Abstract
Stress has many consequences for our wellbeing, both physically and psychologically, underscoring the need to study markers of differential sensitivity to stressful situations. We examined associations between empathy and mentalizing abilities and psycho-physiological responses to a psychosocial stress task. We conducted two highly comparable studies, the first in men (N = 52) and the second in women (N = 72). Each study started with a self-report empathy measure and a mentalizing test [Reading the Mind in the Eyes Test (RMET)] followed by the Trier Social Stress Test (TSST) or a control task. Stress reactivity was confirmed in both men and women with significantly higher levels of cortisol, blood pressure, and subjective stress levels in response to the TSST compared to the control task. Higher accuracy on the RMET significantly predicted higher cortisol and heart rate reactivity, while self-reported empathic concern significantly predicted higher subjective stress reactivity. These associations were found in men, and when men and women were analyzed together. This indicates that higher levels of mentalizing and empathic abilities may confer sensitivity to socially stressful situations. While a moderation analysis indicated no gender differences in these associations, the findings could not be directly replicated in women. This suggests that gender may impact such associations and that replication of the findings in larger samples is warranted.
Keywords: Psychology, Stress, Empathy, Mentalizing abilities, Gender, Cortisol, Heart rate
Psychology; Stress; Empathy; Mentalizing abilities; Gender; Cortisol; Heart rate
1. Introduction
Stress is an increasingly common phenomenon in our ever more technology-driven society. While stress helps us to overcome challenges and to achieve our goals, stress can also have many detrimental health consequences if it continues for a long time (Grippo and Johnson, 2009; Kessler et al., 2010; McEwen, 2003, 2017). When people have to deal with acute stress, this can lead to irritability or aggression (Sandi and Haller, 2015), impulsivity, but also to maladaptive choice behavior (Raio et al., 2020). From earlier studies we know that stress and social behavior are highly interrelated. On the one hand, stress is often activated by social demands and expectations, as it stimulates people's urge to meet these social requirements (Beery and Kaufer, 2015). On the other hand, experiencing feelings of stress often has an impact on how people behave towards others (Raio et al., 2020; Sandi and Haller, 2015). Although several studies looked into the causes and consequences of stress [see e.g. (Gelkopf et al., 2012; Greenberg et al., 2002; Sapolsky, 1994; Schneider et al., 2013)], more knowledge is warranted about differential sensitivity to stressful situations to predict how people will respond to (socially) demanding contexts. The current study will therefore address the question how individual differences in empathy and mentalizing abilities may lead to differential responses in psychological and physiological stress reactivity to acute psychosocial stress.
When a situation is perceived as threatening – e.g. when having to speak in front of an audience without preparation – multiple hormonal systems come into play. Firstly, (nor)adrenalin is released by the autonomic nervous system, leading to increases in heart rate and blood pressure, to enhance energy and focus to deal with the stressor at hand (Gordan et al., 2015). Secondly, with a slight delay, cortisol is released by the hypothalamic-pituitary-adrenal-axis (HPA-axis) to mobilize energy so that homeostasis can be restored (Russell and Lightman, 2019). In the brain, the amygdala is involved in threat detection leading to the physiological stress responses, but also to subjective stress responses, including feelings of tenseness, anxiety and arousal (Joëls et al., 2018; Rodrigues et al., 2009). Chronic activation of these stress systems can ultimately lead to somatic diseases, such as cardiovascular disease, immune-system dysfunction and metabolic disorders (Grippo and Johnson, 2009; McEwen, 2017). In addition, chronic stress can also enhance the development of anxiety disorders and depression (Kessler et al., 2010; McEwen, 2003). Considering the detrimental effects of chronic stress, it is important to elucidate vulnerability and resilience factors to stress, so that interventions can be developed or improved to prevent negative outcomes of prolonged stress exposures.
Empathy and mentalizing abilities are two concepts that play a unique role in enabling successful social interaction. Specifically, the abilities to empathize and mentalize allow us to assess the affective state of another person and help us to understand and adjust to social situations, as well as predict behavior by assessing other people's mental representations (Hooker et al., 2008). Although empathy and mentalizing are beneficial to the development of healthy relationships with the people around us, these abilities may also make us more sensitive to social judgments or critical evaluations by others. That is, high levels of empathy and mentalizing can help us to adjust our behavior in order to, for example, comply with group norms and/or to prevent social rejection (Cialdini and Goldstein, 2004). For these reasons it may be expected that people with high levels of empathy and mentalizing abilities are more sensitive to potentially threatening social situations. For example, in situations where people are being evaluated, a higher stress response may be predicted for individuals who are more aware of, and sensitive to, others' intentions and emotions. However, there is relatively little known about associations between empathy, mentalizing and stress reactivity.
Several studies indicate a possible link between empathy and stress responses (Fairchild et al., 2019, 2008; von Polier et al., 2013). For example, Fairchild and colleagues found that individuals with callous unemotional traits or conduct problems (characterized by antisocial and aggressive behavior) showed low levels of affective empathy and reduced stress responses (Fairchild et al., 2019, 2008; von Polier et al., 2013). A recent study in mice also showed that low empathy-like behavior in male mice was associated with impaired physiological stress reactivity (Laviola et al., 2017). This indicates that reduced sensitivity to others' emotions may be associated with a lower sensitivity to stress. However, it remains unclear whether people with high sensitivity to others' emotions (i.e., high empathic abilities) are inherently more reactive to psychosocial stress. Furthermore, to our knowledge no previous studies have examined whether individual differences in mentalizing abilities can impact stress reactivity. Several studies did examine how acute stress (elicited in a laboratory setting) can influence subsequent social behavior (Buchanan and Preston, 2014; Margittai et al., 2015) and mentalizing (Smeets et al., 2009). However, these studies did not consider potential baseline individual differences in empathy and mentalizing abilities, and how these individual differences may have influenced reactivity to the stressor at hand and possibly post-stressor social behaviors as well.
Hence, in this study we investigate whether individual differences in empathy and mentalizing abilities influence stress reactivity to negative evaluative signals elicited with an often-used psychosocial stress paradigm, the Trier Social Stress Test [TSST (Kirschbaum et al., 1993)]. In the TSST participants are required to perform an unexpected mock job interview and arithmetic task in front of a selection committee giving negative evaluative signals. The TSST allows for assessment of individual differences in both physiological as well as psychological stress reactivity (Frisch et al., 2015). We expect that a better understanding and recognition of social signals from the committee – as measured by self-reported levels of affective and cognitive empathy, and by accuracy on a mentalizing task – are related to increased levels of psychological stress reactivity (measured by changes in anxiety or mood) and physiological stress reactivity (measured by salivary cortisol levels, heart rate, and blood pressure). In sum, we will examine whether the following individual differences are related to psychosocial stress reactivity: 1) the ability to take the perspective of another person (i.e. cognitive empathy; Davis, 1983; Frith and Frith, 2007), 2) one's own feelings in response to others' emotions (i.e. affective empathy; Davis, 1983; Frith and Frith, 2007), and 3) the ability to reason about other peoples' mental state (i.e. mentalizing; Baron-Cohen et al., 2001).
A key factor to be considered in this context is the impact of gender. Gender may impact both the stress response (Kajantie and Phillips, 2006) as well as empathy and mentalizing abilities (Christov-Moore et al., 2014; Overgaauw et al., 2017; Van der Graaff et al., 2014). Several studies using the TSST have shown higher cortisol responses in men compared to women (Liu et al., 2017; Reschke-Hernández et al., 2017; Stephens et al., 2016; Zänkert et al., 2018). These differences may be due to hormonal influences of the menstrual cycle and hormonal contraceptive use (Childs et al., 2010; Kajantie and Phillips, 2006; Kirschbaum et al., 1999). However, other studies (Uhart et al., 2006) indicate these gender differences may also be partly explained by differential HPA-axis stimulation processes, that is, hormonal response patterns may depend on the nature of a stressor. In this regard, Stroud et al. (2002) found that men are more stress sensitive to high-achievement tasks (e.g. cognitive performance), while women are more stress sensitive to social rejection. The TSST includes both cognitive challenge and negative social evaluation (Dickerson and Kemeny, 2004), making it a suitable experimental tool for inducing stress in both men and women. Studies that have investigated gender differences in mentalizing abilities found that women perform slightly better than men (Baron-Cohen et al., 2001; Schiffer et al., 2013). Similarly, men showed lower levels of self-reported empathic abilities than women (Toussaint and Webb, 2005; Van der Graaff et al., 2014). Considering possible gender differences in empathy, mentalizing abilities, and stress reactivity, it is also important to examine associations between these characteristics in both men and women. In this regards, we set out to study whether higher levels of 1) cognitive empathy 2) affective empathy, and 3) mentalizing abilities, are associated with increased psychological and physiological stress reactivity to negative social evaluation as elicited by the TSST in two highly comparable studies in men (study 1) and women (study 2). To our knowledge this is the first study gaining knowledge on whether males and/or females who are being more susceptible to social cues, have a potential higher stress response following acute psychosocial stress.
2. Materials and methods
2.1. Participants
We conducted two studies, the first in men and the second in women. For both studies, Dutch participants between the ages of 17 and 35 were recruited within and around the Faculty of Social Sciences at Leiden University by means of flyers and active approaching. Inclusion criteria were: at least undergraduate level of study, no recent (<1 year) self-reported major physical or psychological problems, and no medication use that might interfere with cortisol measurements (e.g. corticosteroids, benzodiazepines). Men were actively screened during a telephone call before participation, while women filled in a questionnaire on the inclusion criteria during the lab visit. In both studies a stress and control group were included. Our focus was to examine inter-individual differences in stress reactivity and the stress groups were therefore larger than the control groups, as the latter were only included to confirm the stress reactions. To this end, we included 52 men in the first study (stress group: N = 40, control group: N = 12, Mage = 20.7 yrs, SD = 2.8), and 72 women in the second study (stress group: N = 45, control group: N = 27, Mage = 20.3 yrs, SD = 2.9). Heart rate and blood pressure data were missing for one male and cortisol data for one female, both in the TSST condition. In the screening questionnaire five women (3 in the stress and 2 in the control group) reported use of corticosteroid or anti-depressant medication, and were therefore excluded from the analyses (final n = 67). Forty-three (64%) of these women reported use of hormonal contraceptives (30 in the stress and 13 in the control group), which was included as a possible covariate in the stress reactivity analyses as it may affect cortisol reactivity (Liu et al., 2017). Participants were randomly assigned to either the TSST or control condition and were not aware of a possible stress test beforehand and during assessment of the empathy questionnaire and mentalizing task.
2.2. The psychosocial stress test
The Trier Social Stress Test (TSST) was employed, which is designed to induce psychosocial stress in laboratory settings (Kirschbaum et al., 1993). In both studies the TSST consisted of a 5-minute explanation and anticipation period followed by a 5-minute oral presentation. During the oral presentation participants had to convince a committee composed of two or three psychologists of mixed gender of their suitability for a chosen job while being videotaped and voice-recorded. Next, a 5-minute arithmetic task was performed during which the committee gave negative feedback on performance. The TSST has been shown to reliably induce both physiological and psychological stress responses (Frisch et al., 2015). The control groups were instructed to write a job application letter for 10 min after which the same arithmetic task was performed on paper for 5 min, all without social evaluation. Throughout the rest of the test session they followed the same procedure as the TSST groups.
2.3. Measures and materials
2.3.1. Mentalizing
The Reading the Mind in the Eyes Test (RMET) was originally developed as a measure of adults' understanding of higher order Theory of Mind or mentalizing (Baron-Cohen et al., 2001), assessing the ability to accurately recognize others' intentions and emotions. We used a Dutch computerized version of the RMET (van Honk et al., 2011), administered via E-Prime. Participants had to infer mental states from 36 randomly presented photographs of people's eyes. A choice had to be made between four descriptive words to match the eyes. All correct responses were summed to calculate the accuracy score.
2.3.2. Empathy
The Interpersonal Reactivity Index [IRI; (Davis, 1983; De Corte et al., 2007)] is a 28-item self-report measure of empathic abilities. It originally consists of four scales, comprising seven items each. The four scales are: perspective taking (PT; the tendency to adopt another person's perspective), empathic concern (EC; the tendency to experience emotions of warmth, sympathy, and concern), fantasy (FS; the tendency to identify closely with fictitious characters), and personal distress (PD; the tendency to experience discomfort and concern when observing other's negative feelings). Answers are rated on a five point Likert-scale and the maximum sum score per scale is 28, with higher scores reflecting higher empathic abilities. Since we had specific hypotheses on affective and cognitive empathy, we chose to only include the PT and EC scales in this study, as these are most reflective of the affective (EC) and cognitive (PT) aspects of empathy (Davis, 1983). Cronbach's alpha of the EC scale was .77 for men and .68 for women, and of the PT scale .63 for men and .78 for women.
2.3.3. Physiological stress measures
Saliva for cortisol analyses was collected with Salivette collection devices (Sarstedt, Germany) and free cortisol levels were determined with a chemiluminescence immuno assay (IBL-Hamburg, Germany) by the Kirschbaum lab (Technical University of Dresden). Heart rate and systolic and diastolic BP were measured from the non-dominant arm with an automatic blood pressure monitor (Omron R5-I).
2.3.4. Psychological stress measures
Subjective stress experience was measured by changes in anxiety levels in men with the state version of the State-Trait Anxiety Inventory [STAI; (Spielberger, 1983)], including 20 items indicating either anxiety-related emotions or the opposite that were scored on a scale from 1 (not at all) to 4 (very much). After recoding, a sum score was calculated with higher scores reflecting higher anxiety levels. Cronbach's alpha of the STAI before, during, and after the TSST was .78, .95, and .94 respectively. While we intended to replicate the findings on subjective stress reactivity in women, we also wanted to assess a broader spectrum of negative affect than just anxiety. Therefore, in women, the negative affect scale of the Positive and Negative Affect Schedule [PANAS; (Watson et al., 1988)] was used as a subjective stress measure, including 10 items describing negative emotions scored on a scale from 1 (very slightly or not at all) to 5 (extremely). A sum score was calculated with higher scores reflecting higher levels of negative affect. Cronbach's alpha of the NA scale before, during and after the TSST was .88, .94, and .92 respectively. To compare the subjective stress levels between study 1 and 2, STAI and NA scores were standardized to the group mean within each study.
2.4. Procedures
Study 1. After a screening by phone, males completed online questionnaires at home (including the IRI) and then visited the lab for a two-hour test session, starting between noon and 4pm to reduce the impact of the circadian cortisol rhythm. At the start of the test session, 40 min before the start of the stress test, the RMET was administered, after which a working memory and a mimicry test were performed. The results of these two additional tests were ultimately not included in the present study, as the working memory test does not address the central concept of empathy and mentalizing, while the mimicry test was not successful due to technical problems. A baseline measurement of the psycho-physiological stress measures was taken just before the start of the TSST (t = 0 min, [t is time in minutes]). Directly after the TSST, the psychophysiological stress measures were taken again (t = 20 min), as well as 20 min later (t = 40 min).
Study 2. The female participants visited the lab for a two-hour session starting between 9am and 4pm, and completed questionnaires including the IRI and a form regarding inclusion criteria. No women were excluded at that time point, as none reported severe psychological or physical problems that could have interfered with the test procedures. Thirty minutes before the start of the TSST, the RMET was administered, as well as a working memory and attentional bias test. The results of the working memory and attentional bias tests are not included in the present analysis given that neither addresses our core concepts relating to empathy and mentalizing. Again, psychophysiological stress measures were taken at t = 0, t = 20 and t = 40 with regards to the TSST. Due to the large range in starting times for women, start time was also included as a possible covariate in the stress reactivity analyses.
All participants gave informed consent before start of the studies, and at the end of their test session participants were debriefed and received a monetary reward (6.50 euros per hour) or study credits for participation. The Psychology Research Ethics Committee of the Faculty of Social and Behavioral Sciences at Leiden University approved the study protocols, which were performed according to the World Medical Association Declaration of Helsinki.
2.5. Statistical analyses
We first performed descriptive analyses of the predictors for each study. To examine possible baseline gender differences, datasets of study 1 and 2 were combined and t-tests were performed. To assess whether the TSST was successful in eliciting stress, we first measured stress reactivity in men and women separately by performing Repeated Measures (RM-) MANOVAs with all stress measures (cortisol, heart rate, systolic BP, diastolic BP and subjective stress) as the multivariate outcomes measure, time (t = 0, t = 20 and t = 40) as within-subjects factor, and group allocation (TSST vs. control) as between-subjects factor in study 1 and 2 (see Figures 1[a-e] and 2[a-e] for the reactivity scores). In case of violations of sphericity, Greenhouse-Geisser corrected values are reported. For women, time of day and oral contraceptive use were examined as possible covariates in the RM-ANOVAs. Cortisol values were log transformed before analyses due to skewness of the data, but raw values are shown in the figures. Afterwards a RM-MANOVA including gender as a between-subjects factor was performed to examine possible gender differences in stress reactivity.
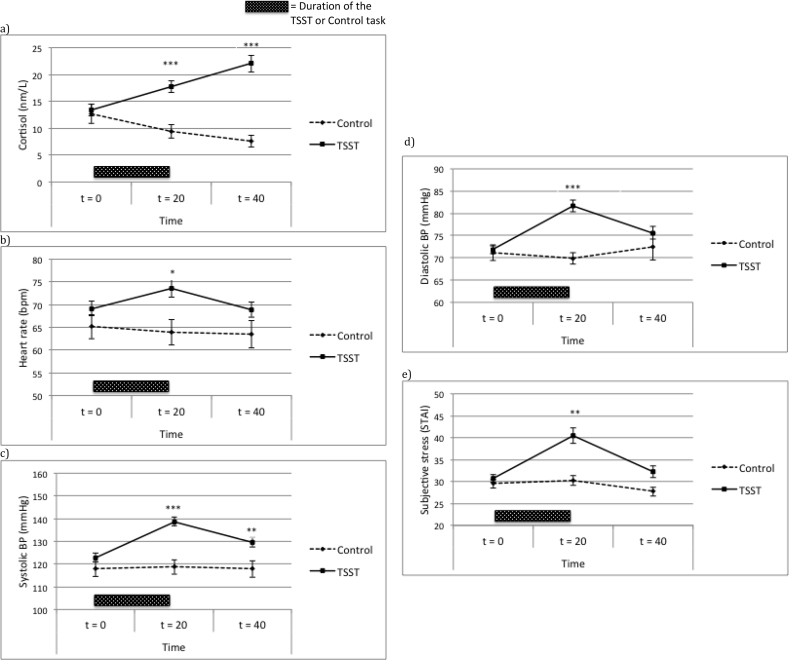
Figure 1.
Stress measure levels (a–e: Mean ± SEM) in the male TSST and control groups over time. Group differences per time point are indicated with ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. TSST = Trier Social Stress Test, BP = Blood Pressure. Duration of the TSST or control task.
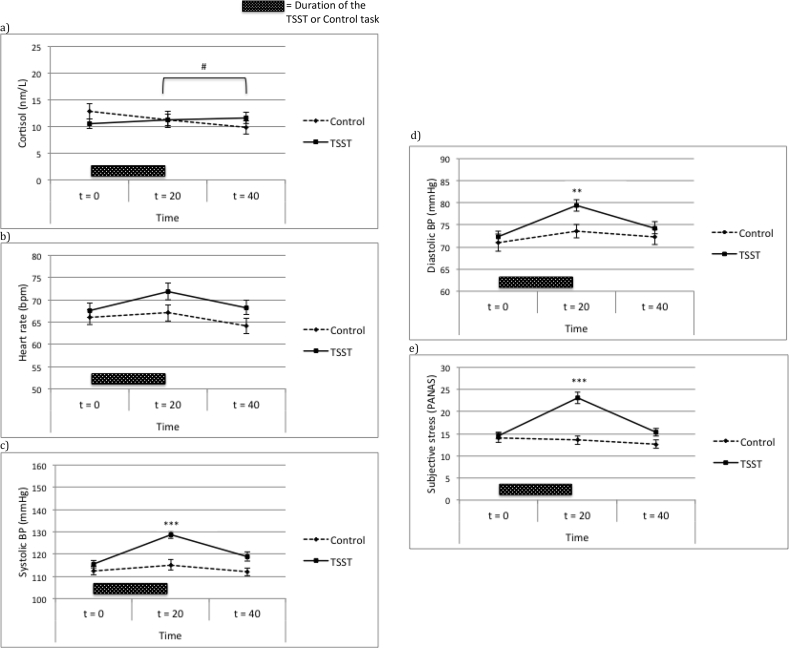
Figure 2.
Stress measure levels (a–e: Mean ± SEM) in the female TSST and control groups over time. Group differences per time point are indicated with ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. The significant increase in cortisol levels over time in the TSST group is indicated with #p < 0.05. TSST = Trier Social Stress Test, BP = Blood Pressure. Duration of the TSST or control task.
Next, to test our hypotheses that higher levels of mentalizing abilities (RMET accuracy) and higher levels of self-reported cognitive (PT scale of the IRI) and affective empathy (EC scale of the IRI) would predict increased stress reactivity, we performed multivariate regression analyses to test the predictive value of the RMET and the two IRI scales on stress reactivity using cortisol, heart rate, systolic BP, diastolic BP, and subjective stress reactivity as the multivariate outcome measures in study 1 (n = 52) and 2 (n = 67) separately. Reactivity scores were calculated by regressing the highest post-TSST stress levels (based on the group means for each stressor, i.e., t = 40 for cortisol and t = 20 for the other four stress measures) on pre-TSST stress levels and group allocation, and then saving the standardized regression residuals. By using these regression residuals we control for initial baseline differences between participants and their effect on reactivity (Ramsay and Lewis, 2003), and the standardized values reflect deviations in reactivity from the estimated group average (Tollenaar et al., 2011).
In case of differences in associations between empathy, mentalizing abilities, and stress reactivity between men and women, we combined the datasets from study 1 and 2 to examine whether gender statistically moderated the effect of empathy and mentalizing abilities on stress reactivity. This was done because conclusions about differences between males and females require the presence of an interaction between the independent variable (the two IRI scales and RMET accuracy) and gender (Nieuwenhuis et al., 2011).
All analyses were performed in IBM SPSS 24 and alpha was set at .05. Because three predictors were included within each regression analyses (the RMET and 2 IRI scales), the alpha for the individual predictors was Bonferroni corrected to .017 (.05/3) in the multivariate regression analyses. In the univariate post-hoc analyses for the five stress measures, the alpha for the individual outcome measures was Bonferroni corrected to .01 (.05/5). Partial eta squared (eta) is given as a measure of effect size where applicable.
3. Results
3.1. Descriptive statistics
Table 1 shows the means, SDs, and ranges on the predictor variables and start time for male participants (study 1) and female participants (study 2). Independent t-tests show that women scored significantly higher than men on both the PT and EC scales of the IRI (ps < .05), but not on the RMET (p = .091). Furthermore, the start time for women was on average earlier in the day (p < .001). Interrelations amongst the predictors were low enough (rs < .27, ps > .05), excluding the possibility of collinearity.
Table 1.
Descriptive statistics of study 1 (men) and study 2 (women).
| Study 1 Men (N = 52) |
Study 2 Women (N = 67) |
Gender difference |
|||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | p-value | |
| Start time | 14:34 | 1:08 | 12:41–16:36 | 12:46 | 2:21 | 9:05–16:33 | <.001 |
| RMET | 26.6 | 3.0 | 22–33 | 27.6 | 3.4 | 20–34 | .091 |
| EC | 16.9 | 5.0 | 6–28 | 19.9 | 3.7 | 8–27 | <.001 |
| PT | 16.8 | 4.3 | 7–26 | 18.6 | 4.4 | 6–27 | .022 |
Notes. RMET = Reading the Mind in the Eyes Test, EC = Empathic Concern Scale of the Interpersonal Reactivity Index, PT = Perspective Taking Scale of the Interpersonal Reactivity Index.
3.2. Manipulation check of the TSST: stress reactivity
The RM-MANOVA in study 1 indicated that men in the TSST responded with higher stress levels than men in the control condition [time∗condition effect: F(10, 190) = 9.82, p < .001, eta = .34]. Univariate post-hoc tests showed the interaction between time and condition was significant for cortisol [F(1.7, 84.3) = 30.27, p < .001, eta = .38], systolic BP [F(2, 98) = 10.18, p < .001, eta = .17], diastolic BP [F(1.8, 6.0) = 9.50, p < .001, eta = .16], and subjective stress [F(1.7, 83.5) = 3.95, p = .030, eta = .073], but not heart rate [F(2, 98) = 2.80, p = .066, eta = .054]. The interaction between time and condition was due to the findings that cortisol and systolic blood pressure were significantly increased at t = 20 and t = 40 in the stress group compared to the control group (all ps < .01) while they were similar at baseline. Diastolic blood pressure, heart rate and subjective stress levels were significantly higher in the stress group compared to the control group at t = 20 (all ps < .05), while they were similar at baseline. Hence, the TSST successfully elicited a response on all psychophysiological outcomes in the male participants compared to the control group, although the effect in heart rate was least pronounced; see Figure 1(a-e).
The RM-MANOVA in study 2 indicated that women in the TSST responded with higher stress levels than women in the control condition [time∗condition effect: F(10, 242) = 7.90, p < .001, eta = .25]. Univariate post-hoc tests showed the interaction between time and condition was significant for cortisol [F(1.4, 84.0) = 15.54, p < .001, eta = .20], systolic BP [F(2, 126) = 11.86, p < .001, eta = .16], diastolic BP [F(1.8, 114.6) = 3.97, p = .025, eta = .059], and subjective stress: F(2, 126) = 14.46, p < .001, eta = .19], but not for heart rate [F(1.73, 108.87] = 2.34, p = .108, eta = .036). The interaction between time and condition was due to the findings that systolic blood pressure was significantly increased at t = 20 and t = 40 in the stress group compared to the control group (ps < .05), while similar at baseline. Diastolic blood pressure and subjective stress were significantly higher in the stress group compared to the control group at t = 20 (ps < .05), while similar at baseline. No differences between the stress and control group were found in cortisol level and heart rate levels at any of the time points (all ps > .05). However, paired sample t-tests showed significant decreases in cortisol levels over time from t = 0 to t = 20 and from t = 20 to t = 40 (t(24) = 5.25, p < .001 and t(24) = 4.03, p < .001 respectively) in the control group. In contrast, the stress group showed a significant increase in cortisol levels from t = 20 to t = 40 [t(40) = 2.36, p = .023]. Overall, the TSST successfully elicited a psychophysiological stress response in the female participants compared to the control group on all stress measures except heart rate; see Figure 2(a-e). Time of day and contraceptive use did not significantly affect psychophysiological responses over time (all interactions with time; p > .05), and were hence not included as covariates in the regression analyses.
In addition, a RM-MANOVA was performed on the dataset combining both study 1 and 2, to examine the impact of gender on stress reactivity. The three-way interaction between time, condition and gender was significant [F(10, 446) = 2.67, p = .003, eta = .057], which was due to the impact of gender on cortisol reactivity only (univariate test for cortisol: F(1.60, 180.6) = 10.14, p < .001, eta = .082).
3.3. The role of empathy and mentalizing in stress reactivity
A multivariate regression analysis was first performed in men (Study 1), with the RMET and the PT and EC scales as predictors and the five stress reactivity scores as dependent variables. The overall multivariate model including all three predictors and five stress reactivity scores as outcome did not reach significance, F(15, 138) = 1.57, p = .089, eta = .146. Within this model, the EC and PT scale did not predict stress reactivity, F(5,44) = 2.00, p = .097 eta = .185 and F(5,44) = .67, p = .65, eta = .071, respectively. RMET accuracy did predict stress reactivity [F(5,44) = 2.82 p = .027, eta = .242], although this effect was no longer significant after correction for multiple testing (p > .017). We did explore the univariate tests per stress reactivity outcome, to assess whether specific stress measures would be more sensitive to mentalizing or empathy than others. Post-hoc univariate tests showed that higher accuracy on the RMET predicted higher levels of cortisol reactivity (beta = .33, t = 2.92, p = .005) and heart rate reactivity (beta = .11, t = 2.26, p = .023), although the latter effect did not survive correction for multiple testing (p > .01). Furthermore, higher scores on the EC scale significantly predicted higher subjective stress reactivity (beta = .076, t = 2.73, p = .009), see Table 2.
Table 2.
Univariate beta coefficients of the RMET and IRI scales in predicting cortisol, heart rate, BP, and subjective stress reactivity, and the multivariate F-values per predictor.
| Study 1 | Males |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cortisol reactivity |
Heart rate reactivity |
Systolic BP reactivity |
Diastolic BP reactivity |
Subjective stressa reactivity |
||||||||
| Beta | p | Beta | p | Beta | p | Beta | p | Beta | p | F(5,44) | ||
| RMET | .133 | .005∗∗ | .110 | .023∗ | .003 | .958 | .018 | .720 | .026 | .568 | 2.82∗ | |
| EC | -.013 | .630 | .023 | .426 | .009 | .763 | -.017 | .568 | .076 | .009∗∗ | 2.00 | |
| PT | -.031 | .354 | -.036 | .289 | -.013 | .710 | .027 | .442 | -.045 | .180 | .669 | |
| Study 2 | Females | |||||||||||
| Cortisol reactivity | Heart rate reactivity | Systolic BP reactivity | Diastolic BP reactivity | Subjective stressa reactivity | ||||||||
| Beta | p | Beta | p | Beta | p | Beta | p | Beta | p | F(5,58) | ||
| RMET | .036 | .316 | .067 | .061 | .041 | .246 | -.012 | .728 | -.054 | .138 | 1.51 | |
| EC | -.022 | .552 | .006 | .858 | -.021 | .556 | -.027 | .445 | .043 | .235 | .468 | |
| PT | -.039 | .198 | -.050 | .102 | -.041 | .175 | -.064 | .034∗ | -.036 | .236 | 1.74 | |
Notes: Reactivity scores are post-stressor levels (based on highest group mean) regressed on pre-stressor levels and group allocation (TSST versus control). TSST = Trier Social Stress Test, BP = Blood Pressure, EC = Empathic Concern Scale of the Interpersonal Reactivity Index, PT = Perspective Taking Scale of the Interpersonal Reactivity Index, RMET = Reading the Mind in the Eyes Test, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
State anxiety in men; Negative Affect in women.
The multivariate regression analysis was repeated in women (Study 2). The overall multivariate model including all three predictors and five stress reactivity scores as outcome was not significant, F(15, 180) = 1.39, p = .16, eta = .140). Within this model, neither the EC or PT scale, nor the RMET predicted stress reactivity [F(5,58) = 1.51, p = .202, eta = .105, F(5,58) = .47, p = .80, eta = .039, and F(5,58) = 1.74, p = .14, eta = .13, respectively]. While explorative univariate posthoc analyses indicated that higher scores on the PT scale predicted reduced diastolic blood pressure reactivity, this effect did not survive correction for multiple testing (beta = -.064, t = -2.17, p = .034), see Table 2.
Different effects were found in men and women with regard to the predictive value of the RMET and IRI scales on stress reactivity. We therefore also performed a multivariate regression analysis on the combined data set including both Study 1 and 2, to examine whether gender would moderate the impact of mentalizing and empathy on stress reactivity. The overall multivariate model in this combined data set, including all three predictors and five stress reactivity scores as outcome was significant, F(15, 336) = 2.157, p = .008, eta = .088. Within this model, the RMET significantly predicted stress reactivity, F(5,110) = 3.491, p = .006, eta = .137, but the EC and PT scales did not [F(5,110) = 2.108, p = .070, eta = .087 and F(5,110) = 1.785, p = .122, eta = .075, respectively]. Interestingly, gender did not moderate the impact of the RMET or IRI scales on stress reactivity (all ps > .27), or on any of the univariate tests per stress outcome (all ps > .048). In line with the findings in men, the univariate posthoc tests showed that the association of the RMET was specific for cortisol and heart rate reactivity (beta = .072, t = 2.59 p = .011 and beta = .082, t = 2.99, p = .003, respectively), and the EC scale significantly predicted subjective stress reactivity (beta = .060, t = 2.81, p = .006), see Table 3.
Table 3.
Univariate beta coefficients of the RMET and IRI scales in predicting cortisol, heart rate, BP, and subjective stress reactivity, and the multivariate F-values per predictor in the combined data set (Study 1 and 2).
| Males & Females | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cortisol reactivity |
Heart rate reactivity |
Systolic BP reactivity |
Diastolic BP reactivity |
Subjective stressa reactivity |
||||||||
| Beta | p | Beta | p | Beta | p | Beta | p | Beta | p | F(5,110) | ||
| RMET | .072 | .011∗ | .082 | .003∗∗ | .029 | .308 | .004 | .890 | -.027 | .334 | 3.49∗∗ | |
| EC | -.015 | .477 | .015 | .491 | .000 | .982 | -.017 | .449 | .060 | .006∗∗ | 2.11 | |
| PT | -.033 | .139 | -.044 | .046 | -.032 | .158 | -.024 | .289 | -.040 | .071 | 1.79 | |
Notes: Reactivity scores are post-stressor levels (based on highest group mean) regressed on pre-stressor levels and group allocation (TSST versus control). TSST = Trier Social Stress Test, BP = Blood Pressure, EC = Empathic Concern Scale of the Interpersonal Reactivity Index, PT = Perspective Taking Scale of the Interpersonal Reactivity Index, RMET = Reading the Mind in the Eyes Test, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
State anxiety in men; Negative Affect in women.
4. Discussion
In this study we investigated whether individual differences in empathy and mentalizing abilities are associated with stress reactivity to negative social evaluation elicited with a psychosocial stress test in both men (study 1) and women (study 2). Our results indicate that higher levels of mentalizing abilities, operationalized by higher accuracy on the RMET, are associated with higher cortisol and heart rate reactivity. Furthermore, self-reported affective empathy (empathic concern) was associated with higher subjective stress reactivity. These findings were specifically found in men (study 1) and were not directly replicated in women (study 2). However, when the two study samples were analyzed together, these associations were significant for the full group without moderation by gender. In sum, we show for the first time that higher levels of mentalizing abilities and affective empathy may confer sensitivity to socially stressful situations, leading to higher heart rate, cortisol, or subjective stress responses, most prominently in men. These findings are partly in line with our hypotheses and with prior studies showing a co-occurrence of low levels of stress reactivity and low levels of empathy in those with callous unemotional traits or conduct problems (Fairchild et al., 2019, 2008; von Polier et al., 2013). Overall, these outcomes suggest that individuals who are being more susceptible to social cues and who experience a high affect intensity in reaction to others' emotions have a potential higher stress response. This can in turn result in more serious health effects after prolonged exposure to stress, emphasizing the importance of taking these traits into account when people encounter a continuation of stressful events.
Stress was successfully elicited in both men and women, as indicated by increased levels of cortisol, blood pressure, and subjective stress in response to the TSST in comparison with the control groups. Only heart rate response was similar for females in both the TSST and control group. This lack of a difference in heart rate response between groups seems due to a slight increase in heart rate in the control condition as well. The control condition consisted of a non-socially matched procedure including a written job interview and calculation test, which may still have induced some arousal. With these results we replicate previous studies including the TSST in adult men and women (Campbell and Ehlert, 2012; Kirschbaum et al., 1993). Interestingly, while some studies have failed to show a cortisol response in women using the TSST (Liu et al., 2017), we did find a significant interaction effect between condition and time. This was mainly due to a decrease in cortisol levels over time in the control group, while the stress group showed a minor increase in cortisol after the TSST. In men the interaction was due to a clear increase in cortisol during and after the TSST. Hormonal responses beyond cortisol may therefore be of interest to study in the context of gender differences and the association between social cognition and stress.
The associations we found between stress reactivity, self-reported empathy, and mentalizing abilities may be gender specific, with stronger indications for these associations in men than women. A possible explanation for the fact that we predominantly found these associations in men could be differences in coping strategies. Previous studies have described different coping styles between men and women; men are more prone to use problem-focused coping strategies, whereas women use more emotion-focused coping strategies (Kelly et al., 2008). Additionally, according to the study by Tamres et al. (2002), women have a tendency to seek emotional support in stressful situations, whereas men are more likely to avoid situations causing stress or to deny the presence of a stressor. In socially stressful situations, women may be better able to use their emotion-focused skills to cope with the stressor compared to men. For example, when confronted with the intentions and evaluations of someone else – such as a committee evaluating your performance – women have been found to show more accurate social responses due a better self-other distinction (Tomova et al., 2014). Hence, while women may be more aware of the negative evaluations by others due to their higher levels of empathy, they may at the same time use emotion-focused coping strategies to deal with them (Kelly et al., 2008). As such, the impact of empathy and mentalizing on stress reactivity in women may be balanced with effective emotion-focused coping skills. However, for men, higher levels of empathy and mentalizing abilities may make them more sensitive and prone to negative social evaluations, which distracts them from effective problem-solving strategies. Future studies should include detailed measures of trait and state coping styles to elucidate these mechanisms and possible gender differences in stress reactivity. Another reason for the non-significant findings in women could be that – in general – women report higher levels of empathy [see (Rueckert, 2011) and our data], which could lead to less variability in scores compared to men. This might have reduced the power to find associations with stress reactivity. Future studies could use the current paradigm but include a larger mixed gender sample, to further test the influence of low or high levels of empathy and mentalizing abilities on stress sensitivity in men and women.
A possible underlying mechanism for the association between mentalizing and physiological stress reactivity may be related to the oxytocin system. We know from animal studies that oxytocin, i.e. a triggering hormone in approaching behavior within social situations, is also released within the brain when facing threatening situations (Heinrichs et al., 2009; Neumann and Slattery, 2015). A prior study, including only men, found that oxytocin administration increased males' accuracy on the RMET (Domes et al., 2007). Higher levels of endogenous oxytocin may possibly be related to higher trait levels of mentalizing, as well as behavioral and physiological responses during socially stressful situations (Bartz et al., 2011; Heinrichs et al., 2009), and may hence explain associations between mentalizing, empathy, and stress reactivity during a stressful situation.
In addition, a study by Tomova et al. (2017) measuring the association between acute stress and empathy for pain, showed that under the stress of solving difficult tasks, participants showed enhanced neural activation in the affective empathy brain network (anterior insula, anterior midcingulate cortex) when empathizing with unknown others suffering from physical pain. The findings of our present study add to this knowledge by demonstrating that this association also works in the opposite direction; being more stress sensitive because of higher levels of mentalizing abilities may lead to higher psychological and physiological stress reactivity, and should therefore be taken into account as a possible risk factor for stress-related symptomatology. Likewise, the influence of baseline or trait empathy and mentalizing abilities should be taken into account in studies looking at the impact of acute stress on social behavior and cognition. Individual differences in trait empathy and mentalizing may influence the reactivity to a laboratory-induced stressor, and thereby both directly (due to their underlying abilities) and indirectly (via the stress response) influence post-stressor performance on tasks that require mentalizing or empathic responses. It is yet unknown whether the ability to estimate and feel the intentions and emotions of evaluators during a social evaluative stressor like the TSST is related to a person's ability to handle the stressful situation and how they may adjust their behavior afterwards accordingly. Future studies in this area are warranted.
Interestingly, accuracy on the RMET was not related to either self-reported affective empathy or cognitive empathy in our study. A prior study (Melchers et al., 2015) also demonstrated absent correlations between self-reported empathy and indirectly measured empathy (i.e. by using tasks). It could be that indirect measures give more information about biological mechanisms like stress reactivity. This may also explain why the RMET is a better predictor for physiological stress reactivity compared to self-reported empathy in our study. Additionally, mentalizing abilities might be more relevant to stress reactivity, as both the TSST and the RMET require – although with a different purpose – the ability to read other people's intentions and emotions. In contrast, self-reported empathy may more closely relate to self-reported emotions during stress, which may explain the association we found between affective empathy and subjective stress.
Also important to address are the limitations of the present study. Men and women were tested within 2 different studies, including similar tests and protocols but also with slight differences in set-up (e.g. other committee members, different measures of subjective stress). It is therefore possible that the gender differences we observed in the separate analyses could be study dependent. When the samples were merged, gender did not moderate the associations between empathy, mentalizing ability, and stress reactivity, and the association between the RMET and heart rate reactivity was even significant including corrections for multiple testing. This may be due to the larger sample size when combining the studies, and thus to higher power to detect such associations. However, from a more conservative point of view, the results from our first study in men could not be replicated in our second study in women, stressing the need for replication of these findings in a study including both men and women. Also of note, all participants performed the RMET shortly before the TSST and a direct influence of such an emotion-focused test on TSST reactivity cannot be directly ruled out. Ideally, a stress group should be included that did not receive this test beforehand. In addition, our choice to include the IRI and the RMET was predominantly based on the fact that both measures are well validated and often used. However, using self-reports to measure empathy, like the IRI, has often led to gender differences that were not found in studies testing empathy in a more indirect way (Rueckert, 2011). The RMET is a more indirect measure of mentalizing abilities, which has its benefits when it comes to possible gender differences as a result of using self-reports and demand characteristics. However, the RMET also has its disadvantage because it does not include contextual information as it only presents the eye region of faces. Future studies might consider using more ecologically valid mentalizing measures (Achim et al., 2013) and implement paradigms like the one designed by Dziobek et al. (2006: the Movie Assessment for Social Cognition) in order to test the broader concept of mentalizing. Furthermore, with regards to the subjective stress measures, the STAI and negative affect scale of the PANAS may not optimally reflect a stressed state. While the STAI measures anxiety levels, which are expected to increase in response to stress, it is conceptually not a perfect stress measure. The PANAS includes a broader range of negative states, e.g. also representing feelings of anger in addition to anxiety and distress. Little consensus is found in the literature on how to best measure state levels of stress and a possible alternative is the use of Visual Analogue Scales (Lesage et al., 2012).
Our goal was to examine individual differences in stress reactivity and for this reason we chose to include most participants in the experimental stress conditions with relatively small groups in the control conditions. This may have resulted in low power to detect stress reactivity in the TSST compared to the control group and a higher influence of possible outliers. However, except for heart rate measures in females, we showed statistically significant reactivity of our stress measures to the TSST in both men and women, with heart rate reactivity still variable enough to be used as a stress outcome. Furthermore, due to the multiple stress measures, chances for type 1 errors are possible and replication is needed in larger samples, including both men and women, to further establish gender differences in the association between empathy, mentalizing abilities, and stress. Also, generalizability beyond students is limited, because students may possibly perform higher on a mentalizing task than the general population, while psychology students may specifically over-report on empathic abilities. Inclusion of participants with broader educational levels would be of interest.
Additionally, because we did not manipulate empathy or mentalizing abilities, we cannot necessarily conclude that higher levels of these traits cause stress sensitivity. That is, empathy, mentalizing abilities, and stress sensitivity may be expressions of underlying un-examined traits. Such traits include possible genetic variations (in e.g. the oxytocin system) or neural mechanisms implicated in social behavior, such as the amygdala and anterior insula (Petrovic et al., 2008; Singer et al., 2008). Finally, differences in stress sensitivity might also be explained by the biopsychosocial model, which postulates that individuals tend to make a trade-off between their level of competence (e.g. empathic skills) and the situational demands in case of acute stress. This situational appraisal influences physiological responses and subsequently individuals' performance and could be susceptible to individual and/or gender differences (Blascovich et al., 1999; Folkman et al., 1986). Future studies should further address these underlying mechanisms.
This is the first study to examine associations between empathy, mentalizing abilities, and stress reactivity in men and women. In future studies, it will be of interest to study the mechanisms through which mentalizing abilities are associated with reactivity to social stress, while taking gender differences into consideration. Potential mediators could be: differences in emotion regulation and coping strategies, situational appraisal, and fear of negative evaluation. Furthermore, investigating how empathy, mentalizing abilities, and stress reactivity in chronic stressful situations change over time could provide further insight into cause-effect relationships. Moreover, it would be interesting to deepen our understanding of stress in relation to hypo- and hypermentalizing (Sharp and Vanwoerden, 2014). Hypomentalizing can be explained as less Theory of Mind (ToM), which characterizes individuals having an insufficient mental state reasoning resulting in incorrect mental state attributions. Hypermentalizing can be interpreted as an excessive form of ToM where individuals make assumptions about a person's mental state that go far beyond what can be observed, and which often leads to a misinterpretation of the other person's behavior. Based on our results, it seems that hypermentalizing could play a role in how stress sensitive people are. It would be interesting to specifically test this, and also whether individuals who have a tendency towards hypomentalizing would be better protected against a stress-inducing environment.
To conclude, while sensitivity to social signals is generally considered a good social quality, it may also indicate a vulnerability to social evaluations, especially in men, which could become problematic in chronically stressful situations (Grippo and Johnson, 2009; Kessler et al., 2010; McEwen, 2017). Therefore, our findings should be further investigated in order to explore whether empathy, mentalizing abilities, and gender should be taken into account when training people in how to cope with stressful situations. Such insights are likely to be of relevance in creating healthy psychological work environments in a world of continually rising expectations and demands. With this study, we have taken an important step towards a better understanding of the role of empathy and mentalizing abilities, i.e. a more socially sensitive constitution, in psychological and physiological stress reactivity.
Declarations
Author contribution statement
M. Tollenaar: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
S. Overgaauw: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank Oscar Rabouw, Valerie Bakker, Mirjam Been, Evelyne Provily, and Claire Wielenga for their help in collecting the data for study 1, and Steven van Veelen, Johannes Osterloh, and Selai Mandozai for their help in collecting the data for study 2.
References
- Achim A.M., Guitton M., Jackson P.L., Boutin A., Monetta L. On what ground do we mentalize? Characteristics of current tasks and sources of information that contribute to mentalizing judgments. Psychol. Assess. 2013 doi: 10.1037/a0029137. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Hill J., Raste Y., Plumb I. The “Reading the Mind in the Eyes” test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatry Allied Discip. 2001;42(2):241–251. [PubMed] [Google Scholar]
- Bartz J.A., Zaki J., Bolger N., Ochsner K.N. Social effects of oxytocin in humans: context and person matter. Trends Cognit. Sci. 2011;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Beery A.K., Kaufer D. Stress, social behavior, and resilience: insights from rodents. Neurobiol. Stress. 2015 doi: 10.1016/j.ynstr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blascovich J., Mendes W.B., Hunter S.B., Salomon K. Social “facilitation” as challenge and threat. J. Pers. Soc. Psychol. 1999 doi: 10.1037//0022-3514.77.1.68. [DOI] [PubMed] [Google Scholar]
- Buchanan T.W., Preston S.D. Stress leads to prosocial action in immediate need situations. Front. Behav. Neurosci. 2014;8:5. doi: 10.3389/fnbeh.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J., Ehlert U. Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Childs E., Dlugos A., De Wit H. Cardiovascular, hormonal, and emotional responses to the TSST in relation to sex and menstrual cycle phase. Psychophysiology. 2010 doi: 10.1111/j.1469-8986.2009.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christov-Moore L., Simpson E.A., Coudé G., Grigaityte K., Iacoboni M., Ferrari P.F. Empathy: gender effects in brain and behavior. Neurosci. Biobehav. Rev. 2014 doi: 10.1016/j.neubiorev.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cialdini R.B., Goldstein N.J. Social influence: compliance and conformity. Annu. Rev. Psychol. 2004;55(1):591–621. doi: 10.1146/annurev.psych.55.090902.142015. [DOI] [PubMed] [Google Scholar]
- Davis M.H. Measuring individual differences in empathy: evidence for a multidimensional approach. J. Pers. Soc. Psychol. 1983;44(1):113–126. [Google Scholar]
- De Corte K., Buysse A., Verhofstadt L.L., Roeyers H., Ponnet K., Davis M.H. Measuring empathic tendencies: reliability and validity of the Dutch version of the interpersonal reactivity Index. Psychol. Belg. 2007;47:235–260. http://www.ingentaconnect.com/search/download?pub=infobike://acad/psyb/2007/00000047/00000004/art00002&mimetype=application/pdf Retrieved from. [Google Scholar]
- Dickerson S.S., Kemeny M.E. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004 doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Michel A., Berger C., Herpertz S.C. Oxytocin improves “mind-reading” in humans. Biol. Psychiatr. 2007;61(6):731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Dziobek I., Fleck S., Kalbe E., Rogers K., Hassenstab J., Brand M.…Convit A. Introducing MASC: a movie for the assessment of social cognition. J. Autism Dev. Disord. 2006;36:623–636. doi: 10.1007/s10803-006-0107-0. [DOI] [PubMed] [Google Scholar]
- Fairchild G., Hawes D.J., Frick P.J., Copeland W.E., Odgers C.L., Franke B.…De Brito S.A. Conduct disorder. Nat. Rev. Dis. Prim. 2019;5(1):43. doi: 10.1038/s41572-019-0095-y. [DOI] [PubMed] [Google Scholar]
- Fairchild G., van Goozen S.H.M., Stollery S.J., Brown J., Gardiner J., Herbert J., Goodyer I.M. Cortisol diurnal rhythm and stress reactivity in male adolescents with early-onset or adolescence-onset conduct disorder. Biol. Psychiatr. 2008 doi: 10.1016/j.biopsych.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman S., Lazarus R.S., Gruen R.J., DeLongis A. Appraisal, coping, health status, and psychological symptoms. J. Pers. Soc. Psychol. 1986 doi: 10.1037//0022-3514.50.3.571. [DOI] [PubMed] [Google Scholar]
- Frisch J.U., Häusser J.A., Mojzisch A. The Trier Social Stress Test as a paradigm to study how people respond to threat in social interactions. Front. Psychol. 2015;6:14. doi: 10.3389/fpsyg.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C.D., Frith U. Social cognition in humans. Curr. Biol. 2007 doi: 10.1016/j.cub.2007.05.068. [DOI] [PubMed] [Google Scholar]
- Gelkopf M., Berger R., Bleich A., Silver R.C. Protective factors and predictors of vulnerability to chronic stress: a comparative study of 4 communities after 7 years of continuous rocket fire. Soc. Sci. Med. 2012 doi: 10.1016/j.socscimed.2011.10.022. [DOI] [PubMed] [Google Scholar]
- Gordan R., Gwathmey J.K., Xie L.-H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015 doi: 10.4330/wjc.v7.i4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg N., Carr J.A., Summers C.H. Integrative and Comparative Biology. 2002. Causes and consequences of stress. [DOI] [PubMed] [Google Scholar]
- Grippo A.J., Johnson A.K. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress (Amsterdam, Netherlands) 2009;12(1):1–21. doi: 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M., von Dawans B., Domes G. Oxytocin, vasopressin, and human social behavior. Front. Neuroendocrinol. 2009;30(4):548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Hooker C.I., Verosky S.C., Germine L.T., Knight R.T., D’Esposito M. Mentalizing about emotion and its relationship to empathy. Soc. Cognit. Affect. Neurosci. 2008 doi: 10.1093/scan/nsn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M., Karst H., Sarabdjitsingh R.A. The stressed brain of humans and rodents. Acta Physiol. 2018;223(2) doi: 10.1111/apha.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajantie E., Phillips D.I.W. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006 doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kelly M.M., Tyrka A.R., Price L.H., Carpenter L.L. Sex differences in the use of coping strategies: predictors of anxiety and depressive symptoms. Depression Anxiety. 2008 doi: 10.1002/da.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., McLaughlin K.A., Green J.G., Gruber M.J., Sampson N.A., Zaslavsky A.M.…Williams D.R. Childhood adversities and adult psychopathology in the WHO world mental health surveys. Br. J. Psychiatry. 2010;197(5):378–385. doi: 10.1192/bjp.bp.110.080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C., Pirke K.M., Hellhammer D.H. The “Trier Social Stress Test” - a tool for investigating psychobiological stress responses in a labor atory setting. Neuropsychobioloy. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum Clemens, Kudielka B.M., Gaab J., Schommer N.C., Hellhammer D.H. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal Axis. Psychosom. Med. 1999;61(2):154–162. doi: 10.1097/00006842-199903000-00006. http://www.psychosomaticmedicine.org/content/61/2/154.long Retrieved from. [DOI] [PubMed] [Google Scholar]
- Laviola G., Zoratto F., Ingiosi D., Carito V., Huzard D., Fiore M., Macrì S. Low empathy-like behaviour in male mice associates with impaired sociability, emotional memory, physiological stress reactivity and variations in neurobiological regulations. PloS One. 2017 doi: 10.1371/journal.pone.0188907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F.X., Berjot S., Deschamps F. Clinical stress assessment using a visual analogue scale. Occup. Med. 2012 doi: 10.1093/occmed/kqs140. [DOI] [PubMed] [Google Scholar]
- Liu J.J.W., Ein N., Peck K., Huang V., Pruessner J.C., Vickers K. Sex differences in salivary cortisol reactivity to the Trier Social Stress Test (TSST): a meta-analysis. Psychoneuroendocrinology. 2017;82:26–37. doi: 10.1016/j.psyneuen.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Margittai Z., Strombach T., van Wingerden M., Joëls M., Schwabe L., Kalenscher T. A friend in need: time-dependent effects of stress on social discounting in men. Horm. Behav. 2015;73:75–82. doi: 10.1016/j.yhbeh.2015.05.019. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Mood disorders and allostatic load. Biol. Psychiatr. 2003;54(3):200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Neurobiological and systemic effects of chronic stress. Chronic Stress. 2017;1 doi: 10.1177/2470547017692328. 247054701769232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers M., Montag C., Markett S., Reuter M. Assessment of empathy via self-report and behavioural paradigms: data on convergent and discriminant validity. Cognit. Neuropsychiatry. 2015 doi: 10.1080/13546805.2014.991781. [DOI] [PubMed] [Google Scholar]
- Neumann I.D., Slattery D.A. Oxytocin in general anxiety and social fear: a translational approach. Biol. Psychiatr. 2015 doi: 10.1016/j.biopsych.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S., Forstmann B.U., Wagenmakers E.-J. Erroneous analyses of interactions in neuroscience: a problem of significance. Nat. Neurosci. 2011 doi: 10.1038/nn.2886. [DOI] [PubMed] [Google Scholar]
- Overgaauw S., Rieffe C., Broekhof E., Crone E.A., Güroglu B. Assessing empathy across childhood and adolescence: validation of the empathy questionnaire for children and adolescents (EmQue-CA) Front. Psychol. 2017 doi: 10.3389/fpsyg.2017.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P., Kalisch R., Singer T., Dolan R.J. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J. Neurosci. 2008 doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raio C.M., Konova A.B., Otto A.R. Trait impulsivity and acute stress interact to influence choice and decision speed during multi-stage decision-making. Sci. Rep. 2020 doi: 10.1038/s41598-020-64540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay D., Lewis M. Reactivity and regulation in cortisol and behavioral responses to stress. Child Dev. 2003 doi: 10.1111/1467-8624.7402009. [DOI] [PubMed] [Google Scholar]
- Reschke-Hernández A.E., Okerstrom K.L., Bowles Edwards A., Tranel D. Sex and stress: men and women show different cortisol responses to psychological stress induced by the Trier social stress test and the Iowa singing social stress test. J. Neurosci. Res. 2017;95(1–2):106–114. doi: 10.1002/jnr.23851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues S.M., LeDoux J.E., Sapolsky R.M. The influence of stress hormones on fear circuitry. Annu. Rev. Neurosci. 2009;32(1):289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Rueckert L. Psychology of Empathy. 2011. Gender differences in empathy. [Google Scholar]
- Russell G., Lightman S. The human stress response. Nat. Rev. Endocrinol. 2019 doi: 10.1038/s41574-019-0228-0. [DOI] [PubMed] [Google Scholar]
- Sandi C., Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat. Rev. Neurosci. 2015;16(5):290–304. doi: 10.1038/nrn3918. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. Individual differences and the stress response. Semin. Neurosci. 1994 doi: 10.1007/978-1-4899-2064-5_31. [DOI] [PubMed] [Google Scholar]
- Schiffer B., Pawliczek C., Müller B.W., Gizewski E.R., Walter H. Why don’t men understand women? Altered neural networks for reading the language of male and female eyes. PloS One. 2013 doi: 10.1371/journal.pone.0060278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T.R., Lyons J.B., Khazon S. 2013. Emotional Intelligence and resilience. Personality and Individual Differences. [Google Scholar]
- Sharp C., Vanwoerden S. Social cognition: empirical contribution: the developmental building blocks of psychopathic traits: revisiting the role of theory of mind. J. Pers. Disord. 2014 doi: 10.1521/pedi.2014.28.1.78. [DOI] [PubMed] [Google Scholar]
- Singer T., Snozzi R., Bird G., Petrovic P., Silani G., Heinrichs M., Dolan R.J. Effects of oxytocin and prosocial behavior on brain responses to direct and vicariously experienced pain. Emotion. 2008 doi: 10.1037/a0014195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets T., Dziobek I., Wolf O.T. Social cognition under stress: differential effects of stress-induced cortisol elevations in healthy young men and women. Horm. Behav. 2009;55(4):507–513. doi: 10.1016/j.yhbeh.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D. State-trait anxiety inventory (STAI) Mind Garden. 1983;94061(650):261–3500. [Google Scholar]
- Stephens M.A.C., Mahon P.B., McCaul M.E., Wand G.S. Hypothalamic–pituitary–adrenal axis response to acute psychosocial stress: effects of biological sex and circulating sex hormones. Psychoneuroendocrinology. 2016;66:47–55. doi: 10.1016/j.psyneuen.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud L.R., Salovey P., Epel E.S. Sex differences in stress responses: social rejection versus achievement stress. Biol. Psychiatr. 2002 doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Tamres L.K., Janicki D., Helgeson V.S. Sex differences in coping behavior: a meta-analytic review and an examination of relative coping. Pers. Soc. Psychol. Rev. 2002 [Google Scholar]
- Tollenaar M.S., Beijers R., Jansen J., Riksen-Walraven J.M.A., De Weerth C. Maternal prenatal stress and cortisol reactivity to stressors in human infants. Stress. 2011;14(1) doi: 10.3109/10253890.2010.499485. [DOI] [PubMed] [Google Scholar]
- Tomova L., Majdandžić J., Hummer A., Windischberger C., Heinrichs M., Lamm C. Increased neural responses to empathy for pain might explain how acute stress increases prosociality. Soc. Cognit. Affect. Neurosci. 2017 doi: 10.1093/scan/nsw146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomova L., Von Dawans B., Heinrichs M., Silani G., Lamm C. Is stress affecting our ability to tune into others? Evidence for gender differences in the effects of stress on self-other distinction. Psychoneuroendocrinology. 2014;43:95–104. doi: 10.1016/j.psyneuen.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Toussaint L., Webb J.R. Gender differences in the relationship between empathy and forgiveness. J. Soc. Psychol. 2005 doi: 10.3200/SOCP.145.6.673-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M., Chong R.Y., Oswald L., Lin P.I., Wand G.S. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 2006 doi: 10.1016/j.psyneuen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Van der Graaff J., Branje S., De Wied M., Hawk S., Van Lier P., Meeus W. Perspective taking and empathic concern in adolescence: gender differences in developmental changes. Dev. Psychol. 2014;50(3):881–888. doi: 10.1037/a0034325. [DOI] [PubMed] [Google Scholar]
- van Honk J., Schutter D.J., Bos P. a, Kruijt A.-W., Lentjes E.G., Baron-Cohen S. Testosterone administration impairs cognitive empathy in women depending on second-to-fourth digit ratio. Proc. Natl. Acad. Sci. U.S.A. 2011;108:3448–3452. doi: 10.1073/pnas.1011891108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Polier G.G., Herpertz-Dahlmann B., Konrad K., Wiesler K., Rieke J., Heinzel-Gutenbrunner M.…Vloet T.D. Reduced cortisol in boys with early-onset conduct disorder and callous-unemotional traits. BioMed Res. Int. 2013 doi: 10.1155/2013/349530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L. a, Tellegen a. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Zänkert S., Bellingrath S., Wüst S., Kudielka B.M. HPA axis responses to psychological challenge linking stress and disease: what do we know on sources of intra- and interindividual variability? Psychoneuroendocrinology. 2018 doi: 10.1016/j.psyneuen.2018.10.027. [DOI] [PubMed] [Google Scholar]