Abstract
Diabetes Mellitus is an endocrine disorder which causes insulin deficiency. Medicinal plants are documented to be efficacious in the management of the disease. The current research set to determine the phytochemicals present, anti-oxidant activity and investigate the potency of Eriobotrya japonica against α-amylase inhibition. The leaves of the plant were extracted sequentially. The different extracts were evaluated for the presence of phytochemicals and their potential anti-oxidant and α-amylase inhibition activity. Methanol with the highest polarity, gave the highest yield of 20% and hexane with the lowest polarity have the lowest yield of 2.09%. This trend resembled that observed for the total flavanoid and total phenolic content analysis which gave values of 0.3822 mg QAE/mg and 3.810 mg GAE/mg respectively for methanolic and hexane extracts. The extracts of methanol recorded higher DPPH free radical scavenging activity of 87% and gave the lowest IC50 of 0.5336 which was below that of ascorbic acid used as a control. Hexane extract had a higher α-amylase inhibitory activity of 24% at 1 μg/ml as compared to other extracts. Generally hexane extracts of Eriobotrya japonica exhibits mild inhibitory activity against α-amylase enzyme which is recommended than the conventional therapy which maximally inhibits the enzyme causing major side effects. The results obtained herein support the use of the plant as an anti-diabetic agent at higher concentrations.
Keywords: Food science, Food analysis, Pharmaceutical chemistry, Diabetes, α-amylase, Hyperglycaemia, Phytochemicals, Monosaccharides
Food science; Food analysis; Pharmaceutical chemistry; Diabetes; α-amylase; Hyperglycaemia; Phytochemicals; Monosaccharides.
1. Introduction
Diabetes mellitus is an acquired or inherited chronic disease that develops when there is insulin deficiency or the organs in the body are not sensitive to insulin produced or both. The latter often results in increased glucose levels in the blood stream which damages the body system (Vadivelan et al., 2019). Hyperglycaemia often damages the body's natural antioxidant defence system thus inducing oxidative stress. High glucose level induces oxidative stress through processes such as glucose protein glycation, Advanced glycation end (AGE) products formation and auto-oxidation. Oxidative stress has also been reported to cause diabetes, thus one of the ways in which diabetes can be prevented is by improving the antioxidant system (Wang and Zhao, 2019). The disease can be categorised as type one diabetes mellitus (T1DM) and type two diabetes mellitus (T2DM) depending on several factors. The prevalence of T2DM is documented to be a worldwide problem with about 90–95% of total patients (Gunsel et al., 2020a, 2020b). About 463 million people were suffering from the disease in 2019 and this figure is expected to increase to more than half a million in the next two decades (Guariguata et al., 2014). As the number of diabetic patients is projected to increase worldwide, so are the costs required to for treatment of the disease (Anyanwu et al., 2019). Type 2 diabetes can be managed through prescribed medication such as hydrolysing carbohydrates inhibitors (Acarbose and miglitol), which decreases the exaggerated increase in the glucose level that follows a meal (post prandial hyperglycaemia) (Nebih et al., 2020; Gulcin et al., 2020). This is achieved by slowing down the rate in which glucose is absorbed into the blood system blocking the enzymes needed for hydrolysing complex carbohydrates (starch) to absorbable monosaccharides (Turkan et al., 2020). Alpha amylase located in the saliva and in the pancreatic juice, helps in the digestion of polysaccharides into glucose and maltose. Inhibiting alpha amylase is achieved through binding of polyphenols onto the enzyme through hydrogen bonding. Ideal treatment for diabetes is one that maintain normal glucose level both at fasting and prandial state with minimum or no side effects (Bicer et al., 2020; Gunsel et al., 2020a; Altay et al., 2019). Currently used medications such as metfomin, glebenclamide and galvasmet among others have been associated with side effects such abdominal pains and their inability to maintain normal glucose level (Anyanwu et al., 2019). The prescribed medication has also been reported to be costly and is not easily accessible for people staying in rural areas (Anyanwu et al., 2019). To overcome the drawback associated with all the prescribed medication for type 2 diabetes, patients are going back to the use of natural products for their primary health care. Natural products have been reported to be safer and effective compared to pharmaceutical drugs, however minimum scientific or medical evaluation have been done to evaluate their efficacy (Sanni et al., 2019).
Loquats scientifically known as Eriobotrya japonica belongs to the family Rosaceae. E. japonica is native to China, however it is cultivated in many regions of the world such as South Africa, India and South America. The plant possess pharmacological activities, including antioxidant, anti-cancer and anti-hyperglycaemic activity (Ahumada et al., 2017; Harborne, 1998). However, not much has been reported on the plants ability to inhibit α-amylase the carbohydrate hydrolysing enzyme. Therefore this study envisages performing phytochemical screening of the crude plant extract and evaluating the anti-oxidant and α-amylase inhibitory activity of E. Japonica.
2. Materials and methods
2.1. Collection of plant material
The leaves of the plant were collected in Vanderbijlpark, South Africa during the month of August 2018 and deposited at the botanical gardens in Pretoria, South Africa. The entire study took about six months to be completed. The material was dusted then cleaned with running tap water before being dried at room temperature. The dried leaves were then ground into fine powder in readiness for extraction.
2.2. Sample preparation for extraction
The air dried and ground leaves of E. japonica (10g) were extracted with 100 ml of solvents of increasing polarity for 24 h in an orbital shaker. The crude plant extracts were then filtered and concentrated to a constant weight. The crude extracts were further weighed to evaluate the % yield.
2.3. Qualitative phytochemical analysis
Qualitative screening of phytochemicals of E. japonica extract was done to detect the presence of tannins, phenols, saponins, flavonoids, alkaloids, proteins, carbohydates, steroids and glycocides using the standards method of Harborne (1998).
2.4. Determination of total phenolic content
This was determined by the folin ciocalteu method with slight modifications (Kim et al., 2010; Wolfe et al., 2003). Approximately, 1 mL of the crude plant extract was mixed with 5 mL Folin Ciocalteu reagent, followed by addition of 20% w/v of Na2CO3. The mixture was then incubated for 30 min and the absorbance measured at 720 nm. A calibration curve prepared from gallic acid which was the standard was used to calculate the total phenolic content in mg/g gallic acid equivalence.
2.5. Determination of total flavonoids
Total flavonoid content (TFC) from the plant extracts were determined using the procedure of Ramamoorthy and Bono (2007). Briefly, 0.5 mL of the crude extract was mixed with 0.5 mL of 2% methanolic solution aluminium chloride (AlCl3). The appearance of a yellow colour illustrates the presence of phenols in the plant extract. Absorbance was measured using UV-Vis spectrophotometer at 720 nm. The TFC was expressed as quercetin equivalent (mg/g), and this was calculated from the calibration curve.
2.6. In-vitro anti-oxidant assays
2.6.1. 1.1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging assay
The free radical scavenging activity of E. Japonica extracts were measured using DPPH as a free radical model following documented methods (Bursal and Gulcin, 2011; Polat Kose et al., 2015; Pothitirat et al., 2009). Plant extract with different concentration ranging from (0.125–1 μg/mL) was prepared from the stock solutions. Approximately, 3 mL of 5 μg/mL DPPH was mixed with 1 mL of the plant extract and incubated in the dark for 30 min at room temperature. Ascorbic acid was used as the standard while the absorbance was measured at 517 nm using UV-vis spectrophotometer. The free radical scavenging activity of each extract was evaluated by comparing its absorbance with that of a blank solution. The ability to scavenge the DPPH radical was expressed as percentage inhibition and calculated using Eq. (1) below.
| (1) |
2.6.2. Ferric reducing anti-oxidant power (FRAP) assay
The ferric reducing power of the crude extracts of E. japonica was evaluated following documented methods (Gulcin, 2006, 2007). To approximately 3.0 mL of phosphate buffer, 1.0 mL of the crude extract and 1% w/v potassium ferricynide was added to the mixture, the mixture was incubated at 50 °C for 20 min. The pH of the mixture lowered by adding several drops of 10% trichloroacetic acid. To 1 mL of the mixture, 1 mL of distilled water and 0.5 mL of 0.1% iron chloride were added. The absorbance of the mixture was measured at 700 nm and Eq. (2) below was used to calculate the FRAP.
| (2) |
2.6.3. Inhibition of α –amylase by the crude extracts
Iodine-starch test was conducted, to test the plants ability to inhibit α –amylase enzyme. This was done according to the method developed by Bashary and Khatik (2019). The procedure depends on the formation of a blue or violet complex when iron reacts with starch acarbose that was used as a standard. Working solutions were made by mixing 0.2 mL of the plant extracts with 0.4 mL of the 1% starch before incubation at 37 °C. Specific amounts of α –amylase was added to the mixture and further incubated for 15 min. This was then terminated by adding 0.8 mL of 0.1 M HCl, followed by addition 200 μL of 1 μM iodine solution. The % inhibition was calculated using Eq. (3) below.
| (3) |
3. Results and discussion
3.1. Quantitative screening pf phytochemicals
Qualitative phytochemical screening results are presented in Table 1. The identified phenols, flavonoids and tannins in the extracts is of interest since it is reported in literature that these bioactive compounds are responsible for the α-amylase inhibitory activity and its antioxidant activity.
Table 1.
Phytochemical screening results of the E. japonica leave extracts.
| Phytochemicals | Extracts |
|||
|---|---|---|---|---|
| Methanol | Acetone | Ethyl acetate | Hexane | |
| Phenols | + | + | + | + |
| flavonoids | + | + | + | + |
| Tannins | + | + | - | - |
| Saponins | - | + | - | - |
| Alkaloids | + | + | + | + |
| Proteins | + | + | + | + |
| Carbohydrates | + | + | + | + |
| Glycosides | + | + | + | + |
| Steroids | + | + | + | + |
Key: (+) = Present, (-) = Absent.
3.2. Surface morphological analysis of the crude extracts
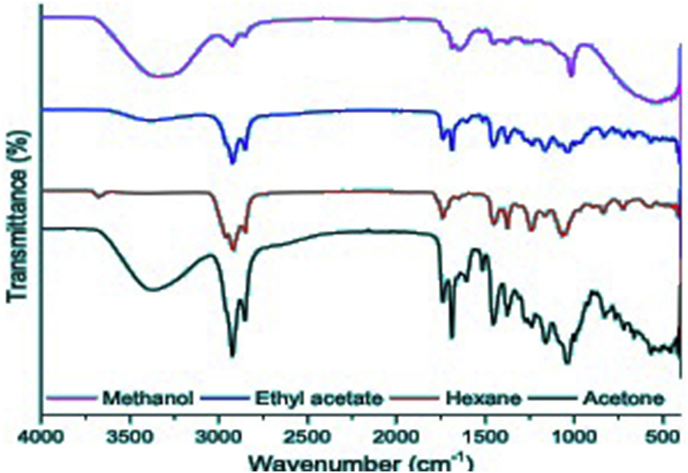
The changes in chemical functionality were obtained by FTIR spectroscopy which was used for the analysis of the functional groups after extraction using different solvents. The FTIR spectra of methanol, ethyl acetate, hexane and acetone extracts of E.japonica are shown in Figure 1. A prominent hydroxyl group peak at 3342 cm−1 was observed in all the crude extracts, except for the hexane crude extracts. The OH− group was not detected and this might be attributed to the poor polarity of hexane. A peak at 1050, 1715 and 2990 cm−1 is due to C–O–C, C=O and C–H respectively.
Figure 1.
Fourier Transform Infrared sprectra of methanol, acetone, ethyl acetate and hexane extracts of Eriobotrya japonica.
3.3. Quantitative phytochemical screening
The total phenols, flavonoids and yield of the various extracts are presented in Table 2. There are several methods available for obtaining phytochemicals (Stalikas, 2007). Methanol, ethyl acetate, acetone and hexane extract showed differences in terms of their yield. It was observed that the yield of methanol extract was higher compared to that of acetone, ethyl acetate and hexane, thus the higher the polarity the more the extraction yield. Methanol has a relative polarity of 0.762, Acetone 0.355 while the one for hexane is 0.009. These differences may be due to increased solubility of carbohydrates and proteins in the higher polar solvent than in the other solvents (Zielinski and Kozlowska, 2000). Even though both methanol and acetone are polar, methanol is considered more polar than acetone since methanol is capable of dipole-dipole interaction and hydrogen bonding while acetone is only capable of hydrogen bonding. These results are in agreement with Diem et al. (2014) who studied the effects of selected solvents on phytochemicals and antioxidant efficacy of L. aromatica. Similar trends in the yields were reported in his study and he attributed the observations to the above reasons.
Table 2.
The yield, total phenols and total flavonoid content of E. japonica extracts.
| Extract | Yield (%) | Total phenols (mg GAE/g) | Total flavonoids (mg QCE/g) |
|---|---|---|---|
| Methanol | 20.20 | 3.810 ± 0.0036 | 0.3833 ± 0.0016 |
| Acetone | 9.59 | 3.680 ± 0.0019 | 0.1464 ± 0.0010 |
| Ethyl acetate | 6.79 | 1.606 ± 0.0012 | 0.0394 ± 0.0011 |
| Hexane | 2.09 | 1.2334 ± 0.0027 | 0.0098 ± 0.0006 |
GAE = gallic acid equivalent, QCE = quercetin, value ± SD, n = 3.
The phenolic compounds in leave extract varied from 1.233 ± 0.0027 to 3.810 ± 0.0036 mg GAE/g with the hexane extract having the least (P ≤ 0.05) amount among the studied solvents. The results obtained herein are very low compared to those obtained by Song et al. (2010), who reported 31.47 ± 0.48 mg GAE/g in leaves of one of the Eriobotrya japonica cultivars. Hong et al. (2008), equally obtained values of 54.9 ± 2.40 mg GAE/g in leaves from different cultivars. The total flavonoid content in this study ranged from 0.0098 ± 0.0006 to 0.3822 ± 0.0065 mg QAE/g. There was also a difference (P ≤ 0.05) on the flavonoid levels of the different extracts. Just like for total phenols, methanol extract recorded the highest flavonoid content while hexane gave the lowest. Zhou et al., (2011) recorded flavonoid content of 3.01 ± 0.13 mg from the golden nugget cultivar of the Eriobotrya japonica from China; however, this value was higher than the flavonoid content reported in this study. These great differences observed may be significantly influenced by the cultivar type and environmental factors (Ahumada et al., 2017). Phenolics have essential function in the adaptation of plants to stress and UV-light radiation, among other factors, increasing its biosynthesis under stress conditions (Oh et al., 2009). Eriobotrya japonica is normally grown in regions of warmer sub-tropical climates like Japan, China, Brazil and Korea (Ahumada et al., 2017), therefore, its cultivation in colder conditions or the four seasons observed in South Africa could likely explain the lower contents of the total phenols and flavonoids. Furthermore the sampling of the leaves of the Eriobotrya japonica was done during winter season which could have heavily contributed to the low levels recorded.
3.4. Antioxidant activity
Several methods are documented that assist in the determination of the anti-oxidative capability of plant species. The challenges of oxidation and reduction processes have made it difficult to have a single method capable of summarizing the anti-oxidative profile of any plant sample (Parejo et al., 2002). Several methods are recommended inassessing the anti-oxidative property for an appropriate conclusion (Cetin Cakmak and Gulcin, 2019; Gulcin, 2020; Taslimi and Gulcin, 2018). In this study, DPPH and FRPA were used to determine the anti-oxidative power of the Eriobotrya japonica crude extracts. The results of the former are presented in Table 3. Methanol was observed to have the highest free radical scavenging activity of 87.06 ± 1.50% at a concentration of 10 μg/ml. This was closely followed by acetone crude extract while ethyl acetate and hexane extracts showed very poor anti-oxidative activity. The DPPH free radical scavenging for the studied extracts depended on the dosage used. The IC50 value was calculated from the dosage curve. Hexane extract recorded the highest IC50 value of 1.5422 mg/L while methanol extract had the lowest value of 0.5336 mg/L. The reference standard used was Ascorbic acid which recorded an IC50 figure of 0.7155 mg/L.
Table 3.
IC50 values for antioxidant activity of the Eriobotrya japonica.
| Extracts | IC50 mg/L |
|
|---|---|---|
| DPPH radical scavenging | Ferric reducing anti-oxidant power | |
| Methanol | 0.5336 | 0.2341 |
| Acetone | 0.5376 | 0.3020 |
| Ethyl acetate | 1.1290 | 1.2342 |
| Hexane | 1.5422 | 1.7233 |
| Ascorbic acid | 0.7155 | 0.3214 |
The reduction potential of the different extracts of Eriobotrya japonica leaves at a concentration of 0.5 mg/L is shown in Table 3. The methanol extract still showed the highest reducing power just like in the DPPH free radical scavenging activity. The increase in polarity of the solvent was observed to increase the reducing power which was also directly proportional with the increase in the concentration of the phenols and flavonoids. Antioxidant activity and the phenolic content are documented to have a positive correlation (Aklima et al., 2014; Velioglu et al., 1998). It is observed herein that, an increase in the concentration of the total phenols is inversely proportional to the reciprocal of IC50 values and this indicated an increase in the phenolic content led to an increase in the anti-oxidative activities (Duh et al., 1999). Correlation was also found between phenolic content and reducing power. The antioxidants can be reducing agents capable of inactivating the oxidants during acid-base reactions which causes the reactant to be reduced and not oxidizing another species. The presence of the antioxidants which act as reductants in samples, causes the reduction of the Fe3+/ferricyanide complex to the ferrous form (Tuba and Gulcin, 2008; Gulcin, 2009, 2010).
3.5. Inhibition of α-amylase enzyme using E. japonica leaf extracts
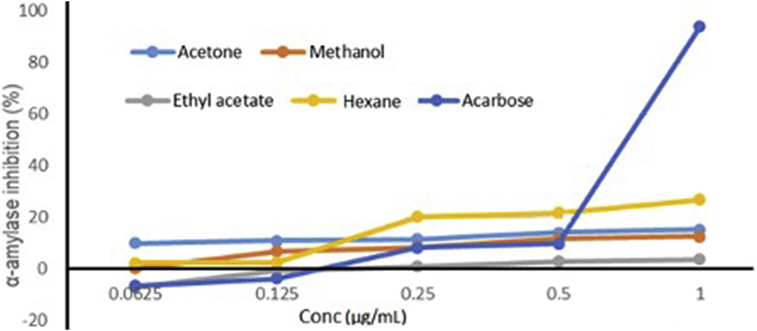
All the crude extracts of E. japonica from different solvents were observed to have α-amylase inhibitory activity that is concentration dependent as shown in Figure 2. Hexane extract was found to have a higher inhibitory activity of about 24% against the α-amylase enzyme at 1 μg/mL. These results however, show little inhibition activity against α-amylase. Higher inhibition activity was observed in acarbose which was used as a standard and showed an inhibition close to 100%.
Figure 2.
Inhibitory activity of α-amylase using E. japonica leaf extract prepared from different solvents.
The higher inhibitory activity of the α-amylase observed using hexane extract has been reported in several other studies from different plants (Vadivelan et al., 2019). The inhibition activity of α-amylase inhibition using different extracts of Asparagus racemosus Willd have been reported. In all the extracts, dosage was a factor that affected the inhibition of α-amylase with n-hexane having the highest inhibition activity despite showing lower levels of the flavonoids and phenols. In their study, Acarbose still showed the highest inhibition of α-amylase. These researchers attributed the potential of anti-diabetic activity of this herb several active phytochemicals ranging from terpenes and flavonoids among many other, though at this point it cannot be clear (Vadivelan et al., 2019). The current study equally showed that the leaves of E. Japonica, are potential inhibitors of α-amylase and thus can be used for T2DM.
Several other researches have documented phenolic compounds exercising anti-diabetic activity by inhibiting α-amylase and α-glucosidase (Aklima et al., 2014). Finding inhibitors of α-amylase from natural products may be more safer than the conventional therapy (Kim et al., 2005). Phenolic synergies have been documented to assist in inhibiting α –amylase thus contributes towards managing T2DM (Kwon et al., 2006; Apostolidis et al., 2007). Ahumada et al., (2017) also found a high correlation (P < 0.05) between in vitro property of α-amylase inhibitory with the antioxidant capacity evaluated by the DPPH. He indicate that phenolic compounds and flavonoids such as quercetin derivatives found in higher levels of the E. Japonica flowers would be related not only to their high antioxidant capacity, but also to its high inhibitory activity against α-amylase.
The α –amylase inhibition helps increase the rate in which complex starch is broken down to glucose however, the more the inhibition for instance by Acarbose, the more the side effects like intestinal disorder (Kwon et al., 2006). Inhibition of α-amylase in excess by Acarbose causes a release of starch in its undigested form into the lower gastrointestinal tract whereby abnormal fermentation of the undigested starch by the intestinal micro flora takes place, causing abdominal discomforts and diarrhoea. Thus, moderate inhibition of α–amylase is desired. This study therefore considers the moderate inhibition of α–amylase by hexane extract of E. Japonica, might not induce side effects caused by presently used prescribed medication for type 2 diabetes.
4. Conclusion
Hexane, acetone, ethyl acetate and methanol plant extracts were found to contain major compounds such as phenols, flavonoids and tannins. Methanol extracts were also found to have higher phenolic and flavonoid content followed by acetones crude extracts. The DPPH free radical scavenging was observed to be higher in the methanol extract. On the other hand, α–amylase inhibition of the enzyme was higher with the hexane extracts. The inhibition of about 24% observed by the hexane extract is considered to be mild which is recommended to avoid the side effects associated with maximum inhibition of the enzyme.
4.1. Significance statement
This study discovered the leaves of E. Japonica plant from South Africa has mild inhibitory effects on α-amylase which is recommended for managing T2DM. This study will help the researchers to uncover the critical areas of diabetes management using natural products that that will help the populace. Thus a new drug can be obtained by combining these extract with one that will completely inhibit α-glucosidase.
Declarations
Author contribution statement
Lebogang Mogole: Performed the experiments; Analyzed and interpreted the data.
Wesley Omwoyo: Analyzed and interpreted the data; Wrote the paper.
Fanyana Mtunzi: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to thank the Research Directorate and the Department of Chemistry, Vaal University of Technology for the support offered to accomplish this study.
References
- Ahumada J., Fuentealba C., Olaeta J., Undurraga P., Pedreschi R., Shetty K., Chirinos R., Campos D., Ranilla L. Bioactive compounds of loquat (Eriobotrya japonica Lindl.) cv. Golden Nugget and analysis of in vitro functionality for hyperglycemia management. Ciena Invest. Agraria. 2017;44(3):272–284. [Google Scholar]
- Aklima J., Mojumder S., Sikdar D. Total phenolic content, reducing power, antioxidative and anti-amylase activities of five Bangladeshi fruits. Int. Food Res. J. 2014;21(1):119–124. [Google Scholar]
- Altay A., Tohma H., Durmaz L., Taslimi P., Korkmaz M., Gülçin İ., Koksal E. Preliminary phytochemical analysis and evaluation of in vitro antioxidant, antiproliferative, antidiabetic and anticholinergics effects of endemic Gypsophila taxa from Turkey. J. Food Biochem. 2019;43(7) doi: 10.1111/jfbc.12908. [DOI] [PubMed] [Google Scholar]
- Anyanwu G.O., Iqbal J., Khan S.U., Zaib S., Rauf K., Onyeneke E. Antidiabetic activities of chloroform fraction of Anthocleista vogelii Planch root bark in rats with diet and alloxan-induced obesity-diabetes. J. Ethnopharmacol. 2019;229:293–302. doi: 10.1016/j.jep.2018.10.021. [DOI] [PubMed] [Google Scholar]
- Apostolidis E., Kwon Y., Shetty K. Inhibitory potential of herb, fruit and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov. Sci. Emerg. Tech. 2007;8(1):46–54. [Google Scholar]
- Bashary R., Khatik G.L. Design, and facile synthesis of 1,3 diaryl-3-(arylamino)propan-1-one derivatives as the potential alpha-amylase inhibitors and antioxidants. Bioorg. Chem. 2019;82:156–162. doi: 10.1016/j.bioorg.2018.10.010. [DOI] [PubMed] [Google Scholar]
- Biçer A., Kaya R., Yakali G., Gültekin M.S., Turgut Cin G., Gülçin İ. Synthesis of novel β-amino carbonyl derivatives and their inhibition effects on some metabolic enzymes. J. Mol. Struct. 2020;1204:1274–1283. [Google Scholar]
- Bursal E., Gulcin I. Polyphenol contents and in vitro antioxidant activities of lyophilized aqueous extract of kiwifruit (Actinidia deliciosa) Food Res. Int. 2011;44(5):1482–1489. [Google Scholar]
- Çetin Çakmak K., Gülçin İ. Anticholinergic and antioxidant activities of usnic acid-An activity-structure insight. Toxicol. Rep. 2019;6:1273–1280. doi: 10.1016/j.toxrep.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diem D., Angkawijaya A., Tran-Nguyen P., Huynh L., Soetaredjo F., Ismadji S., Ju Y. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014;2(2):296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duh P., Tu Y., Yen G. Antioxidant activity of water extract of harng Tyur (Chrysanthemum morifolium Ramat.) Lebensm. Wiss.u- Technol. 1999;32:269–277. [Google Scholar]
- Guariguata L., Whiting D.R., Hambleton I., Beagley J., Linnenkamp U., Shaw J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014;103(2):137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Gulcin I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid) Toxicology. 2006;217(2-3):213–220. doi: 10.1016/j.tox.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Gulcin I. Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa. Amino Acids. 2007;32:431–438. doi: 10.1007/s00726-006-0379-x. [DOI] [PubMed] [Google Scholar]
- Gulcin I. Antioxidant activity of L-Adrenaline: an activity-structure insight. Chem. Biol. Interact. 2009;179(2-3):71–80. doi: 10.1016/j.cbi.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Gulçin I. Antioxidant properties of resveratrol: a structure-activity insight. Innovat. Food Sci. Emerg. Technol. 2010;11:210–218. [Google Scholar]
- Gulcin I. Antioxidants and antioxidant methods-An updated overview. Arch. Toxicol. 2020;94(3):651–715. doi: 10.1007/s00204-020-02689-3. [DOI] [PubMed] [Google Scholar]
- Gülçin İ., Gören A.C., Taslimi P., Akyuz B., Tüzün B. Anticholinergic, antidiabetic and antioxidant activities of Anatolian pennyroyal (Mentha pulegium)-Analysis of its polyphenol contents by LC-MS/MS. Biocatal. Agricul. Biotech. 2020;23:1014–1041. [Google Scholar]
- Günsel A., Bilgiçli A.T., Barut B., Taslimi P., Özel A., Gulçin İ., Bıyıklıoğlu Z., Yarasir M.N. Synthesis of water soluble tetra-substituted phthalocyanines: investigation of DNA cleavage, cytotoxic effects and metabolic enzyme inhibition. J. Mol. Struct. 2020;214:1282. -100. [Google Scholar]
- Günsel A., Yaşa Atmaca G., Taslimi P., Bilgiçli A.T., Gulçin İ., Erdoğmuş A., Yaraşır M.N. Synthesis, characterization, photo-physicochemical and biological properties of water-soluble tetra-substituted phthalocyanines: antidiabetic, anticancer and anticholinergic potentials. J. Photochem. Photobiol. Chem. 2020;396:1125–1131. [Google Scholar]
- Harborne B. Phytochemical methods; A guide to modern techniques of plant analysis. J. Chem. Inf. Model. 1998;3:71–92. [Google Scholar]
- Hong Y., Lin S., Jiang Y., Ashraf M. Variation in contents of total phenolics and flavonoids and antioxidant activities in the leaves of Eriobotrya species. Plant Foods Hum. Nutr. 2008;63:200–204. doi: 10.1007/s11130-008-0088-6. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Kwon Y.S., Chun W.J., Kim T.Y., Sun J., Yu C.Y., Kim M.J. Rhus verniciflua Stokes flavonoid extracts have anti-oxidant, anti-microbial and α-glucosidase inhibitory effect. Food Chem. 2010;120(2):539–543. [Google Scholar]
- Kim Y., Jeong Y., Wang M., Lee W., Rhee H. Inhibitory effect of pine extracts on α-glucosidase activity and postprandial hyperglycemia. Nutrition. 2005;21(6):756–761. doi: 10.1016/j.nut.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Kwon Y., Vattem D., Shetty K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac. J. Clin. Nutr. 2006;15:107–118. [PubMed] [Google Scholar]
- Nebih L., Suleyman A., Cuneyt T., Parham T., Mesut I., Sukru B., Gulcin I., Mustafa D. Synthesis, characterization, inhibition effects, and molecular docking studies as acetylcholinesterase, α-glycosidase, and carbonic anhydrase inhibitors of novel benzenesulfonamides incorporating 1,3,5-triazine structural motifs. Bioorg. Chem. 2020;100:1038–1097. doi: 10.1016/j.bioorg.2020.103897. [DOI] [PubMed] [Google Scholar]
- Oh M.M., Carey E.E., Rajashekar C. Environmental stresses induce health-promoting phytochemicals in lettuce. Plant Physiol. Biochem. 2009;47:578–583. doi: 10.1016/j.plaphy.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Parejo I., Viladomat F., Bastida J., Rosas-Romero A., Flerlage N., Burillo J., Codina C. Comparison between the radical scavenging activity and antioxidant activity of six distilled and no distilled Mediterranean herbs and aromatic plants. J. Agric. Food Chem. 2002;50:6882–6890. doi: 10.1021/jf020540a. [DOI] [PubMed] [Google Scholar]
- Polat Kose L., Gülçin İ., Gören A.C., Namiesnik J., Martinez-Ayala A.L., Gorinstein S. LC-MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind. Crop. Prod. 2015;74:712–721. [Google Scholar]
- Pothitirat W., Chomnawang M.T., Supabphol R., Gritsanapan W. Comparison of bioactive compounds content, free radical scavenging and anti-acne inducing bacteria activities of extracts from the mangosteen fruit rind at two stages of maturity. Fitoterapia. 2009;80(7):442–447. doi: 10.1016/j.fitote.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy P.K., Bono A. Antioxidant activity, total phenolic and flavonoid content of Morinda citrifolia fruit extracts from various extraction processes. J. Eng. Sci. Technol. 2007;2(1):70–80. [Google Scholar]
- Sanni O., Erukainure O.L., Chukwuma C.I., Koorbanally N.A., Ibeji C.U., Islam M. Azadirachta indica inhibits key enzyme linked to type 2 diabetes in vitro, abates oxidative hepatic injury and enhances muscle glucose uptake ex vivo. Biomed. Pharmacother. 2019;109:734–743. doi: 10.1016/j.biopha.2018.10.171. [DOI] [PubMed] [Google Scholar]
- Song F.L., Gan R.Y., Zhang Y., Xiao Q., Kuang L., Li B. Total phenolic contents and antioxidant capacities of selected Chinese medicinal plants. Int. J. Mol. Sci. 2010;11:2362–2372. doi: 10.3390/ijms11062362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalikas C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Separ. Sci. 2007;30:3268–3295. doi: 10.1002/jssc.200700261. [DOI] [PubMed] [Google Scholar]
- Taslimi P., Gulçin İ. Antioxidant and anticholinergic properties of olivetol. J. Food Biochem. 2018;42(3) [Google Scholar]
- Tuba A., Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Turkan F., Atalar M.N., Aras A., Gulçin İ., Bursal E. ICP-MS and HPLC analyses, enzyme inhibition and antioxidant potential of Achillea schischkinii Sosn. Bioorg. Chem. 2020;94:1033–1043. doi: 10.1016/j.bioorg.2019.103333. [DOI] [PubMed] [Google Scholar]
- Vadivelan R., Gopala R., Kannan R. Antidiabetic potential of Asparagus racemosus Willd leaf extracts through inhibition of α-amylase and α-glucosidase. J. Tradit. Complementary Med. 2019;9(1):1–4. doi: 10.1016/j.jtcme.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velioglu S., Mazza G., Gao L., Oomah B. Antioxidant activity and total phenolics in selected fruits and vegetables, and grain products. J. Agric. Food Chem. 1998;46:4113–4117. [Google Scholar]
- Wang K.J., Zhao J.L. Corn silk (Zea mays L.), a source of natural antioxidants with α-amylase, α-glucosidase, advanced glycation and diabetic nephropathy inhibitory activities. Biomed. Pharmacother. 2019;110:510–517. doi: 10.1016/j.biopha.2018.11.126. [DOI] [PubMed] [Google Scholar]
- Wolfe K., Wu X., Liu R. Antioxidant activity of Apple peels. J. Agric. Food Chem. 2003;51:509–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- Zhou C., Sun C., Chen K., Li X. Flavonoids, phenolics, and antioxidant capacity in the flower of Eriobotrya japonica lindl. Int. J. Mol. Sci. 2011;12:2935–2945. doi: 10.3390/ijms12052935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski H., Kozłowska H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J. Agric. Food Chem. 2000;48:2008–2016. doi: 10.1021/jf990619o. [DOI] [PubMed] [Google Scholar]