Highlights
-
•
Inflammatory conditions cause multilocular thymic cyst.
-
•
Causes of the inflammation include autoimmune diseases or malignant tumors.
-
•
Evaluating the cause of inflammation in multilocular thymic cyst is essential.
-
•
First reported multilocular thymic cyst with preclinical rheumatoid arthritis.
-
•
Early treatment of rheumatoid arthritis prevents irreversible disability.
Abbreviations: MTC, multilocular thymic cyst; RA, rheumatoid arthritis; ACPA, anti-cyclic citrullinated peptide antibody; CT, computed tomography; sIL-2r, soluble interleukin-2 receptor; MRI, magnetic resonance imaging; POD, postoperative day; RF, rheumatoid factor; MALT lymphoma, extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue
Keywords: Multilocular thymic cyst, Anti-cyclic citrullinated peptide antibody, Preclinical rheumatoid arthritis, Case report
Abstract
Introduction
Multilocular thymic cyst (MTC) is a rare condition of an acquired multilocular cystic lesion caused by inflammation and often associated with autoimmune diseases or malignant tumors. We present a patient with MTC and asymptomatic rheumatoid arthritis (RA), which is termed preclinical RA.
Presentation of case
A 60-year-old man underwent a computed tomography scan, which revealed an 8.5 cm multilocular cystic lesion in the anterior mediastinum. The tumor had a lower intensity on T1-weighted imaging and a higher intensity on T2-weighted imaging. The imaging did not only suggest an MTC, but also the possibility of a thymoma with cystic degeneration, or lymphoma. We performed an extended thymectomy via median sternotomy. The lesion was diagnosed as MTC based on histopathological findings. Laboratory tests were performed for the purpose of screening for autoimmune diseases. He was diagnosed with preclinical RA, since the anti-cyclic citrullinated peptide antibody (ACPA) was positive.
Discussion
Specificity of ACPA is recorded in over 90% of patients with RA; ACPA is positive in about 40% of patients with preclinical RA. As patients with preclinical RA are more likely to develop RA, careful follow-up is required. Early diagnosis and treatment of RA can prevent destruction of joints, thereby preventing irreversible disability.
Conclusion
In patients with MTC, evaluating the cause of the inflammation, such as autoimmune diseases, is essential. Further studies are required to investigate the relationship between MTC and preclinical RA.
1. Introduction
Thymic cysts account for 3%–5% of mediastinal tumors, and most of them are caused by congenital unilocular cysts [1,2]. Multilocular thymic cyst (MTC) is a rare type of an acquired multilocular cystic lesion caused by inflammation [3]. Inflammatory conditions that result in the development of MTCs include autoimmune diseases (rheumatoid arthritis [RA] [4], systemic lupus erythematosus [1], Sjögren’s syndrome [2], and myasthenia gravis [5]), tumors (thymoma, thymic cancer, lymphoma, and seminoma [3]), and human immunodeficiency virus infection [6]. RA is one of the most common autoimmune diseases. A period of asymptomatic progression of RA is termed preclinical RA [7]. Herein, we report the case of a resection of MTC in a patient with preclinical RA. To our knowledge, this is the first report on a patient with MTC and preclinical RA.
This work has been reported in line with the SCARE criteria [8].
2. Presentation of case
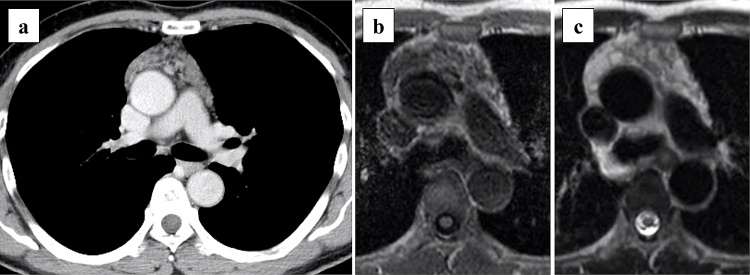
A computed tomography (CT) scan was performed on a 60-year-old man with hypertension and a history of 40 pack-years of smoking, which revealed an 8.5 cm multilocular cystic lesion in the anterior mediastinum (Fig. 1a). He was taking olmesartan medoxomil and benidipine hydrochloride for his hypertension. He had a family history of hypertension but no autoimmune diseases. The tumor markers, carcinoembryonic antigen, cytokeratin-19 fragment, and α-fetoprotein, were within normal limits, and the soluble interleukin-2 receptor (sIL-2 r) was 850 U/mL (<496 U/mL). The anti-acetylcholine receptor antibody was within normal limits. Magnetic resonance imaging (MRI) showed that the tumor had a lower intensity on T1-weighted imaging and had a higher intensity on T2-weighted imaging (Fig. 1b, c). Imaging findings did not only suggest a thymic cystic disease, but also suggested the possibility of a thymoma with cystic degeneration, or lymphoma. We considered that surgical resection was required for the purpose of diagnosis and treatment, and his informed consent was obtained.
Fig. 1.
Imaging findings of multilocular thymic cyst.
(a) Enhanced computed tomography image showing a multilocular cystic anterior mediastinal tumor. Magnetic resonance imaging showing that the tumor has (b) a lower intensity on a T1-weighted image and (c) a higher intensity on a T2-weighted image.
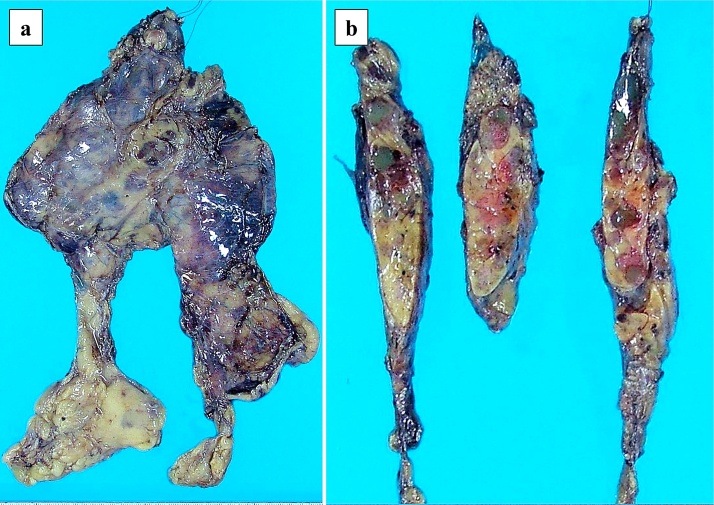
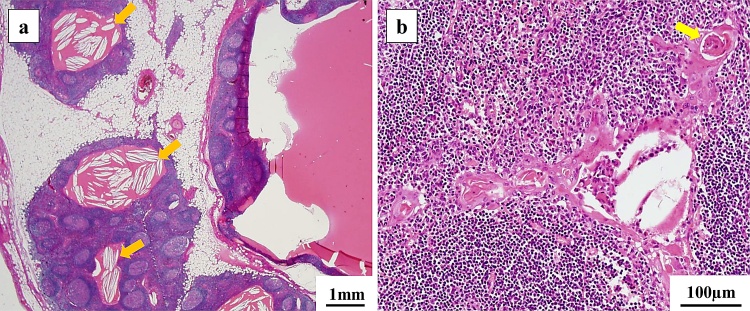
We performed an extended thymectomy using medium sternotomy. The operation time was 200 min, and the amount of blood loss was 45 mL. Chest drains were placed in bilateral thoracic cavities. No bleeding or air leakage were observed postoperatively, and the chest drains were removed on postoperative day (POD) 1. He experienced fever with chills on POD 6, and a CT scan revealed a left pleural effusion. We treated the pleural effusion using thoracentesis; however, no bacteria were cultured. The postoperative course was uneventful, and he was discharged on POD 14. Macroscopic findings of the tumor revealed multiple 1-cm cysts in the thymus, and these contained gelatinous substances (Fig. 2a, b). Histopathological findings revealed multiple cysts with partially squamous or columnar epithelium containing eosinophilic substances and cholesterol crystals in the thymic tissue (Fig. 3a, b). Hassall’s corpuscles and reactive lymphoid hyperplasia with prominent germinal centers were observed around the cysts. No tumorous lesion was observed, and the lesion was diagnosed as MTC. Laboratory tests were performed for the purpose of screening for autoimmune diseases. The rheumatoid factor (RF) was 10 U/mL (<15 U/mL), and the anti-cyclic citrullinated peptide antibody (ACPA) was 26.7 U/mL (<4.5 U/mL). RA was suggested by a rheumatologist. Since no joint symptoms were observed, he was diagnosed with preclinical RA.
Fig. 2.
Macroscopic findings of multilocular thymic cyst.
(a, b) Multiple 1-cm cysts are observed in the thymus, and these contain gelatinous substances.
Fig. 3.
Histopathological findings of multilocular thymic cyst.
(a, b) Multiple cysts with partially squamous or columnar epithelium containing eosinophilic substances and cholesterol crystals (orange arrows) are observed in the thymic tissue. Hassall's corpuscles (yellow arrow) and reactive lymphoid hyperplasia with prominent germinal centers are observed around the cysts.
At a 7-month follow-up, no joint symptoms were observed, and CT examination showed no recurrence of the MTC.
3. Discussion
MTC results from the cystic transformation of medullary duct epithelium-derived structures (including Hassall's corpuscles), induced by an acquired inflammatory process [3]. The main histopathological features of MTC include the following [3]: multiple cystic cavities partially lined by squamous, columnar, or cuboidal epithelium; non-neoplastic thymic tissue continuous with the cyst wall; and severe acute or chronic inflammation accompanied by fibrovascular proliferation, necrosis, hemorrhage, cholesterol granuloma formation, and reactive lymphoid hyperplasia with prominent germinal centers. CT scans show findings of a well-defined tumor consisting of cyst and solid parts [1]. MRI findings consist of the following: a low intensity MTC on T1-weighted imaging and a high intensity MTC on T2-weighted imaging [9]. The imaging findings of MTC and extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) in the thymus are similar [10]. In this case, the possibility of MALT lymphoma was considered based on imaging findings and the elevation of sIL-2 r levels. The preoperative diagnosis of MTC is not easily, and surgical resection is performed for the purpose of diagnosis and treatment. In patients with MTC, extended thymectomy is recommended because there are some reported cases of MTC with malignant tumors such as thymic carcinoma and lymphoma, and recurrence caused by incomplete resection of the multilocular cyst [2]. A MTC is caused by inflammatory conditions, such as autoimmune diseases or malignant tumors; therefore, evaluating the cause of the MTC is essential [2]. It has been reported that the symptoms of autoimmune diseases improve after resection of the MTC [2,4]; MTC itself may influence the development of autoimmune diseases. Even in cases of MTC with malignant tumors, the development of the MTC is thought to be due to the inflammation that accompanies the tumors rather than the tumors themselves [3].
In this case, the RF was within normal limits, but the ACPA was positive, suggesting concurrent preclinical RA. The prevalence of RA is 0.5%–1% [11]. In patients with RA, the synovial membrane of joints becomes inflamed, causing joint pain, swelling, and destruction. Early diagnosis and treatment of RA can avert or substantially slow down the progression of joint damage in up to 90% of patients, thereby preventing irreversible disability [12]. RF can be detected in 70%–80% of patients with RA and in relatively high percentages in other autoimmune diseases, infections, and in healthy individuals [13]. While RF was the only biomarker of RA in the past, currently, ACPA is recognized as a more valuable serologic marker of RA [14]. It is reported that the sensitivity and specificity of ACPA are 64%–81% and 90%–99%, respectively, in patients with RA [15]. In addition, ACPA is positive in 33.7%–40% of patients with preclinical RA, while ACPA is positive within 4.8 years before being diagnosed with RA [16,17]. As patients with preclinical RA are more likely to develop RA, it is necessary to embark on check-ups as well as early treatment from the onset of RA.
4. Conclusion
In patients with MTC, evaluating the cause of the inflammation is essential. MTCs are often associated with autoimmune diseases, and further studies are required to investigate the relationship between MTC and preclinical RA.
Conflicts of interest
All authors have no conflicts of interest.
Sources of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Ethics board approval was not required for our paper as case reports our exempted from ethical approval in our institute.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Nobutaka Kawamoto carried out the operation, acquired the data, and drafted the manuscript.
Riki Okita conducted the entire study.
Hidetoshi Inokawa assisted in the operation.
Tomoyuki Murakami diagnosed the patient based on the pathological findings.
Kazunori Okabe supervised the writing of the manuscript.
All authors have read and approved the final manuscript.
Registration of research studies
We consider that this case report does not fall into the category that requires registration of research studies. Hence, we have not registered it in a publicly accessible database.
Guarantor
Nobutaka Kawamoto.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgements
We thank Dr. Teppei Hashimoto, Dr. Masataro Hayashi, and Dr. Masanori Okada for attending to the patient postoperatively.
We thank Editage (http://www.editage.jp) for English language editing.
Contributor Information
Nobutaka Kawamoto, Email: kawamotonobutaka@gmail.com.
Riki Okita, Email: riki0716okita@yahoo.co.jp.
Hidetoshi Inokawa, Email: h_inokawa@hotmail.com.
Tomoyuki Murakami, Email: tomondenali@hotmail.co.jp.
Kazunori Okabe, Email: okabe-oka@umin.ac.jp.
References
- 1.Choi Y.W., McAdams H.P., Joen S.C., Hong E.K., Kim Y.H., Im J.G. Idiopathic multilocular thymic cyst: CT features with clinical and histopathologic correlation. AJR Am. J. Roentgenol. 2001;177:881–885. doi: 10.2214/ajr.177.4.1770881. [DOI] [PubMed] [Google Scholar]
- 2.Kondo K., Miyoshi T., Sakiyama S., Shimosato Y., Monden Y. Multilocular thymic cyst associated with Sjögren’s syndrome. Ann. Thorac. Surg. 2001;72:1367–1369. doi: 10.1016/s0003-4975(00)02706-5. [DOI] [PubMed] [Google Scholar]
- 3.Suster S., Rosai J. Multilocular Thymic cyst: an acquired reactive process. Study of 18 cases. Am. J. Surg. Pathol. 1991;15:388–398. [PubMed] [Google Scholar]
- 4.Matsumoto S., Mori Y., Takiya H., Iwata H., Shirahashi K. Multilocular thymic cyst associated with rheumatoid arthritis. Kyobu Geka. 2012;65:205–208. (Japanese article with English abstract) [PubMed] [Google Scholar]
- 5.Yano M., Numanami H., Akiyama T., Taguchi R., Furuta C., Iwakoshi A. Thoracoscopic thymectomy for large thymic cyst: myasthenia gravis with thymoma concealed by thymic cyst. Surg. Laparosc. Endosc. Percutan. Tech. 2019;29:e34–6. doi: 10.1097/SLE.0000000000000641. [DOI] [PubMed] [Google Scholar]
- 6.Leonidas J.C., Berdon W.E., Valderrama E., Neveling U., Schuval S., Weiss S.J. Human immunodeficiency virus infection and multilocular thymic cysts. Radiology. 1996;198:377–379. doi: 10.1148/radiology.198.2.8596835. [DOI] [PubMed] [Google Scholar]
- 7.Deane K.D. Preclinical rheumatoid arthritis (autoantibodies): an updated review. Curr. Rheumatol. Rep. 2014;16:419. doi: 10.1007/s11926-014-0419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A., Orgill D.P., For the SCARE Group The SCARE 2018 statement: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M., Endo M., Adachi S., Kono M. Multilocular thymic cyst: MRI findings. AJR Am. Roentgenol. 1994;164:479–480. doi: 10.2214/ajr.163.2.8037066. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu K., Ishii G., Nagai K., Yokose T., Ishizawa K., Tamaru J. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) in the thymus: report of four cases. Jpn. J. Clin. Oncol. 2005;35:412–416. doi: 10.1093/jjco/hyi105. [DOI] [PubMed] [Google Scholar]
- 11.Willemze A., Trouw L.A., Toes R.E., Huizinga T.W. The influence of ACPA status and characteristics on the course of RA. Nat. Rev. Rheumatol. 2012;8:144–152. doi: 10.1038/nrrheum.2011.204. [DOI] [PubMed] [Google Scholar]
- 12.Aletaha D., Smolen J.S. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320:1360–1372. doi: 10.1001/jama.2018.13103. [DOI] [PubMed] [Google Scholar]
- 13.Smolen J.S. Autoantibodies in rheumatoid arthritis. In: Venrooij W.J., Maini R.N., editors. Manual of Biological Markers of Disease. Kluwer Academic Publishers; The Netherlands: 1996. pp. C1.1/1–C1.1/18. [Google Scholar]
- 14.Corrao S., Argano C., Calvo L., Pistone G. The challenge of using the rheumatoid arthritis diagnostic criteria in clinical practice. Intern. Emerg. Med. 2015;10:271–275. doi: 10.1007/s11739-015-1206-8. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura K., Sugiyama D., Kogata Y., Tsuji G., Nakazawa T., Kawano S. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann. Intern. Med. 2007;146:797–808. doi: 10.7326/0003-4819-146-11-200706050-00008. [DOI] [PubMed] [Google Scholar]
- 16.Rantapää-Dahlqvist S., de Jong B.A., Berglin E., Hallmans G., Wadell G., Stenlund H. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 17.Nielen M.M., van Schaardenburg D., Reesink H.W., van de Stadt R.J., van der Horst-Bruinsma I.E., de Koning M.H. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]