Abstract
Background
Patient-reported outcomes (PROs) can help to reduce uncertainties about hepatitis C virus (HCV) treatment with direct-acting antivirals (DAAs) among people who inject drugs and increase treatment uptake in this high-risk group. Besides clinical data, this study analyzed for the first time PROs in a real-world sample of patients on opioid agonist treatment (OAT) and HCV treatment with DAAs.
Methods
HCV treatment data including virological response, adherence, safety, and PROs of 328 German patients on OAT were analyzed in a pragmatic prospective cohort study conducted from 2016 to 2018. Clinical effectiveness was defined as sustained virological response (SVR) at week 12 after end of treatment and calculated in per-protocol (PP) and intention-to-treat (ITT) analyses. Changes over time in PROs on health-related quality of life, physical and mental health, functioning, medication tolerability, fatigue, concentration, and memory were analyzed by repeated-measures analyses of variances (ANOVAs).
Results
We found high adherence and treatment completion rates, a low number of mainly mild adverse events, and high SVR rates (PP: 97.5% [n = 285]; ITT: 84.5% [n = 328]). Missing SVR data in the ITT sample were mainly caused by patients lost to follow-up after treatment completion. Most PROs showed statistically significant but modest improvements over time, with more pronounced improvements in highly impaired patients.
Conclusions
This real-world study confirms that DAA treatment among OAT patients is feasible, safe, and effective. PROs show that all patients, but particularly those with higher somatic, mental, and social burden, benefit from DAA treatment.
Keywords: direct-acting antivirals, hepatitis C virus, opioid substitution treatment, patient-reported outcome measures
Injection drug use is a major risk factor for hepatitis C virus (HCV) infection. In most countries, more than half of the people who inject drugs (PWID) are HCV antibody positive [1], and similar rates are reported among patients on opioid agonist treatment (OAT) [2, 3]. As most of the HCV-related burden results from chronic hepatitis C infections (CHCs) [3], the treatment of OAT patients with CHC is of utmost relevance.
There are excellent therapeutic opportunities within the setting of OAT, as the frequent treatment provider–patient contact allows for continuous monitoring and sustainable HCV management [4]. HCV treatment with direct-acting antivirals (DAAs) among OAT patients is not only feasible and safe, but also results in sustained virological response (SVR) rates comparable to those of non-PWID populations [5–8]. Despite the proven virological effectiveness, HCV treatment uptake among OAT patients is still limited [2, 9]. Besides provider-level barriers [10], patient-related barriers include fear of invasive medical interventions and concerns with regard to DAA side effects [11–13].
Beyond virological response, patient-reported outcomes (PROs) provide important information on overall health, symptoms, burden of disease, and response to treatment and are an important tool to improve patient-centered care, as they are free from third-party interpretation [14]. Thus, more than virological effectiveness alone, improvements in PROs may help to reduce patients’ fears and increase HCV treatment uptake in OAT settings and beyond [10, 15].
Clinical trials and cohort studies [16–18] have shown improved PROs like health-related quality of life, functioning, work productivity, fatigue, depression, and activity among non-PWID populations on DAA treatment. For OAT patients, comparable data are only available from a post hoc analysis of data collected from phase 3 clinical trials [19], and due to their strict inclusion criteria, the findings might be of limited generalizability. Given this, more real-world data are needed to inform patients and providers on PROs among PWID treated with DAA [20, 21]. The aim of the “Interferon-Free Antiviral Treatment of Chronic Hepatitis C Virus Infection Among Opioid Substituted Patients” (INFO) study was to assess the real-world effectiveness, safety, and PROs of DAA treatment among OAT patients in Germany.
METHODS
Study Design and Population
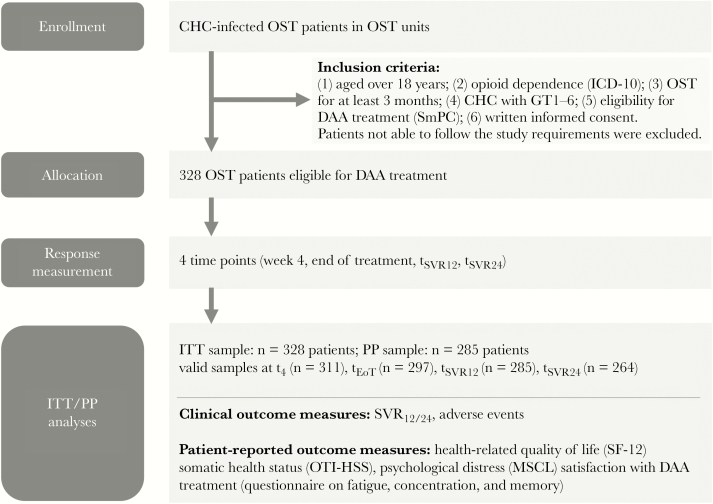
HCV treatment data of OAT patients were collected in a prospective cohort study under clinical routine conditions. Inclusion criteria comprised minimum age of 18 years, opioid dependence according to ICD-10, OAT for at least 3 months, CHC infection with virus genotypes 1–6, eligibility for DAA treatment according to the respective summary of product (SmPC), and written informed consent (Figure 1). Both HCV treatment–naïve and –experienced patients were eligible. Patients with severely impaired cognitive functioning impeding study participation were excluded. Overall, 328 OAT patients from 19 OAT units (on average 17.3 patients per unit, ranging from 3 to 40) spread across 8 out of 16 German federal states participated. Patient recruitment took place between January 2016 and December 2017, and data collection was finished in October 2018 (last follow-up assessment).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of the INFO study. Abbreviations: CHC, chronic hepatitis C virus infection; DAA, direct-acting antivirals; GT, genotype; ITT, intention to treat (all patients with first dose); MSCL, Mini Symptom Checklist; OST, opioid substitution treatment; OTI-HSS, Opiate Treatment Index Health Symptom Scale; PP, per protocol (only patients with complete data for SVR12 or SVR24); SmPC, summary of product; SVR12/24, sustained virological response at week 12/24 after treatment.
Clinical and Patient-Reported Outcome Measures
Clinical effectiveness was defined as a sustained virological response (SVR) at week 12 or week 24 after the end of treatment (SVR12/24). Safety end points were all adverse events (AEs) collected between treatment initiation and week 12 after the end of treatment (tSVR12). An AE was defined as any new untoward medical occurrence or worsening of a preexisting medical condition of a patient. Clinicians were asked to rate AEs for their intensity (low, medium, high) and for their assumed relationship with the antiviral medication using the categories “no causal relationship,” “unlikely,” “possible,” “probable,” and “certain.” Clinicians provided categorical ratings on medication adherence and tolerability at week 4 (t4) and at the end of treatment (tEoT). Moreover, clinicians rated patients’ functioning and illness severity using the Global Assessment of Functioning (GAF) scale [22] and the Clinical Global Impression scales (CGI-S and CGI-I) [23].
Patient-reported outcomes were collected before (t0), at week 4 (t4), at the end (tEoT), and 12 and 24 weeks after the end of DAA treatment (tsvr12, tsvr24). The 12-item Short Form Health Survey (SF-12) [24] was used to measure health-related quality of life on 2 T-standardized composite scores: the Physical Component Summary (PCS) and the Mental Component Summary (MCS). We used the German version [25], and we imputed up to 2 missing items per person with the respective mean sample weights, using the method described in Perneger et al. [26]. The Mini Symptom Checklist (MSCL) [27] is the most recent German version of the 18-item Brief Symptom Inventory (BSI-18), contains 3 subscores (depression, anxiety, somatization) and a Global Severity Index (GSI), and is T-standardized on a recent German norm sample. The Opiate Treatment Index Health Symptom Scale (OTI-HSS) is a measure of physical health, comprising a checklist of 50 symptoms that opioid users often experience [28]. At t4, patients were asked to provide feedback on medication tolerability on the basis of a 4-point Likert scale from “very good” to “poor.” At t4 and tEoT, patients gave a 5-item assessment on therapy side effects using 4-point scales (1 = agree, 2 = rather agree, 3 = rather disagree, 4 = disagree) (Table 2). In addition, treatment-experienced patients were asked at baseline to retrospectively assess their previous treatment (Supplementary Table 1). To measure fatigue, concentration, and memory, we self-constructed a 12-item questionnaire (Supplementary Table 2), as preexisting instruments were either too long or did not cover all 3 concepts. Patients were asked to fill in the questionnaires at every time point (t0–tsvr24), except for treatment-related questions on side effects and medication tolerability, which were only asked at t4 and tEoT.
Table 2.
Course of HCV Therapy, Patient- and Clinician-Reported Outcomes—Total Sample
| Clinician-Reported | T0 | T4 | EoT | SVR12 | SVR24 |
|---|---|---|---|---|---|
| Medication adherence (n = 301–288), % | — | Very good: 90.4 Good: 9.0 Fair: 0.7 |
Very good: 86.5 Good: 13.2 Fair: 0.3 |
- | — |
| CGI-S (n = 324–256) | 3.06 (1.52) | 2.95 (1.52) | 2.91 (1.50) | 2.82 (1.51) | 2.80 (1.49) |
| CGI-I (n = 280–224) | — | 3.76 (0.64) | 3.65 (0.71) | 3.51 (0.75) | 3.46 (0.78) |
| GAF | 66.34 (17.00) | 68.14 (17.36) | 70.27 (17.39) | 71.14 (16.66) | 71.84 (16.82) |
| Medication tolerability, clinician-reported (n = 318–305), % | — | Very good: 70.1 Good: 27.0 Moderate: 2.2 Poor: 0.6 |
Very good: 70.5 Good: 24.6 Moderate: 4.9 |
— | — |
| Patient-Reported | T0 | T4 | EoT | SVR12 | SVR24 |
| Medication tolerability, patient-reported (n = 310), % | — | Very good: 53.5 Good: 39.0 Moderate: 7.1 Poor: 0.3 |
— | — | — |
| Much bothered by side effectsa | — | 3.54 (0.75) | 3.40 (0.90) | — | — |
| Feeling exhausted and weaka | — | 2.99 (1.11) | 3.03 (1.12) | — | — |
| Being sad all the timea | — | 3.47 (0.88) | 3.46 (0.90) | — | — |
| Got done less than I wanteda | — | 3.01 (1.12) | 3.04 (1.14) | — | — |
| Problems thinking straighta | — | 3.35 (0.94) | 3.32 (0.96) | — | — |
| SF-12, Physical Component Summary (n = 317–241) |
43.66 (9.32) | 44.95 (9.14) | 45.12 (9.35) | 45.44 (9.10) | 45.47 (9.05) |
| SF-12, Mental Component Summary (n = 317–241) | 42.35 (11.26) | 45.21 (11.14) | 45.35 (11.18) | 45.73 (11.35) | 45.68 (10.93) |
| MSCL, total score (GSI; n = 327–242) | 58.87 (9.32) | 56.54 (10.27) | 55.96 (11.14) | 55.41 (10.72) | 55.45 (10.87) |
| MSCL, subscale somatization (n = 327–242) | 57.69 (9.63) | 56.56 (9.69) | 56.00 (10.22) | 54.68 (10.11) | 54.94 (10.16) |
| MSCL, subscale depression (n = 327–242) | 58.16 (8.72) | 55.61 (9.62) | 55.41 (9.84) | 55.23 (9.81) | 55.38 (9.92) |
| MSCL, subscale anxiety (n = 327–242) | 57.44 (10.95) | 55.37 (11.32) | 54.89 (11.47) | 54.64 (10.80) | 54.95 (11.33) |
| OTI-HSS (n = 326–237) | 12.17 (7.62) | 11.14 (7.75) | 11.14 (8.32) | 10.14 (8.06) | 10.23 (8.13) |
| Fatigue, concentration, and memory, total scoreb (n = 325–243) | 0.79 (0.71) | 0.72 (0.77) | 0.68 (0.74) | 0.63 (0.68) | 0.66 (0.73) |
| Fatigue, concentration, and memory, subscale fatigueb (n = 325–243) | 0.93 (0.89) | 0.85 (0.94) | 0.80 (0.96) | 0.70 (0.82) | 0.72 (0.87) |
| Fatigue, concentration, and memory, subscale concentration and memoryb (n = 325–243) | 0.69 (0.71) | 0.62 (0.75) | 0.60 (0.71) | 0.58 (0.66) | 0.62 (0.70) |
Data are presented as means and standard deviations or %. Categories of clinician-rated medication adherence: “very good” (100% of the medication taken), “good” (at least 90%), “fair” (at least 80%), “poor” (<80%),
Abbreviations: CGI-I, Clinical Global Impression–improvement (ranging from 1 = “very much improved” to 7 = “very much worsened”); CGI-S, Clinical Global Impression–severity (ranging from 1 = “not at all ill” to 7 = “extremely ill”; GAF = Global Assessment of Functioning (0–100; higher scores indicate better functioning); GSI, Global Severity Index; HCV, hepatitis C virus; MCS, Mental Component Summary; MSCL, Mini Symptom Checklist, with GSI (higher scores indicate worse mental health); OTI-HSS, Opiate Treatment Index–health symptoms scale (higher scores indicate worse physical health); PCS, Physical Component Summary; SF-12, Short Form Health Assessment, consisting of PCS and MCS (higher scores indicate better quality of life).
a“Altogether, how do/did you feel during HCV treatment?” Response options: 1 = agree, 2 = rather agree, 3 = rather disagree, 4 = disagree (n = 310–287).
bHigher scores indicate higher impairments, ranging from 0 = “not at all” to 4 = “very much.”
Patient Consent Statement
The study was conducted in accordance with Good Clinical Practice (GCP), as defined by the International Conference on Harmonisation. For each patient, a written consent was obtained. The design of the work was approved by the Ethics Committee of the Medical Association of Hamburg (reference number PV4603). Secondary ethical votes were approved by local ethical committees in other federal states. The study was registered at clinicaltrials.gov (NCT02969668).
Statistical Analyses
Baseline sample characteristics and safety data were analyzed descriptively. For SVR rates, 95% confidence intervals were calculated. All patients with a first dose of DAA were included in the intention-to-treat (ITT) analysis, whereas the per-protocol (PP) sample comprised only patients with complete data for SVR12/24 (n = 285). PRO and clinician ratings were first analyzed descriptively using the valid samples at each measurement point from baseline until tsvr24, and second, they were tested for significant changes over time with repeated-measures analyses of variances (ANOVAs). In addition, we assessed group × time interactions on health-related quality of life (SF-12) by comparing patients with low baseline levels (PCS/ MCS < 40) with the remaining sample. For the self-constructed items on fatigue, concentration, and memory, we performed exploratory factor analyses (Varimax rotation) to decide on the number of (sub)scales, and we determined the internal consistencies (Cronbach’s alpha) of the final scales. Among treatment-experienced patients, we compared ratings on treatment tolerability for the previous and the current treatment with dependent-sample t tests. All analyses were performed with IBM SPSS statistics, version 22.
RESULTS
Baseline Sample Characteristics
The 328 OAT patients had a mean age of 44.5 (range: 26–69) years, were predominately male (77.4%), and had been infected with HCV for about 13 years on average (Table 1). Around 80% were treatment-naïve, 18.6% had previously been treated with interferon-based protocols (Table 1). Around two-thirds of the patients were treated with (levo-)methadone, and, according to the last 2 urine tests, around a third were actively using illicit drugs (Table 1).
Table 1.
Sociodemographic and Clinical Characteristics of the Patient Sample at Baseline (n = 328)
| % or Mean (SD), Range | |
|---|---|
| Male (n = 328) | 77.4 |
| Age (n = 328) | 44.5 (8.4), 26–69 |
| Having children (n = 291) | 47.4 |
| Living together with children (n = 321) | 10.3 |
| Relationship (n = 280) | |
| Single | 60.7 |
| Relationship, not living together | 11.8 |
| Relationship, living together | 27.5 |
| Employment (n = 324) | |
| Employed (regular full- or part-time) | 20.4 |
| Unemployed/disability pension | 67.6 |
| Occasional/other | 12.0 |
| Living situation (n = 324) | |
| Own flat/with partner | 65.4 |
| Institutional | 19.8 |
| With relatives/friends | 8.6 |
| Other/temporary accomodation | 5.6 |
| Homeless | 0.6 |
| Caucasian ethnicity (n = 322) | 98.8 |
| German citizenship (n = 320) | 86.6 |
| Migration background (n = 255) | 23.2 |
| German language skills (n = 327) | |
| Very good/native speaker | 85.6 |
| Good | 11.0 |
| Poor | 3.4 |
| Duration of OAT with their current physician (n = 289), y | 4.2 (4.6), 0–23 |
| Overall duration of OAT (n = 311), y | 11.3 (7.3), 0–35 |
| Substitution medication (n = 325) | |
| D-/L-methadone (liquid or tablets) | 67.7 |
| Buprenorphine (with or without naloxone) | 20.0 |
| Other (eg, slow-release morphine, diamorphine) | 12.4 |
| (Estimated) duration of HCV infection (n = 309), y | 13.7 (7.6), 1–38 |
| Liver cirrhosis (n = 328) | |
| Cirrhosis, decompensated | 2.7 |
| Cirrhosis, compensated | 11.0 |
| Cirrhosis, not specified | 2.7 |
| No cirrhosis | 75.0 |
| Unclear | 8.5 |
| Liver fibrosis (n = 328) | |
| Metavir score F4/cirrhosis | 16.5 |
| Metavir score F1–F3 | 23.5 |
| No fibrosis | 11.6 |
| Unclear | 48.5 |
| HCV genotype (n = 327) | |
| 1 | 49.8 |
| 2 | 2.4 |
| 3 | 42.5 |
| 4 | 5.2 |
| HIV status (n = 328) | |
| Positive | 4.0 |
| Negative | 83.8 |
| Unknown | 12.2 |
| Previously treated for HCV (n = 328) | |
| Never treated | 79.9 |
| Interferon-based | 18.6 |
| Interferon-free | 0.6 |
| Unclear | 0.9 |
| Antiviral medication (n = 328) | |
| Sofosbuvir & velpatasvir | 41.5 |
| Sofosbuvir & ledipasvir | 29.3 |
| Glecaprevir & pibrentasvir | 12.2 |
| Elbasvir & grazoprevir | 10.1 |
| Ombitasvir, paritaprevir, ritonavir, & dasabuvir | 4.6 |
| Other | 2.4 |
| Treatment duration (planned; n = 326) | |
| 8 wk | 27.0 |
| 12 wk | 70.6 |
| >12 wk | 2.4 |
| Treatment duration (weeks between t0 and EoT; n = 311) | |
| Up to 10 wk | 22.8 |
| >10–14 wk | 62.1 |
| >14–18 wk | 10.3 |
| >18 wk | 4.8 |
| Past 2 urine samples: ≥1 positive sample | |
| Cocaine (n = 287) | 16.4 |
| Benzodiazepines (n = 285) | 30.2 |
| Opiates (n = 287) | 34.8 |
| Amphetamines (n = 245) | 0.8 |
| Global Assessment of Functioning score (n = 326) | |
| ≤30 (unable to function in almost all areas) | 1.5 |
| 31–40 (major impairment in several areas) | 6.1 |
| 41–50 (serious impairment) | 9.8 |
| 51–60 (moderate impairment) | 21.8 |
| 61–70 (mild impairment) | 21.8 |
| 71–80 (only slight impairment) | 21.2 |
| 81–90 (good functioning) | 11.7 |
| 91–100 (superior functioning) | 6.1 |
Percentages are based on valid numbers, which are indicated in parentheses. Active drug use: ≥1 positive urine samples in the past 12 weeks.
Abbreviations: HCV, hepatitis C virus; OAT, opioid agonist treatment.
Global functioning scores (GAF) at baseline indicate a high heterogeneity of the sample. Around two-thirds of the patients fell into the medium categories “moderate/mild/slight impairment,” 17.5% were rated as “serious impairment” or worse, and a similar proportion (17.8%) showed “good” or “superior” functioning (Table 1).
Virological Response
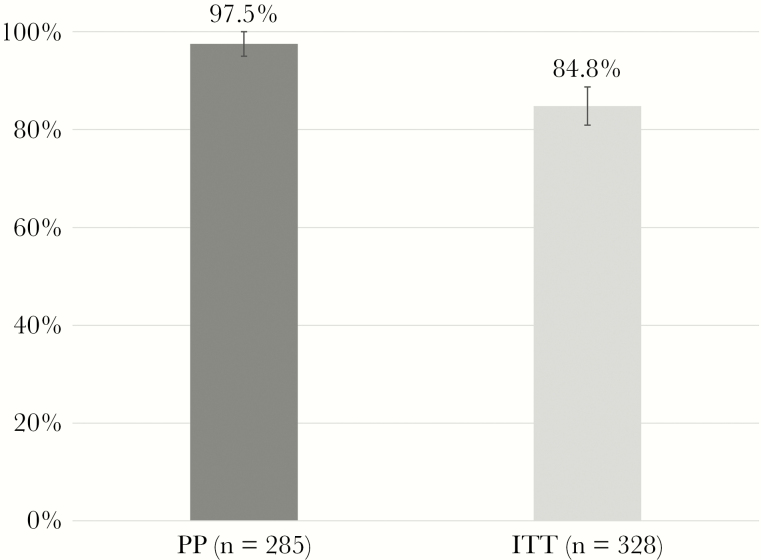
Most patients were treated with a sofosbuvir-based protocol, mostly for 12 weeks (Table 1). Seven patients (2.1%) dropped out before week 4, 9 patients (2.7%) were lost before tEoT, and 27 patients (8.2%) left the study after EoT. Moreover, there were 21 subjects (6.4%) with partially missing RNA test data who finished the study. A number of 274 patients with SVR12 data plus another 11 patients with missing SVR12 but valid SVR24 data resulted in a sample of 285 patients for the primary outcome measure, SVR12/24. Seven out of 285 patients had no SVR, which is an SVR12/24 rate of 97.5% (95% CI 95.0%–99.0%) in the PP sample (n = 285) (Figure 2). For the ITT sample (n = 328), the assumption of nonresponse for all 43 missing individuals resulted in an SVR12/24 rate of 84.8% (95% CI, 80.4%–88.5%) (Figure 2). However, 27 of these 43 missing individuals (62.8%) had completed DAA treatment, and 25 of them were HCV-RNA negative at tEoT. In total, among all HCV-RNA-negative patients at EoT (n = 287), 89.9% remained negative until SVR12, 1.4% (n = 4) did not achieve SVR, and 8.7% (n = 25) dropped out.
Figure 2.
Sustained virological response rates (SVR12/24). Abbreviations: ITT, intention to treat (all patients with first dose); PP, per protocol (only patients with complete data for SVR12/24).
Safety
Between baseline and tEoT, 151 AEs from 76 patients (23.2% of the total sample) were documented. The most frequently reported AEs were nausea (19×), headaches (14×), fatigue (13×), sleeping problems (9×), anemia (6×), loss of appetite (5×), diarrhea (5×), and heartburn/gastroesophageal reflux disease (5×). Clinicians provided intensity ratings for 139 of these 151 AEs, resulting in 33 AEs (23.7%) with low, 81 AEs (58.3%) with medium, and 25 AEs (18.0%) with high intensity. A causal relationship with the antiviral medication was assessed for 92 of 151 AEs, resulting in 22.8% “no causal relationship,” 25.0% “unlikely,” 31.5% “possible,” 16.3% “probable,” and 4.3% “certain.” The 19 AEs probably or certainly related to DAA medications were anemia (5×), fatigue (4×), nausea (3×), and loss of appetite (2×).
Between tEoT and tSVR12, another 65 AEs from 31 patients were reported. In total, between baseline and tSVR12, this resulted in 216 AEs from 85 persons. Moreover, 6 SAEs were reported between baseline and tSVR12, among them 3 deaths (due to drug overdose, suicide, aneurysm), suicidal thoughts, 1 hospitalization, and 1 pregnancy. No causal relationship between all SAEs and the antiviral treatment was reported.
Adherence and Tolerability (Patient- and Clinician-Reported)
Medication adherence (n = 288) at tEoT was mainly rated as “very good”; that is, patients reported having taken all of the medication (Table 2). Around 90% of both patients and clinicians stated that medication tolerability was either “good” or “very good” (Table 2). The high tolerability ratings are supported by the results of the 5 additional items on therapy side effects and depression, with mean values between 3 (rather disagree) and 4 (disagree) (Table 2). In comparison with previous HCV treatments (94% interferon-based), treatment-experienced patients reported substantially reduced side effects while on the current DAA treatment (Supplementary Table 2).
Physical and Mental Health Outcomes
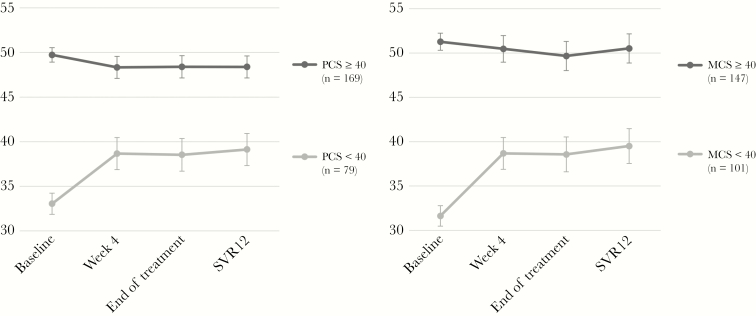
Health-related quality of life, as measured with the SF-12, was clearly reduced compared with the general population [24, 25], who have mean values (SD) of approximately 50 (10). In our sample, baseline mean PCS (SD) was 43.7 (9.3), and the mean MCS (SD) was 42.4 (11.3). During the course of treatment, MCS showed small but significant improvement (Tables 2 and 3) persisting after treatment, whereas PCS did not change over time. Patients with clearly reduced health-related quality of life at baseline (PCS or MCS < 40) showed a considerable level of improvement, mainly between baseline and t4 (Figure 3).
Table 3.
Course of HCV Therapy, Patient- and Clinician-Reported Outcomes—Per-Protocol Sample (t0- SVR12)
| Clinician-Reported | T0 | T4 | EoT | SVR12 | Time Effect in Repeated-Measures ANOVA |
|---|---|---|---|---|---|
| CGI-S (n = 270) | 3.02 (1.55) | 2.94 (1.53) | 2.93 (1.50) | 2.83 (1.52) | F (2.60, 698.68) = 6.03, P = .001 |
| GAF (n = 268) | 66.45 (17.49) | 68.50 (17.59) | 70.15 (17.39) | 70.97 (16.72) | F (2.45, 653.70) = 23.27, P < .001 |
| Patient-Reported | T0 | T4 | EoT | SVR12 | Time Effect in Repeated-Measures ANOVA |
| SF-12, PCS (n = 248) | 44.42 (9.44) | 45.25 (9.28) | 45.25 (9.42) | 45.44 (9.20) | n.s. |
| SF-12, MCS (n = 248) | 43.19 (11.34) | 45.62 (10.88) | 45.10 (11.44) | 45.99 (11.39) | F (2.85, 704.52) = 7.28, P < .001 |
| MSCL, total score (GSI; n = 262) | 58.31 (9.37) | 56.16 (10.24) | 55.78 (11.13) | 55.24 (10.76) | F (2.78, 725.10) = 15.44, P < .001 |
| MSCL, subscale somatization (n = 262) | 57.18 (9.61) | 56.20 (9.74) | 55.64 (10.09) | 54.63 (10.14) | F (2.84, 741.17) = 8.71, P < .001 |
| MSCL, subscale depression (n = 262) | 57.82 (8.74) | 55.15 (9.53) | 55.16 (10.01) | 55.05 (9.83) | F (2.84, 740.14) = 17.00, P < .001 |
| MSCL, subscale anxiety (n = 262) | 56.58 (11.01) | 54.81 (11.17) | 54.90 (11.47) | 54.45 (10.84) | F (3, 783) = 6.42, P < .001 |
| OTI-HSS (n = 255) | 11.57 (7.37) | 10.96 (7.71) | 10.87 (8.09) | 10.13 (8.00) | F (2.88, 731.00) = 6.00, P = .001 |
| Fatigue, concentration, and memory, total score (n = 261)a | 0.76 (0.70) | 0.68 (0.75) | 0.66 (0.75) | 0.61 (0.67) | F (2.86, 743.99) = 6.85, P < .001 |
| Fatigue, concentration, and memory, subscale fatigue (n = 261)a | 0.88 (0.89) | 0.81 (0.93) | 0.78 (0.98) | 0.69 (0.82) | F (2.81, 731.28) = 6.35, P < .001 |
| Fatigue, concentration, and memory, subscale concentration and memory (n = 261)a | 0.67 (0.69) | 0.58 (0.73) | 0.57 (0.70) | 0.56 (0.65) | F (2.87, 739.69) = 4.74, P = .003 |
Data are presented as means and standard deviations. Abbreviations: CGI-S, Clinical Global Impression–severity (ranging from 1 = “not at all ill” to 7 = “extremely ill”); GAF, Global Assessment of Functioning (0–100; higher scores indicate better functioning); GSI, Global Severity Index; MCS, Mental Component Summary; MSCL, Mini Symptom Checklist, with GSI (higher scores indicate worse mental health); OTI-HSS, Opiate Treatment Index–health symptoms scale (higher Scores indicate worse physical health); PCS, Physical Component Summary; SF-12, Short Form Health Assessment, consisting of PCS and MCS (higher scores indicate better quality of life).
aHigher scores indicate higher impairments, ranging from 0 = “not at all” to 4 = “very much.”
Figure 3.
Estimated marginal means of the Physical Composite Score (PCS) and Mental Composite Score (MCS) means of the 12-item Short-Form Health Survey (SF-12) for patients with valid data for all measurement points between baseline and tSVR12. Groups are divided according to their baseline PCS/MCS levels (<40 vs ≥40 points).
In the Mini-SCL (MSCL), the Global Severity Index (GSI) for the total sample at baseline (SD) was 58.87 (9.32), which indicates, compared with the population mean (SD) of 50 (10), a clearly increased symptom load (t (326) = 17.21; P < .001), consistent over all subscales: somatization, depression, and anxiety (Table 2). In the course of the DAA treatment, all MSCL scores modestly improved, mainly between baseline and t4 (Tables 2 and 3). Patients with higher psychological distress at baseline showed higher improvements over time.
According to OTI-HSS at baseline, patients reported, on average (SD), 12.17 (7.62) physical health symptoms. Similar to other PROs, there were modest improvements over time (Tables 2 and 3); these improvement were more prominent in patients with higher symptom loads at baseline.
The self-constructed 12 items on fatigue, concentration, and memory were divided into 2 subscales: 1 subscale for fatigue and 1 subscale for concentration and memory, as supported by exploratory factor analyses (Supplementary Table 1). Mean values include all patients with no more than 1 missing value per subscale or 2 missing values in total. At baseline, the mean of the total scale “fatigue, concentration, and memory” (SD) was 0.79 (0.71), indicating a mean impairment between 0 (not at all) and 1 (a little) (Table 2); 8.5% of the sample had a minimum value of 0.00, indicating no impairment at all. At the other end of the spectrum, 9.8% had a mean score of 2 or higher, and the maximum value (n = 1) was 3.17. Overall, patients scored higher on the fatigue subscale than on the subscale on concentration and memory (Table 2). Over time, fatigue, concentration, and memory showed modest improvements (Tables 2 and 3), with higher improvements in patients who had more impairments at baseline.
The clinician-reported severity rating CGI-S had, at baseline, a mean score (SD) of 3.06 (1.52) and showed modest improvement over time.
DISCUSSION
The aim of this prospective cohort study was to analyze the clinical effectiveness, safety, and PROs of DAA treatment among OAT patients under the conditions of clinical routine treatment in Germany.
The first main study outcome was that DAA treatment among OAT patients is feasible, safe, and results in high SVR rates. Feasibility of DAA treatment in OAT settings is confirmed by the high adherence and treatment completion rates in our sample, also in accordance with previous studies [29, 30]. Compared with clinical data from IFN-ribavirin-based treatment among OAT patients [31] and even compared with previous clinical trials on DAA treatment among OAT patients [32], we found a low prevalence of AEs. Between baseline and EoT, around a quarter of patients reported on average 2 mainly mild AEs like nausea, headache, or fatigue, which are deemed reversible after treatment completion [19]. The high adherence and completion rates and the low number of mainly mild AEs went along with high SVR rates (PP, 97.5%; ITT, 84.5%), comparable to those found in previous studies [5, 8], also from Germany [6]. The lower rate in our ITT sample is a consequence of the conservative assumption treating all missing data on SVR like treatment failures. An alternative, probably more realistic assumption considers that more than half of our dropouts (27 out of 43) completed treatment, and 25 of these 27 patients were RNA-negative at EoT. Given that in the total sample virtually all patients who were RNA-negative at EoT did achieve SVR (98.6%), we have reason to assume SVR in at least 24 of these dropouts. This assumption would result in an SVR12/24 rate of 92.1% for the ITT sample.
The second main outcome of this study was that DAA treatment results in improved PROs among OAT patients. In contrast to interferon- and ribavirin-based protocols [31], health-related quality of life does not deteriorate during DAA treatment. Still, compared with the findings of a post hoc analysis of phase 3 clinical trial data on PROs among OAT patients in antiviral treatment [19], the improvements in our study were modest, especially with regard to the PCS of the SF-12. However, as smaller improvements were also found in other real-world populations [33, 34], 1 explanation might be that the exclusion of “difficult-to-treat” patients from registration trials results in better PROs. In our sample, health-related quality of life (PCS and MCS) at baseline was substantially reduced compared with the general population, which is consistent with a recent German large-scale study among OAT patients [35].
Reasons why patients with higher baseline HRQoL impairments reported significant improvements on DAA treatment may be 2-fold. On the one hand, statistical effects need to be considered, such as regression to the mean or ceiling effects among those with very low or very high baseline levels, respectively. On the other hand, CHC is a systemic disease, and a number of metabolic, autoimmune, and neuro-psychiatric extrahepatic manifestations (EHMs) associated with CHC have been described that also affect patients’ health-related quality of life but can be improved or eliminated after successful antiviral treatment [36]. Given this, patients with higher baseline HRQoL impairments might have experienced a stronger increase in HRQoL during and after treatment due to improvements in EHM. In general, the fact that SVR reduces the risk of EHM [37, 38] is another reason why individuals with CHC should be treated, including patients with opioid use disorders.
With regard to the other PROs (physical/mental health, fatigue, cognitive impairment), we also found consistent but modest improvements over time, as well as substantial improvements among those with higher impairments. Similar to HRQoL, these findings need to be addressed in further research. The information that especially patients with high self-reported health burden will benefit from PROs on DAA treatment could be relevant for both patients and providers.
Some limitations need to be considered. First of all, as shown in Table 2, no data on the stage of liver disease from more than half of the patients could be obtained, which impeded further analyses on PROs depending on the severity of the liver disease. However, the relevance of the stage of liver disease on HRQoL during DAA treatment might not be as important as presumed. Recent studies show that patients with early and advanced fibrosis have comparable improvements, and other factors like sociodemographic characteristics or psychiatric comorbidities might have a higher impact on HRQoL [35, 39, 40]. Another limitation is that that patient-reported data on fatigue, concentration, and memory need to be carefully interpreted, as they were not assessed with a validated instrument.
CONCLUSIONS
DAA treatment among PWIDs is feasible, safe, and effective. Besides clinical effectiveness, health-related quality of life and other PROs improve during DAA treatment, in particular among those OAT patients with higher somatic, mental, and social burden. These findings may reduce uncertainties about HCV treatment with DAAs in OAT settings in clinical routine treatment, for both patients and providers. Given the high prevalence rates of CHC infections among OAT patients and the excellent therapeutic opportunities in this setting, more investments are needed to improve the linkage to HCV care.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Elke Rühling and Dr. Philipp Hiller for supporting the data processing.
Financial support. This work was supported by Bristol-Myers Squibb GmbH (unrestricted educational grant). Bristol-Myers Squibb GmbH had no role in the design of the study, the collection, analysis, or interpretation of the data, or the writing of the manuscript.
Potential conflicts of interest. Dr. Schulte reports grants and nonfinancial support from Camurus AB, Sweden; personal fees and nonfinancial support from AbbVie GmbH, Germany; outside the submitted work. Dr. Christensen reports personal fees from BMS GmbH, Germany; AbbVie GmbH, Germany; Janssen-Cilag GmbH, Germany; ViiH GmbH, Germany; Gilead GmbH, Germany; and MSD GmbH, Germany; outside the submitted work. Dr. Cimander reports personal fees from BMS GmbH, Germany; AbbVie GmbH, Germany; Camurus GmbH, Germany; Indivior GmbH, Germany; Gilead GmbH, Germany; MSD GmbH, Germany; Mundipharma GmbH, Germany; Sanofi-Aventis GmbH, Germany; Spectrum Therapeutics; Tilray and Hexal GmbH, Germany; outside the submitted work. Mr Görne and Dr. Khaykin report personal fees from Sanofi-Aventis GmbH, Germany; AbbVie GmbH, Germany; Janssen-Cilag GmbH, Germany; ViiH GmbH, Germany; Gilead GmbH, Germany; and MSD GmbH, Germany; outside the submitted work. Mr. Walcher reports personal fees from Sanofi-Aventis GmbH, Germany; AbbVie GmbH, Germany; Janssen-Cilag GmbH, Germany; ViiH GmbH, Germany; Gilead GmbH, Germany; MSD GmbH, Germany; Camurus GmbH, Germany; Indivior GmbH, Germany; and Hexal GmbH, Germany; outside the submitted work. Dr. Mauss reports personal fees from AbbVie GmbH, Germany; Janssen-Cilag GmbH, Germany; ViiH GmbH, Germany; Gilead GmbH, Germany; MSD GmbH, Germany; and Myr Pharmaceuticals; outside the submitted work. Prof. Scherbaum reports personal fees and nonfinancial support from AbbVie GmbH, Germany; personal fees from Camurus GmbH, Germany; personal fees from Indivior GmbH, Germany; personal fees from Medice; personal fees from MSD GmbH, Germany; personal fees and nonfinancial support from Mundipharma GmbH, Germany; personal fees from Sanofi-Aventis GmbH, Germany; personal fees and nonfinancial support from Janssen-Cilag GmbH, Germany; personal fees and nonfinancial support from Hexal GmbH, Germany; outside the submitted work. Dr. Verthein reports personal fees from Mundipharma GmbH, Germany; grants from Camurus AB, Sweden; nonfinancial support from Camurus GmbH, Germany; outside the submitted work. Prof. Reimer reports grants from BMS GmbH, Germany; during the conduct of the study. Prof. Reimer reports personal fees and nonfinancial support from AbbVie GmbH, Germany; personal fees from BMS GmbH, Germany; MSD GmbH, Germany; Janssen-Cilag GmbH, Germany; and Gilead GmbH, Germany; outside the submitted work. Ms. Schmidt, Dr. Strada, Mr. Manthey, Prof. Rehm, and Prof. Schäfer have nothing to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Degenhardt L, Peacock A, Colledge S, et al. . Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 2017; 5:e1192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schulte B, Schmidt CS, Strada L, et al. . Hepatitis C virus prevalence and incidence in a large nationwide sample of patients in opioid substitution treatment in Germany: a prospective cohort study. Clin Infect Dis 2020; 10:2199–205. [DOI] [PubMed] [Google Scholar]
- 3. Meijerink H, White RA, Løvlie A, et al. . Modelling the burden of hepatitis C infection among people who inject drugs in Norway, 1973-2030. BMC Infect Dis 2017; 17:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ti L, Socías ME, Wood E, et al. . The impact of methadone maintenance therapy on access to regular physician care regarding hepatitis C among people who inject drugs. PLoS One 2018; 13:e0194162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Macias J, Morano LE, Tellez F, et al. . Response to direct-acting antiviral therapy among ongoing drug users and people receiving opioid substitution therapy. J Hepatol 2019; 71:45–51. [DOI] [PubMed] [Google Scholar]
- 6. Christensen S, Buggisch P, Mauss S, et al. . Direct-acting antiviral treatment of chronic HCV-infected patients on opioid substitution therapy: still a concern in clinical practice? Addiction 2018; 113:868–82. [DOI] [PubMed] [Google Scholar]
- 7. Scherz N, Bruggmann P, Brunner N. Direct-acting antiviral therapy for hepatitis C infection among people receiving opioid agonist treatment or heroin assisted treatment. Int J Drug Policy 2018; 62:74–7. [DOI] [PubMed] [Google Scholar]
- 8. Graf C, Mucke MM, Dultz G, et al. . Efficacy of direct-acting antivirals for chronic hepatitis C virus infection in people who inject drugs or receive opioid substitution therapy: a systematic review and meta-analysis. Clin Infect Dis 2020; 70(11):2355–65. [DOI] [PubMed] [Google Scholar]
- 9. Midgard H, Bramness JG, Skurtveit S, et al. . Hepatitis C treatment uptake among patients who have received opioid substitution treatment: a population-based study. PLoS One 2016; 11:e0166451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grebely J, Drolet M, Nwankwo C, et al. . Perceptions and self-reported competency related to testing, management and treatment of hepatitis C virus infection among physicians prescribing opioid agonist treatment: the C-SCOPE study. Int J Drug Policy 2019; 63:29–38. [DOI] [PubMed] [Google Scholar]
- 11. Crowley D, Cullen W, Laird E, et al. . Exploring patient characteristics and barriers to hepatitis C treatment in patients on opioid substitution treatment attending a community based fibro-scanning clinic. J Transl Int Med 2017; 5:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Madden A, Hopwood M, Neale J, Treloar C. Beyond interferon side effects: what residual barriers exist to DAA hepatitis C treatment for people who inject drugs? PLoS One 2018; 13:e0207226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bryant J, Rance J, Hull P, et al. . Making sense of “side effects”: counterpublic health in the era of direct-acting antivirals. Int J Drug Policy 2019; 72:77–83. [DOI] [PubMed] [Google Scholar]
- 14. Bingham CO 3rd, Noonan VK, Auger C, et al. . Montreal Accord on Patient-Reported Outcomes (PROs) use series - paper 4: patient-reported outcomes can inform clinical decision making in chronic care. J Clin Epidemiol 2017; 89:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Madden A, Hopwood M, Neale J, Treloar C. Acceptability of patient-reported outcome and experience measures for hepatitis C treatment among people who use drugs. Patient 2019; 12:259–65. [DOI] [PubMed] [Google Scholar]
- 16. Younossi ZM, Stepanova M, Gordon S, et al. . Patient-reported outcomes following treatment of chronic hepatitis C virus infection with sofosbuvir and velpatasvir, with or without voxilaprevir. Clin Gastroenterol Hepatol 2018; 16:567–74.e6. [DOI] [PubMed] [Google Scholar]
- 17. Kracht PAM, Lieveld FI, Amelung LM, et al. . The impact of hepatitis C virus direct-acting antivirals on patient-reported outcomes: a Dutch prospective cohort study. Infect Dis Ther 2018; 7:373–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Evon DM, Sarkar S, Amador J, et al. . Patient-reported symptoms during and after direct-acting antiviral therapies for chronic hepatitis C: the PROP UP study. J Hepatol 2019; 71:486–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stepanova M, Thompson A, Doyle J, et al. . Hepatitis C virus-infected patients receiving opioid substitution therapy experience improvement in patient-reported outcomes following treatment with interferon-free regimens. J Infect Dis 2018; 217:1033–43. [DOI] [PubMed] [Google Scholar]
- 20. Marcellin F, Roux P, Protopopescu C, et al. . Patient-reported outcomes with direct-acting antivirals for the treatment of chronic hepatitis C: current knowledge and outstanding issues. Expert Rev Gastroenterol Hepatol 2017; 11:259–68. [DOI] [PubMed] [Google Scholar]
- 21. Mahajan R. Real world data: additional source for making clinical decisions. J Appl Basic Med Res 2015; 5:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bell CC. DSM-IV: diagnostic and statistical manual of mental disorders. JAMA 1994; 272:828–9. [Google Scholar]
- 23. Guy W. The Clinical Global Impression Scale. The ECDEU Assessment Manual for Psychopharmacology Revised. Rockville, MD: US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. [Google Scholar]
- 24. Ware J Jr, Kosinski M, Keller SD. A 12-item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34:220–33. [DOI] [PubMed] [Google Scholar]
- 25. Morfeld M, Kirchberger I, Bullinger M. SF-36 Fragebogen zum Gesundheitszustand: Deutsche Version des Short Form-36 Health Survey. Göttingen: Hogrefe; 2011. [Google Scholar]
- 26. Perneger TV, Burnand B. A simple imputation algorithm reduced missing data in SF-12 health surveys. J Clin Epidemiol 2005; 58:142–9. [DOI] [PubMed] [Google Scholar]
- 27. Franke GH, Jaeger S, Glaesmer H, et al. . Psychometric analysis of the Brief Symptom Inventory 18 (BSI-18) in a representative German sample. BMC Med Res Methodol 2017; 17:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Darke S, Ward J, Zador D, Swift G. A scale for estimating the health status of opioid users. Br J Addict 1991; 86:1317–22. [DOI] [PubMed] [Google Scholar]
- 29. Butner JL, Gupta N, Fabian C, et al. . Onsite treatment of HCV infection with direct acting antivirals within an opioid treatment program. J Subst Abuse Treat 2017; 75:49–53. [DOI] [PubMed] [Google Scholar]
- 30. Cunningham EB, Hajarizadeh B, Amin J, et al. . Adherence to once-daily and twice-daily direct acting antiviral therapy for hepatitis C infection among people with recent injection drug use or current opioid agonist therapy. Clin Infect Dis. In press. [DOI] [PubMed] [Google Scholar]
- 31. Reimer J, Schmidt CS, Schulte B, et al. . Psychoeducation improves hepatitis C virus treatment during opioid substitution therapy: a controlled, prospective multicenter trial. Clin Infect Dis 2013; 57(Suppl 2):S97–104. [DOI] [PubMed] [Google Scholar]
- 32. Grebely J, Feld JJ, Wyles D, et al. . Sofosbuvir-based direct-acting antiviral therapies for HCV in people receiving opioid substitution therapy: an analysis of phase 3 studies. Open Forum Infect Dis 2018; 5:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saeed S, Moodie EEM, Strumpf E, et al. ; Canadian Co-Infection Cohort Study Investigators Real-world impact of direct acting antiviral therapy on health-related quality of life in HIV/hepatitis C co-infected individuals. J Viral Hepat 2018; 25:1507–14. [DOI] [PubMed] [Google Scholar]
- 34. Loo N, Lawitz E, Alkhouri N, et al. . Ombitasvir/paritaprevir/ritonavir + dasabuvir +/- ribavirin in real world hepatitis C patients. World J Gastroenterol 2019; 25:2229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strada L, Schmidt CS, Rosenkranz M, et al. . Factors associated with health-related quality of life in a large national sample of patients receiving opioid substitution treatment in Germany: a cross-sectional study. Subst Abuse Treat Prev Policy 2019; 14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuna L, Jakab J, Smolic R, et al. . HCV extrahepatic manifestations. J Clin Transl Hepatol 2019; 7:172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rossi C, Jeong D, Wong S, et al. ; BC Hepatitis Testers Cohort Team Sustained virological response from interferon-based hepatitis C regimens is associated with reduced risk of extrahepatic manifestations. J Hepatol 2019; 71:1116–25. [DOI] [PubMed] [Google Scholar]
- 38. Mahale P, Engels EA, Li R, et al. . The effect of sustained virological response on the risk of extrahepatic manifestations of hepatitis C virus infection. Gut 2018; 67:553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cossais S, Schwarzinger M, Pol S, et al. . Quality of life in patients with chronic hepatitis C infection: severe comorbidities and disease perception matter more than liver-disease stage. PLoS One 2019; 14:e0215596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Younossi ZM, Stepanova M, Afdhal N, et al. . Improvement of health-related quality of life and work productivity in chronic hepatitis C patients with early and advanced fibrosis treated with ledipasvir and sofosbuvir. J Hepatol 2015; 63:337–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.