Abstract
Exposures to hazardous chemicals including formaldehyde are harmful to human health. In this study, the authors investigate the protective effects of pumpkin seed oil (PSO) extract against formaldehyde-induced major organ damages in mice. Administration of formaldehyde (FA) caused significant elevation of serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), serum creatinine, etc. Histopathological examinations of liver, kidney, and brain tissues showed the degenerations of those organs. Mice pretreated with PSO extract significantly attenuated the FA-induced elevation of SGOT (39.0 ± 1.30 vs 20.5 ± 0.65 IU/L; FA-group vs PSO treatment group), SGPT (91.8 ± 1.65 vs 51.0 ± 1.29 IU/L), serum creatinine (1.05 ± 0.07 vs 0.65 ± 0.07 IU/L), and preserved the normal histology of organ tissues. The FA-induced elevation of malondialdehyde (MDA) in the brain, liver, and kidneys was suppressed by pretreatment with PSO extract. The extract also attenuated the FA-induced reduction of endogenous antioxidant pools. In vitro phytochemical analyses showed that PSO extract possesses free radical scavenging and total antioxidant activities due to the presence of phenolic and flavonoid compounds. Thus, PSO extract has significant protective effects against FA-induced organ toxicities by scavenging oxidative stress and inhibiting lipid peroxidation.
Keywords: Pumpkin seed oil, Free radical scavenging, Formaldehyde, Lipid peroxidation, Antioxidant activities, Chemistry, Food science, Agricultural science, Biological sciences, Health sciences
Pumpkin seed oil; Free radical scavenging; formaldehyde; Lipid peroxidation; antioxidant activities; Chemistry; Food Science; Agricultural Science; Biological Sciences; Health Sciences.
1. Introduction
Exposures to hazardous chemicals and pesticides that are harmful to human health are increasing day by day. Chemicals and pesticides commonly used in the agricultural area sometimes contaminate fruits, vegetables, and drinking water. Dangerous chemicals such as formaldehyde (FA), carbide, etc. are used illegally for the preservation of fruits, vegetables, fishes, etc. [1, 2]. Moreover, FA is widely used in the industrial and medical fields, and employees in these sectors are frequently exposed to it. This chemical is hazardous to major organs like the brain, liver, kidney, pancreas, lung, etc. [3, 4, 5].
FA is a very reactive one-carbon compound that can react with various cellular components such as lipids, proteins, and nucleic acids [6]. FA quickly diffuses into many tissues, including the brain, testes, and liver after intraperitoneal, oral, or nasal administration [7]. It can cause gene mutations, carcinogenesis, a neurodegenerative disorder, digestive complications, and spermatogenetic inhibition [8, 9]. FA sharply increases free radicals in the human body (e,g. hepatic cells) by acting as a pro-oxidant or by reducing antioxidant levels and contributing to the progression of a variety of chronic diseases [10]. FA is often synthesized by the natural biochemical reactions that are unavoidable. Various marine organisms together with fish develop FA progressively in post-mortem condition by the reduction of dimethylamine oxide [11, 12, 13]. The maximum value of naturally occurring FA is up to 60 mg/kg in some foods like fruits and marine fish [14, 15]. FA concentrations in fish have higher extreme values of 220–290 mg/kg [16, 17]. The maximum acceptable exposure level of FA to humans is 100 mg/kg [18]. However, no specific antidote is available for the treatment of FA-induced organ toxicity. Treatment against FA-induced toxicity is mainly the supportive care of the various organ systems and multiple approaches are required for the proper management. Daily intake of natural foods and supplements that are rich in bio-active compounds can effectively suppress hazardous FA-induced organ damages. Natural compounds having antioxidant properties are classified as secondary plant metabolites, e.g., polyphenols (phenolic acids, flavonoids). The supplementation of foods that contain these compounds in large extents appears to play an important role in prophylaxis against many diseases. Phenolic compounds act as an antioxidant via different modes, such as by 'scavenging' free radicals as well as enhancing the activity of other antioxidants, e.g., fat-soluble vitamins [19].
Pumpkin (Cucurbita maxima; Family-Cucurbitaceae) is one of the most popular vegetables worldwide. The nutrition value of pumpkin seed is familiar. The major nutritionally related components of pumpkin seeds are proteins (30–51 %) and oils (up to 40 %). They are also rich in carbohydrates and microelements. The seed extract contains triglycerides with palmitic, stearic, oleic, and linoleic acid as the dominant fatty acids. Other important components present in the pumpkin oils are tocopherols, sterols, phospholipids, and hydrocarbons. Animal studies have shown that pumpkin, pumpkin seeds, pumpkin seed powder, and pumpkin juice can reduce blood sugar [20, 21]. The pumpkin seed oil exhibits anti-inflammatory effects [22], reduces elevated blood pressure, and blood cholesterol levels [23, 24]. Pumpkin seed oil can be obtained by following several methods such as solvent extraction [25], aqueous enzymatic extraction [26], microwave-assisted aqueous enzymatic extraction [27], cold-pressing extraction [28], or supercritical CO2 extraction methods [29]. The modern way of processing vegetable oil is solvent extraction, which produces higher yields and is quicker and less expensive. Solvent extraction as employed in the United States is the most efficient means of deriving vegetable oil from the seeds [30]. In terms of oil yield, extraction efficiency, and oil qualities, petroleum ether or n-hexane is a good choice of solvent for the extraction of pumpkin seed oils [31].
In the present study, for the first time, we investigated the protective effect of pumpkin seed oils as well as the signalling molecular mechanisms of the protective effect against FA-induced organ damages in mice model.
2. Materials and methods
2.1. Chemicals
Formaldehyde was purchased from Merck (Darmstadt, Germany). Catechin hydrate, malondialdehyde tetrabutylammonium salt, thiobarbituric acid (TBA), 5, 5′-dithiobis 2-nitrobenzoic acid (dTNB) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ascorbic acid and vitamin E were obtained as the generous gift samples from Square Pharmaceuticals Ltd., Dhaka, Bangladesh. SGOT and SGPT assay kits were purchased from Human (Germany). Pyrogallol, gallic acid, Folin-Ciocalteu reagent (FCR), 2, 2-diphenyl-1-picrylhydrazyl, KOH, xylene, hematoxylin, and eosin were purchased from Loba Chemie (India). All other chemicals were of analytical grade and procured from reliable commercial suppliers.
2.2. Collection and extraction of pumpkin seed oil
Pumpkin seeds were collected from the local market and authenticity was confirmed as Cucurbita species by a taxonomist, Department of Botany, University of Rajshahi, Bangladesh. After proper drying and grinding into a coarse powder, it was soaked into petroleum ether for 72 h with occasional shaking and stirring for the extraction of oils. The acid value of extracted oil was determined according to AOAC [32] standard to ensure the quality of extracted oils.
2.3. In vitro chemical analysis of pumpkin seed oil extract
-
i)
Analysis of total phenolic and flavonoid contents
The total phenolic (TP) content of the pumpkin seed oil (PSO) extract was determined by Folin-Ciocalteu reagent (FCR) according to the method of Kumar et al. [33]. Briefly, 0.5 ml (1 mg/ml) of PSO extract was mixed with 2.5 ml of FCR (diluted tenfold) and 2.5 ml of sodium carbonate (75 g/l) solution. The resultant mixture was incubated for 30 min at 25 °C to complete the reaction, and the absorbance was measured at 760 nm by UV–Visible spectrophotometer. The total phenolic content was calculated by comparison with the standard calibration curve of gallic acid, and results were presented as mg of gallic acid equivalents (mg of GAE) per gram (g) dry weight of the extract. On the other hand, the total flavonoid content of the PSO extract was estimated as described by Zhishen et al. [34], which was modified by Alpona et al. [35]. Briefly, 0.5 ml (1 mg/ml) of PSO extract was mixed with 2 ml of distilled water and then with 0.15 ml of NaNO2 solution (15%). The mixture was then incubated for 6 min, and 0.15 ml of AlCl3 solution (10%) was added to the mixture, which was again allowed to stand for another 6 min. Then 2 ml of NaOH solution (4%) was added to the mixture and adjusted the final volume to 5 ml by distilled water. The mixture was then mixed properly and allowed to stand for another 15 min. The final solution's absorbance was measured at 510 nm. The total flavonoid content was calculated by comparison with the standard calibration curve of catechin, and results were presented as mg of catechin equivalents (mg of CAE)/g dry weight of the extract. All tests were conducted in triplicate.
-
ii)
In vitro DPPH free radical scavenging activity assay
The PSO extract was tested for the free radical scavenging activity on the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical following the method of Pan et al. [36]. Briefly, 0.2 ml of PSO extract at different concentrations (1, 2, 4, 8, 16, 32 and 64 μg/ml, respectively) was added to 8 ml of 0.004% (w/v) DPPH solution. Both PSO extract and DPPH were dissolved in 95% ethanol. After 30 min of incubation at room temperature, the absorbance was read against a blank at 517 nm using a UV-visible spectrophotometer (Shimadzu, Tokyo, Japan). The per cent inhibition (I %) of DPPH free radical was calculated by the following way:
| I% = [(A blank-A sample)/A blank] ×100 |
Here, A blank is the absorbance of the reaction mixture containing all reagents except the test compound (blank solution), whereas A sample is the absorbance of the reaction mixture containing the test compound. The tested compound concentration, which delivered 50% inhibition, (IC50, stated in μg/ml) was calculated from the graph plotted as inhibition percentage against the extract concentration. Ascorbic acid was used as a standard free radical scavenging agent.
-
iii)
In vitro total antioxidant activity assay
The total antioxidant activity of the extract was assessed by the phosphomolybdenum method as described previously [37]. Briefly, 0.5 ml of PSO extract at different concentrations (6.25, 12.5, 25, 50, and 100 μg/ml) was mixed with 3 ml of phosphomolybdenum solution (0.6 M H2SO4, 28 mM Na3PO4 and 4 mM ammonium molybdate). The mixture was incubated in a water bath at 95 °C for 90 min and subsequently cooled at room temperature. The absorbance of the solution was measured at 695 nm against blank and the values were presented as equivalent to mg of catechin/g of dried extract. The experiment was conducted in triplicate, and results were reported as mean ± SEM.
2.4. Experimental animals
Thirty-two Swiss Albino mice of average weight 32.88 g and age six weeks were purchased from the animal centre, Department of Biochemistry and Molecular Biology, Rajshahi University, Rajshahi, Bangladesh. They were kept in polypropylene mice cages all over the study. They were housed in a temperature-controlled (24 ± 1 °C) room with 60–70% humidity and standardized light/dark (12/12 h) cycles. They were adapted for 1 week and fed with standard mice diet and tap water ad libitum. The animal handling guidelines were approved by the Institutional Animal, Medical Ethics, Biosafety, and Biosecurity Committee of the University of Rajshahi, Bangladesh.
2.5. Experimental design
The experimental mice were randomly allocated into four groups of eight members each as follows: The treatment schedule was for four weeks (28 days).
| Group 1: | The control group received normal drinking water. |
| Group 2: | The formaldehyde (FA)-induced group received 10 mg/kg body weight FA intraperitoneally (IP) once a day [38]. |
| Group 3: | The treatment group received FA plus PSO extract (4 ml/kg) orally once a day [39, 40]. |
| Group 4: | The standard group received FA plus vitamin E (4 IU/kg) orally once a day [41, 42]. |
2.6. Evaluation of biochemical markers for liver, kidneys, and brain
Blood samples were collected directly from the heart after the end of the experimental schedule, and serum was isolated by centrifuging the whole blood at 2300×g for 10 min. SGOT and SGPT enzyme levels were determined by using assay kits obtained from Human, Germany, according to the methods of Reitman and Frankel [43]. Serum creatinine was estimated by Jaffe's method [44], glutathione (GSH) was determined according to the methods of Ellman GL and Wu G [45, 46], SOD was determined according to the method of Marklund and Marklund [47], and catalase activity was assayed following the method of Kar and Mishra [48]. Lipid peroxidation of tissue homogenate was determined by measuring thiobarbituric acid reactive substances (TBARS) according to the method of Preuss et al. [49]. The protein concentration of serum and tissue homogenates was estimated by the standard Lowry method [50].
2.7. Histopathological study of liver, kidney and brain tissues
After sacrifice liver, kidney, and brain tissues were separated, washed in cold saline water, and fixed in 10% neutral buffered formalin. For histopathology, tissues were embedded in paraffin and several sections of tissues were transversely cut (5μm) using a microtome. Then, slides were deparaffinized in p-xylene and washed with decreasing order of ethanol (100%, 90%, 80%, 70%, and 50%) and finally rinsed under tap water. Slides were then stained by hematoxylin-eosin and viewed under a light microscope (Olympus IX71, Japan) connected to a computer.
2.8. Statistical analysis
All data are presented as the mean ± SEM (standard error of the mean). Variances among groups were calculated by one-way analysis of variance (ANOVA) or Student t-test where it was necessary. Values at p < 0.05 were considered statistically significant. Statistical analysis was carried out by using a statistical software package Graph Pad Prism 7.0 (San Diego, CA, USA).
3. Results
3.1. In vitro chemical assay of pumpkin seed oil extract
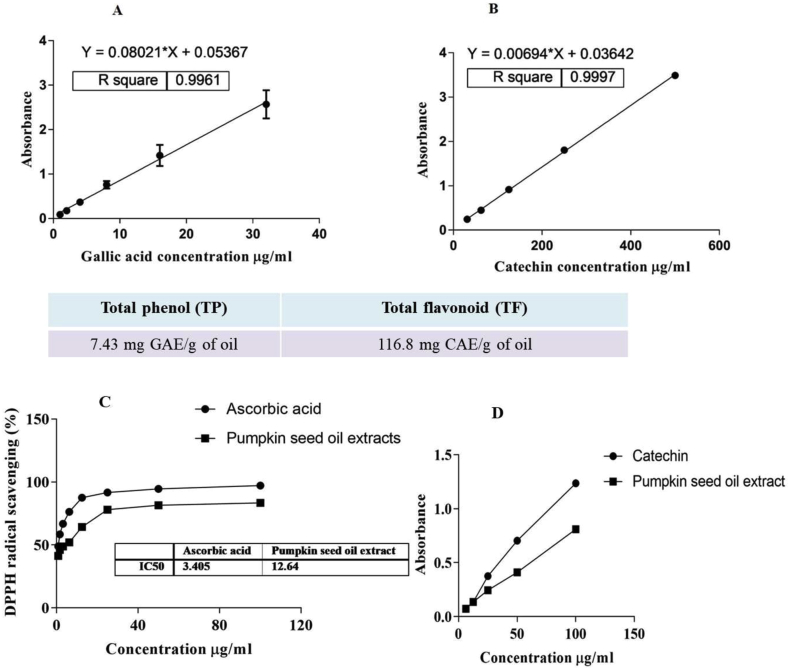
The acid value of petroleum ether-extracted pumpkin seed oil (PSO) was 1.80 ± 0.2 mg/KOH/g of oil. The acid value is a relative measure of rancidity as free fatty acids are normally formed during the decomposition of triglycerides. This value is concordant with previous reports [51]. Hence, the extracted PSO was free from decomposed fats. The total phenolic content of the PSO extract, calculated from the calibration curve (R2 = 0.9961, Figure 1A), was 7.43 mg gallic acid equivalent (GAE)/g of oil and the total flavonoid content (R2 = 0.9997, Figure 1B) was 116.8 mg catechin equivalent/g of oil. As shown in Figure 1C, PSO extract scavenged DPPH free radicals in a concentration-dependent manner. The maximum scavenging (84.5 ± 0.4%) of DPPH free radicals was observed by 100 μg of PSO extract. Whereas the same dose (100 μg) of ascorbic acid scavenged the maximum of 97.2 ± 0.2% DPPH free radicals. Besides, 50% inhibitory concentration (IC50) values of DPPH free radical neutralizing activities were found to be 3.41 μg/ml and 12.64 μg/ml for ascorbic acid and PSO extract, respectively. Figure 1D showed that PSO extract has antioxidant activity. Catechin was used as a standard antioxidant agent. The results are concordant with the presence of total phenolic and total flavonoid contents in PSO extract.
Figure 1.
Calibration curve for standard (A) gallic acid and (B) catechin. (C) DPPH free radical scavenging activity of PSO extract. Ascorbic acid was used as a standard free radical scavenging agent (D) Total antioxidant activity of the PSO extract. Catechin was used as a standard antioxidant agent.
3.2. Effects of PSO extract on SGOT, SGPT and serum creatinine
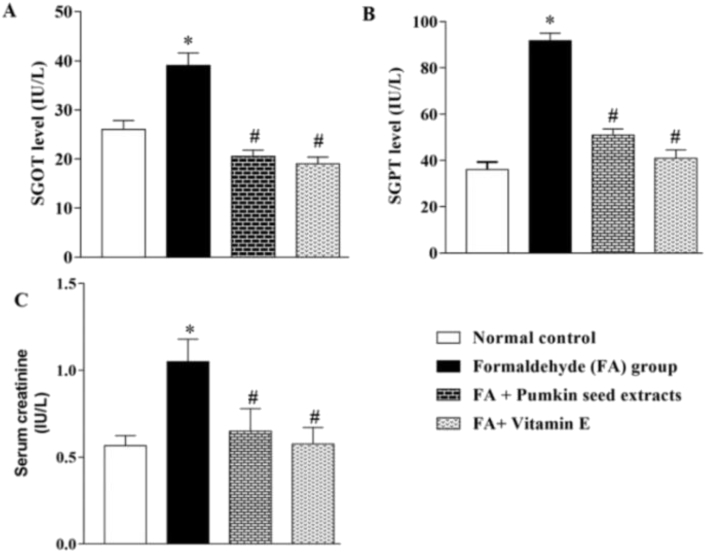
Estimation of serum GOT, and GPT are good indicators for the evaluation of liver health. These enzymes are elevated in the case of damaged/injured liver. On the other hand, the serum creatinine level indicates kidney health. Twenty-eight days after the treatment period, blood samples were collected and estimated those biochemical parameters. As shown in Figure 2A, B, C, intraperitoneal injection of FA caused a significant elevation of SGOT (26.0 ± 0.91 vs 39.0 ± 1.30; normal control vs FA group), SGPT (36.0 ± 1.68 vs 91.8 ± 1.65), and serum creatinine (0.57 ± 0.03 vs 1.05 ± 0.07), respectively. Mice pretreated with PSO extract suppressed the FA-induced elevation of SGOT (39.0 ± 1.30 vs 20.5 ± 0.65; FA-group vs PSO treatment group), SGPT (91.8 ± 1.65 vs 51.0 ± 1.29), and serum creatinine (1.05 ± 0.07 vs 0.65 ± 0.07) significantly. The standard antioxidant agent, vitamin E suppressed the FA-induced elevation of SGOT (39.0 ± 1.30 vs 19.0 ± 0.71; FA-group vs vitamin E treatment group), SGPT (91.8 ± 1.65 vs 41.0 ± 1.78), and serum creatinine (1.05 ± 0.07 vs 0.58 ± 0.05). The results suggest that the magnitude of suppression of PSO extract was quite similar to that of vitamin E. The level of SGOT, SGPT, and serum creatinine were expressed as IU/L.
Figure 2.
Effect of PSO extract on FA-induced elevated biochemical parameters. Mice pretreated with PSO extract significantly attenuated the FA-induced elevation of (A) SGOT (B) SGPT, and (C) serum creatinine. ∗ indicates a significant elevation of blood biomarkers (SGOT, SGPT, and serum creatinine) in the FA-induced group as compared to the control group. # indicates a significant attenuation of the elevation of biomarkers in the treatment group as compared to the FA-induced group. Values at p < 0.05 were considered statistically significant. All experiments were repeated three times for reproducibility.
3.3. Effect of PSO extract on FA-induced lipid peroxidation
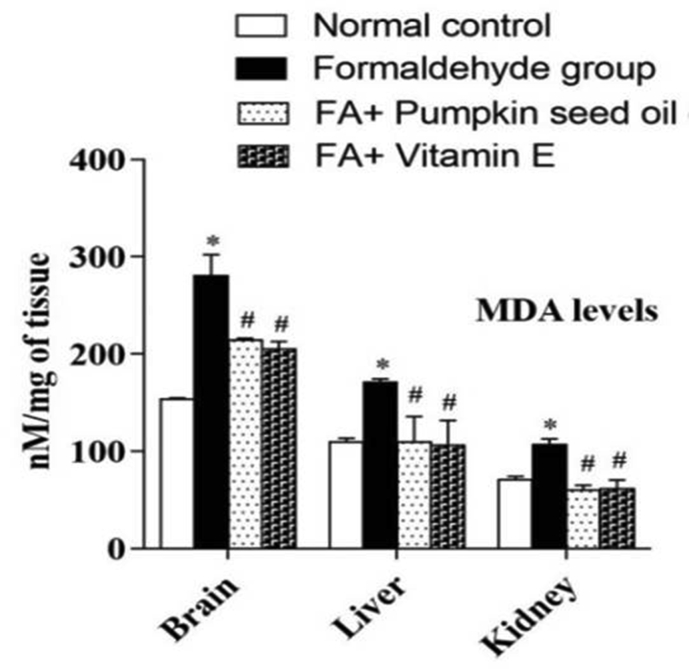
Lipid peroxidation comprises the oxidative degradation of polyunsaturated fatty acids to malondialdehyde (MDA). We compared MDA levels in the brain, liver, and kidney tissues with or without pretreatment of PSO extract to investigate the protective effects against oxidative lipid peroxidation. As shown in Figure 3, MDA level in the brain, liver, and kidney tissue homogenates of the normal control group was 153.91 ± 0.90, 109.62 ± 3.93 and 70.58 ± 3.91 nM/mg of tissue proteins, respectively, whereas, in FA-induced group, the level of MDA was 280.06 ± 1.54, 171.15 ± 3.32 and 107.24 ± 5.66 nM/mg of tissue proteins, respectively. But in the PSO pretreated group, it was 214.04 ± 2.06, 109.87 ± 26.10, and 59.70 ± 5.51 nM/mg of tissue proteins, respectively. In the case of standard antioxidant, vitamin E pretreated group, the level was 205.26 ± 7.47, 106.54 ± 25.24, and 61.41 ± 8.99, respectively. Statistical analysis has shown that the level of MDA was significantly higher in the FA-induced group as compared to the control group, which were significantly attenuated by the pretreatment of PSO extract with FA. The Student t-test was used for comparison between two groups. Values at p < 0.05 were considered statistically significant.
Figure 3.
Effect of PSO extract on FA-induced lipid peroxidation. Mice pretreated with PSO extract significantly attenuated the FA-induced elevation of malondialdehyde (MDA) levels. ∗ indicates a significant elevation of MDA in the FA-induced group as compared to the control group. # indicates a significant attenuation of the elevation of MDA in the treatment group as compared to the FA-induced group. Values at p < 0.05 were considered statistically significant.
3.4. Effect of PSO extract on endogenous antioxidant enzyme activity
The living body possesses endogenous antioxidant defence systems to prevent the formation of free radicals and to decrease their damaging effect. The antioxidant defence systems include-catalase (CAT), superoxide dismutase (SOD), glutathione (GSH), etc. We estimated serum and tissues (liver, kidney, and brain) CAT, SOD, and GSH levels according to the methods as described in the “materials and method section” after the scheduled experimental periods with or without PSO extract pretreatment. As shown in Table 1, FA-induction caused a significant reduction of serum and tissue endogenous antioxidant enzymes level as compared to the control group whereas pretreatment of PSO extract significantly attenuated the FA-induced reduction of those antioxidant enzymes level, suggesting that FA caused the elevation of oxidative stress that exhausted the endogenous antioxidant pools. However, supplementation with PSO extract maintained the endogenous antioxidant levels within the normal range, presumably by neutralizing the excess oxidative stress.
Table-1.
Effects of PSO extract on endogenous antioxidant enzyme activity.
| Treatment | CAT(μmol/mg) |
SOD(U/mg) |
GSH(U/mg) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Serum | Liver | Kidney | Serum | Liver | Brain | Kidney | Liver | Kidney | |
| N. Control | 1.32 ± 0.01 | 93.90 ± 2.0 | 94.73 ± 2.30 | 2.66 ± 0.10 | 4.32 ± 0.211 | 4.99 ± 0.05 | 4.54 ± 0.07 | 111.93 ± 4.0 | 71.13 ± 2.20 |
| FA-induced | 0.42 ± .03∗ | 42.24 ± 0.97∗ | 36.43 ± 9.65∗ | 1.75 ± 0.11∗ | 2.52 ± 0.10∗ | 3.71 ± 0.33∗ | 3.92 ± 0.16 | 39.41 ± 4.63∗ | 41.70 ± 5.31∗ |
| FA + PSO | 1.12 ± 0.01# | 62.75 ± 3.29# | 89.05 ± 5.58# | 2.55 ± 0.15# | 3.71 ± 0.06# | 6.52 ± 0.05# | 4.30 ± 0.13 | 130.03 ± 2.2# | 70.90 ± 1.68# |
| FA + Vit E | 1.24 ± 0.03# | 65.56 ± 1.0# | 85.58 ± 5.12# | 2.65 ± 0.15# | 4.04 ± 0.07# | 6.35 ± 0.20# | 4.34 ± 0.1 | 136.12 ± 1.1# | 104.38 ± 2.4# |
Effect of PSO extract on endogenous antioxidant enzyme activity. ∗ indicates significant changes in endogenous antioxidant enzyme levels as compared to the control group. # indicates significant attenuation of the FA-induced changes of endogenous antioxidant enzymes in the treatment group as compared to the FA-induced group. Values are mean ± SEM of eight animals' data. Data were considered statistically significant when p < 0.05.
3.5. Histopathological study of liver, kidney and brain tissues
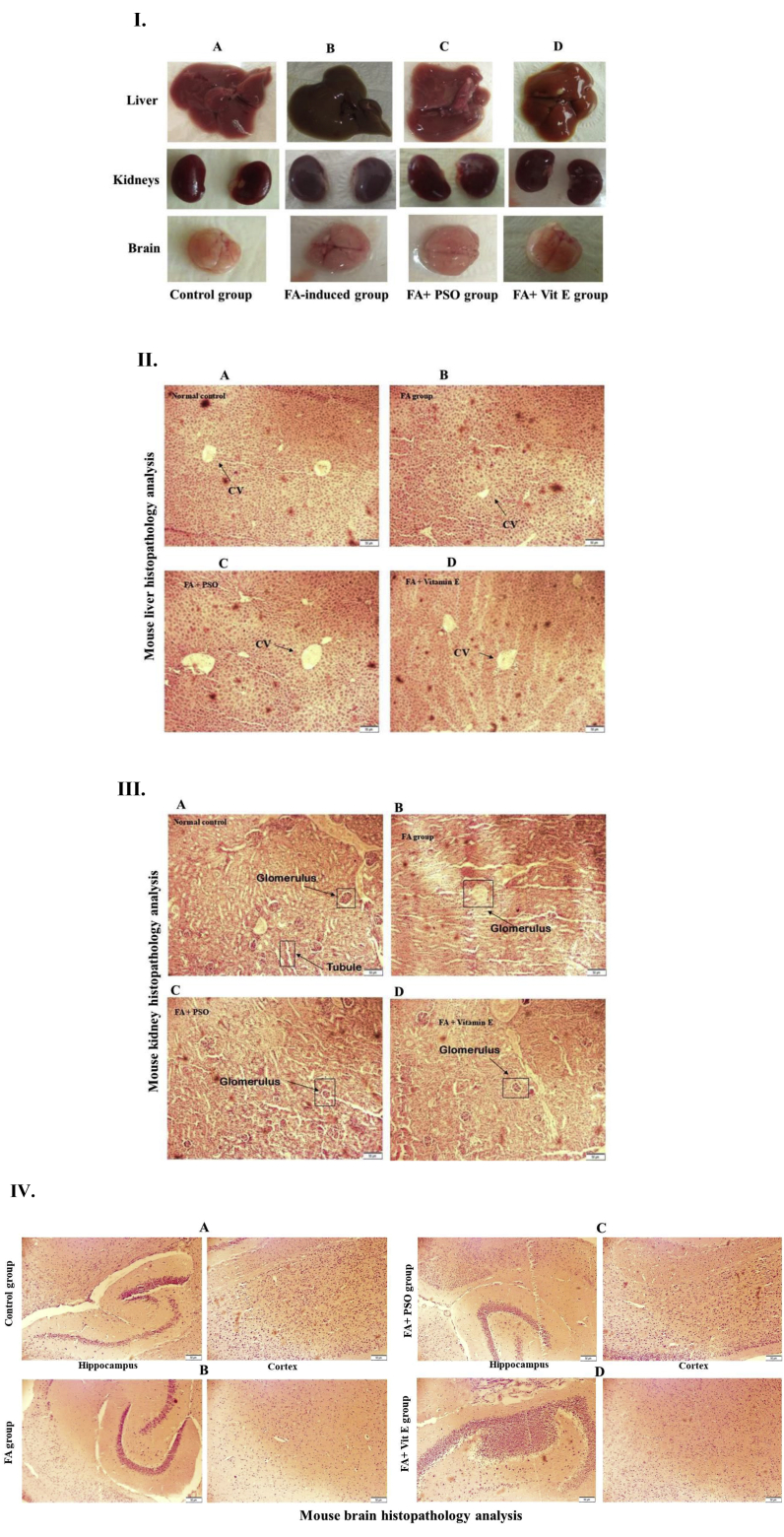
Twenty-eight days after the treatment period, animal's organs were dissected and investigated macroscopically. As shown in Figure 4 (I), snapshots of liver, kidney, and brain have shown that FA administration results in the blackish discolouration of the organs probably due to the oxidative damages. However, those organs, dissected from the PSO extract pretreatment group was similar to the organs of the control group. These observations are concordant with the above biochemical evaluations, suggesting that PSO extract has a protective effect against FA-induced organ damages. The histopathological studies of liver, kidney, and brain tissues under the microscope showed varying degrees of cellular changes in the FA-induced mice group as compared to the control group. In liver tissue of the control group as shown in Figure 4 (II) A, the lobular structure remained unaltered, central vein were intact, sinusoids and Kupffer cells were normal. However, in the liver tissue of the FA-induced group, there was congestion of central vein and swelling of hepatocytes (Figure 4 (II) B). We found that the pretreatments of PSO extract in the FA-induced mice group, the liver tissues were protected with central vein and normalized hepatocytes (Figure 4 (II) C). In Figure 4 (II) D, the standard vitamin E treatment group also showed the protective effects against FA-induced hepatic damages. On the other hand, in case of the histopathological analyses of kidney tissues, we found that glomerular and tubular structures were disorganized in the FA group's mice (Figure 4 (III) B) when compared to the control group (Figure 4 (III) A), which were protected by the pretreatment with PSO extract (Figure 4 (III) C) and standard antioxidant, vitamin E (Figure 4 (III) D). The results suggest that PSO extract has protective effects against FA-induced kidney damages. Besides, from the histopathological analyses of mouse brain tissues, we found that structure of hippocampus became altered, and the number of astrocytes in the cortex were decreased in FA group's mice (Figure 4 (IV) B) as compared to the control group (Figure 4 (IV) A). In PSO extract pretreated group, there was no significant recovery of the structure of the hippocampus, but the number of astrocytes increased (Figure 4 (IV) C). In the vitamin E group, we found a little improvement in the hippocampus part and increased number of astrocytes (Figure 4 (IV) D).
Figure 4.
Histopathological analysis of mouse liver, kidneys, and brain. I. Macroscopic observations of mouse liver, kidneys, and brain. Representative snapshots of liver, kidneys, and brain, showing normal visual colour in (A) control group, which became blackish discolouration in (B) FA-induced group. However, mice pretreated with (C) PSO extract and (D) vitamin E protected the FA-induced oxidative changes to blackish discolouration. Snapshots were taken by Canon Powershot sx100 (Japan). II. Mouse liver histopathology analysis. (A) A photomicrograph of a liver section from the control group, showing a compact configuration of hepatic tissue with the intact central vein (B) A photomicrograph of a liver section from FA-induced group, showing congestion of central vain and swelling of hepatocytes. However, liver sections from (C) PSO pretreated group, and (D) standard antioxidant, vitamin E treated group, showing a nearly normal architecture of hepatocytes with the central vein. (H&E, ×20, scale bar, 50 μm). III. Mouse renal histopathology analysis. (A) A photomicrograph of a renal tissue section from the control group, showing a normal architecture of glomerulus and renal tubular structure. (B) A photomicrograph of a renal tissue section from the FA-induced group, showing degeneration of glomerulus and tubular structure, which were protected in (C) PSO extract pretreated group and (D) standard antioxidant, vitamin E treated group. (H&E, ×20, scale bar, 50 μm). IV. Mouse brain histopathology analysis. (A) Photomicrographs of a brain tissue section from the control group, showing the normal organization of hippocampus and astrocytes in the cortex. (B) Photomicrographs of a brain tissue section from FA-induced group, showing the deformed architecture of hippocampus and less number of astrocytes in the cortex. Representative photomicrographs from (C) PSO extract pretreated group, and (D) vitamin E pretreated group, showing no significant protection of hippocampal degeneration but the number of astrocytes in the cortex was increased as compared to the FA-induced group. (H&E, ×10, scale bar, 50 μm).
4. Discussion
Formaldehyde (FA) is legally used in the medical sector for preserving dead bodies in hospital mortuaries, dissection room in medical colleges, fixation of biopsy sample for medical examinations, disinfection of hospital rooms, operation theatres, and few surgical instruments. But this is sometimes used unethically for preserving foods and daily commodities. The widespread use of FA is dangerous to public health. In the present study, we aimed to investigate the protective effect of PSO extract against formaldehyde (FA)-induced damages of major organs (liver, kidney, brain).
Phytochemical screening of PSO extract has shown the presence of phenolic and flavonoid compounds (Figure 1A, B). Phenolic compounds have redox properties, which allow them to act as antioxidants [52]. As their hydroxyl groups enable their free radical scavenging ability, the total phenolic concentration might be used as a basis for the evaluation of the antioxidant activity. Plant flavonoids have antioxidant activity in vitro and also act as antioxidants in vivo [53, 54]. FA-induction (10 mg/kg b.w. IP) to Swiss Albino mice caused the abnormalities of several biochemical parameters such as elevation of serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), and serum creatinine. Glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) are two enzymes that are synthesized and stored solely in the liver. However, during injury and inflammation of liver cells, these enzymes are released from the liver and raise the concentration in blood as SGOT and SGPT. Therefore, SGOT and SGPT are good indicators of liver injury and/or inflammation. Serum creatinine is a biomarker for the determination of kidney inflammation and injury [55]. During the experimental period, we observed the slight reduction of animals' body weight, decreased food and water intake, and appearance of some clinical symptoms such as depression, lethargy, etc. (data not shown). Similar clinical signs were investigated by Bhatt et al. in rats [56]. Mice pretreatment with PSO extract has shown to decrease the FA-induced elevation of SGOT, SGPT, and serum creatinine significantly (Figure 2), suggesting the liver and kidney protective effects of PSO extract against FA-induced damages. FA disturbs the oxidant-antioxidant balance, by the production of reactive oxygen species (ROS), resulting in oxidative stress [57]. The process of lipid peroxidation is a type of oxidative stress [58]. Malondialdehyde is a measure of lipid peroxidation. In the present study, the MDA level in the brain, liver, and kidney tissues was significantly higher in the FA-treated group than the control group. In brain tissue, the MDA level was higher than the liver and kidney tissues. The result suggests that oxidative stress is an important mechanism of organ damages. Zhang et al. [59] reported that FA caused the increment of ROS that caused the toxicity of the central nervous system [60]. FA-induced elevation of MDA was significantly attenuated in the group pretreated with PSO extract and vitamin E (Figure 3). Histopathological observations also suggested that FA induction caused the damages of the liver, kidneys, and brain (Figure 4). Excessive ROS production results in several deleterious events, including an irreversible oxidative modification of lipids, proteins, and carbohydrates [61, 62]. Besides, it induces apoptosis in hepatocytes and the release of inflammatory cytokines, thereby increasing the expression of adhesion molecules and the infiltration of leukocytes. A combination of all of these processes causes massive tissue destruction in the liver [63, 64].
To find the molecular mechanisms for the protective effects of PSO extract against FA-induced major organ damages, we investigated the endogenous protective antioxidant levels. Biochemical analyzes have shown that FA-induction caused the reduction of endogenous antioxidants such as CAT, SOD, and GSH. And treatment with PSO extract preserved the antioxidant pools. The present data of GSH results in the experimental groups are in agreement with previous reports [65, 66], who found that GSH is an antioxidant and powerful nucleophile. It has been mentioned that the antioxidant activity of PSO extract is due to the presence of the phenolic group [67]. In this study, we found that in the liver and kidney, the activity of GSH is more than the brain. In the FA treated group, there is a significant level of decrease of GSH. After FA treatment, the level of ROS increased, and liver GSH plays an important role in protecting membrane lipids from ROS [68]. It is rapidly oxidized by oxidants released from the metabolism or degrading process. This depletion may be the reason for low GSH values in the liver, brain, and kidney. PSO extract possesses strong antioxidant properties in experimental animals [69]. PSO administration showed a significant elevation of serum glutathione levels in albino rats [70]. Tissue glutathione depletion seems to be responsible for the induction of lipid peroxidation [71]. In our experiment, we found that CAT activity decreased in the FA treated group. A decreased activity of CAT might be due to the consumption of enzymes as a result of ROS. Decreased CAT activity could be attributed to cross-linking and inactivation of the enzyme protein in the lipid peroxidases [72]. From the results of our study, we found that CAT activity is more significant in the liver than the kidney and brain. SOD is the most important mitochondrial antioxidant enzymes and provides defence against superoxide anions. We observed in our study that SOD activity was decreased in the FA-induced group. In the PSO and vitamin E pretreated group, this level was increased. SOD's located in the cytosol and mitochondria, catalytically convert the superoxide radicals into oxygen and H2O2. Another assumption of depletion of SOD is the excess activation of phagocytes and the production of superoxide radicals [73] that can harm surrounding tissue either by a powerful oxidizing action or by the formation of hydrogen peroxide and hydroxyl radical from ROS. ROS initiates lipid peroxidation and destroys membrane. This destruction may initiate an inflammatory reaction process. The present study found that PSO extract pretreatment under FA-induced toxic circumstances significantly protected the liver and brain structure and restore liver functions. This protective effect of PSO extract could be the result of direct free radical scavenging properties [74].
5. Conclusion
FA caused the damages of major organs such as the brain, liver, kidneys, etc. in Swiss Albino mice. FA-induced organ damages were due to lipid peroxidation and an excessive load of oxidative stress that depleted the endogenous antioxidative defence mechanisms. However, pretreatment of mice with PSO extract protected the FA-induced organ damages. The PSO extract protected the organs by scavenging excess oxidative stress and prevented lipid peroxidation induced by FA. The endogenous antioxidant pools were also preserved in PSO treated mice. Pumpkin seed oil (PSO) is edible, cheap, and easily available, especially in Bangladesh. Hence, it can be a useful supplement in our daily life to protect our major organs and to maintain a healthy life. PSO can be used as an adjuvant for the formulation of functional foods. However, further study necessitates for its proper application.
Declarations
Author contribution statement
R. I. Khan: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
M. Paul, S.U. Sohag and A. Khan: Performed the experiments.
R. K. Barman: Analyzed and interpreted the data.
M.I.I. Wahed: Contributed reagents, materials, analysis tools or data.
Funding statement
This research received grant partly from University Grants Commission of Bangladesh, the People's Republic of Bangladesh under the supervision of Science Faculty, University of Rajshahi (Grant No-1042/5/52/UGC/Science-07/18-19) and was partly supported by the Department of Pharmacy, University of Rajshahi, Bangladesh.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Bhowmik S., Begum M., Hossain M.A., Rahman M., Nowsad A.K.M.A. Determination of formaldehyde in wet marketed fish by HPLC analysis: a negligible concern for fish and food safety in Bangladesh. Egyptian Journal of Aquatic Research. 2017;43:245–248. [Google Scholar]
- 2.Hoque M.S., Jacxsen L., De-Meulenaer B., Nowsad A.K.M.A. Quantitative risk assessment for formalin treatment in fish preservation: food safety concern in local market of Bangladesh. Procedia Food Science. 2016;6:151–158. [Google Scholar]
- 3.Söğüt S., Songur A., Özen O.A., Özyurt H., Sarsılmaz M. Does the subacute (4-week) exposure to formaldehyde inhalation lead to oxidant/antioxidant imbalance in rat liver? Eur. J. Gen. Med. 2004;1(3):26–32. [Google Scholar]
- 4.Hipkiss A.R. Depression, diabetes, and dementia: formaldehyde may Be a common causal agent; could carnosine, a pluripotent peptide, Be protective? Aging and Disease. 2017;8(2):128–130. doi: 10.14336/AD.2017.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Türkoğlu A.O., Sarsılmaz M., Çolakoğlu N., Zararsız I., Kuloğlu T., Pekmez H., U T. Formaldehyde-induced damage in lungs and effects of caffeic acid phenethyl ester: a light microscopy study. Eur. J. Gen. Med. 2008;5(3):152–156. [Google Scholar]
- 6.Cheng G., Shi Y., Sturla S.J., Jalas J.R., Mcintee E.J., Villata P.W., Wang M., Hecht S.S. Reactions of FA plus acetaldehyde with deoxyguanosine and DNA: formation of cyclic deoxyguanosine adducts and Formaldehyde cross-links. Chem. Res. Toxicol. 2003;16(2):145–152. doi: 10.1021/tx025614r. [DOI] [PubMed] [Google Scholar]
- 7.Smith A.E. Formaldehyde. Occup. Med. 1992;42(2):83–88. doi: 10.1093/occmed/42.2.83. [DOI] [PubMed] [Google Scholar]
- 8.Ames B.N. Mutagenesis and carcinogenesis: endogenous and exogenous factors. Environ. Mol. Mutagen. 1989;16:66–77. doi: 10.1002/em.2850140614. [DOI] [PubMed] [Google Scholar]
- 9.Jiménez-Villarreala J., Betancourt-Martíneza N.D., Carranza-Rosalesb P., Viveros-Valdezc E., Guzmán-Delgadod N.E., López-Márqueze F.C., Martínezaa J.M. Formaldehyde induces DNA strand breaks on spermatozoa and lymphocytes of wistar rats. Cytol. Genet. 2017;51(1):65–73. [PubMed] [Google Scholar]
- 10.Clemens D.L., Jerrells T.R. Ethanol consumption potentiates viral pancreatitis and may inhibit pancreas regeneration: preliminary findings. Alcohol. 2004;33(3):183–189. doi: 10.1016/j.alcohol.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Sotelo C.G., Pineiro C., Perez-Martin R.I. Denaturation of fish proteins during frozen storage: role of formaldehyde. Z. Lebensm. Unters. Forsch. 1995;200:14–23. doi: 10.1007/BF01192902. [DOI] [PubMed] [Google Scholar]
- 12.Harard N.F., Simpson B.K. Marcel Dekker; New York, USA: 2000. Seafood Enzymes. [Google Scholar]
- 13.Badii F., Howell N.K. Changes in the texture and structure of cod and haddock fillets during frozen storage. Food Hydrocolloids. 2002;16:313–319. [Google Scholar]
- 14.Noordiana N., Fatimah A.B., Farhana Y.C.B. Formaldehyde content and quality characteristics of selected fish and seafood from wet markets. Int Food Res J. 2011;18:125–136. [Google Scholar]
- 15.Bianchi F., Careri M., Musci M., Mangia A. Fish and food safety, determination of formaldehyde in 12 fish species by SPME extraction, and GC–MS analysis. Food Chem. 2007;100:1049–1053. [Google Scholar]
- 16.Weng X., Chon C.H., Jiang H., Li D. Rapid detection of formaldehyde concentration in food on a polydimethylsiloxane (PDMS) microfluidic chip. Food Chem. 2009;114:1079–1089. [Google Scholar]
- 17.EFSA Endogenous formaldehyde turnover in humans compared with exogenous contribution from food sources. EFSA J. 2014;12:3550. [Google Scholar]
- 18.Rahman M.M., Gilmour S., Saito E., Sultana P., Shibuya K. Self-reported illness and household strategies for coping with health-care payments in Bangladesh. Bull. World Health Organ. 2013;91:449. doi: 10.2471/BLT.12.115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin D., Xiao M., Zhao J., Li Z., Xing B., Li X., Kong M., Li L., Zhang Q., Liu Y. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules. 2016;21:1374. doi: 10.3390/molecules21101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makni M., Fetoui H., Gargouri N.K., Garoui el M., Zeghal N.J. Antidiabetic effect of flax and pumpkin seed mixture powder: effect on hyperlipidemia and antioxidant status in alloxan diabetic rats. Diabetes Complications. 2011;25(5):339–345. doi: 10.1016/j.jdiacomp.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Caili F., Huan S., Quanhong L. A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods Hum. Nutr. 2006;61(2):73–80. doi: 10.1007/s11130-006-0016-6. [DOI] [PubMed] [Google Scholar]
- 22.Bardaa S., Halima N.B., Aloui F., Mansour R.B., Jabeur H., Bouaziz M., Sahnoun Z. Oil from pumpkin (Cucurbita pepoL.) seeds: evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 2016;15:73. doi: 10.1186/s12944-016-0237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuhair H.A., Abd El-Fattah A.A., El-Sayed M.I. Pumpkin-seed oil modulates the effect of felodipine and captopril in spontaneously hypertensive rats. Pharmacol. Res. 2000;41(5):555–563. doi: 10.1006/phrs.1999.0622. [DOI] [PubMed] [Google Scholar]
- 24.Zuhair H.A., Abd el-Fattah A.A., Abd el Latif H.A. Efficacy of simvastatin and pumpkin-seed oil in the management of dietary-induced hypercholesterolemia. Pharmacol. Res. 1997;35(5):403–408. doi: 10.1006/phrs.1997.0148. [DOI] [PubMed] [Google Scholar]
- 25.AOAC . eighteenth ed. Association of Official Analytical Chemists; Washington, DC, USA: 2010. (Official Methods of Analysis). [Google Scholar]
- 26.Qi Q. Study on extraction of pumpkin seed oil by aqueous enzymatic method. Journal of Anhui Agriculture Science. 2012;40:7410–7413. [Google Scholar]
- 27.Jiao J., Li Z.G., Gai Q.Y., Li X.J., Wei F.Y., Fu Y.J., Ma W. Microwave-assisted aqueous enzymatic extraction of oil from pumpkin seeds and evaluation of its physicochemical properties, fatty acid compositions, and antioxidant activities. Food Chem. 2014;147:17–24. doi: 10.1016/j.foodchem.2013.09.079. [DOI] [PubMed] [Google Scholar]
- 28.Rabrenović B.B., Dimić E.B., Novaković M.M., Tešević V.V., Basić Z.N. The most important bioactive components of cold-pressed oil from different pumpkin (Cucurbita pepo L.) seeds. Food Sci. Technol. 2014;55(2):521–527. [Google Scholar]
- 29.Mitra P., Ramaswamy H.S., Chang K.S. Pumpkin (Cucurbita maxima) seed oil extraction using supercritical carbon dioxide and physicochemical properties of the oil. J. Food Eng. 2009;95:208–213. [Google Scholar]
- 30.Johnson L.A. Oil recovery from soybeans, Chemistry. In: Johnson L.A., White P.J., Galloway R., editors. Soybeans, Production, Processing, and Utilization. AOCS Press; Urbana, IL: 2008. pp. 331–375. [Google Scholar]
- 31.Odewole M.M., Sunmonu M.O., Obajemihi O.I., Owolabi T.E. Extraction of oil from fluted pumpkin seed (telfairia occidentalis) by solvent extraction method. Annals Food Science and Technology. 2015;16(2):372–378. [Google Scholar]
- 32.AOAC . 2002. Official Methods of Analysis Association of Analytical Chemists, Washington D.C. [Google Scholar]
- 33.Kumar K.S., Ganesan P.V., Kumar R.S. Antioxidant potential of solvent extracts of Kappaphycus alvarezii (Doty) Doty–edible seaweed. Food Chem. 2007;107:289–295. [Google Scholar]
- 34.Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–559. [Google Scholar]
- 35.Khatun A., Rana M.M., Khan M.R.I., Wahed M.I.I., Habib M.A., Uddin M.N., Sathi Z.S., Ruhul-Amin A.R.M., Anisuzzaman A.S.M. Molecular mechanism of formalin- induced toxicity and its management. Am. J. Life Sci. 2015;3(2):85–92. [Google Scholar]
- 36.Pan Y., Wang K., Huang S., Wang H., Mu X., He C., Ji X., Zhang J., Huang F. Antioxidant activity of microwave-assisted extract of longan (Dimocarpus Longan Lour.) peel. Food Chem. 2008;106(3):1264–1270. [Google Scholar]
- 37.Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a Phosphomolybdenum Complex: specific application to the determination of vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 38.Naghdi M., Maghbool M., Seifalah-Zade M., Maryam Mahaldashtian M., Makoolati Z., Kouhpayeh S.A., Ghasemi A., Fereydouni N. Effects of common fig (Ficus carica) leaf extracts on sperm parameters and testis of mice intoxicated with formaldehyde. Evidence-Based Comple Altern Med. 2016;9:2016. doi: 10.1155/2016/2539127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abou-Zeid S.M., Abu Bakr H.O., Mohamed M.A., El-Bahrawy A. Ameliorative effect of pumpkin seed oil against emamectin induced toxicity in mice. Biomed. Pharmacother. 2018;98:242–251. doi: 10.1016/j.biopha.2017.12.040. [DOI] [PubMed] [Google Scholar]
- 40.Elfikya S.A., Elelaimyb I.A., Hassana A.M., Ibrahimb H.M., Elsayada R.I. Protective effect of pumpkin seed oil against genotoxicity induced by azathioprine. The Journal of Basic and Applied Zoology. 2012;65:289–298. [Google Scholar]
- 41.Bakar E., Ulucam E., Cerkezkayabekir A. Investigation of the protective effects of proanthocyanidin and vitamin E against the toxic effect caused by formaldehyde on the liver tissue. Environ. Toxicol. 2015;30:1406–1415. doi: 10.1002/tox.22010. [DOI] [PubMed] [Google Scholar]
- 42.Gulec M., Gurel A., Armutcu F. Vitamin E protects against oxidative damage caused by formaldehyde in the liver and plasma of rats. Mol. Cell. Biochem. 2006;290:61–67. doi: 10.1007/s11010-006-9165-z. [DOI] [PubMed] [Google Scholar]
- 43.Reitman A., Frankel S. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28:e56. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 44.Delanghe J.R., Speeckaert M. Creatinine determination according to Jaffe—what it stands for? Nephrol. Dial. Transplant. 2012:1–4. doi: 10.1093/ndtplus/sfq211. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ellman G.L. Tissue sulphydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 46.Wu G. Glutathione metabolism and its implications for health. J. Nutr. 2004;134(3):489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 47.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 48.Kar M., Mishra D. American Society of Plant Biologists; 1976. Catalase, Peroxidase, and Polyphenol Oxidase Activities during rice Leaf Senescence; p. 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Preuss H.G., Jarrel S.T., Scheckenbach S., Anderson R.A. Comparative effects of chromium, vanadium, and Gymnema Sylvestre on sugar-induced blood pressure elevations in SHR. J Coll Nutr. 1998;17(2):116e123. doi: 10.1080/07315724.1998.10718736. [DOI] [PubMed] [Google Scholar]
- 50.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 51.Raczyk M., Siger A., Radziejewska-Kubzdela E., Ratusz K., Rudzińska M. Roasting pumpkin seeds and changes in the composition and oxidative stability of cold pressed oils. Acta Sci Pol Technol Aliment. 2017;16(3):293–301. doi: 10.17306/J.AFS.0494. [DOI] [PubMed] [Google Scholar]
- 52.Soobrattee M.A., Neergheen V.S., Luximon-Ramma A., Aruoma O.I., Bahorun O.T. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res Fundam Mol. 2005;579:200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 53.Geetha S., Sai-Ram M., Mongia S.S., Singh V., Ilavazhagan G. Evaluation of antioxidant activity of leaf extract of sea buckthorn (Hippophae rhamnoides L.) on chromium (VI) induced oxidative stress in albino rats. J. Ethnopharmacol. 2003;87:247–251. doi: 10.1016/s0378-8741(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 54.Shimoi K., Masuda S., Shen B., Furugori M., Kinze N. Radioprotective effects of antioxidative plant flavonoids in mice. Mutat Res Fund Mol. 1996;350:153–161. doi: 10.1016/0027-5107(95)00116-6. [DOI] [PubMed] [Google Scholar]
- 55.Keller C.R., Odden M.C., Fried L.F., Newman A.B., Angleman S., Green C.A., Cummings S.R., Harris T.B., Shlipak M.G. Kidney function and markers of inflammation in elderly persons without chronic kidney disease: the health, aging, and body composition study. Kidney Int. 2007;71(3):239–244. doi: 10.1038/sj.ki.5002042. [DOI] [PubMed] [Google Scholar]
- 56.Bhatt H.V., Panchal G.M. Behavioural change in rats due to chronic oral and systemic formaldehyde. Indian J. Physiol. Pharmacol. 1992;36:270–272. [PubMed] [Google Scholar]
- 57.Zhang B., Shi Y., Chen X., Dai J., Jiang Z., Li N., Zhang Z. Protective effect of curcumin against formaldehyde-induced genotoxicity in A549 cell lines. J. Appl. Toxicol. 2013;33:1468–1473. doi: 10.1002/jat.2814. [DOI] [PubMed] [Google Scholar]
- 58.Niki E. Lipid peroxidation products as oxidative stress biomarkers. Biofactors. 2008;34(2):171–180. doi: 10.1002/biof.5520340208. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y., Liu X., McHale C., Li R., Zhang L., Wu Y., Ye X., Yang X., Ding S. Bone marrow injury induced via oxidative stress in mice by inhalation exposure to formaldehyde. PloS One. 2013;8(9) doi: 10.1371/journal.pone.0074974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arici S., Karaman S., Dogru S., Cayli S., Arici A., Suren M., Karaman T., Kaya Z. Central nervous system toxicity after acute oral formaldehyde exposure in rabbits: an experimental study. Hum. Exp. Toxicol. 2014;33(11):1141–1149. doi: 10.1177/0960327113514098. [DOI] [PubMed] [Google Scholar]
- 61.Leung T.M., Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J. Hepatol. 2013;58(2):395–398. doi: 10.1016/j.jhep.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 62.Parveen K., Khan M.R., Mujeeb M., Siddigui W.A. Protective effects of Pycnogenol on hyperglycemia-induced oxidative damage in the liver of type 2 diabetic rats. Chem. Biol. Interact. 2010;186:219–227. doi: 10.1016/j.cbi.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 63.Welt K., Weiss J., Martin R., Dettmer D., Hermsdorf T., Asayama K. Ultrastructural, immunohistochemical and biochemical investigations of the rat liver exposed to experimental diabetes and acute hypoxia with and without application of Ginkgo extract. Exp. Toxicol. Pathol. 2004;55(5):341–345. doi: 10.1078/0940-2993-00337. [DOI] [PubMed] [Google Scholar]
- 64.Wei Y., Chen P., de Bruyn D., Zhang W., Bremer E., Helfrich W. Carbon monoxide-releasing molecule-2 (CORM-2) attenuates acute hepatic ischemia-reperfusion injury in rats. BMC Gastroenterol. 2010;10:42. doi: 10.1186/1471-230X-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown L.A., Harris F.L., Ping X., Gauthier T.W. Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability? Alcohol. 2004;33(3):191–197. doi: 10.1016/j.alcohol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Mallikarjuna K., Chetan P., Reddy K.S., Rajendra W. Ethanol toxicity: rehabilitation of hepatic antioxidant defense system with dietary ginger. Fitoterapia. 2008;79(3):174–178. doi: 10.1016/j.fitote.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 67.Hatapakki B.C., Suresh H.M., Bhoomannavar V., Shivkumar S.I. Effect of Cascia auriculata Linn flowers against alloxan-induced diabetes in rats. J. Nat. Remedies. 2005;5(2):132–136. [Google Scholar]
- 68.Kobayashi T., Robinson M.K.R.V. Glutathione depletion alters hepatocellular high-energy phosphate metabolism. J. Surg. Res. 1993;54(3):189–195. doi: 10.1006/jsre.1993.1030. [DOI] [PubMed] [Google Scholar]
- 69.Gerber G.S. Phytotherapy of benign prostatic hyperplasia. Curr. Urol. Rep. 2002;3(4):285–291. doi: 10.1007/s11934-002-0050-3. [DOI] [PubMed] [Google Scholar]
- 70.Ahmed H.H., Abdel-Rahman M., Ali R.S., Abdel Moniem A.E. Protective effect of Ginkgo biloba extract and pumpkin seed oil against neurotoxicity of rotenone in adult male rats. J. Appl. Sci. Res. 2009;5(6):622–635. [Google Scholar]
- 71.Lewis D.A. Vol. 35. Birkhauser, Verlag; Basel: 1989. (Anti-inflammatory Drugs from Plants and marine Sources). [PubMed] [Google Scholar]
- 72.Sayed H., Seif A. Ameliorative effect of pumpkin oil (Cucurbita pepo L.) against alcohol-induced hepatotoxicity and oxidative stress in albino rats. Beni-Suef University Journal of Basic and Applied Sciences. 2014;3(3):178–185. [Google Scholar]
- 73.Wills E.D., Gillham B., Papachristodoulou D.K., Thomas J.H. Vol. 77. Butterworth-Heinemann; Oxford: 1997. p. 351. (Wills' Biochemical Basis of Medicine). [Google Scholar]
- 74.Gurel A., Coskun O., Armutcu F., Kanter M., Ozen O.A. Vitamin E against oxidative damage caused by formaldehyde in frontal cortex and hippocampus: biochemical and histological studies. J. Chem. Neuroanat. 2005;29:173–178. doi: 10.1016/j.jchemneu.2005.01.001. [DOI] [PubMed] [Google Scholar]