Abstract
Hepatocellular carcinoma (HCC) is one of the most common causes of cancer-related death around the world due to advanced clinical stage at diagnosis, high incidence of recurrence and metastasis after surgical treatment. It is in urgent need to create appropriate animal models to explore the mechanism, patterns, risk factors, and therapeutic strategies of HCC metastasis and recurrence. However, most of the established models lack the phenotype of invasion and metastasis in patient, or have unstable phenotype. To establish HCC models with stable metastasis phenotype requires profound understanding in cancer metastasis biology and scientific methodology. Over the past 3 decades, HCC models with stable metastasis have been extensively studied. This paper reviewed the history and development of HCC animal models and cell models, focusing on the screening and maintaining of metastatic potential and phenotype. In-depth studies using these models vastly promote the understanding of cellular and molecular mechanisms and development of therapeutic strategies on HCC metastasis.
Keywords: Animal model, Cell model, Chemically induced HCC model, Genetically engineered mouse model, Hepatocellular carcinoma, Metastasis, Patient derived xenograft model
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of cancer death in male, and the sixth leading cause in female around the world. China accounts for about 50% of total cases and death.1 Metastasis is the most fundamental biological characteristic and main cause of treatment failure and cancer death.2 Even in small HCC, the metastasis and recurrence rate can reach up to 50%.3, 4, 5 Most of the HCC metastasizes to other organs through blood vessel, with lymphatic metastasis being the second most frequent route. Direct spreading, invasion or implantation is also found in HCC metastasis.
The extrahepatic hematogenous metastasis usually occurs in early stage and involves large extent of organs or tissues. Zhu et al6 found that extrahepatic organ metastasis through hematogenous route happened in more than 60% of HCC patients, with lung being the most frequently involved organ (approximately 90% in all hematogenous metastasis). Liu et al7 performed autopsy on 56 primary HCC patients, and revealed 85 organ metastases and 78 lymph node metastases. In an autopsy study of 387 patients with 14 various primary cancers, the metastatic rate of HCC was 51.8%. In another autopsy study of 240 HCC patients in Japan,8 the intrahepatic metastasis rate was 78.5%, hematogenous metastatic rate 59.5%, portal vein tumor thrombus 57.9%, lymph node metastasis 32.9%, locally direct spreading 21.3%, hepatic vein tumor thrombus 12.1%, biliary tract invasion 4.2%, and perihepatic dissemination 3.8%. Among organs involved, pulmonary metastatic rate was 49.2% (118/240). These data suggested that lung was a more favorable organ of extrahepatic metastasis in HCC. Therefore, one of the vital emphasis on the study of HCC metastasis should be the mechanism of pulmonary metastasis.
Experimental HCC models with metastasis are essential for exploring mechanism of HCC metastasis and elucidating the contributing factors. However, the invasion and metastasis characteristics in clinical patients are not always able to be replicated in animal models or cell lines. The features of both tumor specimens and conditions of host animal for developing metastasis models are fundamental issues to be solved during mechanism studies of cancer metastasis. Therefore, the significance of establishing HCC model with metastasis is more than just constructing a laboratory platform. Instead, it is important to explore the basic laws and mechanism of HCC metastasis, so that HCC model with metastasis can be established by certain scientific approaches rather than by chances. This review is intended to summarize the progress on the construction of HCC animal and cell models with metastasis and/or metastatic potential.
HCC animal model with metastasis
Spontaneous HCC models
Spontaneous tumor model develops naturally in animals without any intentional intervention, reflecting the accumulation of environmental carcinogens, cancer-promoting substances and tumor susceptibility, replicating human HCC in terms of antigenicity, growth pattern, cell kinetics and differentiation.9 However, there are many uncontrollable factors in spontaneous HCC model. First, the susceptibility varies among different species, with C3H mice and CBA mice being more susceptible to spontaneous HCC.10 Second, even in the same species, the incidence and the time of tumor development could be largely different between male mice and female mice.11 Third, the histopathology of tumor is markedly heterogeneous. In a study of 1,000 wild rats, only one cavernous hemangioma, two fibromas and one adenofibroma were found, showing great variance within only four models.12 In terms of metastasis, pulmonary metastasis was not observed until 24 months, with an incidence rate of only 4.5%.9 To sum up, spontaneous HCC model is usually characterized by low incidence, long incubation period, great phenotype heterogeneity, and low spontaneous metastasis rate. Therefore, spontaneous HCC model is currently not a favorable option, except for the purpose of providing tumor source for transplantation models.13
Chemically induced HCC models
Exposure of chemical, physical, biological, and other carcinogenic factors are critical links in the pathogenesis of HCC due to the essential function of xenobiotic detoxification in liver. Among the carcinogenic risk factors, chemical agents are the most frequently used method to induce HCC model, because it is technically easy to perform and can replicate human HCC in terms of pathogenetic mechanism and clinical-pathological presentation. Dimethylnitrosamine (DMN), diethylnitrosamine (DEN), N-nitrosomorpholine (NMOR), N-butyl-N-(4-hydroxybutyl) nitrosamine, 1,2-dimethylhydrazine, and 2,2′-dihydroxy-di-N-propylnitrosamine are several kinds of frequently used chemical carcinogens for the inducing of HCC models.14 Metastasis in chemically induced HCC models mostly occurred through blood vessels.
As early as 1956, Magee et al15 firstly reported that DMN was a promising chemical agent to induce hepatic tumor accompanied with metastasis in rats (Table 1). Using this method, researchers have been devoting to the construction of HCC model with hematogenous metastasis. Later, based on the three-stage assumption (initiation, promotion, and progression) in liver carcinogenesis, a two-step method (initiation and promotion) were innovated to produce HCC model, mostly composed of the combination of DEN or other chemical compounds as the initiating agents and phenobarbital as the promoter.16 In 1983, Dennis et al17 demonstrated that partial hepatectomy was also a promising promoting stimulus in inducing liver cancer. In the late 1980s, it was reported that NMOR could induce HCC oncogenesis and spontaneous pulmonary metastasis in F344 rats by adding it to drinking water.18
Table 1.
Synopsis of chemically induced HCC model with metastasis.
| Chemical agents | Characteristics | Metastasis | Comments | References (Year) |
|---|---|---|---|---|
| DMN induced model | Months 6.5–10: primary hepatic tumor development | Pulmonary (7/20) and intra-abdominal (3/20) metastasis | The first to provide methods for chemical agent induced HCC model | Magee et al (1956)15 |
| DEN and 2-AAF + PH induced model | Months 8: 68%–71% HCC; | 9.1%–16.7% metastatic rate | Starting the method of “two-step” (initiation, promotion) model; DEN alone could not induce carcinogenesis; |
Solt et al (1983)17 |
| [PH + B(a)P] + [2-AAF + CCl4] induced model | Months 18: 82% (14/17) HCC | 23.5% (4/17) pulmonary metastasis | ||
| [PH + 1,2-DMH] + [2-AAF + CCl4] induced model | Months 18: 67% (8/12) HCC | 16.7% (2/12) pulmonary metastasis | ||
| DEN induced model | Months 11-: 100% HCC | 25%–50% metastasis rate in B6C3F1 mice; 0%–33% metastasis rate in C3AF1 mice |
Revealing heterogeneity between different species; Younger mice have faster HCC development than older mice |
Vesselinovitch et al (1984)20 |
| NMOR induced model | Months 25: 63% (15/24) HCC (NMOR 100 mg/L); Months 40: 67% (16/24) HCC (NMOR 40 mg/L) |
NMOR 100 mg/L: 4.2% metastatic rate; NMOR 40 mg/L: 29.2% metastatic rate |
Focusing on the dose response in F344 rats when administered with NMOR | Lijinsky et al (1988)18 |
| DEN + PB induced model | Months 10: 30% (6/20) HCC | NA | PB shortened the time to HCC appearance | Klaunig et al (1988)23 |
| DEN + NMOR induced model | Months 4: 100% (15/15) HCC | Months 7: 100% (15/15) pulmonary metastasis | Providing an optimal method for the inducing of HCC with significant pulmonary metastasis | Masui et al (1997)14 |
| DEN + NMOR induced model | Months 2: 60% (9/15) HCC; Months 4: 100% (13/13) HCC; Months 5.5: 94% (17/18) HCC |
Months 2: 0% (0/15) pulmonary metastasis; Months 4: 69% (9/13) pulmonary metastasis; Months 5.5: 84% (16/19) pulmonary metastasis |
Established relatively stable HCC model for studies on metastasis; Providing experience for metastatic model construction |
Futakuchi et al (1999)19 |
| NNM induced model | Months 6.8: 100% (15/15) HCC; | Months 7: 87% (13/15) metastasis rate | WS/Shi was the most sensitive species to NNM compared with SD/gShi, and F344/DuCrj rats | Murai et al (2000)24 |
| DEN + PB induced model | Months 5: 67% (6/9) macroscopic hepatic masses; Months 9: 100% (9/9) macroscopic hepatic masses |
NA | PB did not influence the incidence of macroscopic hepatic masses | Goldsworthy et al (2002)25 |
| DEN + NMOR induced model | Months 3.5: HCC was observed; Months 5: 100% HCC |
Months 5.5: first pulmonary metastasis; Months 9: 60% pulmonary metastasis; Months 10: 100% pulmonary metastasis |
Modifying the experimental protocol to improve survival and to establish a better animal metastasis model | Yoshino et al (2005)22 |
| DEN in GDF-15 deleted mice | Months 6: 80% (16/20) HCC | No metastasis | GDF-15 had no apparent effect on HCC tumor formation rate, growth rate, or invasiveness in DEN-induced HCC | Zimmers et al (2008)26 |
| DEN in ATM mutated mice | Months 1.3: development of HCC; Months 12: 100% HCC |
Pulmonary metastasis in 50% of ATM+/+ and 52% of ATM+/− mice; Months 12: 100% metastasizing HCC in wild type or ATM+/- mice |
Hepatocarcinogenesis is abrogated in ATM-deficient mice | Teoh et al (2010)27 |
| DEN + PB | Months 8: microscopically and macroscopically detectable tumors; Months 14: HCC |
NA | Exploring the tumor genomes of DEN induced HCC for the first time; Beta-catenin mutation and activation of the Wnt/β-catenin pathway were not involved in tumor initiation of this model |
Aleksic et al (2011)28 |
Abbreviations: HCC, hepatocellular carcinoma; DMN, dimethylnitrosamine; DEN, diethylnitrosamine; 2-AAF, 2-acetylaminofluorene; PH, partial hepatectomy; B(a)P, benzo(a)pyrene; 1,2-DMH, 1,2-dimethylhydrazine; NMOR, nitrosomorpholine; PB, phenobarbital; NNM, N-nitrosomorpholine; GDF-15, Growth/differentiation factor-15; ATM, ataxia telangiectasia mutated.
However, tumor incidence rate in corresponding chemical agents was the major observation target, instead of HCC metastasis. In the late 1990s, Masui et al14 was the first to demonstrate that pulmonary metastatic rate of DEN could be significantly enhanced to 100% by increasing the dosage of NMOR to 120 ppm. In 1999, Futakuchi et al19 further demonstrated that combination of DEN and NMOR was the most efficient method to induce HCC with significant pulmonary metastasis. The HCC incidence rate and pulmonary metastatic rate reached 94%–100% and 69%–84% respectively, with histopathology being moderately differentiated HCC and moderately to poorly differentiated carcinoma. One important molecular feature of this model is the progressive decline in cadherin expression during the transformation from adenocarcinoma to HCC, and eventually to metastatic cancer, which mimicked the malignant biological behavior in patient.
As listed in Table 1, the phenotype of pulmonary metastasis was frequently observed in chemically induced HCC model. However, the development of HCC and potential of metastasis vary with the changes of age, gender and species of mice,20,21 as well as the dosage of chemo-toxic agents. Even DEN plus NMOR in F344 rats was a relatively mature method, slight changes in age (6 weeks vs. 6 weeks vs. 5 weeks), DEN (100 mg/kg, 4 weeks vs. 100 mg/kg, single intraperitoneal injection vs. 100 mg/kg, single intraperitoneal injection), and NMOR (120 ppm, 24 weeks vs. 120 ppm, 22 weeks vs. 40 ppm, 14 weeks) vastly influence the time of pulmonary metastasis and incidence rate.14,19,22 Nevertheless, DEN plus NMOR was still a more effective and stable protocol for chemically induced HCC model with significant pulmonary metastasis when comparing other counterparts in Table 1.
Patient derived xenograft (PDX) HCC model
In 1969, Rygaard proved for the first time that T cell development in mice were obstructed due to thymic aplasia and cellular immunodeficiency caused by congenital gene mutation, which made the grafting of xenogeneic skin tissue and tumor possible.29,30 PDX model of HCC started in the mid-1970s. In 1976, Shimosato for the first time reported the establishment of murine HCC model.31 Tumor was grafted subcutaneously in the back of BALB/c nude mice using cuff technique. The tumor volume reached 40 mm × 30 mm × 15 mm 28 days later, accompanied with local lymphatic metastasis. In 1979, Hirohashi et al32 studied several subcutaneous HCC nude mouse models by consecutive passaging, demonstrating that most of the HCC were able to maintain primary histological features during passaging. This HCC model mimicked patient in terms of protein expression level and correlation among tumor growth speed, histological differentiation and alpha-fetoprotein (AFP) expression level, indicating that PDX model was a promising and reliable platform in the future studies of HCC.
At the same period, studies on PDX models were also carried out at Liver Cancer Institute of Fudan University (former Liver Cancer Institute of Shanghai Medical University). In 1982, the earliest HCC nude mouse model in China, LTNM1 and LTNM2,33,34 were established by subcutaneous grafting of AFP-positive tumor sample to the neck and back of nude mice via trocar. Later in 1986, 2 heterotopic subcutaneous nude rat models, LTNR1 and LTNR2, were constructed.35 Compared with the HCC nude mouse model, the nude rat model had the advantages of more tumor nodules with more adequate blood supply, and was more convenient for experimental operation. But both nude mouse and nude rat subcutaneous models lacked the feature of infiltration or metastasis, failing to fully reflect all the malignant biological behaviors of human HCC. Therefore, subcutaneous model was still a transitional model before optimal model was established.
The advantages and disadvantages of different transplantation methods, including subcutaneous, intraperitoneal, and intrahepatic nude rat models were compared and concluded by Bao et al.36 Subcutaneous model had the advantages of high successful rate, high growth speed, which was suitable for batch experiment. However, the deficiency on the microenvironment, biological behavior of infiltration and metastasis greatly limits the application of subcutaneous models in HCC study. Intraperitoneal model is inconvenient for the measurement and observation of tumor hiding deep in the peritoneal cavity, but the incidence rate of infiltration, metastasis, and ascites is significantly elevated compared with subcutaneous model. Intrahepatic model possesses the advantages of short latent period and rapid growth. Besides, it replicates human HCC, showing characteristics of infiltration, metastasis, ascites, and tumor loci. Intrahepatic model might bring practical significance for the exploration of interventional treatments, for example, intubation, ligation, and embolism.
By the mid-1990s, owing to the aforementioned well-rounded studies, the theoretical basis and experimental method have been gradually concluded and refined to produce ideal HCC PDX model with significant phenotype of metastasis. Improved experimental protocol has been put into practice, including the following three major aspects. (1) Tumor source: HCC specimen was obtained from the metastatic loci rather than the primary tumor, so as to selectively increase the metastatic potential of original tumor tissue; (2) Implantation method: To provide a more favorable microenvironment for tumor growth and the expression of metastatic feature, tumor samples were grafted orthotopically to murine liver; and (3) Serial selection: Several rounds of in vivo selection by continuously grafting tumor into liver was applied to further promote and stabilize the metastatic potential of HCC. With the refined methods, HCC PDX models with high-metastatic potential were gradually constructed and put into HCC studies. HCC PDX models can be further divided into three types based on the features of dominant metastasis behaviors, including HCC model with hematogenous metastasis, HCC model with intrahepatic metastasis, and HCC model with lymphatic metastasis (Table 2).
Table 2.
Synopsis of patient-derived xenograft HCC model with metastasis.
| High metastatic HCC Model | Characteristics | Metastasis | Comments | References (Year) |
|---|---|---|---|---|
| Hematogenous metastasis model | ||||
| LCI-D20 | Days 10 ± 1.5: 100% transplant-ability (96/96) for 18 passages | Weeks 6–8: all transplanted mice died of serious metastasis; Spontaneous metastasis to lung (78/78), liver (78/78), and lymph node (78/78) |
Having significant and stable phenotype of hematogenous metastasis; Played a vital role in the exploration of HCC metastasis and recurrence |
Sun et al (1996)40 |
| LCI-D35 | Low metastatic ability | No metastasis in the liver, lung and lymph node | Used as a control compared to LCI-D20; The biological features remained unchanged until 59 passages |
Sun et al (1995, 1996, 2001)38,39,41 |
| Intrahepatic metastasis model | ||||
| Subcutaneous liver metastatic HCC model | Weeks 2: latent period; Weeks 4: 1.0 cm in average diameter |
Weeks 4: significant liver metastasis in 100% mice (5/5); 100% liver metastasis after the third generation and even after 18 generations |
Non-orthotopic intrahepatic metastasis model | Aruga et al (1993)46 |
| HCC cell lines inoculated HCC model | Li7 and KYN-2 cell lines: multiple small liver tumors | Li7 and KYN-2 cell lines: vascular tumor thrombi and intrahepatic metastasis; | Rho/p160ROCK signaling pathway is critical in intrahepatic metastasis of human HCC. | Genda et al (1999)62 |
| Mouse HCC tumor implanted model | 100% transplant-ability | Weeks 4: 100% (7/7) intrahepatic metastasis; and 28.6% (2/7) pulmonary metastasis | Reflecting the steps of HCC metastasis; Intrahepatic tumor presented time-dependent growth pattern; |
Sawada et al (2002)63 |
| Lymphatic metastasis model | ||||
| Lymphatic metastasis model with H22 tumor cells | Constructed by injecting cells obtained by repeated lymphatic system screen; Cell subclones with different metastatic potential was isolated from lymphatic metastatic models |
100% (10/10) lymph node metastasis | First established HCC model with significant lymphatic metastasis, and heterogeneity inside this model was further illustrated | Ling et al (1984, 1990)51,55 |
| Lymphatic metastasis model with Hca-F cells | No metastasis to other organs except for lymph node | High metastatic rate during the 17 generations (80%–100%) | Lymphatic metastatic models with stable and high metastatic rates | Li et al (1998)58 |
| HCC model with lymph node-specific metastasis | Using lymph node metastasis from HCCLM3 as the tumor source; Using fluorescent stereomicroscopy to detect metastasis loci |
Weeks 6: 100% lymph node metastasis | Applied convenient method to detect tiny metastasis loci | Tao et al (2011)59 |
| Annexin A7 down-regulated lymphatic metastasis model | Downregulation of the Annexin A7 gene significantly inhibit lymph node metastasis | 100% lymph node metastasis rate | Illustrating the potential of lymph node metastasis in terms of gene expression | Jin et al (2013)60 |
Abbreviations: HCC, hepatocellular carcinoma.
HCC model with hematogenous metastasis
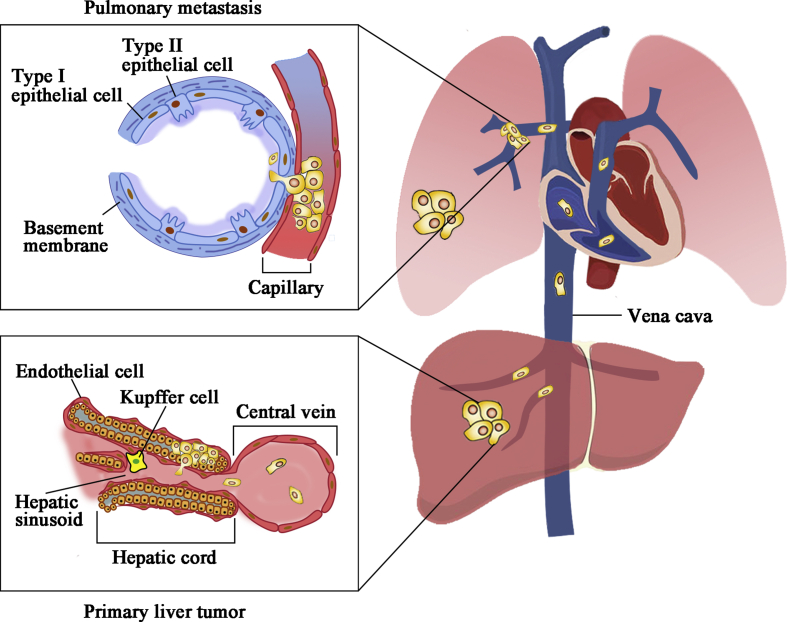
In clinical conditions, the most frequent site of distant metastasis is the lung, due to dissemination of tumor cells via the blood stream, hemodynamic characteristics of the liver and the intrinsic biological features of the tumor, for example, increased proliferation, invasion, and motility (Fig. 1).2,37
Figure 1.
Primary HCC tumor metastasizes to the lung via bloodstream in clinical settings. Experimental models of HCC established by orthotopic implantation of intact tumor tissue into nude mice optimally mimic human primary HCC, as the tumor microenvironment is also transplanted, creating similar liver and lung microenvironments favorable for the growth of metastatic cells.
In 1996, high metastatic LCI-D20 model of HCC was constructed by Liver Cancer Institute of Fudan University (Table 2).38, 39, 40 Surgical specimens from 30 patients were used for transplantation, among which one case from a 39-year old man showed pulmonary metastasis. LCI-D20 was constructed via in vivo clonal selection by repeated “lung foci to liver”.40,41 LCI-D20 mimicked biological behaviors of human HCC, including local-regional growth and invasion, and spontaneous metastasis to lung, liver, and lymph node. This model manifested 100% transplant-ability and the high metastatic ability was maintained to more than 120 passages, with short passage duration of about 20 days. Both LCI-D20 and the patient showed Edmonson Grade-II HCC microscopically, accompanying with the expression of AFP and hepatitis virus-B surface antigen (HBsAg). At the same period, an orthotopic HCC model with low metastatic potential, LCI-D35, was established for comparative studies.38,39 To this point, HCC PDX models in accordance with clinical patient and more relevant to clinical setting were successfully established. LCI-D20 was now widely applied in the study of HCC recurrence and metastasis, for example, the studies of antisense oligonucleotides and gene transfection,42 extracellular matrix,43 intercellular cell adhesion molecule (ICAM),44 anti-angiogenesis, and inducers for cellular differentiation.45
HCC model with intrahepatic metastasis
HCC metastasis mostly started with intrahepatic spreading. Therefore, it's important to establish HCC model with intrahepatic metastasis. In 1993, Aruga et al46 firstly introduced a subcutaneous HCC model with spontaneous liver metastasis. However, the established subcutaneous model might not fully reflect the process of intrahepatic metastasis. Kuriyama et al47 grafted carbocyanine dye (DiI)-labeled HCC cells under the capsule of liver tissue. Fluorescence microscopy and laser confocal microscopy revealed that cancer cells invaded portal vein nearby, but did not invade central vein. However, cancer cells with DiI label did not show the sign of migration. The author repeated the experiment above after inducing cirrhosis using intraperitoneal injection of thioacetamide. The speed cancer cells spread to the portal vein area was significantly elevated in mice with cirrhosis than in normal mice,48 which indicated that HCC cells were prone to invade portal vein, especially under the circumstances of liver cirrhosis. Kuriyama again proved that cirrhosis is an important contributing factor for HCC development and metastasis. With the successful experiment, HCC model with intrahepatic metastasis were widely applied in the interventional experiments, for example heat treatment and radiofrequency ablation.49,50
HCC model with lymphatic metastasis
The ascitic type HCC model H22 possesses stable tumorgenicity, but not fully presenting the characteristics of metastasis. In 1984, tumor cells from H22 model were injected subcutaneously into the foot pad of the inbred 615 mice, and extensive lymphatic metastasis occurred 21 days later, including ipsilateral axillary lymph nodes, bilateral inguinal lymph nodes, bilateral axillary lymph nodes, lumbar and renal hilar lymph node.51 After 20 rounds of repeated in vivo lymphatic system selection, the lymphatic metastasis rate was elevated and even involved paratracheal lymph nodes.52 Using in vivo screening for 25 rounds, the ascites of H22–F25 was cultured in vitro to establish a mouse HCC cell line H22–F25/L.53,54
In 1990, Ling further isolated five cell clones (16A3, 16C3, 22C5, H7, and A2) from H22–F25/L cell line,55 including one high metastatic cell line (16A3) and one low metastatic cell line (A2). The subclone of 16A3 and A2 were able to preserve the metastasis potential within 20 passages. However, the high-metastatic potential of 16A3 was relatively unstable and could be preserved within 20 consecutive in vitro passages, but might change significantly following further passages.56 To get cell lines with stable metastasis potential, Ling suggested to freeze primary cultured cells as much as possible or to further separate subclones with high metastasis potential.57 By applying limited dilution method, 8 subclones were isolated, among which HCa-F25/CL16A3-F (F) and HCa–F25/CL16A3-D (D) had the highest metastasis rate.
In the following 2 decades, several lymphatic metastatic models have been established, using similar method to Ling et al (Table 2).58,59 Using gene knock up and knock down technique, Jin et al60 demonstrated that knock down of Annexin A7 expression could significantly induce lymphatic metastasis in HCC model. In the treatment study, Liu et al61 showed that oxyresveratrol could prevent tumor growth and lymphatic metastasis by inhibiting tumor angiogenesis and lymph-angiogenesis.
The current status and development of HCC animal models
Chemically induced and PDX models are currently not meeting the demand of the research on hepatocarcinogenesis mechanism in specific liver disease context. On the one hand, the histopathological process and mutation profile vary in different liver disease, requiring HCC model induced by corresponding liver diseases or gene mutation. On the other hand, PDX models using immunocompromised mice lack one or more major human immune cells, and was not able to replicate full human immune response. Therefore, humanized mouse models with minimal differences between human and mouse immune system are developed to provide an ideal model for reliable translational studies of immunotherapy in HCC.
Since the first genetically engineered mouse (GEM) model was established by Chisari64 in 1989, the techniques in GEM model construction have been gradually improved. Nowadays, there are mainly four kinds of methods, including mouse embryo manipulation, Cre-Lox recombination, hydrodynamic injection and clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR associated protein 9 (Cas9).65 GEM model related studies have been focusing on the role of specific gene and the interaction of different genes in hepatocarcinogenesis. Through the knock in or knock out of HCC-associated genes, the process of tumor development including hepatitis, hepatocyte hyperplasia, hepatic adenoma and HCC can be highly replicated from clinical HCC (Synopsis of genetically engineered HCC model has been listed in Supplementary Table 1). Therefore, GEM models are ideal and important tools in the exploration of the mechanism of hepatocarcinogenesis. However, the metastatic potential of GEM HCC models is not clear yet, with only few reports mentioning metastasis status, but lacking detailed illustration (Supplementary Table 1).66, 67, 68, 69, 70, 71 In the future development of GEM HCC models, the knock in of metastasis regulator genes, such as MET,72 could be a promising way to facilitate the study on the metastasis mechanism of HCC. Apart from GEM models, several liver disease-associated HCC models have been successfully established, including HBV/HCV models,64,73, 74, 75, 76, 77, 78, 79 alcohol models80, 81, 82 and non-alcoholic fatty liver disease or non-alcoholic steatohepatitis model (methionine and choline-deficient diet model83, 84, 85, 86; choline-deficient l-amino-defined diet model87, 88, 89; high fat diet model89, 90, 91, 92; fast food diet model93,94).
Although the development of liver disease-associated HCC model has mimicked different disease settings to the most extent, most of them failed to replicate full immune response, as well as the feature of metastasis, which is still not sufficient for translational studies, especially preclinical immunotherapy. Up to now, several techniques have been developed to mimic tumor microenvironment, one of which is to transplant human hematopoietic stem cells and precursor cells into the marrow of sub-lethally irradiated mice. And several models for immunotherapy had been successfully established.95,96 However, an ideal model replicating patient tumor microenvironment and immune response have not been established, requiring further technical advancement. The application of GEM models, liver disease-associated HCC models and humanized HCC models could devote to the construction of HCC animal models which might replicate human HCC to the most extent. However, further emphasis is need on the phenotype selection of HCC metastasis in the above three models.
Cell models
High metastatic human HCC cell lines: MHCC97
Before the 2000s, there were already many kinds of human HCC cell lines, for example, BEL-7402,97 PLC/PRF/598 and SMMC-7721.99 But most of them lack the phenotype of metastasis or haven't been demonstrated. The metastatic ability, organ affinity, and stability of unexamined cell lines were often indeterminate, though they had some metastatic potential. Therefore, it is inaccurate to name all human HCC cell lines metastatic cell lines. And common HCC cell lines were usually not suitable for experimental studies of HCC metastasis.
It is a complex task to develop metastatic HCC cell lines. However, it is also a compulsory requirement for the studies in HCC metastasis, for example, the procedure of metastasis, metastatic mechanism, the interaction between cancer cells and host animals, the relationship between metastasis, adhesion molecular, oncogenes and their products, and the interventional studies in HCC metastasis. Thus, metastatic HCC cell lines are fundamental experimental materials for the studies of metastatic HCC. The Liver Cancer Institute of Fudan University has been devoted to the development of metastatic HCC cell lines, and Dr. Tian was the first to create the groundbreaking work in this field, establishing human HCC cell line MHCC97 with spontaneously high metastatic potential.100,101
High and low metastatic potential HCC cell lines with similar genetic background
The successful establishment of MHCC97 cell line had significantly promoted the studies in the biological features of human HCC. All kinds of cancers, including HCC, develop the diversity of differentiation and phenotype caused by cellular mutations under the influence of genetic instability of cancer cells plus the selective pressure from host and other environmental factors. Heterogeneity pertaining to solid tumor tissue or cell lines refers to the fact that they are composed of a variety of cancer cell clones. It is valuable to screen cell strains of different biological features from a heterogeneous cell line, so that the cell strains can be used for comparative experiments, drug test, and differentiation-inducing therapy. Not all cancer cells in the primary tumor tissues possess the abilities of invasion and metastasis, but some specific subclones do. Even in such subclone groups, the variation in the metastatic potential may still exist. If considering the influences from the host animal and environment, the differences of metastatic phenotype between different cell subclones may be even more significant. MHCC97 cell line isolated from metastatic LCI-D20 had greater metastatic potential, but it was still a heterogeneous cell group consisted of a variety of cell subclones, including cancer cells with high metastatic ability, low metastatic ability, and even no metastatic ability. Isolating and comparing the difference of cell subgroups with different metastatic phenotype would be helpful for the studies of the mechanism and the finding of molecular marker of HCC metastasis.
Our research group performed in vitro monoclonal cell culture on MHCC97. Cell strains from single cells were performed initial screening in nude mice to further identify targeted cell strains. We eventually established cell clone with high metastatic potential (MHCC97-H) and low metastatic potential (MHCC97-L).102 Comparing these 2 cell clones, MHCC97-H had smaller cell size and faster in vitro and in vivo growth rate. The result of Boyden chamber in vitro invasion assay showed that the number of penetrating cells through the artificial basement membrane in MHCC97-H was significantly higher than MHCC97-L.
At the same period, based on the constructed model,19 Ogawa et al103 increased the dosages of DEN to 200 mg/kg and NMOR to 120 ppm. HCC model with pulmonary metastasis was successfully induced on week 24. Four monoclonal cell lines (C1, C2, C5F, and C6) were isolated and established from one murine tumor nodule. Another two cell lines, N1 and L2 were isolated from another primary HCC and pulmonary metastasis respectively. These six cell lines showed similar histopathology after subcutaneous inoculation, but varied vastly in metastatic phenotype when transplanted in different ways. When inoculating cells to KSN nude mice subcutaneously at the dosage of 5 × 106/0.2 ml, C5F monoclonal cell line had the highest pulmonary metastatic rate (89%, 8/9) 5–7 weeks after inoculation. When inoculating cells through tail vein, the N1 and L2 cell line had the highest rates (100%, 12/12 and 3/3 respectively) of pulmonary metastasis, while C5F had the lowest metastatic rate (0%, 0/8). When inoculated by intraperitoneal injection, C1, C6, N1, and L2 caused obvious hemorrhagic ascites and intraperitoneal dissemination, with infiltration in hepatic capsule in N1 cell line and pulmonary metastasis in C6 and C5F. Lower expression level of KAI-1 and heparinase genes in C5F were observed compared with other cell lines, indicating heterogeneity in metastatic model.
HCC cell models selected by different rounds of pulmonary metastasis in nude mice
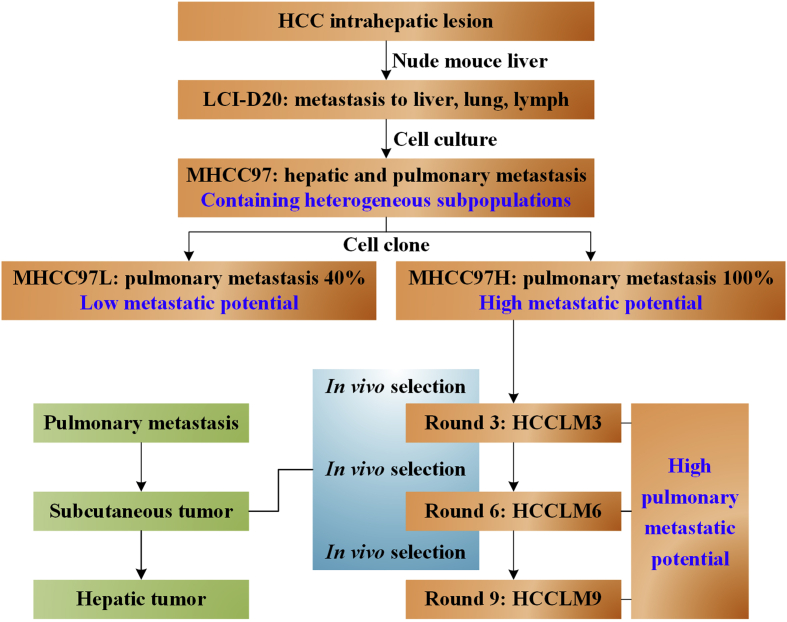
On the basis of the work finished above, our team inoculated MHCC97-H to nude mice to further screen by three, six, and nine rounds of pulmonary metastasis selection. Pulmonary metastasis from the third, sixth, and ninth round screens were harvested to establish cell strains, named HCCLM3, HCCLM6, and HCCLM9 (Fig. 2), with unique characteristics of high pulmonary metastatic rate.104 All the three cell strains presented as polygonal epithelial cancer cells, with similar diameter for around 50 μm, rich cytoplasm, large and round nucleus with homogeneous light red staining, clear nuclear membrane and two to seven large and clear nucleoli. Cells were arranged with single layer and mosaic way, and cytoplasmic bulge were observed between cells. Immunohistochemistry showed positive for AFP, albumin, cytokeratin 8, P16, and negative for P53, nm23, HBsAg. Genetic examination showed HBV DNA integration in cellular genome. Sub-triploid karyotype were found in both cell strains, with the number of chromosomes ranged from 55 to 58 for HCCLM3, and 54 to 57 for HCCLM6.
Figure 2.
The establishment procedure of MHCC97L, MHCC97H and three high pulmonary metastatic potential cell clones by sequential in vivo selection. MHCC97H cell clones isolated from LCI-D20 metastasis was inoculated to BALB/c nude mice, and the pulmonary metastasis were re-inoculated into nude mice for three, six and nine rounds of in vivo pulmonary metastasis selection.
The application of human HCC cell lines with metastatic potential
HCC metastatic cell lines have been widely applied since they were established, mainly in the field of mechanism exploration and prediction of HCC metastasis.
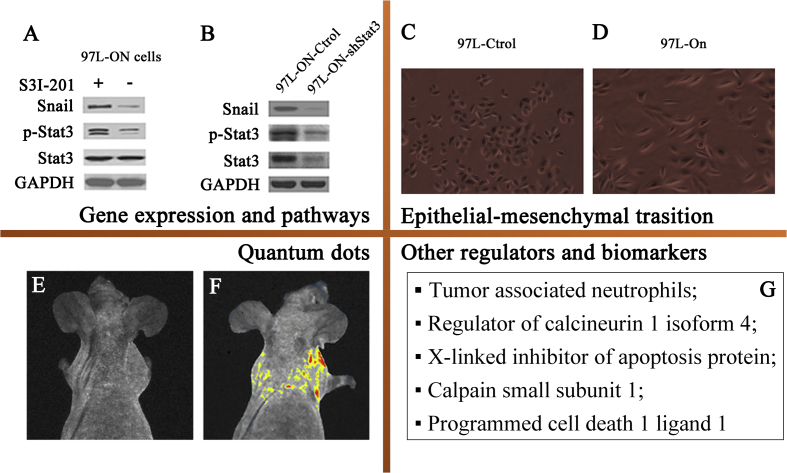
In the mechanism studies, HCC metastatic cell lines were mainly applied to explore the roles of gene expression, pathways, and epithelial mesenchymal transition (EMT) in HCC metastasis (Fig. 3).
-
(1)
Gene expression and pathways: KAI1 gene downregulation was associated with many cancerous invasion and metastasis. Yang et al105 transfected sense and antisense KAI1 expression plasmid to the MHCC97-H cell lines, discovering that KAI1 gene played a role in inhibiting metastasis of HCC cells. Yin et al106 had proved that co-expression of stemness factors Oct 4 and Nanog were related to metastatic potential of HCC cells. In the following study, Yin further demonstrated that co-expression of genes Oct4 and Nanog contribute to EMT change to promote tumor migration, invasion/metastasis through Stat 3/Snail signaling.107
Figure 3.
The application of human HCC cell lines with metastatic potential. A: Oct 4/Nanog induced Snail expression was significantly inhibited by a specific Stat 3 inhibitor S3I-201; B: Knockdown of Stat3 reversed Oct-4/Nanog-induced overexpression of Snail in 97 L-ON cells, indicating that co-expression of genes Oct4 and Nanog might modulate metastatic potential by Stat 3/Snail signaling pathway. Picture A and B were cited from Yin et al79 C and D: MHCC97 L cells (C) underwent dramatic morphologic changes into mesenchymal, fibroblast-like phenotype (D) after treatment with ectopic Oct4 and Nanog expressions. Picture C and D were cited from Yin et al79 E and F: Comparing to the control group (E), the whole-body imaging showed that the quantum dots fluorescence was localized in the pulmonary metastasis of the model (F), showing the promising function of quantum dots for predicting HCC metastasis. Picture E and F were cited from Chen et al85 G: Other regulators and biomarkers.
In the pathway studies, Zhang et al108 proved that cancer-associated fibroblasts (CAFs) can transfer miRNA to HCC cells, by which way miR-320a was able to inhibit MHCC97-H cell proliferation, migration, and metastasis by binding to the direct downstream target PBX3. Thus, miR-320a-PBX3 is an antitumor pathway by inhibiting the activation of MAPK pathway. The loss of exosomal miR-320a contributes to the malignant characteristics of HCC. Zhao et al109 knockdown ST6Gal-I in MHCC97-H cell lines, and found that proliferation, migration, invasion, and tumor volume in PDX model decreased. In vitro study revealed that ST6Gal-I may enhance HCC tumor-genesis and metastasis via the modulation of Wnt/β-catenin signaling pathways.
-
(2)
EMT: EMT was reported to be facilitated by many regulators, and had been shown to be an important mechanism in HCC metastasis and invasion.107,108,110,111 Xu et al110 demonstrated that the depletion of SIN1 in MHCC97-H and HCCLM3 cell lines inhibited Akt phosphorylation, leading to downregulation of Snail, Vimentin, MMP9, and N-cadherin and up-regulation of E-cadherin. And the restoration of Snail expression promoted invasion and migration of HCC cells, demonstrating the promoting role of EMT in HCC cell metastasis. Besides, EMT might also be regulated by Oct 4, Nanog through Stat 3/Snail signaling,107 CAFs through miR-320a-PBX3 pathway,107 and ACA11 through PI3K/AKT pathway.111
-
(3)
Quantum dots (QD): It's hard to predict or make early diagnosis on HCC metastasis due to the lack of specific biomarker probe. The establishment of HCC metastatic cell lines made it possible to develop specific and sensitive biomarker probe. QD is one kind of organic dye characterized by broad excitation spectra, size-tunable fluorescence, high photostability, and long fluorescence lifetime.112 And it is proved to be stable, specific, and biocompatible for ultrasensitive fluorescence imaging of molecular targets in established HCC model system.113 In the further study, Wang et al114 labeled HCCLM9 and MHCC97-L cell lines with 6 potential aptamers, and found that LY-1 was a promising molecular probe for the prediction or early diagnosis of HCC.
-
(4)
Other regulators and biomarkers: Zhou et al115 reported that HCCLM3 formed larger tumors when co-injected with tumor associated neutrophils (TANs). And the mechanism study demonstrated that TANs might promote tumor growth, progression, and resistance to sorafenib through recruiting macrophages and Treg cells to HCC. Jin et al116 proved that regulator of calcineurin 1 isoform 4 (RCAN1.4) significantly reduced proliferation, migration, and invasive activity of HCCLM3. The downregulation of RCAN1.4 may promote the metastasis of HCC. Besides, including X-linked inhibitor of apoptosis protein,117 calpain small subunit 1 (Capn4),118 programmed cell death 1 ligand 1 (PD-L1),119 CD24,120 CD151121, and β-catenin122 were also possible molecular biomarkers in the diagnosis and therapeutic target of HCC.2
Summary
As presented above, there are few models applicable in the studies of HCC metastasis. However, the construction of HCC model with metastasis is far more difficult than routine tumor models. Because, the establishing of HCC model with metastasis is equal to establishing an effective, reliable, and stable experimental system combining in vivo and in vitro studies, which provides macroscopic observation system replicating the process of metastasis from primary organ to distant organ. Among the limited models, HCC model with spontaneous metastasis, represented by LCI-D20, has several specific advantages. First, the tumor sample used for grafting originated form a patient, with biological behaviors closest to clinical conditions. Second, the model had initially formed a relatively complete system. Human HCC cell line MHCC97 was isolated from LCI-D20, and was successfully isolated for the establishing of MHCC97-H and MHCC-97 L cell strains with different metastatic potential. Furthermore, HCCLM3 and HCCLM6 cell strains from the metastasis of MHCC97-H were isolated and cultured, whose metastatic ability was stronger and biological behavior was closer to clinical course of HCC. This system provided a good foundation for the mechanism studies of HCC metastasis. Third, the studies using this system had produced some encouraging findings, such as the relationship between angiogenesis and metastasis, the hypothesis of multiple genes and multiple stages during metastasis, and the basic and clinical studies on the anti-metastasis of interferon. Throughout the history of the development of the HCC model system with metastasis, it can be seen that every progress in model improvement will greatly promote the understanding of the mechanisms of HCC metastasis and the exploration of new prevention and treatment strategies. The direction of future efforts should be focused on the design of scientific and effective experimental protocols. For example, high-throughput analysis techniques can be applied to find HCC metastasis-related genes and proteins and explore the metastatic rules of HCC models with metastasis. And the final destination is the development of a panel of gene-based biomarkers to predict HCC metastasis and design molecular targeted therapies. Besides, GEM models, liver disease-associated HCC models, and humanized models are models more identical with human HCC and HCC metastasis regarding to the liver disease context.
Funding
This work was supported by Beijing Municipal Administration of Hospitals’ Ascent Plan [grant number DFL20180701]; Special Fund for the Capital Characteristic Clinical Medicine Development Project [grant number Z161100000516077]; Beijing Municipal Grant for Medical Talents Group on Peritoneal Surface Oncology [grant number 2017400003235J007]; Beijing Health and Science Technology Achievement and Appropriate Technology Promotion Project [grant number 2018-TG-27] Beijing Natural Science Foundation [grant number 7172108].
Conflict of interests
The authors declare no conflict of interests.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2019.12.008.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Li Y., Tang Z.Y., Hou J.X. Hepatocellular carcinoma: insight from animal models. Nat Rev Gastroenterol Hepatol. 2011;9(1):32–43. doi: 10.1038/nrgastro.2011.196. [DOI] [PubMed] [Google Scholar]
- 3.Tang Z.Y., Yu Y.Q., Zhou X.D., Ma Z.C., Wu Z.Q. Progress and prospects in hepatocellular carcinoma surgery. Ann Chir. 1998;52(6):558–563. [PubMed] [Google Scholar]
- 4.Tang Z., Zhou X., Lin Z. Surgical treatment of hepatocellular carcinoma and related basic research with special reference to recurrence and metastasis. Chin Med J (Engl) 1999;112(10):887–891. [PubMed] [Google Scholar]
- 5.Tang Z.Y. Surgery of hepatocellular carcinoma with special reference to studies on metastasis and recurrence. Gastroenterol Today. 2000;4:191–195. [Google Scholar]
- 6.Zhu S.N. Pathological morphology and biological characteristics of liver cancer. In: Tang Z.Y., Ye Y.Q., editors. Primary Liver Cancer. 2 ed. Shanghai Scientific & Technical Plulishers; Shanghai: 1998. pp. 132–154. [Google Scholar]
- 7.Liu F.S., Wang Q.L. Clinicopathological basis of human malignant tumor invasion and metastasis. In: Gao J., editor. Invasion and Metastasis of Cancer - Basic Research and Clinical. Beijing Medical University, China Union Medical University, Union Press; Beijing: 1996. pp. 272–282. [Google Scholar]
- 8.Yuki K., Hirohashi S., Sakamoto M., Kanai T., Shimosato Y. Growth and spread of hepatocellular carcinoma. A review of 240 consecutive autopsy cases. Cancer. 1990;66(10):2174–2179. doi: 10.1002/1097-0142(19901115)66:10<2174::aid-cncr2820661022>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.Wu L., Tang Z.Y., Li Y. Experimental models of hepatocellular carcinoma: developments and evolution. J Cancer Res Clin Oncol. 2009;135(8):969–981. doi: 10.1007/s00432-009-0591-7. [DOI] [PubMed] [Google Scholar]
- 10.He L., Tian D.A., Li P.Y., He X.X. Mouse models of liver cancer: progress and recommendations. Oncotarget. 2015;6(27):23306–23322. doi: 10.18632/oncotarget.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soga M., Kishimoto Y., Kawamura Y., Inagaki S., Makino S., Saibara T. Spontaneous development of hepatocellular carcinomas in the FLS mice with hereditary fatty liver. Cancer Lett. 2003;196(1):43–48. doi: 10.1016/s0304-3835(03)00213-1. [DOI] [PubMed] [Google Scholar]
- 12.McCoy G.W. A preliminary report on tumors found in wild rats. J Media Res. 1909;21(2):285–296. [PMC free article] [PubMed] [Google Scholar]
- 13.Wu L., Tang Z.Y., Li Y. Research progress in liver cancer model. Chin J Exp Surg. 2009;26:815–816. [Google Scholar]
- 14.Masui T., Nakanishi H., Inada K. Highly metastatic hepatocellular carcinomas induced in male F344 rats treated with N-nitrosomorpholine in combination with other hepatocarcinogens show a high incidence of p53 gene mutations along with altered mRNA expression of tumor-related genes. Cancer Lett. 1997;112(1):33–45. doi: 10.1016/s0304-3835(96)04543-0. [DOI] [PubMed] [Google Scholar]
- 15.Magee P.N., Barnes J.M. The production of malignant primary hepatic tumours in the rat by feeding dimethylnitrosamine. Br J Canc. 1956;10(1):114–122. doi: 10.1038/bjc.1956.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fausto N., Campbell J.S. Mouse models of hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):87–98. doi: 10.1055/s-0030-1247135. [DOI] [PubMed] [Google Scholar]
- 17.Solt D.B., Cayama E., Tsuda H., Enomoto K., Lee G., Farber E. Promotion of liver cancer development by brief exposure to dietary 2-acetylaminofluorene plus partial hepatectomy or carbon tetrachloride. Cancer Res. 1983;43(1):188–191. [PubMed] [Google Scholar]
- 18.Lijinsky W., Kovatch R., Riggs C., Walters P. Dose response study with N-nitrosomorphline in drinking water of F344 rats. Cancer Res and Treat. 1988;48:2089–2095. [PubMed] [Google Scholar]
- 19.Futakuchi M., Hirose M., Ogiso T. Establishment of an in vivo highly metastatic rat hepatocellular carcinoma model. Jpn J Cancer Res. 1999;90(11):1196–1202. doi: 10.1111/j.1349-7006.1999.tb00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vesselinovitch S.D., Koka M., Mihailovich N., Rao K.V. Carcinogenicity of diethylnitrosamine in newborn, infant, and adult mice. J Cancer Res Clin Oncol. 1984;108(1):60–65. doi: 10.1007/BF00390974. [DOI] [PubMed] [Google Scholar]
- 21.Heindryckx F., Colle I., Van Vlierberghe H. Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol. 2009;90(4):367–386. doi: 10.1111/j.1365-2613.2009.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshino H., Futakuchi M., Cho Y.M. Modification of an in vivo lung metastasis model of hepatocellular carcinoma by low dose N-nitrosomorpholine and diethylnitrosamine. Clin Exp Metastasis. 2005;22(5):441–447. doi: 10.1007/s10585-005-2807-9. [DOI] [PubMed] [Google Scholar]
- 23.Klaunig J.E., Pereira M.A., Ruch R.J., Weghorst C.M. Dose-response relationship of diethylnitrosamine-initiated tumors in neonatal balb/c mice: effect of phenobarbital promotion. Toxicol Pathol. 1988;16(3):381–385. doi: 10.1177/019262338801600310. [DOI] [PubMed] [Google Scholar]
- 24.Murai T., Mori S., Hosono M. Induction of hepatocellular carcinoma with high metastatic potential in WS/Shi rats: discovery of an inbred strain highly susceptible to the liver carcinogen N-nitrosomorpholine. Oncol Res. 2000;12(3):121–126. doi: 10.3727/096504001108747594. [DOI] [PubMed] [Google Scholar]
- 25.Goldsworthy T.L., Fransson-Steen R. Quantitation of the cancer process in C57BL/6J, B6C3F1 and C3H/HeJ mice. Toxicol Pathol. 2002;30(1):97–105. doi: 10.1080/01926230252824770. [DOI] [PubMed] [Google Scholar]
- 26.Zimmers T.A., Jin X., Gutierrez J.C. Effect of in vivo loss of GDF-15 on hepatocellular carcinogenesis. J Cancer Res Clin Oncol. 2008;134(7):753–759. doi: 10.1007/s00432-007-0336-4. [DOI] [PubMed] [Google Scholar]
- 27.Teoh N., Pyakurel P., Dan Y.Y. Induction of p53 renders ATM-deficient mice refractory to hepatocarcinogenesis. Gastroenterology. 2010;138(3):1155–1165. doi: 10.1053/j.gastro.2009.11.008. e1151-1152. [DOI] [PubMed] [Google Scholar]
- 28.Aleksic K., Lackner C., Geigl J.B. Evolution of genomic instability in diethylnitrosamine-induced hepatocarcinogenesis in mice. Hepatology. 2011;53(3):895–904. doi: 10.1002/hep.24133. [DOI] [PubMed] [Google Scholar]
- 29.Rygaard J. Immunobiology of the mouse mutant "Nude". Preliminary investigations. Acta Pathol Microbiol Scand. 1969;77(4):761–762. [PubMed] [Google Scholar]
- 30.Rygaard J., Povlsen C.O. Heterotransplantation of a human malignant tumour to "Nude" mice. Acta Pathol Microbiol Scand. 1969;77(4):758–760. doi: 10.1111/j.1699-0463.1969.tb04520.x. [DOI] [PubMed] [Google Scholar]
- 31.Shimosato Y., Kameya T., Nagai K. Transplantation of human tumors in nude mice. J Natl Cancer Inst. 1976;56(6):1251–1260. doi: 10.1093/jnci/56.6.1251. [DOI] [PubMed] [Google Scholar]
- 32.Hirohashi S., Shimosato Y., Kameya T. Production of alpha-fetoprotein and normal serum proteins by xenotransplanted human hepatomas in relation to their growth and morphology. Cancer Res. 1979;39(5):1819–1828. [PubMed] [Google Scholar]
- 33.Tang Z.Y., Ma Z.Q., Xue Q. Transplantation of human hepatocellular carcinoma in nude mice: I. Establishment of model and its serological and morphological features. Fudan Univ J Med Sci. 1982;9:21–26. [Google Scholar]
- 34.Ma Z.C., Tang Z.Y., Xu Y.D. Study on the transplantation model of human liver cancer in nude mice II. Establishment of human liver cancer tissue model of LTNM2 nude mice and observation of tumor growth. Chin J Oncol. 1985;7:405–407. [PubMed] [Google Scholar]
- 35.Ma Z.C., Tang Z.Y., Xue Q., Xu Y.D. Establishment of two nude rat liver cancer models and observation of their biological characteristics. Tumor. 1986;6:161–162. [Google Scholar]
- 36.Bao Y.M., Tang Z.Y., Ma Z.C., Xue Q., Pan W.S. Comparative study on subcutaneous, intraperitoneal and intrahepatic transplantations in nude rat bearing hepatocallular carcinoma (HCC) Chin J Clin Oncol. 1989;11(5):329–331. [PubMed] [Google Scholar]
- 37.Li Y., Tian B., Yang J. Stepwise metastatic human hepatocellular carcinoma cell model system with multiple metastatic potentials established through consecutive in vivo selection and studies on metastatic characteristics. J Cancer Res Clin Oncol. 2004;130(8):460–468. doi: 10.1007/s00432-004-0564-9. [DOI] [PubMed] [Google Scholar]
- 38.Sun F.X., Tang Z.Y., Liu K.D., Ye S.L., Xue Q. Growth characteristics and metastatic potential of orthotopic transplantation model of human hepatocarcinoma in nude mice. Natl Med J China (Peking) 1995;75(11):673–675. [Google Scholar]
- 39.Sun F.X., Tang Z.Y., Liu K.D. Highly metastatic model of human hepatocellular carcinoma established in nude mice using orthotopic organ selection of metastatic variant from patient specimens. Chin J Clin Oncol. 1996;18(2):109–112. [PubMed] [Google Scholar]
- 40.Sun F.X., Tang Z.Y., Lui K.D. Establishment of a metastatic model of human hepatocellular carcinoma in nude mice via orthotopic implantation of histologically intact tissues. Int J Cancer. 1996;66(2):239–243. doi: 10.1002/(SICI)1097-0215(19960410)66:2<239::AID-IJC17>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 41.Tang Z.Y., Sun F.X., Tian J. Metastatic human hepatocellular carcinoma models in nude mice and cell line with metastatic potential. World J Gastroenterol. 2001;7(5):597–601. doi: 10.3748/wjg.v7.i5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao Y., Tang Z.Y., Ye S.L., Liu K.D., Sun F.X., Huang Z. Modulation of apoptosis, tumorigenesity and metastatic potential with antisense H-ras oligodeoxynucleotides in a high metastatic tumor model of hepatoma: LCI-D20. Hepato-Gastroenterology. 2000;47(32):365–370. [PubMed] [Google Scholar]
- 43.Bu W., Tang Z.Y., Sun F.X. Effects of matrix metalloproteinase inhibitor BB-94 on liver cancer growth and metastasis in a patient-like orthotopic model LCI-D20. Hepato-Gastroenterology. 1998;45(22):1056–1061. [PubMed] [Google Scholar]
- 44.Sun J.J., Zhou X.D., Liu Y.K. Inhibitory effects of synthetic beta peptide on invasion and metastasis of liver cancer. J Cancer Res Clin Oncol. 2000;126(10):595–600. doi: 10.1007/pl00008470. [DOI] [PubMed] [Google Scholar]
- 45.Sun J.J., Zhou X.D., Liu Y.K., Wu Z.Q., Shi J.Y., Gao D.M. Effect of CDA-II on prevention and therapy for metastases and recurrence of liver cancer in nude mice. Chin J Hepat Surg. 1999;5(1):14–16. [Google Scholar]
- 46.Aruga A., Takasaki K., Hanyu F. Establishment and characterization of liver metastatic model of human hepatoma in nude mice. Hepatol Res. 1993;1(3):138–145. [Google Scholar]
- 47.Kuriyama S., Yamazaki M., Mitoro A. Analysis of intrahepatic invasion of hepatocellular carcinoma using fluorescent dye-labeled cells in mice. Anticancer Res. 1998;18(6a):4181–4188. [PubMed] [Google Scholar]
- 48.Kuriyama S., Yamazaki M., Mitoro A. Hepatocellular carcinoma in an orthotopic mouse model metastasizes intrahepatically in cirrhotic but not in normal liver. Int J Cancer. 1999;80(3):471–476. doi: 10.1002/(sici)1097-0215(19990129)80:3<471::aid-ijc22>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 49.Tan L., Chen S., Wei G. Sublethal heat treatment of hepatocellular carcinoma promotes intrahepatic metastasis and stemness in a VEGFR1-dependent manner. Cancer Lett. 2019;460:29–40. doi: 10.1016/j.canlet.2019.05.041. [DOI] [PubMed] [Google Scholar]
- 50.Zhang N., Zhu H., Dong Y.H., Wang L. Establishment of an insufficient radiofrequency ablation orthotopic nude mouse model of hepatocellular carcinoma to study the invasiveness and metastatic potential of residual cancer. Oncol Lett. 2019;18(3):2548–2553. doi: 10.3892/ol.2019.10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ling M.Y., Zhang Y., Zhang W.S., Zhu F.C. Establishment of an experimental model for lymphatic metastasis with transplantable ascites hepatomas (h22) in mice. Chin J Pathol. 1984;13(3):190–192. [Google Scholar]
- 52.Ling M.Y., Wang M.H., Zhang C., Liu X.F. An observation on the morphology and metastatic potential to the ascites hepatomas (h22) in mice. J Dalian Med Univ. 1987;9(1):27–32. [Google Scholar]
- 53.Ling M.Y., Liu X.F., Guan S.Q., Zheng H.Z., Gu S.Z. The establishment and some biological characteristics of a murine ascites hepatoma cell line H22-F25/L. J Dalian Med Univ. 1989;11 [Google Scholar]
- 54.Ling M.Y., Liu X.F., Guan S.Q., Yang L., Zheng H.Z., Gu S.Z. Establishment of a murine ascites hepatoma cell line H22-F25/L and its biological characteristics. Chin J Oncol. 1991;13(1):13–15. [PubMed] [Google Scholar]
- 55.Ling M.Y., Liu X.F., Guan S.Q. Study on the isolation and characterization of clones with different metastatic potential from mice hepatocellular carcinoma. Natl Med J China (Peking) 1990;70(6):315–318. [Google Scholar]
- 56.Chen S., Ling M.Y., Liu X.F., Zhang C.H. The stability of highly and lowly metastatic clonal cells from murine ascites hepatocarcinoma. J Dalian Med Col. 1992;14(1):32–37. [Google Scholar]
- 57.Ling M.Y., Wang M.H., Guo L.L., Wang B. Comparison for the metastatic phenotype of two murine hepatocarcinoma subclonal cell lines. J Dalian Med Uni. 1994;16(2):124–129. [Google Scholar]
- 58.Li H.F., Ling M.Y., Xie Y., Xie H. Establishment of a lymph node metastatic model of mouse hepatocellular carcinoma Hca-F cells in C3H/Hej mice. Oncol Res. 1998;10(11–12):569–573. [PubMed] [Google Scholar]
- 59.Tao Z.H., Wu W.Z., Wang X.L. The establishment of a systematic site-specific metastasis model of human hepatocellular carcinoma in nude mouse. Chin J Hepatol. 2011;19(2):110–113. doi: 10.3760/cma.j.issn.1007-3418.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 60.Jin Y., Wang S., Chen W. Annexin A7 suppresses lymph node metastasis of hepatocarcinoma cells in a mouse model. BMC Canc. 2013;13:522. doi: 10.1186/1471-2407-13-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y., Ren W., Bai Y. Oxyresveratrol prevents murine H22 hepatocellular carcinoma growth and lymph node metastasis via inhibiting tumor angiogenesis and lymphangiogenesis. J Nat Med. 2018;72(2):481–492. doi: 10.1007/s11418-018-1173-2. [DOI] [PubMed] [Google Scholar]
- 62.Genda T., Sakamoto M., Ichida T. Cell motility mediated by rho and Rho-associated protein kinase plays a critical role in intrahepatic metastasis of human hepatocellular carcinoma. Hepatology. 1999;30(4):1027–1036. doi: 10.1002/hep.510300420. [DOI] [PubMed] [Google Scholar]
- 63.Sawada S., Murakami K., Yamaura T., Mitani N., Tsukada K., Saiki I. Therapeutic and analysis model of intrahepatic metastasis reflects clinical behavior of hepatocellular carcinoma. Jpn J Cancer Res. 2002;93(2):190–197. doi: 10.1111/j.1349-7006.2002.tb01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chisari F.V., Klopchin K., Moriyama T. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell. 1989;59(6):1145–1156. doi: 10.1016/0092-8674(89)90770-8. [DOI] [PubMed] [Google Scholar]
- 65.Brown Z.J., Heinrich B., Greten T.F. Mouse models of hepatocellular carcinoma: an overview and highlights for immunotherapy research. Nat Rev Gastroenterol Hepatol. 2018;15(9):536–554. doi: 10.1038/s41575-018-0033-6. [DOI] [PubMed] [Google Scholar]
- 66.Mauad T.H., van Nieuwkerk C.M., Dingemans K.P. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994;145(5):1237–1245. [PMC free article] [PubMed] [Google Scholar]
- 67.Reiberger T., Chen Y., Ramjiawan R.R. An orthotopic mouse model of hepatocellular carcinoma with underlying liver cirrhosis. Nat Protoc. 2015;10(8):1264–1274. doi: 10.1038/nprot.2015.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horie Y., Suzuki A., Kataoka E. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Investig. 2004;113(12):1774–1783. doi: 10.1172/JCI20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watanabe S., Horie Y., Kataoka E. Non-alcoholic steatohepatitis and hepatocellular carcinoma: lessons from hepatocyte-specific phosphatase and tensin homolog (PTEN)-deficient mice. J Gastroenterol Hepatol. 2007;22(Suppl 1):S96–S100. doi: 10.1111/j.1440-1746.2006.04665.x. [DOI] [PubMed] [Google Scholar]
- 70.Keng V.W., Villanueva A., Chiang D.Y. A conditional transposon-based insertional mutagenesis screen for genes associated with mouse hepatocellular carcinoma. Nat Biotechnol. 2009;27(3):264–274. doi: 10.1038/nbt.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Q., Yu W.N., Chen X. Spontaneous hepatocellular carcinoma after the combined deletion of Akt isoforms. Cancer Cell. 2016;29(4):523–535. doi: 10.1016/j.ccell.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stauffer J.K., Scarzello A.J., Andersen J.B. Coactivation of AKT and β-catenin in mice rapidly induces formation of lipogenic liver tumors. Cancer Res. 2011;71(7):2718–2727. doi: 10.1158/0008-5472.CAN-10-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dunsford H.A., Sell S., Chisari F.V. Hepatocarcinogenesis due to chronic liver cell injury in hepatitis B virus transgenic mice. Cancer Res. 1990;50(11):3400–3407. [PubMed] [Google Scholar]
- 74.Lakhtakia R., Kumar V., Reddi H., Mathur M., Dattagupta S., Panda S.K. Hepatocellular carcinoma in a hepatitis B 'x' transgenic mouse model: a sequential pathological evaluation. J Gastroenterol Hepatol. 2003;18(1):80–91. doi: 10.1046/j.1440-1746.2003.02902.x. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y., Cui F., Lv Y. HBsAg and HBx knocked into the p21 locus causes hepatocellular carcinoma in mice. Hepatology. 2004;39(2):318–324. doi: 10.1002/hep.20076. [DOI] [PubMed] [Google Scholar]
- 76.Ye H., Zhang C., Wang B.J. Synergistic function of Kras mutation and HBx in initiation and progression of hepatocellular carcinoma in mice. Oncogene. 2014;33(43):5133–5138. doi: 10.1038/onc.2013.468. [DOI] [PubMed] [Google Scholar]
- 77.Kamegaya Y., Hiasa Y., Zukerberg L. Hepatitis C virus acts as a tumor accelerator by blocking apoptosis in a mouse model of hepatocarcinogenesis. Hepatology. 2005;41(3):660–667. doi: 10.1002/hep.20621. [DOI] [PubMed] [Google Scholar]
- 78.Moriya K., Yotsuyanagi H., Shintani Y. Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J Gen Virol. 1997;78(Pt 7):1527–1531. doi: 10.1099/0022-1317-78-7-1527. [DOI] [PubMed] [Google Scholar]
- 79.Moriya K., Fujie H., Shintani Y. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4(9):1065–1067. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- 80.Tsukamoto H., French S.W., Benson N. Severe and progressive steatosis and focal necrosis in rat liver induced by continuous intragastric infusion of ethanol and low fat diet. Hepatology. 1985;5(2):224–232. doi: 10.1002/hep.1840050212. [DOI] [PubMed] [Google Scholar]
- 81.Ueno A., Lazaro R., Wang P.Y., Higashiyama R., Machida K., Tsukamoto H. Mouse intragastric infusion (iG) model. Nat Protoc. 2012;7(4):771–781. doi: 10.1038/nprot.2012.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bertola A., Mathews S., Ki S.H., Wang H., Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013;8(3):627–637. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lieber C.S., DeCarli L.M. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res. 1982;6(4):523–531. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- 84.Ip E., Farrell G., Hall P., Robertson G., Leclercq I. Administration of the potent PPARalpha agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology. 2004;39(5):1286–1296. doi: 10.1002/hep.20170. [DOI] [PubMed] [Google Scholar]
- 85.Rinella M.E., Green R.M. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol. 2004;40(1):47–51. doi: 10.1016/j.jhep.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 86.Dela Peña A., Leclercq I., Field J., George J., Jones B., Farrell G. NF-kappaB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology. 2005;129(5):1663–1674. doi: 10.1053/j.gastro.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 87.Nakae D., Mizumoto Y., Andoh N. Comparative changes in the liver of female Fischer-344 rats after short-term feeding of a semipurified or a semisynthetic L-amino acid-defined choline-deficient diet. Toxicol Pathol. 1995;23(5):583–590. doi: 10.1177/019262339502300504. [DOI] [PubMed] [Google Scholar]
- 88.Kodama Y., Kisseleva T., Iwaisako K. c-Jun N-terminal kinase-1 from hematopoietic cells mediates progression from hepatic steatosis to steatohepatitis and fibrosis in mice. Gastroenterology. 2009;137(4):1467–1477. doi: 10.1053/j.gastro.2009.06.045. e1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hebbard L., George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2011;8(1):35–44. doi: 10.1038/nrgastro.2010.191. [DOI] [PubMed] [Google Scholar]
- 90.Deng Q.G., She H., Cheng J.H. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology. 2005;42(4):905–914. doi: 10.1002/hep.20877. [DOI] [PubMed] [Google Scholar]
- 91.Ito M., Suzuki J., Tsujioka S. Longitudinal analysis of murine steatohepatitis model induced by chronic exposure to high-fat diet. Hepatol Res. 2007;37(1):50–57. doi: 10.1111/j.1872-034X.2007.00008.x. [DOI] [PubMed] [Google Scholar]
- 92.Ibrahim S.H., Hirsova P., Malhi H., Gores G.J. Animal models of nonalcoholic steatohepatitis: eat, delete, and inflame. Dig Dis Sci. 2016;61(5):1325–1336. doi: 10.1007/s10620-015-3977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Charlton M., Krishnan A., Viker K. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol. 2011;301(5):G825–G834. doi: 10.1152/ajpgi.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chheda T.K., Shivakumar P., Sadasivan S.K. Fast food diet with CCl4 micro-dose induced hepatic-fibrosis-a novel animal model. BMC Gastroenterol. 2014;14:89. doi: 10.1186/1471-230X-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shultz L.D., Goodwin N., Ishikawa F., Hosur V., Lyons B.L., Greiner D.L. Human cancer growth and therapy in immunodeficient mouse models. Cold Spring Harb Protoc. 2014;2014(7):694–708. doi: 10.1101/pdb.top073585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou Q., Facciponte J., Jin M., Shen Q., Lin Q. Humanized NOD-SCID IL2rg–/– mice as a preclinical model for cancer research and its potential use for individualized cancer therapies. Cancer Lett. 2014;344(1):13–19. doi: 10.1016/j.canlet.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 97.Shen D.W., Chen R.M. Human hepatocellular carcinoma cells cultivated in vitro. In: Tang Z.Y., editor. Subclinical Hepatocellular Carcinoma. Springer; Berlin: 1985. pp. 336–349. [Google Scholar]
- 98.Alexander J., Bey E., Macnab G. Establishment of human hepatoma cell line whic h produces hepatitis B surface antigen (HbsAg) In: Neiburgs H.E., editor. Prevention and Detection of Cancer. Marcel Dekker; New York: 1977. pp. 321–331. [Google Scholar]
- 99.Dong R.C. Establishment of a human hepatocarcinoma cell line SMMC-7721 and in itial observations on its biologic characteristics. In: Tang Z.Y., Wu M.C., Xia S.S., editors. Primary Liver Cancer. Springer; Berlin: 1989. pp. 145–153. [Google Scholar]
- 100.Tian J., Tang Z.Y., Ye S.L., Liu Y.K., Lin Z.Y. Establishment of a human hepatocellular carcinoma (HCC) cell line with high metastatic potential (MHCC97) and its biological characteristics. Chin J Clin Oncol. 1998;20(6):405–407. [PubMed] [Google Scholar]
- 101.Tian J., Tang Z.Y., Ye S.L. New human hepatocellular carcinoma (HCC) cell line with highly metastatic potential (MHCC97) and its expressions of the factors associated with metastasis. Br J Canc. 1999;81(5):814–821. doi: 10.1038/sj.bjc.6690769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y., Tang Z.Y., Ye S.L. Establishment of cell clones with different metastatic potential from the metastatic hepatocellular carcinoma cell line MHCC97. World J Gastroenterol. 2001;7(5):630–636. doi: 10.3748/wjg.v7.i5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ogawa K., Nakanishi H., Takeshita F. Establishment of rat hepatocellular carcinoma cell lines with differing metastatic potential in nude mice. Int J Cancer. 2001;91(6):797–802. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1140>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 104.Li Y., Tang Y., Ye L. Establishment of a hepatocellular carcinoma cell line with unique metastatic characteristics through in vivo selection and screening for metastasis-related genes through cDNA microarray. J Cancer Res Clin Oncol. 2003;129(1):43–51. doi: 10.1007/s00432-002-0396-4. [DOI] [PubMed] [Google Scholar]
- 105.Yang J.M., Peng Z.H., Si S.H., Liu W.W., Luo Y.H., Ye Z.Y. KAI1 gene suppresses invasion and metastasis of hepatocellular carcinoma MHCC97-H cells in vitro and in animal models. Liver Int. 2008;28(1):132–139. doi: 10.1111/j.1478-3231.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 106.Yin X., Li Y.W., Zhang B.H. Coexpression of stemness factors Oct 4 and Nanog predict liver resection. Ann Surg Oncol. 2012;19(9):2877–2887. doi: 10.1245/s10434-012-2314-6. [DOI] [PubMed] [Google Scholar]
- 107.Yin X., Zhang B.H., Zheng S.S. Coexpression of gene Oct 4 and Nanog initiates stem cell characteristics in hepatocellular carcinoma and promotes epithelial-mesenchymal transition through activation of Stat 3/Snail signaling. J Hematol Oncol. 2015;8:23. doi: 10.1186/s13045-015-0119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Z., Li X., Sun W. Loss of exosomal miR-320a from cancer-associated fibroblasts contributes to HCC proliferation and metastasis. Cancer Lett. 2017;397:33–42. doi: 10.1016/j.canlet.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 109.Zhao Y., Wei A., Zhang H. α2,6-Sialylation mediates hepatocellular carcinoma growth in vitro and in vivo by targeting the Wnt/β-catenin pathway. Oncogenesis. 2017;6(5):e343. doi: 10.1038/oncsis.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 110.Xu J., Li X., Yang H., Chang R., Kong C., Yang L. SIN1 promotes invasion and metastasis of hepatocellular carcinoma by facilitating epithelial-mesenchymal transition. Cancer. 2013;119(12):2247–2257. doi: 10.1002/cncr.28023. [DOI] [PubMed] [Google Scholar]
- 111.Wu L., Zheng J., Chen P., Liu Q., Yuan Y. Small nucleolar RNA ACA11 promotes proliferation, migration and invasion in hepatocellular carcinoma by targeting the PI3K/AKT signaling pathway. Biomed Pharmacother. 2017;90:705–712. doi: 10.1016/j.biopha.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 112.Jaiswal J.K., Mattoussi H., Mauro J.M., Simon S.M. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat Biotechnol. 2003;21(1):47–51. doi: 10.1038/nbt767. [DOI] [PubMed] [Google Scholar]
- 113.Chen L.D., Liu J., Yu X.F. The biocompatibility of quantum dot probes used for the targeted imaging of hepatocellular carcinoma metastasis. Biomaterials. 2008;29(31):4170–4176. doi: 10.1016/j.biomaterials.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 114.Wang F.B., Rong Y., Fang M. Recognition and capture of metastatic hepatocellular carcinoma cells using aptamer-conjugated quantum dots and magnetic particles. Biomaterials. 2013;34(15):3816–3827. doi: 10.1016/j.biomaterials.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 115.Zhou S.L., Zhou Z.J., Hu Z.Q. Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology. 2016;150(7):1646–1658. doi: 10.1053/j.gastro.2016.02.040. e1617. [DOI] [PubMed] [Google Scholar]
- 116.Jin H., Wang C., Jin G. Regulator of calcineurin 1 gene isoform 4, down-regulated in hepatocellular carcinoma, prevents proliferation, migration, and invasive activity of cancer cells and metastasis of orthotopic tumors by inhibiting nuclear translocation of NFAT1. Gastroenterology. 2017;153(3):799–811. doi: 10.1053/j.gastro.2017.05.045. e733. [DOI] [PubMed] [Google Scholar]
- 117.Shi Y.H., Ding W.X., Zhou J. Expression of X-linked inhibitor-of-apoptosis protein in hepatocellular carcinoma promotes metastasis and tumor recurrence. Hepatology. 2008;48(2):497–507. doi: 10.1002/hep.22393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bai D.S., Dai Z., Zhou J. Capn4 overexpression underlies tumor invasion and metastasis after liver transplantation for hepatocellular carcinoma. Hepatology. 2009;49(2):460–470. doi: 10.1002/hep.22638. [DOI] [PubMed] [Google Scholar]
- 119.Gao Q., Wang X.Y., Qiu S.J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15(3):971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 120.Yang X.R., Xu Y., Yu B. CD24 is a novel predictor for poor prognosis of hepatocellular carcinoma after surgery. Clin Cancer Res. 2009;15(17):5518–5527. doi: 10.1158/1078-0432.CCR-09-0151. [DOI] [PubMed] [Google Scholar]
- 121.Shi G.M., Ke A.W., Zhou J. CD151 modulates expression of matrix metalloproteinase 9 and promotes neoangiogenesis and progression of hepatocellular carcinoma. Hepatology. 2010;52(1):183–196. doi: 10.1002/hep.23661. [DOI] [PubMed] [Google Scholar]
- 122.Liu L., Zhu X.D., Wang W.Q. Activation of beta-catenin by hypoxia in hepatocellular carcinoma contributes to enhanced metastatic potential and poor prognosis. Clin Cancer Res. 2010;16(10):2740–2750. doi: 10.1158/1078-0432.CCR-09-2610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.