Abstract
Liver cancer, mostly hepatocellular carcinoma (HCC), is the second leading cause of cancer mortality globally. Most patients were diagnosed at an advanced stage, and systemic therapy is the standard of care. All the approved systemic therapies for HCC are molecular targeted therapies with anti-angiogenic effects targeting the vascular endothelial growth factor signaling pathway. Sorafenib and lenvatinib are the first-line treatment, and regorafenib, ramucirumab, and cabozantinib are second-line treatment options. Although anti-PD-1 antibodies, including nivolumab and pembrolizumab, demonstrated promising anti-tumor effects as monotherapy for advanced HCC in phase II clinical trials, both failed in phase III studies. Anti-angiogenic treatment remains the backbone of systemic therapy for HCC. In this review, we summarized the approved anti-angiogenic medicines and discussed the potential strategies to improve the efficacy of anti-angiogenic therapy, including combination therapy with other treatments, and discussed the approaches to overcome the drawbacks of anti-angiogenic therapies.
Keywords: Anti-angiogenic therapy, Combinational therapy, Hepatocellular carcinoma, Molecular targeted therapy, Systemic therapy
Abbreviations: HCC, hepatocellular carcinoma; TACE, transcatheter chemoembolization; OS, overall survival; PFS, progression-free survival; RECIST, Response Evaluation Criteria in Solid Tumors; ICI, immune checkpoint inhibitor; ORR, objective response rate; PR, partial response; CR, complete response; TKI, tyrosine kinase inhibitor; PD-1, program death-1; PD-L1, program death-1 ligand
Background of systemic therapy
Primary liver cancer is the second leading cancer-related death in China.1 Although the incidence and mortality of liver cancer are declining in China,2,3 owing to the introduction of vaccination for newborns against the hepatitis B virus (HBV),4 it is increasing in the US and Europe.5 More than 90% of primary liver cancers are hepatocellular carcinoma (HCC). Survival after diagnosis of HCC is more miserable than many other types of cancer.5 In China, the 5-year survival of HCC is 12.1%, the second-lowest among all types of cancer.6 Only treatments for HCC are discussed in this Review. Most of the HCC patients were diagnosed at an advanced stage in most regions around the world including China,7 and systemic treatment is the standard of care for them. In most patients, HCC is associated with chronic liver injuries from chronic hepatitis virus infection, alcohol abuse or non-alcoholic liver steatosis hepatitis. These chronic liver injuries not only complicate treatment choice but also compete for the effect of tumor progression on patient survival.8 Treatment, therefore, needs to balance anti-tumor effects and harm to liver parenchyma. The result of systemic therapy for advanced-stage HCC was disappointing until the approval of sorafenib in 2008. Since then, no systemic treatment was found to be superior or equivalent to sorafenib until the approval, within the last two years, of several agents for systemic therapy of advanced-stage or unresectable HCC.9
Tumor angiogenesis in HCC and the rational of anti-angiogenic therapy
HCC is a typically hyper-vascular tumor. The characteristics of abundant and tortuous vessels distinguish HCC from benign lesions in angiography and imaging.10 With this feature, transcatheter chemoembolization (TACE), which is to starve tumors with embolism, is the standard of care for intermediate-stage HCC. Because of the genetic heterogeneity of tumor cells, anti-tumor cell therapy, e.g., chemotherapy, is not successful in HCC, to target the relatively stable vascular cells seems more rationale. Vascular endothelial growth factor (VEGF) is an essential angiogenic cytokine and plays a critical role in tumor angiogenesis.11 VEGF signaling pathway also plays a vivid role in tumor angiogenesis and tumor progression in HCC.12 Moreover, VEGF was found to be the common regulation of stromal cells, including fibroblasts, macrophages, and endothelial cells, by heterogeneous tumor cells.13 All the approved systemic therapies in the US, EU, and China are molecular targeted therapy with the primary mechanism of anti-angiogenesis targeting the VEGF and its receptors. Other signaling pathways with pro-angiogenic effects, such as platelet-derived growth factor (PDGF)/PDGF receptors (PDGFR), fibroblast growth factor (FGF)/FGF receptors (FGFR), angiopoetin/Tie, and endoglin (CD105), were also studied in HCC, and most multi-targeted tyrosine kinase inhibitors (TKIs) evaluated for the treatment for HCC covered these receptors.14
First-line
Sorafenib
Sorafenib is an oral TKI with anti-angiogenic and anti-proliferation effects by targeting VEGF receptors (VEGFRs), PDGFR, and Raf kinases.15 Sorafenib has been approved for the treatment of advanced-stage or unresectable HCC for more than 10 years in China and most parts of the world. Two trials conducted outside and within Asia have shown the efficacy of sorafenib in extending patient survival.16,17 Sorafenib became a standard of care recommended by the guidelines from almost all regions, and management of its toxicities, such as hand-foot syndrome, has improved its tolerance.18 After approval, It has been estimated that the overall survival (OS) of patients with advanced-stage HCC has been extended from 6.5 months to 8.5–8.9 months in Asian patients and from 10.7 months to 11.8–15.1 months in non-Asian patients, probably because of the improvement in the management of toxicities associated with sorafenib.19 Several reports demonstrated sorafenib-induced toxicities, such as diarrhea, hypertension, and hand-foot syndrome, were associated with better tumor response.20 However, attempts to identify a molecular biomarker for selection of patients sensitive to sorafenib has failed. Monotherapy with other anti-angiogenic therapies (such as sunitinib,21 brivanib,22 and linifanib23), or selective internal radiotherapy with yttrium-90 resin microspheres (SARAH and SIRveNIB studies24,25) had been shown not to be superior to sorafenib in head-to-head phase III trials until the REFLECT trial26 demonstrated that lenvatinib is not inferior to sorafenib in improving patient survival.
Combination treatment with sorafenib and locoregional therapy has been intensively investigated. However, most trials failed to demonstrate the additional benefit of sorafenib over TACE versus TACE alone in patients with intermediate-stage HCC, such as the SPACE study.27 Also, adding TACE to sorafenib treatment did not further improve the OS with sorafenib monotherapy.28 Recently, the results from the TACTICS trial demonstrated that TACE plus sorafenib is more effective in prolonging progression-free survival (PFS) than TACE alone in patients with unresectable HCC, but the overall survival (OS) data were not reported.29 The major differences in trial design may be that the development of new lesions was not a criterion for stopping TACE as long as the lesion could be treated with TACE. This strategy gave patients more opportunities to receive TACE, which would prolong treatment duration for both arms.29 A recent single-center randomized control trial (RCT) conducted in China demonstrated that the effect of sorafenib and hepatic arterial infusion chemotherapy (HAIC) using oxaliplatin, 5-fluorouracil, and leucovorin is better than sorafenib alone in patients with tumor invasion to portal vein in terms of OS and PFS and produced a much better objective response rate.30 However, a similar regimen (HAIC plus sorafenib) was not proved to be more effective than sorafenib alone for patients with unresectable HCC (BCLC-B and C stages).31 Therefore, the effect of the combination of sorafenib with other locoregional treatment needs more investigations.
Lenvatinib
Lenvatinib is also a multi-kinase inhibitor targeting VEGFRs 1–3, PDGFR, FGFR, RET, and KIT.32 Lenvatinib was approved for advanced HCC in 2018 based on the REFLECT study, a non-inferior designed open-labeled control trial.26 The objective response rate (ORR) of lenvatinib treatment was 18.8% (RECIST v1.1) or 40.6% (mRECIST) judged by masked independent image review.26 A real-world study demonstrated that therapeutic response and adverse events after taking lenvatinib were similar to those of the REFLECT trial, regardless of previous TKI therapies,33 and the immunomodulatory activity of lenvatinib has also been revealed in an experimental study34 and a clinical study.35
Although the trial demonstrated that lenvatinib provided a similar survival benefit to sorafenib, the higher ORR of lenvatinib is essential to encourage patients to stay on treatment and tolerate the toxicities and for physicians to monitor the effectiveness of treatment. The higher ORR also inspired the thought of down-staging treatment for initially unresectable HCC or neoadjuvant therapy for resectable HCC.
Second line
Regorafenib
Regorafenib is another multi-target TKI, targeting VEGFRs, Tie-2, PDGFR-β, FGFRs, Kit, and Ret. The RESORCE trial36 was conducted in patients who tolerated sorafenib but progressed on sorafenib treatment. The median OS in regorafenib treated patients was 10.6 months compared to 7.8 months in the placebo group (HR = 0.61, P < 0.0001), and PFS was increased from 1.5 months to 3.1 months by regorafenib treatment (HR = 0.46, P < 0.0001); the ORR in regorafenib treated patients was 7% compared with 3% in the placebo group (P = 0.020, RECIST v1.1). Regorafenib is the first second-line treatment after sorafenib showing an OS benefit. The incidence of treatment-related grade 3 or 4 adverse events was 50%, including hand-foot syndrome, infection, hypertension, and fatigue.
One study showed sequential treatment using sorafenib and regorafenib may result in a median OS of 28 months in patients with advanced HCC.37 For BCLC-B stage patients, TACE is the first recommended treatment, while refractory disease is commonly observed. At this time, sequential treatment with sorafenib and regorafenib may be introduced to TACE-resistant BCLC-B stage patients to achieve more prolonged survival.
Cabozantinib
Cabozantinib is a multi-kinase inhibitor targeting VEGFR-2, MET, and AXL. A randomized control study demonstrated cabozantinib treatment resulted in a longer OS (10.2 versus 8.0 months, HR = 0.76, P = 0.005) and PFS (5.2 versus 1.9 months, HR = 0.44, P < 0.001) compared with placebo in patients with advanced HCC as a second-line treatment.38 An interesting finding from this study was that the HR for death was 0.69 in patients with HBV-related HCC and 1.11 in patients with HCV-related HCC, which suggests that anti-tumor effects of cabozantinib may be more potent for HBV-related HCC.
The molecular targets of cabozantinib, MET and AXL, have a role in treatment resistance to anti-angiogenesis therapies,39 which is consistent with the effect of cabozantinib as a second-line treatment for HCC. Compared with regorafenib, cabozantinib resulted in longer PFS (5.2 vs. 3.4 months, per RECIST 1.1 36,38), while the grade 3 and 4 adverse events were more common, including hypertension, diarrhea, and hand-foot syndrome.
Ramucirumab
Ramucirumab is an antibody targeting VEGFR-2 but not a TKI. VEGFR-2 mainly expresses on endothelial cells and is the receptor for the ligand VEGF-A, C, D. VEGFR-2 mediates the majority of the downstream effects of VEGF in tumor angiogenesis.40 In the REACH trial, in patients with advanced HCC who have been treated with sorafenib without success, prespecified subgroup analysis revealed that patients with serum alpha-fetoprotein (AFP) ≥ 400 ng/ml might benefit from ramucirumab treatment.41 The following REACH-2 trial was, therefore, explicitly conducted in patients with serum AFP ≥400 ng/ml, and the results demonstrated that OS and PFS were significantly better than the placebo arm.42
The grade 3 or higher adverse events associated with ramucirumab treatment were very low. The median treatment intensity was 98% in the ramucirumab treated group, suggesting that most patients received a full dose of ramucirumab, and adverse events leading to treatment discontinuation occurred in only 11% of patients. Hypertension and hyponatremia were the only grade 3 or higher treatment-emergent adverse events that were noted in 5% or more of patients.42
Other anti-angiogenic agents
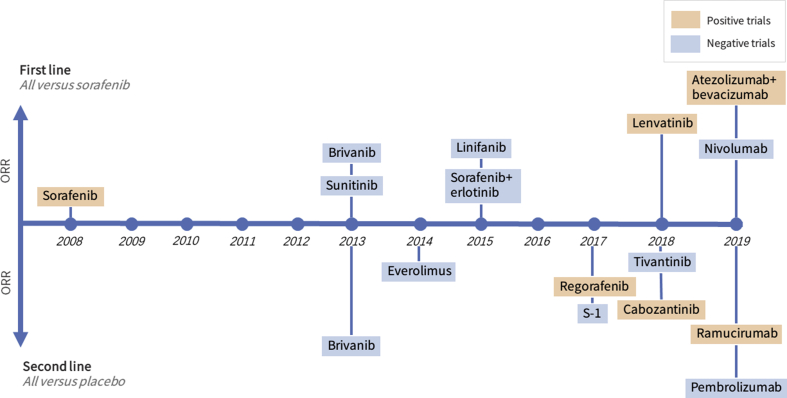
Other agents were also evaluated for the treatment of advanced or unresectable HCC, including sunitinib as first-line,21 brivanib as first-line22 or second-line,43 linifanib,23 however, all the trials did not meet the primary endpoint because of lower anti-tumor effects or higher toxicity as compare with sorafenib or placebo (Fig. 1). Thalidomide is a proved drug with anti-angiogenic effect, however, its antitumor effects for HCC were modest in phase II clinical trials.44, 45, 46, 47 Bevacizumab is a monoclonal antibody that blocks VEGF-A. In a phase II study, bevacizumab at 5–10 mg/kg every two weeks did show anti-tumor activity in HCC patients with an ORR of 13%, and 65% of patients were progression-free at six months.48 However, severe bleeding occurred in 11% of the HCC patients and held back further phase III studies. However, in more carefully selected HCC patients, when combined with atezolizumab, an anti-PD-L1 antibody, bevacizumab at a dose of 15 mg/kg q3weeks showed acceptable tolerability with promising results; ORR was 29.9% (pooled analysis of arms A and F1) in a phase Ib clinical trial in 164 HCC patients.49 The combination was further investigated as first-line treatment compared with sorafenib in a phase III study (IMbrave150 study, NCT03434379). Also, bevacizumab was in development with duravalumab (an anti-PD-L1 antibody) in combination with TACE (EMERALD-1, NCT03778957) or as adjuvant therapy (EMERALD-2 study, NCT03847428).
Figure 1.
Timeline of phase III clinical trials evaluating systemic therapies for unresectable or advanced hepatocellular carcinoma in the last decade. ORR, objective response rate, the proportion of partial response and complete response per RECIST v1.1 criteria, except for brivanib which was evaluated by mRECIST criteria. Note that donafenib was also studied in a phase III trial, but the ORR was not reported.
Three TKIs with antiangiogenic agents manufactured by Chinese pharmaceutical companies were also in clinical development for HCC. Donafenib (targeting Raf and VEGFRs) (NCT02645981), apatinib (targeting VEGFR2) (NCT02329860) have been investigated in phase III studies. Most recently, the company announced that donafenib showed more potent anti-tumor efficacy than sorafenib in the phase 3 study for patients with advanced HCC.50 Anlotinib (targeting VEGFRs, EGFR, and FGFR) also showed a durable anti-tumor activity (ORR 4.6% and median time to progression 4.0–5.5 months) and manageable toxicity in a phase II study.51
Anti-angiogenic therapy in early-stage HCC
The STORM trial to evaluate the effect of adjuvant sorafenib treatment after resection or ablation on early-stage HCC (BCLC stage 0-A) with a high risk of tumor recurrence did not reach its primary endpoint.52 The median treatment duration in the sorafenib arm was 12.5 months (22.2 months in the control arm), and 1-year discontinuation rate was 49% (35% in the control arm), suggesting long-term treatment with sorafenib is challenging, especially in the absence of a target lesion. Furthermore, more than 60% of patients were not the target population for receiving adjuvant anti-tumor treatment because the 2-year recurrence rate is less than 40% in the control arm. “Wrong stage and wrong dose” were the major criticisms for this trial.53 However, a small trial demonstrated that sorafenib improved patient OS and decreased tumor recurrence rate only in patients with a higher risk of tumor recurrence.54
Beyond anti-angiogenesis
In all phase III studies that led to the approval of molecular targeting therapies, the median OS for patients with advanced or unresectable HCC was about one year,16,17,26 and there may be a ceiling of efficacy for anti-angiogenic treatments.55 Strategies for improving the survival bar of these drugs are hot spots in clinical development. However, all combinational therapies with sorafenib, including systemic chemotherapy (doxorubicin,56 or capecitabine and oxaliplatin57), hepatic arterial infusion chemotherapy,31 tigatuzumab (a death receptor-5 agonist),58 erlotinib (an EGFR inhibitor),59 and TACE,28 have failed to improve patient OS compared with sorafenib monotherapy.
The combination of anti-angiogenic therapy and an anti-PD-1 antibody
Immune checkpoint inhibitors (ICIs) may be promising for combination therapy with sorafenib and other anti-angiogenic drugs because the major toxicity profiles of TKIs and ICIs do not overlap. There're early-phase clinical studies in HCC and late-phase studies in other solid tumors have shown that the toxicity of these two categories combination is manageable. More importantly, there may be synergistic biological effects between anti-angiogenesis and ICI agents. Intratumoral VEGF overexpression exerts an immunosuppression microenvironment in tumors by accumulation of regulatory T cells, myeloid-derived suppressor cells, immunosuppressive cytokines, inhibiting DC maturation and production of IDO,60,61 inhibiting T cell infiltration,62 and upregulating the expression of immune checkpoints on CD8+ T cells.63 Most recently, VEGF was also found to reprogramming tumor microenvironment to promote an immune-suppressive environment that favors tumor progression and provides a rationale for combination therapy of ICI and anti-angiogenesis therapy.13 Anti-angiogenesis treatment may also increase the efficacy of immunotherapies by targeting angiopoietin-2 and hepatocyte growth factor pathways, while immunotherapies may increase the effectiveness of anti-angiogenesis treatment by eliciting antibody-dependent cytotoxicity on endothelial cells followed by destructing tumor vasculature.64 An animal study found that lenvatinib showed a more potent anti-tumor effect in immunocompetent mice than in immunodeficient mice, and the combination of lenvatinib and anti-PD-1 antibody resulted in a higher response rate compared with either treatment alone in immunocompetent mice.34
In a phase Ib study evaluating the safety of lenvatinib in combination with pembrolizumab in 67 evaluable patients with unresectable HCC (NCT03006926)65, no new adverse event was identified, with a confirmed ORR of 40.3% (27/67). Another phase I study combining SHR-1210 and apatinib in patients with advanced solid tumors, including HCC, showed manageable toxicity, with 50% of patients with evaluable HCC (8/16) achieving a PR.66 Beyond acceptable safety and tolerability, a promising synergic effect of ICI and anti-angiogenic therapies has shown in several phase III studies in other solid tumors, like RCC, which is also characterized as a hyper-vascular tumor. TKIs, namely sunitinib or sorafenib, are standard treatments for advanced and metastatic RCC. The combination of axitinib (a TKI targeting VEGFRs) and pembrolizumab67 or avelumab (a PD-L1 antibody)67 showed superior anti-tumor effects to sunitinib in 2 phase III studies in RCC. The combination of lenvatinib and pembrolizumab showed promising anti-cancer activity in a phase II study in RCC, with the ORR as high as 66.7%, and the mPFS as 17.7 months.68 The successful experience in RCC has shed light on drug development for HCC, and the combination of TKI and ICI can be anticipated to improve HCC outcomes further. The FDA has granted a breakthrough therapy designation for the combination of pembrolizumab and lenvatinib for the treatment of both RCC and HCC.69 The highest ORR was reported in several small trials testing combination treatment of anti-angiogenesis agents with PD-1 antibodies, which are summarized in Table 1. Further evaluation of the efficacy and safety in phase III clinical trials is warranted as a top priority in drug development for advanced-stage or unresectable HCC by the pharmaceutical companies. The ongoing large phase III clinical trials, most of which concerned combination therapy with anti-angiogenesis and ICI in HCC patients, are listed in Table 2. Most recently, the combination of atezolizumab and bevacizumab showed improved OS and PFS in compare with sorafenib in a phase III clinical trial (IMbrave150 study) both in global cohort of HCC patients70 and in Chinese patients.71
Table 1.
Safety and efficacy of combination treatment with anti-angiogenic therapy and anti-PD-1/PD-L1 antibody in patients with advanced HCC.
| Combinations | Number of patients | ORR (RECIST v1.1) | mPFS (months) | Grade 3/4 AE |
|---|---|---|---|---|
| apatinib + camrelizumab66 | 16 (2nd line) | 50% | 5.8 | NA |
| lenvatinib + pembrolizumab65 | 67 evaluable (63 as 1st line) | 40.3% | 9.5 | 80.6% |
| bevacizumab + atezolizumab49 | 104 (1st line) arm A | 36% | 7.3 | 38% |
| 60 (1st line) arm F1 | 20% | 5.6 | 37% | |
| axitinib + avelumab83 | 22 (1st line) | 13.6% | 5.5 | 72.7% |
| cabozantinib + nivolumab + ipilimumab84 | 35 (1st line) | 26% | 6.8 | 71% |
| cabozantinib + nivolumab84 | 36 (1st line) | 17% | 5.5 | 42% |
| lenvatinib + nivolumab85 | 30 (1st line) | 54.2% | 7.39 | 60% |
| regorafenib + pembrolizumab86 | 23 (1st line) | 30% | NA | NA |
ORR, objective response rate; mPFS, median progression-free survival; AE, adverse events; NA, not available.
Table 2.
Ongoing phase 3 clinical trials of anti-angiogenic therapy with or without anti-PD-1/PD-L1 antibody for advanced or unresectable hepatocellular carcinoma.
| Trial | Lines | Arms | Clinicaltrials.gov identifier | Sponsor |
|---|---|---|---|---|
| ZGDH3 | First line | donafenib vs sorafenib | NCT02645981 | Zelgen |
| LEAP-002 | First line | lenvatinib + pembrolizumab vs lenvatinib | NCT03713593 | MSD + Eisai |
| COSMIC-312 | First line | cabozantinib + atezolizumab vs sorafenib vs cabozantinib | NCT03755791 | Exelixis |
| SHR-1210-III-310 | First line | camrelizumab + apatinib vs sorafenib | NCT03764293 | Hengrui |
| ORIENT-32 | First line | sintilimab + IBI305 vs sorafenib | NCT03794440 | Innovent |
Overcome the “opposite effects” of anti-angiogenic therapy
The “opposite effects”, rather than the “adverse effects”, is an off-target effect of an anti-tumor agent that increases the invasiveness of tumor cells and may partly counteract the antitumor effects. In preclinical studies, although anti-angiogenic therapies showed potent anti-tumor effects, they were also found to facilitate tumor metastasis.72,73 Our research found that sorafenib promotes invasiveness and the metastatic potential of orthotopic tumors in HCC mouse models by down-regulating the expression of HTATIP2,74 and aspirin minimized the pro-metastasis effect of sorafenib by up-regulating HTATIP2 in tumor cells.75 Antiangiogenic therapy also acts upon the host,76 and the changes in the host or metastatic target organ may facilitate tumor metastasis. Sorafenib was found to suppress host immune response by inhibiting NK cells' proliferation and cytotoxic effects.77 Sorafenib treatment in HCC mouse models recruits more macrophages by elevation of colony-stimulating factor-1, stromal-derived factor 1α, and VRGF expression from tumor cells. The depletion of macrophages by clodrolip or zoledronic acid in combination with sorafenib significantly inhibited tumor progression compared with mice treated with sorafenib alone.78 Host macrophages in organs other than liver may also affected by anti-angiogenic therapy. Our in vivo study found that two anti-angiogenic agents (sunitinib and sorafenib) facilitated tumor cell survival in blood stream and promoted lung metastasis by down-regulation the expression of interleukin-12b in macrophages and dendritic cells from host organs. Supplement with recombinant IL-12b or restoration of IL-12b expression by low-dose zoledronic acid alleviated the pro-metastasis effects of sorafenib or sunitinib.79 To overcome the opposite effects of anti-angiogenic therapy may become a promising approach to further increase the efficacy of anti-angiogenic therapy.
New targets for anti-angiogenic therapy
Most anti-angiogenic drugs are targeting the VEGF signaling pathway in tumor endothelial cells. However, emerging studies indicated that tumor endothelial cells are also heterogeneous. Our previous study found that CD105 (endoglin)-positive HCC endothelial cells showed increased apoptosis resistance, motility, and proangiogenic properties as compared with endothelial cells from non-tumor liver tissue. These cells acquired more resistance to chemotherapeutic agents and sorafenib than their counterparts without CD105 expression in normal liver tissue.80 The combination of TRC105 (an anti-endoglin antibody) and sorafenib demonstrated encouraging evidence of efficacy, including a 25% partial response rate and a durable response in HCC patients with measurable disease in an early-phase clinical trial.81,82
Prospective
Nowadays, anti-angiogenic therapy is the backbone of systemic treatment for advanced or unresectable HCC. Based on the ongoing clinical trials, anti-angiogenic treatment will remain the first-line therapy in the near future. The combination of anti-angiogenic therapy with an anti-PD-1 antibody showed promising efficacy in the early phase clinical trials, and will hopefully be the first-line treatment in the future. For Chinese patients, drug development by local pharmaceuticals will provide them with more affordable medications. Based on the ongoing clinical trials (Table 2), not only the systemic therapy for patients with advanced HCC will be changed by combinational therapy or novel molecular targeted therapy, the treatment of early-stage and intermediate-stage HCC will also be largely changed with the emerging agents or strategies. For the patients with early-stage HCC, a first widely-accepted adjuvant therapy may be an ICI or an anti-angiogenic treatment with low toxicity shortly, and the efficacy of TACE for the patients with intermediate-stage HCC will be also improved by the combination with ICIs and an anti-angiogenic agent.
Conflict of interest
HCS received a lecture fee from Bayer, Eisai, and MSD.
Acknowledgments
This work was supported by the Leading Investigator Program of Shanghai municipal government (17XD1401100), the National Key Basic Research Program (973 Program; 2015CB554005), and the National Natural Science Foundation of China (81372655, 81472224 and 81672326) to HCS.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Zhou M., Wang H., Zeng X. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–1158. doi: 10.1016/S0140-6736(19)30427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou M., Wang H., Zhu J. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387(10015):251–272. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 3.Chen W., Zheng R., Baade P.D. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Liang X., Bi S., Yang W. Evaluation of the impact of hepatitis B vaccination among children born during 1992-2005 in China. J Infect Dis. 2009;200(1):39–47. doi: 10.1086/599332. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 6.Zeng H., Chen W., Zheng R. Changing cancer survival in China during 2003–15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6(5):e555–e567. doi: 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- 7.Park J.W., Chen M., Colombo M. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 2015;35(9):2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medavaram S., Zhang Y. Emerging therapies in advanced hepatocellular carcinoma. Exp Hematol Oncol. 2018;7:17. doi: 10.1186/s40164-018-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu X.D., Sun H.C. Emerging agents and regimens for hepatocellular carcinoma. J Hematol Oncol. 2019;12(1):110. doi: 10.1186/s13045-019-0794-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun H.C., Tang Z.Y. Angiogenesis in hepatocellular carcinoma: the retrospectives and perspectives. J Canc Res Clin Oncol. 2004;130(6):307–319. doi: 10.1007/s00432-003-0530-y. [DOI] [PubMed] [Google Scholar]
- 11.Dvorak H.F. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20(21):4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 12.Sampat K.R., O'Neil B. Antiangiogenic therapies for advanced hepatocellular carcinoma. Oncol. 2013;18(4):430–438. doi: 10.1634/theoncologist.2012-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma L., Hernandez M.O., Zhao Y. Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer. Cancer Cell. 2019;36(4):418–430. doi: 10.1016/j.ccell.2019.08.007. e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin S., Li A., Yi M., Yu S., Zhang M., Wu K. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J Hematol Oncol. 2019;12(1):27. doi: 10.1186/s13045-019-0718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilhelm S.M., Adnane L., Newell P., Villanueva A., Llovet J.M., Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Canc Therapeut. 2008;7(10):3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PubMed] [Google Scholar]
- 16.Llovet J.M., Ricci S., Mazzaferro V. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 17.Cheng A.L., Kang Y.K., Chen Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 18.Ren Z., Zhu K., Kang H. Randomized controlled trial of the prophylactic effect of urea-based cream on sorafenib-associated hand-foot skin reactions in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33(8):894–900. doi: 10.1200/JCO.2013.52.9651. [DOI] [PubMed] [Google Scholar]
- 19.Faivre S., de Gramont A., Raymond E. Learning from 7 Years of experience with sorafenib in advanced HCC: sorafenib better than sorafenib? Targeted Oncol. 2016;11(4):565–567. doi: 10.1007/s11523-016-0427-8. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Rahman O., Lamarca A. Development of sorafenib-related side effects in patients diagnosed with advanced hepatocellular carcinoma treated with sorafenib: a systematic-review and meta-analysis of the impact on survival. Expet Rev Gastroenterol Hepatol. 2017;11(1):75–83. doi: 10.1080/17474124.2017.1264874. [DOI] [PubMed] [Google Scholar]
- 21.Cheng A.L., Kang Y.K., Lin D.Y. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31(32):4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 22.Johnson P.J., Qin S., Park J.W. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31(28):3517–3524. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 23.Cainap C., Qin S., Huang W.T. Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33(2):172–179. doi: 10.1200/JCO.2013.54.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vilgrain V., Pereira H., Assenat E. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18(12):1624–1636. doi: 10.1016/S1470-2045(17)30683-6. [DOI] [PubMed] [Google Scholar]
- 25.Chow P.K.H., Gandhi M., Tan S.B. SIRveNIB: selective internal radiation therapy versus sorafenib in asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol. 2018;36(19):1913–1921. doi: 10.1200/JCO.2017.76.0892. [DOI] [PubMed] [Google Scholar]
- 26.Kudo M., Finn R.S., Qin S. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 27.Lencioni R., Llovet J.M., Han G. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64(5):1090–1098. doi: 10.1016/j.jhep.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Park J.-W., Kim Y.J., Kim D.Y. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol. 2019;70(4):684–691. doi: 10.1016/j.jhep.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 29.Kudo M., Ueshima K., Ikeda M. Randomized, open label, multicenter, phase II trial comparing transarterial chemoembolization (TACE) plus sorafenib with TACE alone in patients with hepatocellular carcinoma (HCC): TACTICS trial. J Clin Oncol. 2018;36(4_suppl):206. [Google Scholar]
- 30.He M., Li Q., Zou R. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7):953–960. doi: 10.1001/jamaoncol.2019.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kudo M., Ueshima K., Yokosuka O. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3(6):424–432. doi: 10.1016/S2468-1253(18)30078-5. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto Y., Matsui J., Matsushima T. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6:18. doi: 10.1186/2045-824X-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiraoka A., Kumada T., Kariyama K. Clinical features of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions: multicenter analysis. Cancer Med. 2019;8(1):137–146. doi: 10.1002/cam4.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimura T., Kato Y., Ozawa Y. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Canc Sci. 2018;109(12):3993–4002. doi: 10.1111/cas.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Y.Y., Tan C.T., Chen C.W., Ou D.L., Cheng A.L., Hsu C. Immunomodulatory effects of current targeted therapies on hepatocellular carcinoma: implication for the future of immunotherapy. Semin Liver Dis. 2018;38(4):379–388. doi: 10.1055/s-0038-1673621. [DOI] [PubMed] [Google Scholar]
- 36.Bruix J., Qin S., Merle P. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 37.Finn R.S., Merle P., Granito A. Outcomes with sorafenib (SOR) followed by regorafenib (REG) or placebo (PBO) for hepatocellular carcinoma (HCC): results of the international, randomized phase 3 RESORCE trial. J Clin Oncol. 2017;35(4_suppl):344. [Google Scholar]
- 38.Abou-Alfa G.K., Meyer T., Cheng A.L. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou L., Liu X.D., Sun M. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. 2016;35(21):2687–2697. doi: 10.1038/onc.2015.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hicklin D.J., Ellis L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23(5):1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 41.Zhu A.X., Park J.O., Ryoo B.-Y. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 42.Zhu A.X., Kang Y.-K., Yen C.-J. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 43.Llovet J.M., Decaens T., Raoul J.L. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31(28):3509–3516. doi: 10.1200/JCO.2012.47.3009. [DOI] [PubMed] [Google Scholar]
- 44.Zhu A.X., Fuchs C.S., Clark J.W. A phase II study of epirubicin and thalidomide in unresectable or metastatic hepatocellular carcinoma. Oncol. 2005;10(6):392–398. doi: 10.1634/theoncologist.10-6-392. [DOI] [PubMed] [Google Scholar]
- 45.Patt Y.Z., Hassan M.M., Lozano R.D. Thalidomide in the treatment of patients with hepatocellular carcinoma: a phase II trial. Cancer. 2005;103(4):749–755. doi: 10.1002/cncr.20821. [DOI] [PubMed] [Google Scholar]
- 46.Chuah B., Lim R., Boyer M. Multi-centre phase II trial of Thalidomide in the treatment of unresectable hepatocellular carcinoma. Acta Oncol. 2007;46(2):234–238. doi: 10.1080/02841860600702076. [DOI] [PubMed] [Google Scholar]
- 47.Lin A.Y., Brophy N., Fisher G.A. Phase II study of thalidomide in patients with unresectable hepatocellular carcinoma. Cancer. 2005;103(1):119–125. doi: 10.1002/cncr.20732. [DOI] [PubMed] [Google Scholar]
- 48.Siegel A.B., Cohen E.I., Ocean A. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26(18):2992–2998. doi: 10.1200/JCO.2007.15.9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee M., Ryoo B.-Y., Hsu C.-H. LBA39 Randomised efficacy and safety results for atezolizumab (Atezo) + bevacizumab (Bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC) Ann Oncol. 2019;30(suppl ment_5) [Google Scholar]
- 50.Donafenib tosylate achieved statistical significance for its primary endpoint in phase III clinical study of first-line treatment for advanced hepatocellular carcinoma (in Chinese) 2020. http://www.zelgen.com/xinwenzhongxin/2020/01-01/69.html
- 51.Zhou A., Sun Y., Zhang W. 751P Anlotinib for advanced hepatocellular carcinoma: interim results from the phase II ALTER0802 study. Ann Oncol. 2019;30(suppl ment_5) [Google Scholar]
- 52.Bruix J., Takayama T., Mazzaferro V. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16(13):1344–1354. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 53.Kelley R.K. Adjuvant sorafenib for liver cancer: wrong stage, wrong dose. Lancet Oncol. 2015;16(13):1279–1281. doi: 10.1016/S1470-2045(15)00296-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang S.N., Chuang S.C., Lee K.T. Efficacy of sorafenib as adjuvant therapy to prevent early recurrence of hepatocellular carcinoma after curative surgery: a pilot study. Hepatol Res. 2014;44(5):523–531. doi: 10.1111/hepr.12159. [DOI] [PubMed] [Google Scholar]
- 55.Abou-Alfa G.K., Venook A.P. The antiangiogenic ceiling in hepatocellular carcinoma: does it exist and has it been reached? Lancet Oncol. 2013;14(7):e283–e288. doi: 10.1016/S1470-2045(13)70161-X. [DOI] [PubMed] [Google Scholar]
- 56.Abou-Alfa G.K., Shi Q., Knox J.J. Assessment of treatment with sorafenib plus doxorubicin vs sorafenib alone in patients with advanced hepatocellular carcinoma: phase 3 CALGB 80802 randomized clinical trial. JAMA Oncol. 2019;5(11):1582–1588. doi: 10.1001/jamaoncol.2019.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yau T.C., Tang V., Leung R.C.-Y. Randomized phase II trial of sorafenib, capecitabine and oxaliplatin (SECOX) versus single agent sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2019;37(4_suppl l) 365-365. [Google Scholar]
- 58.Cheng A.-L., Kang Y.-K., He A.R. Safety and efficacy of tigatuzumab plus sorafenib as first-line therapy in subjects with advanced hepatocellular carcinoma: a phase 2 randomized study. J Hepatol. 2015;63(4):896–904. doi: 10.1016/j.jhep.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Zhu A.X., Rosmorduc O., Evans T.R. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33(6):559–566. doi: 10.1200/JCO.2013.53.7746. [DOI] [PubMed] [Google Scholar]
- 60.Terme M., Colussi O., Marcheteau E., Tanchot C., Tartour E., Taieb J. Modulation of immunity by antiangiogenic molecules in cancer. Clin Dev Immunol. 2012;2012:492920. doi: 10.1155/2012/492920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hato T., Zhu A.X., Duda D.G. Rationally combining anti-VEGF therapy with checkpoint inhibitors in hepatocellular carcinoma. Immunotherapy. 2016;8(3):299–313. doi: 10.2217/imt.15.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohm J.E., Gabrilovich D.I., Sempowski G.D. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101(12):4878–4886. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- 63.Voron T., Colussi O., Marcheteau E. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212(2):139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khan K.A., Kerbel R.S. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol. 2018;15(5):310–324. doi: 10.1038/nrclinonc.2018.9. [DOI] [PubMed] [Google Scholar]
- 65.Llovet J., Shepard K.V., Finn R.S. 747P A phase Ib trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC): updated results. Ann Oncol. 2019;30(suppl ment_5) [Google Scholar]
- 66.Xu J., Zhang Y., Jia R. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res. 2019;25(2):515–523. doi: 10.1158/1078-0432.CCR-18-2484. [DOI] [PubMed] [Google Scholar]
- 67.Rini B.I., Plimack E.R., Stus V. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 68.Lee C.-H., Motzer R.J., Makker V. 847O A phase 1b/2 trial of lenvatinib plus pembrolizumab in patients with renal cell carcinoma. Ann Oncol. 2017;28(suppl l_5) [Google Scholar]
- 69.FDA Grants Breakthrough Therapy Designation to Pembrolizumab Plus Lenvatinib in Advanced HCC. 2019. https://www.ascopost.com/News/60276 [Google Scholar]
- 70.Cheng A.-L., Qin S., Ikeda M. LBA3 IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC) Ann Oncol. 2019;30(suppl ment_9) [Google Scholar]
- 71.Qin S., Ren Z., Feng Y. 2020. OP02-03 Efficacy and safety of atezolizumab + bevacizumab vs sorafenib in Chinese patients with unresectable HCC in the phase III IMbrave150 study. Paper presented at: Liver Cancer Summit; 6-8 February, 2020; Prague, Czech Republic. [Google Scholar]
- 72.Loges S., Mazzone M., Hohensinner P., Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15(3):167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 73.Ebos J.M., Lee C.R., Cruz-Munoz W., Bjarnason G.A., Christensen J.G., Kerbel R.S. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15(3):232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang W., Sun H.C., Wang W.Q. Sorafenib down-regulates expression of HTATIP2 to promote invasiveness and metastasis of orthotopic hepatocellular carcinoma tumors in mice. Gastroenterology. 2012;143(6):1641–1649. doi: 10.1053/j.gastro.2012.08.032. e1645. [DOI] [PubMed] [Google Scholar]
- 75.Lu L., Sun H.C., Zhang W. Aspirin minimized the pro-metastasis effect of sorafenib and improved survival by up-regulating HTATIP2 in hepatocellular carcinoma. PloS One. 2013;8(5) doi: 10.1371/journal.pone.0065023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kerbel R.S., Ebos J.M. Peering into the aftermath: the inhospitable host? Nat Med. 2010;16(10):1084–1085. doi: 10.1038/nm1010-1084. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Q.B., Sun H.C., Zhang K.Z. Suppression of natural killer cells by sorafenib contributes to prometastatic effects in hepatocellular carcinoma. PloS One. 2013;8(2) doi: 10.1371/journal.pone.0055945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang W., Zhu X.D., Sun H.C. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16(13):3420–3430. doi: 10.1158/1078-0432.CCR-09-2904. [DOI] [PubMed] [Google Scholar]
- 79.Zhu X.D., Sun H.C., Xu H.X. Antiangiogenic therapy promoted metastasis of hepatocellular carcinoma by suppressing host-derived interleukin-12b in mouse models. Angiogenesis. 2013;16(4):809–820. doi: 10.1007/s10456-013-9357-6. [DOI] [PubMed] [Google Scholar]
- 80.Xiong Y.Q., Sun H.C., Zhang W. Human hepatocellular carcinoma tumor-derived endothelial cells manifest increased angiogenesis capability and drug resistance compared with normal endothelial cells. Clin Cancer Res. 2009;15(15):4838–4846. doi: 10.1158/1078-0432.CCR-08-2780. [DOI] [PubMed] [Google Scholar]
- 81.Duffy A.G., Ma C., Ulahannan S.V. Phase I and Preliminary phase II study of TRC105 in combination with sorafenib in hepatocellular carcinoma. Clin Cancer Res. 2017;23(16):4633–4641. doi: 10.1158/1078-0432.CCR-16-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raghav K.P.S., Lee R.T., Paluri R.K. An open-label phase Ib/2 trial of TRC105 plus sorafenib in patients with advanced/metastatic hepatocellular carcinoma (HCC) ( NCT01806064) J Clin Oncol. 2019;37(4_suppl):268. [Google Scholar]
- 83.Kudo M., Motomura K., Wada Y. First-line avelumab + axitinib in patients with advanced hepatocellular carcinoma: results from a phase 1b trial (VEGF Liver 100) J Clin Oncol. 2019;37(15_suppl):4072. doi: 10.1159/000514420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yau T., Zagonel V., Santoro A. Nivolumab (NIVO) + ipilimumab (IPI) + cabozantinib (CABO) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. J Clin Oncol. 2020;38(suppl 4):478. [Google Scholar]
- 85.Kudo M., Ikeda M., Motomura K. A phase Ib study of lenvatinib (LEN) plus nivolumab (NIV) in patients (pts) with unresectable hepatocellular carcinoma (uHCC): study 117. J Clin Oncol. 2020;38(suppl 4):513. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El-Khoueiry A.B., Kim R.D., Harris W.P. Phase Ib study of regorafenib (REG) plus pembrolizumab (PEMBRO) for first-line treatment of advanced hepatocellular carcinoma (HCC) J Clin Oncol. 2020;38(suppl 4):564. [Google Scholar]