Abstract
Cardiovascular diseases are described as disorders of heart and vessels that involve stroke and coronary heart diseases. People in the Middle East converged to complementary medicine as an economic alternative to expensive healthcare services. Crataegus monogyna Jacq. (Lindm.) Rosacea is among the most commonly used herb for the treatment of declining cardiac performance, hypertension, and arrhythmias. Previously, we had shown that Crataegus Spp. (Hawthorn) extract increased the tendency of bleeding among patients undergoing coronary artery bypass grafting. Herein, the effects of Crataegus Spp. extract on oxidative stress, cardiac and hematological parameters were evaluated in Sprague Dawley rats. Male rats were randomly assigned into four groups. Group 1 served as control while groups 2–4 served as the experimental groups and were administered extract at doses of 100, 200, and 500 mg/kg. All the doses were given orally once/day and the treatment was continued for three weeks. Hawthorn treatment resulted in a significant decrease in the liver thiobarbituric acid reactive substances level in a dose-dependent manner compared to the control (1.258 (3, 24); P < 0.0001). We found a significant increase in the cardiac antithrombin III among hawthorn treated group compared to the control (4.18 (3, 24); P < 0.0001). On the other hand, hawthorn treatment decreased significantly the liver factor-X level (0.1341 (3, 22); P < 0.0001), while no significant changes were seen in soluble-platelet endothelial cell adhesion molecule-1 (P-value = 0.0599). In conclusions, hawthorn extract possesses an antioxidant effect and blood-thinning properties. Hence, we recommend attention when using this herbal extract with other anticoagulation and/or antiplatelet drugs or undergoing major cardiac surgery.
Keywords: Hawthorn, Crataegus monogyna Jacq. (Lindm.) Rosacea, Hemostasis, Oxidative biomarkers, Rats, Ethnopharmacology, Botany, Bioactive plant product, Cardiovascular system, Pharmacology, Clinical toxicology, Alternative medicine
Hawthorn; Crataegus monogyna Jacq. (Lindm.) Rosacea; Hemostasis; Oxidative biomarkers; Rats; Ethnopharmacology; Botany; Bioactive plant product; Cardiovascular system; Pharmacology; Clinical toxicology; Alternative medicine.
1. Introduction
Herbal medicines have been used by different cultures all over the world for the prevention and treatment of a wide array of health problems, including cardiovascular disease [1, 2]. There is a steady growth in the market of herbal medicines worldwide, with estimated annual global sales exceeding US $ 60 billion [3]. In the United States, total retail sales of herbal dietary supplements have increased by 8.5% in 2017 marking the 14th consecutive year of growth [4]. Furthermore, the WHO estimated that about 80% of the people in developing countries rely on plant-derived traditional medicines for their primary health care needs [5]. The increased use of herbal products could be attributed to many factors, including the wide-spread of chronic illnesses, the general desire for good health, the increased costs of conventional medications, and the erroneous belief among the public that herbal products are safer than synthetic drugs [6].
Wild edible fruits including hawthorn have been an indispensable part of human life for ages. Ever since ancient times, their fruits, leaves, seeds, even roots and branches have been used to meet personal and social needs such as severing food and beautifying the planet [7, 8]. Some of them may also possess medicinal properties, and are therefore used in treatment of ailments as a part of herbal medicine [9, 10]. Crataegus preparations are among the most commonly used herbal medicines worldwide for the treatment of declining cardiac performance according to Stage II of the New York Heart Association (NYHA) [11]. Hawthorn flowers and berries have been used traditionally as cardiac tonics and mild diuretics [2]. Hawthorn, which belongs to the family rosacea, is a shrub or small tree with thorny twigs that includes about 260 species native to Europe, East Asia, and eastern North America [2, 11]. Crataegus oxyacantha and C. monogyna are the most commonly used species for the preparation of hawthorn-based medicines [12]. Although the therapeutic efficacy for hawthorn leaves and flowers is well-established, the German Commission E did not approve therapeutic use of the fruit [1, 12]. Most of the clinical and pharmacological studies of hawthorn reported the use of hydro-alcoholic dry extracts of the flowers and leaves, herb-to-extract ratio 4–7:1 (w/w), using ethanol 45% (v/v) or methanol 70% (v/v) [11].
Hawthorn flowers, berries and leaves contain a range of bioactive constituents with flavonoids (e.g. vitexin, hyperoside, and rutin) and catechin/epicatechin derived oligomeric procyanidins being the most important. Other constituents include triterpene acids (ursolic, oleanolic, and crategolic acids) and amines (e.g. phenylethylamine, O-methoxy-phenylethylamine, and tyramine). Hawthorn preparations are standardized based on their flavonoid and oligomeric procyanidin content, which are reported to possess a set of cardiovascular effects, including coronary vasodilation, inotropic, and diuretic effects [13, 14, 15, 16, 17, 18, 19]. Extensive human and animal studies have shown hawthorn to be of value in the treatment of atherosclerosis, hypertension, and congestive heart failure [2, 11, 20, 21, 22, 23]. Additionally, the extract of Crataegus, a mixture of procyanidins and flavonoids obtained from hawthorn, increased the production of cGMP and caused a relaxation in vascular tone [24].
Hemostasis is a finely-regulated dynamic process that maintains fluidity of the blood, repairing vascular injury, and reducing blood loss while preventing vessel occlusion and inadequate perfusion of vital organs [25]. Endothelial dysfunction linked with different CVDs is interrelated to the local formation of reactive oxygen species (ROS). The ROS induce several changes in the structure and function in the hemostatic components. The proteins and lipids are considered the main preliminary targets in endothelial cells, plasma and platelets. During a vascular injury, the endothelial cell layer rapidly undergoes a series of modifications resulting in a more procoagulant phenotype [26]. Hence, the body will activate the clotting factors cascade in order to prevent bleeding. For example, factor Xa along with factor Va develops the prothrombinase complex on the activated cell surfaces, which catalyzes the conversion of prothrombin into thrombin [27, 28]. Thrombin, in turn, activates upstream clotting factors, primarily factors V, VIII, and XI, resulting in amplification of thrombin generation [29]. On the other hand, the endogenous anticoagulant Antithrombin III is a glycoprotein produced by the liver causes an inactivation of the serine proteases clotting factors and compensate for any disorder of blood coagulation [30]. Thus, disturbing the balance of oxidant/antioxidant system and clotting factors/endogenous anticoagulants could produce changes in the body hemostasis. In the current study, we hypothesized that Crataegus extract maintains the cardiac hemostasis in rats via its antioxidants properties.
Haydari et al (2017) had shown that the hydroalcoholic extract of Crataegus fruit holds antihypertensive effects, which could be partially due to the antioxidant and nitric oxide releasing properties in a hypertensive rat model [31]. Recently, Halver and colleagues addressed in their study the potential contributions of Crataegus extracts to cardiopoietic differentiation from stem cells [32]. Moreover, evidence from the literature proposed the potential use of hawthorn extract as an immunomodulatory substance [33]. On the other hand, Shatoor et al (2012) reported that the aqueous hawthorn extract significantly altered the bleeding time as identified by the levels of PFA-100 and thromboxane B2, suggesting a significant antiplatelet activity in Wistar albino rats [34]. Additionally, we showed previously that hawthorn (Crataegus Spp.) increased the tendency of bleeding among patients undergoing coronary artery bypass grafting [35]. Hence, we aimed to investigate the potential effects of Crataegus Spp. extract on oxidative stress, cardiac and hematological parameters in Sprague Dawley rats.
2. Materials and methods
2.1. Hawthorn flower and leaf extract
Dry extract of hawthorn leaves (Crataegus monogyna Jacq. (Lindm.) Rosacea) with flowers, dry-to-extract ratio 4–7:1, ethanol 45% (v/v) was obtained from Finzelberg GmbH & Co. KG, Germany. Total vitexin (HPLC) calculated as vitexin after hydrolysis was found to be 2.14%, while flavonoids calculated as hyperoside analogues was found as 1.26%. The extract powder was kept in tight-closure container and away from moisture. The standardized hawthorn leaves and flowers (Crataegus monogyna Jacq. (Lindm.) Rosacea) extract was provided as a generous gift from Dr. Rainer Kunz from Finzelberg GmbH & Co. KG.
2.2. Animals and treatment
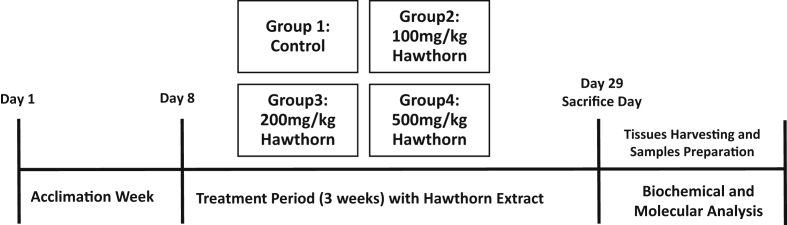
Adult young male Sprague Dawley rats (weight: 180–270 g, baseline) were used in these experiments. The animals were obtained from the animal house at Jordan University of Science and Technology (Irbid, Jordan). Animals were allowed one week to acclimate before any experimental manipulation. The Animal Care and Use Committee (ACUC) at Jordan University of Science and Technology, Irbid, Jordan approved animals work and experimental procedures. We used Sprague Dawley rats because they are the widely utilized to study long-term pharmacological interventions including long-term survival experiments. Moreover, they are reproducible, easily maneuvered, ethically appropriate, physiologically descriptive of human pathologies. The animals were housed in plastic cages (3–4 rats per each cage) under hygienic conditions with a pathogen-free environment and maintained at 24 °C and 12 h light/dark cycle, with food (standard rodent chow; Sahel Horan Co., Ramtha, Jordan) and tap water available ad libitum. Figure 1 represents a schematic depiction of the experimental design.
Figure 1.
A schematic depiction of the experimental design.
Rats were randomly assigned into four groups (n = 5–8 rats/group): Group 1 was a control (n = 5), rats received agar solution by oral gavage. Groups 2 (n = 8), 3 (n = 7), and 4 (n = 8) were the hawthorn treatment groups and were given a single daily dose of 100, 200, 500 mg/kg, of hawthorn extract, respectively. The selected doses were based on the amount of extract approved for their antiplatelet effect in rats according to previously published literature [34]. Interestingly, they elucidated that hawthorn extract showed different patterns of effect depending on the dose used. They showed that the extract significantly altered the bleeding time and the closure time, suggesting significant platelet function inhibition. These effects were observed with doses between 100 – 500 mg/kg, whereas the higher dose produced opposite effects on these parameters. The amount administered to the animal in the current study could reasonably be expected to be achieved in the human population. All experimental animal procedures where conducted at animal house (Jordan University of Science and Technology) during daytime. The extract powder was dissolved in agar solution and given in a single daily dose orally using oral gavage needle. The solution were prepared freshly according to the animal's weight. The treatment was continued for 3 weeks. Weights of the animals were measured every other day during the experiment time and a final reading upon decapitation. Additionally, the international normalized ratio (INR) level was measured two times during the experiment for each rat; at the baseline and at the euthanization using the CoaguChek XS PT/INR monitoring system (Roche).
Two hours after the last dosing, rats were sacrificed by decapitation. Livers and hearts obtained from each animal were removed immediately and washed with sterile phosphate-buffered saline over a glass plate filled with crushed ice. The liver and heart weights were measured and the organ collected in prelabeled 1.5-mL eppendorf tubes, then placed in liquid nitrogen. Samples were frozen at -80 °C until the time of tissue processing.
2.3. Biochemical analysis
For serum preparation, blood samples were collected in plain tubes, centrifuged at 3400 rpm for 12 min, and then, serum was collected to measure blood hemostasis parameters. The platelet, WBC, RBCs counts, hematocrits, and hemoglobin levels were measured (baseline, and at the end of treatment). The blood sample was withdrawn using tail vein procedure (baseline reading) and upon decapitation (end of the treatment) by well-trained personnel. Any hemolysed samples were excluded from the analysis. The measurements were taken in collaboration with the veterinary clinic at Jordan University of Science and Technology (Irbid, Jordan) using Celltac Alpha MEK-6550 analyzer (Nihon Kohden; Europe).
2.4. Molecular analysis
Rat tissues (hearts and livers) were homogenized using an electrical homogenizer (Polytron PT 1200E Homogenizer; Kincmetica INC, USA) in ice-cold lysis phosphate buffer containing: 8 g NaCl, 0.2 g KCl, 0.24 g KH2PO4, and 1.44 g Na2HPO4, 5 mM EDTA (5 mmol/L as preservative), in 1 L of distilled water mixed with fresh protease inhibitor cocktail (Sigma–Aldrich Corp., St. Louis, MO). Homogenates were centrifuged at 16,000 rcf at 4 °C for 15 min to remove the insoluble materials. All the work was carried out over crushed ice. Total protein concentration was assayed using the BioRAD procedure (Hercules, California, USA) at 595 nm.
The following biochemical markers were measured in order to evaluate the anticoagulant effect of hawthorn extract using commercially available ELISA kits: soluble platelet endothelial cell adhesion molecule 1 (sPECAM-1; MBS265996, MyBioSource, San Diego, USA), coagulation Factor X (MBS266437, MyBioSource, San Diego, USA), and antithrombin III (MBS269809, MyBioSource, San Diego, USA). Additionally, the levels of oxidative stress biomarkers glutathione peroxidase (GPx) (Sigma–Aldrich, St. Louis, MO), and thiobarbituric acid reactive substances (TBARS) (Cayman Chem, Ann Arbor, MI) were measured using commercially available ELISA kits following the instructions provided by the manufacturer. Assay plates were read at specified wavelengths using Epoch Biotek microplate reader (BioTek, Winooski, VT, USA).
2.5. Statistical analysis
All statistics were carried out using the GraphPad Prism (version 7.0, USA). One way ANOVA test followed by Tukey's multiple test was used to determine whether differences between control and various treatment groups were statistically significant. All data were presented as mean ± SEM with a P-value of less than 0.05 considered statistically significant.
3. Results
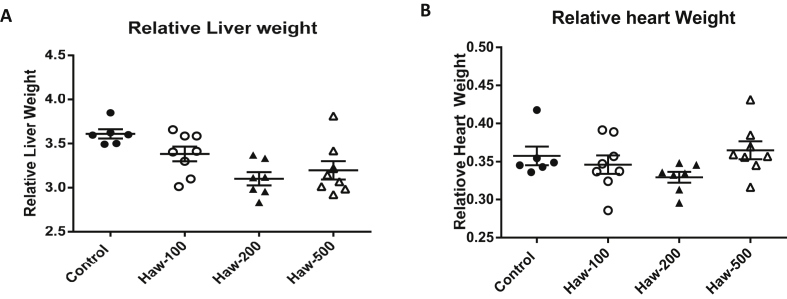
During the three week of the treatment, the animals' weights were measured every other day. There was no statistical significance in the rats’ weights or weight gained between the beginning and the end of the treatment period (0.2586 [3, 24]; P-value = 0.0856). There were no significant changes in the relative heart weight of treated rats in relation to control groups (P-value = 0.156; Figure 2-B). However, 100 and 200 mg/kg of hawthorn treatment produced a significant decrease in the relative weight of the liver compared to the control (P-value = 0.0067; Figure 2-A). At 500 mg/kg of hawthorn treatment, there was no statistically remarkable difference in the relative weight of the liver in relation to the control (Figure 2-A). No animal mortality were recorded in the current study.
Figure 2.
Hawthorn treatment decreased the relative liver weight with no change in the relative heart weight of Sprague Dawley rats. (A) the effect of hawthorn on rats liver weight. (B) the effect of hawthorn extract on rats' heart weights. Hawthorn treatment did not change the relative heart weight between hawthorn-treated groups and control group. Data are presented as the mean ± SEM.
We next investigated whether hawthorn treatment could affect the hematological parameters, including platelet count, WBC, RBC, haemoglobin concentrations (HB), and hematocrit (HCT) levels. The results showed that there were no significant differences in these parameters between hawthorn treated groups and the control group (P-value > 0.05; Table 1).
Table 1.
The effect of hawthorn extract treatment on the hemostatic parameters (platelet count, WBC, RBC, HB and HCT) of Sprague Dawley rats.
| Groups | WBC (×103/μl) | RBC (×106/μl) | HB (g/dl) | HCT (%) | Platelet Count (×103/μl) |
|---|---|---|---|---|---|
| Control | 14.20 ± 1.38 | 7.486 ± 0.03 | 15.52 ± 0.16 | 40.54 ± 0.46 | 766.6 ± 43.60 |
| Hawthorn-100mg | 16.83 ± 1.45 | 7.247 ± 0.31 | 14.33 ± 0.64 | 38.50 ± 1.87 | 745.4 ± 62.95 |
| Hawthorn-200mg | 15.77 ± 1.06 | 7.990 ± 0.26 | 15.93 ± 0.32 | 42.97 ± 1.03 | 823.0 ± 45.94 |
| Hawthorn-500mg | 16.73 ± 1.42 | 7.554 ± 0.21 | 15.41 ± 0.24 | 40.70 ± 0.99 | 762.6 ± 38.24 |
White Blood Cells (WBC), Red Blood Cells (RBC), Hemoglobin (HB), and Hematocrit (HCT). Control (n = 5); Hawthorn-100 (n = 8); Hawthorn-200 (n = 7); Hawthorn-500 (n = 8).
Data are presented as the Mean ± SEM.
The effect of hawthorn treatment on various coagulating/anticoagulants factors and endothelial adhesion molecule are presented in Table 2. The measured parameters includes coagulant factors (heart factor X, and liver factor X), the endogenous anticoagulant factors (liver and heart antithrombin III), and soluble platelet endothelial cell adhesion molecule-1 (CD31; Table 2). Our results showed a significant increase in the heart antithrombin III in hawthorn-treated group compared to the control group. This effect was noticed clearly at the lower dose (100 mg) and it was significantly different compared to others (200 and 500 mg). However, this effect was not seen when we investigated the levels of liver antithrombin III among all treated groups compared to the control group (P-value > 0.05; Table 2). On the other hand, hawthorn treatment significantly decreased the liver factor X level (P-value < 0.0001) with no remarkable difference in heart factor X (P-value > 0.05; Table 2). Upon studying the endothelial adhesion molecule, the current study showed a decrease in the serum sPECAM-1 levels for hawthorn-treated groups, but these changes were not statistically remarkable (P-value = 0.0599; Table 2).
Table 2.
The effect of hawthorn extract treatment on selected coagulating and anticoagulants factors of Sprague Dawley rats.
| Groups | Control | Hawthorn-100mg | Hawthorn-200mg | Hawthorn-500mg | P-Value |
|---|---|---|---|---|---|
| Serum SPECAM-1 | 1.111 ± 0.044 | 0.792 ± 0.053 | 0.847 ± 0.092 | 0.873 ± 0.129 | 0.0599 |
| Antithrombin III (Heart) | 34.29 ± 1.11 | 141.6 ± 20.87∗ | 55.12 ± 7.96# | 58.12 ± 8.21# | <0.0001 |
| Heart Factor X | 0.205 ± 0.010 | 0.387 ± 0.120 | 0.165 ± 0.049 | 0.102 ± 0.031# | 0.0529 |
| Antithrombin III (Liver) | 12.98 ± 1.497 | 15.45 ± 1.940 | 11.78 ± 0.979 | 11.11 ± 1.091 | 0.1551 |
| Liver Factor X | 2.893 ± 0.136 | 1.671 ± 0.123∗ | 2.089 ± 0.106∗ | 1.314 ± 0.119∗ | <0.0001 |
indicates significant difference from control.
indicates significant difference from Hawthorn-100mg.
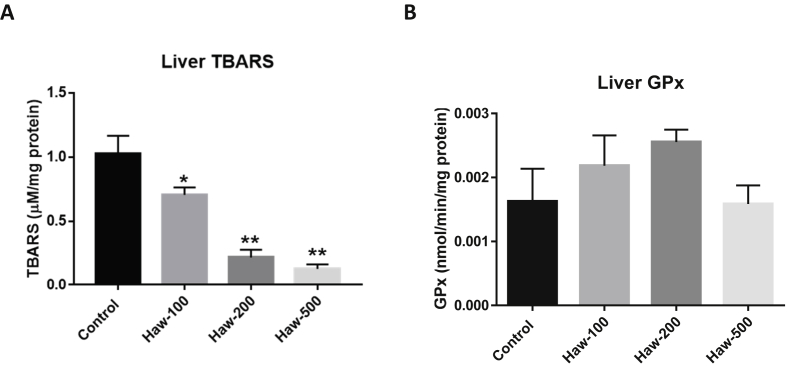
To link the effect of hawthorn on inflammatory process with oxidative/antioxidants parameters, we examined the effect of hawthorn treatment on oxidative stress biomarkers including liver thiobarbituric acid reactive substances (TBARS) and the antioxidant marker liver glutathione peroxidase (GPx). Our results showed that hawthorn treatment resulted in a significant decrease in the liver TBRAS level in a dose-dependent manner compared to the control group (P-value < 0.0001; Figure 3-A). No remarkable changes have been shown in the GPx levels between hawthorn-treated animals and the control group (P-value = 0.262; Figure 3-B).
Figure 3.
Hawthorn treatment decreased the liver thiobarbituric acid reactive substances (TBARS) level without changes in the glutathione peroxidase (GPx) level in Sprague Dawley rats. (A) the effect of hawthorn on the liver TBRAS level. (B) the effect of hawthorn extract on the liver glutathione peroxidase level. Data are presented as the mean ± SEM.
4. Discussion
Cardiovascular diseases (CVDs) are still the leading global healthcare problem. In high-income countries, attitudes to the impediment and treatment of CVDs have reduced the death rates due to CVDs through the previous 20–30 years [36]. Nowadays, different healthcare services in developing countries are overstressed by growing claims to deal with heart diseases, cancer, stroke, diabetes mellitus, and chronic respiratory diseases [36, 37]. Thus, people in the Middle East region focused on alternative medicine as a complementary option from engaging with expensive healthcare services. The reasons for the extensive use of herbs are multifaceted, but the thought that ‘natural’ can be connected with ‘safe’ is indeed an important factor. In this study, we investigated the effect of Crataegus monogyna Jacq. (Lindm.) on cardiac hemostasis and oxidative biomarkers in Sprague Dawley rats.
We reported in the current study a significant reduction in the relative liver weights for rats treated with hawthorn extract with no alteration in the levels of hematological parameters such as platelet count, WBC, RBC, HB, and HCT. This is suggesting that hawthorn extract, through its well-documented pharmacological activities, improves general health and exert hepatoprotective effect with no probable toxicities. These findings come in agreement with a previously published study, which showed that hawthorn extract was capable to produce a hepatoprotective effect and defend from hypercholesterolemia-induced oxidative stress [38]. Moreover, the findings of the present study support the literature in which it showed that the administration of hawthorn extract attenuated the levels of liver thiobarbituric acid reactive substances (TBARS) in a dose-dependent manner, prohibiting lipid peroxidation and producing an antioxidant effect. TBARS is one of the most commonly used assays for assessing the lipid peroxidation end-product malondialdehyde, a reactive aldehyde generated via lipid peroxidation of the polyunsaturated fatty acids. Significantly lower levels of TBARS were measured in all groups treated with hawthorn extract compared to the control (with the higher dose (500 mg/kg) produced the greatest reduction effect). Lipid peroxidation amends membrane flexibility, enzyme activity, and integrity of cell structure. The enhancement of antioxidant defense mechanisms decreases oxidative stress in the liver and provides a reasonable explanation for the maintained hemostasis accompanying hawthorn treatment. We also elucidated a non-significant increase in the levels of liver GPx in hawthorn treated group. Thus, it appears that hawthorn extract does not affect all biomarkers and antioxidant enzymes involved in oxidative stress pathways equivalently. Therefore, further examinations into mechanisms of this effect would be our future work theme. Ultimately, this may require higher doses of extract or a longer period of treatment, and this could open the door for further future studies to support the potential use of hawthorn for its antioxidant and hepatoprotective effects.
The findings of the present study showed that the hydroalcoholic extract of hawthorn alters the levels of specific clotting factors (factor X and antithrombin III) in a specific approach that is highly dependent on the administered dose and tissue type. The levels of liver factor X were significantly lower in all hawthorn-treated groups, while this reduction in the levels of factor X was not seen in heart tissues. Subsequently, hawthorn reduced the synthesis process of factor X in the liver, which could produce the blood-thinning properties. This is consistent with a previously published study elucidated that hawthorn extract exhibited a blood-thinning activity in rats [7]. Furthermore, it was evident in the current study that hawthorn extract possessed blood-thinning properties through the significant increase in the cardiac levels of antithrombin III. This effect was obviously seen with the 100 mg of hawthorn extract. The medicinal activity of hawthorn extracts is attributed to its high levels of flavonoids and phenolic compounds [31, 38]. This is reminiscent that the hawthorn extract produced a dose-dependent effect on the cardiac antithrombin III levels, which could clinically produce effect similar to the heparins’ effect in potentiation the antithrombin III.
The present study revealed that hawthorn extract maintain cardiac hemostasis, has antioxidant and blood-thinning properties in an experimental animal. However, the mechanisms underlying these outcomes should be further investigated. Worth mentioning here and based on the findings of the current study we suggest that the administration of hawthorn extract should be monitored when using along with other anticoagulation and/or antiplatelet agents or in patients undergoing major surgery. This study is opening the door for future studies to investigate more in depth the mechanistic approach of hawthorn pharmacodynamic activity and possibility of extracting new pharmacological agents. Therefore, future work is warranted, testing different durations or doses of hawthorn, to establish whether it might be possible also to provide a greater enhancement of antioxidant capacity in the liver.
5. Conclusion
The data presented in current study revealed that hawthorn extracts possess antioxidant activity through reducing the liver TBARS levels in a dose-dependent manner. Additionally, hawthorn extract has blood-thinning properties through inhibiting factor X synthesis and augmenting the cardiac levels of antithrombin III. Until supplementary validation of the applicability of these findings in clinical trials, we recommend attention when using this herb extract along with other anticoagulation and/or antiplatelet drugs or undergoing major cardiac surgery.
Declarations
Author contribution statement
A.M. Rababa'h and T. El-Elimat: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
O.N. Al Yacoub: Performed the experiments; Wrote the paper.
M. Rabab'ah: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
S.E.I. Altarabsheh, S. Alazzam and K. H. Alzoubi: Contributed reagents, materials, analysis tools or data; Wrote the paper.
S. Deo: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
A. Al-Azayzih: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
A. Zayed: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Financial Support was via grant number 306/2017 from the Deanship of Research at the Jordan University of Science and Technology to AR.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank Jordan University of Science and Technology for Financial Support via grant number 306/2017 from to AR.
References
- 1.Barnes J., Anderson L.A., Phillipson J.D. third ed. Pharmaceutical Press; London: UK: 2007. Herbal Medicines. [Google Scholar]
- 2.Pizzorno J.E., Murray M.T. Elsevier Health Sciences; 2012. Textbook of Natural Medicine. [Google Scholar]
- 3.Nirmal S.A, Pal S C, Otimenyin S.O., Aye T., Elachouri M., Kumar Kundu S., Rajarajan Amirthalingam Thandavarayan R.A., Mandal S.C. Contribution of herbal products In global market. Pharma Rev. 2013:95–104. [Google Scholar]
- 4.Smith T., Kawa K., Eckl V. Herbal supplement sales in US increased 8.5% in 2017, topping $8 billion. Herbal Gram. 2018;119:62–71. [Google Scholar]
- 5.Farnsworth N.R., Soejarto D.D. Global importance of medicinal plants. In: Synge H., Akerele O., Heywood V., editors. Conservation of Medicinal Plants. Cambridge University Press; Cambridge: 1991. pp. 25–52. [Google Scholar]
- 6.Bandaranayake W.M. Modern Phytomedicine. Wiley-VCH Verlag GmbH & Co. KGaA; 2006. Quality control, screening, toxicity, and regulation of herbal drugs; pp. 25–57. [Google Scholar]
- 7.Senica M., Stampar F., Mikulic-Petkovsek M. Different extraction processes affect the metabolites in blue honeysuckle (Lonicera caerulea L. subsp. edulis) food products. Turk. J. Agric. For. 2019;43:576–585. [Google Scholar]
- 8.Vijayan K.C.S., Ercisli S., Ghosh P.D. NaCI induced morpho-biochemical and anatomical changes in mulberry (Morus spp.) Plant Growth Regul. 2008;56:61–69. [Google Scholar]
- 9.Deshmukh B.S.S.V. Fruits in wilderness: a potential of local food resource. Int. J. Pharma Bio Sci. 2010;2:1–5. [Google Scholar]
- 10.Hazarika T.K.S.T. Wild edible fruits of Manipur, India: associated traditional knowledge and implications to sustainable livelihood. Genet. Resour. Crop Evol. 2018;65:319–332. [Google Scholar]
- 11.Blumenthal M., Brinckmann J.A., Wollschlaeger B. Thieme Publishers Series: American Botanical Council; 2003. The ABC Clinical Guide to Herbs. [Google Scholar]
- 12.Schulz V., Hänsel R., Blumenthal M. fifth ed. Springer-Verlag Berlin Heidelberg; 2004. Rational Phytotherapy: A Reference Guide for Physicians and Pharmacists. [Google Scholar]
- 13.Leuchtgens H. Crataegus Special Extract WS 1442 in NYHA II heart failure. A placebo controlled randomized double-blind study. Fortschr. Med. 1993;111:352–354. [PubMed] [Google Scholar]
- 14.Jayalakshmi R., Thirupurasundari C.J., Devaraj S.N. Pretreatment with alcoholic extract of Crataegus oxycantha (AEC) activates mitochondrial protection during isoproterenol - induced myocardial infarction in rats. Mol. Cell. Biochem. 2006;292:59–67. doi: 10.1007/s11010-006-9218-3. [DOI] [PubMed] [Google Scholar]
- 15.Ranjbar K., Zarrinkalam E., Salehi I. Cardioprotective effect of resistance training and Crataegus oxyacantha extract on ischemia reperfusion-induced oxidative stress in diabetic rats. Biomed. Pharmacother. 2018;100:455–460. doi: 10.1016/j.biopha.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Schwinger R.H., Pietsch M., Frank K. Crataegus special extract WS 1442 increases force of contraction in human myocardium cAMP-independently. J. Cardiovasc. Pharmacol. 2000;35:700–707. doi: 10.1097/00005344-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Veveris M., Koch E., Chatterjee S.S. Crataegus special extract WS 1442 improves cardiac function and reduces infarct size in a rat model of prolonged coronary ischemia and reperfusion. Life Sci. 2004;74:1945–1955. doi: 10.1016/j.lfs.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 18.Wang X., Liang Y., Shi J. Crataegus special extract WS 1442 effects on eNOS and microRNA 155. Planta Med. 2018;84:1094–1100. doi: 10.1055/a-0601-7083. [DOI] [PubMed] [Google Scholar]
- 19.Brixius K., Willms S., Schwinger R.H. [The basis for treatment of cardiovascular diseases: cellular and molecular mechanisms of Crataegus extract] Pharmazie Unserer Zeit. 2005;34:48–51. doi: 10.1002/pauz.200400104. [DOI] [PubMed] [Google Scholar]
- 20.Koch E., Malek F.A. Standardized extracts from hawthorn leaves and flowers in the treatment of cardiovascular disorders – preclinical and clinical studies. Planta Med. 2011;77:1123–1128. doi: 10.1055/s-0030-1270849. [DOI] [PubMed] [Google Scholar]
- 21.Holubarsch C.J., Colucci W.S., Meinertz T. Survival and prognosis: investigation of Crataegus extract WS 1442 in congestive heart failure (SPICE)-rationale, study design and study protocol. Eur. J. Heart Fail. 2000;2:431–437. doi: 10.1016/s1388-9842(00)00109-4. [DOI] [PubMed] [Google Scholar]
- 22.Holubarsch C.J., Colucci W.S., Meinertz T. The efficacy and safety of Crataegus extract WS 1442 in patients with heart failure: the SPICE trial. Eur. J. Heart Fail. 2008;10:1255–1263. doi: 10.1016/j.ejheart.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Koch E., Kohler S., Malek F.A. [The results of experimental and clinical studies. Cardio- and vasoprotective actions of standardized Crataegus extract] Pharmazie Unserer Zeit. 2005;34:52–57. doi: 10.1002/pauz.200400105. [DOI] [PubMed] [Google Scholar]
- 24.Kim S.H., Kang K.W., Kim K.W. Procyanidins in crataegus extract evoke endothelium-dependent vasorelaxation in rat aorta. Life Sci. 2000;67:121–131. doi: 10.1016/s0024-3205(00)00608-1. [DOI] [PubMed] [Google Scholar]
- 25.Lasne D., Jude B., Susen S. From normal to pathological hemostasis. Can. J. Anaesth. 2006;53:S2–11. doi: 10.1007/BF03022247. [DOI] [PubMed] [Google Scholar]
- 26.Gimbrone M.A., Jr., Garcia-Cardena G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tripodi A. Thrombin generation assay and its application in the clinical laboratory. Clin. Chem. 2016;62:699–707. doi: 10.1373/clinchem.2015.248625. [DOI] [PubMed] [Google Scholar]
- 28.Asada Y., Yamashita A., Sato Y. Pathophysiology of atherothrombosis: mechanisms of thrombus formation on disrupted atherosclerotic plaques. Pathol. Int. 2020;70:309–322. doi: 10.1111/pin.12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishnaswamy S. The transition of prothrombin to thrombin. J. Thromb. Haemostasis : JTH. 2013;11(Suppl 1):265–276. doi: 10.1111/jth.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu E., Moosavi L. StatPearls. Treasure Island (FL) 2019. Biochemistry, antithrombin III. [Google Scholar]
- 31.Haydari M.R., Panjeshahin M.R., Mashghoolozekr E. Antihypertensive effects of hydroalcoholic extract of crataegus azarolus subspecies aronia fruit in rats with renovascular hypertension: an experimental mechanistic study. Iran. J. Med. Sci. 2017;42:266–274. [PMC free article] [PubMed] [Google Scholar]
- 32.Halver J., Wenzel K., Sendker J. Crataegus extract WS(R)1442 stimulates cardiomyogenesis and angiogenesis from stem cells: a possible new Pharmacology for hawthorn? Front. Pharmacol. 2019;10:1357. doi: 10.3389/fphar.2019.01357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lis M., Szczypka M., Suszko-Pawlowska A. Hawthorn (crataegus monogyna) phenolic extract modulates lymphocyte subsets and humoral immune response in mice. Planta Med. 2020;86:160–168. doi: 10.1055/a-1045-5437. [DOI] [PubMed] [Google Scholar]
- 34.Shatoor A.S., Soliman H., Al-Hashem F. Effect of Hawthorn (Crataegus aronia syn. Azarolus (L)) on platelet function in albino Wistar rats. Thromb. Res. 2012;130:75–80. doi: 10.1016/j.thromres.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Rababa'h A.M., Altarabsheh S.E., Haddad O. Hawthorn herb increases the risk of bleeding after cardiac surgery: an evidence-based approach. Heart Surg. Forum. 2016;19:E175–179. doi: 10.1532/hsf.1570. [DOI] [PubMed] [Google Scholar]
- 36.Leong D.P., Joseph P.G., McKee M. Reducing the global burden of cardiovascular disease, Part 2: prevention and treatment of cardiovascular disease. Circ. Res. 2017;121:695–710. doi: 10.1161/CIRCRESAHA.117.311849. [DOI] [PubMed] [Google Scholar]
- 37.Gaziano T.A. Reducing the growing burden of cardiovascular disease in the developing world. Health Aff. 2007;26:13–24. doi: 10.1377/hlthaff.26.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rezaei-Golmisheh A., Malekinejad H., Asri-Rezaei S. Hawthorn ethanolic extracts with triterpenoids and flavonoids exert hepatoprotective effects and suppress the hypercholesterolemia-induced oxidative stress in rats. Iran. J. Basic Med. Sci. 2015;18:691–699. [PMC free article] [PubMed] [Google Scholar]