Abstract
The dibenzoxepines derivatives have found a broad application in biological and pharmaceutical fields as new prospective drugs. So, the molecule (3aS,12bS)-5-Chlor-2-methyl-2,3,3a,12b-tetrahydro-1H-dibenzo[2,3:6,7]oxepino[4,5-c]pyrrol has been characterized by DFT (Density Functional Theory) approach to predict the important properties of it. The minimum energy conformer has been found by PES (Potential Energy Surface) and then the structure is optimized. Further, the structure is characterized spectroscopically by FT-IR and FT-Raman techniques to know the functional group and chemically active atoms. The geometrical parameters, PED (Potential Energy Distribution) assignments have also been reported. The electronic properties of the title compound have been explained by UV-Vis and HOMO-LUMO analyses that describe the charge transfer between the atoms of the molecule. Molecular Electrostatic Potential (MEP), Electron Localization Function (ELF) and Localized Orbital Locator (LOL) have been depicted to know the chemically active regions. The electrophilic and nucleophilic regions have been shown by Fukui functions. The Non-Linear Optics (NLO) for non-linear optical effects and the Natural Bond Orbital (NBO) for charge delocalization were studied. To study the biological activity of the title compound, molecular docking has been performed which suggests that the title molecule may act as a membrane permeable inhibitor.
Keywords: Analytical chemistry, Materials chemistry, Theoretical chemistry, DFT, FT-IR, FT-Raman, NBO, Fukui functions, Docking
Analytical chemistry, Materials chemistry, Theoretical chemistry, DFT; FT-IR; FT-Raman; NBO; Fukui functions; Docking
1. Introduction
(3aS,12bS)-5-Chlor-2-methyl-2,3,3a,12b-tetrahydro-1H-dibenzo[2,3:6,7]oxepino[4,5-c]pyrrol (5TDOP) is a dibenzoxepine derivative compound. It has two benzene rings that are attached with oxepino[4,5-c]pyrrole ring with electrophilic functional groups (C–O, N–C and C–Cl). Oxepin is a heterocycle derivative that is acted as tricyclic antidepressant. This contains oxygen and three double bonds with seven-membered ring structure. In addition, dibenzoxepines and pyrrole derivatives play an essential role in wide range of biological activities such as DNA binding, antimicrobial, antioxidant, enzyme/receptor interactions, antidepressant, bipolar disorder, plasma therapy etc. [1, 2, 3, 4, 5]. 5TDOP compound has heterocyclic intermediate substituent pyrrole which is widely used in pharmaceuticals [2], production of agrochemicals, synthesis of dyes, photographic chemicals etc. It has aromatic nature which is a main feature of organic compound. Usually it reacts with electrophiles. Miller et al [6] reported that 5TDOP is an antipsychotic drug that has the aforesaid substituents. Antipsychotic drugs (APDs) are used for the treatment of schizophrenia and are also commonly used in the treatment of bipolar disorder with psychotic symptoms that is referred to bipolar I disorder. Now a days, many of the people are affected by bipolar I mental disorder due to stress. 5TDOP is a drug for Schizophrenia and bipolar disorders. The diseases namely schizophrenia, bipolar disorders cause serious neuropsychiatric disorders along with substantial health risks in patients [7,8]. 5TDOP drug is approved by FDA for schizophrenia acute treatment and for the acute treatment of manic related to bipolar I disorder.
Raghunath Reddy Anugu, Prathama S. Mainkar et al [9] presented the ireland-claisen rearrangement strategy for the synthesis of schizophrenia drug, (+)-asenapine. Vaishali M. Gambhire et al [10] reported the enhanced oral delivery with asenapine maleate that taking from the solid lipid nanoparticles (pharmacokinetic and brain distribution evaluations). The compound has biological and chemical importance. So, to characterize and to know the properties of the 5TDOP, the spectroscopy and quantum chemical calculations are considered since that is not reported in literature. In the present study, the experimental and the theoretical spectroscopic (FT-IR, FT-Raman and UV-Vis) characterizations for 5TDOP have been reported. Molecular geometry, vibrational frequencies, molecular surface analysis and chemical reactivities are calculated using density functional theory with B3LYP method with 6–311++G(d,p) basis set. The stability of molecule was studied by Potential Energy Scan analysis (PES). The electronic properties are found by UV-Vis and HOMO-LUMO analyses with their fundamental chemical parameters. The molecular reactivities of electrophilic and nucleophilic characters are calculated using Fukui function and MEP surface analyses. In addition, the biological activity of antipsychotic nature in bipolar disorders for 5TDOP has been explained by molecular docking (protein-ligand) analysis.
2. Experimental methods
The solid form of 5TDOP that has purity of 98% has been purchased from the Sigma Aldrich Company. This pure 5TDOP has been taken to do characterization namely FT-IR, FT-Raman and UV-Vis analysis. The FT-IR spectrum has been precisely recorded from the range 4000 to 400 cm−1 by Perkin Elmer FT-IR Spectrum instrument which has resolution of 1.0 cm−1. The FT-Raman spectrum of 5TDOP has been recorded by using BRUCKER RFS 27 instrument with Nd: YAG Laser of 100 mW from the range 4000 to 100 cm−1 in which the resolution is maintained as 2 cm−1. The UV-Vis absorption spectrum of 5TDOP has been obtained with the usage of DMSO solvent from 200 to 600 nm by the spectrometer of Perkin Elmer LAMBDA 950 UV. These characterizations have been carried out at the institute of Sophisticated Analytical Instruments Facility (SAIF), IIT (M), Chennai, India.
3. Computational methods
Generally, DFT and HF quantum computational methods are utilized to predict the quantum states in many electron systems that are available in the molecule. The second method not contains the detail electronic correlation details [11]. So, for the present study, DFT technique which is more accurate and reliable was used to characterize 5TDOP. Gaussian 09W program was used to predict DFT calculations on 5TDOP by B3LYP/6-311++G(d,p) set for the characterization of the structure, electronic, chemical, biological and other properties [12, 13, 14]. The geometric parameters namely bond lengths and bond angles were obtained and the structure of 5TDOP has been depicted by Gauss View 5.0 [15]. The fundamental modes of vibrations using vibrational spectral calculations are done by VEDA software for the small 5TDOP molecule [16]. The UV-Vis analysis has been explained by using the calculations through TD-DFT method of Gaussian 09W output. The unscaled spectral values are scaled by using scaling factor 0.961 scaling factor which gives better agreement with the experimental values. HOMO-LUMO and MEP analyses were depicted using Gauss View 5.0 to explain the 5TDOP [17]. Furthermore, Multiwfn software [18] was used to explain electronic and chemical properties by DOS, LOL and ELF. The property namely NLO was obtained by Gaussian 09W output. AUTODOCK 1.4.6 and PYMOL software were used for the docking between the selected proteins [19] and 5TDOP to explain the biological behaviour.
4. Results and discussion
4.1. Geometrical parameters
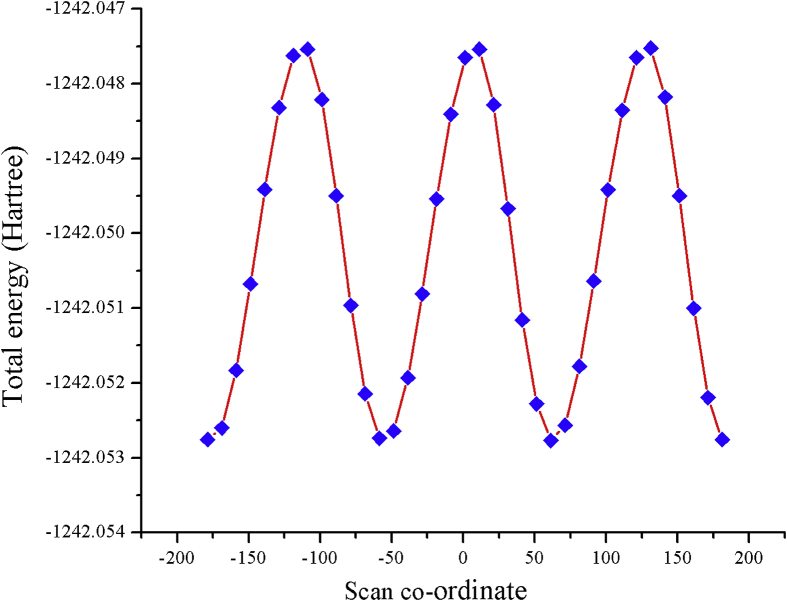
The molecule 5TDOP has a molecular formula C17H16ClNO with a molecular weight of 285.768 g/mol. Potential Energy Scan analysis (PES) is most successful approach for finding the molecular stability through conformation analysis [20]. The stability of 5TDOP is analyzed by PES using HF/6-31G(d,p) basis set which is given in Figure 1. PES has been processed and 37 energy conformers have been obtained with the rotation angles to predict stable structure. In Figure 1, blue dots represent the 37 energy conformer and 37 conformations are tabulated in Table 1. The total energy has been obtained as -1242.0527 Hartree with the angle of 183.62°. This minimum energy conformer of 5TDOP has been taken for the further characterization to predict the structural, electronic, chemical, biological properties of the molecule.
Figure 1.
PES (Potential Energy Surface) graph of 5TDOP.
Table 1.
Potential energy surface analysis of 5TDOP.
| No. of Conformations | Scan coordinate (angle) | Total energy (hartree) |
|---|---|---|
| 1 | -178.7623 | -1242.0528 |
| 2 | -168.7623 | -1242.0526 |
| 3 | -158.7623 | -1242.0518 |
| 4 | -148.7623 | -1242.0507 |
| 5 | -138.7623 | -1242.0494 |
| 6 | -128.7623 | -1242.0483 |
| 7 | -118.7623 | -1242.0476 |
| 8 | -108.7623 | -1242.0475 |
| 9 | -98.7623 | -1242.0482 |
| 10 | -88.7623 | -1242.0495 |
| 11 | -78.7623 | -1242.0510 |
| 12 | -68.7623 | -1242.0521 |
| 13 | -58.7623 | -1242.0527 |
| 14 | -48.7623 | -1242.0526 |
| 15 | -38.7623 | -1242.0519 |
| 16 | -28.7623 | -1242.0508 |
| 17 | -18.7623 | -1242.0495 |
| 18 | -8.7623 | -1242.0484 |
| 19 | 1.2377 | -1242.0477 |
| 20 | 11.2377 | -1242.0475 |
| 21 | 21.2377 | -1242.0483 |
| 22 | 31.2377 | -1242.0497 |
| 23 | 41.2377 | -1242.0512 |
| 24 | 51.2377 | -1242.0523 |
| 25 | 61.2377 | -1242.0528 |
| 26 | 71.2377 | -1242.0526 |
| 27 | 81.2377 | -1242.0518 |
| 28 | 91.2377 | -1242.0506 |
| 29 | 101.2377 | -1242.0494 |
| 30 | 111.2377 | -1242.0484 |
| 31 | 121.2377 | -1242.0477 |
| 32 | 131.2377 | -1242.0475 |
| 33 | 141.2377 | -1242.0482 |
| 34 | 151.2377 | -1242.0495 |
| 35 | 161.2377 | -1242.0510 |
| 36 | 171.2377 | -1242.0522 |
| 37 | 181.2377 | -1242.0528 |
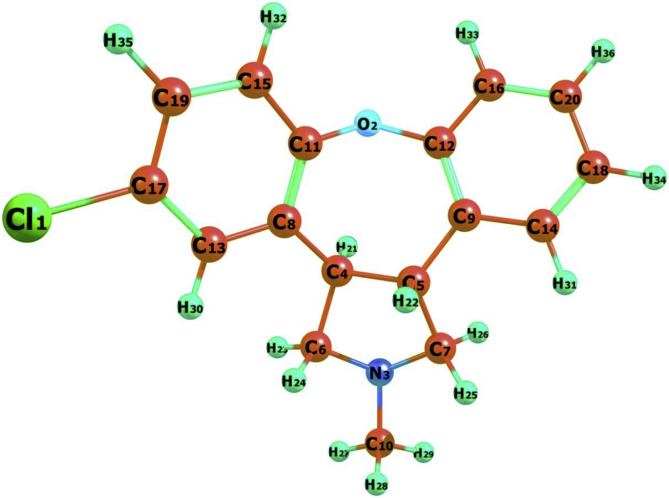
Geometrical parameters play most significant role in theoretical investigations on organic compounds because; it is found the stability of the molecules [17,20]. The optimized geometrical parameters of 5TDOP is calculated by density functional theory with the help of B3LYP methods and 6-311++G(d,p) set of functions. At present, many of the researchers reported B3LYP/6-311++G(d,p) method of geometrical parameter calculations on many compounds that are in very good agreement with the experimental ones. In the present study, the geometrical parameters in terms of bond length and bond angles are calculated and the values are reported in Table 2. 5TDOP possesses various types of bond lengths (C–C, C–Cl, C–H, C–N, and C–O) in which two major part of benzene rings R1, R2 and oxepino [4,5-c]pyrrole rings are present. The optimized molecular structure along with bond lengths and bond angles is shown in Figure 2.
Table 2.
Optimized geometrical parameters (bond length (Å) and bond angle (o)) of 5TDOP by 6–311++G(d,p) basis set.
| Bond length(Å) |
Bond angle(°) |
||||
|---|---|---|---|---|---|
| Bonds | B3LYP | Experimental∗ | Bonds | B3LYP | Experimental∗ |
| Cl1–C17 | 1.761 | 1.744 | N3–C7–H26 | 110.0 | - |
| O2–C11 | 1.385 | 1.209 | N3–C10–H27 | 109.9 | - |
| O2–C12 | 1.391 | - | N3–C10–H28 | 112.9 | - |
| N3–C6 | 1.458 | 1.458 | N3–C10–H29 | 109.9 | 109.6 |
| N3–C7 | 1.472 | 1.474 | C5–C4–C6 | 101.4 | - |
| N3–C10 | 1.449 | 1.463 | C5–C4–C8 | 111.8 | 117.8 |
| C4–C5 | 1.551 | - | C5–C4–H21 | 108.3 | 108.5 |
| C4–C6 | 1.526 | - | C4–C5–C7 | 103.3 | - |
| C4–C8 | 1.503 | 1.504 | C4–C5–C9 | 116.3 | 116.8 |
| C4–H21 | 1.096 | - | C4–C5–H22 | 106.1 | 108.5 |
| C5–C7 | 1.555 | - | C6–C4–C8 | 119.1 | 119.4 |
| C5–C9 | 1.517 | 1.514 | C6–C4–H21 | 107.6 | 108.5 |
| C5–H21 | 1.097 | - | C4–C6–H23 | 113.3 | 116.5 |
| C6–H23 | 1.094 | - | C4–C6–H24 | 110.8 | 109.3 |
| C6–H24 | 1.107 | - | C8–C4–H21 | 108.2 | 108.5 |
| C7–H25 | 1.102 | - | C4–C8–C11 | 117.1 | 117.6 |
| C7–H26 | 1.094 | - | C4–C8–C13 | 124.6 | 121.8 |
| C8–C11 | 1.399 | 1.388 | C7–C5–C9 | 115.8 | 118.6 |
| C8–C13 | 1.396 | 1.388 | C7–C5–H22 | 107.4 | - |
| C9–C12 | 1.405 | 1.403 | C5–C7–H25 | 110.2 | - |
| C9–C14 | 1.404 | 1.400 | C5–C7–H26 | 112.2 | - |
| C10–H27 | 1.093 | - | C9–C5–H22 | 107.3 | - |
| C10–H28 | 1.107 | - | C5–C9–C12 | 124.4 | 125.3 |
| C10–H29 | 1.093 | - | C5–C9–C14 | 118.9 | 118.8 |
| C11–C15 | 1.390 | - | H23–C6–H24 | 107.9 | 107.9 |
| C12–C16 | 1.397 | - | H25–C7–H26 | 107.5 | 107.5 |
| C13–C17 | 1.392 | - | C11–C8–C13 | 118.3 | - |
| C13–H30 | 1.082 | - | C8–C11–C13 | 121.5 | 121.9 |
| C14–C18 | 1.390 | - | C8–C11–C17 | 120.1 | - |
| C14–H31 | 1.084 | - | C8–C13–H30 | 120.5 | - |
| C15–C19 | 1.393 | - | C12–C9–C14 | 116.7 | 117.8 |
| C15–C19 | 1.083 | 0.930 | C9–C12–C16 | 121.1 | - |
| C16–C20 | 1.388 | 1.380 | C9–C14–C18 | 122.6 | - |
| C16–H33 | 1.083 | 0.930 | C9–C14–H31 | 118.5 | 119.0 |
| C17–C19 | 1.389 | 1.385 | H27–C10–H28 | 108.2 | - |
| C18–C20 | 1.393 | - | H27–C10–H29 | 107.7 | - |
| C18–C34 | 1.084 | 0.970 | H28–C10–H29 | 108.0 | - |
| C19–H35 | 1.082 | 0.960 | C11–C15–C19 | 120.0 | - |
| C20–H36 | 1.084 | 0.970 | C11–C15–H32 | 119.2 | - |
| Bond angle(°) | C12–H16–C20 | 120.7 | - | ||
| Cl1–C17–C13 | 119.1 | 117.3 | C12–C16–H33 | 117.9 | - |
| Cl1–C17–C19 | 119.5 | 119.7 | C17–C13–H30 | 119.4 | - |
| C11–O2–C12 | 122.8 | 121.9 | C13–C17–C19 | 121.4 | - |
| O2–C11–C8 | 120.6 | 120.4 | C18–C14–H31 | 118.9 | - |
| O2–C11–C15 | 117.7 | - | C14–C18–C20 | 119.4 | - |
| C2–C12–C9 | 125.1 | 125.3 | C14–C18–H34 | 120.0 | - |
| C2–C12–C16 | 113.7 | C19–C15–H32 | 120.8 | - | |
| C6–N3–C7 | 108.1 | 109.5 | C15–H19–C17 | 118.8 | - |
| C6–N3–C10 | 115.6 | 111.8 | C15–C19–H35 | 120.8 | - |
| N3–C6–C4 | 102.0 | - | C20–H16–H33 | 121.4 | - |
| N3–C6–H23 | 110.7 | 109.5 | C16–C20–C18 | 119.4 | - |
| N3–C6–H24 | 112.2 | 109.5 | C16–C20–H36 | 119.9 | - |
| C7–N3–C10 | 115.1 | 110.5 | C17–C19–H35 | 120.5 | - |
| N3–C7–C5 | 105.4 | - | C20–C18–H34 | 120.6 | - |
| N3–C7–H25 | 111.6 | 109.6 | C18–C20–H36 | 120.7 | - |
Taken from Ref [9].
Figure 2.
Optimized structure of 5TDOP using B3LYP/6-311++G(d,p) basis set.
Oxepino [4,5-c]pyrrole ring has higher C–C bond lengths compare to benzene rings R1, R2. The pyrrole ring C–C bond lengths are calculated at C5–C7/1.555, C5–C4/1.551, C4–C6/1.526, C5–C9/1.517, C4–C8/1.503Å and benzene rings C–C bond lengths varying from 1.388 to 1.405 Å. The Oxepino and benzene ring R1 are attached with C=C bond with higher bond length value C9–C12/1.405Å and benzene ring R1 C=C bond lengths are gradually decreased (C14–C18/1.390, C20–C16/1.388 Å). The C=C and C–C bond lengths of benzene ring R2 are lower than benzene R1 and the differences are clearly visualized in Figure 2. The benzene ring R2 has substitution of Cl atom that changes the nature of the benzene. The longest bond length is presented between C–Cl bond at 1.761Å and shortest bonds are between C–H bonds (1.082–1.084 Å) for 5TDOP molecule. So, 5TDOP bond length values can be increased and decreased because, pyrrole ring and Cl substitutions affects the surrounding bonds (ring R1 and R2).
4.2. Vibrational spectral analysis
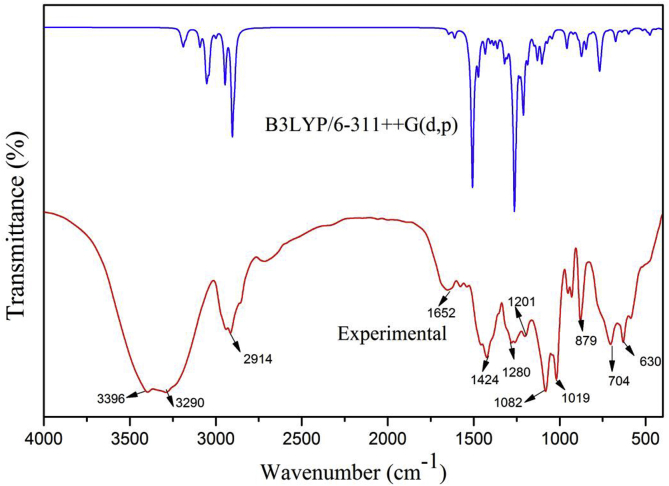
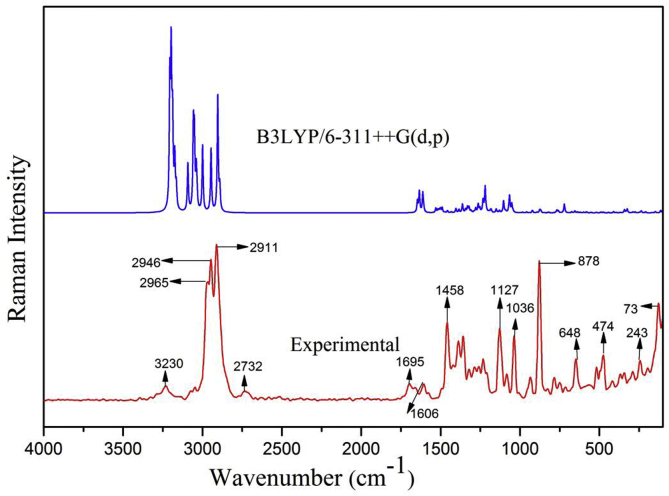
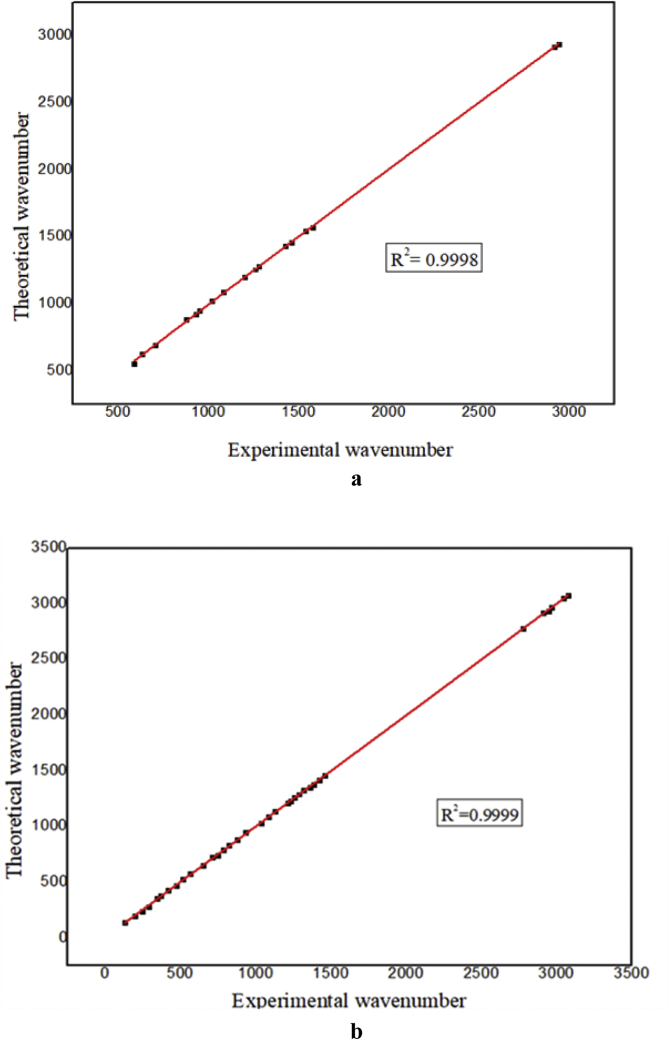
The vibrational spectroscopy (FT-IR and FT-Raman) is efficient tool for structural conformational analysis of organic molecules [21]. 5TDOP consists 26 atoms with 86 normal modes of vibrations since it has non-linear molecular structure. It has 2 benzene rings, one methyl and two methylene group in it. The atoms oxygen, nitrogen and chlorine play an important role in structural elucidation. The experimental FT-IR and FT-Raman were recorded and theoretical vibrational frequency, IR and Raman intensities are calculated using 6-311++G(d,p) basis set and the values are listed in Table 3. Each vibrational mode with Potential Energy Distributions (PED) has been calculated using Veda 4 program. Figures 3 and 4 shows the comparative experimental and theoretical FT-IR and FT-Raman spectra and the correlation graph of Figure 5a, b represents the FTIR and FT-Raman. The scaled values of 5TDOP have been obtained from unscaled values after multiplying with the scaling factor 0.961 [22]. These scaled values are in agreement with the observed values that are reported in Table 3.
Table 3.
Calculated vibrational frequencies (cm−1) assignments of 5TDOP (Experimental, theoretical by B3LYP/6-311++G(d,p) set).
| Mode no | Experimental wave number (cm−1) |
Theoretical wave number(cm−1) |
IIRc | I Raman |
Assignments (PED)a | |||
|---|---|---|---|---|---|---|---|---|
| FTIR | FT-RAMAN | Unscaled | Scaledb | Relative | Absoluted | |||
| 102 | - | - | 3206 | 3081 | 0 | 555 | 13 | γCH(88) |
| 101 | - | - | 3204 | 3079 | 1 | 50 | 1 | γCH(90) |
| 100 | - | 3075(w) | 3197 | 3073 | 4 | 704 | 16 | γCH(88) |
| 99 | - | - | 3191 | 3067 | 1 | 129 | 3 | γCH(96) |
| 98 | - | - | 3188 | 3064 | 8 | 340 | 8 | γCH(87) |
| 97 | - | 3047(w) | 3175 | 3051 | 4 | 251 | 6 | γCH(86) |
| 96 | - | - | 3165 | 3041 | 1 | 110 | 3 | γCH(91) |
| 95 | - | 2965(s) | 3092 | 2971 | 10 | 258 | 6 | γCH(100) |
| 94 | 2939(m) | 2947(s) | 3057 | 2938 | 16 | 439 | 10 | γCH(92) |
| 93 | - | - | 3051 | 2932 | 18 | 385 | 9 | γCH(95) |
| 92 | - | - | 3039 | 2921 | 20 | 214 | 5 | γCH(91) |
| 91 | 2914(m) | 2911(s) | 3034 | 2916 | 1 | 67 | 2 | γCH(94) |
| 90 | - | - | 2999 | 2882 | 4 | 392 | 9 | γCH(91) |
| 89 | - | - | 2946 | 2831 | 33 | 393 | 9 | γCH(95) |
| 88 | - | - | 2904 | 2791 | 61 | 751 | 18 | γCH(90) |
| 87 | - | 2774(w) | 2891 | 2778 | 25 | 153 | 4 | γCH(92) |
| 86 | - | - | 1646 | 1582 | 3 | 262 | 6 | γCC(59) |
| 85 | 1577(m) | - | 1634 | 1570 | 1 | 494 | 12 | γCC(55) |
| 84 | - | - | 1612 | 1549 | 4 | 458 | 11 | γCC(45) |
| 83 | 1538(m) | - | 1607 | 1544 | 1 | 92 | 2 | γCC(51) |
| 82 | - | - | 1530 | 1470 | 4 | 116 | 3 | βHCH(81) |
| 81 | 1461(s) | 1458(s) | 1517 | 1458 | 6 | 62 | 1 | βHCH(55) |
| 80 | - | - | 1515 | 1456 | 2 | 29 | 1 | βHCH(72) |
| 79 | - | - | 1507 | 1448 | 91 | 59 | 1 | βHCC(16) |
| 78 | - | - | 1501 | 1443 | 5 | 99 | 2 | βHCH(76)+τHCCC(10) |
| 77 | 1424(s) | - | 1490 | 1432 | 9 | 137 | 3 | βHCH(83) |
| 76 | - | 1423(m) | 1472 | 1415 | 23 | 14 | 0 | βHCC(52) |
| 75 | - | - | 1457 | 1400 | 1 | 60 | 1 | βHCN(82) |
| 74 | - | 1387(s) | 1433 | 1377 | 13 | 32 | 1 | γCC(23)+βHCC(11) |
| 73 | - | 1358(s) | 1404 | 1349 | 6 | 126 | 3 | βHCC(50) |
| 72 | - | 1320(m) | 1384 | 1330 | 7 | 101 | 2 | βHCC(62) |
| 71 | - | - | 1362 | 1309 | 9 | 279 | 7 | τHCCC(29) |
| 70 | - | 1285(m) | 1343 | 1290 | 2 | 108 | 3 | βHCC(37) |
| 69 | 1280(m) | - | 1331 | 1279 | 1 | 192 | 4 | βHCC(38)+γCC(23) |
| 68 | - | - | 1321 | 1270 | 16 | 182 | 4 | βHCC(16)+γCC(49) |
| 67 | 1258(m) | 1259(m) | 1309 | 1258 | 3 | 56 | 1 | γCC(32) |
| 66 | - | - | 1305 | 1254 | 7 | 23 | 1 | βHCC(45) |
| 65 | - | - | 1289 | 1239 | 1 | 37 | 1 | βHCC(44) |
| 64 | - | 1232(m) | 1279 | 1229 | 14 | 156 | 4 | βHCC(45) |
| 63 | - | 1212(m) | 1263 | 1214 | 100 | 298 | 7 | γCC(56) |
| 62 | 1201(m) | - | 1253 | 1204 | 28 | 158 | 4 | γCC(19)+τHCCC(37) |
| 61 | - | - | 1233 | 1185 | 9 | 349 | 8 | γCC(36)+βHCC(14) |
| 60 | - | - | 1231 | 1183 | 4 | 140 | 3 | βHCN(69) |
| 59 | - | - | 1219 | 1172 | 11 | 1004 | 24 | γCC(42) |
| 58 | - | - | 1211 | 1163 | 43 | 49 | 1 | γCC(30) |
| 57 | - | - | 1196 | 1150 | 2 | 44 | 1 | βHCC(37) |
| 56 | - | - | 1186 | 1139 | 14 | 82 | 2 | βHCC(27) |
| 55 | - | 1127(vs) | 1181 | 1135 | 2 | 98 | 2 | βHCC(68) |
| 54 | - | - | 1149 | 1105 | 6 | 151 | 4 | βHCN(18) |
| 53 | 1082(s) | 1083(m) | 1131 | 1087 | 10 | 40 | 1 | βHCN(51) |
| 52 | - | - | 1128 | 1084 | 7 | 64 | 1 | βHCC(44) |
| 51 | - | - | 1103 | 1060 | 18 | 536 | 13 | βHCC(38) |
| 50 | - | - | 1091 | 1048 | 7 | 71 | 2 | γCC(40) |
| 49 | - | 1034(s) | 1072 | 1030 | 6 | 37 | 1 | γCC(28) |
| 48 | 1019(s) | - | 1066 | 1025 | 0 | 816 | 19 | γCC(51) |
| 47 | - | - | 1051 | 1010 | 2 | 430 | 10 | γCC(23)+τHCCC(19) |
| 46 | - | - | 1043 | 1002 | 4 | 70 | 2 | γCC(37)+τHCCC(11) |
| 45 | 952(m) | - | 988 | 949 | 0 | 3 | 0 | τHCCH(89) |
| 44 | - | 934(m) | 984 | 945 | 0 | 29 | 1 | γCC(10)+τHCCC(23) |
| 43 | 929(m) | - | 961 | 923 | 1 | 6 | 0 | τHCCC(78) |
| 42 | - | - | 958 | 921 | 1 | 40 | 1 | τHCCC(76)+βHCC(10)+γCC(11) |
| 41 | - | - | 958 | 920 | 10 | 6 | 0 | γCC(11)+γCC(15) |
| 40 | 879(s) | 876(vs) | 922 | 886 | 2 | 153 | 4 | βHCC(43) |
| 39 | - | - | 894 | 859 | 4 | 39 | 1 | βHCC(10)+τHCNC(35) |
| 38 | - | - | 877 | 843 | 4 | 43 | 1 | γNC(15)+τHCCCl(56) |
| 37 | - | - | 875 | 841 | 7 | 142 | 3 | γNC(14)+τHCCCl(11) |
| 36 | - | 824(w) | 869 | 835 | 8 | 146 | 3 | γNC(61) |
| 35 | - | - | 846 | 813 | 11 | 13 | 0 | γCC(11)+τHCCC(46) |
| 34 | - | 783(m) | 820 | 788 | 2 | 51 | 1 | γCC(23)+τHCCC(15) |
| 33 | - | 749(m) | 775 | 745 | 9 | 25 | 1 | βHCC(15)+τHCCC(11) |
| 32 | - | - | 767 | 737 | 19 | 211 | 5 | τHCCC(48) |
| 31 | - | 713(w) | 758 | 729 | 7 | 148 | 3 | τHCCC(64) |
| 30 | 704(s) | - | 721 | 692 | 1 | 759 | 18 | γCC(22)+τHCNC(16)+τHCCC(13) |
| 29 | - | 648(m) | 675 | 648 | 8 | 101 | 2 | γClC(43) |
| 28 | 630(s) | - | 654 | 629 | 0 | 180 | 4 | βHCC(47) |
| 27 | - | - | 638 | 613 | 2 | 91 | 2 | βHCC(50) |
| 26 | - | - | 621 | 597 | 1 | 62 | 1 | βHCC(49) |
| 25 | - | 565(w) | 599 | 576 | 4 | 86 | 2 | βCCC(43) |
| 24 | 588(s) | - | 580 | 558 | 1 | 129 | 3 | βHCC(15)+τHCCC(19) |
| 23 | - | 517(m) | 552 | 530 | 0 | 72 | 2 | βHCC(10)+τHCCC(34) |
| 22 | - | - | 518 | 498 | 2 | 88 | 2 | τHCNC(40) |
| 21 | - | 474(s) | 490 | 471 | 2 | 127 | 3 | βCCC(37) |
| 20 | - | - | 475 | 456 | 4 | 123 | 3 | τHCCC(12)+γClC(18) |
| 19 | - | 417(w) | 442 | 425 | 1 | 81 | 2 | τHCCC(53) |
| 18 | - | - | 410 | 394 | 1 | 244 | 6 | τHCNC(35)+τHCNC(10) |
| 17 | - | 367(m) | 392 | 377 | 2 | 73 | 2 | τHCCCl(36) |
| 16 | - | 343(m) | 368 | 354 | 0 | 124 | 3 | τHCNC(30) |
| 15 | - | - | 342 | 328 | 1 | 770 | 18 | βHCC(21) |
| 14 | - | - | 331 | 319 | 0 | 180 | 4 | τHCNC(26) |
| 13 | - | - | 324 | 312 | 2 | 1026 | 24 | τHCNC(48) |
| 12 | - | 289(m) | 289 | 278 | 0 | 280 | 7 | τHCNC(27)+βHCC(10) |
| 11 | - | - | 275 | 264 | 2 | 356 | 8 | βHCC(23) |
| 10 | - | 243(m) | 247 | 237 | 0 | 250 | 6 | τHCNC(65) |
| 9 | - | - | 236 | 227 | 0 | 886 | 21 | τHCNC(40) |
| 8 | - | 195(m) | 209 | 201 | 0 | 608 | 14 | τHCCC(26) |
| 7 | - | - | 170 | 163 | 0 | 267 | 6 | τHCCC(42) |
| 6 | - | 127(s) | 147 | 142 | 0 | 1331 | 31 | τHCNC(13) |
| 5 | - | - | 113 | 109 | 0 | 4267 | 100 | τHCCC(57) |
| 4 | - | - | 97 | 93 | 0 | 2487 | 58 | τHCCC(68) |
| 3 | - | - | 77 | 74 | 0 | 3696 | 87 | τHCCC(62) |
| 2 | - | - | 58 | 56 | 0 | 4022 | 94 | τHCCC(57) |
| 1 | - | - | 36 | 35 | 0 | 4150 | 97 | βCOC(28)+τHCNC(14) |
γ-stretching,γa-Symmetrical stretching,γas-asymmetrical stretching, β-inplane bending,ω-outplane bending, τ-torsion, vs-very strong, s-strong, m-medium, w-weak.
Scaling factor: 0.961 for B3LYP/6-311+G(d,p).
Relative absorption intensities normalized with highest peak absorption equal to 100.
Relative Raman intensities normalized to 100.
Figure 3.
Experimental and theoretical FT-IR spectrum of 5TDOP.
Figure 4.
Experimental and theoretical FT-Raman spectrum of 5TDOP.
Figure 5.
Correlation graph of (Fig. a represents the FT-IR and Fig. b represents the FT-Raman).
4.2.1. C–H vibrations
The aromatic, heterocyclic asymmetric and symmetric stretching C–H vibrations were found in the region of wave numbers 3000-2800 cm−1 [23,24]. In the current study, C–H stretching vibrations were observed at 3081 to 2778 cm−1 by B3LYP/6-311++G(d,p) basis set. The corresponding experimental FT-IR bands were observed at 2939(m), 2914(m) and the FT-Raman spectrum bands at 3075(w), 3047(w), 2965(s), 2947(s), 2911(s), 2774(w) at with the highest PED contribution value as 100% respectively. Similarly, the bending or bond deformation modes of vibrations appeared in the region between 1500 and 1300 cm−1 [25]. The theoretical frequencies were found to be in 1458, 1432 cm−1 which has close agreement with recorded FT-IR spectrum bands at 1461, 1424 cm−1 and with the strong intensity FT-Raman bands at 1458 cm−1 with 55 and 83 % of PED values. In general, in-plane bending of C–C–H vibrations are present as a number of strong to weak intensity bands in the region of 1300–1000 cm−1 [26]. The in plane bending vibrations for 5TDOP have been noted experimentally in FT-Raman spectrum as 1320, 1232, 1127 cm−1. These values have good agreement with theoretical results that are obtained at 62, 45, 68% PED values. The H–C–H bending vibrations of 5TDOP have been observed at 1461(s) and 1424(s) cm−1 in FT-IR spectrum with theoretical values at 1458 and 1432 cm−1 with the PED values of 55 and 83%. The H–C–H bending vibration of 5TDOP has been observed at 1458(s) cm−1 in FT-Raman spectrum with theoretical value at 1458 cm−1 with the PED value of 55%.
4.2.2. C–C vibrations
The stretching vibrations (C=C bond) are supposed to be in the region 1650–800 cm−1 [27, 28, 29, 30, 31]. The C=C vibrations of 5TDOP are presented at the benzene rings (two in number) region. The theoretical values of 5TDOP were obtained in the range of 1570, 1544, 1214, 1030, 1025 cm−1 by B3LYP/6–311++G(d,p) method. The recorded bands which are of different intensities were observed at 1577(m), 1538(m), 1019(s) in FT-IR spectrum. The FT-Raman bands were identified at 1212(m), 1034(s) cm−1. It shows that the theoretical values are in good agreement with the experimental data. The ring C–C–C vibrations of 5TDOP have been observed at 565(w) and 474(s) cm−1 in FT-Raman spectrum with the corresponding theoretical values at 576 and 471 cm−1 with the PED values of 43 and 37%. The observed values of 5TDOP have agreement with the scaled values of theoretical values.
4.2.3. C–N and C–Cl vibrations
The atom N3 is shared with the carbon atoms C6, C7 and C10 through single bond. The atoms C6 and C7 are attached with the methylene groups such as C6–H23–H24 and C7–H25–H26. The atom C10 is attached with the methyl group such as C10–H27–H28–H29. Due to this reason, the stretching N–C vibrations [32] have been observed at 824(w) cm−1 in FT-Raman spectrum with the theoretical value of 835 cm−1 with the PED value of 61%. The stretching mode of C–Cl vibrations is generally obtained in the region 760–505 cm−1 [33]. The atom Cl1 is attached with the carbon atom C17. The Cl1–C17 stretching vibration has been observed in FT-Raman spectrum as 648(m) cm−1 with the theoretical value as 648 cm−1 with 43% of PED.
4.2.4. Other vibrations
The bending H–C–N vibration is observed at 1082(s) cm−1 in FT-IR spectrum and 1083(m) cm−1 in FT-Raman spectrum with the corresponding theoretical value of 1087 cm−1 along with 51% of PED. The torsion H–C–C–C vibrations of 5TDOP have been measured at 1201(m) and 588(s) cm−1 in FT-IR spectrum with the theoretical values at 1204 and 558 cm−1. The torsion H–C–C–C vibrations of 5TDOP have been measured at 934(m), 783(m), 749(m), 713(w), 517(m), 417(w) and 195(m) cm−1 in FT-Raman spectrum with the theoretical values at 945, 788, 745, 729, 530, 425 and 201 cm−1. The torsion H–C–N–C vibration of 5TDOP has been measured at 704(s) cm−1 in FT-IR spectrum with the theoretical value at 692 cm−1. The torsion H–C–N–C vibrations of 5TDOP has been measured at 343(m), 289(m), 243(m) and 127(s) cm−1 in FT-Raman spectrum with the theoretical values at 354, 278, 237, 142 cm−1. The other theoretical H–C–C–C, H–C–N–C and H–C–C–Cl torsion vibrations have been reported in Table 3. Thus the vibration wavenumbers were estimated in theoretical approach has been favorable agreement with the recorded FT-IR and FT-Raman values.
4.3. Electronic properties
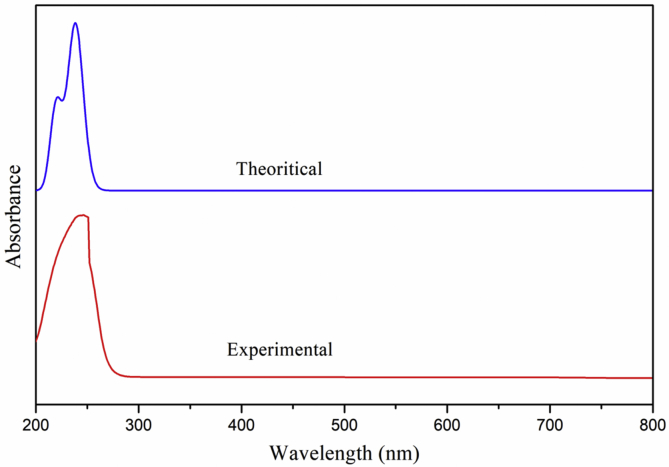
The experimental UV-Vis spectrum of 5TDOP has been performed in DMSO solution and the theoretical UV-Vis spectrum has been computed using TD-DFT/6-311++G(d,p) basis set by employing the same DMSO as a solvent. The results of both experimentally and theoretically (TD-DFT) calculated absorption wavelength (λ), excitation energies (E) and oscillator strengths (f) are the major factors during the electronic transitions and these are depicted in Table 4. As shown in Figure 6 the theoretical absorption values at 239, 236, 220 nm are associated with the 0.0412, 0.0216, 0.0328 oscillator frequencies and its major contributions are HOMO-1->LUMO (34%), HOMO-2->LUMO (18%), HOMO->LUMO (27%), HOMO->LUMO+1 (23%) along with the experimental value at 245 nm. The energy gap (ΔE) values of 5TDOP have found to be 5.2014 (H-1->LUMO (34%)), 5.2675 (H-2->LUMO (18%)), 5.6506 eV (HOMO->LUMO (27%)) with the corresponding experimental value 5.0740 eV. Thus, the simulated UV-Vis spectrum and experimental peaks are in good agreement with each other.
Table 4.
UV-Vis absorption spectrum of 5TDOP (Experimental, Theoretical by B3LYP/6-311++G(d,p) set).
| Experimental |
TD-DFT/6-311++G(d,p) |
|||||
|---|---|---|---|---|---|---|
| λcal (nm) | Band gap (eV) | λcal (nm) | Band gap (eV) | Energy (cm−1) | f | Assignment |
| 245 | 5.0740 | 239 | 5.2014 | 41803 | 0.0412 | H-4->L+2 (11%), H-1->LUMO (34%), H-1->L+2 (10%) |
| - | - | 236 | 5.2675 | 42323 | 0.0216 | H-2->LUMO (18%), H-1->L+2 (10%) |
| - | - | 220 | 5.6506 | 45501 | 0.0328 | HOMO->LUMO (27%), HOMO->L+1 (23%), HOMO->L+3 (16%) |
Figure 6.
Experimental and theoretical UV-Vis absorption spectrum of 5TDOP.
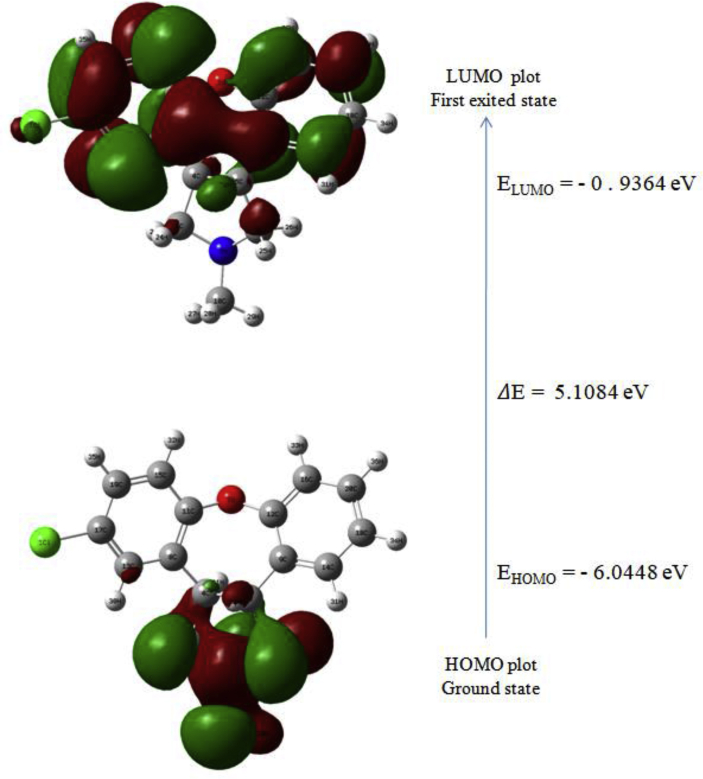
FMOs play an important role during molecular interactions. Further, the energy difference between the molecular orbitals provides a significant perspective about the optical, electronic properties and chemical reactivity of the molecule under investigation [34, 35, 36]. The HOMO (highest occupied molecular orbital) is the orbital which is signified as an electron donor (filled state) and therefore the LUMO (lowest unoccupied molecular orbital) symbolize the electron acceptor (empty state). The HOMO and LUMO energies and their orbital energy gap were computed using the B3LYP/6-311++G(d,p) technique. The simulated FMO's were depicted in Figure 7 in which a positive phase is shown in green and the negative is shown in red that represents the charge transfer within the molecule. The calculated band gap energy value Energy gap (eV) = 5.1084 eV of 5TDOP confirms the chemical stability of the molecule. Further, the HOMO (MO) and LUMO (MO) values are related to the ionization potential (electron-donating) and electron affinity (electron-accepting) of the compound. The Global molecular reactivity descriptors such as Electronegativity, Chemical hardness, Chemical softness (S), chemical potential (μ) and electrophilicity index (ω) electronic charge were predicted for 5TDOP and given in the Table 5. The parameters determined using the relation as,
| Chemical Potential = 1/2 (ELUMO + EHOMO) | (1) |
| Electro negativity = -1/2 (ELUMO + EHOMO) | (2) |
| Chemical hardness = 1/2 (ELUMO-EHOMO) | (3) |
| Electrophilicity index = μ2/2η | (4) |
| Chemical Softness = 1/ η | (5) |
Figure 7.
Frontier molecular orbital diagram (HOMO-LUMO) of 5TDOP with band gap values.
Table 5.
Calculated energy values of 5TDOP by B3LYP/6-311++G(d,p) by B3LYP/6-311++G(d,p) method.
| Basis set | B3LYP/6-311+G(d,p) |
|---|---|
| HOMO(eV) | -6.0448 |
| LUMO(eV) | -0.9364 |
| Ionization potential | 6.0448 |
| Electron affinity | 0.9364 |
| Energy gap(eV) | 5.1084 |
| Electronegativity | 3.4906 |
| Chemical potential | -3.4906 |
| Chemical hardness | 2.5542 |
| Chemical softness | 0.1958 |
| Electrophilicity index | 2.3851 |
| Electronic charge | 1.3666 |
| Electron donating capability (ω-) | 4.4496 |
| Electron accepting capability (ω+) | 0.9591 |
The 5TDOP has the ionization potential value (6.0448) which is much higher than the value of electron affinity (0.9364) that makes a finding of greater electron donor capability (ω-) of header compound as compared to electron acceptor capability (ω+) values. All these values may lead the biological activity of 5TDOP.
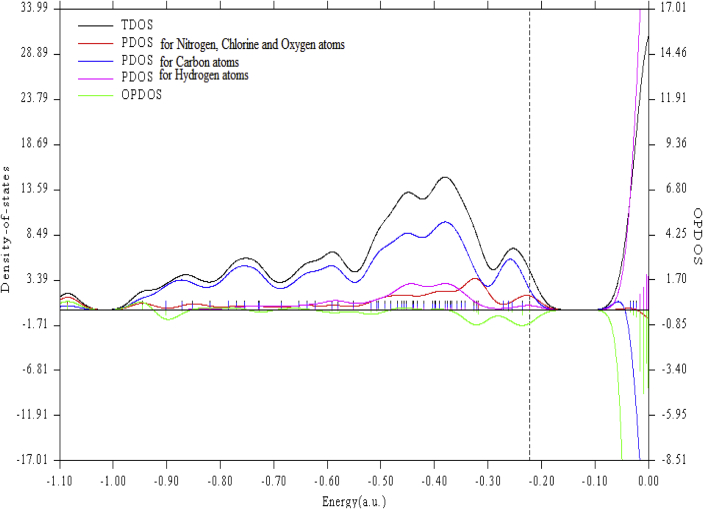
The Density of States graph of 5TDOP has been obtained by Multiwfn software to predict the electrical property [20] of the molecule. The band gap value that obtained in DOS is in agreement with the band gap values of UV-Vis analysis and HOMO-LUMO analysis. The small band gap of 5TDOP indicates the interaction between the atoms that are important in electronic and NLO property. Figure 8 represents the DOS diagram of 5TDOP in which black curve indicate total density of states, red curve indicate the density of states due to electronegative atoms (such as Cl, N and O atoms), blue curve depict the density of states due to carbon atoms, pink curve represents the density of states due to hydrogen atoms, green curve indicate the overlapping density of states of the molecule. The dotted long black line separates the HOMO and LUMO orbital which is called as band gap. The small vertical blue line indicates the HOMO (filled) orbitals of 5TDOP. The transmission from HOMO to LUMO has been shown clearly in Figure 8 that shows the charge transfer with in the molecule. The HOMO transitions are started from 0.22 a.u. (-0.22∗27.211 = 5.986 eV). One of the LUMO transition values is noted as 0.05 a. u. (0.05∗27.211 = 1.3 eV). Hence the DOS figure shows the HOMO-LUMO transitions for the molecule.
Figure 8.
Density of States graph of 5TDOP with TDOS (Total Density of States), PDOS (Partial Density of States), OPDOS (Overlapping Density of states).
4.4. Natural Bond Orbital (NBO) analysis
Natural bond orbital analysis is a significant tool for investigating the inter and intra molecular interactions between the bonds of atoms [37, 38, 39]. The donor–acceptor interactions, bonding type, electron density and stabilization energy have been calculated on ASN molecule at DFT/B3LYP method with 6–311++G(d,p) basis set using second-order perturbation theory and an important donor-acceptor interaction values are listed in Table 6. The higher stabilization energy (E2) is involved in the enormous charge transfer of donor–acceptor interactions with in the molecular system. In the present study, the higher stabilization energy is calculated in LP(Cl), LP(O) and LP(N) donors that are interacted with the neighboring antibonding acceptors π∗(C17–C19), π∗(C9–C12), and π(C6–H24), with the energies of 13.26, 13.95, and 7.86 kcal/mol for 5TDOP. The NBO and MEP analysis results indicate that the maximum electrophilic potential atoms are donating the electrons to the surrounding acceptors.
Table 6.
Second order perturbation theory analysis of Fock matrix in NBO basis for 5TDOP.
| Donor(i) | ED/e | Acceptor(i) | ED/e | aE(2) (kJ mol−1) | bE(j)-E(i) (a.u.) | cF(i,j) (a.u.) |
|---|---|---|---|---|---|---|
| LP(1)Cl1 | 1.99124 | π(C13–C17) | 0.02877 | 1.87 | 1.48 | 0.047 |
| π(C17–C19) | 0.02892 | 1.80 | 1.50 | 0.047 | ||
| LP(2)Cl1 | 1.96892 | π(C13–C17) | 0.02877 | 4.59 | 0.87 | 0.056 |
| π(C17–C19) | 0.02892 | 4.61 | 0.89 | 0.057 | ||
| LP(3)Cl1 | 1.92449 | π∗(C17–C19) | 0.40260 | 13.26 | 0.34 | 0.066 |
| LP(1)O2 | 1.94950 | π(C8–C11) | 0.03625 | 5.77 | 1.04 | 0.069 |
| π(C9–C12) | 0.03680 | 7.57 | 1.04 | 0.079 | ||
| π(C11–C15) | 0.02557 | 1.12 | 1.04 | 0.031 | ||
| π∗(C11–C15) | 0.36148 | 1.84 | 0.50 | 0.030 | ||
| π(C12–C16) | 0.02435 | 1.35 | 1.02 | 0.033 | ||
| LP(2)O2 | 1.87009 | π(C8–C11) | 0.03625 | 1.34 | 0.95 | 0.033 |
| π∗(C9–C12) | 0.37986 | 13.95 | 0.43 | 0.074 | ||
| π(C11–C15) | 0.02557 | 3.66 | 0.95 | 0.054 | ||
| π∗(C11–C15) | 0.36148 | 9.46 | 0.42 | 0.060 | ||
| π(C12–C16) | 0.02435 | 1.51 | 0.93 | 0.034 | ||
| LP(1)N3 | 1.88664 | π(C6–H23) | 0.01671 | 2.77 | 0.69 | 0.040 |
| π(C6–H24) | 0.03457 | 7.86 | 0.66 | 0.066 | ||
| π(C7–H25) | 0.02398 | 5.27 | 0.67 | 0.054 | ||
| π(C7–H26) | 0.02033 | 4.31 | 0.69 | 0.050 | ||
| π(C10–H27) | 0.00872 | 1.27 | 0.68 | 0.027 | ||
| π(C10–H28) | 0.02639 | 8.24 | 0.67 | 0.068 | ||
| π(C10–H29) | 0.00866 | 1.25 | 0.68 | 0.027 |
E(2) means energy of hyper conjugative interaction (stabilization energy).
Energy difference between donor and acceptor i and j NBO orbitals.
F(i,j) is the Fock matrix element between i and j NBO orbitals.
4.5. Fukui analysis
The Fukui functions are widely used for molecular reactivity analysis such as electrophilic, nucleophilic and free radical attacks in a molecular system [40]. The individual atomic sites of 5TDOP is calculated using natural population analysis (NPA) with B3LYP/6-311++G(d,p) basis set. The Fukui functions are calculated from following equations
| (6) |
| (7) |
| (8) |
In these equations, is the atomic charge (evaluated from Natural population analysis, electrostatic derived charge, etc.) at the rth atomic site with the neutral (N), anionic (N+1), cationic (N-1) chemical species. The dual descriptor is the best view of nucleophilicity and electrophilicity characters. Recently, Morrel et al [41] calculated and reported the nucleophilic, nucleophilic characters of organic molecules using dual descriptor analysis by using the following equation.
| (9) |
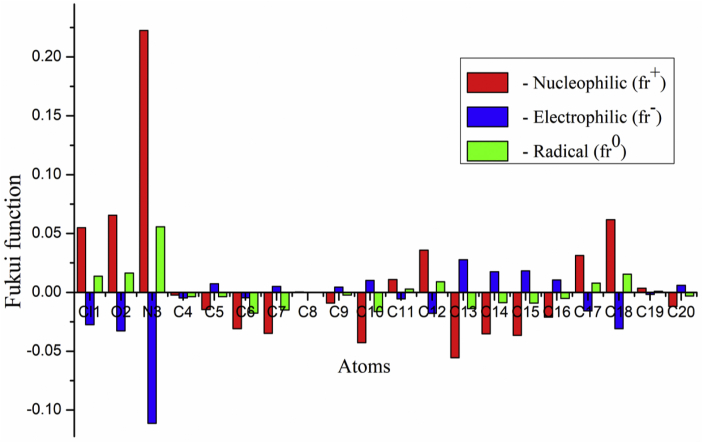
In the present study, the Fukui functions and the dual descriptors are displayed in Figures 9 and 10. In the Figure 9, nucleophilic, electrophilic and radical attacks have been represented by red, blue and Green colours. The nucleophilic attacks have been found around Cl1, O2, N3 C11, C12, C17, C18 and C19 atoms for 5TDOP. The electrophilic attacks have been found around C5, C6, C7, C9, C10, C13, C14, C15, C16 and C20 atoms. The results for the positive and the negative regions have been reported in Table 7.
Figure 9.
Fukui function graph for natural charges of 5TDOP.
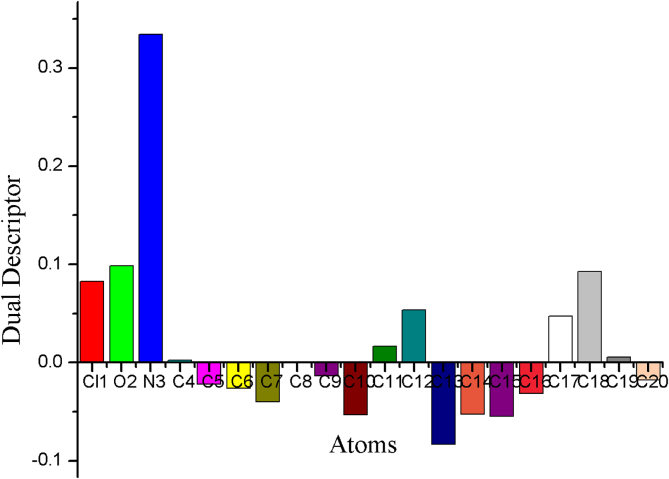
Figure 10.
Fukui function graph for dual descriptor of 5TDOP.
Table 7.
Fukui functions of 5TDOP with NPA atomic charges.
| Atoms | Natural charges |
Fukui functions |
Dual descriptor (Δr) | ||||
|---|---|---|---|---|---|---|---|
| N | N +1 | N-1 | fr + | fr - | fr0 | ||
| Cl1 | 0.0353 | 0.0903 | 0.0628 | 0.0550 | -0.0275 | 0.0138 | 0.0825 |
| O2 | 0.0057 | 0.0712 | 0.0384 | 0.0655 | -0.0327 | 0.0164 | 0.0982 |
| N3 | 0.0063 | 0.2289 | 0.1176 | 0.2226 | -0.1113 | 0.0557 | 0.3339 |
| C4 | -0.0018 | -0.0042 | 0.0030 | -0.0024 | -0.0048 | -0.0036 | 0.0024 |
| C5 | 0.0107 | -0.0039 | 0.0034 | -0.0146 | 0.0073 | -0.0037 | -0.0219 |
| C6 | 0.0054 | -0.0254 | 0.0100 | -0.0308 | -0.0046 | -0.0177 | -0.0262 |
| C7 | 0.0112 | -0.0237 | 0.0062 | -0.0349 | 0.0050 | -0.0150 | -0.0399 |
| C8 | 0.0023 | 0.0027 | 0.0025 | 0.0004 | -0.0002 | 0.0001 | 0.0006 |
| C9 | 0.0233 | 0.0142 | 0.0188 | -0.0091 | 0.0045 | -0.0023 | -0.0136 |
| C10 | 0.0158 | -0.0270 | 0.0056 | -0.0428 | 0.0102 | -0.0163 | -0.0530 |
| C11 | 0.0085 | 0.0195 | 0.0140 | 0.0110 | -0.0055 | 0.0028 | 0.0165 |
| C12 | -0.0062 | 0.0296 | 0.0117 | 0.0358 | -0.0179 | 0.0090 | 0.0537 |
| C13 | 0.0669 | 0.0115 | 0.0392 | -0.0554 | 0.0277 | -0.0139 | -0.0831 |
| C14 | 0.0359 | 0.0007 | 0.0183 | -0.0352 | 0.0176 | -0.0088 | -0.0528 |
| C15 | 0.0618 | 0.0252 | 0.0435 | -0.0366 | 0.0183 | -0.0092 | -0.0549 |
| C16 | 0.0459 | 0.0249 | 0.0354 | -0.0210 | 0.0105 | -0.0053 | -0.0315 |
| C17 | 0.0115 | 0.0428 | 0.0272 | 0.0313 | -0.0157 | 0.0078 | 0.0470 |
| C18 | 0.0112 | 0.0729 | 0.0421 | 0.0617 | -0.0309 | 0.0154 | 0.0926 |
| C19 | 0.0201 | 0.0236 | 0.0219 | 0.0035 | -0.0018 | 0.0009 | 0.0053 |
| C20 | 0.0371 | 0.0251 | 0.0311 | -0.0120 | 0.0060 | -0.0030 | -0.0180 |
The Dual descriptor states that, if Δƒ() >0, then the location is prone to nucleophilic attack, if Δƒ()<0, then the location is prone to electrophilic attack [37,40]. The higher nucleophilicity has been calculated at a lone pair of nitrogen and oxygen atoms. The Figure 10 represents NPA atomic charges that predict the reactivity order for the nucleophilic case as N3 > O2 > C18 > Cl1 > C12 > C17 > C11 > 19 > C4 atoms, electrophilic case as C13 > C15 > C14 > Cl0 > C7 > C16 > C6 > C5 > C20 > C9 atoms and radical case as C8 atom. The nucleophilic attacks usually play major role in biochemistry field. Here, the donation of electrons with attraction (electrophilic) and partially electrophilic characters in surrounding atomic sites are reported. In the molecular reactivity analysis of the Fukui functions, the dual descriptor is very well with the results from the MEP analysis of the 5TDOP molecule.
4.6. MEP, LOL, and ELF analysis
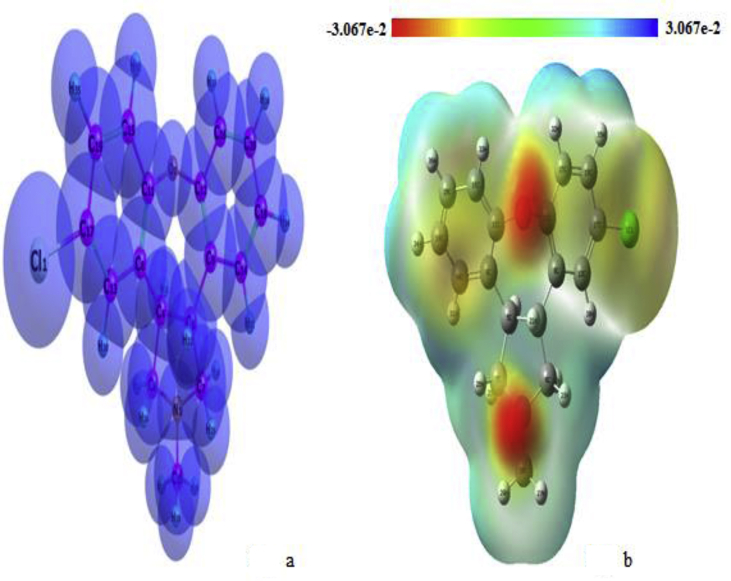
Molecular electrostatic potential surface analysis is the most successful approach to investigate electrophilic, nucleophilic and radical attack. It is very useful to analyze the molecular reactivity in organic, inorganic, polymer materials and also to identify the bio-molecular nature [42,43]. In the present study, the MEP surface of an ASN molecule is visualized by Gauss View 5.0 program and shown in Figure 11. In recent years, most of the researchers are used to visualize MEP surface using Gauss View 5.0 program in order to explain the different molecular electrostatic potential surfaces (in different colors for electrophilic, radial and nucleophilic attack that are presented by red, green and blue colors). The higher nucleophilic potential (Red color) is presented around N and O region in oxepino[4,5-c]pyrrole ring, partially nucleophilic (yellow colour) potential is presented around Cl and C atoms in Dibenzazepine (iminostilbene) ring and electrophilic potential is presented in C (pyrrole ring) and H atoms for 5TDOP molecule. The higher electrostatic potentials are concerned with an enormous amount of charge transfer during the intra and inter-molecular interactions in ASN molecule.
Figure 11.
Molecular electrostatic potential diagram of 5TDOP (Fig. a represents the Van der Waals sphere for every atom, Fig. b represents the MEP surface).
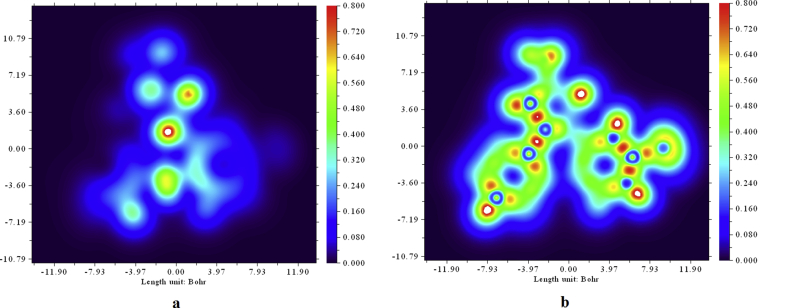
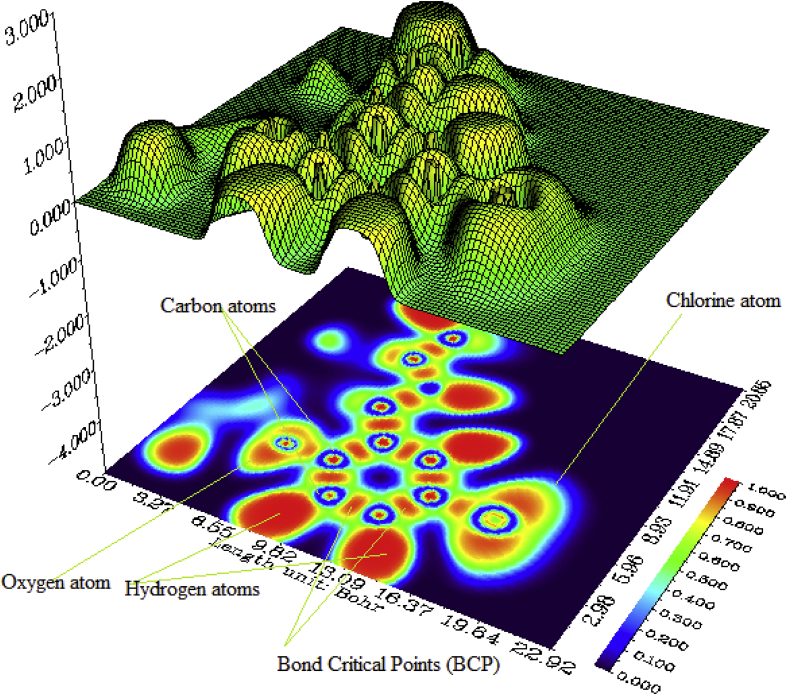
The Localized Orbital Locator (LOL) contributed by atoms [42] of 5TDOP has been explained through a color filled Plane map. LOL is one of the unique descriptors to know chemical bonding between the atoms of the molecule. The LOL plane map has been shown in Figure 12a and Figure 12b to know the chemically significant regions of 5TDOP. The benzene ring is shown by a blue color circle and BCP (Bond Critical Point) has been shown by red with yellow path at where delocalization of electrons of 5TDOP can be seen. Figure 12a represents the LOL due to electronegative atoms and Figure 12b represents the LOL due to whole atoms of the molecule.
Figure 12.
LOL (Localized orbital locator) of 5TDOP (Fig. a for electronegative atoms, Fig. b for whole molecule).
The Electron Localization Function (ELF) [45,20] of 5TDOP has been visualized Figure 13 with the Relief map which is a green map at the top of the topology image. Relief map shows the interactive interface of every atom in 5TDOP. Every atom is denoted by a peak (nuclei) with its electron distribution in the surroundings. The bottom map is ELF color filled map of 5TDOP. The BCP's, Chlorine, hydrogen, oxygen and carbon atoms have clearly shown in the figure with the electronic distribution. The lone pair at O and Cl atoms, electron depletion layer between inner and outer shell (blue circle around the nuclei) have also been shown in figure. Thus, these topology analyses suggest that the molecule 5TDOP is chemically active and hence it may possess biological applications.
Figure 13.
ELF (Electron localization function) with relief map of 5TDOP.
4.7. NLO properties
Buckingham's definitions are usually used to calculate the NLO properties such as the dipole moment (μ), polarizability (α), and the first static hyperpolarizability (β) of the selected molecule through DFT calculations from Gaussian output [44]. The polarizability and the hyperpolarizability are the important tools in pharmacology, drug designing and in industrial fields as quantitative structure activity relationship tools [45]. The atoms that are in 5TDOP share its electrons unequally. Therefore, a difference in electronegativity creates dipole moment.
When the external electric field is weak and homogenous, this expansion becomes:
| E = E0 – μαFα – ½ (ααβFαFβ) – ½ (βαβγFαFβFγ) + … | (10) |
where E0 is the energy of the unperturbed molecules, Fα the field at the origin μα, ααβ and βαβγ are the components of dipole moments, polarizability and the first hyperpolarizability [48-50]. The total static dipole moment μ, the mean polarizability α0, the anisotropy of the polarizability Δα and the mean polarizability β0, using the x,y,z components are defined as:
| μ = (μx2+ μy2+ μz2)1/2 | (11) |
| α = (αxx+ αyy+ αzz)/3 | (12) |
| Δα = 2−1/2 [(αxx – αyy)2 + (αyy – αzz)2 + (αzz – αxx)2 + 6αxx2]1/2 | (13) |
| β0 = (βx2 + βy2 + βz2)1/2 | (14) |
And
βx = βxxx + βxyy + βxzz
βx = βyyy + βyxx + βyzz
βx = βzzz + βzxx + βzyy
The dipole moment of the title molecule is 0.8516 which is a non-zero value that shows the larger value in electronegativity difference. The NLO parameters are shown in Table 8 in which the β value is compared to the reference NLO material urea. The β value of urea is 0.927 × 10−30 e.s.u and the β value of 5TDOP is 5.7285X10−30 e.s.u with the α of 3.2499 × 10−23 e.s.u. So, the title molecule may possess good NLO activity to produce second order non-linear effects. The polarizability of the molecule represents the changes in electron distribution. According to NBO analysis which is mentioned earlier, π- π∗ transactions indicate the intramolecular interaction that increases the polarizability value. The minimum band gap energy from HOMO-LUMO analysis also indicates the increase in NLO property of 5TDOP. Thus, 5TDOP may have electronic and biological significance.
Table 8.
The values of calculated dipole moment μ (D), polarizability (α0), first order hyperpolarizability (βtot) components of 5TDOP.
| Parameters | B3LYP/6-311++G(d,p) | Parameters | B3LYP/6-311++G(d,p) |
|---|---|---|---|
| μx | -0.57599 | βxxx | -400.17730 |
| μy | -0.19195 | βxxy | -114.56300 |
| μz | 0.59710 | βxyy | 9.24335 |
| μ(D) | 0.85160 | βyyy | -25.70450 |
| αxx | 272.59830 | βzxx | 135.45640 |
| αxy | 8.42020 | βxyz | 8.39180 |
| αyy | 238.46580 | βzyy | 102.77320 |
| αxz | 2.61330 | βxzz | -65.19154 |
| αyz | -12.16064 | βyzz | 36.23500 |
| αzz | 146.81810 | βzzz | 231.66410 |
| α0 (e.s.u) | 3.2499 × 10−23 | βtot (e.s.u) | 5.7285X10−30 |
| Δα(e.s.u) | 7.1938 x10−23 |
4.8. Molecular docking analysis
The biological activities of title compound are estimated by molecular docking analysis. Molecular docking bioactive conformation is helpful for drug design with organic molecules. It is a preliminary analysis that reduces time and cost with simply identified protein-ligand energy interactions [46,47].
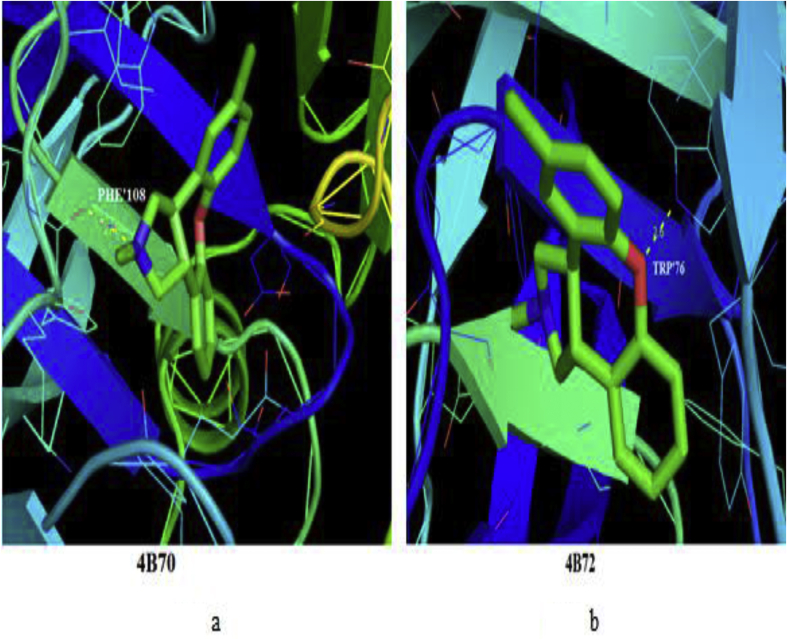
The 5TDOP molecule has the form of aminoimidazoles as BACE-1 Inhibitors. The aminoimidazoles as BACE-1 Inhibitors affect human beings and cause Alzheimer's disease. The Alzheimer's disease is a neurological disorder in which the death of brain cells causes memory loss and cognitive decline. Harald Hampel et al reported that BACE-1 Inhibitor is a most important drug target for slowing down Aβ (β-amyloid peptide) production in early Alzheimer's disease (AD). The BACE-1 inhibition has direct consequences in the Alzheimer's disease (AD) pathology without largely affecting viability [1]. Thus, the activity of BACE-1 inhibition is satisfactory to interpreting schizophrenia like phenotypes [2]. Additional, chiriano et al [3] stated that the possible frameworks of aminoimidazole turned to be an validate its capability to interact with the BACE-1 inhibitor. Thus, the BACE-1 Inhibitors chosen with suitable protein targets such as 4B70, 4B72 are downloaded from the protein data bank. The protein-ligand interactions, binding energy, bond distance, estimate inhibition constant and RMSD values are calculated for the 5TDOP molecule and the values have been listed in Table 9. The lowest binding energies have been observed as -6.31 kcal/mol with 4B70 protein and as -6.58 kcal/mol with 4B72 protein. The estimated inhibition constant has been calculated as 23.78 μm and 14.92 μm during the protein-ligand docking analysis. The lowest binding energies of the ligand 5TDOP molecule with the selected proteins are clearly illustrated in Figure 14 that the title molecule 5TDOP (antiaminoimidazoles) may inhibit BACE-1 and hence it may be a biologically significant molecule.
Table 9.
Molecular docking analysis of 5TDOP with proteins for membrane permeable inhibitor.
| Protein (PDB ID) | Bonded residues | Bond distance (Å) | Estimated inhibition constant (μm) | Binding energy (kcal/mol) | Intermolecular energy (kcal/mol) | Reference RMSD (Å) |
|---|---|---|---|---|---|---|
| 4B70 | PHE′108 | 2.4 | 23.78 | -6.31 | -6.71 | 20.571 |
| 4B72 | 2.6 | 14.92 | -6.58 | -7.50 | 22.573 |
Figure 14.
Molecular docking simulation of 5TDOP with the selected proteins (Fig. a for 4B70 and Fig. b for 4B72 proteins).
4.9. Conclusion
The aromatic, heterocyclic dibenzoxepine derivative molecule has been chosen. The molecular stability has been performed through PES conformation analysis. The minimum energy conformer structure has been optimized for the structural parameters and vibrational modes of the molecule. The FT-IR, FT-Raman and bond length values were obtained theoretically by DFT/B3LYP/6-311++G(d,p) and compared with experimental values. Charge transformation within 5TDOP was calculated using frontier molecular orbitals. The band gap energy obtained between HOMO and LUMO is 5.0740 eV which is closely related to UV-Vis value. The transition from HOMO to LUMO shows the maximum distribution of electrons. The stabilization energies are calculated in LP(O), LP(Cl) and LP(N) donors that are interacted with the neighboring anti-bonding acceptors. The second order non-linear optical effects of 5TDOP have been reported by hyperpolarizability. Furthermore, the non-zero value of the dipole moment indicates that the molecule is highly polar. The nucleophilic and electrophilic nature of the molecule has been explained by MEP and Fukui reactivity receptors. ELF and LOL highlight the chemically significant region of 5TDOP. The estimated binding energy with hydrogen bond lengths using 4B70 and 4B72 proteins have been calculated which shows that 5TDOP can act as a membrane permeable inhibitor.
Declarations
Author contribution statement
S. Sevvanthi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
S. Muthu: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
M. Raja: Performed the experiments; Analyzed and interpreted the data.
S. Aayisha, S. Janani: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Kakkar S., Tahlan S., Lim S.M., Ramasamy K., Mani V., A Shah S.A., Narasimhan B. Benzoxazole derivatives: design, synthesis, and biological evaluation. Chem. Cent. J. 2018;12(1) doi: 10.1186/s13065-018-0459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaur R., Rani V., Abbot V. Recent synthetic and medicinal perspectives of pyrroles: an overview. J. Pharm. Chem. Chem. Sci. 2017;1(1):17–32. [Google Scholar]
- 3.Boer T., Meulman E., Meijering H., Wieling J., Dogterom P., Lass H. Quantification of asenapine and three metabolites in human plasma using liquid chromatography-tandem mass spectrometry with automated solid-phase extraction: application to a phase I clinical trial with asenapine in healthy male subjects. Biomed. Chromatogr. 2011;26(2):156–165. doi: 10.1002/bmc.1640. [DOI] [PubMed] [Google Scholar]
- 4.Protti M., Vignali A., Sanchez Blanco T., Rudge J., Bugamelli F., Ferranti A., Mercolini L. Enantioseparation and determination of asenapine in biological fluid micromatrices by HPLC with diode array detection. J. Sep. Sci. 2018;41(6):1257–1265. doi: 10.1002/jssc.201701315. [DOI] [PubMed] [Google Scholar]
- 5.Patel N.P., Sanyal M., Sharma N., Patel D.S., Shrivastav P.S., Patel B.N. Determination of asenapine in presence of its inactive metabolites in human plasma by LC-MS/MS. J. Pharm. Sci. 2018;8(5):341–347. doi: 10.1016/j.jpha.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller C., Pleitez O., Anderson D., Mertens-Maxham D., Wade N. Asenapine (Saphris(R)): GC-MS method validation and the postmortem distribution of a new atypical antipsychotic medication. J. Anal. Toxicol. 2013;37(8):559–564. doi: 10.1093/jat/bkt076. [DOI] [PubMed] [Google Scholar]
- 7.Citrome L., Walling D., Zeni C., Komaroff M., Park A. O50. Efficacy and safety of an asenapine transdermal patch (asenapine transdermal system, HP-3070) in the treatment of adults with schizophrenia: a phase 3, randomized, double-blind, placebo-controlled, 6-week inpatient study. Biol. Phychiatry. 2019;85(10):S126. [Google Scholar]
- 8.Karaca S.A., Ugur D.Y. A stability indicating ion-pair lc method for the determination of asenapine in pharmaceuticals. J. Chil. Chem. Soc. 2017;62(1):3325–3329. [Google Scholar]
- 9.Anugu R.R., Mainkar P.S., Sridhar B., Chandrasekhar S. The Ireland–Claisen rearrangement strategy towards the synthesis of the schizophrenia drug, (+)-asenapine. Org. Biomol. Chem. 2016;14(4):1332–1337. doi: 10.1039/c5ob02047f. [DOI] [PubMed] [Google Scholar]
- 10.Gambhire V.M. Enhanced oral delivery of asenapine maleate from solid lipid nanoparticles: pharmacokinetic and brain distribution evaluations. Asian J. Pharm. 2018;12(3) [Google Scholar]
- 11.Becke A.D. Perspective: fifty years of density-functional theory in chemical physics. J. Chem. Phys. 2014;140(18):18A301. doi: 10.1063/1.4869598. [DOI] [PubMed] [Google Scholar]
- 12.Kohn W., Sham L.J. Self-consistent equations including exchange and correlation effects. APS. 1965;140:A1133–A1138. [Google Scholar]
- 13.Becke A.D. Density-functional thermochemistry. III. The role of exact exchange. Chem. Phys. 1993;98(7):5648–5652. [Google Scholar]
- 14.Frisch M.J., Trucks G.W., Zakrzewski V.G., Ortiz, Cioslowski J., Fox D.J. Gaussian, Inc.; Wallingford CT: 2009. Gaussian 09, Revision E.01. [Google Scholar]
- 15.Dennington R., Keith T., Millam J. Semichem Inc.; Shawnee Mission, KS: 2009. GaussView. Version 5. [Google Scholar]
- 16.Jamroz M.H. VEDA 4 Program Warsaw; Poland: 2004. Vibrational Energy Distribution Analysis. [Google Scholar]
- 17.Aayisha S., Renuga Devi T.S., Janani S., Muthu S., Raja M., Sevvanthi S. DFT, molecular docking and experimental FT-IR, FT-Raman, NMR inquisitions on “4-chloro-N-(4,5-dihydro-1H-imidazol-2-yl)-6-methoxy-2-methylpyrimidin-5-amine”: alpha-2-imidazoline receptor agonist antihypertensive agent. J. Mol. Struct. 2019;1186:468–481. [Google Scholar]
- 18.Lu T., Chen F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 2012;33:580–592. doi: 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- 19.Azam S., Abbasi S. Molecular docking studies for the identification of novel melatoninergic inhibitors for acetylserotonin-O-methyltransferase using different docking routines. Theor. Biol. Med. Model. 2013;10(1):63. doi: 10.1186/1742-4682-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aayisha S., Renuga Devi T.S., Janani S., Muthu S., Raja M., Sevvanthi S., Raajaraman B.R. Vibrational and computational analysis for molecular structure properties of N-(2-(trifluoromethyl)phenyl)acetamide: density functional theory approach. Spectrosc. Lett. 2019;52(9):563–576. [Google Scholar]
- 21.Karunakaran V., Balachandran V. FT-IR, FT-Raman spectra, NBO, HOMO–LUMO and thermodynamic functions of 4-chloro-3-nitrobenzaldehyde based on ab initio HF and DFT calculations. Spectrochim. Acta A. 2012;98:229–239. doi: 10.1016/j.saa.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Abraham Christina Susan, Prasana Johanan Christian, Muthu S., Fathima Rizwana B., Raja M. Quantum computational studies, spectroscopic (FT-IR, FT-Raman and UV-Vis) profiling, natural hybrid orbital and molecular docking analysis on 2,4 Dibromoaniline. J. Mol. Struct. 2018;1160:393–405. [Google Scholar]
- 23.Furie K., Mohcek V., Bonifacic M., Stefanic I. Raman spectroscopic study of H2O and D2O water solutions of glycine. J. Mol. Struct. 1992;267:39–44. [Google Scholar]
- 24.Sevvanthi S., Muthu S., Raja M. Quantum mechanical, spectroscopic studied and molecular docking analysis on 5,5-diphenylimidazolidine-2,4-dione. J. Mol. Struct. 2017;1149:487–498. [Google Scholar]
- 25.Prabakaran A., Muthu S. Normal coordinate analysis and vibrational spectroscopy (FTIR and FT-Raman) studies of (S)-2-amino-3-(3,4-dihydroxyphenyl)-2-methylpropanonic acid using ab initio HF and DFT method. Spectrochim. Acta A. 2012;99:90–96. doi: 10.1016/j.saa.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Varsanyi G. Academic Kiaclo; Budapest: 1973. Assignment for Vibrational Spectra of Seven Hundred Benzene Derivatives. [Google Scholar]
- 27.Mohan J. Narosa Publishimg House; New Delhi: 2001. Organic Spectroscopy Principles and Aplications. [Google Scholar]
- 28.Gunasekaran S., Kumaresan S., Arunbalaji R., Anand G., Seshadri S., Muthu S. Vibrational assignments and electronic structure calculations for 6-thioguanine. J. Raman Spectrosc. 2019;40:1675–1681. [Google Scholar]
- 29.Barnes A.J., Majid M.A., Stuckey M.A., Gregory P., Stead C.V. Quantum mechanicl study and spectroscopic (FT-IR, FT-Raman, 13C, 1H, UV) study, first order hyperpolarizability, NBO analysis, HOMO and LUMO analysis of 4-[(4-aminobenzene) sulfonyl]aniline by ab initio HF and density functional method. Spectrochim. Acta A. 1985;4:629–635. doi: 10.1016/j.saa.2012.02.056. [DOI] [PubMed] [Google Scholar]
- 30.Rajamani T., Muthu S., Karabacak M. Electronic absorption, vibrational spectra, nonlinear optical properties, NBO analysis and thermodynamic properties of N-(4-nitro-2-phenoxyphenyl) methanesulfonamide molecule by ab initio HF and density functional methods. Spectrochim. Acta A. 2013;108:186–196. doi: 10.1016/j.saa.2013.01.090. [DOI] [PubMed] [Google Scholar]
- 31.Karabacak M., karaka C., Atac A., Eskici M., Karanfil A. Synthesis, analysis of spectroscopic and nonlinear optical properties of the novel compound: (S)-N-benzyl-1-phenyl-5-(thiophen-3-yl)-4-pentyn-2-amine. Spectrochim. Acta A. 2012;97:556–567. doi: 10.1016/j.saa.2012.05.087. [DOI] [PubMed] [Google Scholar]
- 32.Gunasekaran S., Ponnambalam U., Muthu S., Ponnusamy S. Vibrational and normal coordinate analysis of pyrazinamide. Asian J. Chem. 2004;16(23):1513–1518. [Google Scholar]
- 33.George S. third ed. Wiley; New York: 2001. Infrared and Raman Characteristic Group Frequencies, Tables and Charts. [Google Scholar]
- 34.Kaschula C.H., Egan T.J., Hunter R., Basilico N., Parapini S., Taramelli D., Pasini E., Monti D. Structure− activity relationships in 4-aminoquinoline antiplasmodials. The role of the group at the 7-position. J. Med. Chem. 2002;45(16):3531–3539. doi: 10.1021/jm020858u. [DOI] [PubMed] [Google Scholar]
- 35.Muruganantham N., Sivakumar R., Anbalagan N., Gunasekaran V., Leonard J.T. Synthesis, anticonvulsant and antihypertensive activities of 8-substituted quinoline derivatives. Biol. Pharm. Bull. 2004;27(10):1683–1687. doi: 10.1248/bpb.27.1683. [DOI] [PubMed] [Google Scholar]
- 36.Sevvanthi S., Muthu S., Raja M. Molecular docking, vibrational spectroscopy studies of (RS)-2-(tert-Butylamino)-1-(3-chlorophenyl)propan-1-one: a potential Adrenaline uptake inhibitor. J. Mol. Struct. 2018;1173:251. [Google Scholar]
- 37.Raja M., Raj Muhamed R., Muthu S., Suresh M. Synthesis, spectroscopic (FT-IR, FT-Raman, NMR, UV–Visible), NLO, NBO, HOMO-LUMO, Fukui function and molecular docking study of (E)-1-(5-bromo-2-hydroxybenzylidene)semicarbazide. J. Mol. Struct. 2017;1141:284–298. [Google Scholar]
- 38.Muthu S., Uma Maheswari J. Quantum mechanical study and spectroscopic (FT-IR, FT-Raman, 13C, 1H, UV) study, first order hyperpolarizability, NBO analysis, HOMO and LUMO analysis of 4-[(4-aminobenzene) sulfonyl] aniline by ab initio HF and density functional method. Spectrochim. Acta A. 2012;92:154–163. doi: 10.1016/j.saa.2012.02.056. [DOI] [PubMed] [Google Scholar]
- 39.Swarnalatha N., Gunasekaran S., Muthu S., Nagarajan M. Molecular structure analysis and spectroscopic characterization of 9-methoxy-2H-furo[3,2-g]chromen-2-one with experimental (FT-IR and FT-Raman) techniques and quantum chemical calculations. Spectrochim. Acta A. 2015;137:721–729. doi: 10.1016/j.saa.2014.08.125. [DOI] [PubMed] [Google Scholar]
- 40.Anju L.S., Aruldhas D., Hubert Joe I., Balachandran S. Density functional theory, spectroscopic and hydrogen bonding analysis of fenoxycarb–water complexes. J. Mol. Struct. 2019;1201:127201. [Google Scholar]
- 41.Morell C., Grand A., Toro-Labbé A. New dual descriptor for chemical reactivity. J. Phys. Chem. A. 2005;109(1):205–212. doi: 10.1021/jp046577a. [DOI] [PubMed] [Google Scholar]
- 42.Rizwana B.F., Muthu S., Prasana J.C., Abraham C.S., Raja M. Spectroscopic (FT-IR, FT-Raman) investigation, topology (esp, elf, lol) analyses, charge transfer excitation and molecular docking (dengue, hcv) studies on ribavirin. Chem. Data Collections. 2018;17–18:236–250. [Google Scholar]
- 43.Raja M., Muhamed R.R., Muthu S., Suresh M. Synthesis, spectroscopic (FT-IR, FT-Raman, NMR, UV–Visible), first order hyperpolarizability, NBO and molecular docking study of (E)-1-(4-bromobenzylidene)semicarbazide. J. Mol. Struct. 2017;1128:481–492. [Google Scholar]
- 44.Fernando D.V., David A.S., Yoshinari T., Xavier A., Angel R., Steven G.L., John J.R. Basis set effects on the hyperpolarizability of CHCl_3: Gaussian-type orbitals, numerical basis sets and real-space grids. J. Chem. Phys. 2010;133 doi: 10.1063/1.3457362. [DOI] [PubMed] [Google Scholar]
- 45.Silva J.R.A., Lameira J., Alves C.N. Insights for design of Tripanosoma cruzi GAPDH inhibitors: a QM/MM MD of 1,3-bisphospho-D-glyceric acid analogs. Int. J. Quant. Chem. 2012;112:3398–3402. [Google Scholar]
- 46.Ramesh P., Lydia Caroline M., Muthu S., Narayana B., Raja M., Aayisha S. Spectroscopic and DFT studies, Structural determination, Chemical properties and Molecular docking of 1-(3-Bromo-2-thienyl)-3-[4-(dimethylamino)-phenyl]prop-2-en-1-one. J. Mol. Struct. 2019;1200:127123. [Google Scholar]
- 47.Liang X., Jiang J., Xue X., Huang L., Ding X., Nong D. Synthesis, characterization, photoluminescence, anti-tumor activity, DFT calculations and molecular docking with proteins of zinc(ii) halogen substituted terpyridine compounds. Dalton Trans. 2019;48(28):10488–10504. doi: 10.1039/c8dt04924f. [DOI] [PubMed] [Google Scholar]