Abstract
Tumor recurrence rate after surgery or ablation of hepatocellular carcinoma (HCC) is as high as 70%. However, there are no widely accepted adjuvant therapies; therefore, no treatment has been recommended by guidelines from the American Association for the Study of Liver Disease or the European Association for the Study of the Liver. All the registered trials failed to find any treatment to prolong recurrence-free survival, which is the primary outcome in most studies, including sorafenib. Some investigator-initiated studies revealed that anti-hepatitis B virus agents, interferon-α, transcatheter chemoembolization, chemokine-induced killer cells, and other treatments prolonged patient recurrence-free survival or overall survival after curative therapies. In this review, we summarize the current status of adjuvant treatments for HCC and explain the challenges associated with designing a clinical trial for adjuvant therapy. Promising new treatments being used as adjuvant therapy, especially anti-PD-1 antibodies, are also discussed.
Keywords: Adjuvant therapy, Anti-PD-1 antibody, Clinical trial, Hepatocellular carcinoma, Molecular targeted therapy, Recurrence-free survival
Abbreviations: HCC, hepatocellular carcinoma; TACE, transcatheter chemoembolization; OS, overall survival; RFS, recurrence-free survival; RECIST, Response Evaluation Criteria in Solid Tumors; ICI, immune checkpoint inhibitor; ORR, objective response rate; PR, partial response; CR, complete response; TKI, tyrosine kinase inhibitor; PD-1, program death-1; PD-L1, program death-1 ligand; CIK, chemokine-induced killer cells; RCT, randomized clinical trial
Introduction
Liver cancer is the second leading cause of absolute years of life lost according to the Global Burden of Disease study.1 In China, liver cancer ranks second among cancer-related mortalities.2 Hepatocellular carcinoma (HCC) accounts for 85–90% of patients with liver cancer. Most patients are not amenable to curative therapies because they were diagnosed at an advanced stage or complicated with advanced liver disease. However, curative treatment is the first choice for the treatment for HCC, because it presents the best chance for long-term survival. Curative therapies for HCC include liver transplantation, liver resection, and ablation. As the pattern of tumor recurrence following liver transplantation is different from that following liver resection or ablation, only the adjuvant therapies after liver resection or ablation are discussed in this review.
Although the recurrence rate is as high as 70% in 5 years after curative surgery,3 there are no widely accepted adjuvant therapies for HCC, and the guidelines from the American Association for the Study of Liver Disease and the European Association for the Study of the Liver do not recommend any adjuvant therapies.4,5 Investigator-initiated clinical studies suggested that interferon (IFN)-α,6 chemokine-induced killer cells,7 transcatheter chemoembolization (TACE),8 and anti-hepatitis B virus (HBV) agents9,10 lower the incidence of tumor recurrence and/or prolong survival in patients after curative therapies for HCC; however, all the registered studies, including sorafenib,11 PI-88,12 and CMS-024, failed to extend recurrence-free survival (RFS). Recently, the face of systemic therapy for advanced HCC was largely changed by several molecular targeted agents and anti-PD-1 antibodies.13 More and more pharmaceutical companies are focusing on the development of adjuvant therapies. In this review, we review the current status of adjuvant therapies for HCC, and try to explain the challenges and opportunities associated with the development of an adjuvant therapy.
The rationale of adjuvant therapy
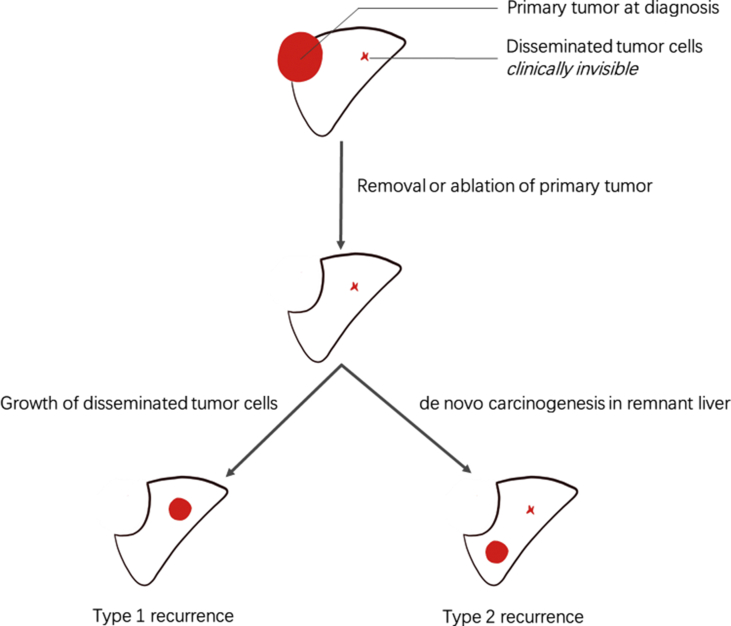
Generally, post-operative tumor recurrence includes intrahepatic tumor metastasis from the primary tumor (type 1 recurrence) and de novo carcinogenesis in the remnant liver tissue (multi-centric occurrence, type 2 recurrence) because of hepatitis or liver cirrhosis from any cause (Fig. 1). Type 1 recurrence usually appears within 1 or 2 years after resection and is associated with the aggressiveness of the primary tumor; type 2 recurrence may occur later, usually after 1–2 years, and is associated with underlying liver disease.14,15 The different risk factors for the two types of recurrence suggested that the target should be different when designing adjuvant therapy for each. For intrahepatic metastasis, the primary strategy should target disseminated tumor cells that have spread from the primary tumor.16 For de novo carcinogenesis, the strategy is to control the progression of hepatitis or cirrhosis (Table 1). An adjuvant therapy targeting type 2 recurrence is not generally suitable for HCC with various etiology. For example, oral anti-HBV nucleotide (nucleoside) analog (NA) decreased tumor recurrence rate for HBV-related HCC,9,10,17 but not for hepatitis C virus (HCV)-related HCC or HCC without hepatitis virus infection.
Figure 1.
Two putative types of tumor recurrence after curative therapy for hepatocellular carcinoma. After surgical removal or ablation of primary tumors, there are two putative types of tumor recurrence. The first one was the growth from the disseminated tumor cells that have been spread from the primary tumor before curative therapy (type 1 recurrence). The other one was from de novo carcinogenesis in the residual liver because of hepatitis or liver cirrhosis (type 2 recurrence).
Table 1.
The strategies to be applied to prevent type 1 and type 2 tumor recurrence.
| Type 1 recurrence | Type 2 recurrence | |
|---|---|---|
| Key mechanism | Growth of disseminated micro-metastasis | de novo carcinogenesis |
| Target population | patients with a high risk of disease recurrence | all patients, especially patients with low risk of disease recurrence |
| Types of treatment | antitumor agents, without regarding for etiology | anti-inflammation agents in the residual liver, concerning the etiology (viral hepatitis or other) |
| Treatment period | short, 1–2 years after surgery | long, life-long |
| Treatment-related toxicity events | toxicity events could be higher | low |
| Examples | tyrosine kinase inhibitor, anti-PD-1 antibody | anti-HBV agents, agents with anti-inflammation effects |
The current status of adjuvant therapies
Although many guidelines do not recommend any adjuvant treatment, the investigator-initiated studies did suggest that some medicines effectively prolong the RFS or overall survival (OS) for HCC patients who underwent curative therapies. All the treatments evaluated as adjuvant therapy for HCC are listed in Table 2, including systemic therapy (tyrosine kinase inhibitor [TKI], IFN, chemotherapy, immunotherapy, and anti-HBV agents), locoregional therapy, traditional Chinese medicine, radiotherapy, and other regimens.
Table 2.
Randomized clinical trials that evaluated an adjuvant therapy for hepatocellular carcinoma.
| Types of treatment | Arms | No. of patients | Improved RFSa | Improved OS | Ref. |
|---|---|---|---|---|---|
| TKI | sorafenib vs. placebo | 1114 | no | no | 11 |
| muparfostat (PI-88) vs. placebo | 519 | no | – | 67 | |
| peretinoin vs. placebo | 377 | no | – | 68 | |
| IFN | IFN-α vs. no treatment, for HBV-related HCC | 236 | no | yes | 6 |
| IFNα-2b vs. no treatment, for viral hepatitis-related HCC | 268 | no | no | 69 | |
| IFNα vs. no treatment, for HCV-related HCC | 30 | yes | – | 70 | |
| IFNα-2b vs. no treatment, | 86 | no | no | 21 | |
| Chemotherapy | Capecitabine vs. no treatment | 60 | yes | no | 71 |
| tegafur/uracil vs. no treatment | 117 | no | no | 72 | |
| tegafur/uracil vs. no treatment | 159 | no | no | 73 | |
| epirubicin + carmofur vs. no treatment | 56 | no | no | 74 | |
| Immunotherapy | CIK vs. no treatment | 230 | yes | yes | 7 |
| autologous dendritic cells vs. no treatment | 156 | no | no | 75 | |
| CIK vs. no treatment | 200 | no | no | 76 | |
| CIK 3 courses vs. CIK 6 courses vs. no treatment | 127 | yes | no | 77 | |
| Autologous formalin-fixed tumor vaccine vs. no treatment | 41 | yes | yes | 78 | |
| Adoptive immunotherapy vs. no treatment | 150 | yes | no | 79 | |
| recombinant interleukin-2 and lymphokine-activated killer cells + adriamycin vs. adriamycin alone | 24 | no | no | 80 | |
| Locoregional therapy | TACE vs. no treatment | 280 | yes | yes | 8 |
| TACE vs. no treatment | 250 | yes | yes | 24 | |
| TACE vs. no treatment | 115 | yes | yes | 81 | |
| HAIC vs. no treatment | 135 | yes | – | 82 | |
| epirubicin hydrochloride + TACE vs. no treatment | 66 | no | no | 83 | |
| pre- and post-operative targeting locoregional chemotherapy-immunotherapy vs. no treatment | 36 | yes | – | 84 | |
| TCM | Huaier granule vs. no treatment | 1044 | yes | yes | 85 |
| Intravenous Cinobufacini + oral Jiedu granule vs. TACE | 364 | yes | yes | 86 | |
| Radiotherapy | external beam radiotherapy vs. no treatment | 119 | no | no | 87 |
| iodine-125 brachytherapy vs. no treatment | 68 | yes | yes | 46 | |
| intra-arterial iodine-131-lipiodol vs. single unlabeled lipiodol | 58 | yes | no | 88 | |
| intra-arterial iodine-131-lipiodol vs. no treatment | 103 | no | no | 89 | |
| intra-arterial iodine-131-lipiodol vs. no treatment | 43 | yes | yes | 90,91 | |
| Anti-HBV | telbivudine vs. no treatment, for serum HBV-DNA < 2000 IU/mL | 200 | yes | yes | 18 |
| adefovir vs. no treatment, for serum HBV-DNA > 2000 IU/mL | 200 | yes | yes | 9 | |
| Lamivudine, adefovir dipivoxil, or entecavir vs. no treatment, for serum HBV-DNA > 500 copies/mL | 163 | yes | yes | 10 | |
| Others | vitamin K2 analog (menatetrenone) vs. no treatment | 101 | yes | no | 92 |
| polyprenoic acid vs. placebo | 89 | yes | – | 93 |
RFS, recurrence-free survival; OS, overall survival; TKI, tyrosine kinase inhibitor; TCM, traditional Chinese medicine; TACE, transcatheter chemoembolization; CIK, chemokine-induced killer cells.
If RFS was not reported, recurrence rate or time to recurrence were evaluated.
Oral anti-HBV agents
A longitudinal study that included a randomized clinical trial (RCT) found that post-operative nucleotide (nucleoside) analog (NA) use decreased tumor recurrence and decreased the incidence of HCC-related death10 in HCC patients with serum HBV-DNA > 500 copies/mL who underwent curative surgery. Another RCT conducted in the same center found that adefovir for patients with serum HBV-DNA >2000 IU/mL9 or telbivudine for those with serum HBV-DNA < 2000 IU/mL18 also prolonged RFS and OS in patients with HBV-related HCC. NA is now the standard of care for chronic hepatitis B patients with positive serum HBV-DNA and HCC. For HBV-related HCC patients with active HBV replication, NA therapy initiated before surgery is now a clinical routine for the purpose of not only lowering the incidence of perioperative reactive of HBV but also lowering the rate of HCC recurrence after surgery.19
Interferon (IFN)-α
In preclinical studies, IFN-α was found to decrease the incidence of tumor recurrence after removal of orthotopically implanted liver tumors.20 Then, an RCT was conducted in patients who underwent curative resection for HBV-related HCC, and found that adjuvant IFN-α prolonged OS (63.8 vs. 38.8 months, p = 0.0003) and RFS (31.2 vs. 17.7 months, p = 0.142) compared with those without adjuvant therapy after surgery.6 In another RCT conducted in Hong Kong, China, IFN-α also showed a trend to prolong the survival time of patients with HBV-related HCC after surgery.21 In a meta-analysis of 9 RCTs and 5 cohort studies, IFN was found to decrease the mortality and recurrence rates in HCV-related HCC, and to decrease mortality but not reduce the recurrence rate in HBV-related HCC.22 A subsequent study revealed that patients whose tumors had low microRNA-26 expression had a better response to adjuvant IFN-α therapy than those who had high expression of microRNA-26.23 Based on these findings, another multi-centric RCT was conducted in patients with low expression of microRNA-26 in the resected tumor samples (NCT01681446).
Transcatheter arterial chemoembolization (TACE)
TACE is the standard of care for patients with intermediate-stage HCC. There's some rationale for TACE to prevent tumor recurrence, e.g., TACE in a combination of digital subtraction angiography and chemo-embolism, therefore, post-operative TACE could find the residual lesions that could not be detected by MRI or CT scan, and immediately embolism could prevent the residual lesion from growing into a clinically visible tumor. In a single-center RCT, patients with HBV-related HCC who had an intermediate or high risk of recurrence after curative surgery were randomized to receive adjuvant TACE therapy or no therapy. Patients who received adjuvant TACE had a higher 3-year RFS (56.0% vs. 42.1%, p = 0.01) and a higher 3-year OS rate than those in the control group (85.2% vs. 77.4%, p = 0.04).8 Another single-center RCT in patients with a solitary tumor ≥5 cm and microvessel invasion also found that adjuvant TACE improved RFS (17.45 vs. 9.27 months, HR = 0.70, p = 0.020) and OS (44.29 vs. 22.37 months, HR = 0.68, p = 0.029).24 Based on these two studies, adjuvant therapy with TACE has become the standard of care for patients with a high risk of tumor recurrence after surgery in some centers.
Anti-PD-1 antibodies
Two anti-PD-1 antibodies, nivolumab and pembrolizumab, have been approved for second-line treatment of advanced HCC by the U.S. Food and Drug Administration, based on the results of two single-arm studies.25,26 Both of these agents demonstrated an objective response rate (ORR) for advanced HCC of about 14%–20% (RECIST v1.1) as a first or second-line treatment with a much lower grade 3 or higher treatment-related adverse effects (19%–26%) than TKIs (sorafenib, 50%; cabozantinib, 68%27,28). However, in the placebo-controlled study (KEYNOTE-240) pembrolizumab as a second-line treatment failed to show statistical significance to improve OS or RFS compared with a placebo.29 Also, in the head-to-head RCT, CheckMate-459, where nivolumab was compared with sorafenib as first-line therapy for advanced HCC, it failed to improve OS.30 Although both anti-PD-1 antibodies failed at their primary endpoints, they did show anti-tumor efficacy and low toxic profile in the phase 3 trials, which were consistent with the findings from the single-arm studies. In the CheckMate 459 study, the frequency of grade 3 or 4 adverse events in the nivolumab arm were about half that of the sorafenib arm (22% vs. 49%).30 Quality of life also significantly favors nivolumab at almost all the time points. Therefore, anti-PD-1 antibodies may be ideal as adjuvant therapies. In fact, almost all the ongoing phase 3 clinical trials for adjuvant therapies (Table 3) evaluate an anti-PD-1 antibody with or without an anti-angiogenic agent. Both nivolumab and pembrolizumab were investigated as adjuvant therapy for HCC (NCT03383458 and NCT03867084). For other solid tumors, immune checkpoint inhibitors (ICIs) have been approved as adjuvant therapy—for example, ipilimumab (an anti-CTLA4 antibody) and pembrolizumab have been approved for melanoma.31,32 It has been predicted that, in the HCC market, adjuvant therapy will account for a half of nivolumab's total sales in 2027.33
Table 3.
The ongoing phase 3 clinical trials evaluating an adjuvant therapy for hepatocellular carcinoma.
| Trial | Arms | Clinicaltrials.gov identifier | Sponsor |
|---|---|---|---|
| CheckMate 9DX | nivolumab vs. placebo | NCT03383458 | BMS |
| KEYNOTE-937 | pembrolizumab vs. placebo | NCT03867084 | MSD |
| EMERALD-2 | durvalumab + bevacizumab vs. durvalumab + placebo vs. placebo | NCT03847428 | AstraZeneca |
| JUPITER 04 | toripalimab (JS001) vs. placebo | NCT03859128 | Junshi |
| IMbrave050 | atezolizumab + bevacizumab vs. no therapy | NCT04102098 | Roche |
The combinational use of neoadjuvant therapy and adjuvant therapy with ICIs produced a remarkable pathological complete response rate. In a phase II study evaluating nivolumab with or without ipilimumab in patients with resectable HCC, the rate of pathologic complete response was 4/14 (29%), with 2 cases in the monotherapy with nivolumab group and 2 cases in the nivolumab plus ipilimumab group.34 As therapy with ICIs is entering the neoadjuvant and adjuvant settings for other resectable solid tumors, such as lung cancer, melanoma, and glioblastoma,35, 36, 37, 38 the usage of these agents may be expanded to more settings in the treatment of HCC.
Molecular-targeted agents
Although several molecular targeting agents have been approved for advanced HCC, the development as adjuvant therapy did not go as expected. The failure of the STORM trial, which evaluated sorafenib as adjuvant therapy in patients who have undergone curative surgery or ablation,11 cast a shadow over the development of adjuvant treatment. The STORM trial is the largest clinical trial to date for adjuvant therapy in the field of HCC. In the STORM trial, 1114 patients who underwent curative treatments were assigned to sorafenib or a placebo group. As for the primary endpoint, the median RFS was not different between the two groups (33.3 vs. 33.7 months, HR = 0.940, p = 0.26). “Wrong stage and wrong dose” were the major criticisms for this trial.39 When the trial was started, sorafenib was the only approved agent for advanced HCC. Nowadays, there are several other choices for advanced HCC, including lenvatinib,40 regorafenib,28 cabozantinib,27 and ramucirumab.41 Lenvatinib has a more potent antitumor activity in comparison with sorafenib (the ORR was 40.6% for lenvatinib compared with 12.4% for sorafenib, as evaluated by masked independent imaging review, per mRECIST42). Ramucirumab has a high safety profile; treatment discontinuation because of adverse events were recorded in 18% of the patients, and hypertension and hyponatremia were the only grade 3 or higher treatment-emergent adverse events of 5% or more.43 In comparison with sorafenib, lenvatinib and ramucirumab may be more suitable for expansion to adjuvant use. However, neither agent is under investigation as adjuvant therapy.
The development of a TKI as an adjuvant therapy also seems unlikely for other solid tumors. The NCCN guidelines for other gastrointestinal cancers, including esophageal and esophagogastric junction cancers, gastric cancer, pancreatic adenocarcinoma, colon cancer, and rectal cancer, did not recommend any TKI as adjuvant therapy after curative therapy. The effects of adjuvant treatment with sunitinib for renal cancer carcinoma were also not conclusive. Although the S-TRAC trial established that a 1-year treatment with sunitinib significantly prolonged the RFS for patients who underwent nephrectomy for renal cancer carcinoma,44 experts did not recommend patients receive adjuvant therapy because of the discrepancy between the S-TRAC trial and the ASSURE trial and because of adverse events with sunitinib.37 We should also balance the benefit and the toxicity of a TKI in the adjuvant setting for an individual patient.
Chemotherapy and radiotherapy
In contrast to other solid malignancies, chemotherapy plays a limited role in the treatment of advanced HCC, except that oxaliplatin plus fluorouracil/leucovorin (FOLFOX4 regimen) was found to prolong patient survival with borderline significance compared with doxorubicin.45 As summarized in Table 1, chemotherapy with capecitabine, tegafur/uracil, or epirubicin plus carmofur were investigated as adjuvant treatment; however, all of them failed to prolong patient survival and only capecitabine was found to prolong patient RFS.
External radiotherapy has no role in adjuvant therapies for HCC. However, when localized radiotherapies are introduced, such as localized implantation with radioactive seeds46 or trans-arterial radioembolization, they may prolong patient RFS and/or OS with controversial data (Table 1).
The major concerns in designing a clinical trial evaluating an adjuvant therapy
All the registered trials in adjuvant settings with a large sample size failed at their primary endpoint. Based on the data of these negative studies and positive trials for other solid tumors, we will discuss the major concerns in designing a clinical trial for adjuvant therapy for HCC. We will discuss these questions based on the PICO framework47 with modification.
Patient selection
Patients who have undergone curative therapy, including surgical resection or ablation, without residual tumor on imaging examination, should be the target population in a trial for adjuvant therapy. However, these patients are quite heterogenic, with different levels of risk of tumor recurrence.
Risk stratification. Although “high risk” or “intermediate risk” of tumor recurrence was used in different adjuvant clinical trials, the criteria of risk stratification were different. In general, large tumor size, multiple nodules, microvessel or gross vascular invasion, and pre-operative elevated serum alpha-fetoprotein were widely accepted risk factors for recurrence. However, there is no consensus on risk stratification. Our retrospective study has found that a “Shanghai Score”48 that integrated 14 clinical parameters could stratify patients with different risks of disease recurrence, and only patients with high risk could benefit from adjuvant TACE, while patients with moderate or high risk could benefit from adjuvant IFN-α. In the design of prospective clinical trials, the scoring system could be integrated into patient stratification, and the score could also be employed to identify a history control for a single-arm phase Ib-II study.
Molecular stratification for targeted therapies. The risk stratification system based on clinicopathological features was a compromise. In the era of targeted oncogene therapy, the ultimate aim was molecular stratification based on the diver oncogene. Currently, this stratification seems unrealistic because there are also no well-accepted molecular stratifications for the approved agents for advanced HCC, including anti-angiogenic agents or anti-PD-1 antibodies. Although some features were found to predict the efficacy of anti-PD-1 antibodies, including PD-L1 expression in tumor tissue and tumor mutation burden, none of them could predict the efficacy in HCC. Ji et al studied the pooled tumor tissue from two RCTs evaluating IFN-α as adjuvant therapy6,21 and found that microRNA-26 expression could predict the effects of IFN-α.23 However, these results need to be validated in a prospective clinical trial (NCT01681446).
A larger sample size. The sample size of an adjuvant trial is always larger than a trial for systemic therapy for advanced disease. For example, in a trial for pembrolizumab for advanced stage melanoma, where active control was used as a control, the sample size for each arm was 27849; in the adjuvant settings, a placebo was used as a control, and the sample size for each arm was more than 500.31 Also, in a clinical trial in which ipilimumab was compared with active control for advanced melanoma, the sample size was 137,50 whereas in the adjuvant settings, where a placebo was used as the control, the sample size of each arm was 476.32 In a trial in which sunitinib was compared with active control for advanced renal cell carcinoma, the sample size was 375,51 whereas in advanced settings, where a placebo was used as the control, the sample size for each arm was 308–647.44,52 At the follow-up period in a clinical trial, if the RFS in the control arm is 50%, the anti-recurrence effects of an investigated agent will be not useful in another 50% of patients without recurrence. Therefore, in theory, the sample size should be enlarged in an RCT for adjuvant therapy as compared with those for advanced disease. Therefore, multi-centric cooperation is necessary.
Intervention
The intervention for intrahepatic metastasis and de novo carcinogenesis should be different (Fig. 1 and Table 1). To suppress the growth of disseminated tumor cells, an anti-tumor agent should be used; to prevent carcinogenesis, a chemoprevention agent is more appropriate. All the approved agents for advanced or unresectable HCC were anti-tumor agents, with the major mechanism of anti-angiogenic and anti-proliferation effects. These agents may be more suitable to prevent metastasis. However, there are other concerns about adjuvant therapy.
The treatment magnitude of an agent. Although sorafenib prolonged the OS with an HR of about 0.7, the ORR is very low (SHARP study 2%53 and Oriental study 3.3%54). Major anti-tumor effects of sorafenib were shown in the endpoint of progression-free survival. Therefore, the low magnitude may be the crucial drawback of sorafenib when used in adjuvant therapy. Lenvatinib showed a much higher ORR than sorafenib in the REFLECT study—the ORR for lenvatinib is 40.6% vs. 12.4% (mRECIST) or 18.8% vs. 6.5% (RECIST v1.1) as compared with sorafenib.42 The higher anti-tumor effects of lenvatinib in advanced-stage HCC should rationally converse to higher RFS rates in an adjuvant trial. More recently, the three most intensively studied PD-1 antibodies for advanced-stage HCC with a sample size larger than 100, including nivolumab, pembrolizumab, and camrelizumab,25,26,55 showed an ORR of 13.8%–20% and a more acceptable safety profile than TKIs, and nivolumab and pembrolizumab were tested in phase 3 adjuvant studies, we could expect a positive trial with caution.
Treatment-related toxicity and drug adherence. In the STORM trial, the drug exposure in the advanced stage was similar between the sorafenib and the placebo group (5.3 vs. 4.3 months),53 whereas in the adjuvant settings the drug exposure was much lower in the sorafenib group than the placebo group (12.5 vs. 22.2 months).11 Without encouragement from a decrease or maintenance in tumor size or serum tumor markers (alpha-fetoprotein or protein induced by vitamin K absence-II), the patients were more likely to discontinue when toxicity events occurred. Also, the median duration of treatment was much shorter than that of median RFS. In the STORM trial,11 the duration of therapy was 12.5–22.2 months, whereas the median RFS was 33.3–33.7 months, meaning that most of the recurrence occurred out of drug exposure. In an adjuvant trial, the treatment period was always set at 1–1.5 years. Therefore, patients with a higher risk of recurrence should be recruited to these trials to let more recurrence events occur during exposure to the investigated agent. Longer-term exposure should produce a higher efficacy. For example, for estrogen receptor-positive early breast cancer, adjuvant therapy with endocrine therapy with tamoxifen for 5 years reduced breast cancer mortality. Continuing tamoxifen to 10 years rather than stopping at 5 years further reduced the recurrence rate and breast cancer mortality.56 Compared with TKIs, an anti-PD-1 antibody seems to be more suitable as an adjuvant therapy because of low toxicity. In the CheckMate-459 study, health-related quality of life of the patients in the nivolumab arm was significantly better than that in the sorafenib arm at almost all time points until 2 years after the initiation of therapy.30 Therefore, treatment adherence of an anti-PD-1 antibody would be better than sorafenib, and the median duration of exposure was predicted to be more than 1 year.
Furthermore, the biomarker study of the STORM trial revealed that patients with negative pERK seem to benefit from sorafenib treatment, which suggested that the results may be caused by chance.57 To predict the RFS in these patients was difficult; only tumor pERK staining and microvessel invasion were independently associated with poor prognosis. For those with early-stage HCC, the biomarkers from adjuvant liver tissue were more predictive than those from tumor tissue,58 suggesting that the non-tumor tissue may play a crucial role in the recurrence of HCC. Therefore, targeting the non-tumor tissue may be another choice for those with type 2 recurrence. Oral anti-HBV NAs were ideal as a chemoprevention therapy to prevent type 2 recurrence.
To this end, ICI seems more suitable than TKI when used as adjuvant therapy. In the treatment of advanced cancers, the duration of response of an ICI was always much longer than those of a TKI mainly because the activated T cells can maintain memory of their target.59 When an anti-PD-1 antibody was used as adjuvant therapy, continuing separation of the survival curve could be observed after the discontinuation of treatment. When adjuvant pembrolizumab for resected melanoma was stopped after 1 year, the cumulative survival curves of RFS continue to separate without a crossover,31 indicating the memorial effects of anti-PD-1 antibodies. The efficacy of TKIs may manifest when the treatment is ongoing; however, after drug discontinuation, the anti-recurrent effects did not sustain. Also, in the adjuvant therapy for melanoma, the cumulative survival curve of RFS crossed over after TKI discontinuation after 1 year.60 Adjuvant sunitinib or pazopanib for renal cancer carcinoma also showed a similar trend.44,61
Comparison/control
There's no standard of adjuvant therapy, and international guidelines do not recommend any adjuvant therapy. Nowadays, in the design of clinical trials for adjuvant therapy, a placebo or no treatment could be used as a control. However, in clinical practice, different centers may incorporate different adjuvant therapies for the management of patients with a high risk of tumor recurrence after surgery based on their single-center studies, especially for the centers where TACE, chemokine-induced killer cells, or other anti-tumor therapies have been proven to prevent tumor recurrence effectively. In the conduction of a clinical trial of adjuvant therapy, we should persuade the investigators to accept no treatment or placebo as a control. Or, the randomization should be at the ratio of 2:1, to encourage more patients to participate in an adjuvant trial.
Outcome: RFS
Adjuvant therapies aim to decrease the incidence of tumor recurrence, including intrahepatic and extrahepatic metastasis, or to prolong long-term survival. To prolong patient survival is the gold standard of anti-tumor therapy. However, RFS is a well-accepted surrogate outcome in an adjuvant trial because treatment strategies vary widely after tumor recurrence, which dilutes the effects of adjuvant therapy.
Recurrence or death are two major events for the evaluation of RFS, and in most patients recurrence occurs first. Therefore, the diagnosis of tumor recurrence should be done with care, and a follow-up plan should be strictly implemented. In registered studies, a blinded independent central review is always incorporated. However, due to limited resources, blinded independent central review is not always available in an investigator-initiated clinical trial. In most clinical trials, the diagnosis of recurrence was referred to the guidelines of the diagnosis of HCC. However, serial evaluation of imaging study with or without serial serum tumor marker increase, the diagnosis of tumor recurrence may find lesions less than 1 cm. For studies with an arm with no treatment, the follow-up plan should be the same as the treatment arm in order not to delay the recurrence diagnosis and therefore delay the RFS time in the control arm.
Adjuvant therapy for Chinese patients
China has the highest tumor burden of HCC in the world. New cases of liver cancer in China (370,000 in 201562) make up more than half of the new cases per year globally (597 thousand).63 Although the age-standardized incidence rate of liver cancer has decreased in China because of the prevention of HBV infection,64 more patients were diagnosed at an early stage, and with the advances of surgical technique, more patients will receive curative therapy. According to Chinese guidelines for the diagnosis and treatment of liver cancer,19 some patients in the BCLC B stage (Chinese stage IIa and IIb) or partly BCLC C stage (Chinese stage IIIa) are candidates for liver resection. These patients are at a higher risk of disease recurrence than patients with BCLC 0 or A stage, and adjuvant therapies for these patients are of higher value. Indeed, Chinese investigators have found various treatments that could be used as adjuvant therapy.
Most Chinese patients have an HBV infection background. Anti-HBV therapy is now widely used in HBV-related HCC patients in China. The REFLECT trial showed lenvatinib might be more effective in hepatitis B virus-infected HCC patients,42 whereas sorafenib may be more effective in hepatitis C virus-infected HCC patients.65 In the phase 3 trial of cabozantinib for second-line therapy for advanced HCC,27 the HR for death was 0.69 in HBV-related HCC patients and 1.11 in HCV-related HCC patients, which suggests that cabozantinib may be more potent for HBV-related HCC. These two agents may be more suitable for HBV-related HCC patients used as a neoadjuvant or adjuvant therapy.
Prospects
HCC is different from other malignancies because the major etiology varies widely among different regions. There is no world widely accepted adjuvant therapy for HCC. Agents targeting type 2 recurrence should be different among various etiologies, whereas agents targeting type 1 recurrence (intrahepatic metastasis) should be universally applicable among different etiologies. As ongoing phase 3 trials are conducted (Table 3), we hope that there will be one approved adjuvant therapy for HCC shortly. Four anti-PD-1 antibodies with low price tags manufactured by Chinese pharmaceutical companies make these drugs more affordable for Chinese patients. An anti-PD-1 antibody, toripalimab, is under investigation as adjuvant therapy for HCC in China (NCT03859128). Also, donafenib, a TKI, showed more potent anti-tumor efficacy than sorafenib in a phase 3 study for patients with advanced HCC (NCT02645981).66 We also expect that a more widely available adjuvant therapy is coming to Chinese patients. These anti-tumor agents may help to lower the incidence of type 1 recurrence. Anti-HBV agents are now intensively used for patients with HBV-related HCC in China; therefore, type 2 recurrence is expected to be lower in the near future.
Conflict of interest
All authors declare no conflict of interest.
Acknowledgments
This work was supported by the Leading Investigator Program of Shanghai municipal government (17XD1401100), the National Key Basic Research Program (973 Program; 2015CB554005) from the Ministry of Science and Technology of the People's Republic of China, and the National Natural Science Foundation of China (81372655, 81472224 and 81672326) to HCS.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Global Burden of Disease Cancer C., Fitzmaurice C., Abate D. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–1768. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou M., Wang H., Zeng X. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–1158. doi: 10.1016/S0140-6736(19)30427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruix J., Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver Electronic address eee, European association for the study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Heimbach J.K., Kulik L.M., Finn R.S. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 6.Sun H.C., Tang Z.Y., Wang L. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV-related hepatocellular carcinoma: a randomized clinical trial. J Canc Res Clin Oncol. 2006;132(7):458–465. doi: 10.1007/s00432-006-0091-y. [DOI] [PubMed] [Google Scholar]
- 7.Lee J.H., Lee J.-H., Lim Y.-S. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148(7):1383–1391. doi: 10.1053/j.gastro.2015.02.055. e1386. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z., Ren Z., Chen Y. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Canc Res. 2018;24(9):2074–2081. doi: 10.1158/1078-0432.CCR-17-2899. [DOI] [PubMed] [Google Scholar]
- 9.Huang G., Lau W.Y., Wang Z.G. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann Surg. 2014;261(1):56–66. doi: 10.1097/SLA.0000000000000858. [DOI] [PubMed] [Google Scholar]
- 10.Yin J., Li N., Han Y. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol. 2013;31(29):3647–3655. doi: 10.1200/JCO.2012.48.5896. [DOI] [PubMed] [Google Scholar]
- 11.Bruix J., Takayama T., Mazzaferro V. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16(13):1344–1354. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 12.Liu C.J., Lee P.H., Lin D.Y. Heparanase inhibitor PI-88 as adjuvant therapy for hepatocellular carcinoma after curative resection: a randomized phase II trial for safety and optimal dosage. J Hepatol. 2009;50(5):958–968. doi: 10.1016/j.jhep.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Zhu X.D., Sun H.C. Emerging agents and regimens for hepatocellular carcinoma. J Hematol Oncol. 2019;12(1):110. doi: 10.1186/s13045-019-0794-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Portolani N., Coniglio A., Ghidoni S. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243(2):229–235. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poon R.T., Fan S.T., Ng I.O., Lo C.M., Liu C.L., Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89(3):500–507. [PubMed] [Google Scholar]
- 16.Xie D.Y., Fan H.K., Ren Z.G., Fan J., Gao Q. Identifying clonal origin of multifocal hepatocellular carcinoma and its clinical implications. Clin Transl Gastroenterol. 2019;10(2):e00006. doi: 10.14309/ctg.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang G., Lai E.C., Lau W.Y. Posthepatectomy HBV reactivation in hepatitis B-related hepatocellular carcinoma influences postoperative survival in patients with preoperative low HBV-DNA levels. Ann Surg. 2013;257(3):490–505. doi: 10.1097/SLA.0b013e318262b218. [DOI] [PubMed] [Google Scholar]
- 18.Huang G., Li P.P., Lau W.Y. Antiviral therapy reduces hepatocellular carcinoma recurrence in patients with low HBV-DNA levels: a randomized controlled trial. Ann Surg. 2018;268(6):943–954. doi: 10.1097/SLA.0000000000002727. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J., Sun H.C., Wang Z. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 edition) Liver Canc. 2018;7(3):235–260. doi: 10.1159/000488035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L., Tang Z.Y., Qin L.X. High-dose and long-term therapy with interferon-alfa inhibits tumor growth and recurrence in nude mice bearing human hepatocellular carcinoma xenografts with high metastatic potential. Hepatology. 2000;32(1):43–48. doi: 10.1053/jhep.2000.8525. [DOI] [PubMed] [Google Scholar]
- 21.Lo C.M., Liu C.L., Chan S.C. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg. 2007;245(6):831–842. doi: 10.1097/01.sla.0000245829.00977.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W., Song T.Q., Zhang T. Adjuvant interferon for early or late recurrence of hepatocellular carcinoma and mortality from hepatocellular carcinoma following curative treatment: a meta-analysis with comparison of different types of hepatitis. Mol Clin Oncol. 2014;2(6):1125–1134. doi: 10.3892/mco.2014.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji J., Shi J., Budhu A. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med. 2009;361(15):1437–1447. doi: 10.1056/NEJMoa0901282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei W., Jian P.E., Li S.H. Adjuvant transcatheter arterial chemoembolization after curative resection for hepatocellular carcinoma patients with solitary tumor and microvascular invasion: a randomized clinical trial of efficacy and safety. Canc Commun. 2018;38(1):61. doi: 10.1186/s40880-018-0331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Khoueiry A.B., Sangro B., Yau T. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu A.X., Finn R.S., Edeline J. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 27.Abou-Alfa G.K., Meyer T., Cheng A.L. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruix J., Qin S., Merle P. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESOURCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 29.Finn R.S., Ryoo B.-Y., Merle P. Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC) J Clin Oncol. 2019;37(15_suppl):4004. [Google Scholar]
- 30.Yau T., Park J.W., Finn R.S. LBA38_PR CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC) Ann Oncol. 2019;30(suppl ment_5) [Google Scholar]
- 31.Eggermont A.M.M., Blank C.U., Mandala M. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 32.Eggermont A.M., Chiarion-Sileni V., Grob J.J. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845–1855. doi: 10.1056/NEJMoa1611299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dawkins J., Webster R.M. The hepatocellular carcinoma market. Nat Rev Drug Discov. 2019;18(1):13–14. doi: 10.1038/nrd.2018.146. [DOI] [PubMed] [Google Scholar]
- 34.Kaseb A.O., Pestana R.C., Vence L.M. Randomized, open-label, perioperative phase II study evaluating nivolumab alone versus nivolumab plus ipilimumab in patients with resectable HCC. J Clin Oncol. 2019;37(4_suppl l) 185-185. [Google Scholar]
- 35.Cloughesy T.F., Mochizuki A.Y., Orpilla J.R. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–486. doi: 10.1038/s41591-018-0337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blank C.U., Rozeman E.A., Fanchi L.F. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24(11):1655–1661. doi: 10.1038/s41591-018-0198-0. [DOI] [PubMed] [Google Scholar]
- 37.Amaria R.N., Reddy S.M., Tawbi H.A. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24(11):1649–1654. doi: 10.1038/s41591-018-0197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forde P.M., Chaft J.E., Smith K.N. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976–1986. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelley R.K. Adjuvant sorafenib for liver cancer: wrong stage, wrong dose. Lancet Oncol. 2015;16(13):1279–1281. doi: 10.1016/S1470-2045(15)00296-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cainap C., Qin S., Huang W.T. Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33(2):172–179. doi: 10.1200/JCO.2013.54.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu A.X., Park J.O., Ryoo B.-Y. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 42.Kudo M., Finn R.S., Qin S. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 43.Zhu A.X., Kang Y.-K., Yen C.-J. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 44.Ravaud A., Motzer R.J., Pandha H.S. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med. 2016;375(23):2246–2254. doi: 10.1056/NEJMoa1611406. [DOI] [PubMed] [Google Scholar]
- 45.Qin S., Bai Y., Lim H.Y. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31(28):3501–3508. doi: 10.1200/JCO.2012.44.5643. [DOI] [PubMed] [Google Scholar]
- 46.Chen K., Xia Y., Wang H., Xiao F., Xiang G., Shen F. Adjuvant iodine-125 brachytherapy for hepatocellular carcinoma after complete hepatectomy: a randomized controlled trial. PloS One. 2013;8(2):e57397. doi: 10.1371/journal.pone.0057397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang X., Lin J., Demner-Fushman D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu Symp Proc. 2006:359–363. [PMC free article] [PubMed] [Google Scholar]
- 48.Sun H.C., Xie L., Yang X.R. Shanghai score: a prognostic and adjuvant treatment-evaluating system constructed for Chinese patients with hepatocellular carcinoma after curative resection. Chin Med J. 2017;130(22):2650–2660. doi: 10.4103/0366-6999.218019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robert C., Schachter J., Long G.V. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 50.Hodi F.S., O'Day S.J., McDermott D.F. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Motzer R.J., Hutson T.E., Tomczak P. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 52.Haas N.B., Manola J., Uzzo R.G. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016;387(10032):2008–2016. doi: 10.1016/S0140-6736(16)00559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Llovet J.M., Ricci S., Mazzaferro V. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 54.Cheng A.L., Kang Y.K., Chen Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 55.Qin S.-K., Ren Z.-G., Meng Z.-Q. LBA27 A randomized multicentered phase II study to evaluate SHR-1210 (PD-1 antibody) in subjects with advanced hepatocellular carcinoma (HCC) who failed or intolerable to prior systemic treatment. Ann Oncol. 2018;29(suppl l_8) mdy424.029. [Google Scholar]
- 56.Davies C., Pan H., Godwin J. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pinyol R., Montal R., Bassaganyas L. Molecular predictors of prevention of recurrence in HCC with sorafenib as adjuvant treatment and prognostic factors in the phase 3 STORM trial. Gut. 2019;68(6):1065–1075. doi: 10.1136/gutjnl-2018-316408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoshida Y., Villanueva A., Kobayashi M. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359(19):1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Long G.V., Hauschild A., Santinami M. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813–1823. doi: 10.1056/NEJMoa1708539. [DOI] [PubMed] [Google Scholar]
- 61.Motzer R.J., Haas N.B., Donskov F. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma. J Clin Oncol. 2017;35(35):3916–3923. doi: 10.1200/JCO.2017.73.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng R., Sun K., Zhang S. Report of cancer epidemiology in China, 2015 [in Chinese] Chin J Oncol. 2019;41(1):19–28. doi: 10.3760/cma.j.issn.0253-3766.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 63.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 64.Liu Z., Mao X., Jiang Y. Changing trends in the disease burden of primary liver cancer caused by specific etiologies in China. Cancer Med. 2019;8(12):5787–5799. doi: 10.1002/cam4.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jackson R., Psarelli E.E., Berhane S., Khan H., Johnson P. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol. 2017;35(6):622–628. doi: 10.1200/JCO.2016.69.5197. [DOI] [PubMed] [Google Scholar]
- 66.Donafenib tosylate achieved statistical significance for its primary endpoint in phase III clinical study of first-line treatment for advanced hepatocellular carcinoma (in Chinese) 2020. http://www.zelgen.com/xinwenzhongxin/2020/01-01/69.html
- 67.Chen P.J., Lee P.H., Han K.H. 624PD A phase III trial of muparfostat (PI-88) as adjuvant therapy in patients with hepatitis virus related hepatocellular carcinoma (HV-HCC) after resection. Ann Oncol. 2017;28(suppl l_5) mdx369.008-mdx369.008. [Google Scholar]
- 68.Okita K., Izumi N., Matsui O. Peretinoin after curative therapy of hepatitis C-related hepatocellular carcinoma: a randomized double-blind placebo-controlled study. J Gastroenterol. 2015;50(2):191–202. doi: 10.1007/s00535-014-0956-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen L.T., Chen M.F., Li L.A. Long-term results of a randomized, observation-controlled, phase III trial of adjuvant interferon Alfa-2b in hepatocellular carcinoma after curative resection. Ann Surg. 2012;255(1):8–17. doi: 10.1097/SLA.0b013e3182363ff9. [DOI] [PubMed] [Google Scholar]
- 70.Kubo S., Nishiguchi S., Hirohashi K. Effects of long-term postoperative interferon-alpha therapy on intrahepatic recurrence after resection of hepatitis C virus-related hepatocellular carcinoma. A randomized, controlled trial. Ann Intern Med. 2001;134(10):963–967. doi: 10.7326/0003-4819-134-10-200105150-00010. [DOI] [PubMed] [Google Scholar]
- 71.Xia Y., Qiu Y., Li J. Adjuvant therapy with capecitabine postpones recurrence of hepatocellular carcinoma after curative resection: a randomized controlled trial. Ann Surg Oncol. 2010;17(12):3137–3144. doi: 10.1245/s10434-010-1148-3. [DOI] [PubMed] [Google Scholar]
- 72.Ishizuka M., Kubota K., Nemoto T. Administration of adjuvant oral tegafur/uracil chemotherapy post hepatocellular carcinoma resection: a randomized controlled trial. Asian J Surg. 2016;39(3):149–154. doi: 10.1016/j.asjsur.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 73.Hasegawa K., Takayama T., Ijichi M. Uracil-tegafur as an adjuvant for hepatocellular carcinoma: a randomized trial. Hepatology. 2006;44(4):891–895. doi: 10.1002/hep.21341. [DOI] [PubMed] [Google Scholar]
- 74.Ono T., Nagasue N., Kohno H. Adjuvant chemotherapy with epirubicin and carmofur after radical resection of hepatocellular carcinoma: a prospective randomized study. Semin Oncol. 1997;24(2 suppl 6) S6-18-S16-25. [PubMed] [Google Scholar]
- 75.Lee J.H., Tak W.Y., Lee Y. Adjuvant immunotherapy with autologous dendritic cells for hepatocellular carcinoma, randomized phase II study. OncoImmunology. 2017;6(7):e1328335. doi: 10.1080/2162402X.2017.1328335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu L., Wang J., Kim Y. A randomized controlled trial on patients with or without adjuvant autologous cytokine-induced killer cells after curative resection for hepatocellular carcinoma. OncoImmunology. 2016;5(3):e1083671. doi: 10.1080/2162402X.2015.1083671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hui D., Qiang L., Jian W., Ti Z., Da-Lu K. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig Liver Dis. 2009;41(1):36–41. doi: 10.1016/j.dld.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 78.Kuang M., Peng B.G., Lu M.D. Phase II randomized trial of autologous formalin-fixed tumor vaccine for postsurgical recurrence of hepatocellular carcinoma. Clin Canc Res. 2004;10(5):1574–1579. doi: 10.1158/1078-0432.ccr-03-0071. [DOI] [PubMed] [Google Scholar]
- 79.Takayama T., Sekine T., Makuuchi M. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356(9232):802–807. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
- 80.Kawata A., Une Y., Hosokawa M. Adjuvant chemoimmunotherapy for hepatocellular carcinoma patients. Adriamycin, interleukin-2, and lymphokine-activated killer cells versus adriamycin alone. Am J Clin Oncol. 1995;18(3):257–262. doi: 10.1097/00000421-199506000-00014. [DOI] [PubMed] [Google Scholar]
- 81.Zhong C., Guo R.P., Li J.Q. A randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for Stage III A hepatocellular carcinoma. J Canc Res Clin Oncol. 2009;135(10):1437–1445. doi: 10.1007/s00432-009-0588-2. [DOI] [PubMed] [Google Scholar]
- 82.Asahara T., Itamoto T., Katayama K. Adjuvant hepatic arterial infusion chemotherapy after radical hepatectomy for hepatocellular carcinoma--results of long-term follow-up. Hepato-Gastroenterology. 1999;46(26):1042–1048. [PubMed] [Google Scholar]
- 83.Lai E.C., Lo C.M., Fan S.T., Liu C.L., Wong J. Postoperative adjuvant chemotherapy after curative resection of hepatocellular carcinoma: a randomized controlled trial. Arch Surg. 1998;133(2):183–188. doi: 10.1001/archsurg.133.2.183. [DOI] [PubMed] [Google Scholar]
- 84.Lygidakis N.J., Pothoulakis J., Konstantinidou A.E., Spanos H. Hepatocellular carcinoma: surgical resection versus surgical resection combined with pre- and post-operative locoregional immunotherapy-chemotherapy. A prospective randomized study. Anticancer Res. 1995;15(2):543–550. [PubMed] [Google Scholar]
- 85.Chen Q., Shu C., Laurence A.D. Effect of Huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial. Gut. 2018;67(11):2006–2016. doi: 10.1136/gutjnl-2018-315983. [DOI] [PubMed] [Google Scholar]
- 86.Zhai X.F., Liu X.L., Shen F., Fan J., Ling C.Q. Traditional herbal medicine prevents postoperative recurrence of small hepatocellular carcinoma: a randomized controlled study. Cancer. 2018;124(10):2161–2168. doi: 10.1002/cncr.30915. [DOI] [PubMed] [Google Scholar]
- 87.Yu W., Wang W., Rong W. Adjuvant radiotherapy in centrally located hepatocellular carcinomas after hepatectomy with narrow margin (<1 cm): a prospective randomized study. J Am Coll Surg. 2014;218(3):381–392. doi: 10.1016/j.jamcollsurg.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 88.Dumortier J., Decullier E., Hilleret M.N. Adjuvant intraarterial lipiodol or (1)(3)(1)I-lipiodol after curative treatment of hepatocellular carcinoma: a prospective randomized trial. J Nucl Med. 2014;55(6):877–883. doi: 10.2967/jnumed.113.131367. [DOI] [PubMed] [Google Scholar]
- 89.Chung A.Y., Ooi L.L., Machin D. Adjuvant hepatic intra-arterial iodine-131-lipiodol following curative resection of hepatocellular carcinoma: a prospective randomized trial. World J Surg. 2013;37(6):1356–1361. doi: 10.1007/s00268-013-1970-4. [DOI] [PubMed] [Google Scholar]
- 90.Lau W.Y., Lai E.C., Leung T.W., Yu S.C. Adjuvant intra-arterial iodine-131-labeled lipiodol for resectable hepatocellular carcinoma: a prospective randomized trial-update on 5-year and 10-year survival. Ann Surg. 2008;247(1):43–48. doi: 10.1097/SLA.0b013e3181571047. [DOI] [PubMed] [Google Scholar]
- 91.Lau W.Y., Leung T.W., Ho S.K. Adjuvant intra-arterial iodine-131-labelled lipiodol for resectable hepatocellular carcinoma: a prospective randomised trial. Lancet. 1999;353(9155):797–801. doi: 10.1016/s0140-6736(98)06475-7. [DOI] [PubMed] [Google Scholar]
- 92.Mizuta T., Ozaki I., Eguchi Y. The effect of menatetrenone, a vitamin K2 analog, on disease recurrence and survival in patients with hepatocellular carcinoma after curative treatment: a pilot study. Cancer. 2006;106(4):867–872. doi: 10.1002/cncr.21667. [DOI] [PubMed] [Google Scholar]
- 93.Muto Y., Moriwaki H., Ninomiya M. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N Engl J Med. 1996;334(24):1561–1567. doi: 10.1056/NEJM199606133342402. [DOI] [PubMed] [Google Scholar]