Abstract
The need for light intensity has made dye degradation very costly for industry. In this work, Fenton reagent was used for the efficient degradation of an aqueous solution of dye without the need for a light source. The influences of the pH of the media, the initial concentrations of Fe2+, H2O2, and methylene blue (MB) dye; in addition to temperature on the oxidation of MB dye were studied. The optimum amounts of the Fenton reagent were 4mM of Fe2+ and 70mM of H2O2 at 20 mg/L of dye. The optimum ratio of 0.05 of Fe2+/H2O2 was found to give the best result for the decolorization of dye. The Fenton process was effective at pH 3 with a maximum dye decolorization efficiency of 98.8% within 30 min of reaction, corresponding to a COD removal of 85%. The decolorization process was thermodynamically feasible, spontaneous, and endothermic. The activation energy (Ea) was 33.6 kJ/mol suggesting that the degradation reaction proceeded with a low energy barrier.
Keywords: Physical chemistry, Interactions, Fenton's reagent, Dye, Thermodynamic, Kinetics
Physical chemistry; Interactions; Fenton's reagent; Dye; Thermodynamic; Kinetics.
1. Introduction
The use of dye in industry is increasing and thus the amount of industrial wastewater that is produced from different processes including the paper and printing industries (Pang and Abdullah, 2013). Wastewater stemming from processes such as dyeing has continued to have an adverse impact (Saha et al., 2012). Therefore, their toxicity and recalcitrance to degradation pose a huge challenge to removal technologies. To combat this menace of the pollution problem, it seems more desirable to reduce dyes to a harmless form before discharging into the mainstream.
Different actions are widely utilized to treat the dye-containing wastewater, including physical, such as membrane treatment (Pang and Abdullah, 2013), adsorption (Bello et al., 2008; Saleh, 2020; Saleh and Ali, 2018), chemicals such as coagulation (Dalvand et al., 2011), and chemical oxidation (Turhan et al., 2012) or those enhanced with light (Liu et al., 2013), electricity (Bensalah et al., 2013), ultrasonic (Ghauch et al., 2016), biological methods (Mezohegyi et al.,2008), and the combination of several methods (Prato-Garcia et al., 2013). However, there are some disadvantages, such as the cost and time required, recycling, etc (Arulkumar et al., 2011). On the other hand, some processes like chemical methods could end up with negative side products adding more pollution to the environment due to the use of reagents (Pang and Abdullah, 2013). Also, although biological degradation is cheap, it is ineffective owing to toxicity and the recalcitrant nature of dyes to biospecies (Ji et al., 2011).
The scientific and research community have shown great interest in the cleaning of industrial effluents using methods called “Advanced Oxidation Processes (AOPs)”, not only as a result of environmental concern tackled by legislative characteristics but also due to the thriving of advanced oxidation schemes to overcome pollutant ‘phase transfer’ issues. AOPs offer an extremely reactive oxidant, specifically hydroxyl radicals (HO·), which are capable of destroying organic pollutants. Among various AOPs, the use of the Fenton process is an effective method for organic pollutant oxidation. Fenton's reagent is considered as homogeneous catalytic oxidation method using hydrogen peroxide, as well as iron (II) ions in acidic media, to cause a complex redox reaction for producing HO· with an oxidation potential of about 2.8 V (Metcalf and Eddy, 2003). These are strong oxidants compared to ozone 2.08 V and hydrogen peroxide 1.78 V.
| The overall reaction is: H2O2 + Fe2+ → Fe3+ + HO− + HO· | (1) |
The HO· is capable of quickly degrading organic pollutants (RH) and causing decomposition of the molecules by H- abstraction, as well as addition into C─C unsaturated bonds
| RH + HO· → R· + H2O | (2) |
In this study, we aimed at investigating the efficiency of degrading aqueous solutions of MB dye by a new Fenton process with the optimization of several experimental conditions. The results obtained prove that this reported procedure is cost effective and promising for the efficient treatment of industrial effluents.
2. Materials and methods
2.1. Chemicals
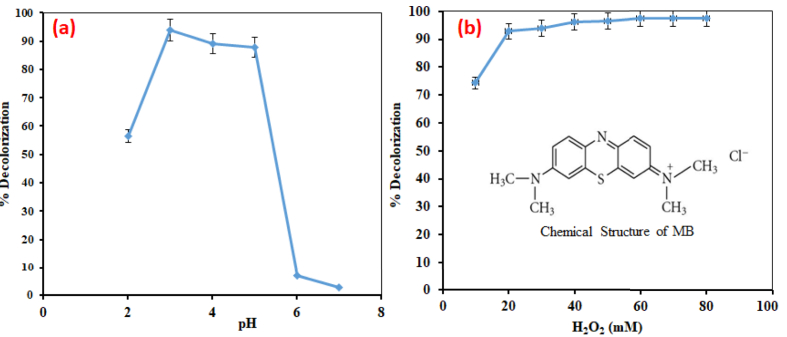
Hydrogen peroxide (30% w/v H2O2), sulphuric acid (98% H2SO4), iron (II) sulphate (FeSO4.7H2O), and sodium hydroxide (99.9% NaOH) were obtained from the Sigma-Aldrich Chemicals Company (United States). Methylene Blue dye (CI 52015) was purchased from Merck (Germany). It is water-soluble (MW: 319.85 g/mol) with a chemical structure depicted in Figure 1.
Figure 1.
Influence of pH (a) Influence of H2O2 dose (b) on the decolorization percentage of MB; Operating conditions: (a) [H2O2] = 30 mM, [Fe2+] = 4 mM, reaction time = 60 min, [MB] = 20 ppm, T = 25 °C; (b) pH = 3, [Fe2+] = 4 mM, reaction time = 60 min, [MB] = 20 ppm, T = 25 °C.
2.2. Fenton experiments
The decolorization of MB dye was carried out in a batch operation. The dye solution was prepared in deionized water. The batch tests of the Fenton degradation process were performed in a 500 mL glass beaker at different experimental conditions including a Fenton dosage of 10–80 mM H2O2, 2–5mM FeSO4, (pH = 2–7), and temperature of 298–318 K. The temperature was controlled through a thermostat. For each experiment, the reactor was loaded with 250 mL of MB aqueous solution, to which 5 mL of the ferrous catalyst was added, the pH value was adjusted with 0.1 M H2SO4 or 0.1 M NaOH under stirring. The reactions were initiated by adding 5 mL of H2O2 and the time was recorded. The reaction was halted by using 0.5 mL of 10 M NaOH (Xiang-Rong et al., 2004). The absorbance of the dye solution was taken and recorded before and after each oxidative degradation experiment. All of the results were estimated by obtaining the numerical mean of the results of triplicate experiments.
The UV-Vis spectrum of each MB solution was determined by a Cecil 7200 model spectrophotometer with a quartz cuvette 1 cm in length. The range from 200 to 800 nm was scanned and a calibration curve was created using the maximum absorbance wavelength of the dye solution. On the other hand, the MB degradation was computed by Eq. (3) below.
| (3) |
where A0 and At are the absorbance of MB after treatment time t in minutes, at corresponding tax respectively. The expanse of the mineralization of the MB by the Fenton process was determined by chemical oxygen demand (COD) measurements. COD was determined by the closed reflux method as outlined in the standard method (APHA, 1998). The mineralization of MB was assessed for COD reduction of the treated solutions.
3. Results and discussion
3.1. pH effect on fenton oxidation
Fenton oxidation processes are dependent on pH which plays a key role in the HO· generation mechanism in the Fenton reactions as indicated in Eq. (1). The HO· are efficiently formed in acidic media (Neamtu et al., 2003). The influence of pH on the decolorization of MB dye by the Fenton procedure is depicted in Figure 1a. Clearly, pH has a considerable effect on the dye degradation. At pH above 5, very weak color degradation of less than 10% was produced. At high pH, ferrous ions are not stable and form ferric ions with a tendency to generate a colloidal ferric hydroxo complex [Fe(OH)3] (Ensign et al., 2003). The production of HO· gets slower due to the development of this species which impeded further reaction of Fe2+ and H2O2. At a pH of 3–5, the degradation percentage obtained was greater than 80%. Under these conditions, sufficient HO· is formed and Fe2+ is more soluble in aqueous media at a pH from 2-5 (Arslan-Alaton et al., 2008). In this work, higher degradation was observed at pH 3, where degradation was 93.9% at 60 min. The optimal pH of 3 obtained in this work was in close agreement with previous studies (Saeedah, 2013; Jafari et al., 2014). However, when the pH dropped to 2, a substantial decline in degradation efficiency was noticed compared to that of pH 3. At pH 2, the decolorization percentage was 56.6%. This occurrence could be explained by the high excess of hydrogen ions, behaving as an HO· radical scavenger according to the reaction scheme (4) below.
| HO· + H++ e−→H2O | (4) |
3.2. Influence of H2O2 concentrations
H2O2 plays an important role as an oxidizing reagent in the presence of iron. To make the Fenton process compete with other processes, it is necessary that the applications depict a cost-effective operation that involves a better control of H2O2 dosages. The aim of the test was to optimize the Fenton degradation process to obtain the highest performance under an optimum H2O2 dosage. The effect of H2O2 concentrations on the process was studied in H2O2 concentrations of 10 mM and 80 mM while keeping the ferrous ion dose, pH of solution and temperature at 4 mM, 3 and 298 K respectively. There is a correlation between the degradation efficiency of the dye and H2O2 concentrations in the Fenton process as shown in Figure 1b below. The results designate that the degradation efficiency appreciated from 74.3 to 96.3% with an increase in H2O2 concentration from 10 mM to 30 mM after 60 min of the reaction. The intensification in the color degradation efficiency of the Fenton process was due to an increase in HO· concentration by the addition of H2O2. The further enhancement from 30 mM to 40 mM initiated no noteworthy change in color degradation, as it only improved by 0.1%. Doubling the concentration of H2O2 to 80 mM only improved the efficiency by 1.2%. The highest degradation efficiency of 97.6% was achieved with a 70 mM H2O2 dose which was 0.1% higher than that obtained at an H2O2 dose of 80mM. This small difference could be explained by the fact that at high H2O2 concentrations, scavenging of HO· will take place and the result is the formation of perhydroxyl radicals, HO2·, which are less reactive than HO· and thus affect the degradation of the dye (Bergendahl and Thies, 2004). An optimal H2O2 dose of 50 mM for the removal of methyl violet was reported with a degradation efficiency of 95.5% (Saeedah, 2013) while the best color degradation efficiency of 99.0% with 44 mM H2O2 for the removal of Basic Blue 3 was reported (Elham and Mina, 2014).
3.3. Effect of Fe2+ concentration
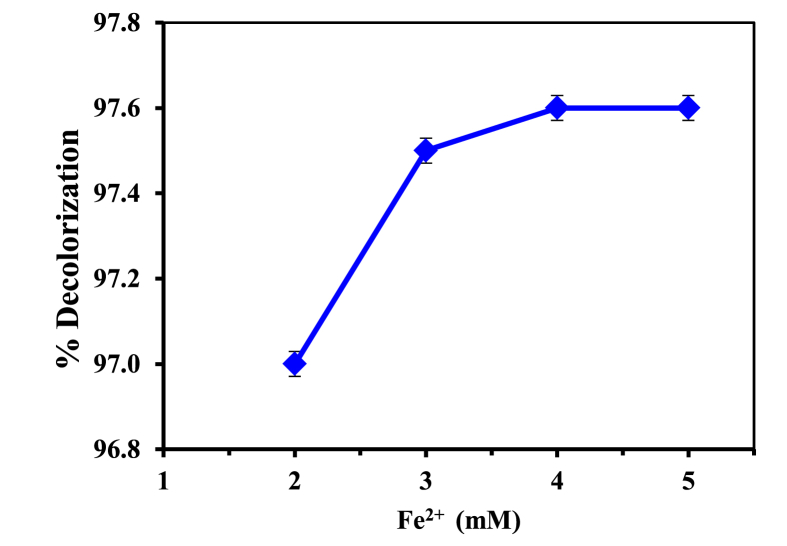
The concentrations of ferrous ions affect the Fenton process. Generally, advanced oxidation of organic pollutants is more efficient when ferrous ions are at 2–5 mM concentrations. This range is sufficient for HO· to be produced where Fe2+ ions are still highly soluble at a pH of 2–5 (Arslan-Alaton et al., 2008; Askarniya et al., 2020). Iron solubility is one of the obvious aspects of Fenton oxidation since the rate of HO· formation is proportional to Fe2+ concentration. The effect of the addition of Fe2+ was studied. Different doses of ferrous catalyst from 2 to 5 mM were tested as shown in Figure 2. No remarkable difference was observed in degradation with increasing ferrous concentrations. The addition of Fe2+ from around 2 mM–4 mM raised color degradation from 97.0% to 97.6 %. However, at 4 mM–5 mM, color degradation remained constant with a further increase of Fe2+ concentrations. The lower removal efficiency of Fe2+ at low concentrations was due to the lower HO· radical formation for oxidation. On the other hand, the excess of Fe2+ ions scavenged the formed HO· which can decrease the color degradation.
Figure 2.
Influence of Fe2+ concentration on decolorization percentage of MB; Operating conditions: pH = 3, [H2O2] = 70 mM, reaction time = 60 min, [MB] = 20 ppm, T = 25 °C.
The results obtained were in line with the literature report (Tarr, 2003), in which an increase in iron (II) salt concentration increases the removal rate of the organic pollutants but only to the point where the subsequent addition of Fe becomes insignificant. The concentration of Fe2+ 4 mM or 5 mM can be used as an optimum dosage within this treatment. The results were in line with the findings of (Agustina and Ang, 2012) in which 4mM was stated as the optimal ferrous dose in the degradation of Reactive Blue 4.
3.4. Fenton ratio
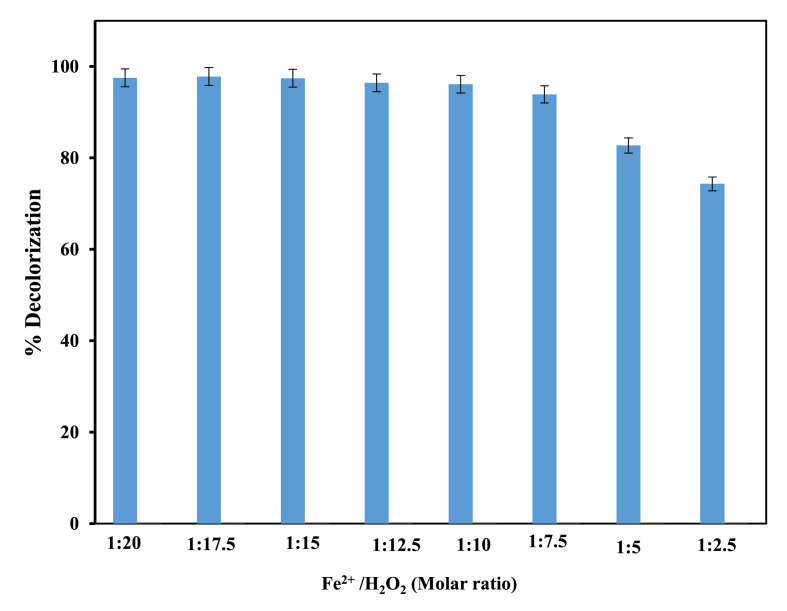
Both the iron (II) ion (Fe2+) and H2O2 do not react solely to form HO· but also are scavengers of HO·. The ratio of [Fe2+]/[H2O2] should influence the rate of HO· formation and scavenging. Therefore, it is vital to use optimum [Fe2+]/[H2O2]. To obtain the optimal initial concentration ratio of [Fe2+]/[H2O2] on the removal of MB dye, the fixed concentration of 4 mM of ferrous ion and 20 ppm of the dye was studied at different hydrogen peroxide concentrations to give molar ratios 1:2.5–1:20. In this work, it was observed that the best molar ratio is at the value 1:17.5, Figure 3. The results were in close agreement with what the literature reported on Fenton treatment regarding the degradation of azo dyes which identified that the effective condition was established at an iron-to –hydrogen peroxide ratio of 1:20 (Neamtu et al., 2003; Matyszczak et al., 2020).
Figure 3.
Influence of [Fe2+]/[H2O2] ratio on decolorization percentage of MB dye; Operating conditions: pH = 3, [Fe2+] = 4 mM, reaction time = 60 min, [MB] = 20 ppm, T = 25 °C.
3.5. Influence of MB concentrations
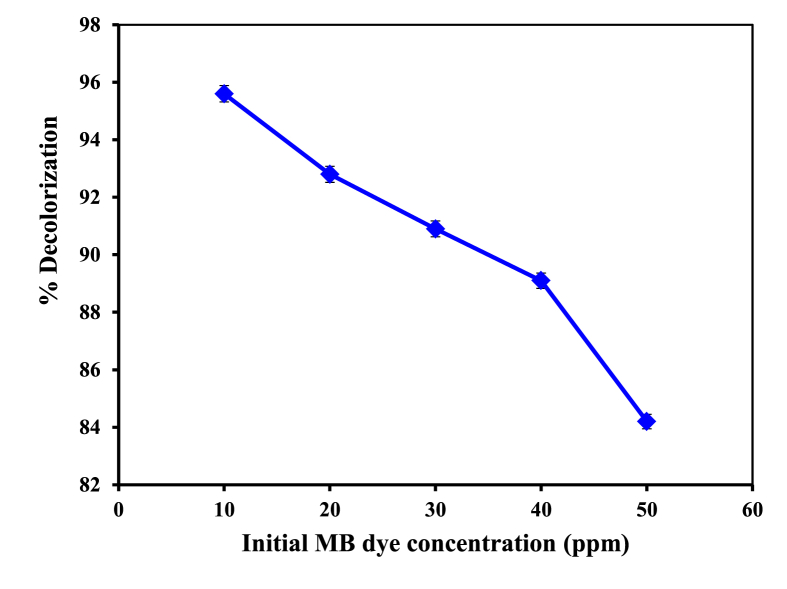
Dye concentrations are a very crucial specification in wastewater management. The influence of initial MB dye concentrations on its decolonization was investigated with in a range of 10–50 ppm of MB dye. The impact of the dye concentration is illustrated in Figure 4. It is evident that the percentage of color degradation drops with a rise in the dye concentrations. Increasing the initial concentration of MB from 10 to 50 ppm decreases the color degradation from 95.6% to 84.2% after 60 min of reaction. The degradation efficiencies were 95.6% (10 ppm), 92.8% (20 ppm), 90.9% (30 ppm), 89.1 % (40 ppm) and 84.2 % (50 ppm) after 1h reaction time (Wang et al., 2019; Lucas and Peres, 2006).
Figure 4.
Influence of dye concentrations on decolorization percentage of MB; Operating conditions: [Fe2+] = 4 mM, [H2O2] = 70 mM, pH = 3, reaction time = 60 min, T = 25 °C.
3.6. Influence of temperature and time
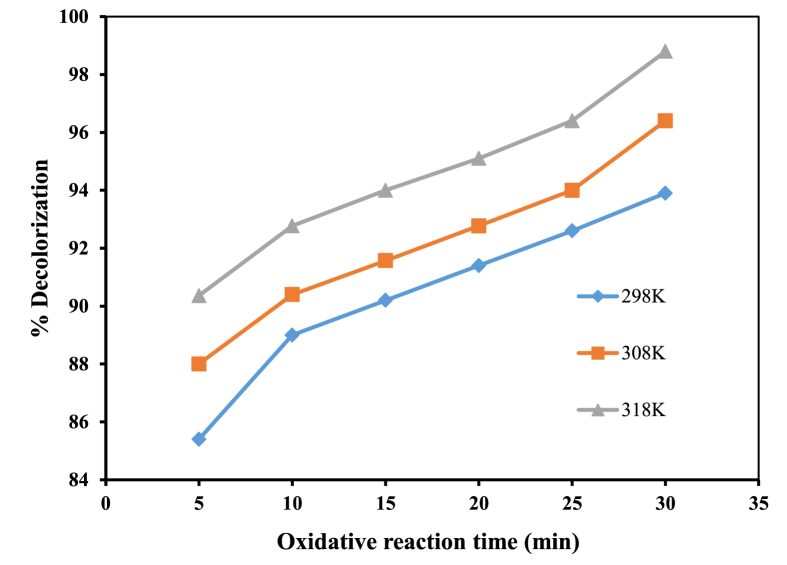
Most commercial applications of Fenton Reagent occur at temperatures between 293 and 313 K. A temperature range of 298–318K was investigated to obtain the effect of temperature on the Fenton treatment. There was a significant temperature effect on the removal of MB efficiency as presented in Figure 5. The removal efficacy of the MB dye was 85–90% after 5 min of treatment for all temperatures. The degradation efficiency of MB was enhanced from around 85 to about 98.8% by increasing temperature from 298 to 318K after 30 min of the oxidation process. This is because the high temperature could increase the reaction rate between H2O2 and iron (II) ion, thus increasing the rate of HO· formation. The results implied that increasing the temperature from 298K to 318K had a positive effect on MB dye degradation. At 318K, percentage of color degradation was 98.8% after 30 min. A further increase in temperature of 10K caused a slight decrease in color degradation from 99.8% (318K) to 95.0% (318K). In this study, the optimal temperature of 45 °C (318K) was obtained in the degradation of MB dye as opposed to some literature reports (Guedes et al., 2003; Ramirez et al., 2005) in which around 30 °C was reported as optimal temperature for Fenton degradation. Also, 50 °C was stated on dyes decolorization by Fenton-like reaction (Medien and Khalil, 2010). However, results in this study were in agreement with the report by Dutta et al. (2001) which stated no remarkable change in the expanse of color removal between 40 °C and 50 °C. There are conflicting reports in the literature, including conflicting reports as regards optimal temperatures, what is common in that degradation efficiency depreciated above the optimal value since H2O2 decomposed to O2 and H2O was noticeable at a temperature >40 °C.
Figure 5.
Influence of temperature with time on the decolorization efficiency of MB; [Fe2+] = 4 mM, [H2O2] = 70 mM, pH = 3.
3.7. Decolorization kinetics
The oxidation of organic pollutants by the HO· radicals can be represented by several reaction mechanisms. General rate law of the key organic molecule is:
| (5) |
The term Oxi represents oxidants other than HO· such as (hydroperoxyl radical HOO·). In order to simplify; the HO· can be regarded as the only active oxidant. The concentration of HO· cannot be measured directly. It is considered to be constant under certain reaction conditions, leading to the pseudo-first order reaction where HO· concentration is a part of the apparent rate constant kapp (Saeedah, 2013).
| (6) |
| (7) |
The absorbance of the detected compound is proportional to its concentration according to the Lambert-Beer equation and as such the absorbance can be used in the rate expression instead of concentration.
| (8) |
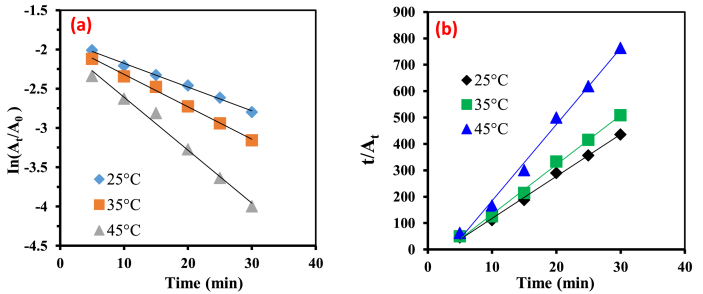
The degradation kinetics of MB by the Fenton process was studied at different contact times varying from 5 to 30 min at different temperatures 298, 308,318 & 318 K. Assuming pseudo-first-order reaction kinetics for MB dye decolorization in water, the decolorization rate constant,, was computed from the slope of the logarithmic plot of color (Absorbance A) vs. treatment time t as presented in Eq. (8). A pseudo first order kinetic plot (Figure 6a) was utilized to define the degradation of the MB dye in this study as reported in previous work (Arslan et al., 2001). The pseudo second order model was also tested as proposed by Ho and McKay (1998) using the following equation. The plot is as shown in Figure 6b.
| (9) |
is the equilibrium absorbance of the dye. The pseudo second order rate constant was obtained from the plot of versus.The data obtained were presented in Table 1. In order to fit the best experimental data, the correlation coefficients of both models were compared. The rate constants obtained from kinetic plots of the pseudo first order model were higher than that of the pseudo second order model. Temperature has a significant impact on the pseudo first order rate constants. Increasing the temperature from 298 to 318K increased k1 from 0.0302 to 0.0674 min−1 (the rate constant increased by a factor of 2). A further increase in temperature by 10K decreased k1 from 0.0674 to 0.0420 min−1. The pseudo second order rate values did not follow a specific trend with a high temperature from about 298.15 to 318.15 K. The retention of negative value for k2 indicated that the experimental data could not be best described by the pseudo second order kinetic model. Given that the pre-exponential factor of the Arrhenius equation is always positive, the rate constant is never negative. The coefficients of correlation of the kinetic study of the pseudo-first order are very close to unity and are more than the pseudo second order values. Thus, the results of the experiments indicated that degradation of MB dye by Fenton process could be best described by the pseudo-first-order kinetic model. This was in close agreement with the studies conducted by previous researchers (Arslan and Balcioglu, 2001; Abou-Gamra, 2014). The reported method is comparable to the literature, Table 2.
Figure 6.
Plots of pseudo first-order (a) pseudo-second-order (b) kinetic model for the degradation of MB.
Table 1.
Rate parameters for the decolorisation of MB by Fenton's Reagent.
| T(K) | Pseudo first order model |
Pseudo second order model |
||
|---|---|---|---|---|
| k1 (min−1) | R2 | k2 (min−1) | R2 | |
| 298 308 318 |
0.0302 0.0413 0.0674 |
0.9947 0.9958 0.9976 |
-6.04 -6.518 -8.03 |
0.890 0.932 0.889 |
Table 2.
Summary of existing and emerging treatment techniques reported in the literature for MB dye removal from wastewater.
| Physical/chemical Method |
Method Description | Core findings | %Colour Reduction |
Reference | Shortcomings |
|---|---|---|---|---|---|
| Photo Fenton process (Microwave assisted Fenton) | Oxidation reaction using H2O2–Fe(II)-MW | Effects of experimental conditions on the Microwave assisted Fenton process was determined. | 93.0 | Liu et al., (2013) | (1)No information was provided on the kinetics & thermodynamics of the degradation process. (2) The high cost of MW irradiation |
| Electro-Fenton process | Oxidation reaction using mainly H2O2–Fe(II)-electric current | Effects of experimental conditions on Electro-Fenton process were investigated. | 98.8 | Guangsen et al., (2014) | (1) Kinetic and thermodynamic studies were not carried out. (2) The high cost of electricity |
| Ozonation | Oxidation reaction using ozone Gas | Evaluation of the effectiveness of indirect ozonation for enhancing the degradability of MB | 94.56 | Turhan et al., (2012) | (1)Information about thermodynamic studies was not supplied. (2)Short half-life (20min) |
| Photocalytic process (TiO2 assisted) | Oxidation of dye mainly by TiO2-UV irradiation | Effects of experimental conditions on the photo catalytic process &kinetics of the degradation process were determined. | >90 | Marziyeh et al., (2012) | (1)The thermodynamics of the photo catalytic process was not studied. (2)Formation of by-products |
| Activated Carbon | Dye removal by adsorption Using: (a) Java Plum leaves |
Experimental conditions, isothermal and thermodynamic studies were used to determine the effectiveness of the adsorption process | 93.45 | Gnana et al., (2014) | (1)Degradation kinetics was not studied. (2)Regeneration difficulties |
| (b) periwinkle shell | Experimental conditions, kinetic and isothermal studies were used to verify the effectiveness of the adsorption process. | >90.0 | Bello et al., (2008) | Information about thermodynamic studies was not supplied | |
| (c)rice husk | Effects of experimental parameters on the adsorption process. | 97.15 | Mohammad et al., (2012) | No information was provided on kinetics and thermodynamics of the degradation process | |
| Fenton's reagent | Oxidation reaction using mainly H2O2–Fe(II) |
Experimental conditions, kinetic and thermodynamic studies were used to verify the effectiveness of the Fenton process. | 98.8 | This work | - |
3.8. Thermodynamic studies for the decolorization of MB dye
To study the effect of reaction temperature on the MB removal, kinetic data were collected at four different temperatures (298, 308, and 318 K). The temperature necessity of the kinetic values of the Fenton oxidation was found by using the Arrhenius Eq. (10) below:
| (10) |
From the plot of the natural logarithm of k against 1/T, with the straight line was obtained. From the slope of the line was deduced. Rate constants may also be related to energy changes by the general form of Erying-Polanyi equation (from transition state theory) somewhat resembles the Arrhenius equation as shown in Eq. (11).
| (11) |
where ΔG‡ is the free energy activation and =
The linear Eyring-Polanyi Equation is given below:
| (12) |
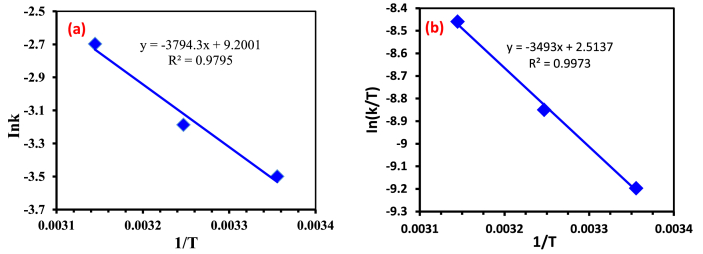
The plot of versusgives a straight line showing slope from which activation enthalpy is calculated. The intercept can be used to find activation entropy. The activation energy (Ea) of the Fenton process was computed from the slope of the Arrhenius plot in Figure 7a to be 31.5 kJ/mol. This low value suggested that the oxidative reaction proceeded with a low energy barrier. The other thermodynamic parameters like activation enthalpy (ΔH‡) and activation entropy (ΔS‡) were found from Erying-Polanyi plot in Figure 7b respectively. The enthalpy of activation (ΔH‡) was found to be 29.0 kJ/mol and the positive value of the enthalpy showed that the oxidative reaction was endothermic. The entropy of activation was 196J/mol and this positive value implied that transition state was highly disordered associated with the ground state. Translational and rotational, as well as vibrational degrees of freedom, can be liberated as they go from ground states to transition states. Therefore, the oxidative degradation reaction preceded rapidly and the reaction was favorable. The values of the Gibb's free energy of activation (ΔG‡), which is well-thought-out as a driving force of the Fenton reaction, were calculated from the enthalpy and the entropy of activation at different varying temperatures (El Haddad et al., 2013). The values were found to be -29.4, -31.3 and -33.3 kJ/mol at the temperatures 298.15, 308.15 and 318.15 K. The retention of negative numbers of the free energy of activation implied that the oxidative reaction using the Fenton reagent for the degradation of MB was spontaneous and the extent of the spontaneity of the reaction increased by increasing the temperature. This result is in line with the proposal that the reaction mechanisms were energetically stable with a rate of Fenton reaction enhanced by the increase in the temperature until the optimum temperature was reached.
Figure 7.
Plots of (a) Ink versus 1/T and (b) ln (k/T) versus 1/T for the degradation of MB.
4. Conclusion
In this work, colour removal was investigated with the influence of process parameters including the pH and temperature of the media and the concentrations of FeSO4, H2O2 and the dye. The process efficacy was studied and established based on the UV-Vis and COD measurements. The optimal conditions included: 70mM of H2O2, 4mM of FeSO4 and a 318 K temperature, in addition to a pH of 3.0 and an initial MB concentration of around 20 mg/L after 30 min. The results indicated that the Fenton process was operative as a color degradation efficiency of 98.8% was attained within 30 min. This corresponds to a COD removal of 85%. Under these optimal conditions, the pseudo-first order removal rate constants are found from batch tests results. The thermodynamic parameters of the Fenton oxidation process were examined and the results showed that the Fenton process is highly spontaneous and endothermic.
Declarations
Author contribution statement
Abdur-Rahim A. Giwa: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Isah A. Bello: Performed the experiments; Analyzed and interpreted the data.
Abdullahi B. Olabintan, Olugbenga S. Bello: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Tawfik A. Saleh: Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abou-Gamra Z.M. Kinetic and thermodynamic study for fenton-like oxidation of amaranth red dye. Adv. Chem. Eng. Sci. 2014;4:285–291. [Google Scholar]
- Agustina T.E., Ang H.M. Decolorization and mineralization of C.I. Reactive blue 4 and C.I. Reactive red 2 by Fenton oxidation process. Int. J. Chem. Environ. Eng. 2012;3(3):141–148. [Google Scholar]
- APHA . 20th ed. American Public Health Association; Washington., USA: 1998. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- Arslan I., Balcioglu I.A. Degradation of remazol black B dye and its simulated dyebath wastewater by advanced oxidation processes in heterogeneous and homogeneous media. Color. Technol. 2001;117:38–42. [Google Scholar]
- Arslan-Alaton I., Gursoy B.H., Schmidt J.E. Advanced Oxidation of acid and reactive dyes: effect of Fenton treatment on aerobic, anoxic and anaerobic processes. Dyes Pigments. 2008;78:117–130. [Google Scholar]
- Arulkumar M., Sathishkumar P., Palvannan T. Optimization of orange G dye adsorption by activated carbon of thespesiapopulnea pods using response surface methodology. J. Hazard Mater. 2011;186:827–834. doi: 10.1016/j.jhazmat.2010.11.067. 2011. [DOI] [PubMed] [Google Scholar]
- Askarniya Z., Sadeghi M., Baradaran S. Decolorization of Congo red via hydrodynamic cavitation in combination with Fenton’s reagent. Chem. Eng. Process-Process Intes. 2020;150:107874. [Google Scholar]
- Bello O.S., Adeogun I.A., Ajaelu J.C., Fehintola E.O. Adsorption of methylene blue onto activated carbon derived from periwinkle shells: kinetics and equilibrium studies. Chem. Ecol. 2008;24(4):285–295. [Google Scholar]
- Bensalah N., Bedoui A., Chellam S., Abdel-Wahab A. Electro-fenton treatment of photographic processing wastewater. Clean. 2013;41:635–644. [Google Scholar]
- Bergendahl J.A., Thies T.P. Fenton's oxidation of MTBE with zero-valent iron. Water Resour. 2004;38:327–334. doi: 10.1016/j.watres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Dalvand A., Gholami M., Joneidi A., Mahmoodi N.M. Dye Removal,Energy consumption and operating cost of electrocoagulation of textile wastewater as a clean process. Clean. 2011;39:665–672. [Google Scholar]
- Dutta K., Mukhopadhyay S., Bhattacharjee S., Chaudhuri B. Chemical oxidation of methylene blue using a fenton-like reaction. J. Hazard Mater. 2001;84:57–71. doi: 10.1016/s0304-3894(01)00202-3. [DOI] [PubMed] [Google Scholar]
- El Haddad M., Regti A., Laamari R., Mamouni R., Saffaj N. Use of Fenton’s reagent as an oxidative process for removing textile dyes from aqueous solutions. J. Mater. Environ. Sci. 2013;5(3):667–674. [Google Scholar]
- Elham K., Mina F. Decolorization and degradation of basic blue 3 and disperse blue 56 dyes using Fenton process. J. App. Chem. Res. 2014;8(3):81–90. [Google Scholar]
- Ensing B., Buda F., Baerends E.J. Fenton-like chemistry in water oxidation catalysis by Fe(III) and H2O2. J. Phys. Chem. 2003;107(30):5722–5731. [Google Scholar]
- Ghauch A., Baydoun H., Dermesropian P. Ultrasonic-assisted adsorption of methylene blue on sumac leaves. Desalination Water Treat. 2016;57(20):9286–9295. (2016) [Google Scholar]
- Gnana K.T., Suggala V.S., King P. Equilibrium and thermodynamic studies of methylene blue biosorption from aqueous solution using Syzygium cumini L. J. Environ. Res. Dev. 2014;8(4):964–976. [Google Scholar]
- Guangsen X., Yonghong L., Xueli G., Congjie G., Haibo X. Electro-fenton degradation of methylene blue using polyacrylonitrile-based carbon fiber brush cathode. Clean. 2014;43(2):229–236. [Google Scholar]
- Guedes A.M.F.M., Madeira L.M.P., Boaventura R.A.R., Costa C.A.V. Fenton oxidation of cork cooking wastewater overall kinetic analysis. Water Res. 2003;37:3061–3069. doi: 10.1016/S0043-1354(03)00178-7. [DOI] [PubMed] [Google Scholar]
- Ho Y.S., McKay G. Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf. Environ. Protect. 1998;76(2):181–191. [Google Scholar]
- Jafari M.H., Edris B., Ahmadreza Y., Mostafa A. Removal of azo dyes from aqueous solution using Fenton and modified Fenton processes. Health Scope. 2014;3(2):15507. [Google Scholar]
- Ji F., Li C.L., Zhang J.H., Deng L. Efficient decolorization of dye pollutants with LiFe(WO4)2 as a reusable heterogeneous fenton-like catalyst. Desalination. 2011;269:284–290. [Google Scholar]
- Liu S.T., Huang J., Ye Y., Zhang A.B., Pan L., Chen X.G. Microwave enhanced Fenton process for the removal of methylene blue from aqueous solution. Chem. Eng. J. 2013;215–216:586–590. [Google Scholar]
- Lucas M.S., Peres J.A. Decolorization of the azo dye reactive black 5 by Fenton and photo-fenton oxidation. Dyes Pigments. 2006;71:236–244. [Google Scholar]
- Marziyeh S., Hassan H., Mohammad M. Experimental study of influencing factors and kinetics in catalytic removal of methylene blue with TiO2 nanopowder. Am. J. Environ. Eng. 2012;2(1):1–7. 2012. [Google Scholar]
- Matyszczak G., Krzyczkowska K., Fidler A. A novel, two-electron catalysts for the electro-Fenton process. J. Water Process Eng. 2020;36:101242. [Google Scholar]
- Medien H.A.A., Khalil S.M.E. Kinetics of the oxidative decolorization of some organic dyes utilizing fenton-like reaction in water. J. King Saud Univ. 2010;22:147–153. [Google Scholar]
- Metcalf, Eddy . fourth ed. McGraw-Hill; 2003. Wastewater Engineering: Treatment and Reuse; pp. 80–93. [Google Scholar]
- Mezohegyi G., Bengoa C., Stuber F., Font J., Fabregat A., Fortuny A. Novel bioreactor design for decolorisation of azo dye effluents. Chem. Eng. J. 2008;143:293–298. [Google Scholar]
- Mohammad A.R., Ruhul Amin S.M., Shafiqul Alam A.M. Removal of methylene blue from waste water using activated carbon prepared from rice husk. Dhaka Univ. J. Sci. 2012;60(2):185–189. [Google Scholar]
- Neamtu M., Yediler A., Siminiceanu I., Kettrup A. Oxidation of commercial reactive azo dye aqueous solutions by the photo-fenton and fenton-like processes. J. Photochem. Photobiol., A. 2003;161:87–93. [Google Scholar]
- Pang Y.L., Abdullah A.Z. Current status of textile industry wastewater management and research progress in Malaysia: a review. Clean. 2013;41:751–764. [Google Scholar]
- Prato-Garcia D., Cervantes F.J., Buitrón G. Azo dye decolorization assisted by chemical and biogenic sulfide. J. Hazard Mater. 2013;250–251:462–468. doi: 10.1016/j.jhazmat.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Ramirez J.H., Costa C.A., Madeira L.M. Experimental design to optimize the degradation of the synthetic dye orange II using Fenton's reagent. Catal. Today. 2005;107–108:68–76. [Google Scholar]
- Saeedah H. Fenton-like oxidation of malachite green solution: kinetic and thermodynamic studies. J. Chem. 2013:1–7. 2013. [Google Scholar]
- Saha P., Das Mishra R., Husk R. Adsorption of safranin on chemically modified rice husk in a upward flow packed bed reactor: artificial neural network modeling. Biotechnol. Adv. 2012;44:7579–7583. [Google Scholar]
- Saleh T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020:101067. [Google Scholar]
- Saleh T.A., Ali I. Synthesis of polyamide grafted carbon microspheres for removal of rhodamine B dye and heavy metals. J. Environ. Chem. Eng. 2018;6(4):5361–5368. [Google Scholar]
- Tarr M. Vol. 46. Marcel Dekker; New York: 2003. Fenton and modifed Fenton methods for pollutant degradation; pp. 381–391. (Chemical Degradation Methods for Wastes and Pollutants). [Google Scholar]
- Turhan K., Durukan I., Ozturkcan A., Turgut Z. Decolorization of textile basic dye in aqueous solution by ozone. Dyes Pigments. 2012;92:897–901. [Google Scholar]
- Wang N., Hu Q., Du X., Xu H., Hao L. Study on decolorization of Rhodamine B by raw coal fly ash catalyzed Fenton-like process under microwave irradiation. Adv. Powder Technol. 2019;30(10):2369–2378. [Google Scholar]
- Xiang-Rong, Hua-Bin, Wen-Hua, Ji-Dong Degradation of dyes in aqueous solutions by Fenton process. Chemosphere. 2004;57:595–600. doi: 10.1016/j.chemosphere.2004.07.030. [DOI] [PubMed] [Google Scholar]