Abstract
Background
Direct-acting antiviral treatment regimens cure >95% of chronic hepatitis C virus (HCV) infections, but recent studies indicate that <25% of patients in the United States receive treatment. Patients need to overcome barriers on the steps of the care continuum in order to be prescribed treatment. We aimed to examine the steps of the HCV care continuum up to prescription of HCV therapy among patients receiving care within a large safety net health care system in Houston, Texas.
Methods
We used electronic medical records to identify patients with positive screening tests for HCV antibodies between July 1, 2017, and June 30, 2018, and abstracted data on their advancement through the care continuum for HCV. We used logistic regression to identify factors associated with patient navigation through the continuum.
Results
Of the 2450 patients screening positive for HCV antibodies, 2016 (82.3%) received quantitative RNA testing, of whom 1081 (53.6%) exhibited chronic infection. Providers referred 915 (84.6%) to specialty care for evaluation, 540 of these patients (50.0%) received their specialist evaluation, and 299 (27.7%) received a prescription for treatment. Patients with history of substance use were less likely to be prescribed treatment (adjusted odds ratio, 0.66; 95% CI, 0.49–0.88).
Conclusions
We found substantial attrition at each stage of the HCV care continuum. In particular, history of substance abuse was a predictor of nonprescription. Challenges in the care continuum motivate increased provider education as well as the adoption of recent innovations in patient care.
Keywords: care continuum, health care access, hepatitis C virus, substance abuse
With the recent introduction of direct-acting antiviral (DAA) therapy, treatment of hepatitis C virus (HCV) infection is all-oral and more efficacious compared with previous interferon-based regimens. Typical treatment regimens require patients to take 3 tablets orally per day for 8 weeks, and >95% of patients are cured (ie, achieve sustained viral response [SVR]) [1, 2]. However, the population-level impact of these curative therapies is threatened by important gaps in the HCV care continuum, the sequence of necessary steps an individual with HCV will experience as they approach cure.
While the continuum for HCV care varies across different health care settings [3–5], it generally involves (1) receiving a screening test for hepatitis C antibodies; (2) receiving a quantitative RNA test to confirm ongoing infection (confirmatory testing); (3) undergoing evaluation for liver disease staging and the creation of a treatment plan; (4) receiving a prescription for treatment; and (5) successfully initiating/completing treatment and achieving SVR.
In such an extended process, patients encounter a variety of barriers to care that may be system-, patient-, and/or provider-related. Following the introduction of DAAs, studies of individual health care environments report that 88%–98% of patients screening positive for HCV antibodies received confirmatory testing [3, 4, 6], while earlier studies indicated that only 63% [7] or as few as 27% [5] received confirmatory testing. The percentage of patients with chronic HCV infection attending and receiving specialist evaluations has varied from 36% to 69% of patients in recent years [3, 4, 6], perhaps as a function of populations served and health plans available in different health care systems. Despite the advent of DAAs, recent studies indicate that only 10% to 24% of patients with chronic HCV infection are prescribed treatment [3, 4, 6], and reported rates of SVR range between 6% and 8% [3, 4].
A variety of sociodemographic variables impede individuals with chronic infection from advancing through the care continuum. While the majority of chronic HCV infections are among people born from 1945 to 1965, contemporary HCV infections are attributable to injection drug use [8, 9]. People with substance use disorder tend to earn less income, demonstrate lower health literacy, and are less likely to have health insurance [10–14]. Additionally, homeless persons exhibit increased prevalence of HCV, likely due to high rates of injection drug use [15]. The prevalence of chronic HCV among the homeless in the United States is estimated to be 20% [16]. The prohibitive price of DAAs, the wholesale acquisition cost of which can reach upwards of $100 000 [17], makes pursuing treatment virtually impossible for most individuals without health coverage. Even among individuals with coverage, insurance companies may still deny coverage of the treatment if the patient is deemed, for example, to have less advanced liver fibrosis, to not meet sobriety restrictions, or to have not had consultation with a specialist [18].
The present study sought to examine contemporary barriers to HCV treatment in a large safety net health care system in Texas by identifying the points at which individuals fail to advance in the care continuum and the risk factors associated with these lapses. Several factors specific to Texas motivate this analysis. Hispanic individuals, a large constituency in Texas and frequent users of the local public health system [19], demonstrate higher rates of HCV than non-Hispanic white individuals, and HCV rates increase with proximity to the US–Mexico border [20]. Additionally, Texas has an estimated HCV prevalence of 1.8% [20], and, perhaps relatedly, the highest rate of hepatocellular carcinoma [21]. Further, this public health system largely serves individuals of minority ethnicity, the under- and uninsured, and the homeless [19], traditionally disenfranchised populations that warrant particular attention in the care continuum.
METHODS
We performed a retrospective cohort study among patients receiving care in the Harris Health System, a safety net public health care system serving Harris County in Houston, Texas. The Harris Health system is comprised of 2 hospitals, 18 community health centers, 3 multispecialty clinics, 10 homeless shelter clinics, and 5 clinics offering homeless eligibility services. More than 60% of patients are uninsured, and 30% utilize public insurance programs [19]. This study was approved by the Institutional Review Boards at Baylor College of Medicine and Harris Health System, and informed consent was waived because the study was retrospective and involved no more than minimal risk.
We identified all Harris Health patients testing positive for HCV antibodies between July 1, 2017, and June 30, 2018, and followed through June 30, 2019. Following the identification of an antibody-positive patient, we manually abstracted information about the patient’s course of evaluation and treatment from the patient’s electronic medical record (EMR). Confirmatory testing was indicated by record of a quantitative HCV RNA test following the patient’s positive antibody test. EMRs indicated whether patients received a referral to a hepatologist in the gastrointestinal (GI) clinic at Harris Health and whether the patient attended the clinic. Finally, if the patient received a treatment plan and prescription for an antiviral drug to treat HCV, the patient was marked as receiving a prescription. Importantly, this record does not indicate whether the patient successfully filled the prescription, completed treatment, or achieved SVR. A patient whose specialist evaluation only resulted in pretreatment counseling, liver imaging, or instructions to return to the clinic at a later date was not considered to have received a prescription unless he or she was prescribed an antiviral drug regimen to treat the HCV infection at a later appointment. We evaluated the EMRs of patients until June 30, 2019, ensuring that all individuals screened positive on and before June 30, 2018, had adequate time to advance through the care continuum (up to 2 years for patients diagnosed in July 2017, and at least 1 year for those diagnosed in June 2018).
In addition to the information on the care continuum, patient sociodemographic data were abstracted from the EMR, including age, sex, race, and ethnicity. Behavioral factors were abstracted from provider notes, including history of homelessness, history of multiple sex partners, use of drugs, and consumption of alcohol. In addition to being included in provider notes, history of homelessness is indicated by the patient’s payer source, with homeless persons enrolled in a dedicated financial plan administered by Harris Health. The study was approved by the Institutional Review Boards of Baylor College of Medicine and Harris Health.
We calculated the percentage of patients who transitioned into each phase of the HCV care continuum overall and by race/ethnicity (Hispanic, non-Hispanic black, non-Hispanic white, other), sex, year of birth (born in 1945–1965: yes vs no), homelessness (yes vs no), documented history of substance use (yes vs no), documented high-risk sexual activity (high risk vs no risk), and heavy alcohol use (yes vs no). First, we used multivariable logistic regression to estimate odds ratios (ORs) and 95% confidence intervals for associations with receiving confirmatory HCV RNA testing and being HCV RNA positive. Next, using only those positive for HCV viral RNA, we performed similar multivariable logistic regression analyses to investigate significant factors associated with a referral to specialist, specialist evaluation, and receipt of HCV treatment. We found no evidence of multicollinearity among regressors, as indicated by a variance inflation factor <5. Patients with missing data were excluded from the logistic regression analyses. All analyses were performed using SAS, version 9.1. Statistical significance was determined at α = .05, and all P values for statistical significance were 2-sided.
RESULTS
Table 1 shows selected characteristics of 2450 patients screened positive for HCV antibodies and included in the primary analysis. The majority of antibody-positive patients were baby boomers (67.0%), with an average age (SD) of 53.7 (11.1) years. The cohort was predominantly male (61.0%) and had higher proportions of patients self-identified as non-Hispanic black (41.7%) and Hispanic (28.2%) than non-Hispanic white (24.6%). Twenty-one percent of patients (n = 513) had a documented history of alcohol use, one-third (n = 820) indicated a history of substance use, 0.94% (n = 23) had a documented history of high-risk sexual activity, and 6.3% (n = 155) were homeless.
Table 1.
Number and Prevalence of Patients Screening Positive for HCV Antibodies (July 31, 2017, and June 30, 2018) and Subsequent Advancement Through the HCV Care Continuum Until June 30, 2019
| Screened HCV Antibody Positive | Received Confirmatory Testing | HCV Viral RNA Positive | Referred to Specialist | Received Specialist Evaluation | Prescribed Treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristicc | No. | No. | % | No. | %a | No. | %b | No. | %b | No. | %b |
| Total | 2450 | 2016 | 82.3 | 1081 | 53.6 | 915 | 84.6 | 540 | 50.0 | 299 | 27.7 |
| Race & ethnicity | |||||||||||
| Hispanic | 690 | 594 | 86.1 | 249 | 41.9 | 213 | 85.5 | 141 | 56.6 | 79 | 31.7 |
| NH black | 1021 | 829 | 81.2 | 502 | 60.6 | 424 | 84.5 | 250 | 49.8 | 139 | 27.7 |
| NH white | 602 | 492 | 81.7 | 296 | 60.2 | 248 | 83.8 | 130 | 43.9 | 73 | 24.7 |
| Other | 137 | 101 | 73.7 | 34 | 33.7 | 30 | 88.2 | 19 | 55.9 | 8 | 23.5 |
| Gender | |||||||||||
| Male | 1495 | 1214 | 81.2 | 746 | 61.4 | 619 | 83.0 | 350 | 46.9 | 202 | 27.1 |
| Female | 954 | 802 | 84.1 | 335 | 41.8 | 296 | 88.4 | 190 | 56.7 | 97 | 29.0 |
| Birth cohort | |||||||||||
| 1945–1965 | 1642 | 1347 | 82.0 | 769 | 57.1 | 659 | 85.7 | 391 | 50.8 | 212 | 27.6 |
| Other | 808 | 669 | 82.8 | 312 | 46.6 | 256 | 82.1 | 149 | 47.8 | 87 | 27.9 |
| Homelessness | |||||||||||
| Homeless | 155 | 135 | 87.1 | 83 | 61.5 | 76 | 91.6 | 40 | 48.2 | 29 | 34.9 |
| Not homeless | 2295 | 1881 | 82.0 | 998 | 53.1 | 839 | 84.1 | 500 | 50.1 | 270 | 27.1 |
| History of substance use | |||||||||||
| Yes | 820 | 676 | 82.4 | 441 | 65.2 | 365 | 82.8 | 195 | 44.2 | 104 | 23.6 |
| No | 1400 | 1141 | 81.5 | 545 | 47.8 | 472 | 86.6 | 294 | 53.9 | 170 | 31.2 |
| Sexual activity | |||||||||||
| Risk activity | 23 | 20 | 87.0 | 15 | 75.0 | 13 | 86.7 | 9 | 60.0 | 4 | 26.7 |
| No risk activity | 2197 | 1797 | 81.8 | 971 | 54.0 | 824 | 84.9 | 480 | 49.4 | 270 | 27.8 |
| Alcohol | |||||||||||
| Alcohol use | 513 | 427 | 83.2 | 273 | 63.9 | 235 | 86.1 | 138 | 50.5 | 73 | 26.7 |
| No alcohol use | 1707 | 1390 | 81.4 | 713 | 51.3 | 602 | 84.4 | 351 | 49.2 | 201 | 28.2 |
Abbreviations: EMR, electronic medical record; HCV, hepatitis C virus; NH, non-Hispanic.
aPercentage of all individuals receiving quantitative RNA testing.
bPercentage of all individuals testing positive for HCV RNA.
cInformation on some risk factors was not recorded in EMRs for some patients.
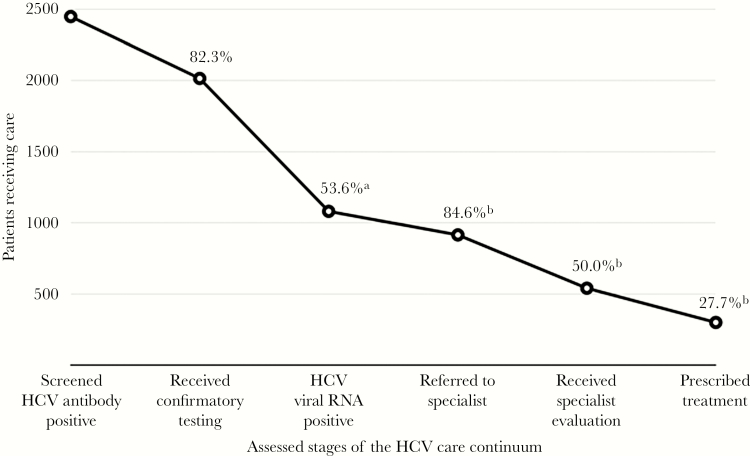
We characterized the numbers and proportions of patients advancing through the care continuum at each stage (Table 1). Among 2450 patients who screened positive for HCV antibodies, 82.3% (n = 2016) received confirmatory testing. Of the 2016 patients who received testing, 53.6% (n = 1081) tested positive for HCV viral RNA. Providers referred 84.6% (n = 915) of patients positive for HCV viral RNA to specialists, but only 50% (n = 540) of those positive for HCV viral RNA received evaluations. Ultimately, 27.7% (n = 299 of 1081) of patients positive for HCV viral RNA were prescribed treatment for HCV (Figure 1).
Figure 1.
Advancement through the hepatitis C virus (HCV) care continuum for patients screening positive for HCV antibodies between July 31, 2017, and June 30, 2018. aPercentage of all individuals receiving quantitative RNA testing. bPercentage of all individuals testing positive for HCV RNA.
In the multivariable model among all positive patients, Hispanics (adjusted OR [aOR], 1.45; 95% CI, 1.05–0.99) and females (aOR, 1.32; 95% CI, 1.05–1.67) were more likely than non-Hispanic whites and males, respectively, to receive confirmatory testing (Table 2). Conversely, of those receiving confirmatory testing, Hispanics (aOR, 0.50; 95% CI, 0.38–0.66) and females (aOR, 0.48; 95% CI, 0.39–0.59) were less likely to have chronic HCV infection compared with non-Hispanic whites and males, respectively. Patients with a documented history of substance use (aOR, 1.69; 95% CI, 1.38–2.09) or alcohol use (aOR, 1.40; 95% CI, 1.10–1.77) were more likely to have chronic HCV infection compared with patients without documented use (Table 2). Compared with patients who were not homeless and had no history of substance use, patients who were homeless and had a history of substance use were 2-fold more likely to be HCV viral RNA positive (aOR, 2.00; 95% CI, 1.18–3.48).
Table 2.
Multivariate Logistic Regression Results for the Effect of Sociodemographic and Behavioral Risk Factors on the Rates of Confirmatory Testing and Chronic HCV Infection
| Received Confirmatory Testing | HCV Viral RNA Positive | |||
|---|---|---|---|---|
| Characteristic | aORa | 95% CI | aORa,b | 95% CIb |
| Race & ethnicity | ||||
| Hispanic | 1.45 | 1.05–1.99 | 0.50 | 0.38–0.66 |
| NH black | 0.97 | 0.74–1.29 | 0.98 | 0.75–1.26 |
| NH white | Ref | Ref | ||
| Other | 0.66 | 0.41–1.07 | 0.47 | 0.28–0.77 |
| Gender | ||||
| Male | Ref | Ref | ||
| Female | 1.32 | 1.05–1.67 | 0.48 | 0.39–0.59 |
| Birth cohort | ||||
| 1945–1965 | 1.05 | 0.82–1.34 | 1.19 | 0.96–1.47 |
| Other | Ref | Ref | ||
| Homelessness | ||||
| Homeless | 1.53 | 0.94–2.64 | 1.16 | 0.77–1.75 |
| Not homeless | Ref | Ref | ||
| History of substance use | ||||
| Yes | 1.06 | 0.84–1.34 | 1.69 | 1.38–2.09 |
| No | Ref | Ref | ||
| Sexual activity | ||||
| Risk activity | 1.37 | 0.46–5.85 | 2.28 | 0.85–7.18 |
| No risk activity | Ref | Ref | ||
| Alcohol | ||||
| Alcohol use | 1.15 | 0.88–1.51 | 1.40 | 1.10–1.77 |
| No alcohol use | Ref | Ref | ||
Abbreviations: aOR, adjusted odds ratio; HCV, hepatitis C virus; NH, non-Hispanic.
aAdjusted odds ratio controlling for all other variables listed.
bCalculated from total number of individuals receiving confirmatory testing.
Compared with males, females with chronic HCV infection were more likely to receive referral to specialty care (aOR, 1.74; 95% CI, 1.16–2.68) and specialist evaluation (aOR, 1.67; 95% CI, 1.26–2.22), but not treatment (aOR, 1.20; 95% CI, 0.88–1.62). Patients with a documented history of substance use were less likely to receive specialist evaluation (aOR, 0.68; 95% CI, 0.52–0.88) and prescriptions for HCV treatment (aOR, 0.66; 95% CI, 0.49–0.88). Conversely, homelessness was associated with an increased rate of specialist referral (aOR, 2.24; 95% CI, 1.06–5.54) and prescription for treatment (aOR, 1.73; 95% CI, 1.02–2.86). The lower likelihood of referral to a specialist and receipt of treatment among patients with a history of substance use were only observed among patients who were also not homeless. Compared with patients who were not homeless and had no history of substance use, patients who were homeless and had a history of substance use were not less likely to be referred to a specialist or receive treatment (aOR, 1.12; 95% CI, 0.58–2.10). Hispanics received specialist evaluations at higher rates than non-Hispanic whites (aOR, 1.84; 95% CI, 1.28–2.65) (Table 3).
Table 3.
Multivariate Logistic Regression Results for the Effect of Sociodemographic and Behavioral Risk Factors on the Rates of Referral to Specialists, Evaluation by Specialists, and Prescription of Treatment for HCV Among Individuals With Chronic HCV Infection
| Received Referral to Specialist | Received Specialist Evaluation | Received Prescription for Treatment | ||||
|---|---|---|---|---|---|---|
| Characteristic | aORab | 95% CI | aORab | 95% CIb | aORab | 95% CIb |
| Race & ethnicity | ||||||
| Hispanic | 1.09 | 0.66–1.80 | 1.84 | 1.28–2.65 | 1.45 | 0.97–2.16 |
| NH black | 0.96 | 0.61–1.49 | 1.26 | 0.91–1.74 | 1.27 | 0.88–1.83 |
| NH white | Ref | Ref | Ref | |||
| Other | 1.12 | 0.40–3.99 | 1.17 | 0.54–2.55 | 0.72 | 0.26–1.76 |
| Gender | ||||||
| Male | Ref | Ref | Ref | |||
| Female | 1.74 | 1.16–2.68 | 1.67 | 1.26–2.22 | 1.20 | 0.88–1.62 |
| Birth cohort | ||||||
| 1945–1965 | 1.48 | 0.99–2.20 | 1.16 | 0.86–1.56 | 0.93 | 0.67–1.30 |
| Other | Ref | Ref | Ref | |||
| Homelessness | ||||||
| Homeless | 2.24 | 1.06–5.54 | 1.28 | 0.78–2.08 | 1.73 | 1.02–2.86 |
| Not homeless | Ref | Ref | Ref | |||
| History of substance use | ||||||
| Yes | 0.72 | 0.50–1.03 | 0.68 | 0.52–0.88 | 0.66 | 0.49–0.88 |
| No | Ref | Ref | Ref | |||
| Sexual activity | ||||||
| Risk activity | Ref | Ref | Ref | |||
| No risk activity | 1.06 | 0.28–6.93 | 1.49 | 0.52–4.55 | 0.95 | 0.26–2.85 |
| Alcohol | ||||||
| Alcohol use | 1.24 | 0.83–1.88 | 1.13 | 0.85–1.51 | 0.98 | 0.26–2.85 |
| No alcohol use | Ref | Ref | Ref | |||
Abbreviations: aOR, adjusted odds ratio; HCV, hepatitis C virus; NH, non-Hispanic.
aAdjusted odds ratio controlling for all other variables listed.
bCalculated from total number of individuals with confirmed chronic HCV infection.
DISCUSSION
In this study of mostly minority, uninsured patients, we found that 82% of patients who screen positive received confirmatory testing for chronic HCV infection. However, among those with chronic infection, only 50% received specialty care and 27.7% were prescribed HCV treatment. The subset of HCV-infected patients with a history of substance abuse in their provider notes was >30% less likely to receive specialty care and treatment than the rest of patients.
The proportion of confirmatory testing in this report (82%) is below other recent studies [4, 6] but exceeds the results seen in a large-scale multistate cohort [7]. Confirmatory testing identified chronic HCV infections in 53.6% of tested patients. Interestingly, this is a lower prevalence rate than other studies [3] or estimates from the Centers for Disease Control and Prevention [8] and may be due to the population-based screening-related sampling frame where false positives are more likely than from targeted testing. Providers referred 84.6% of chronic HCV patients to specialty care, consistent with or above recent studies [3, 4, 6, 7]. The most porous stage of the care continuum proved to be receiving specialist evaluations: only 50% of patients with chronic HCV received specialty care. Rates in other studies vary between 36% and 69% [3, 4]. Finally, less than one-third of patients with chronic HCV were prescribed treatment. While less than optimal, this proportion compares favorably with other studies [3, 6].
People with substance use disorder have an increased risk of incident infection and higher prevalence of chronic HCV infection [8, 9]. In this study, we also showed that patients with substance use disorders were less likely to be referred to specialist care. Importantly, according to clinical guidelines and consensus statements, current or recent injection drug use is not a contraindication to HCV treatment or a reason to deny a patient a referral to specialist evaluation [22–25]. While people with substance use disorder exhibited notably lower SVR rates in interferon-based therapies [26], the advent of DAAs has reduced this disparity [27–29]. The lack of referrals or prescriptions for patients with a documented history of substance use suggests that care providers may benefit from further education on the latest treatment guidelines for chronic HCV, as well as ways to address stigma and bias against patients with substance use disorders that may impact their care.
Interestingly, homeless patients had higher rates of referral to specialty care and prescription for treatment than patients who were not homeless. This is likely attributable to the high quantity of services at Harris Health designed for homeless persons, including dedicated health coverage plans and 10 clinics based in shelters [19]. Additionally, this success underscores the role that safety net health care systems play in eliminating HCV.
The present study highlights potential barriers in the steps of the HCV care continuum. People with substance use disorder exhibit many of the same barriers to treatment as homeless individuals, including low health literacy, inconsistent housing, and a lack of health coverage [10, 13, 30]. As evidenced by success treating HCV in homeless populations, these barriers may be overcome for patients with history of substance use.
The greatest attrition in the care continuum occurred between the referral for specialty care and the receipt of evaluation by a specialist. While exact circumstances varied, records indicate many patients simply failed to attend their scheduled appointment. As the population served by the public health system is primarily low-income and as Harris Health administers specialist evaluation and treatment of HCV exclusively at a dedicated GI clinic, these patients may have difficulty traveling to the specialty clinic located away from their primary care physician. In a study of specialty care among HCV patients, Foster and colleagues found that patients with Medicaid coverage, with no health care coverage, and with limited income experienced greater difficulty receiving specialist evaluations than other patients [31]. As a safety net health care system, these factors are overrepresented in Harris Health’s patient population [19]. The increasing availability of telehealth programs and relative ease of DAA treatment regimens allow primary care providers to manage a growing share of uncomplicated chronic HCV cases, allowing patients to be treated where they are tested [32–34]. Primary care clinics may be more accessible and convenient to patients, increasing attendance. Importantly, individuals who engage in substance use demonstrate high rates of SVR when treated at primary care clinics and at substance use treatment facilities [35, 36], suggesting that integrated models of HCV care may help to address disparities in this population. Further, the adoption of reflex testing aims to reduce the number of patients who never receive confirmatory testing following their positive antibody screening. This institutional practice refers to performing automatic confirmatory testing of all HCV antibody–positive specimens before reporting the screening result to patients, consistent with recommendations by the Centers for Disease Control and Prevention as early as 1998 [37, 38]. Implementation of reflex testing is integral for minimizing false-positive test results, identifying chronic infection, and advancing patients with serologically confirmed infections through the care continuum [38–40]. Harris Health implemented reflex testing following the conclusion of the present study, addressing attrition at this stage.
While this study builds on past research with an expansive and sociodemographically diverse sample in a setting with a large burden of HCV, several constraints limit the interpretation of the results. Importantly, patient behavioral factors impacting treatment were abstracted from provider notes in the patient’s medical record; discrepancies between provider definitions of high-risk sexual behavior or alcohol use may confound the results, as would recall biases. Given that patients with a history of substance abuse were significantly less likely to receive specialty care and treatment, misclassification of exposure due to underreporting of substance use in the notes would have resulted in attenuation of the true effect.
Following prescription for treatment, the next steps in the care continuum are treatment initiation, completion, and finally SVR, all of which remain beyond the scope of this analysis. Patients with a prescription for treatment may still struggle with their coverage providers. Texas Medicaid requires patients to have abstained from drugs or alcohol for at least 3 months and to have advanced fibrosis (Metavir stage F3) before the patient can receive HCV treatment [41]. Notably, these requirements are in contrast with HCV treatment guidelines [22]. Even individuals with private insurance may still be denied coverage for treatment for similar reasons [18].
The present study delineated attrition and associated risk factors at each stage of the HCV care continuum. Many patients are lost at each point of care, though patients with a history of substance use experience the most substantial challenges. Our results highlight a need for increased efforts to adhere to clinical guidelines for the treatment of HCV in these patients. Further, the results motivate the adoption of increasingly popular innovations in HCV health care, including the integration of patient navigators, reflex testing of antibody-positive samples, and the implementation of telehealth programs in primary care provider clinics.
Acknowledgments
Financial support. This work was supported by the Cancer Prevention and Research Institute of Texas (CPRIT) under a Prevention Program Grant (PP160089) to A.P.T.
Potential conflicts of interest. No relevant conflicts of interest exist. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. Shane W. Reader, study concept and design, drafting of the manuscript; Hyunseok Kim, statistical analysis; Hashem B. El-Serag, critical revision of the manuscript; Aaron P. Thrift, obtained funding, critical revision of the manuscript.
References
- 1. American Association for the Study of Liver Diseases. Recommendations for testing, managing, and treating hepatitis C 2019. Available at: https://www.hcvguidelines.org/. Accessed 7 October 2019.
- 2. Dore GJ, Feld JJ. Hepatitis C virus therapeutic development: in pursuit of “perfectovir.” Clin Infect Dis 2015; 60:1829–36. [DOI] [PubMed] [Google Scholar]
- 3. Coyle C, Moorman AC, Bartholomew T, et al. The hepatitis C virus care continuum: linkage to hepatitis C virus care and treatment among patients at an Urban Health Network, Philadelphia, PA. Hepatology 2019; 70:476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hawks L, Norton BL, Cunningham CO, Fox AD. The hepatitis C virus treatment cascade at an urban postincarceration transitions clinic. J Viral Hepat 2016; 23:473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yehia BR, Schranz AJ, Umscheid CA, Lo Re V 3rd. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One 2014; 9:e101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Falade-Nwulia O, Mehta SH, Lasola J, et al. Public health clinic-based hepatitis C testing and linkage to care in Baltimore. J Viral Hepat 2016; 23:366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moorman AC, Gordon SC, Rupp LB, et al. ; Chronic Hepatitis Cohort Study Investigators Baseline characteristics and mortality among people in care for chronic viral hepatitis: the Chronic Hepatitis Cohort Study. Clin Infect Dis 2013; 56:40–50. [DOI] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. Hepatitis C information 2019. Available at: https://www.cdc.gov/hepatitis/hcv/index.htm. Accessed 7 October 2019.
- 9. World Health Organization. What is hepatitis?2019. Available at: https://www.who.int/features/qa/76/en/. Accessed 7 October 2019.
- 10. Chitwood DD, McBride DC, Metsch LR, et al. A comparison of the need for health care and use of health care by injection-drug users, other chronic drug users, and nondrug users. Am Behav Sci 1998; 41:1107–22. [Google Scholar]
- 11. Cronquist A, Edwards V, Galea S, et al. Health care utilization among young adult injection drug users in Harlem, New York. J Subst Abuse 2001; 13:17–27. [DOI] [PubMed] [Google Scholar]
- 12. DeBeck K, Shannon K, Wood E, et al. Income generating activities of people who inject drugs. Drug Alcohol Depend 2007; 91:50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grebely J, Genoway KA, Raffa JD, et al. Barriers associated with the treatment of hepatitis C virus infection among illicit drug users. Drug Alcohol Depend 2008; 93:141–7. [DOI] [PubMed] [Google Scholar]
- 14. Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health 2008; 33:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Linton SL, Celentano DD, Kirk GD, Mehta SH. The longitudinal association between homelessness, injection drug use, and injection-related risk behavior among persons with a history of injection drug use in Baltimore, MD. Drug Alcohol Depend 2013; 132:457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beijer U, Wolf A, Fazel S. Prevalence of tuberculosis, hepatitis C virus, and HIV in homeless people: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:859–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scott JD, Spach DH. Cost and access to direct-acting antiviral agents overview 2019. Available at: https://www.hepatitisc.uw.edu/custom/evaluation-treatment/cost-access-medications. Accessed 7 October 2019.
- 18. Gowda C, Lott S, Grigorian M, et al. Absolute insurer denial of direct-acting antiviral therapy for hepatitis C: a national specialty pharmacy cohort study. Open Forum Infect Dis 2018; 5:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris Health. Facts and figures 2019. Available at: https://www.harrishealth.org/about-us-hh/who-we-are/Pages/statistics.aspx. Accessed 7 October 2019.
- 20. Yalamanchili K, Saadeh S, Lepe R, Davis GL. The prevalence of hepatitis C virus infection in Texas: implications for future health care. Proc (Bayl Univ Med Cent) 2005; 18:3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. White DL, Thrift AP, Kanwal F, et al. Incidence of hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology 2017; 152:812–820.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. American Association for the Study of Liver Diseases and Infectious Disease Society of America. Key populations: identification and management of HCV in people who inject drugs 2018. Available at: https://www.hcvguidelines.org/unique-populations/pwid. Accessed 7 October 2019.
- 23. American Society of Addiction Medicine. Hepatitis C infection 2017. Available at: https://www.asam.org/advocacy/find-a-policy-statement/view-policy-statement/public-policy-statements/2017/04/11/hepatitis-c. Accessed 7 October 2019.
- 24. Substance Abuse and Mental Health Services Administration. Quick guide for clinicians and administrators based on TIP 53: addressing viral hepatitis in people with substance use disorders 2011. Available at: https://store.samhsa.gov/product/TIP-53-Addressing-Viral-Hepatitis-in-People-With-Substance-Use-Disorders/SMA11-4656. Accessed 7 October 2019.
- 25. National Institutes of Health. Consensus development program: management of hepatitis C: 2002 2002. Available at: https://consensus.nih.gov/2002/2002hepatitisc2002116html.htm. Accessed 7 October 2019.
- 26. Dimova RB, Zeremski M, Jacobson IM, et al. Determinants of hepatitis C virus treatment completion and efficacy in drug users assessed by meta-analysis. Clin Infect Dis 2013; 56:806–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burton MJ, Voluse AC, Anthony V. Integrating comprehensive hepatitis C virus care within a residential substance use disorder treatment program. J Subst Abuse Treat 2019; 98:9–14. [DOI] [PubMed] [Google Scholar]
- 28. Ottman AA, Townsend ML, Hashem MG, Britt RB. Impact of substance use disorder on the rate of sustained virological response in veterans with chronic hepatitis C treated with direct-acting antivirals. Ann Pharmacother 2019; 53:581–7. [DOI] [PubMed] [Google Scholar]
- 29. Trabut JB, Barrault C, Charlot H, et al. Integrated care for the use of direct-acting antivirals in patients with chronic hepatitis C and substance use disorder. J Addict Med 2018; 12:346–52. [DOI] [PubMed] [Google Scholar]
- 30. Raven MC, Carrier ER, Lee J, et al. Substance use treatment barriers for patients with frequent hospital admissions. J Subst Abuse Treat 2010; 38:22–30. [DOI] [PubMed] [Google Scholar]
- 31. Foster MA, Xing J, Moorman AC, et al. Frequency of and factors associated with receipt of liver-related specialty care among patients with hepatitis C in the Chronic Hepatitis Cohort Study. Dig Dis Sci 2016; 61: 3469–77. [DOI] [PubMed] [Google Scholar]
- 32. Arora S. Project ECHO: democratising knowledge for the elimination of viral hepatitis. Lancet Gastroenterol Hepatol 2019; 4:91–3. [DOI] [PubMed] [Google Scholar]
- 33. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med 2011; 364: 2199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Syed TA, Bashir MH, Farooqui SM, et al. Treatment outcomes of hepatitis C-infected patients in specialty clinic vs primary care physician clinic: a comparative analysis. Gastroenterol Res Pract 2019; 2019:8434602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Norton BL, Fleming J, Bachhuber MA, et al. High HCV cure rates for people who use drugs treated with direct acting antiviral therapy at an urban primary care clinic. Int J Drug Policy 2017; 47:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Socías ME, Karamouzian M, Parent S, et al. Integrated models of care for people who inject drugs and live with hepatitis C virus: a systematic review. Int J Drug Policy 2019; 72:146–59. [DOI] [PubMed] [Google Scholar]
- 37. Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Morb Mortal Wkly Rep 1998; 47:1–39.9450721 [Google Scholar]
- 38. Centers for Disease Control and Prevention. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. MMWR Morb Mortal Wkly Rep 2003; 52:1–16. [PubMed] [Google Scholar]
- 39. Hirsch AA, Lawrence RH, Kern E, et al. Implementation and evaluation of a multicomponent quality improvement intervention to improve efficiency of hepatitis C screening and diagnosis. Jt Comm J Qual Patient Saf 2014; 40:351–7. [DOI] [PubMed] [Google Scholar]
- 40. Turner BJ, Taylor BS, Hanson JT, et al. Implementing hospital-based baby boomer hepatitis C virus screening and linkage to care: strategies, results, and costs. J Hosp Med 2015; 10:510–6. [DOI] [PubMed] [Google Scholar]
- 41. Center for Health Law and Policy Innovation. Hepatitis C: the state of Medicaid access 2018. Available at: https://www.chlpi.org/wp-content/uploads/2013/12/HCV-State-of-Medicaid-Access-Update-11-8-18.pdf. Accessed 7 October 2019.