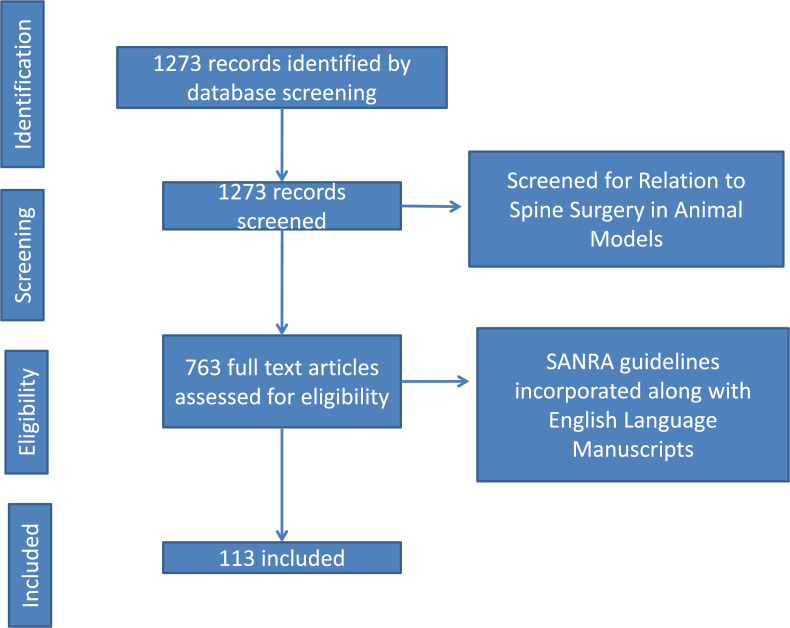
In this review, a PubMed search for all papers stating “spine animal models,” “animal model for spine surgery,” “experimental spine animal models” and ‘Animal Models of Spinal Cord Injury’ were explored. A total of 1273 publications were found, out of which 763 were related to spine surgery in animals. The literature with full-text availability was selected for the review. Scale for the Assessment of Narrative Review Articles (SANRA) guidelines was used to assess the studies. Only English language publications were included which were listed on PubMed. A total of 113 studies were shortlisted after internal validation scoring (Fig. 1). The date of publication of these manuscripts ranged from 1976 till 2019.
Fig. 1.
The Methodology of inclusion of manuscripts through the various database.
2.1. History of spine animal models
Animal models have been used as a surrogate model to study the surgery techniques and physiological changes in the spine and surrounding structures. Large and small animals such as mice, baboons, pigs, and bovines are used to study biomechanics. These quadrupeds and bipeds have a similar trabecular structure of bones due to the loading on these bones. Historically surgeons practiced disc replacement and other surgical techniques on pigs, goats, rabbits, and mice. The advantage of animal models is that they are easily available and can be produced in large numbers.
There are stringent regulations on animal experimentation. There are guidelines for the use and are governed by their respective law of the land and vary from country.13
Different animal models are used to study the motion of the spine to correlate with the human subjects. These animal models include pig, sheep and cow. The selection is based on the region which is being studied and its equivalent range of motion in the human spine.14, 15, 16, 17 For example, to represent the cervical region of the human spine, the porcine models can be used. For the lumbar spine, animal models such as porcine and ovine spine can be used.15, 16 Baboons can be used to study the intervertebral discs as it has asimilar transversal area of humans. But they are limited in use due to low availability, hence, smaller animals such as mice are used.18 Hence, for biomechanical studies, it is important to select an appropriate animal model based on the experimental protocols and research objectives.19
The size of the animal affects the pressure developed in the disc. This results in the change in geometry and material properties of the disc. Even though the scale of pressure generated is different among the animals. The stresses induced due to the various activities are comparable across the various species. The disc pressure produced in animal models is smaller than what is observed in humans. To extrapolate the results, scaling is performed for the animal models to human models.20, 21
Biomechanical studies to evaluate surgical techniques and implants are performed on large animal models. The selection of these models is dependent on morphological features and material properties. Hence, a model such as a sheep, bovine, calf, etc is used. Sheep vertebral body has an oval shape and has a larger width than depth. Pig spines are used for representing the cervical region as it has similar anatomy.18
2.2. The current spine surgery animal models
The literature discusses two kinds of animal models for spine surgery. These range from animal models for disc degeneration to animal models for spinal cord injury. Furthermore, there are a few spontaneous spine animal models that do not require any specific surgery/insult to the animal. These are genetic models and could be made by alteration of the genetic structure of the respective animal/s.
2.3. Animal models for disc degeneration
Degenerated Disc is one of the major risk factors for back pain. The intervertebral disc consists of two regions: nucleus pulposus and annulus fibrosis. Ageing and degeneration cause loss of water-retaining capacity of the nucleus pulposus. This results in loss of shock-absorbing capacity of the disc. Various animal models for disc degeneration have been described in the literature. These range from mice knockout models to rats, rabbits, goats, sheep, dogs, and primates (Table 1). Various mechanisms to induce degeneration have also been described in the literature.Lumbar disc punctures were widely used to create degeneration through an anterior approach. The limitation of this method is that it leads to abdominal pain which affects the behaviour pattern of the mice. Currently, genetic manipulation techniques are widely used, as it helps in understanding the role of specific genes in the degeneration process.
Table 1.
Animal models of spine surgery pertaining to disc degeneration.
| SNo. | Author Name | Animal used | Specific models and/or Techniques used |
|---|---|---|---|
| 1 | J. Sahlman(J. Sahlman22 et al., 2001) | Mice | heterozygous knockout of Col2a1 gene |
| 2 | C. W. Goff (C. W. Goff23 et al., 1957) | Rats and mice | Bipedal animals |
| 3 | M. Higuchi (M. Higuchi19 et al., 1983) | Mice | Changes in the nucleus pulposus of the intervertebral disc |
| 4 | K. Masuda (K. Masuda24et al., 2005) | Rabbit | reproducible disc degeneration by an annulus needle puncture |
| 5 | T. Miyamoto (T. Miyamoto25 et al., 2010) | Rabbit | Intradiscal transplantation of synovial mesenchymal stem cells |
| 6 | M. W. Kroeber (M. W. Kroeber26et al., 2002) | Rabbit | Intervertebral disc compression by percutaneous approach |
| 7 | N. A. Gillett (N. A. Gillett27et al., 1988) | Canine | radiology, histology, and mechanical testing |
| 8 | K. Serigano (K. Serigano et al., 2010) | Canine | mesenchymal stem cell transplantation |
| 9 | N. Bergknut (N. Bergknut28 et al., 2012) | Canine | chondrodystrophic (CD) and nonchondrodystrophic dogs (NCD) translational model for human IVDD research. |
| 10 | Y. Zhang (Y. Zhang29et al., 2011) | Goat | optimal method for inducing goat IVD degeneration |
| 11 | R. J. W. Hoogendoorn(R. J. W. Hoogendoorn14et al., 2008) | Goat | Chondroitinase ABC (CABC) induced goat model |
| 12 | T. H. Smit (T. H. Smit15et al., 2002) | Goat | Biomechanical analysis |
| 13 | R. J. W. Hoogendoorn(R. J. W. Hoogendoorn et al., 2005) | Goat | absence of notochordal cells |
| 14 | C. J. Hunter (C. J. Hunter et al., 2004) | Sheep | comparing the cytomorphology of the nucleus pulposus in mammals commonly used in laboratory |
| 15 | H. J. Wilke (H. J. Wilke16 et al., 1997) | Sheep | quantitative biomechanical properties of the sheep spine and compare with human spine |
| 16 | O. L. Osti (O. L. Osti17 et al., 1990) | Sheep | Peripheral tears of the annulus fibrosus |
| 17 | J. Melrose (J. Melrose29et al., 2012) | Sheep | IVD mechanical destabilization on matrix protein and metalloproteinase gene expression to investigate the pathophysiological mechanisms of lumbar IVDD. |
| 18 | D. Oehme22 (D. Oehme et al., 2014) | Sheep | Pentosapolysulfate with mesenchymal progenitor cells |
| 19 | T. Goldschlager23 (T. Goldschlager et al., 2011) | Sheep | Allogenic mesenchymal precursor cells combined with hydroxyapatite and tricalcium phosphate (HA/TCP) with HA/TCP alone or iliac crest autograft (AG) for cervical interbody fusion |
| 20 | T. Goldschlager24 (T. Goldschlager et al., 2011) | Sheep | To compare the capacity of MPCs and AECs in an ovine model. |
| 21 | T. Goldschlager 25(T. Goldschlager et al., 2010) | Sheep | using MPCs in combination with PPS to produce cartilaginous tissue to replace the intervertebral disc following ACD |
| 22 | F. L. Acosta (F. L. Acosta32et al., 2011) | Porcine | Nucleotomy for IVD deegeneration |
| 23 | G. W. Omlor (G. W. Omlor33et al., 2009) | Goettingenminipigs | Disc remodeling and degeneration after nucleotomy |
| 24 | R. C. Platenberg (R. C. Platenberg34et al., 2001) | Baboon | Spontaneous disc degeneration baboon model |
| 25 | D. J. Nuckley (D. J. Nuckley35 et al., 2008) | Primates | Natural disc degeneration |
| 26 | W. E. Stern (W. E. Stern36et al., 1976) | rhesus monkeys | Collagenase induced IVD degeneration |
| 27 | F. Wei (F. Wei37 et al., 2014) | rhesus monkeys | slowly progressive and reproducible intervertebral disc (IVD) degeneration model by Bleomycin |
Rodent models: Mice and Rat models have been used for a long time for disc degeneration studies. Besides genetic knocking, bipedal mouse and rat models have been created through bilateral mid-humeral surgical and tail amputation.26, 27 Other mechanisms of disc degeneration via tail are by the administration of digestive enzymes or asymmetrical compression (Table 1).
Rabbit Models: The major advantage of a rabbit model is a higher degree of resemblance to human intervertebral discs, cost, and larger size. Compression such as bending, postural changes or cyclical compressions (Table 1).28, 29, 30
Canine models: The CD dogs (Beagles and dachshunds) are well-established models of intervertebral disc degeneration. The administration of intra-discal substances is also easier due to the large size of the disc as compared to the rodent models. Postural changes, chemonucleosis are a few of the techniques used to create the model.31, 32
Goat and Sheep models: The advantages of goat model include anatomical homology with vertebral and disc morphology. The animal is handy, economical and also tolerates the surgery well.14, 15, 16, 17, 33
Porcine model:Porcine models have been widely used in the assessment of biological therapies like stem cells. Their discs have been reported to be similar to human intervertebral discs.Porcine models are also used to study the degeneration due to endplate injuries.34, 35, 36, 37
Primate Model:The baboon and macaque models are used to study the spontaneous disc degeneration. The degeneration are created by intradiscal administration of collagenase and subchondral administration of bleomycin and annulotomy in Rheusus monkey. The primates have a structure similar to human along with mechanical structures compatible with erect posture. The major issue faced in using such models are the ethical concerns and cost.37, 38, 39, 40, 41
Chinese hamster: These are used to study the ageing changes in the intervertebral discs due to diabetes. It was found that diabetic animals had a higher incidence of spondylosis compared to non-diabetic animals.42
2.4. Animal Models of Spinal Cord Injury
Several animal models have been used to study spinal cord injury.43, 44 These range from Contusion models to electrolytic injury. The models have shown sequential evolution from the Allen’s model of contusion injury in 1911to the present-day newer approaches45, 46, 47(Table 2).
Table 2.
Animal models of Spine Surgery pertaining to Spinal Cord Injury.
| Sno. | Author Name | Animal used | Technique used |
|---|---|---|---|
| 1 | Baussart B (Baussart B39 et al., 2006) | Rats | repair processes after spinal cord decompression or neuroprotective treatment |
| 2 | Vijayaprakash K (Vijayaprakash K46et al., 2013) | Rats | Induce contusive type of injury in spinal at the thoracic level with customized instruments |
| 3 | Brösamle C (Brösamle C40 et al., 2007) | Transgenic animals | Spinal cord transection and contusion followed by substance infusion to spinal cord, direct gene therapy, cell based approach, implantation of bridges with rehabilitive training |
| 4 | Gensel JC (Gensel JC41 et al., 2006) | Rats | spinal cord contusion injury using a modified NYU/MASCIS weight drop device |
| 5 | Rahimi-Movaghar V (Rahimi-Movaghar V43et al., 2008) | Rats | Transection model |
| 6 | Jazayeri SB (Jazayeri SB44et al., 2013) | Rats | SCI compression with aneurysmal clip compression |
| 7 | Lim JH (Lim JH45et al., 2007) | mongrel dogs | Balloon inflation model |
| 8 | Dimar JRI (Dimar JRI42et al., 1999) | Rats | Contusion model |
| 9 | Lavrov I (Lavrov I47et al., 2006) | Rats | Transection model |
| 10 | Shah PK (Shah PK48et al., 2012) | Rats | Ischemic model |
| 11 | Schwab ME (Schwab ME et al., 1996) | mammals and humans | Review on current knowledge on the mechanisms involved in degeneration and tissue loss and in axonal regeneration |
| 12 | Lang-Lazdunski L (Lang-Lazdunski L50et al., 2000) | Mice | Ischemic model |
| 13 | Gaviria M (Gaviria M51 et al., 2002) | Mice | Ischemic photochemical model of SCI in mice |
| 14 | Nouri M (Nouri M52et al., 2006) | Rats | Clamping model |
| 15 | Yezierski RP (Yezierski RP53 et al., 1998) | Rats | QUIS induced injury and clinical pathology of SCI |
| 16 | Piao MS (Piao MS54et al., 2009) | Mice | Photochemical SCI |
One of the oldest methods to create injury to the spinal cord was by contusion. In 1911, a study reported the creation of spinal cord injury by dropping a mass from a height onto the surface of a dogs spine. Such a mechanism leads to various alterations in the spinal cord. These range from inflammation to haemorrhagic necrosis. In the cervical spine, unilateral contusion models have been reported to avoid life-threatening injuries in animals.48, 49, 50, 51
The spinal cord injury could also be created by occlusion of the spinal canal. Such models are referred to as compression models of SCI. There are several ways by which these models have been created. These range from aneurysmal clips, balloon induction techniques and calliberated forceps. A few studies have also mentioned the use of a spacer to produce canal narrowing to mimic Antero-posterior compression.51, 52, 53, 54
Lateral displacement of a specific vertebra leads to the development of a dislocation model of spinal cord injury. The spinal cord in animals can also be stretched by using the principle of distraction. In such a model, the opposite traction forces stretch the spinal cord and mimic the spinal cord injury due to various trauma settings.55 A few studies have reported the development of spinal cord injury models by transacting the spinal cord using fine surgical scissors. This leads to complete interruption of spinal signals and mimics acute spinal cord injury. These models have been widely used to study the effects of biomaterials and their biomechanics.56, 57, 58
Aortic problems have been reported to cause spinal cord ischaemia.59 This property has been used to create the ischemic models of spinal cord injury. Temporary aortic occlusion of the spinal cord using aortic clips is an established method to create SCI animal models.59, 60, 61 Besides aortic occlusion, the injury can also be created by inserting excitotoxins. These are referred to as the excitotoxic models of spinal cord injury. In such models, there is little need for laminectomy or mechanical trauma to the cord.62, 63 High electric current has shown to injury the spinal cord in a similar manner as excitotoxins.64 However, it has the advantage of creating restricted damage by using an electrode tip. Such a model is referred to as the Electrolytic injury model.57, 65, 66 A summary of these models has been enlisted in Table 2.
2.5. Genetic animal models of the spine
Several models of knock out mice have been established which lead to spontaneous disc degeneration. Such models have been used to analyze the disc pathologies more physiologically. Furthermore, they lead to fewer morbidities and mortalities in animals as an insult/injury is not required to create a particular model.67, 68
A mutation in the BDL strain of mice leads to muscular atrophy during growth. Such mice present with the kyphoscoliotic condition of the spine.69,70Li et al. have reported that a mutation in GDF5 mice shows disc degeneration.71 A KY BDL strain has been reported to exhibit changes in thoracolumbar as well as cervical discs. CCN2 knock out mice have been developed which show alteration in CCN1 and CCN2 thus affecting the disc structure.72 This has lead to the development of gene knockout technologies and transgenic mouse strains to study disc degeneration.73
A few studies have reported transgenic strains of mice that develop age-dependent disc degeneration.74, 75, 76Wang et al. have reported that breeding Col2a1-CreER(T2) and β-catenin(fx(Ex3)/fx(Ex3)) transgenic mice leads to extensive osteophyte formation, upregulation of mmp13, adamts4, and adamts5 genes.77 This leads to a condition mimicking spondylosis in humans. GDF8 deficient mice have been shown to demonstrate signs of disc degeneration by Hamrick et al.78
Though the knock out models are available only in mice, large animals have been used to study spontaneous disc degenerations. A study by Cho et al. reported that MMP1 expression increases in pigs with ageing and this leads to acceleration of disc degeneration.79 A few of the newer studies have proposed the use of alpacas as the potential model to study disc degeneration.80,81 This is because of several similar properties between the discs of this animal model and humans.
3. Conclusion
The scientists and researchers have ethical and moral obligations to limit the number of animals used in research. Furthermore, it is equally important to limit the sufferings of the animals used in research.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Author names: Dr Shakti A Goel, Dr Vicky Varghese, Dr Teyfik Demir.
Contributor Information
Shakti A. Goel, Email: shaktiagoel@gmail.com.
Vicky Varghese, Email: vvarghese@mcw.edu.
Tyfik Demir, Email: tdemir@etu.edu.tr.
References
- 1.Walsh A.J., Bradford D.S., Lotz J.C. In vivo growth factor treatment of degenerated intervertebral discs. Spine. 2004;29:156–163. doi: 10.1097/01.BRS.0000107231.67854.9F. [DOI] [PubMed] [Google Scholar]
- 2.MohseniSaravi B., Kabirzadeh A., Rezazadeh E. Prevalence and causes of medical absenteeism among staff (case study at Mazandaran University of medical sciences: 2009-2010) Mater Sociomed. 2013;25:233–237. doi: 10.5455/msm.2013.25.233-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grieco A., Molteni G., De Vito G., Sias N. Epidemiology of musculoskeletal disorders due to biomechanical overload. Ergonomics. 1998;41:1253–1260. doi: 10.1080/001401398186298. [DOI] [PubMed] [Google Scholar]
- 4.Nur N.M., Dawal S.Z., Dahari M. International Conference on Industrial Engineering and Operations Management Bali; Indonesia: 2014. The Prevalence of Work Related Musculoskeletal Disorders Among Workers Performing Industrial Repetitive Tasks in the Automotive Manufacturing Companies; pp. 1–8. [Google Scholar]
- 5.Mussi G., Gouveia N. Prevalence of work-related musculoskeletal disorders in Brazilian hairdressers. Occup Med (Lond) 2008;58:367–369. doi: 10.1093/occmed/kqn047. [DOI] [PubMed] [Google Scholar]
- 6.Stucchi G., Battevi N., Cairoli S., Consonni D. The prevalence of musculoskeletal disorders in the retail sector: an Italian cross sectional study on 3380 workers. Med Lav. 2016;107:251–262. [PubMed] [Google Scholar]
- 7.Lipson S.J., Muir H. Volvo award in basic science. Proteoglycans in Experimental Intervertebral Disc Degeneration. Spine. 1980;vol. 6:194–210. doi: 10.1097/00007632-198105000-00002. 1981. [DOI] [PubMed] [Google Scholar]
- 8.Osti O.L., Vernon-Roberts B., Fraser R.D. Volvo award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine. 1990;15:762–767. doi: 10.1097/00007632-199008010-00005. 1990. [DOI] [PubMed] [Google Scholar]
- 9.Hampton D., Laros G., McCarron R., Franks D. Healing potential of the anulusfibrosus. Spine. 1989;14:398–401. doi: 10.1097/00007632-198904000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Kääpä E., Holm S., Han X. Collagens in the injured porcine intervertebral disc. J Orthop Res. 1994;12:93–102. doi: 10.1002/jor.1100120112. [DOI] [PubMed] [Google Scholar]
- 11.Nishida K., Kang J.D., Suh J.K. Adenovirus-mediated gene transfer to nucleus pulposus cells. Implications for the treatment of intervertebral disc degeneration. Spine. 1998;23:2437–2442. doi: 10.1097/00007632-199811150-00016. [DOI] [PubMed] [Google Scholar]
- 12.Goel S.A., Nagpal P., Nagarajan P., Panda A.K., Chhabra H.S. Lateral approach to the lumbar spine of spraguedawley rat: development of a novel animal model for spine surgery. Indian Spine J. 2019;2:134–137. [Google Scholar]
- 13.Nobunaga A.I., Go B.K., Karunas R.B. Recent demographic and injury trends in people served by the model spinal cord injury care systems. Arch Phys Med Rehabil. 1999;80:1372–1382. doi: 10.1016/s0003-9993(99)90247-2. [DOI] [PubMed] [Google Scholar]
- 14.Hoogendoorn R.J.W., Helder M.N., Kroeze R.J., Bank R.A., Smit T.H., Wuisman P.I.J.M. Reproducible long-term disc degeneration in a large animal model. Spine. 2008;33(9):949–954. doi: 10.1097/BRS.0b013e31816c90f0. [DOI] [PubMed] [Google Scholar]
- 15.Osti O.L., Vernon-Roberts B., Fraser R.D. Anulus tears and intervertebral disc degeneration: an experimental study using an animal model. Spine. 1990;15(8):762–767. doi: 10.1097/00007632-199008010-00005. [DOI] [PubMed] [Google Scholar]
- 16.Wilke H.J., Kettler A., Claes L.E. Are sheep spines a valid biomechanical model for human spines? Spine. 1997;22(20):2365–2374. doi: 10.1097/00007632-199710150-00009. [DOI] [PubMed] [Google Scholar]
- 17.Smit T.H. The use of a quadruped as an in vivo model for the study of the spine—biomechanical considerations. Eur Spine J. 2002;11(2):137–144. doi: 10.1007/s005860100346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.F.Galbusera, H.J. Wilke, “Biomechanics of the Spine: Basic Concepts, Spinal Disorders and Treatments”.Academic Press.
- 19.Higuchi M., abe K., Kaneda K. Changes in the nucleus pulposus of the intervertebral disc in bipedal mice: a light and electron microscopic study. Clin Orthop Relat Res. 1983;175 article 251. [PubMed] [Google Scholar]
- 20.S. Reitmaier “Preliminary investigations on intradiscal pressures during daily activities: an in vivo study using the merino sheep”. PloS One 8 (7), e69610. [DOI] [PMC free article] [PubMed]
- 21.Alini M. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17(1):2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oehme D., Ghosh P., Shimmon S. Mesenchymal progenitor cells combined with pentosanpolysulfate mediating disc regeneration at the time of microdiscectomy: a preliminary study in an ovine model. J Neurosurg Spine. 2014;20(6):657–669. doi: 10.3171/2014.2.SPINE13760. [DOI] [PubMed] [Google Scholar]
- 23.Goldschlager T., Rosenfeld J.V., Ghosh P. Cervical interbody fusion is enhanced by allogeneic mesenchymal precursor cells in an ovine model. Spine. 2011;36(8):615–623. doi: 10.1097/BRS.0b013e3181dfcec9. [DOI] [PubMed] [Google Scholar]
- 24.Goldschlager T., Ghosh P., Zannettino A. A comparison of mesenchymal precursor cells and amnion epithelial cells for enhancing cervical interbody fusion in an ovine model. Neurosurgery. 2011;68(4):1025–1035. doi: 10.1227/NEU.0b013e31820d5375. [DOI] [PubMed] [Google Scholar]
- 25.Goldschlager T., Ghosh P., Zannettino A. Cervical motion preservation using mesenchymal progenitor cells and pentosanpolysulfate, a novel chondrogenic agent: preliminary study in an ovine model. Neurosurg Focus. 2010;28(6) doi: 10.3171/2010.3.FOCUS1050. article E4. [DOI] [PubMed] [Google Scholar]
- 26.Sahlman J., Inkinen R., Hirvonen T. Premature vertebral endplate ossification and mild disc degeneration in mice after inactivation of one allele belonging to the Col2a1 gene for type II collagen. Spine. 2001;26(23):2558–2565. doi: 10.1097/00007632-200112010-00008. [DOI] [PubMed] [Google Scholar]
- 27.Goff C.W., Landmesser W. Bipedal rats and mice. The Journal of Bone & Joint Surgery—American. 1957;39(3):616–622. [PubMed] [Google Scholar]
- 28.Masuda K., Aota Y., Muehleman C. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2005;30(1):5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto T., Muneta T., Tabuchi T. Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase-related genes in nucleus pulposus cells in rabbits. Arthritis Res Ther. 2010;12(6) doi: 10.1186/ar3182. article R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroeber M.W., Unglaub F., Wang H. New in vivo animal model to create intervertebral disc degeneration and to investigate the effects of therapeutic strategies to stimulate disc regeneration. Spine. 2002;27(23):2684–2690. doi: 10.1097/00007632-200212010-00007. [DOI] [PubMed] [Google Scholar]
- 31.Gillett N.A., Gerlach R., Cassidy J.J., Brown S.A. Age-related changes in the beagle spine. Acta Orthop. 1988;59(5):503–507. doi: 10.3109/17453678809148772. [DOI] [PubMed] [Google Scholar]
- 32.Bergknut N., Rutges J.P.H.J., Kranenburg H.-J.C. The dog as an animal model for intervertebral disc degeneration? Spine. 2012;37(5):351–358. doi: 10.1097/BRS.0b013e31821e5665. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Drapeau S., An H.S., Markova D., Lenart B.A., Anderson D.G. Histological features of the degenerating intervertebral disc in a goat disc-injury model. Spine. 2011;36(19):1519–1527. doi: 10.1097/BRS.0b013e3181f60b39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holm S., Holm A.K., Ekström L. Experimental disc degeneration due to endplate injury. J Spinal Disord Tech. 2004;17:64–71. doi: 10.1097/00024720-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Omlor G.W., Nerlich A.G., Wilke H.-J. A new porcine in vivo animal model of disc degeneration. Spine. 2009;34:2730–2739. doi: 10.1097/BRS.0b013e3181b723c9. [DOI] [PubMed] [Google Scholar]
- 36.Acosta F.L., Jr., Metz L., Adkisson H.D. Porcine intervertebral disc repair using allogeneic juvenile articular chondrocytes or mesenchymal stem cells. Tissue Eng. 2011;17(23-24):3045–3055. doi: 10.1089/ten.tea.2011.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Omlor G.W., Nerlich A.G., Wilke H.-J. A new porcine in vivo animal model of disc degeneration: response of anulusfibrosus cells, chondrocyte-like nucleus pulposus cells, and notochordal nucleus pulposus cells to partial nucleotomy. Spine. 2009;34(25):2730–2739. doi: 10.1097/BRS.0b013e3181b723c9. [DOI] [PubMed] [Google Scholar]
- 38.Platenberg R.C., Hubbard G.B., Ehler W.J., Hixson C.J. Spontaneous disc degeneration in the baboon model: magnetic resonance imaging and histopathologic correlation. J Med Primatol. 2001;30(5):268–272. doi: 10.1034/j.1600-0684.2001.d01-59.x. [DOI] [PubMed] [Google Scholar]
- 39.Nuckley D.J., Kramer P.A., Del Rosario A., Fabro N., Baran S., Ching R.P. Intervertebral disc degeneration in a naturally occurring primate model: radiographic and biomechanical evidence. J Orthop Res. 2008;26(9):1283–1288. doi: 10.1002/jor.20526. [DOI] [PubMed] [Google Scholar]
- 40.Stern W.E., Coulson W.F. Effects of collagenase upon the intervertebral disc in monkeys. J Neurosurg. 1976;44(1):32–44. doi: 10.3171/jns.1976.44.1.0032. [DOI] [PubMed] [Google Scholar]
- 41.Wei F., Zhong R., Zhou Z. In vivo experimental intervertebral disc degeneration induced by bleomycin in the rhesus monkey. BMC Muscoskel Disord. 2014;15 doi: 10.1186/1471-2474-15-340. article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silberberg R., Gerritsen G. Aging changes in intervertebral discs and spondylosis in Chinese hamsters. Diabetes. 1976;25:477–483. doi: 10.2337/diab.25.6.477. [DOI] [PubMed] [Google Scholar]
- 43.Cheriyan T., Ryan D.J., Weinreb J.H. Spinal cord injury models: a review. Spinal Cord. 2014;52:588–595. doi: 10.1038/sc.2014.91. [DOI] [PubMed] [Google Scholar]
- 44.Kundi S., Bicknell R., Ahmed Z. Spinal cord injury: current mammalian models. Am J Neurosci. 2013;4:1–12. [Google Scholar]
- 45.Schwab M.E., Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- 46.Talac R., Friedman J.A., Moore M.J. Animal models of spinal cord injury for evaluation of tissue engineering treatment strategies. Biomaterials. 2004;25:1505–1510. doi: 10.1016/s0142-9612(03)00497-6. [DOI] [PubMed] [Google Scholar]
- 47.Masri R., Quiton R.L., Lucas J.M., Murray P.D., Thompson S.M., Keller A. Zonaincerta: a role in central pain. J Neurophysiol. 2009;102:181–191. doi: 10.1152/jn.00152.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baussart B., Stamegna J.C., Polentes J., Tadie M., Gauthier P. A new model of upper cervical spinal contusion inducing a persistent unilateral diaphragmatic deficit in the adult rat. Neurobiol Dis. 2006;22:562–574. doi: 10.1016/j.nbd.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 49.Brösamle C., Huber A.B. Cracking the black box–and putting it back together again: animal models of spinal cord injury. Drug Discov Today Dis Model. 2007;3:341–347. [Google Scholar]
- 50.Gensel J.C., Tovar C.A., Hamers F.P.T., Deibert R.J., Beattie M.S., Bresnahan J.C. Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. J Neurotrauma. 2006;23:36–54. doi: 10.1089/neu.2006.23.36. PMID: 16430371. [DOI] [PubMed] [Google Scholar]
- 51.Dimar J.R.I., Glassman S.D., Raque G.H., Zhang Y.P., Shields C.B. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine. 1999;24:1623. doi: 10.1097/00007632-199908150-00002. [DOI] [PubMed] [Google Scholar]
- 52.Rahimi-Movaghar V., Yazdi A., Karimi M. Effect of decompression on complete spinal cord injury in rats. Int J Neurosci. 2008;118:1359–1373. doi: 10.1080/00207450701392340. [DOI] [PubMed] [Google Scholar]
- 53.Jazayeri S.B., Firouzi M., AbdollahZadegan S. The effect of timing of decompression on neurologic recovery and histopathologic findings after spinal cord compression in a rat model. Acta Med Iran. 2013;51:431–437. [PubMed] [Google Scholar]
- 54.Lim J.H., Jung C.S., Byeon Y.E. Establishment of a canine spinal cord injury model induced by epidural balloon compression. J Vet Sci. 2007;8:89–94. doi: 10.4142/jvs.2007.8.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vijayaprakash K., SridharanN An experimental spinal cord injury rat model using customized impact device: a cost-effective approach. J Pharmacol Pharmacother. 2013;4:211–213. doi: 10.4103/0976-500X.114607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lavrov I., Gerasimenko Y.P., Ichiyama R.M. Plasticity of spinal cord reflexes after a complete transection in adult rats: relationship to stepping ability. J Neurophysiol. 2006;96:1699–1710. doi: 10.1152/jn.00325.2006. [DOI] [PubMed] [Google Scholar]
- 57.Shah P.K., Gerasimenko Y., Shyu A. Variability in step training enhances locomotor recovery after a spinal cord injury. Eur J Neurosci. 2012;36:2054–2062. doi: 10.1111/j.1460-9568.2012.08106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edgerton V.R., Tillakaratne N.J., Bigbee A.J., de Leon R.D., Roy R.R. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- 59.Lang-Lazdunski L., Matsushita K., Hirt L., Waeber C., Vonsattel J.-P.G., MoskowitzMA Spinal cord ischemia: development of a model in the mouse. Stroke. 2000;31:208–213. doi: 10.1161/01.str.31.1.208. [DOI] [PubMed] [Google Scholar]
- 60.Gaviria M., Haton H., Sandillon F., PrivatA A mouse model of acute ischemic spinal cord injury. J Neurotrauma. 2002;19:205–221. doi: 10.1089/08977150252806965. [DOI] [PubMed] [Google Scholar]
- 61.Nouri M., Rasouli M., Shafiei S., Tavasoly A., Dehpour A.R., Rahimi-MovagharV Does abdominal aorta clamping, as a method of spinal ischemia in rats, really work? Surg Neurol. 2006;66:332–333. doi: 10.1016/j.surneu.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 62.Yezierski R.P., Liu S., Ruenes G.L., Kajander K.J., Brewer K.L. Excitotoxic spinal cord injury: behavioral and morphological characteristics of a central pain model. Pain. 1998;75:141–155. doi: 10.1016/S0304-3959(97)00216-9. [DOI] [PubMed] [Google Scholar]
- 63.Piao M.S., Lee J.K., Jang J.W., Kim S.H., Kim H.S. A mouse model of photochemically induced spinal cord injury. J Korean NeurosurgSoc. 2009;46:479–483. doi: 10.3340/jkns.2009.46.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Courtine G., Gerasimenko Y., van den Brand R. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Serigano K., Sakai D., Hiyama A., Tamura F., Tanaka M., Mochida J. Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J Orthop Res. 2010;28(10):1267–1275. doi: 10.1002/jor.21147. [DOI] [PubMed] [Google Scholar]
- 66.Steward O., Zheng B., Tessier-LavigneM False resurrections: distinguishing regenerated from spared axons in the injured central nervous system. J Comp Neurol. 2003;459:1–8. doi: 10.1002/cne.10593. [DOI] [PubMed] [Google Scholar]
- 67.Hoogendoorn R.J.W., Helder M.N., Smit T.H., Wuisman P.I.J.M. Notochordal cells in mature caprine intervertebral discs. Eur Cell Mater. 2005;10(3):59. [Google Scholar]
- 68.Hunter C.J., Matyas J.R., Duncan N.A. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat. 2004;205(5):357–362. doi: 10.1111/j.0021-8782.2004.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mason R.M., Palfrey A.J. Intervertebral disc degeneration in adult mice with hereditary kyphoscoliosis. J Orthop Res. 1984;2:333–338. doi: 10.1002/jor.1100020405. [DOI] [PubMed] [Google Scholar]
- 70.Blanco G., Coulton G.R., Biggin A. The kyphoscoliosis (ky) mouse is deficient in hypertrophic responses and is caused by a mutation in a novel muscle-specific protein. Hum Mol Genet. 2001;10:9–16. doi: 10.1093/hmg/10.1.9. [DOI] [PubMed] [Google Scholar]
- 71.Li X., Leo B.M., Beck G., Balian G., Anderson G.D. Collagen and proteoglycan abnormalities in the GDF-5-deficient mice and molecular changes when treating disk cells with recombinant growth factor. Spine. 2004;29:2229–2234. doi: 10.1097/01.brs.0000142427.82605.fb. [DOI] [PubMed] [Google Scholar]
- 72.Bedore J., Sha W., McCann M.R., Liu S., Leask A., Seguin C.A. Impaired intervertebral disc development and premature disc degeneration in mice with notochord-specific deletion of CCN2. Arthritis Rheum. 2013;65:2634–2644. doi: 10.1002/art.38075. [DOI] [PubMed] [Google Scholar]
- 73.Vo N., Niedernhofer L.J., Nasto L.A. An overview of underlying causes and animal models for the study of age-related degenerative disorders of the spine and synovial joints. J Orthop Res. 2013 Jun;31(6):831–837. doi: 10.1002/jor.22204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Millecamps M., Tajerian M., Sage E.H., Stone L.S. Behavioral signs of chronic back pain in the SPARC-null mouse. Spine. 2011;36:95–102. doi: 10.1097/BRS.0b013e3181cd9d75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Millecamps M., Tajerian M., Naso L., Sage E.H., Stone L.S. Lumbar intervertebral disc degeneration associated with axial and radiating low back pain in ageing SPARC-null mice. Pain. 2012;153:1167–1179. doi: 10.1016/j.pain.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 76.Miyagi M., Millecamps M., Danco A.T., Ohtori S., Takahashi K., Stone L.S. ISSLS prize winner: increased innervation and sensory nervous system plasticity in a mouse model of low back pain due to intervertebral disc degeneration. Spine. 2014;39:1345–1354. doi: 10.1097/BRS.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 77.Wang D., Nasto L.A., Roughley P. Spine degeneration in a murine model of chronic human tobacco smokers. Osteoarthritis Cartilage. 2012;20:896–905. doi: 10.1016/j.joca.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McPherron A.C., Lee S.J. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cho H., Park S.H., Lee S., Kang M., Hasty K.A., Kim S.J. Snapshot of degenerative aging of porcine intervertebral disc: a model to unravel the molecular mechanisms. Exp Mol Med. 2011;43:334–340. doi: 10.3858/emm.2011.43.6.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valentine B.A., Saulez M.N., Cebra C.K., Fischer K.A. Compressive myelopathy due to intervertebral disk extrusion in a llama (lama glama) J Vet Diagn Invest. 2006;18:126–129. doi: 10.1177/104063870601800122. [DOI] [PubMed] [Google Scholar]
- 81.Stolworthy D.K., Bowden A.E., Roeder B.L. MRI evaluation of spontaneous intervertebral disc degeneration in the alpaca cervical spine. J Orthop Res. 2015;33:1776–1783. doi: 10.1002/jor.22968. [DOI] [PubMed] [Google Scholar]